Introduction
Atherosclerosis has been shown to cause
cardiovascular diseases that contribute to morbidity and mortality
in developed and developing countries (1,2).
Atherosclerosis is defined as a complex inflammatory response
characterized by the accumulation of lipid in arteries (3,4).
Monocyte/macrophages migrate into the intima and engulf modified
low-density lipoproteins (LDLs) such as oxidized LDL (oxLDL) or
acetyl LDL (Ac-LDL) via scavenger receptors (SRs) and then
transform into foam cells (5–7).
These are the initial events in the development of
atherosclerosis.
Several SRs including SR-AI/II, SR-BI, cluster of
differentiation 36 (CD36), and lectin-like oxidized low-density
lipoprotein receptor-1 (LOX-1) mediate the transport of oxLDL into
macrophages, which results in lipid accumulation and the
transformation of macrophages into foam cells (6,8).
LOX-1 is a type II membrane protein with an
extracellular domain and a short cytoplasmic tail (9). LOX-1 has been reported to be
expressed in endothelial cells, monocyte/macrophages, platelets,
and vascular smooth muscle cells (VSMCs) as well as in renal,
pulmonary and neuronal tissues. LOX-1 expression can be induced by
oxLDL, free radicals (reactive oxygen species), endothelin-1
(ET-1), angiotensin II, advanced glycation end-products (AGEs) and
shear stress (10–12). Furthermore, various pathological
conditions including diabetes mellitus, hypertension, myocardial
ischemia and atherosclerosis contribute to the induction of LOX-1
expression (13,14).
ET-1 has been suggested to be involved in the
pathogenesis of cardiovascular diseases. It is well known that the
plasma level of ET-1 is increased in patients with hypertension and
coronary artery disease (15,16). Studies have demonstrated that
local ET-1 concentrations are increased in the atherosclerotic
plaques (17,18). Furthermore, ET-1 receptor blockade
has been shown to reduce the development of atherosclerotic lesions
in an atherosclerotic animal model, apoE-KO mice (17). Morawietz et al have shown
that ET-1 induces LOX-1 mRNA and protein expression in a time- and
dose-dependent manner in human endothelial cells and promotes oxLDL
uptake (19). ET-1, exclusively
expressed in endothelial cells, enhances the oxidative modification
of LDL via the ETB receptor, which further increases the uptake of
oxLDL in endothelial cells via the LOX-1 receptor leading to the
progression of atherosclerosis (20).
Natural compounds have been demonstrated to inhibit
LOX-1 expression. These compounds include tanshinone II-A (21), curcumin (22), berberine (23), epigallocatechin gallate (EGCG)
(24), and resveratrol (25). Berberine is the primary component
of rhizoma coptidis and is often used as an anti-inflammatory
medicine (26). Berberine has
been shown to significantly inhibit low-density
lipoprotein-cholesterol (LDL-C) synthesis in human hepatocytes by
increasing AMP-activated protein kinase (AMPK) phosphorylation and
AMPK activity (27). In addition,
berberine significantly decreased the expression of LOX-1 and
increased SR-BI expression in a time- and dose-dependent manner
(23).
It is well established that atorvastatin
(3-hydroxy-3-methyl-glutaryl-coenzyme, a reductase inhibitor)
suppresses intracellular cholesterol synthesis and it has been
widely used as an anti-inflammatory drug in the treatment of
atherosclerosis (28).
Atorvastatin has been shown to reduce the activation of
transcription factor NF-κB in cultured VSMCs as well as in
atherosclerotic lesions in rabbit (29).
In the present study, we aimed to investigate the
effect of berberine combined with atorvastatin on atherosclerosis
and the underlying molecular mechanism involved. We found that the
expression of LOX-1 in monocyte/macrophages treated with berberine
(0, 0.1, 1, 10 or 100 nM) combined with atorvastatin (100 nM) was
significantly decreased in a dose-dependent manner. Knockdown of
the ET-1 receptor by small-interfering (siRNA) transfection
significantly reversed the inhibitory effect of berberine on LOX-1
expression in monocyte-derived macrophages (MDMs). A rat model
induced with a high-fat diet (HFD) was also used to analyze the
regulation of LOX-1 expression. Treatment with berberine combined
with atorvastatin markedly influenced physiological parameters,
lipid profile, inflammation and oxidative stress in the rat model.
In addition, the inhibitory effect of berberine on LOX-1 expression
was blocked by an ET-1 receptor antagonist in the rat model.
Materials and methods
Cell culture
MDMs were isolated from peripheral blood monocytes
by adherence to plastic as described previously (30). Blood was layered onto Lymphoprep
(Axis-Shield, Dundee, Scotland) and centrifuged for 30 min at 700
g. The white-blood-cell layer was harvested, washed with PBS and
suspended in RPMI-1640. Cells were counted and then plated at
1×106 cells per 140 mm dish in RPMI-1640 with 5%
heat-inactivated human serum. After 2 h, the plates were washed
three times in RPMI-1640 and then incubated at 37°C overnight. The
cells were left to differentiate into MDMs for 7 days, then washed
with PBS, treated with 5 mM PBS/EDTA at 37°C for 20 min, harvested
gently with a cell scraper, counted and replated on 96- or 6-well
trays at 1×104 and 1×106 cells per well,
respectively, as described previously (31).
Animals
One hundred and twenty 8-week-old male
Sprague-Dawley rats were purchased from Shanghai SLAC Laboratory
Animal Co., Ltd. (Shanghai, China). The animals were housed under
standard conditions of a 12/12 h light/dark cycle at room
temperature with a HFD (MD12033; Mediscience, Ltd., Jiangsu,
China), and free access to water. All animal experimental
procedures were conducted under the guidelines of the National
Health and Medical Research Council for the Care and Use of Animals
for Experimental Purposes in China. All efforts were made to
minimize suffering.
Experimental design
MDMs were plated in triplicate into 12-well cell
culture plates (Takara Biotechnology (Dalian), Co., Ltd., Dalian,
China). The experimental regime consisted of cells undergoing a
preconditioning phase of: i) 100 μl vehicle for 4 h; ii) 0.1 nmol
berberine and 100 nmol atorvastatin for 4 h; iii) 1 nmol berberine
and 100 nmol atorvastatin for 4 h; iv) 10 nmol berberine and 100
nmol atorvastatin for 4 h; v) 100 nmol berberine and 100 nmol
atorvastatin for 4 h; vi) transfection with non-specific siRNA
followed by the addition of 100 μl vehicle for 4 h; vii)
transfection with non-specific siRNA followed by the addition of
100 nmol berberine and 100 nmol atorvastatin for 4 h; viii)
transfection with specific siRNA targeting the ET-1 receptor
followed by the addition of 100 nmol berberine and 100 nmol
atorvastatin for 4 h. One hundred and twenty male Sprague-Dawley
rats were randomly assigned to 6 groups and fed a HFD for 4 months
prior to initiation of mimic atherosclerosis. Subsequently, the
rats were exposed to treatment as follows: i) vehicle for 1 month
(i.v.); ii) 0.1 μmol/kg berberine and 100 μmol/kg atorvastatin for
1 month (i.v.); iii) 1 μmol/kg berberine and 100 μmol/kg
atorvastatin for 1 month (i.v.); iv) 10 μmol/kg berberine and 100
μmol/kg atorvastatin for 1 month (i.v.); v) 100 μmol/kg berberine
and 100 μmol/kg atorvastatin for 1 month (i.v.); vi) 100 μg/kg/min
BQ-788 (i.v.) followed by 100 μmol/kg berberine and 100 μmol/kg
atorvastatin for 1 month (i.v.).
At the end of the treatment, body weight (BW) was
measured and the animals were anesthetized with 10% chloral
hydrate. Blood was collected by cardiac puncture. Organs such as
heart, liver, kidneys and spleen were harvested and weighed.
Knockdown of ET-1 receptor by siRNA
Scrambled siRNA and siRNA targeting the ET-1
receptor were purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). Cells were transfected with scrambled or ET-1
receptor siRNA according to the manufacturer’s instructions.
Briefly, the ET-1 receptor and scrambled siRNAs (30 pmol) were
diluted in 500 μl DMEM and mixed with 5 μl Lipofectamine RNAi MAX
(Invitrogen Life Technologies, Carlsbad, CA, USA). After 15 min
incubation at room temperature, the complexes were added to the
cells to a final volume of 3 ml medium. The cells were then
harvested at the indicated times for further analysis. The
efficiency of the ET-1 receptor siRNA was confirmed by western blot
analysis of Flag expression.
Detection of total cholesterol (TC),
triglycerides (TGs), LDL-C and high-density lipoprotein-cholesterol
(HDL-C)
Blood samples were collected and the levels of TC,
TG, LDL-C, and HDL-C were detected with an automatic biochemistry
analyzer (Hitachi, Tokyo, Japan). The samples were analyzed in
duplicate.
Detection of C-reactive protein (CRP),
malondialdehyde (MDA), glutathione peroxidase (GPx) and superoxide
dismutase (SOD)
The CRP levels were determined with an
ultrasensitive CRP test with a coefficient of variance below 5%
(Sigma, St. Louis, MO, USA). A Biochemical Analysis kit (Nanjing
Jiancheng Biotechnology Co., Ltd., Nanjing, China) was used to
measure MDA content, GPx, and SOD activity according to the
manufacturer’s instructions.
Enzyme-linked immunosorbent assay (ELISA)
analysis for ET-1
The levels of ET-1 protein in serum were analyzed
using a commercially available ELISA (Yanjin Biotechnology Co.,
Shanghai, China) according to the manufacturer’s instructions. The
absorbance was read at 450 nm using a 680XR microplate reader
(Bio-Rad, Hercules, CA, USA). All the samples were analyzed in
duplicate. The standard curve for ET-1 estimation was conducted by
linear regression analysis.
RNA extraction and quantitative reverse
transcription polymerase chain reaction (qRT-PCR)
RNA was extracted from MDMs or monocytes using
TRIzol RNA-extraction reagent (Gibco-BRL, Rockville, MD, USA)
according to the manufacturer’s instructions. Total RNA (5 μg) for
each sample was reverse transcribed into first-strand cDNA for
qRT-PCR analysis. qRT-PCR was performed in a final volume of 10 μl,
which contained 5 μl of SsoFastTM EvaGreen supermix
(Bio-Rad), 1 μl of cDNA (1:50 dilution), and 2 μl each of the
forward and reverse primers (1 mM). The steps in qRT-PCR were
performed as follows: 94°C for 2 min for initial denaturation; 94°C
for 20 sec, 58°C for 15 sec, and 72°C for 15 sec; 2 sec for plate
reading for 40 cycles; and a melt curve from 65 to 95°C. β-actin
was used as a quantitative and qualitative control to normalize the
gene expression. Data were analyzed using the formula: R = 2−
[ΔCT sample−ΔCT control]. All of the primers used in this
experiment are shown in Table
I.
 | Table IList of primers for qPCR
analysis. |
Table I
List of primers for qPCR
analysis.
| Gene | Primers |
|---|
| LOX-1 | F: 5′-GAA CGT TTG
CCT GGG ATT AGT A-3′
R: 5′-CTG GTG GTG AAG TTC CAT TTG G-3′ |
| ET-1 receptor | F:
5′-GATACGACAACTTCCGCTCCA-3′
R: 5′-GTCCACGATGAGGACAATGAG-3′ |
| β-actin | F: 5′-GTG GGG CGC
CCC AGG CACCA-3′
R: 5′-CTC CTT AAT GTC ACG CAC GAT TTC-3′ |
Western blot analysis
Cells were homogenized and lysed with RIPA lysis
buffer (100 mM NaCl, 50 mM Tris-HCl pH 7.5, 1% TritonX-100, 1 mM
EDTA, 10 mM β-glycerophosphate, 2 mM sodium vanadate and protease
inhibitor). Protein concentration was assayed using a micro-BCA
protein kit (Pierce Biotechnology, Inc., Rockford, IL, USA). Forty
micrograms of protein per lane were separated by 12% SDS-PAGE and
electroblotted onto nitrocellulose (Amersham Pharmacia Biotech,
Freiburg, Germany). Non-specific binding was blocked by incubating
with 5% non-fat milk in TBST buffer at room temperature for 1 h.
Immunodetection of LOX-1 and β-actin was conducted using mouse
monoclonal anti-LOX-1 antibody (1:1,000; Santa Cruz Biotechnology,
Inc.), and anti-β-actin (Sigma), respectively. Goat anti-mouse IgG
(1:5,000; Sigma) followed by enhanced chemiluminescence (ECL;
Amersham Pharmacia Biotech, NJ, USA) were used for the detection of
β-actin.
Statistical analysis
Results are expressed as means ± SD. Statistical
significance was analyzed with one-way factorial ANOVA or the
Student’s two-tailed t-test. P<0.05 was considered statistically
significant. Analyses were conducted using SPSS software (SPSS,
Inc., Chicago, IL, USA).
Results
Berberine combined with atorvastatin
downregulates the expression of LOX-1 in MDMs
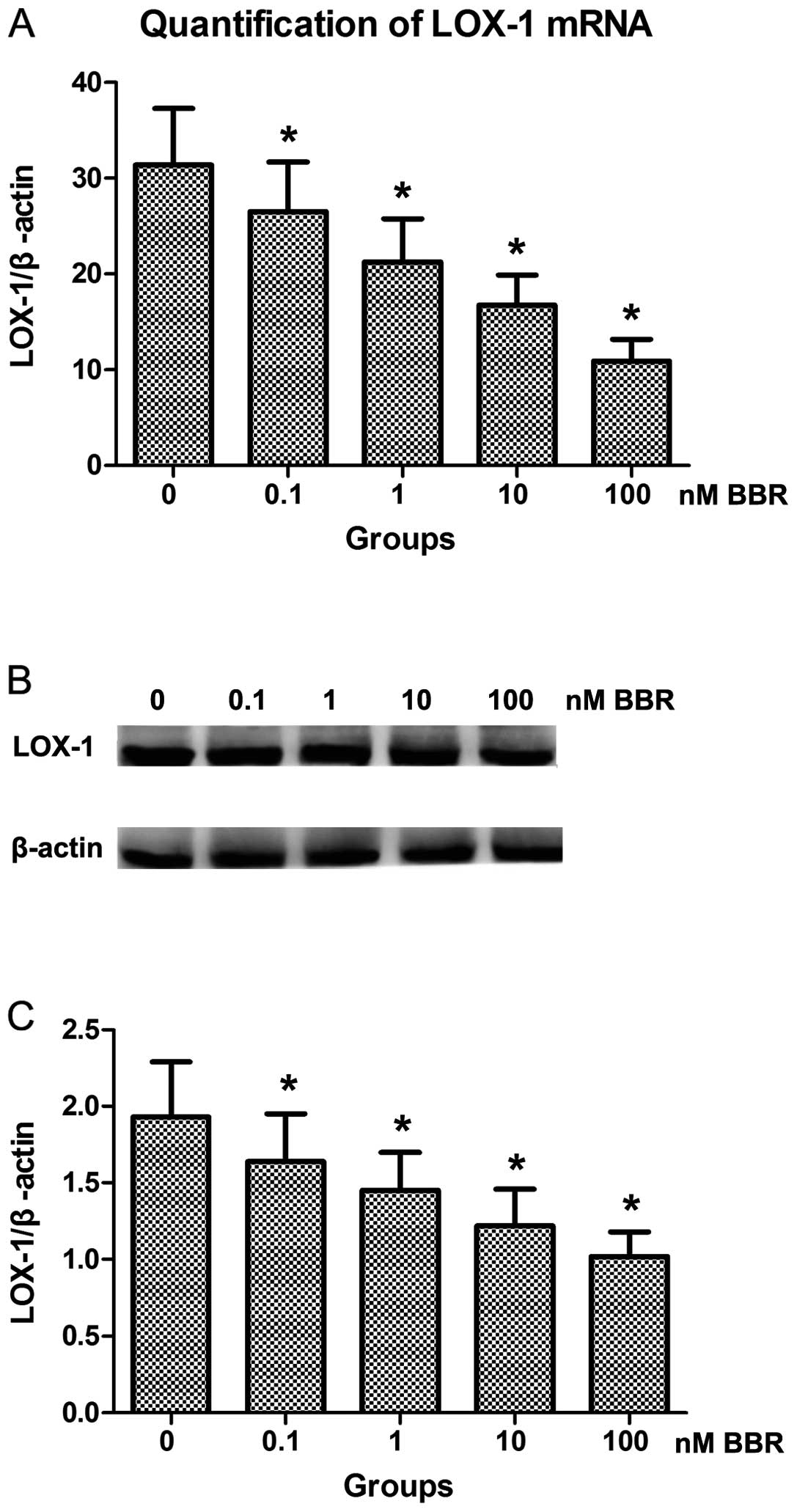
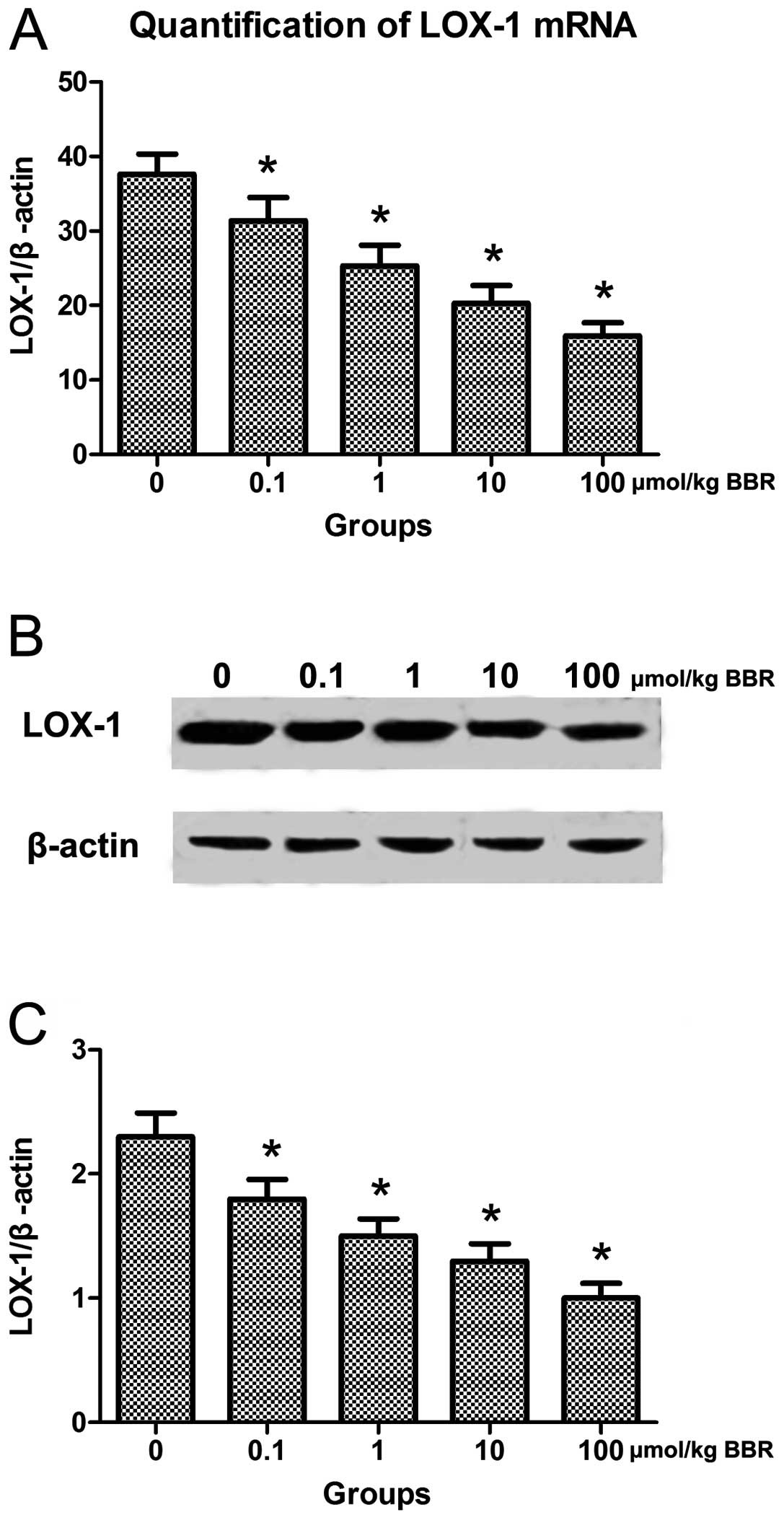
To investigate the effect of berberine combined with
atorvastatin on the expression of LOX-1, the two genes were
analyzed by qRT-PCR and western blot analysis. The qRT-PCR results
showed that the mRNA level of LOX-1 tended to decline as the amount
of berberine increased by 10 to 1,000-fold (Fig. 1A). This result was confirmed by
western blot analysis (Fig. 1B and
C).
The ET-1 receptor mediates the inhibitory
effect of berberine combined with atorvastatin on LOX-1 expression
in MDMs
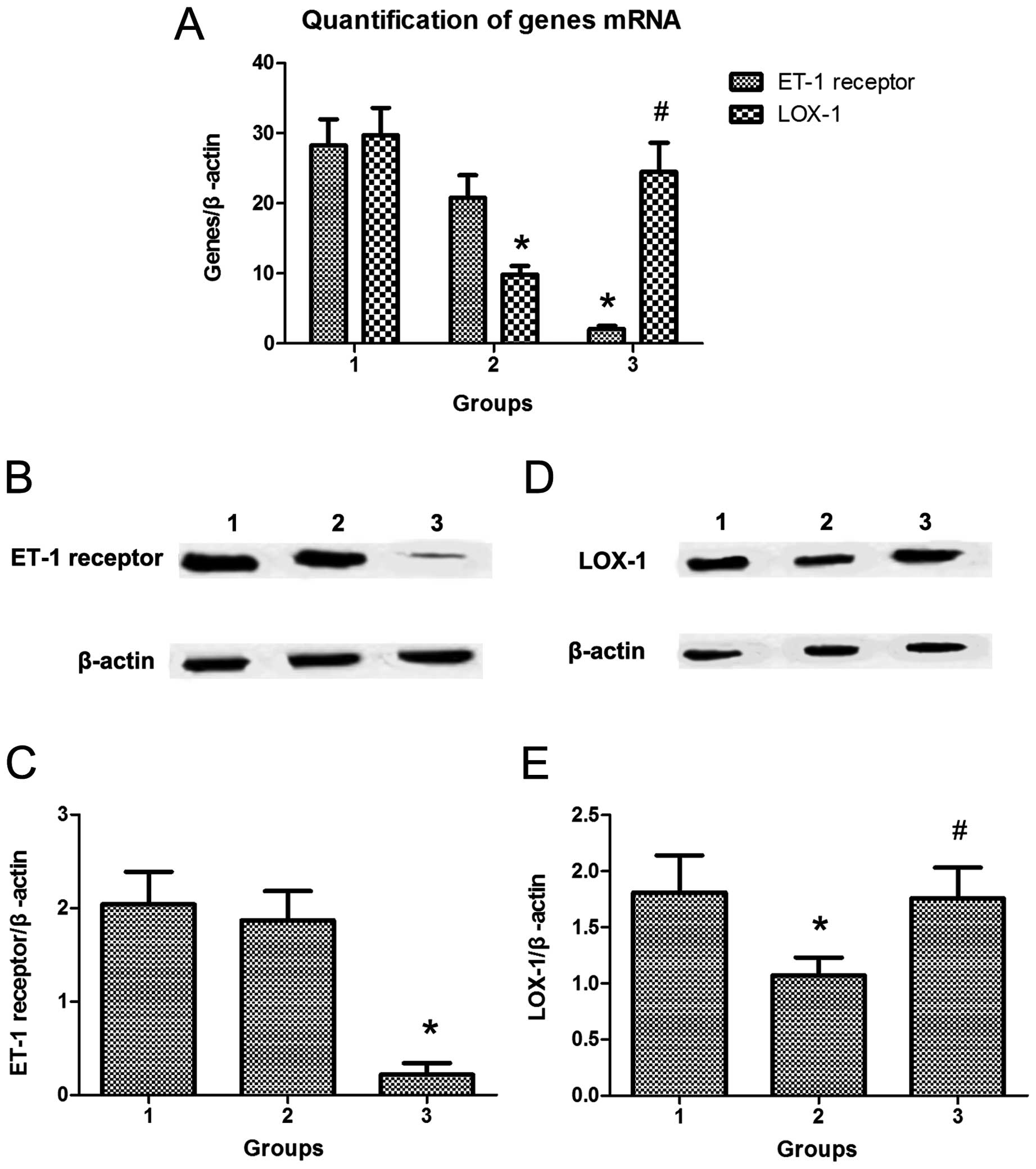
ET-1 has been shown to regulate the expression of
LOX-1 through the ET-1 receptor in endothelial cells. To examine
the involvement of the ET-1 receptor in the regulation of LOX-1
expression in MDMs, we transfected ET-1 receptor siRNA into MDMs.
Following treatment with berberine and atorvastatin, the expression
of LOX-1 mRNA was analyzed by qRT-PCR and western blot analysis.
Transfection with siRNA significantly blocked the inhibitory effect
of berberine combined with atorvastatin on LOX-1 mRNA expression
(Fig. 2A), which was confirmed by
western blot analysis (Fig. 2B and
C).
Berberine combined with atorvastatin
influences physiological parameters in model rats
To explore the effect of berberine combined with
atorvastatin on the physiological parameters of the model rats, the
BW, heart weight (HW), liver weight (LW), spleen weight (SW), and
kidney weight (KW) of rats in different groups were calculated at
the end of the treatment. BW, LW and KW were markedly increased at
concentrations of berberine ≥1 μmol/kg, while HW and SW remained
constant for all the groups. The BW gains were 7.6, 11.4 and 16.1%,
the LW gains were 29.3, 43.9 and 48.8%, and the KW gains were 41.7,
62.5 and 66.7% in the 1, 10 and 100 μmol/kg berberine groups
compared to the control (0 μmol/kg berberine group), respectively
(Table II).
 | Table IIBody and organ weight of animals. |
Table II
Body and organ weight of animals.
| Groups | No. | BW (g) | HW (g) | LW (g) | SW (g) | KW (g) |
|---|
| 0 | 20 | 392.5±22.8 | 2.3±0.7 | 4.1±0.7 | 0.9±0.2 | 2.4±0.6 |
| 0.1 | 20 | 401.3±27.5 | 2.5±0.5 | 4.6±0.6 | 1.1±0.3 | 2.9±0.7 |
| 1 | 20 | 422.7±28.7a | 2.4±0.5 | 5.3±0.8a | 1.2±0.3 | 3.4±0.8a |
| 10 | 20 | 437.6±29.4a | 2.6±0.6 | 5.9±0.6a | 1.1±0.2 | 3.9±0.8a |
| 100 | 20 | 455.9±29.7a | 2.5±0.8 | 6.1±0.9a | 1.3±0.4 | 4.0±1.1a |
Berberine combined with atorvastatin
alters serum TC, TG, LDL-C and HDL-C levels in model rats
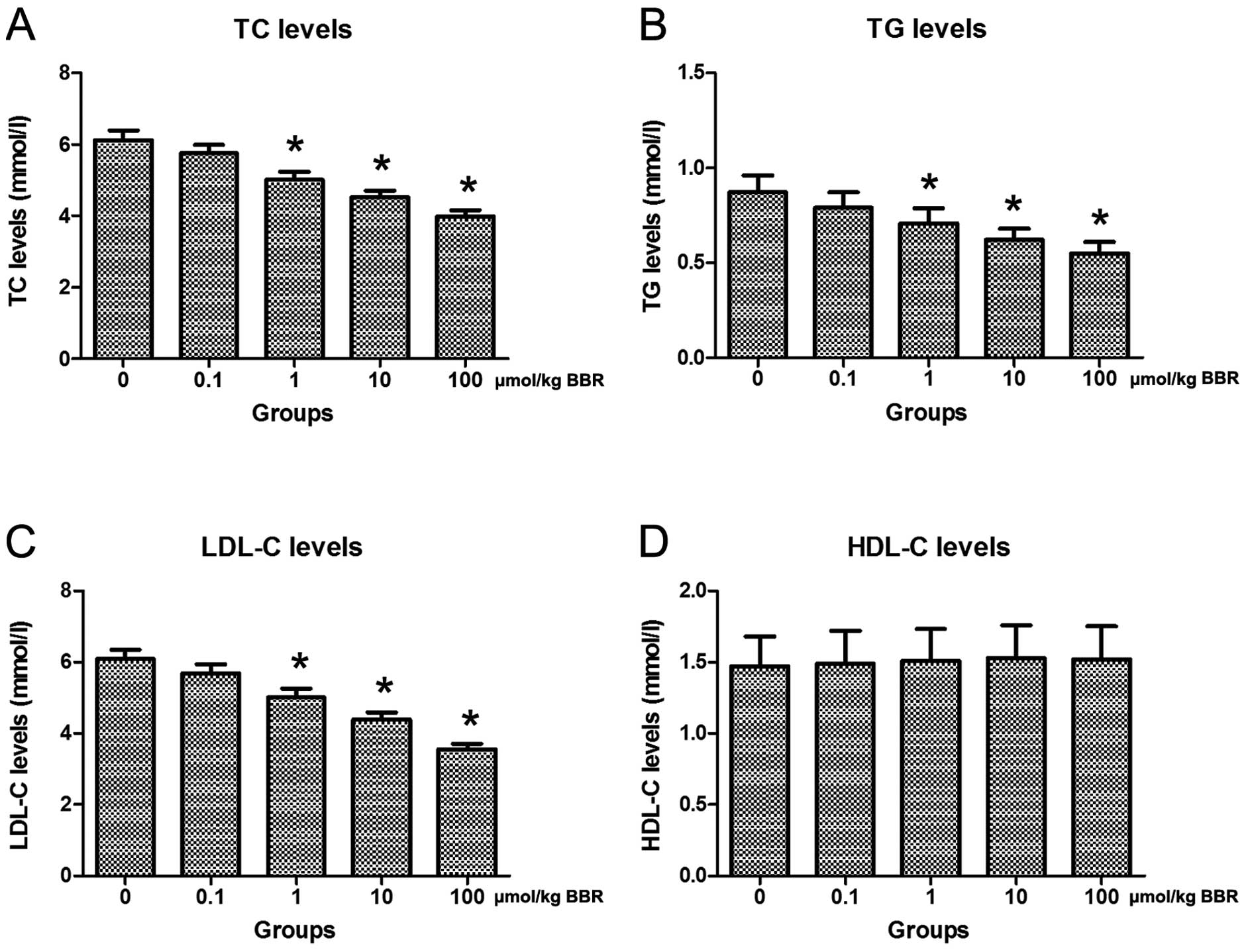
To investigate variations in serum lipid profiles in
model rats treated with berberine and atorvastatin, serum TC, TG,
LDL-C and HDL-C levels were monitored via an automatic biochemistry
analyzer at the end of the treatment. Compared to the control group
(0 μmol/kg), treatment with berberine in combination with
atorvastatin notably decreased serum TC, TG and LDL-C levels in
rats (Fig. 3A–C). However, no
significant difference in the serum level of HDL-C was detected
among rats in the different groups (Fig. 3D).
Berberine combined with atorvastatin
attenuates inflammation and oxidative stress in model rats
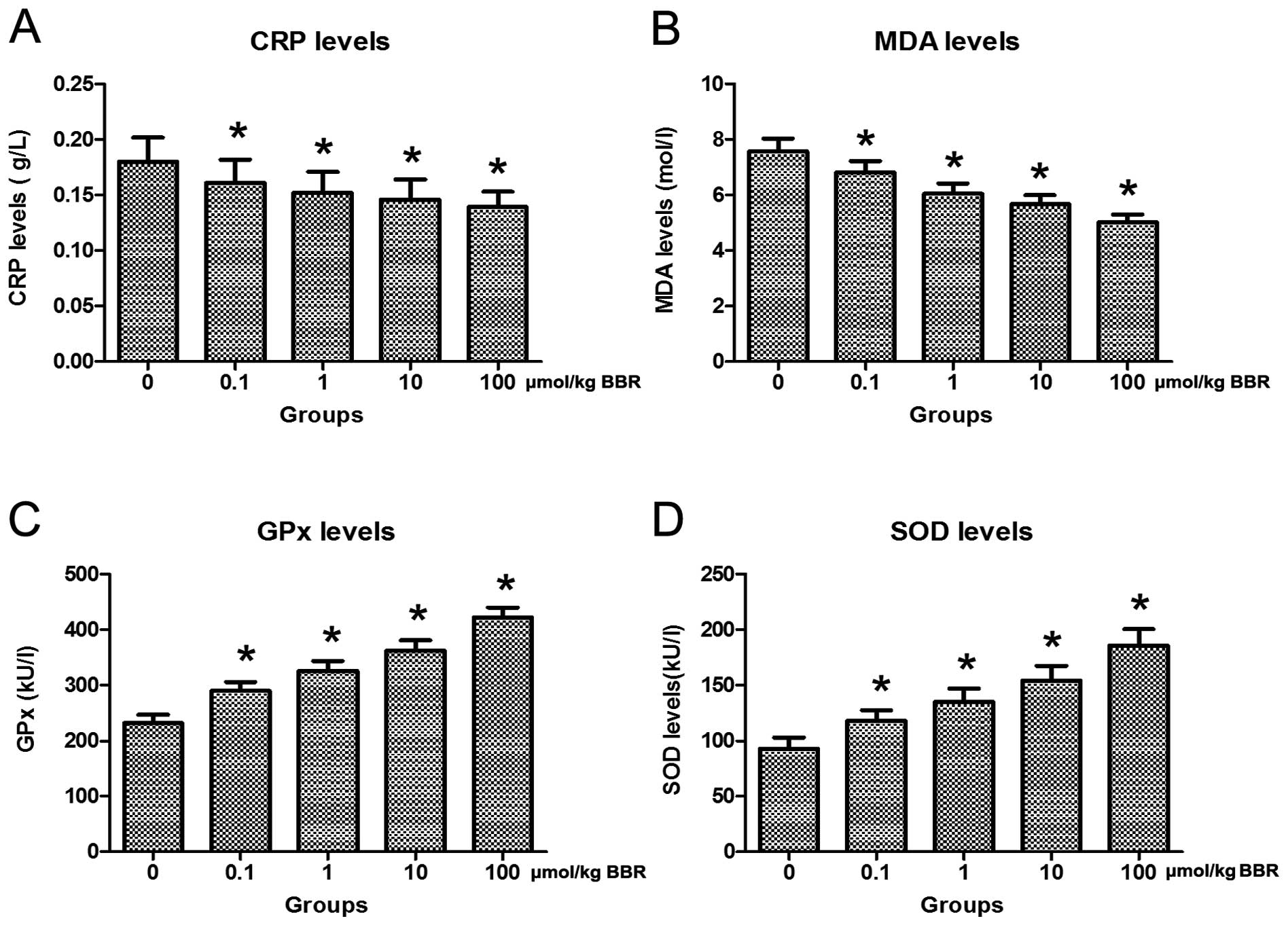
To validate whether treatment with berberine in
combination with atorvastatin affected inflammation and oxidative
stress in model rats, serum CRP, MDA, GPx and SOD were measured
using commercial kits. The results showed that treatment with
berberine in combination with atorvastatin distinctly reduced serum
CRP and MDA levels and promoted serum GPx and SOD levels in the
model rats (Fig. 4).
Berberine combined with atorvastatin
decreases plasma ET-1 level and the expression of LOX-1 in
monocytes in model rats
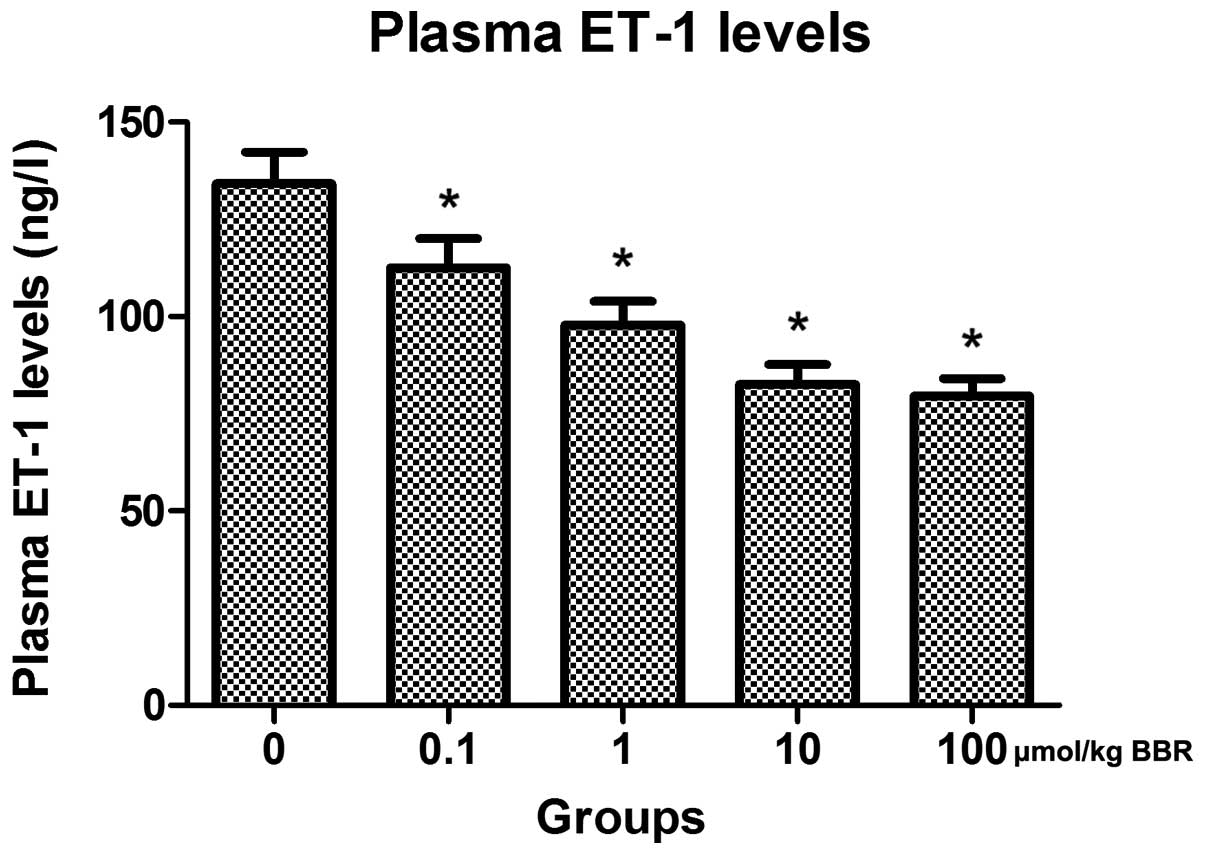
To explore variations in plasma ET-1 levels, ELISA
was performed on rats in each group. Treatment provoked a marked
decrease in the plasma ET-1 level compared to the control group
(Fig. 5). Additionally, the
expression of LOX-1 in monocytes was analyzed by qRT-PCR and
western blot analysis. Compared to the control group, berberine in
combination with atorvastatin significantly downregulated the
expression of LOX-1 in monocytes (Fig. 6).
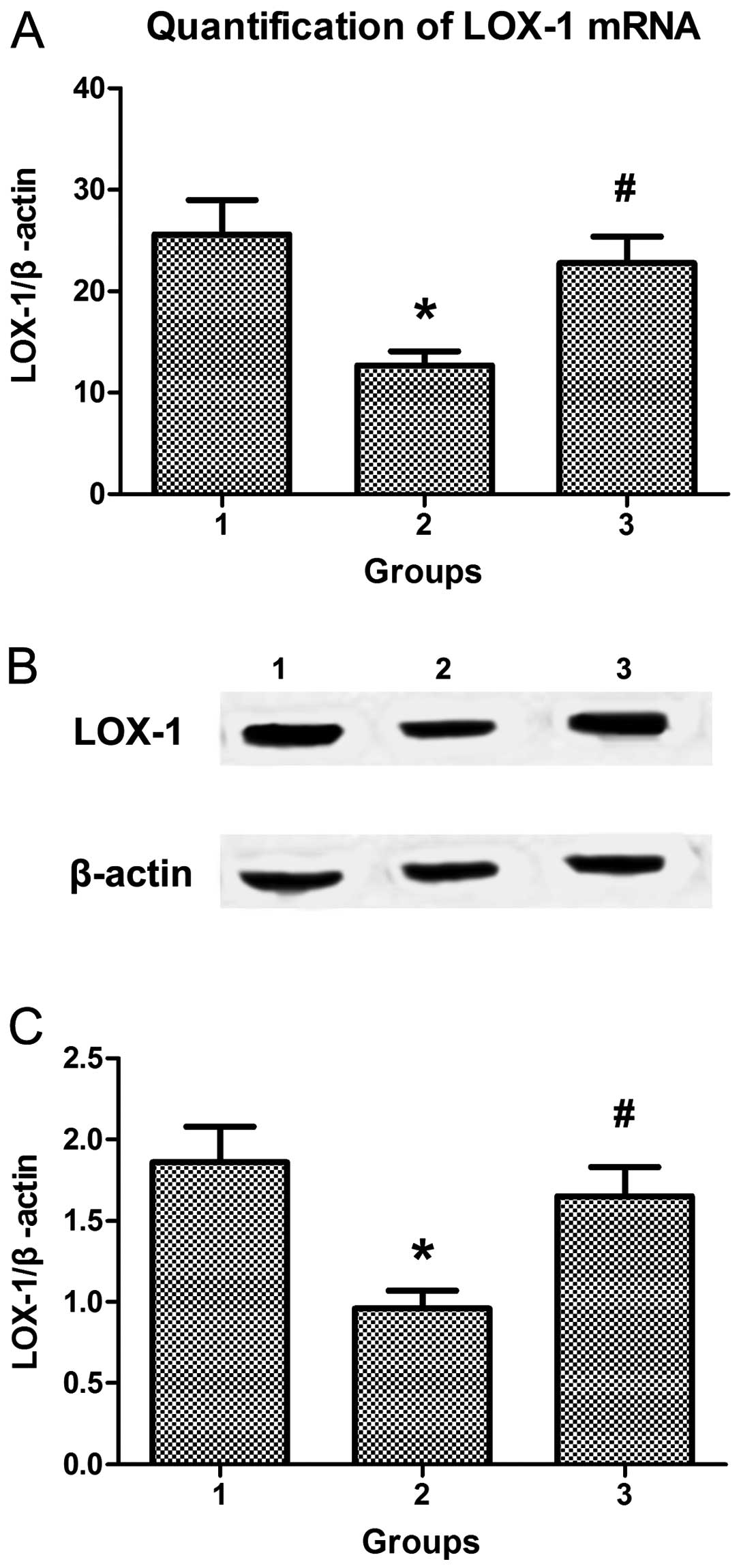
An ET-1 receptor antagonist abolishes the
inhibitory effect of berberine combined with atorvastatin on LOX-1
expression in monocytes from model rats
To examine whether the ET-1 receptor was involved in
the regulation of LOX-1 expression by berberine and atorvastatin,
model rats were preconditioned with an ET-1 receptor antagonist
prior to oral uptake of berberine and atorvastatin. Compared to the
control, treatment with berberine in combination with atorvastatin
led to the downregulation of LOX-1 expression in monocytes. By
contrast, ET-1 receptor antagonist preconditioning eliminated the
inhibitory effect of berberine combined with atorvastatin and
resulted in an increased expression of LOX-1 in monocytes (Fig. 7).
Discussion
Numerous studies have shown that the oxidative
modification of oxLDL is extremely relevant in atherogenesis
(32,33). oxLDL can be vigorously absorbed by
macrophages via receptor-mediated endocytosis, which promotes foam
cell formation (34). These
receptors may include SR-AI/II, SR-BI, CD36, and LOX-1. LOX-1 is
responsible for binding, being internalized, and proteolytically
degrading oxLDL but not acetylated LDL, and thus mediates foam cell
formation in atherosclerotic plaques (35).
Mounting evidence has shown that LOX-1 expression
may be induced by several proinflammatory and proatherogenic
stimuli (36). Anti-inflammatory
drugs have been identified that decrease LOX-1 expression and
regress the progression of foam cell formation. Berberine, as a
primary component of rhizoma coptidis, has been found to be
involved in decreasing lipid deposition and inhibiting the
formation of foam cells in the wall of the aorta (23). In this study, we demonstrated that
berberine combined with atorvastatin treatment suppressed LOX-1
expression in MDMs in a dose-dependent manner, consistent with the
results of Guan et al (23).
ET-1 is a peptide that plays an important role in
the pathophysiology of cardiovascular disease by causing vascular
damage (37). In human
endothelial cells, LOX-1 mRNA and protein expression were induced
by ET-1 (38). When the ET-1B
receptor was blocked by an antagonist, the induction of LOX-1 mRNA
by ET-1 was inhibited (38).
Notably, in rat MDMs, we found the ET-1 receptor plays a crucial
role in the regulation of LOX-1 expression. Transfection of
specific siRNA targeting this receptor into MDMs blocked the
reduction in LOX-1 expression induced by berberine. In a rat model,
injection of berberine and atorvastatin resulted in a decrease in
ET-1 plasma levels. Furthermore, reduction of LOX-1 expression in
monocytes was also induced by treatment with berberine and
atorvastatin. However, preconditioning with the ET-1 receptor
antagonist markedly blocked the inhibition of LOX-1 expression
caused by treatment with berberine and atorvastatin. These results
indicated that berberine may reduce LOX-1 expression through ET-1
receptors both in vitro and in vivo.
Treatment with berberine in combination with
atorvastatin also influenced physiological parameters in the rat
model. The results showed that the gains in BW, LW and KW were
significantly increased as the amount of berberine increased. The
progression of atherosclerosis is intimately associated with
variations in the lipid profile. In this study, the levels of TC,
TG and LDL-C in the rat model were deceased following treatment
with berberine and atorvastatin. Thus, berberine combined with
atorvastatin may be an efficient therapeutic method to treat
atherosclerosis.
Previous studies have demonstrated that HFD induces
inflammation and oxidative stress in rat models (39,40). HFD is sufficient to trigger NADPH
oxidase-related oxidative stress as well as an inflammatory
response, represented by increased PGE2 levels (41), increased COX-1, and in particular
COX-2 expression (42), and
promote NF-κB activation. In this study, berberine in combination
with atorvastatin distinctly reduced CRP and MDA levels as well as
elevating GPx and SOD levels in serum. Thus, berberine may play a
major role in reducing the inflammation and oxidative stress
induced by HFD.
In conclusion, our study has demonstrated that
berberine in combination with atorvastatin effectively
downregulated LOX-1 expression through the ET-1 receptor in
vitro and in vivo. This study may provide new evidence
towards identifying the mechanism of berberine in attenuating foam
cell formation and atherosclerosis progression.
Acknowledgements
This study was supported by the Natural Science
Basic Research Plan in the Shaanxi Province of China (2012JM4004)
and Health Research Project of Lanzhou Military Area Command of
Chinese PLA (CLZ12JA24).
Abbreviations:
|
AGEs
|
advanced glycation end-products
|
|
AMPK
|
AMP-activated protein kinase
|
|
BW
|
body weight
|
|
CD36
|
cluster of differentiation 36
|
|
CRP
|
C-reactive protein
|
|
EGCG
|
epigallocatechin gallate
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
ET-1
|
endothelin-1
|
|
GPx
|
glutathione peroxidase
|
|
HDL-C
|
high-density
lipoprotein-cholesterol
|
|
HFD
|
high-fat diet
|
|
HW
|
heart weight
|
|
KW
|
kidney weight
|
|
LDL
|
low-density lipoprotein
|
|
LDL-C
|
low-density
lipoprotein-cholesterol
|
|
LOX-1
|
lectin-like oxidized low-density
lipoprotein receptor-1
|
|
LW
|
liver weight
|
|
MDA
|
malondialdehyde
|
|
MDMs
|
monocyte-derived macrophages
|
|
oxLDL
|
oxidized low-density lipoprotein
|
|
qRT-PCR
|
quantitative reverse transcription
polymerase chain reaction
|
|
SOD
|
superoxide dismutase
|
|
SRs
|
scavenger receptors
|
|
SW
|
spleen weight
|
|
TC
|
total cholesterol
|
|
TG
|
triglyceride
|
|
VSMCs
|
vascular smooth muscle cells
|
References
|
1
|
Lusis AJ: Atherosclerosis. Nature.
407:233–241. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li J, Chen CX and Shen YH: Effects of
total glucosides from paeony (Paeonia lactiflora Pall) roots
on experimental atherosclerosis in rats. J Ethnopharmacol.
135:469–475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hansson GK: Inflammation, atherosclerosis,
and coronary artery disease. N Engl J Med. 352:1685–1695. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pentikäinen MO, Oörni K, Ala-Korpela M and
Kovanen PT: Modified LDL-trigger of atherosclerosis and
inflammation in the arterial intima. J Intern Med. 247:359–370.
2000.PubMed/NCBI
|
|
5
|
Plihtari R, Kovanen PT and Öörni K:
Acidity increases the uptake of native LDL by human
monocyte-derived macrophages. Atherosclerosis. 217:401–406. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Libby P, Ridker PM and Maseri A:
Inflammation and atherosclerosis. Circulation. 105:1135–1143. 2002.
View Article : Google Scholar
|
|
7
|
Stein S, Lohmann C, Schäfer N, et al:
SIRT1 decreases Lox-1-mediated foam cell formation in
atherogenesis. Eur Heart J. 31:2301–2309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Levitan I, Volkov S and Subbaiah PV:
Oxidized LDL: diversity, patterns of recognition, and
pathophysiology. Antioxid Redox Signal. 13:39–75. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kakutani M, Masaki T and Sawamura T: A
platelet-endothelium interaction mediated by lectin-like oxidized
low-density lipoprotein receptor-1. Proc Natl Acad Sci USA.
97:360–364. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen M, Kakutani M, Naruko T, et al:
Activation-dependent surface expression of LOX-1 in human
platelets. Biochem Biophys Res Commun. 282:153–158. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kataoka H, Kume N, Miyamoto S, et al:
Oxidized LDL modulates Bax/Bcl-2 through the lectinlike Ox-LDL
receptor-1 in vascular smooth muscle cells. Arterioscler Thromb
Vasc Biol. 21:955–960. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mitra S, Goyal T and Mehta JL: Oxidized
LDL, LOX-1 and atherosclerosis. Cardiovasc Drugs Ther. 25:419–429.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamamoto N, Toyoda M, Abe M, et al:
Lectin-like oxidized LDL receptor-1 (LOX-1) expression in the
tubulointerstitial area likely plays an important role in human
diabetic nephropathy. Intern Med. 48:189–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagase M, Hirose S, Sawamura T, Masaki T
and Fujita T: Enhanced expression of endothelial oxidized
low-density lipoprotein receptor (LOX-1) in hypertensive rats.
Biochem Biophys Res Commun. 237:496–498. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rautureau Y and Schiffrin EL: Endothelin
in hypertension: an update. Curr Opin Nephrol Hypertens.
21:128–136. 2012. View Article : Google Scholar
|
|
16
|
Taguchi K and Hattori Y: Unlooked-for
significance of cardiac versus vascular effects of endothelin-1 in
the pathophysiology of pulmonary arterial hypertension. Circ Res.
112:227–229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Watson AM, Li J, Schumacher C, et al: The
endothelin receptor antagonist avosentan ameliorates nephropathy
and atherosclerosis in diabetic apolipoprotein E knockout mice.
Diabetologia. 53:192–203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rodríguez-Pascual F, Busnadiego O, Lagares
D and Lamas S: Role of endothelin in the cardiovascular system.
Pharmacol Res. 63:463–472. 2011.
|
|
19
|
Morawietz H, Rueckschloss U, Niemann B, et
al: Angiotensin II induces LOX-1, the human endothelial receptor
for oxidized low-density lipoprotein. Circulation. 100:899–902.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Böhm F and Pernow J: The importance of
endothelin-1 for vascular dysfunction in cardiovascular disease.
Cardiovasc Res. 76:8–18. 2007.PubMed/NCBI
|
|
21
|
Xu S, Liu Z, Huang Y, et al: Tanshinone
II-A inhibits oxidized LDL-induced LOX-1 expression in macrophages
by reducing intracellular superoxide radical generation and NF-κB
activation. Transl Res. 160:114–124. 2012.PubMed/NCBI
|
|
22
|
Kang B-Y, Khan JA, Ryu S, Shekhar R, Seung
KB and Mehta JL: Curcumin reduces angiotensin II-mediated
cardiomyocyte growth via LOX-1 inhibition. J Cardiovasc Pharmacol.
55:176–183. 2010.
|
|
23
|
Guan S, Wang B, Li W, Guan J and Fang X:
Effects of berberine on expression of LOX-1 and SR-BI in human
macrophage-derived foam cells induced by ox-LDL. Am J Chin Med.
38:1161–1169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ou H-C, Song T-Y, Yeh Y-C, et al: EGCG
protects against oxidized LDL-induced endothelial dysfunction by
inhibiting LOX-1-mediated signaling. J Appl Physiol (1985).
108:1745–1756. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang H-C, Chen T-G, Tai Y-T, Chen T-L,
Chiu W-T and Chen R-M: Resveratrol attenuates oxidized LDL-evoked
Lox-1 signaling and consequently protects against apoptotic insults
to cerebrovascular endothelial cells. J Cereb Blood Flow Metab.
31:842–854. 2011. View Article : Google Scholar
|
|
26
|
Kuo C-L, Chi C-W and Liu T-Y: The
anti-inflammatory potential of berberine in vitro and in vivo.
Cancer Lett. 203:127–137. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brusq J-M, Ancellin N, Grondin P, et al:
Inhibition of lipid synthesis through activation of AMP kinase: an
additional mechanism for the hypolipidemic effects of berberine. J
Lipid Res. 47:1281–1288. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Puato M, Faggin E, Rattazzi M, et al:
Atorvastatin reduces macrophage accumulation in atherosclerotic
plaques: a comparison of a nonstatin-based regimen in patients
undergoing carotid endarterectomy. Stroke. 41:1163–1168. 2010.
View Article : Google Scholar
|
|
29
|
Zhou G, Ge S, Liu D, et al: Atorvastatin
reduces plaque vulnerability in an atherosclerotic rabbit model by
altering the 5-lipoxygenase pathway. Cardiology. 115:221–228. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Simmons G, McKnight À, Takeuchi Y, Hoshino
H and Clapham PR: Cell-to-cell fusion, but not virus entry in
macrophages by T-cell line tropic HIV-1 strains: a V3
loop-determined restriction. Virology. 209:696–700. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McKnight Á, Griffiths DJ, Dittmar M,
Clapham P and Thomas E: Characterization of a late entry event in
the replication cycle of human immunodeficiency virus type 2. J
Virol. 75:6914–6922. 2001.PubMed/NCBI
|
|
32
|
Sitia S, Tomasoni L, Atzeni F, et al: From
endothelial dysfunction to atherosclerosis. Autoimmun Rev.
9:830–834. 2010. View Article : Google Scholar
|
|
33
|
Pawlak K, Mysliwiec M and Pawlak D:
Oxidized LDL to autoantibodies against oxLDL ratio-the new
biomarker associated with carotid atherosclerosis and
cardiovascular complications in dialyzed patients. Atherosclerosis.
224:252–257. 2012. View Article : Google Scholar
|
|
34
|
Howell KW, Meng X, Fullerton DA, Jin C,
Reece TB and Cleveland JC Jr: Toll-like receptor 4 mediates
oxidized LDL-induced macrophage differentiation to foam cells. J
Surg Res. 171:e27–e31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lu J, Mitra S, Wang X, Khaidakov M and
Mehta JL: Oxidative stress and lectin-like ox-LDL-receptor LOX-1 in
atherogenesis and tumorigenesis. Antioxid Redox Signal.
15:2301–2333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pandey H, Arjuman A, Roy KK and Chandra
NC: Reciprocal coordination of a combination oral contraceptive
containing desogestrel+ethinyl estradiol on the expression of LOX-1
and LDLR in placental trophoblast cells. Contraception. 84:e43–e49.
2011.PubMed/NCBI
|
|
37
|
McMurray JJ, Holman RR, Haffner SM, et al;
NAVIGATOR Study Group. Effect of valsartan on the incidence of
diabetes and cardiovascular events. N Engl J Med. 362:1477–1490.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Morawietz H, Duerrschmidt N, Niemann B,
Galle J, Sawamura T and Holtz J: Induction of the oxLDL receptor
LOX-1 by endothelin-1 in human endothelial cells. Biochem Biophys
Res Commun. 284:961–965. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cani PD, Bibiloni R, Knauf C, et al:
Changes in gut microbiota control metabolic endotoxemia-induced
inflammation in high-fat diet-induced obesity and diabetes in mice.
Diabetes. 57:1470–1481. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang X, Dong F, Ren J, Driscoll MJ and
Culver B: High dietary fat induces NADPH oxidase-associated
oxidative stress and inflammation in rat cerebral cortex. Exp
Neurol. 191:318–325. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ju J, Liu Y, Hong J, Huang MT, Conney AH
and Yang CS: Effects of green tea and high-fat diet on arachidonic
acid metabolism and aberrant crypt foci formation in an
azoxymethane-induced colon carcinogenesis mouse model. Nutr Cancer.
46:172–178. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee JY, Sohn KH, Rhee SH and Hwang D:
Saturated fatty acids, but not unsaturated fatty acids, induce the
expression of cyclooxygenase-2 mediated through Toll-like receptor
4. J Biol Chem. 276:16683–16689. 2001. View Article : Google Scholar : PubMed/NCBI
|