Introduction
Stomach cancer is one of the common malignancies
worldwide and is the second most frequent cause of cancer-related
mortality. The ratio of males to females with stomach cancer is
approximately 2:1, with the highest incidence of gastric cancer
(GC) occurring in East Asia, with approximately 70 cases per
100,000 individuals annually (1).
A number of factors have been associated with an increased risk of
stomach cancer, including Helicobacter pylori infection, a
high consumption of salt-preserved food, smoking and air pollution.
Several strategies have been implemented to reduce the severity of
GC; however, the final treatment outcomes have not met
expectations. At the time of diagnosis of GC, the majority cases
already at the advanced stages of tumorigenesis (2).
Damage to the genome alters the expression of
proteins that are associated with various essential cellular
functions. This results in an enhanced malignancy, particularly in
the case of GC. This has been well-known for many years; however,
the identification of effective biomarkers for the detection or the
targeted therapy of cancer remains incomplete. The sex-determining
region of Y-chromosome (SRY)-related high mobility-group box (Sox)
genes belong to the high mobility group (HMG) protein family. These
genes encode proteins or transcription factors similar to the SRY
gene product from a conserved region (3). Together with Sox17 and Sox18, Sox7
belongs to the Sox F gene subfamily (4). Sox7 was first identified in
zebrafish and rats (5,6), with transcriptional regulation
carried out through the methylation of a CpG island at the start
site of the gene (7,8). Previous studies have demonstrated
that the Sox genes can regulate a number of processes, including
gut, B cell, muscle and cardiovascular system development (9–11);
they also participate in a number of biological processes in a
variety of tumors. The expression of Sox7 has been found to be
significantly downregulated in many cancer tissues and cell lines.
Sox7 has also been strongly associated with the Wnt/β-catenin
signaling pathway, tumor prognosis and the clinicopathological
characteristics of cancer (7,8,12–17).
The Wnt/β-catenin signaling pathway regulates a
variety of cellular process, such as cell fate, proliferation,
survival, behavior and migration (18). In this pathway, β-catenin is a key
effector that regulates Wnt/β-catenin targets. It is commonly found
that the accumulation of β-catenin leads to the aberrant activation
of the Wnt/β-catenin pathway in human cancers (19–21). However, to date, there are no
studies focusing on Sox7 expression in GC, apart from a few studies
investigating the association between Sox7 expression and the
Wnt/β-catenin signaling pathway in GC. In the present study, we
focused on the association between the molecular pathology of GC
and survival. Moreover, we investigated the expression levels of
Sox7 and β-catenin in GC tissues, normal mucosal tissues and cell
lines. We also explored the possible association between Sox7 and
β-catenin expression levels and clinicopathological characteristics
of patients with GC.
Materials and methods
Patients and specimens
The patients included in our study had been
diagnosed with GC and had undergone surgical treatment (n=258) at
the General Department of Chinese People’s Liberation Army (PLA)
General Hospital (Beijing, China) between January 2003 and December
2008. None of the patients had undergone chemotherapy or
radiotherapy prior to surgical treatment, nor had they suffered
from other synchronous malignancies. Tumors were evaluated by the
pathological tumor node metastasis (pTNM) staging system according
to the criteria of the American Joint Committee on Cancer (7th
edition) and The Japan Gastric Cancer Association (JGCA)
guidelines. Data relating to the clinicopathological
characteristics of patients and tumors were collected from hospital
records. Follow-up data were collected from the database available
in our department. The follow-up time began on the day of the
primary tumor operation, while the end-point of the overall
survival (OS) analysis was the time of death of the patient or our
last follow-up session. Paraffin-embedded primary GC specimens from
258 patients were obtained, and 80 distal normal gastric tissues
were randomly selected as the normal controls. Another 60 pairs of
freshly frozen GC tissue samples and matched normal mucosal tissue
samples adjacent to the carcinoma were collected from patients
following the above-mentioned criteria. These patients had
undergone surgical excision at the General Surgery Department of
PLA General Hospital from January 2012 to June 2013; the samples
were stored at −80°C until use in western blot analysis. Our study
was conducted with the approval of the Chinese PLA General Hospital
Research Ethics Committee.
Cell culture
The human GC cell lines, AGS and MKN-45, and the
human normal gastric mucosa cell line, GES-1 (obtained from ATCC,
Manassas, VA, USA) were cultured in RPMI 1640 medium (Gibco-BRL,
Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum
(FBS; Gibco-BRL), 100 U/ml penicillin and 100 μg/ml streptomycin,
at 37°C/5% CO2.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to a
standard proteinase K method. Reverse transcription to produce cDNA
was conducted using a reverse transcription kit (Applied
Biosystems, Foster City, CA, USA). We used specific primers and
aliquots (2 μl) of the cDNA as templates, to amplify fragments of
Sox7 (5′-TAAATCAGGGGCCGGGTCG-3′ and 5′-CTTCCACGACTTTCCCAGCA-3′),
β-catenin (5′-ATTG AAGCTGAGGGAGCCAC-3′ and 5′-TCCTGGCCATATCC
ACCAGA-3′) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH;
5′-AGAAGGCTGGGGCTCATTTG-3′ and 5′-AGG GGCCATCCACAGTCTTC-3′) as an
internal control. The thermal cycling conditions we used involved a
denaturation step at 95°C for 10 min, then 30 cycles at 95°C for 30
sec, 58°C for 60 sec and 72°C for 30 sec, with a final extension
step at 72°C for 5 min after the 30th cycle.
Immunohistochemistry (IHC)
Sections (5 μm thick) were cut from paraffin blocks
and IHC was conducted using avidin-biotin-peroxidase complex kits
(Vector Laboratories, Burlingame, CA, USA), according to the
instructions provided by the manufacturer. The sections were
subsequently incubated with polyclonal rabbit anti-human Sox7
(1:250 dilution; R&D Systems, Minneapolis, MN, USA) and
polyclonal rabbit anti-human β-catenin antibodies (1:250; R&D
Systems) at room temperature overnight, in a humidified chamber.
Diaminobenzidine (Vector Laboratories) was used as the chromogen
and the slides were counterstained with Mayer’s hematoxylin. All
sections were examined and scored by two independent investigators,
who were blinded with regard to the sample groups. We analyzed both
the intensity and the extent of positive staining, as previously
described (22,23). Intensity was graded as follows: 0,
no staining; 1, mild intensity; 2, moderate intensity; 3, severe
intensity. The extent of staining was scored as follows: 0, no
positive staining; 1, 1–25% of carcinoma cells was positive; 2,
25–50% was positive; 3, >50% was positive. The final score for a
section was generated by combining the two values for the intensity
and extent of staining. A score of ≤1 was considered negative,
while a score between 2 and 6 was considered positive.
Protein extraction and western blot
analysis
The tissue samples or cells were homogenized in
extraction buffer (100 mM Trizma pH 7.5, 10 mM EDTA, 100 mM NaF, 10
mM sodium pyrophosphate, 10 mM sodium orthovanadate, 2 mM
phenylmethanesulfonyl fluoride and 0.01 mg/ml aprotinin) at 4°C for
15 min. Following homogenization, Triton X-100 was added to a final
concentration of 1% (v/v), and the samples were incubated for 30
min at 4°C and then centrifuged (13,000 × g; 20 min; 4°C). The
concentration of total protein in each sample was determined using
bovine serum albumin (BSA) as a standard. Equal amounts of protein
from each sample were diluted in Laemmli buffer containing
dithiothreitol (DTT; 1 M) and separated by electrophoresis on
polyacrylamide gels (SDS-PAGE). The proteins were then transferred
onto nitrocellulose membranes, which were blocked with 5% skim
milk. The membranes were incubated at room temperature overnight
with the appropriate antibodies [anti-human Sox7 (R&D Systems),
anti-human β-catenin (R&D Systems) and anti-human β-actin
(Sigma St. Louis, MO, USA)] diluted in a basal solution containing
3% skim milk. The membranes were then incubated with the
appropriate corresponding secondary antibody (1:15,000) conjugated
to horseradish peroxidase (HrP) at room temperature for 1 h. After
a final wash, the signals were visualized using an ECL Western
Blotting System kit (GE Healthcare, Little Chalfont,
Buckinghamshire, UK). Signals and band intensities were quantified
by optical densitometry using ImageJ 1.37 software.
Statistical analysis
The correlation between various clinicopathological
characteristics of the patients with GC and Sox7 gene expression
was analyzed using χ2 or Fisher’s exact tests. The
association between Sox7 and β-catenin expression levels was
examined using Spearman’s rank correlation coefficient or Pearson’s
correlation coefficient. The paired-samples t-test was used to
assess the differences in the relative expression of Sox7 and
β-catenin proteins in GC and normal mucosal tissues. The 5-year OS
rate was calculated using the Kaplan-Meier method. The difference
between curves was analyzed using the log-rank test. Multivariate
survival analysis was based on the Cox proportional hazard model. A
P-value <0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using SPSS version 17.0 software (SPSS Inc., Chicago, IL, USA).
Results
Sox7 and β-catenin expression in the GC
cell lines
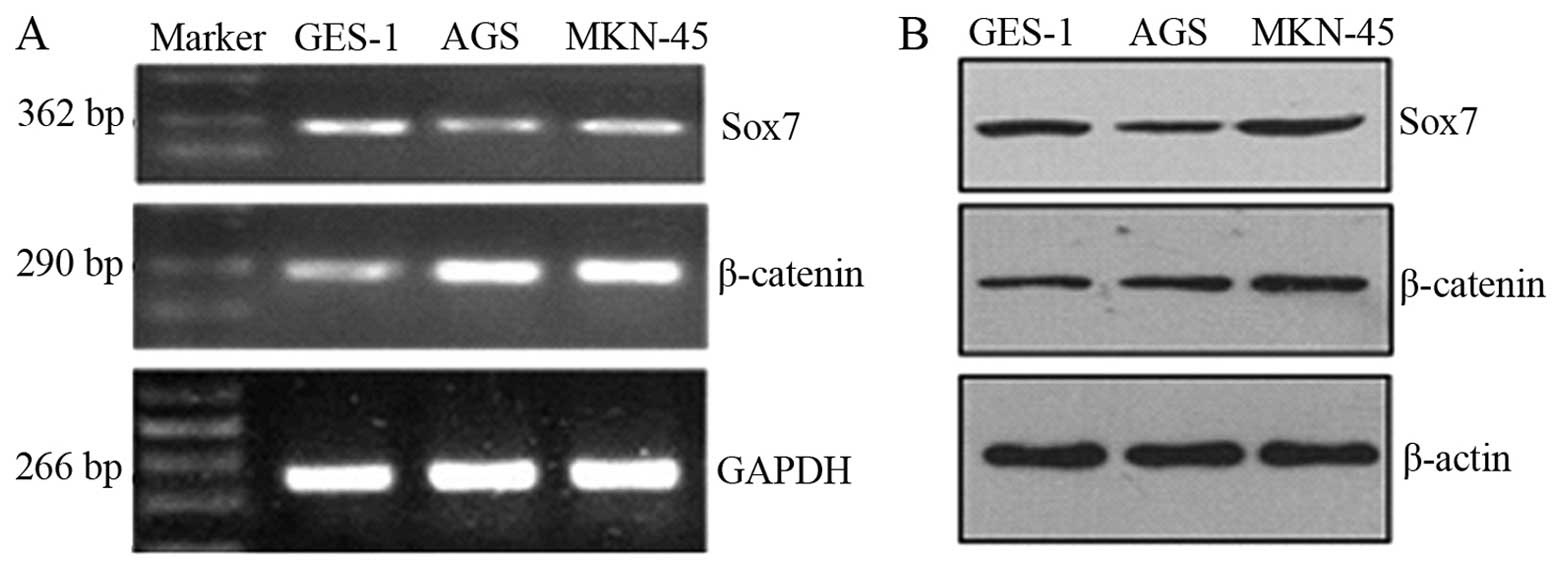
Sox7 expression levels were lower in the tumor cell
lines compared with the GES-1 cells, whereas β-catenin was
overexpressed in the tumor cell lines (Fig. 1A). The western blot analysis
results for the Sox7 and β-catenin proteins in the cell lines
revealed a similar trend (Fig.
1B). The difference in the expression levels was statistically
significant (P<0.05).
IHC analysis of Sox7 and β-catenin
expression in tissues
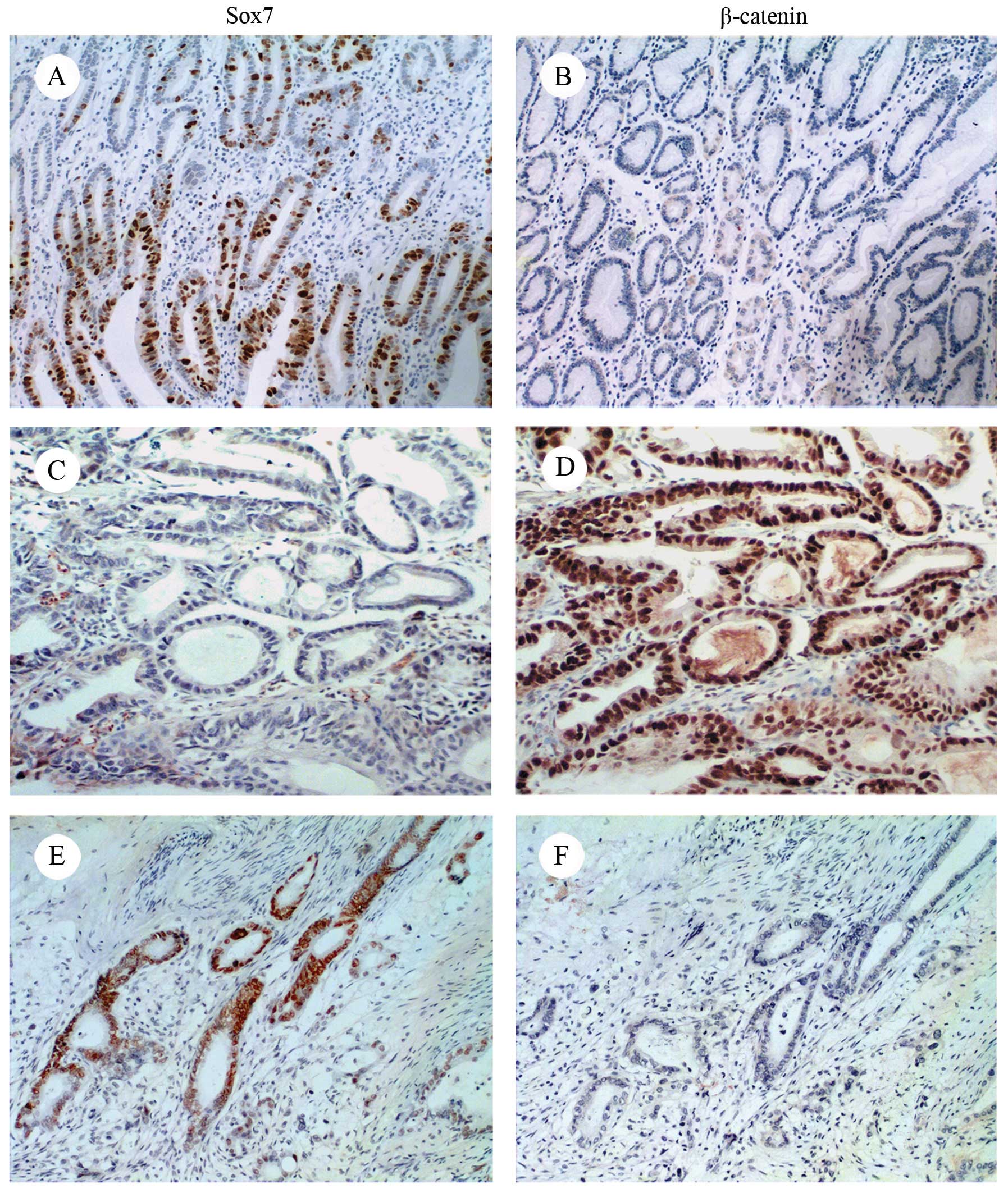
Sox7 expression was downregulated to a greater
extent in the GC tissues compared with the normal mucosal tissues.
Sox7 protein expression was weak or absent in 119 of the GC
samples; in the remaining 139 samples, Sox7 was mainly expressed in
the nucleus, and, to a lesser extent, in the cytoplasm. The
prevalence of Sox7 expression was 86.3% (69/80) in the distal
normal mucosa samples, that is to say, much higher than that in the
cancerous tissue samples. Staining was mainly observed in the
nucleus or cytoplasm (Fig. 2A and
C). By contrast, β-catenin was overexpressed in the GC tissues
(137/258) and weakly expressed or absent in the normal mucosa
(56/80) (Fig. 2B and D).
β-catenin was observed in the nucleus, cytoplasm and cellular
membrane. At sites where Sox7 was expressed, β-catenin was weakly
expressed or absent and, where β-catenin was expressed, Sox7 was
absent (Fig. 2C–F).
Significant positive associations were observed
between Sox7 expression and the differentiation grade (P=0.001),
depth of invasion (P=0.001), lymph node metastasis (P=0.001) and
pTNM stage (P=0.001; Table I).
The expression of β-catenin showed a significant correlation with
the differentiation grade (P=0.001), depth of invasion (P=0.001),
lymph node metastasis (P=0.004), distant metastasis (P=0.012) and
pTNM stage (P=0.001; Table I).
There was a significant association between the downregulated Sox7
expression and the high-level β-catenin expression (P=0.001;
Table II). An analysis of the
association between Sox7 and β-catenin expression by IHC revealed a
significant negative correlation (R=−0.698, P=0.001; Table II).
 | Table IAssociation of Sox7 and β-catenin
expression with clinicopathological characteristics of gastric
cancer patients. |
Table I
Association of Sox7 and β-catenin
expression with clinicopathological characteristics of gastric
cancer patients.
| Sox7 | β-catenin |
|---|
|
|
|
|---|
| Variables | Positive | Negative | P-value | Positive | Negative | P-value |
|---|
| Gender |
| Male | 71 | 80 | 0.488 | 82 | 69 | 0.645 |
| Female | 55 | 52 | | 55 | 52 | |
| Age, years |
| ≤45 | 31 | 33 | 0.286 | 33 | 31 | 0.921 |
| 45–60 | 53 | 44 | | 51 | 46 | |
| >60 | 42 | 55 | | 53 | 44 | |
| Tumor site |
| Upper | 34 | 39 | 0.651 | 42 | 31 | 0.594 |
| Middle | 45 | 40 | | 42 | 43 | |
| Low | 47 | 53 | | 53 | 47 | |
| Tumor size |
| ≤4 cm | 52 | 52 | 0.759 | 51 | 53 | 0283 |
| >4 cm | 74 | 80 | | 86 | 68 | |
| Lauren
classification |
| Intestinal | 94 | 101 | 0.672 | 102 | 93 | 0.555 |
| Diffuse | 23 | 25 | | 25 | 23 | |
| Mixed | 9 | 6 | | 10 | 5 | |
| Histological
type |
|
Adenocarcinoma | 102 | 114 | 0.239 | 115 | 101 | 0.919 |
| Other | 24 | 18 | | 22 | 20 | |
| Differentiation
grade |
| Well-Moderate | 74 | 90 | 0.115 | 101 | 63 | 0.001 |
| Poor | 52 | 42 | | 36 | 58 | |
| Depth of
invasion |
| T1 | 22 | 7 | 0.001 | 5 | 24 | 0.001 |
| T2 | 38 | 33 | | 31 | 40 | |
| T3 | 53 | 58 | | 67 | 44 | |
| T4 | 13 | 34 | | 34 | 13 | |
| Lymph node
metastasis |
| N0 | 52 | 22 | 0.001 | 27 | 47 | 0.004 |
| N1 | 43 | 55 | | 54 | 44 | |
| N2 | 26 | 40 | | 42 | 24 | |
| N3 | 5 | 15 | | 14 | 6 | |
| Distant
metastasis |
| M0 | 120 | 118 | 0.079 | 121 | 117 | 0.012 |
| M1 | 6 | 14 | | 16 | 4 | |
| TNM stage |
| I | 36 | 10 | 0.001 | 7 | 39 | 0.001 |
| II | 63 | 70 | | 76 | 57 | |
| III | 22 | 39 | | 39 | 22 | |
| IV | 5 | 13 | | 15 | 3 | |
 | Table IICorrelation between Sox7 and
β-catenin expression in gastric cancer. |
Table II
Correlation between Sox7 and
β-catenin expression in gastric cancer.
| Sox7 | χ2
test | Correlation
analysis |
|---|
|
|
|
|
|---|
| β-catenin | + | − | χ2 | P-value | R | P-vlaue |
|---|
| + | 22 | 115 | 125.614 | 0.001 | −0.698 | 0.001 |
| − | 104 | 17 | | | | |
Western blot analysis of Sox7 and
β-catenin expression in tissue samples
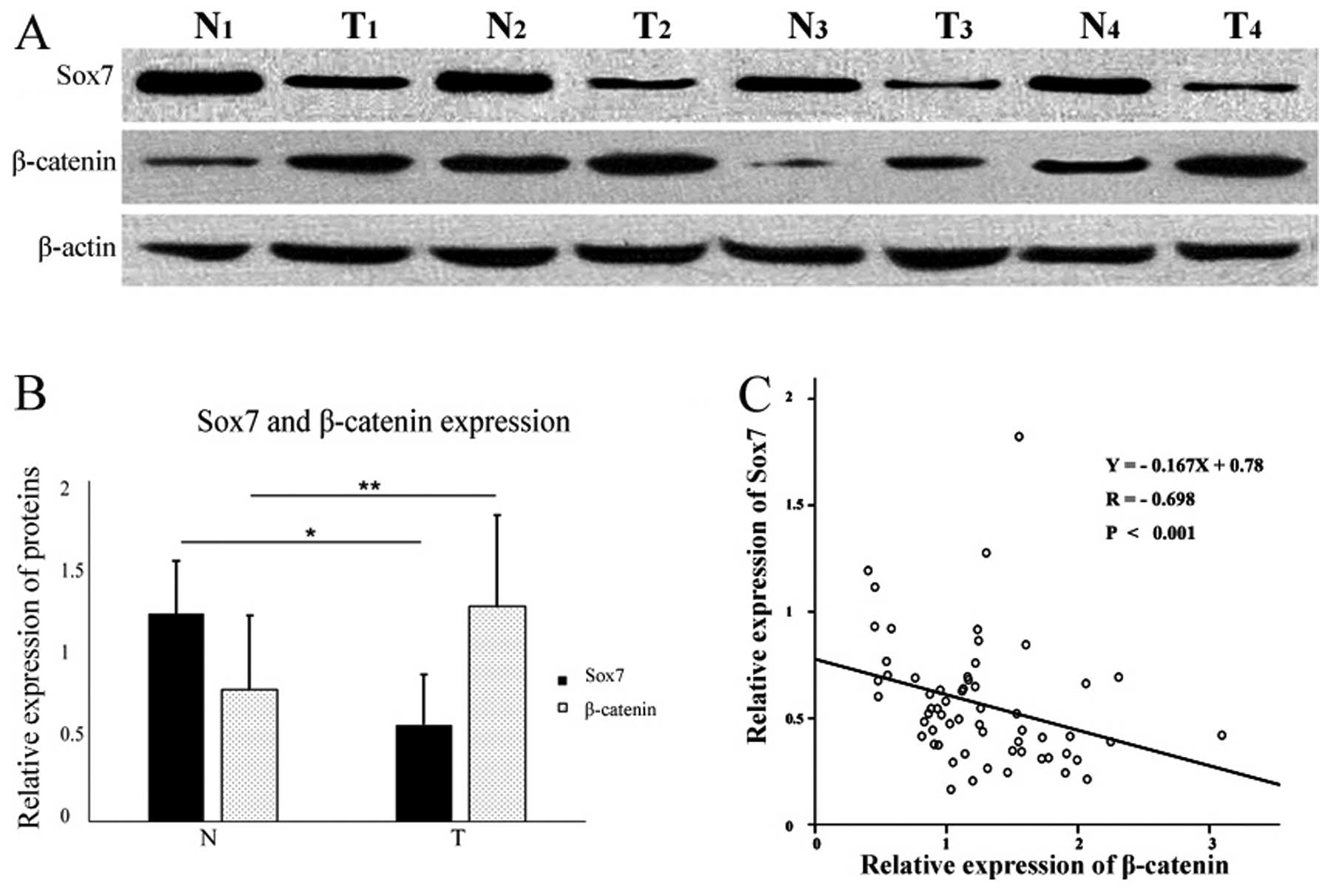
Sox7 protein expression was detected in 29 tumor and
58 normal mucosal tissue specimens (Fig. 3A). The mean relative expression
level of Sox7 was 0.57±0.29 in the tumor samples and 1.23±0.31 in
the normal samples (Fig. 3B).
β-catenin protein expression was detected in 94.7% (56/60) of the
tumor specimens and in 66.7% (40/60) of the normal mucosal tissue
samples (Fig. 3A). The mean
relative expression levels of β-catenin were 1.25±0.53 in the tumor
samples, and 0.77±0.43 in the normal mucosal tissues (Fig. 3B). The relative expression level
of Sox7 protein in the tumor samples was significantly lower
(P<0.05) than that in the matched distal normal mucosal tissues,
whereas β-catenin expression was significantly higher (P<0.05;
Fig. 3B).
Association between Sox7 and β-catenin
expression
IHC staining revealed a correlation between Sox7 and
β-catenin expression. We conducted a linear regression analysis of
our western blot analysis results and found a negative correlation
between the relative expression levels of Sox7 and β-catenin
protein in the GC samples (Y=−0.167X+0.78, P<0.001; Fig. 3C).
Survival analysis
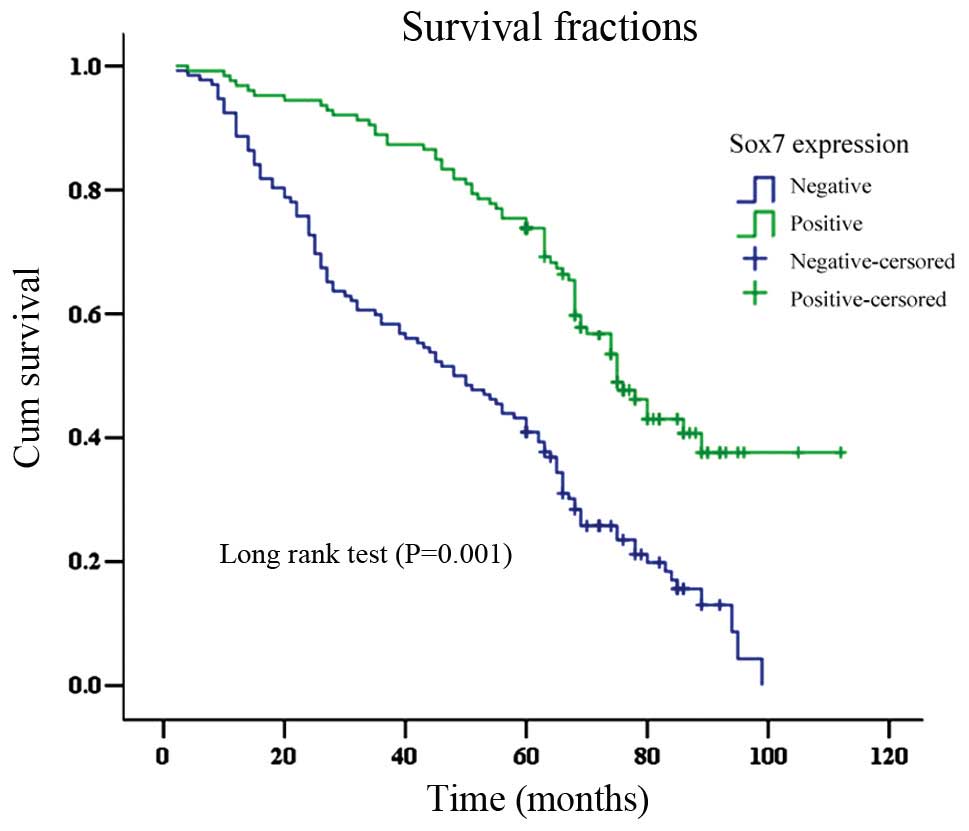
The OS time for patients that were Sox7-negative was
significantly shorter (mean, 49.7±2.6 months) than that for
Sox7-positive cases (78.5±3.0 months; log-rank test, P<0.001;
Fig. 4). The overall 5-year
survival rate for the Sox7-negative group (40.9%) was lower than
that of the Sox7-positive group (69.2%; log-rank =35.99, P=0.001).
Sox7 expression was found to be an independent prognostic factor
(P=0.001) by the Cox proportional hazard model. Other independent
prognostic factors were the Lauren classification, lymph node
metastasis, pTNM stage and β-catenin expression (all P<0.05;
Table III).
 | Table IIICox regression analysis of prognostic
factors in gastric cancer. |
Table III
Cox regression analysis of prognostic
factors in gastric cancer.
| | | | | | 95% CI for HR |
|---|
| | | | | |
|
|---|
| Prognostic
variables | B | SE | Wald test | P-value | HR | Lower | Upper |
|---|
| Gender | −0.166 | 0.168 | 0.979 | 0.322 | 0.847 | 0.609 | 1.177 |
| Age | 0.126 | 0.106 | 1.432 | 0.231 | 1.135 | 0.923 | 1.396 |
| Tumor site | −0.115 | 0.099 | 1.360 | 0.243 | 0.891 | 0.734 | 1.082 |
| Tumor size | −0.167 | 0.170 | 0.965 | 0.326 | 0.846 | 0.607 | 1.180 |
| Lauren
classification | 0.384 | 0.173 | 4.931 | 0.026 | 1.469 | 1.046 | 2.061 |
| Histology type | −0.334 | 0.279 | 1.429 | 0.232 | 0.716 | 0.414 | 1.238 |
| Differentiation
grade | −0.095 | 0.194 | 0.241 | 0.623 | 0.909 | 0.622 | 1.329 |
| Depth of
invasion | 0.250 | 0.143 | 3.059 | 0.080 | 1.284 | 0.970 | 1.698 |
| Lymph node
metastasis | 0.365 | 0.155 | 5.541 | 0.019 | 1.441 | 1.063 | 1.953 |
| Distant
metastasis | 0.173 | 0.367 | 0.222 | 0.638 | 1.189 | 0.579 | 2.440 |
| TNM stage | 0.536 | 0.253 | 4.486 | 0.034 | 1.709 | 1.041 | 2.805 |
| Sox7
expression | −0.404 | 0.205 | 3.888 | 0.049 | 0.668 | 0.447 | 0.998 |
| β-catenin
expression | 0.442 | 0.211 | 4.395 | 0.036 | 1.556 | 1.029 | 2.352 |
Discussion
In order to develop sensitive and specific therapies
against GC, molecular biomarkers must be determined (24). Abnormal expression levels of
members of the Sox gene family usually correlate with malignant and
metastatic tumors (16,25). Based on our findings, we suggest
that Sox7 plays an important role in the inhibition of
carcinogenesis and the progression of GC. The results from a
previous study demonstrated that Sox7 mRNA levels were increased in
the MKN-45 cell line (13). In
the present study, we found that Sox7 expression was decreased in
the GC cell lines. Our findings were confirmed by IHC analyses of
GC tissues, suggesting that normal expression levels of Sox7 play
an important role in suppressing carcinogenesis. Similar results
have been reported for prostate, colorectal, endometrial, lung and
breast cancer (7,8,14,16,17). In our study, Sox7 expression
negatively correlated with the depth of invasion, lymph node
metastasis, distant metastasis and pTNM stage. These findings
suggest that Sox7 expression levels are associated with the
malignancy of GC and possibly play a potential role in predicting
the progression of GC.
Previous findings have suggested that Sox genes are
widespread and play potential roles in regulating the Wnt/β-catenin
signaling pathway during development (26). A number of studies have
demonstrated that the Wnt/β-catenin pathway, in which β-catenin is
a key regulator, is involved in the carcinogenesis, progression and
prognosis of a variety of malignancies, GC in particular (27–30). Mutations in β-catenin are involved
in regulating the occurrence of tumors. Our results demonstrate
that β-catenin is often present in GC tissues, positively
correlating with the differentiation grade, depth of invasion,
lymph node metastasis, distant metastasis and pTNM stage. These
findings further indicate that the Wnt/β-catenin pathway plays an
important role in the progression of GC.
A motif in the Sox7 protein enables it to bind to
β-catenin, enabling their interaction (31). Previous studies have indicated
that the regulation of the Wnt/β-catenin signaling pathway by Sox7
results in the disruption of the transcriptional functions of the
β-catenin/TCF/LEF-1 complex (12). Sox7 has been reported to be an
independent checkpoint for β-catenin function in prostate and colon
epithelial cells (8). In this
study, we investigated the association between Sox7 and β-catenin
using IHC. β-catenin was present in the majority of Sox7-negative
tissues; at most Sox7-positive sites, β-catenin was absent. We
speculated that there was a negative correlation between Sox7 and
β-catenin, which was confirmed by the linear regression analysis of
our western blot analysis results. We therefore suggest that the
interaction between Sox7 and β-catenin plays an important role in
carcinogenesis and the progression of GC. Previous research
suggests that the methylation of a CpG island plays a major role as
a transcriptional regulator of the Sox7 gene (8,16,32); therefore, we hypothesized that the
regulation of β-catenin signaling by targeting or upregulating Sox7
expression may prevent carcinogenesis and control the progression
of GC (33).
Previous similar studies have demonstrated that the
expression of Sox7 in lung and prostate cancer is associated with
the prognosis or survival of patients (16,17,32). In our study, Kaplan-Meier survival
analysis suggested that the reduced expression of the Sox7 gene was
associated with the poor prognosis of GC patients. Sox7-negative GC
patients showed a significantly shorter OS compared with
Sox7-positive patients, suggesting that the downregulated
expression of Sox7 contributes to the malignancy of the tumor. It
may also play a role as a potential biomarker for identifying GC
cases with a poor prognosis.
In conclusion, our data demonstrate that the
expression of Sox7 is lower in GC cell lines compared with their
normal counterparts. We found that Sox7 expression levels in human
tissues were similar to those observed in GC tissues, while the
opposite was observed for β-catenin levels. We also observed a
negative correlation between Sox7 and β-catenin expression levels,
indicating that Sox7 is possibly associated with the Wnt/β-catenin
signaling pathway. Sox7 may be a potential indicator for predicting
the clinical outcome of GC patients. We believe that our findings
can significantly contribute to our understanding of the molecular
mechanisms of GC carcinogenesis and progression.
Acknowledgements
This study was supported by grants from the National
Nature Science Foundation of China (nos. 81272698, 81101883 and
81172368), a grant from the PLA Medical Technology Key Project of
Scientific Research in the 12th Five-Year-Plan (no. BWS12J049), a
grant from PLA medical and health research fund project (no.
11BJZ17), a grant from the Capital Health Research and Development
of Special (no. 2011-5001-01), and a grant from the Major Science
and Technology Project of ‘National Significant New Drug Creation’
from the Major Science and Technology of China (no.
2011ZX09307-001-05).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar
|
|
3
|
Bowles J, Schepers G and Koopman P:
Phylogeny of the SOX family of developmental transcription factors
based on sequence and structural indicators. Dev Biol. 227:239–255.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chew LJ and Gallo V: The Yin and Yang of
Sox proteins: Activation and repression in development and disease.
J Neurosci Res. 87:3277–3287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shiozawa M, Hiraoka Y, Komatsu N, Ogawa M,
Sakai Y and Aiso S: Cloning and characterization of Xenopus
laevis xSox7 cDNA. Biochim Biophys Acta. 1309:73–76. 1996.
View Article : Google Scholar
|
|
6
|
Taniguchi K, Hiraoka Y, Ogawa M, Sakai Y,
Kido S and Aiso S: Isolation and characterization of a mouse
SRY-related cDNA, mSox7. Biochim Biophys Acta. 1445:225–231. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stovall DB, Wan M, Miller LD, et al: The
regulation of SOX7 and its tumor suppressive role in breast cancer.
Am J Pathol. 183:1645–1653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo L, Zhong D, Lau S, et al: Sox7 is an
independent checkpoint for beta-catenin function in prostate and
colon epithelial cells. Mol Cancer Res. 6:1421–1430. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Endo Y, Deonauth K, Prahalad P, Hoxter B,
Zhu Y and Byers SW: Role of Sox-9, ER81 and VE-cadherin in retinoic
acid-mediated trans-differentiation of breast cancer cells. PLoS
One. 3:e27142008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gandillet A, Serrano AG, Pearson S, Lie
ALM, Lacaud G and Kouskoff V: Sox7-sustained expression alters the
balance between proliferation and differentiation of hematopoietic
progenitors at the onset of blood specification. Blood.
114:4813–4822. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kamachi Y and Kondoh H: Sox proteins:
regulators of cell fate specification and differentiation.
Development. 140:4129–4144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chan DW, Mak CS, Leung TH, Chan KK and
Ngan HY: Down-regulation of Sox7 is associated with aberrant
activation of Wnt/b-catenin signaling in endometrial cancer.
Oncotarget. 3:1546–1556. 2012.PubMed/NCBI
|
|
13
|
Katoh M: Expression of human SOX7 in
normal tissues and tumors. Int J Mol Med. 9:363–368.
2002.PubMed/NCBI
|
|
14
|
Hayano T, Garg M, Yin D, et al: SOX7 is
down-regulated in lung cancer. J Exp Clin Cancer Res. 32:172013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Futaki S, Hayashi Y, Emoto T, Weber CN and
Sekiguchi K: Sox7 plays crucial roles in parietal endoderm
differentiation in F9 embryonal carcinoma cells through regulating
Gata-4 and Gata-6 expression. Mol Cell Biol. 24:10492–10503. 2004.
View Article : Google Scholar
|
|
16
|
Zhang Y, Huang S, Dong W, et al: SOX7,
down-regulated in colorectal cancer, induces apoptosis and inhibits
proliferation of colorectal cancer cells. Cancer Lett. 277:29–37.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong WD, Qin GQ, Dai QS, et al: SOXs in
human prostate cancer: implication as progression and prognosis
factors. BMC Cancer. 12:2482012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karim R, Tse G, Putti T, Scolyer R and Lee
S: The significance of the Wnt pathway in the pathology of human
cancers. Pathology. 36:120–128. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Polakis P: Wnt signaling in cancer. Cold
Spring Harb Perspect Biol. 4:2012. View Article : Google Scholar
|
|
21
|
Kolligs FT, Bommer G and Goke B:
Wnt/beta-catenin/tcf signaling: a critical pathway in
gastrointestinal tumorigenesis. Digestion. 66:131–144. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao P, Li Y and Lu Y: Aberrant expression
of CD133 protein correlates with Ki-67 expression and is a
prognostic marker in gastric adenocarcinoma. BMC Cancer.
10:2182010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xi HQ and Zhao P: Clinicopathological
significance and prognostic value of EphA3 and CD133 expression in
colorectal carcinoma. J Clin Pathol. 64:498–503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Filomena A, Saieva C, Lucchetti V, et al:
Gastric cancer surveillance in a high-risk population in tuscany
(Central Italy): preliminary results. Digestion. 84:70–77. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wegner M: From head to toes: the multiple
facets of Sox proteins. Nucleic Acids Res. 27:1409–1420. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kormish JD, Sinner D and Zorn AM:
Interactions between SOX factors and Wnt/beta-catenin signaling in
development and disease. Dev Dyn. 239:56–68. 2010.PubMed/NCBI
|
|
27
|
Willert K and Nusse R: Beta-catenin: a key
mediator of Wnt signaling. Curr Opin Genet Dev. 8:95–102. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu WK, Cho CH, Lee CW, et al:
Dysregulation of cellular signaling in gastric cancer. Cancer Lett.
295:144–153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chan DW, Chan CY, Yam JW, Ching YP and Ng
IO: Prickle-1 negatively regulates Wnt/beta-catenin pathway by
promoting Dishevelled ubiquitination/degradation in liver cancer.
Gastroenterology. 131:1218–1227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qi J and Zhu YQ: Targeting the most
upstream site of Wnt signaling pathway provides a strategic
advantage for therapy in colorectal cancer. Curr Drug Targets.
9:548–557. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takash W, Canizares J, Bonneaud N, et al:
SOX7 transcription factor: sequence, chromosomal localisation,
expression, transactivation and interference with Wnt signalling.
Nucleic Acids Res. 29:4274–4283. 2001. View Article : Google Scholar
|
|
32
|
Li B, Ge Z, Song S, et al: Decreased
expression of SOX7 is correlated with poor prognosis in lung
adenocarcinoma patients. Pathol Oncol Res. 18:1039–1045. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Castillo SD and Sanchez-Cespedes M: The
SOX family of genes in cancer development: biological relevance and
opportunities for therapy. Expert Opin Ther Targets. 16:903–919.
2012. View Article : Google Scholar : PubMed/NCBI
|