Introductions
Various growth factors, also known as cytokines or
steroid hormones, regulate a variety of cellular processes, as well
as the development of tumors, inflammation and wound healing. These
growth factors differ from well-known polypeptide hormones, such as
insulin and adrenocorticotropic hormone, not only in the response
elicited, but also in the mode of delivery from the secreting to
the responding cell. Growth factors also have different cell-type
specificities and different functions (1–4).
For example, epidermal growth factor (EGF), basic fibroblast growth
factor (bFGF) and keratinocyte growth factor (KGF) are thought to
play a role in wound healing and the regulation of class II major
histocompatibility complex, macrophages and lymphocytes. During
this process, inflammation initiates healing. The regulation of
inflammation is so important that homeostatic mechanisms have
evolved to control this process (1,5–9).
Insulin-like growth factor (IGF)-1 displays pleiotropic properties,
including the ability to promote cellular proliferation and
differentiation, as well as processes involved in
metabolism/hypertrophy, such as nutrient transport, energy storage,
gene transcription and protein synthesis (10). Superoxide dismutase (SOD), one of
the most important antioxidants, is an enzyme that catalyzes the
dismutation of superoxide (O2−) into oxygen
and hydrogen peroxide and serves as a key antioxidant in cells
(11). The aforementioned growth
factors, including EGF, bFGF, KGF, IGF-1 and SOD, act by binding to
their respective receptor tyrosine kinases, followed by the
downstream signaling and activation of the protein kinase C, AKT
and ERK signaling pathways. In particular, mitogen-activated
protein kinase (MAPK) signaling, including ERK, plays a critical
role in innate immune responses (8,11,12).
MAPKs are serine/threonine-specific protein kinases
(13–16). They are important upstream factors
that lead to the activation of nuclear factor-κB (NF-κB) (6,17).
They are mainly composed of three subfamily members: ERK, JNK and
p38. The MAPK signaling pathway regulates a wide variety of
cellular events, including complex cellular programs, such as
differentiation, proliferation, apoptosis and processes involved in
immune response (18). The
phosphorylation of MAPKs modulates the expression of a variety of
genes involved in immune and inflammatory responses, including
inducible nitric oxide (NO) synthase (iNOS) and cyclooxygenase-2
(COX-2).
In addition, NF-κB nuclear translocation, as well as
inhibitory factor-κB (IκB) phosphorylation and degradation are
important inflammatory factors. The expression of pro-inflammatory
cytokines is mainly regulated by the NF-κB pathway (19). In unstimulated cells, NF-κB
resides in the cytoplasm as an inactive NF-κB-IκB complex (20).
Although there are several studies focusing on the
growth factors and pathways associated with inflammation (8,12,21), the mechanisms underlying the
inflammatory response, particularly the response to a mixture of
the five aforementioned growth factors, have not been investigated
to date.
In this study, the anti-inflammatory effects of
mixtures of recombinant growth factors (MRGFs) on the generation of
several chemokines, cytokines and enzymes involved in the
inflammatory process, such as inducible iNOS, COX-2, interleukin
(IL)-1β, IL-6, IL-10, IL-12p40, granulocyte-macrophage
colony-stimulating factor (GM-CSF), monocyte chemoattractant
protein-1 (MCP-1), tumor necrosis factor-α (TNF-α) and NO in
lipopolysaccharide (LPS)-stimulated RAW 264.7 cells, were
investigated. We also investigated whether MRGFs affect the
LPS-stimulated ERK and NF-κB signaling pathways.
Materials and methods
Materials
Mixed in the same ratio, recombinant human EGF,
recombinant human bFGF, recombinant human KGF, recombinant human
IGF-1 and recombinant human SOD were provided by Nutrex Technology
Co., Ltd. (Seoul, Korea).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and LPS were purchased from Sigma Chemical Co. (St. Louis, MO,
USA); Dulbecco’s modified Eagle’s medium (DMEM), fetal calf serum
(FBS), trypsin EDTA, phosphate-buffered saline (PBS) and
penicillin/streptomycin were purchased from WelGENE Co. (Daegu,
Korea). Antibodies specific to COX-2, and GAPDH were purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA); iNOS was
purchased from Pharmingen BD Biosciences (San Diego CA, USA).
Antibodies against phosphorylated (p-)ERK, ERK, p-JNK, JNK, p-p38,
p38, NF-κB, p-IκB, IκB and lamin B1 were purchased from Cell
Signaling Technology (Berverly, MA, USA). PD98059 and IKK inhibitor
VII were purchased from Sigma Chemical Co. and Calbiochem (San
Diego, USA), respectively. The secondary antibodies, anti-goat IgG,
anti-mouse IgG and anti-rabbit IgG were purchased from Vector
Laboratories (Burlingame, CA, USA). The iNOS, COX-2 and GAPDH
oligonucleotide primers were obtained from Bioneer, Inc. (Seoul,
Korea).
Culture of RAW 264.7 cells
The RAW 264.7 murine macrophage cells were
maintained at 37°C in a humidified atmosphere of 95% air and 5%
CO2 in DMEM supplemented with 10% heat-inactivated fetal
bovine serum, 100 U/ml penicillin and 10 μg/ml streptomycin.
Cell viability
Cell viability was assessed by MTT assay that was
performed using a slightly modified version of the method described
in the study by Twentyman and Luscombe (22). Seeded RAW 264.7 cells in a 12-well
plate were treated with LPS and MRGFs after 24 h of incubation. The
supernatant was removed and MTT solution was added to each well
followed by further incubation for 4 h. Subsequently, 1.5 ml of
dimethyl sulfoxide (DMSO) were added to each well to solubilize any
deposited formazon. Following incubation for 10 min at room
temperature, the optical density (OD) was determined at 540 nm on
an ELISA plate reader (Thermomax, Molecular Devices, Sunnyvale, CA,
USA).
NO assay
NO production was assessed by measuring nitrite
accumulation. After the RAW 264.7 cells were seeded in a 12-well
plate, LPS (100 ng/ml) and MRGFs at the indicated concentrations
(0.01, 0.1, 1 and 10 μg/ml) were added to the culture medium,
followed by incubation for 24 h. Cells treated with LPS only were
used as controls. The concentration of nitrite in the spent culture
medium was determined using the Griess reaction. Subsequently, 100
μl of supernatant from each well were transferred to a 96-well
plate and mixed with 100 μl of Griess reagent (Thermo Fisher
Scientific, Wilmington, DE, USA) in a separate 96-well plate.
Following incubation for 10 min at room temperature, OD was
determined at 540 nm on an ELISA plate reader.
Cell lysate preparation and western blot
analysis
Treated whole cell extracts were lysed in RIPA
buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 0.5% Triton
X-100, 0.1% sodium dodecyl sulfate (SDS) (Sigma Chemical Co.) and a
protease inhibitor cocktail tablet (Roche Diagnostics,
Indianapolis, IN, USA) for the preparation of cellular extracts.
Cytoplasmic and nuclear proteins were extracted using buffer A
[HEPES 10 mmol/l, pH 7.9, KCl 10 mmol/l, 2 mM EDTA,
phenylmethylsulfonyl fluoride (PMSF) 1 mmol/l, 1 mM EGTA,
dithiothreitol (DTT) 1 mmol/l, aprotinin 1 mg/l and protease
inhibitor cocktail tablet 5 mg/ml] and buffer B (HEPES 20 mmol/l,
pH 7.9, NaCl 420 mmol/l, edetic acid 0.1 mmol/l, egatazic acid 0.1
mmol/l, PMSF 1 mmol/l, DTT 1 mmol/l, aprotinin 1 mg/l and protease
inhibitor cocktail tablet 1 mg/ml), as previously described
(23). The protein concentration
of the extracts was estimated using Bradford reagent (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) with bovine serum albumin as
the standard, as previously described (24).
For western blot analysis, cell lysates containing
20 μg of proteins were resolved by 10–12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis, and transferred onto
polyvinylidene fluoride (PVDF) membranes. The membranes were washed
with Tris-buffered saline (10 mM Tris, 150 mM NaCl) containing
0.05% Tween-20 (TBST) and blocked in TBST containing 5% non-fat
dried milk. The membranes were further incubated with respective
specific antibodies. The membranes were continuously incubated with
appropriate secondary antibodies coupled to horseradish peroxidase
and developed using enhanced chemiluminescence (ECL) western
blotting detection reagents (Amersham Pharmacia Biotech,
Piscataway, NJ, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s
instructions following treatment and quantified using an ND-1000
spectrophotometer (Thermo Fisher Scientific) with a ratio of
absorbance at 260 nm. cDNA was synthesized with 2 μg of denatured
total RNA in a final volume of 20 μl of buffer containing
MgCl2, KCl, dNTPs and oligo(dT) reverse transcriptase by
incubation at 42°C for 60 min. The cDNA obtained was amplified with
the following primers: iNOS forward, 5′-CTA CCT ACC TGG GGA ACA CCT
GGG-3′ and reverse, 5′-GGA GGA GCT GAT GGA GTA GTA GCG G-3′; COX-2
forward, 5′-CTG TAT CCC GCC CTG CTG GTG-3′ and reverse, 5′-ACT TGC
GTT GAT GGT GGC TGT CTT-3′; and GAPDH forward, 5′-GCC AAA AGG GTC
ATC ATC TC-3′ and reverse, 5′-GGT CCT CAG TGT AGC CCA AG-3′.
Application was performed using PCR Master Mix (Takara Bio Inc.,
Shiga, Japan) in a total volume 20 μl. PCR cycling conditions
consisted of denaturation at 94°C for 30 sec, annealing at 60°C for
1 min and extension at 72°C for 30 sec. The products were
electrophoresed for 30 min at 100 V on a 1% agarose gel. Gels were
visualized using the Molecular Imager® Gel Doc™ XR
imaging system (Bio-Rad Laboratories, Inc.).
Real-time PCR
Total RNA and the cDNAs were generated as described
above. Real-time PCR was performed with a C1000™ Thermal Cycler
(Bio-Rad Laboratories, Inc.) using SYBR-Green (Takara Bio Inc.).
Reactive mixtures were incubated for 40 cycles at 95°C for 15 sec,
58°C for 45 sec and 72°C for 20 sec. Gene expression was normalized
to those of the housekeeping gene, GAPDH.
ELISA for the detection of cytokine
production
After the RAW 264.7 cells were seeded in a 24-well
plate, LPS and MRGFs were added to each well and followed by
incubation for 24 h. The cytokine concentrations in the culture
medium were measured using the ELISA kit for each cytokine
(eBioscience, San Diego, CA, USA).
Statistical analysis
Statistical analyses were performed using SPSS
version 18.0 for Windows (SPSS Inc., Chicago, IL, USA). Results are
expressed as the means ± standard deviation, as previously
described (25). Data were
analyzed using one-way ANOVA followed by a Duncan’s test for
multiple comparison; a two-tailed value of P<0.05 was considered
to indicate a statistically significant difference.
Results
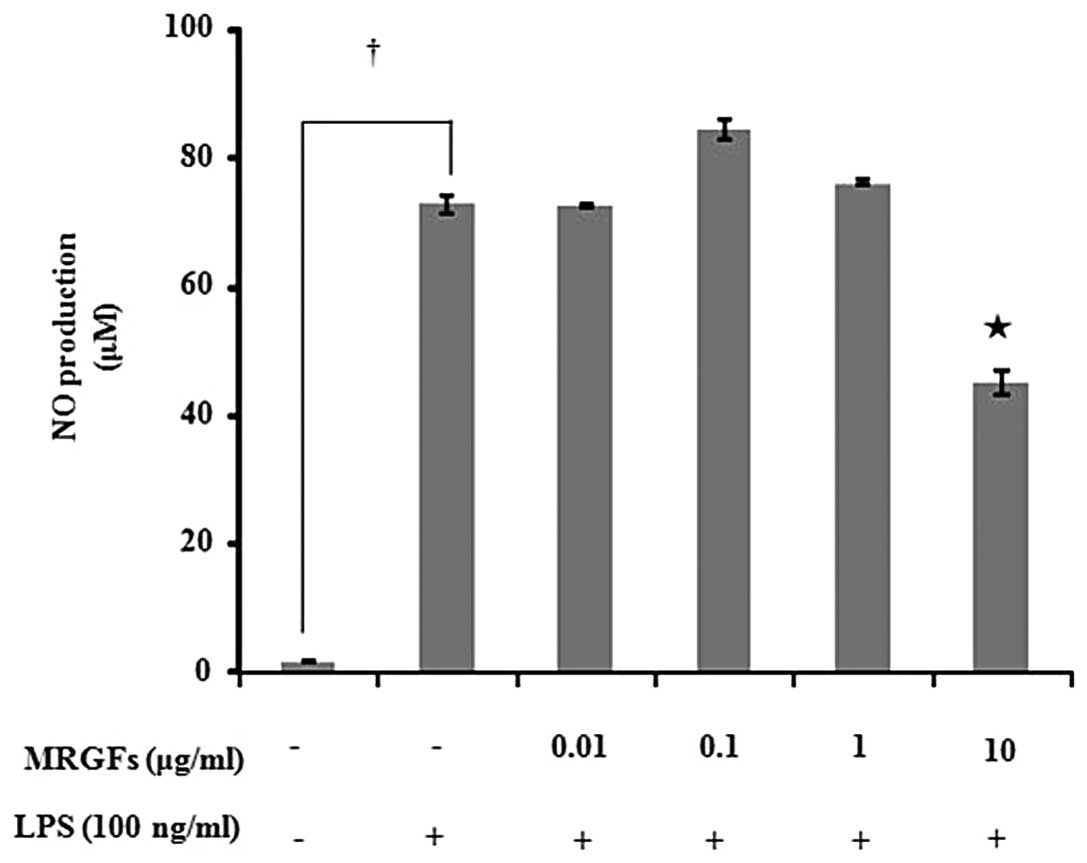
Effect of MRGFs on cell viability and NO
production
After examining the effects of each of the five
growth factors (EGF, bFGF, KGF, IGF-I and SOD) on NO production and
cell viability, we examined the effects of MRGFs on the production
of NO. As none of the five growth factors decreased cell viability
or NO production up to a concentration of 100 ng/ml in the
LPS-stimulated RAW 264.7 cells (data not shown), we prepared MRGFs
by mixing 100 ng/ml of each of the five growth factors in the same
ratio. As the MRGFs did not decrease the viability of the RAW 264.7
cells up to a concentration of 10 μg/ml (data not shown), this
concentration was used in the subsequent experiments. The RAW 264.7
cells were incubated with LPS (100 ng/ml) and MRGFs at
concentrations of 0.01, 0.1, 1 and 10 μg/ml for 24 h, and the NO
production was measured. The levels of NO production following
treatment of the RAW264.7 cells with the MRGFs are shown in
Fig. 1. Following treatment with
10 μg/ml of MRGFs, NO production significantly decreased in the RAW
264.7 cells compared to the LPS-stimulated control (P<0.05).
These results demonstrate that MRGFs inhibit the production of NO
by suppressing iNOS activity.
Inhibitory effects of MRGFs on iNOS and
COX-2 protein and mRNA expression in LPS-stimulated RAW 264.7
cells
We investigated the effects of MRGFs on
LPS-stimulated iNOS and COX-2 protein expression by western blot
analysis and mRNA expression by RT-PCR and real-time PCR. After the
cells were co-treated with LPS (100 ng/ml) and MRGFs at a
concentration of 0.01, 0.1, 1 and 10 μg/ml for 24 and 6 h, we
harvested the protein and mRNA samples. MRGFs at a concentration of
10 μg/ml significantly decreased iNOS and COX-2 protein and mRNA
expression in the LPS-stimulated cells (Fig. 2). Therefore, the MRGFs showed a
significant inhibitory effect on the production of pro-inflammatory
mediators, such as iNOS and COX-2 in the LPS-stimulated RAW 264.7
cells.
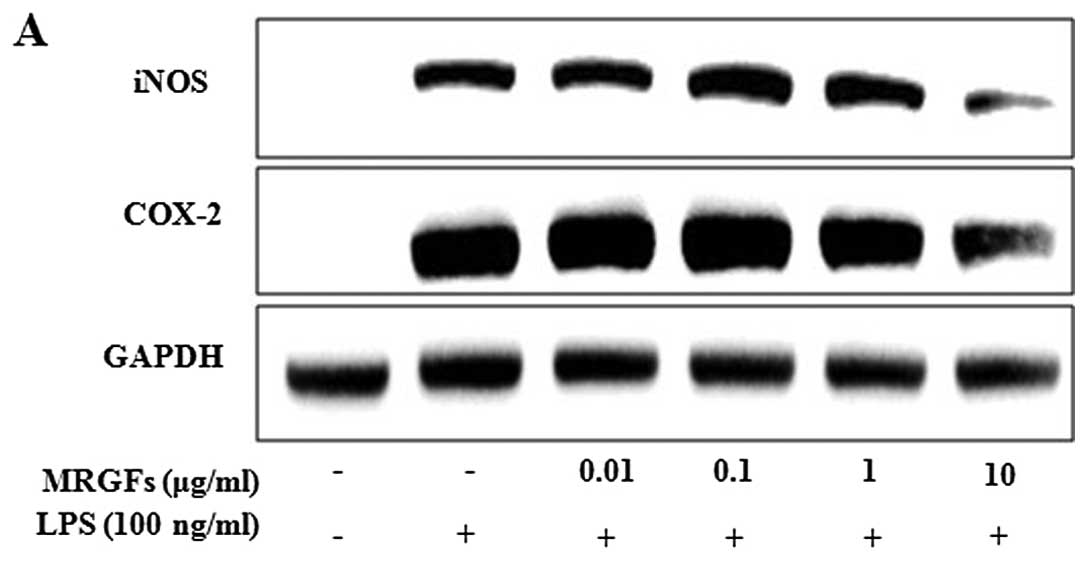 | Figure 2Effects of mixtures of recombinant
growth factors (MRGFs) on the protein and mRNA expression of
inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2)
in RAW 264.7 cells stimulated with lipopolysaccharide (LPS). (A)
RAW 264.7 cells were co-treated with various concentrations of
MRGFs (0, 0.01, 0.1, 1 and 10 μg/ml) and LPS (100 ng/ml) for 24 h.
MRGFs, at 10 μg/ml inhibited the LPS-stimulated protein expression
of iNOS and COX-2, as shown by in western blot analysis. (B and C)
After the cells were co-treated with various concentrations of
MRGFs (0, 0.01, 0.1, 1 and 10 μg/ml) and LPS (100 ng/ml) for 6 h,
total RNA was extracted, and cDNA was synthesized for RT-PCR and
real-time PCR. The results indicated that 10 μg/ml of MRGFs
inhibited the LPS-stimulated mRNA expression of iNOS and COX-2. (C)
Data are the means ± standard deviation (SD) (n=3).
†P<0.05 vs. control; *P<0.05 vs.
LPS-stimulated mRNA expression. |
Inhibitory effects of MRGFs on MAPK
phosphorylation in LPS-stimulated RAW 264.7 cells
We examined the effects of MRGFs on LPS-stimulated
MAPK phosphorylation in the RAW 264.7 cells. After the cells were
stimulated with LPS, western blot analysis was performed to
determine the total and phosphorylated levels of ERK. Compared with
the control, the levels of phosphorylated ERK significantly
increased after the cells were stimulated with LPS for 2 h
(Fig. 3A). The cells were then
co-treated with LPS (100 ng/ml) and MRGFs at a concentration of
0.01, 0.1, 1 and 10 μg/ml for 2 h. At the concentration of 10
μg/ml, the MRGFs inhibited the phosphorylation of ERK, but not that
of JNK and p38 in the LPS-stimulated RAW 264.7 cells (Fig. 3B). To confirm the causal link
between the inhibition of the phosphorylation of MAPKs and MRGFs,
the RAW 264.7 cells were pre-treated with ERK inhibitor (PD98059,
30 μM) for 1 h. Following treatment with LPS (100 ng/ml) and MRGFs
(10 μg/ml) for 2 h, we confirmed that the production of
LPS-stimulated pro-inflammatory mediators, such as iNOS and COX-2,
as well as ERK phosphorylation were inhibited by 30 μM/ml of
PD98059 and 10 μg/ml of MRGFs (Fig.
3C).
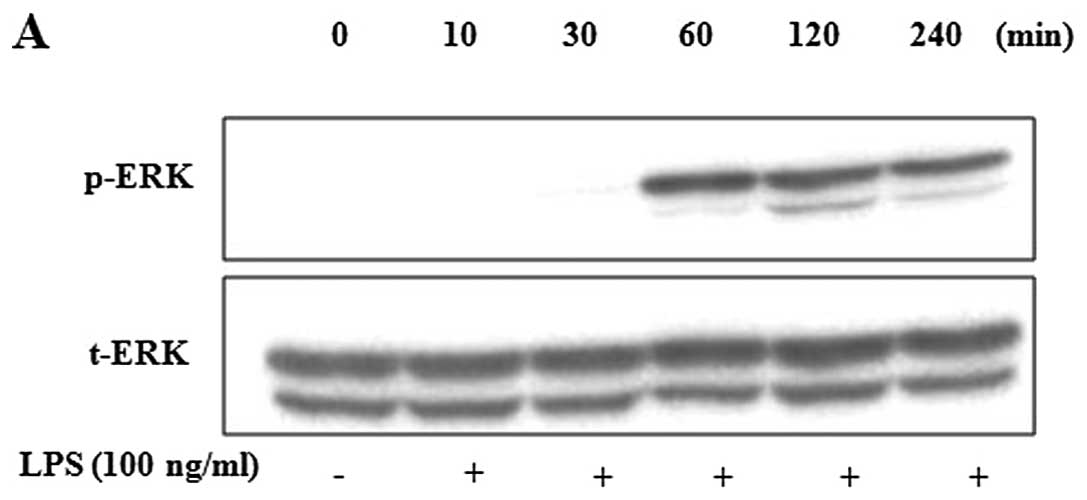 | Figure 3Effects of mixtures of recombinant
growth factors (MRGFs) on the phosphorylation of mitogen-activated
protein kinases (MAPKs). (A) RAW 264.7 cells were incubated with
lipopolysaccharide (LPS) for the indicated time. LPS stimulation
increased the phosphorylation of ERK at 2 h. (B) RAW 264.7 cells
were co-treated with various concentrations of MRGFs (0, 0.01, 0.1,
1 and 10 μg/ml) and LPS (100 ng/ml) for 2 h. MRGFs, at 10 μg/ml,
inhibited LPS-stimulated ERK phosphorylation, as shown by western
blot analysis. (C) After the RAW 264.7 cells were pre-treated with
PD98059 (10, 20 or 30 μM/ml) for 1 h, they were co-treated with LPS
(100 ng/ml) and MRGFs (10 μg/ml) for 2 h. Cell lysates were
analyzed by western blot analysis using various antibodies against
inducible nitric oxide synthase (iNOS) and cyclooxygenase-2
(COX-2). PD98059 (ERK inhibitor) inhibited the LPS-induced
production of iNOS and COX-2 in a dose-dependent manner. The
production of pro-inflammatory mediators, such as iNOS and COX-2,
and ERK phosphorylation, which were induced by LFS were both
inhibited by 30 μM/ml of PD98059 and 10 μg/ml of MRGFs. |
Inhibitory effects of MRGFs on NF-κB
nuclear translocation, and IκB phosphorylation and degradation in
LPS-stimulated RAW 264.7 cells
The cells were co-treated with LPS (100 ng/ml) and
MRGFs at a concentration of 0.01, 0.1, 1 and 10 μg/ml for 4 h. The
effects of MRGFs on NF-κB nuclear translocation, and IκB
phosphorylation and degradation in the cytoplasm and nucleus were
then assessed by western blot analysis. Fig. 4A shows that the LPS-stimulated IκB
phosphorylation and degradation was significantly inhibited by
MRGFs (10 μg/ml) in the cytoplasm of RAW 264.7 cells. Moreover, the
amount of NF-κB in the nucleus was markedly increased upon exposure
to LPS alone, but MRGFs (10 μg/ml) inhibited the LPS-stimulated
nuclear translocation of NF-κB. To confirm whether the MRGFs
suppressed the activation of NF-κB, the cells were pre-treated with
the upstream inhibitor of NF-κB, IKK inhibitor VII, for 2 h and
exposed to LPS (100 ng/ml) and MRGFs for 4 h. We confirmed that the
activation of the LPS-stimulated NF-κB pathway was inhibited by
both 1 μM/ml of IKK inhibitor VII and 10 μg/ml of the MRGFs
(Fig. 4B).
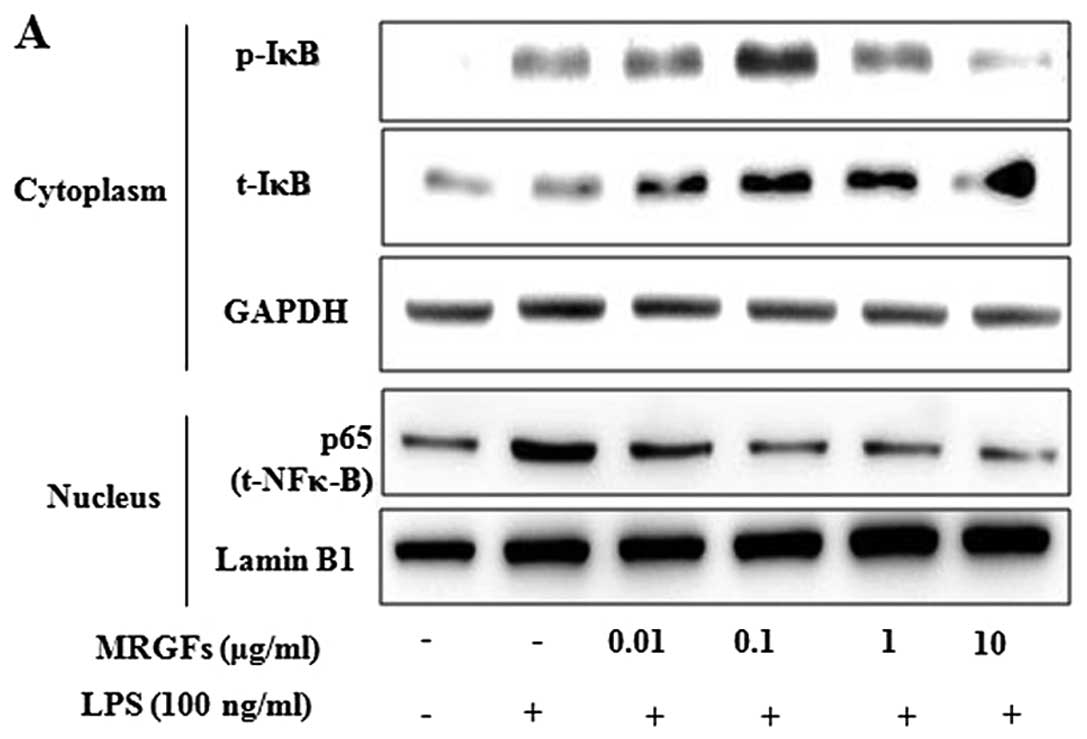 | Figure 4Effects of mixtures of recombinant
growth factors (MRGFs) on lipopolysaccharide (LPS)-stimulated
nuclear factor-κB (NF-κB) activation, and inhibitory factor-κB
(IκB) degradation and phosphorylation in RAW 264.7 cells. (A) After
the RAW 264.7 cells were co-treated with MRGFs (0, 0.01, 0.1, 1 and
10 μg/ml) and LPS (100 ng/ml), the cells were incubated for 4 h.
Cytoplasmic and nuclear extracts of the cells were measured by
western blot analysis. The results indicated that MRGFs (10 μg/ml)
inhibited the LPS-stimulated IκB phosphorylation and degradation in
the cytosol, and NF-κB phosphorylation in the nucleus. (B) After
the RAW 264.7 cells were pre-treated with IKK inhibitor VII (0.1,
0.5 or 1 μM/ml) for 2 h, the cells were co-treated with MRGFs (10
μg/ml) and LPS (100 ng/ml) for 4 h. The cell lysates were analyzed
by western blot analysis using various antibodies against p-IκBα,
IκBα and NF-κB. IKK inhibitor VII (1 μM/ml) and MRGFs (10 μg/ml)
inhibited the LPS-induced IκB phosphorylation and degradation in
the cytosol, and NF-κB phosphorylation in the nucleus. |
Inhibitory effects of MRGFs on
inflammatory cytokine production in LPS-stimulated RAW 264.7
cells
Inflammatory cytokines, such as IL-1β, IL-6 and
TNF-α, were released as a result of the activation of the NF-κB
pathway in the LPS-stimulated RAW 264.7 cells. We have already
confirmed that MRGFs inhibit the activation of the NF-κB pathway in
the above-mentioned results. The effects of the MRGFs on the
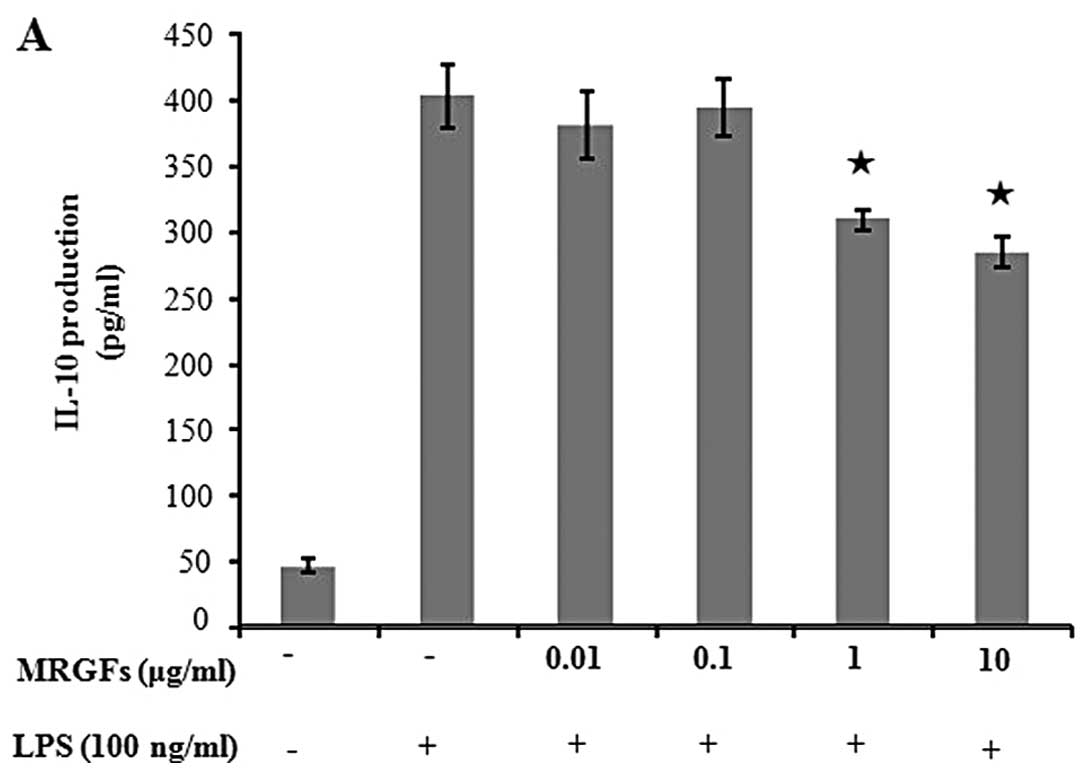
release of cytokines were assessed by ELISA. Table I shows the inhibitory effects of
the MRGFs on the production of inflammatory cytokines (IL-1β, IL-6,
IL-10, IL-12p40, GM-CSF, MCP-I and TNF-α) in the LPS-stimulated RAW
264.7 cells. Specifically, MRGFs, at a concentration of 10 μg/ml,
decreased the production of IL-10, IL-12p40, GM-CSF, MCP-I and
TNF-α in the LPS-stimulated RAW 264.7 cells (Fig. 5). These results indicate that
MRGFs can modulate the synthesis of several cytokines involved in
the inflammatory process.
 | Table IEffect of MRGFs on pro-inflammatory
cytokine production in LPS-stimulated RAW 264.7 cells. |
Table I
Effect of MRGFs on pro-inflammatory
cytokine production in LPS-stimulated RAW 264.7 cells.
|
Pro-inflammatorymediator (pg/ml) | Control | LPS 100 ng | Concentration
(μg/ml) of MRGFs (administered with LPS) |
|---|
|
|---|
| 0.01 | 0.1 | 1 | 10 |
|---|
| IL-1β | 7.18±0.72 | 71.88±3.29 | 69.76±1.36 | 67.43±3.18 | 72.76±3.85 | 62.23±12.81 |
| IL-6 | 8.27±5.17 | 1064.17±103.81 | 1055.74±51.34 | 1008.82±91.78 | 983.42±57.75 | 951.45±87.95 |
| IL-10 | 46.32±5.24 | 402.60±24.32 | 381.04±24.64 | 393.50±20.91 | 309.34±7.04a |
284.58±11.80a |
| IL-12p40 | 102.65±7.20 | 882.71±36.55 | 932.01±132.61 | 954.55±52.16 | 765.64±125.92 |
514.27±76.87a |
| GM-CSF | 295.91±27.69 | 2007.73±100.71 | 1858.30±96.60 | 1836.32±128.42 | 1500.67±461.97 |
959.34±288.08a |
| MCP-1 | 1539.87±377.35 | 3013.51±273.40 | 3224.93±238.52 | 2732.03±361.92 | 2433.15±321.58 |
2089.91±344.59a |
| TNF-α | 216.79±142.11 | 1981.54±98.78 | 1900.50±51.69 | 1854.94±81.89 |
1634.96±22.18a |
1128.78±46.50a |
Discussion
The major finding of this study is that MRGFs play
an important role in the LPS-stimulated inflammatory response.
Previous studies (1–4) have demonstrated that various growth
factors regulate a variety of cellular processes, such as cell
growth, differentiation and proliferation, by binding to specific
high-affinity cell membrane receptors. A number of studies have
found a correlation between growth factors and wound healing,
cancer and DNA synthesis in various cells (13,16). Nonetheless, little information is
available regarding the molecular mechanisms underlying the
anti-inflammatory effects of various combinations of growth
factors.
The activation of macrophages plays an important
role in the initiation and propagation of inflammatory responses by
inflammatory mediators (26).
Therefore, LPS-stimulated macrophage activation increased the
production of cytokines, such as IL-1β, TNF-α, GM-CSF and NO, which
is modulated by the upregulation of iNOS (27). iNOS and COX-2 are often present
together, share a number of similarities and play fundamental roles
in similar pathophysiological conditions, such as inflammation and
cancer (28,29).
The MAPK and NF-κB signaling pathways are important
in the regulation of inflammatory mediators. Members of the MAPK
family, including ERK, JNK and p38, are frequently involved in
LPS-stimulated inflammation and play a critical role in the
regulation of cell growth and differentiation, and in the control
of cellular responses to cytokines and stresses.
The transcription factor, NF-κB, controls a number
of inflammatory mediators, such as iNOS, COX-2 and cytokines, and
is important for immunity and inflammation (30). Previous studies have reported that
the LPS-induced stimulation of NF-κB signaling activity leading to
the activation of MAPK is a major mechanism underlying NO
production by iNOS (31). When
RAW 264.7 cells are stimulated by LPS, IκB is phosphorylated and
separated from NF-κB, resulting in the translocation of NF-κB to
the nucleus (32).
Cytokines can be used as markers of inflammation
(33). The cytokines, TNF-α and
IL-1β, are closely related to each other and share many biological
activities, i.e., pyrogenicity, activation of T lymphocytes,
stimulation of fibroblast proliferation and neutrophil activation
(34). IL-10 is a pleiotropic
cytokine that modulates the adaptive immune-related cell function.
It possesses immune stimulatory properties, including the ability
to activate T cells, B cells, natural killer (NK) cells and mast
cells (35,36). In addition, previous studies have
demonstrated that IL-6, MCP-1, GM-CSF and IL-12p40 are related
inflammatory mediators (35). In
particular, IL-12p40 has been reported to markedly upregulate the
expression of TNF-α and induce the expression of iNOS in a
dose-dependent manner (37).
In the present study, we treated LPS-stimulated RAW
264.7 cells with various concentrations of MRGFs. The aim was to
elucidate the pharmacological, biological and inhibitory effects of
MRGFs, which include EGF, bFGF, KGF, IGF-I and SOD, on the
production of inflammatory mediators in macrophages. Our data
clearly indicated that MRGFs suppressed the production of NO, iNOS
and COX-2 in the LPS-stimulated RAW 264.7 cells. In addition,
inhibiting the phosphorylation of ERK and NF-κB decreased the
production of these inflammatory mediators. Furthermore, our data
demonstrated that treatment with MRGFs decreased the production of
inflammatory cytokines, such as IL-10, IL-12p40, GM-CSF, MCP-I and
TNF-α, in LPS-stimulated RAW 264.7 cells.
In conclusion, MRGFs have the potential to decrease
the production of inflammatory mediators, such as iNOS, COX-2,
IL-10, IL-12p40, GM-CSF, MCP-I and TNF-α, by inhibiting the
phosphorylation of the ERK and the activation of the NF-κB
signaling pathways. These findings suggest that MRGFs may prevent
inflammatory diseases by suppressing MAPK- and NF-κB-mediated
inflammation.
Acknowledgements
This study was financially supported by the Ministry
of Knowledge Economy (MKE) and the Korea Institute for Advancement
of Technology (KIAT) through the Inter-ER Cooperation Projects.
Abbreviations:
|
NO
|
nitric oxide
|
|
MRGFs
|
mixtures of recombinant growth
factors
|
|
MAPK
|
mitogen-activated protein kinase
|
|
LPS
|
lipopolysaccharide
|
|
NF-κB
|
nuclear factor-κB
|
|
IκB
|
inhibitory factor-κB
|
References
|
1
|
Goustin AS, Leof EB, Shipley GD and Moses
HL: Growth factors and cancer. Cancer Res. 46:1015–1029. 1986.
|
|
2
|
Childs CB, Proper JA, Tucker RF and Moses
HL: Serum contains a platelet-derived transforming growth factor.
Proc Natl Acad Sci USA. 79:5312–5316. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Taguchi M, Moran SL, Zobitz ME, et al:
Wound-healing properties of transforming growth factor β (TGF-β)
inducible early gene 1 (TIEG1) knockout mice. J Musculoskelet Res.
11:63–69. 2008.
|
|
4
|
Hollwy RW and Kiernan JA: Control of the
initiation of DNA synthesis in 3T3 cells: serum factors. Proc Natl
Acad Sci USA. 71:2908–2911. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martin P: Wound healing - aiming for
perfect skin regeneration. Science. 276:75–81. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matt P, Schoenhoff F, Habashi J, et al:
Circulating transforming growth factor-beta in Marfan syndrome.
Circulation. 120:526–532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim HS: Assignment1 of the human basic
fibroblast growth factor gene FGF2 to chromosome 4 band q26 by
radiation hybrid mapping. Cytogenet Cell Genet. 83:731998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barrientos S, Stojadinovic O, Golinko MS,
Brem H and Tomic-Canic M: Growth factors and cytokines in wound
healing. Wound Repair Regen. 16:585–601. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rotolo S, Ceccarelli S, Romano F, Frati L,
Marchese C and Angeloni A: Silencing of keratinocyte growth factor
receptor restores 5-fluorouracil and tamoxifen efficacy on
responsive cancer cells. PLoS One. 3:e25282008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schultz G, Rotatori DS and Clark W: EGF
and TGF-alpha in wound healing and repair. J Cell Biochem.
45:346–352. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee JA, Song HY, Ju SM, et al:
Differential regulation of inducible nitric oxide synthase and
cyclooxygenase-2 expression by superoxide dismutase in
lipopolysaccharide stimulated RAW 264.7 cells. Exp Mol Med.
41:629–637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Andres C, Hasenauer J, Ahn HS, et al:
Wound-healing growth factor, basic FGF, induces Erk1/2-dependent
mechanical hyperalgesia. Pain. 154:2216–2226. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weston CR, Lambright DG and Davis RJ:
Signal transduction. MAP kinase signaling specificity. Science.
296:2345–2347. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su B and Karin M: Mitogen-activated
protein kinase cascades and regulation of gene expression. Curr
Opin Immunol. 8:402–411. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Herlaar E and Brown Z: p38 MAPK signalling
cascades in inflammatory disease. Mol Med Today. 5:439–447. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chan-Hui PY and Weaver R: Human
mitogen-activated protein kinase kinase kinase mediates the
stress-induced activation of mitogen-activated protein kinase
cascades. Biochem J. 336:599–609. 1998.PubMed/NCBI
|
|
17
|
Carter AB, Knudtson KL, Monick MM and
Hunninghake GW: The p38 mitogen-activated protein kinase is
required for NF-kappaB-dependent gene expression. The role of
TATA-binding protein (TBP). J Biol Chem. 274:30858–30863. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pearson G, Robinson F, Beers Gibson T, et
al: Mitogen-activated protein (MAP) kinase pathways: regulation and
physiological functions. Endocr Rev. 22:153–183. 2001.PubMed/NCBI
|
|
19
|
Francisco V, Costa G, Figueirinha A, et
al: Anti-inflammatory activity of Cymbopogon citratus leaves
infusion via proteasome and nuclear factor-κB pathway inhibition:
contribution of chlorogenic acid. J Ethnopharmacol. 148:126–134.
2013.
|
|
20
|
Kim HG, Shrestha B, Lim SY, et al:
Cordycepin inhibits lipopolysaccharide-induced inflammation by the
suppression of NF-kappaB through Akt and p38 inhibition in RAW
264.7 macrophage cells. Eur J Pharmacol. 545:192–199. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
O’Connor JC, McCusker RH, Strle K, Johnson
RW, Dantzer R and Kelley KW: Regulation of IGF-I function by
proinflammatory cytokines: at the interface of immunology and
endocrinology. Cell Immunol. 252:91–110. 2008.
|
|
22
|
Twentyman PR and Luscombe M: A study of
some variables in a tetrazolium dye (MTT) based assay for cell
growth and chemosensitivity. Br J Cancer. 56:279–285. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reddy DB and Reddanna P: Chebulagic acid
(CA) attenuates LPS-induced inflammation by suppressing NF-kappaB
and MAPK activation in RAW 264.7 macrophages. Biochem Biophys Res
Commun. 381:112–117. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jeong Y and Mangelsdorf DJ: Nuclear
receptor regulation of stemness and stem cell differentiation. Exp
Mol Med. 41:525–537. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tilg H, Wilmer A, Vogel W, et al: Serum
levels of cytokines in chronic liver diseases. Gastroenterology.
103:264–274. 1992.PubMed/NCBI
|
|
27
|
ter Steege JC, van de Ven MW, Forget PP,
Brouckaert P and Buurman WA: The role of endogenous IFN-gamma,
TNF-alpha and IL-10 in LPS-induced nitric oxide release in a mouse
model. Cytokine. 10:115–123. 1998.
|
|
28
|
Wu KK: Inducible cyclooxygenase and nitric
oxide synthase. Adv Pharmacol. 33:179–207. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Albini A and Sporn MB: The tumour
microenvironment as a target for chemoprevention. Nat Rev Cancer.
7:139–147. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barnes PJ and Karin M: Nuclear
factor-kappaB: a pivotal transcription factor in chronic
inflammatory diseases. N Engl J Med. 336:1066–1071. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim SH, Park HS, Lee MS, et al: Vitisin A
inhibits adipocyte differentiation through cell cycle arrest in
3T3-L1 cells. Biochem Biophys Res Commun. 372:108–113. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kao SJ, Lei HC, Kuo CT, et al:
Lipoteichoic acid induces nuclear factor-kappaB activation and
nitric oxide synthase expression via phosphatidylinositol 3-kinase,
Akt, and p38 MAPK in RAW 264.7 macrophages. Immunology.
115:366–374. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Heinrich PC, Castell JV and Andus T:
Interleukin-6 and the acute phase response. Biochem J. 265:621–636.
1990.PubMed/NCBI
|
|
34
|
Flamand L, Gosselin J, D’Addario M, et al:
Human herpesvirus 6 induces interleukin-1 beta and tumor necrosis
factor alpha, but not interleukin-6, in peripheral blood
mononuclear cell cultures. J Virol. 65:5105–5110. 1991.PubMed/NCBI
|
|
35
|
Yuk SS, Lim EM, Lee JY, et al:
Antiinflammatory effects of Epimedium brevicornum water extract on
lipopolysaccharide-activated RAW264.7 macrophages. Phytother Res.
24:1781–1787. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mocellin S, Marincola F, Rossi CR, Nitti D
and Lise M: The multifaceted relationship between IL-10 and
adaptive immunity: putting together the pieces of a puzzle.
Cytokine Growth Factor Rev. 15:61–76. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jana M, Dasgupta S, Saha RN, Liu X and
Pahan K: Induction of tumor necrosis factor-alpha (TNF-alpha) by
interleukin-12 p40 monomer and homodimer in microglia and
macrophages. J Neurochem. 86:519–528. 2003. View Article : Google Scholar : PubMed/NCBI
|