Introduction
It is widely recognized that tubular lesions
participate in the progression of chronic kidney disease (CKD), and
there is compelling evidence that the severity of tubular damage
has a more significant correlation with the reduction in creatinine
clearance as compared with glomerular damage scores (1,2).
Renal tubulointerstitial inflammation plays a central role in the
loss of renal function in CKD by promoting the expression of
pro-inflammatory cytokines, the generation of reactive oxygen
species (ROS) and cell apoptosis, ultimately leading to renal
fibrosis (3,4). Consistently, the extent of
inflammation positively correlates with kidney function and may be
used to predict long-term prognosis in some clinical settings
(5).
The levels of high-density lipoprotein (HDL)
inversely correlate with cardiovascular events by mediating reverse
cholesterol transport and exerting potent antioxidant,
anti-inflammatory and antithrombotic effects (6,7).
However, simply increasing the amount of circulating HDL does not
reduce the risk of developing coronary heart disease (CHD), or
CHD-related deaths and total deaths (8). Previous studies have demonstrated
that HDL is susceptible to damaging structural modifications,
including oxidation in atherosclerosis and diabetes in the presence
of systemic inflammation (9,10).
The oxidative modification of HDL not only deprives HDL of
important protective functions, but even transforms it into a
pro-oxidant and pro-inflammatory agent (9,11).
In the serum of patients with CKD, HDL has also been reported to be
in a state of enhanced susceptibility to oxidative modification,
particularly in diabetic nephropathy (12,13). However, the role of oxidized HDL
in mediating renal tubular damage remains unclear.
Knowing that tubular lesions play an important role
in the progression of CKD, in the present study, we aimed to
clarify the role of oxidized HDL in inducing inflammatory responses
in renal tubular cells, as well as the molecular mechanisms
involved.
Materials and methods
HDL oxidation and cell culture
Native HDL (Chemicon International, Inc., Billerica,
MA, USA) was incubated with CuSO4 (20 μmol/l final
concentration) in 1 mol/l PBS pH 7.4 at a lipoprotein concentration
of 1 mg protein/ml for 24 h at 37°C. Oxidation was terminated by
the addition of ethylenediaminetetraacetic acid (EDTA) (200 μmol/l
final concentration) and confirmed by measurements of
thiobarbituric acid reactive substances (TBARS) and oxidized HDL
was sterilized by passing it through a 0.22-μm filter, as
previously described (14).
Human renal proximal tubule epithelial cells (HK-2)
were purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA) and cultured in keratinocyte serum-free medium
(K-SFM) (Gibco, Grand Island, NY, USA) supplemented with 5 ng/ml
epidermal growth factor (EGF) and 40 mg/ml of bovine extract
(Gibco), as well as 100 U/ml of penicillin and 100 Ug/ml of
streptomycin. The cells were placed in an atmosphere of 5%
CO2-95% air at 37°C, and passaged by trypsinization
(0.25% trypsin, 0.02% EDTA) following the formation of a confluent
monolayer and placed in a serum-free medium 24 h prior to
stimulation. Oxidized HDL or native HDL was added to the medium at
the indicated concentrations for 24 h, and used for various
analyses.
Transfection of CD36 short interference
RNA (siRNA)
The HK-2 cells were transfected with 100 nM of CD36
siRNA (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and the
transfection procedure was performed using Lipofectamine 2000
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions. Briefly, 1 day prior to transfection,
the cells were plated in growth medium without antibiotics so that
they would be 50% confluent at the time of transfection.
Subsequently, CD36 siRNA or scrambled control plasmid
oligomer-Lipofectamine 2000 complexes were prepared, added to each
well containing cells and medium and mixed gently by rocking the
plate back and forth. The cells were incubated for 24 h and then
treated with various doses of oxidized HDL or native HDL.
Measurement of intracellular ROS
Measurement of intracellular ROS was based on the
ROS-mediated conversion of non-fluorescent
2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) into
dichlorodihydrofluorescein (DCFH), as previously described
(15,16). The fluorescence intensity reflects
enhanced oxidative stress. Following treatment as described above,
the HK-2 cells were incubated with DCFH-DA in medium at 37°C for 45
min and then washed with PBS. Following incubation, the DCFH
fluorescence of the cells from each well was imaged under a
fluorescence microscope and analyzed using AxioVision 4.5 software
(Carl Zeiss, Jena, Germany).
Apoptosis assay
Annexin V-fluorescein isothiocyanate/propidium
iodide (FITC/PI) staining was used to detect the apoptosis induced
by treatment with oxidized HDL in HK-2 cells. The HK-2 cells were
harvested by centrifugation at 300 × g for 5 min, followed by 2
washes with cold PBS. The cells were stained using an Annexin
V-FITC/PI staining kit (Roche Diagnostics, Indianapolis, IN, USA)
according to the manufacturer’s instructions. The early stages of
apoptosis were assayed using a flow cytometer (BD Biosciences, San
Jose, CA, USA).
Migration assays
The migratory function of the HK-2 cells was
measured using 24-well-modified Boyden chambers (Corning Life
Sciences, Oneonta, NY, USA). Following transfection and treatment
as described above, the cells (1×104) were detached,
resuspended and seeded onto the filters (8-μm pore size) in the top
compartment of the chamber. Following incubation at 37°C for 24 h,
the membrane was washed with PBS and fixed with 4% paraformaldehyde
for 15 min at room temperature. Non-migrating cells were gently
removed from the upper side of the Transwell, and the membrane of
the Transwell filter was then stained using hexamethyl
pararosaniline solution for a further 15 min, and the upper surface
of the filters was carefully wiped with a cotton-tipped applicator.
For quantification, the migrated cells were counted in 6 random
microscopic fields (×40) in a blinded manner.
Real-time reverse transcription PCR
Total RNA was extracted using the RNase mini kit
(Invitrogen Life Technologies) and was reverse-transcribed. The
primer sequences were as follows: tumor necrosis factor-α (TNF-α)
forward, 5′-TGCTT GTTCCTCAGCCTCTT; and reverse, 5′-GGTTTGCTACAA
CATGGGCT; monocyte chemoattractant protein-1 (MCP-1) forward,
5′-CCCCAGTCACCTGCTGTTAT; and reverse, 5′-AGATCTCCTTGGCCACAATG;
regulated upon activation normal T cell expressed and secreted
(RANTES) forward, 5′-GAAGGAAGTCAGCATGCCTC; and reverse, 5′-AGCC
GATTTTTCATGTTTGC; CD36 forward, 5′-GAGAGCCT GTGCCTCATTTC; and
reverse, 5′-GACTGGCTCCAGAGT CTTGC; 18S forward,
5′-CGCACGGCCGGTACAGTGAA; and reverse, 5′-GGGAGAGGAGCGAGCGACCA.
Real-time PCR was performed using SYBR-Green PCR Master mix (Toyobo
Co., Ltd., Osaka, Japan) and Rotor-Gene-3000. A real-time PCR
system (Corbett Research Pty, Ltd., Sydney, Australia) was used
according to the manufacturer’s instructions. In brief, the PCR
amplification reaction mixture (25 μl) contained 1 μl cDNA, 0.4 mM
sense and antisense primer and 12.5 μl SYBR-Green I. After the
initial denaturation at 95°C for 10 min, the reaction was cycled 35
times. Each cycle consisted of denaturation at 95°C for 30 sec,
primer annealing at 60°C for 30 sec and primer extension at 72°C
for 30 sec. The results are presented as the relative expression of
TNF-α, MCP-1, RANTES and CD36 normalized to the expression of
18S.
Enzyme-linked immunosorbent assay
(ELISA)
The protein levels of TNF-α, MCP-1 and RANTES under
the different experimental conditions were determined in the cell
supernatants using commercial ELISA kits (R&D Systems, Inc.,
Minneapolis, MN, USA) according to the manufacturer’s instructions,
and each protein level was normalized with the cell numbers, as
previously described (17).
Western blot analysis
The HK-2 cells were washed twice with cold PBS and
lysed in protein extract buffer (1 ml protein extract buffer with
10 μl mixture of protease inhibitors and 10 μl 100-mM PMSF) at 4°C
for 30 min. The lysates were centrifuged at 14,000 × g and 4°C for
10 min. The supernatant was collected and the concentration of
total soluble protein was quantified using the BCA protein assay
kit (Pierce Biotechnology, Inc., Rockford, IL, USA). The extracts
were employed for immunoblot analysis. Cellular proteins were
electrophoresed through a 10% SDS-PAGE gel before transferring to
PVDF membranes. After blocking for 1 h at room temperature in
blocking buffer (1% gelatin in PBS with 0.05% Tween-20), the
membranes were incubated for 16 h with monoclonal mouse anti-human
β-actin antibody (1:1,000; Santa Cruz Biotechnology); monoclonal
mouse anti-human CD36 antibody (1:1,000; Abcam, Cambridge, UK),
monoclonal mouse anti-human poly(ADP-ribose) polymerase (PARP)
antibody, polyclonal rabbit anti-human Src antibody, polyclonal
rabbit anti-human phosphorylated (phospho)-Src (tyr416) antibody,
polyclonal rabbit anti-human phospho-Src (tyr527) antibody,
monoclonal mouse anti-human extracellular regulated kinase (ERK)1/2
antibody, monoclonal mouse anti-human phospho-ERK1/2 antibody,
monoclonal mouse anti-human p38 antibody, monoclonal mouse
anti-human phospho-p38 antibody, monoclonal mouse anti-human c-Jun
N-terminal kinase (JNK) antibody, monoclonal mouse anti-human
phospho-JNK antibody, monoclonal mouse anti-human p65 antibody and
monoclonal mouse anti-human phospho-p65 antibody (1:1,000; Cell
Signaling Technology, Danvers, MA, USA) in PBS Tween-20. The
membranes were washed and incubated for 1 h at room temperature
with a secondary antibody for 1 h at room temperature and then
visualized by enhanced chemiluminescence detection reagents.
Relative intensities of the protein bands were analyzed using
ImageJ software.
Statistical analysis
The results are expressed as the means ± standard
deviation (SD) from at least 3 experiments. Significance was
determined by analysis of variance (ANOVA) and a t-test using
StatView 4.0. Differences with P-values <0.05 were considered
statistically significant.
Results
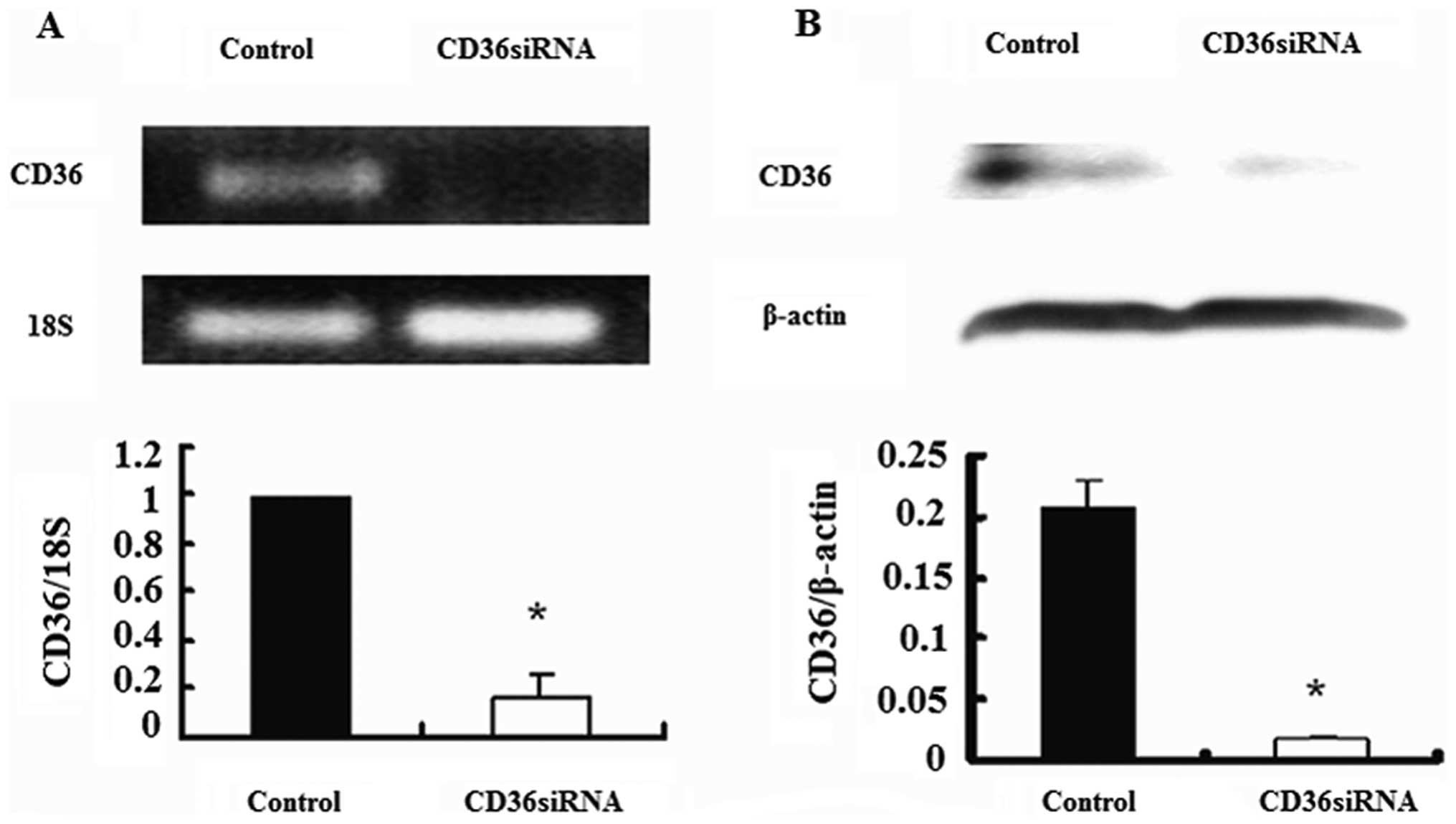
CD36 siRNA suppresses the expression of
CD36 in HK-2 cells
In order to elucidate the role of CD36 in the
impairment of cellular function induced by oxidized HDL, we
transfected the cells with CD36 siRNA. As shown in Fig. 1, 100 nM CD36 siRNA effectively
suppressed CD36 mRNA (approximately 80% inhibition) and protein
expression (approximately 90% inhibition) in the HK-2 cells. Hence,
CD36 siRNA was applied in the following experiments.
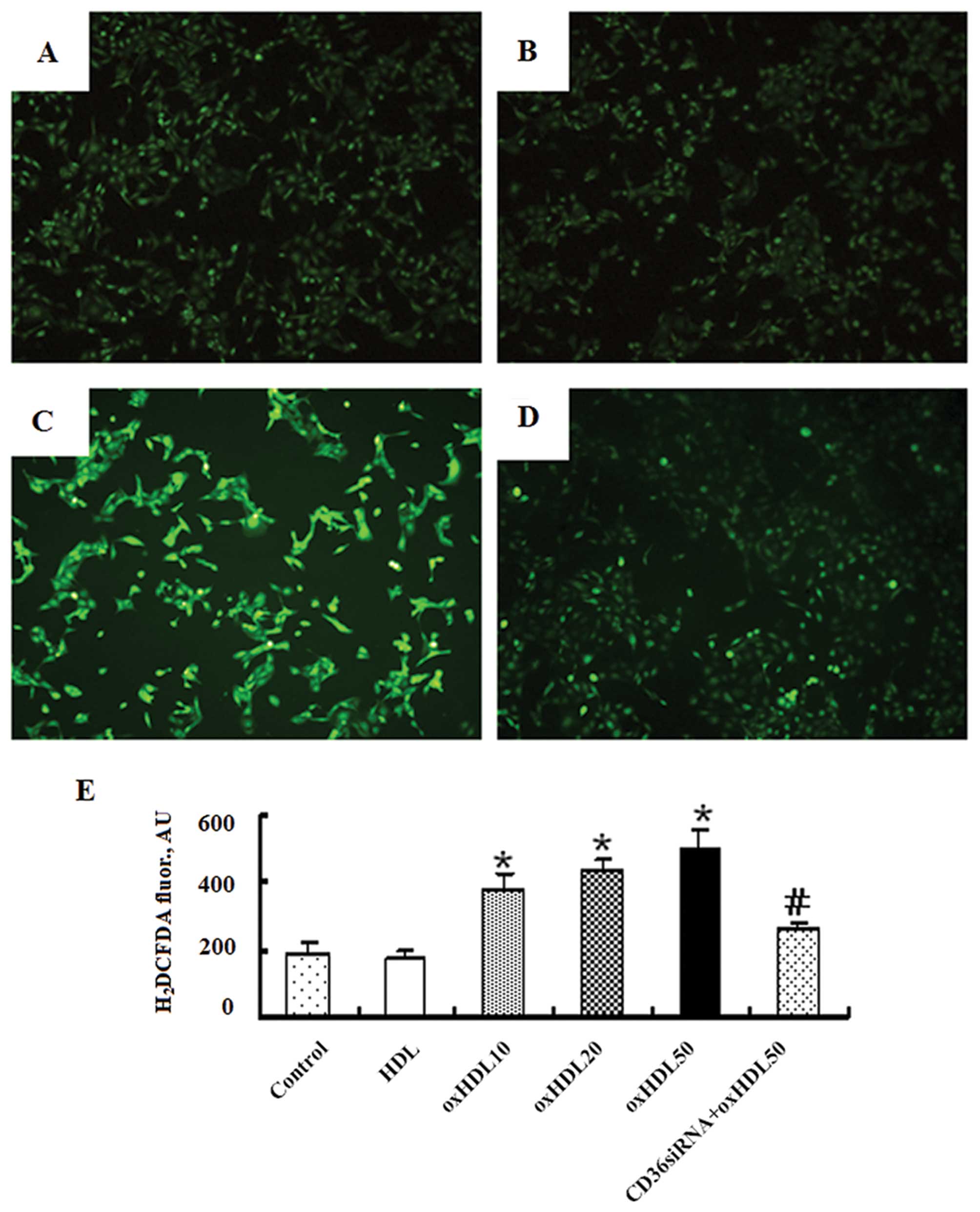
Oxidized HDL increases ROS production in
HK-2 cells
The HK-2 cells were co-incubated with various
concentrations of oxidized HDL (0, 10, 20 and 50 μg/ml), HDL (50
μg/ml) or oxidized HDL (50 μg/ml) plus CD36 siRNA (100 nM).
Oxidized HDL has been shown to induce oxidative stress in human
umbilical vein endothelial cells by promoting the generation of
intracellular ROS (18). As shown
in Fig. 2, oxidized HDL increased
the generation of ROS in the HK-2 cells in a
concentration-dependent manner and this effect was markedly
attenuated by CD36 siRNA, while native HDL had no effect on ROS
production.
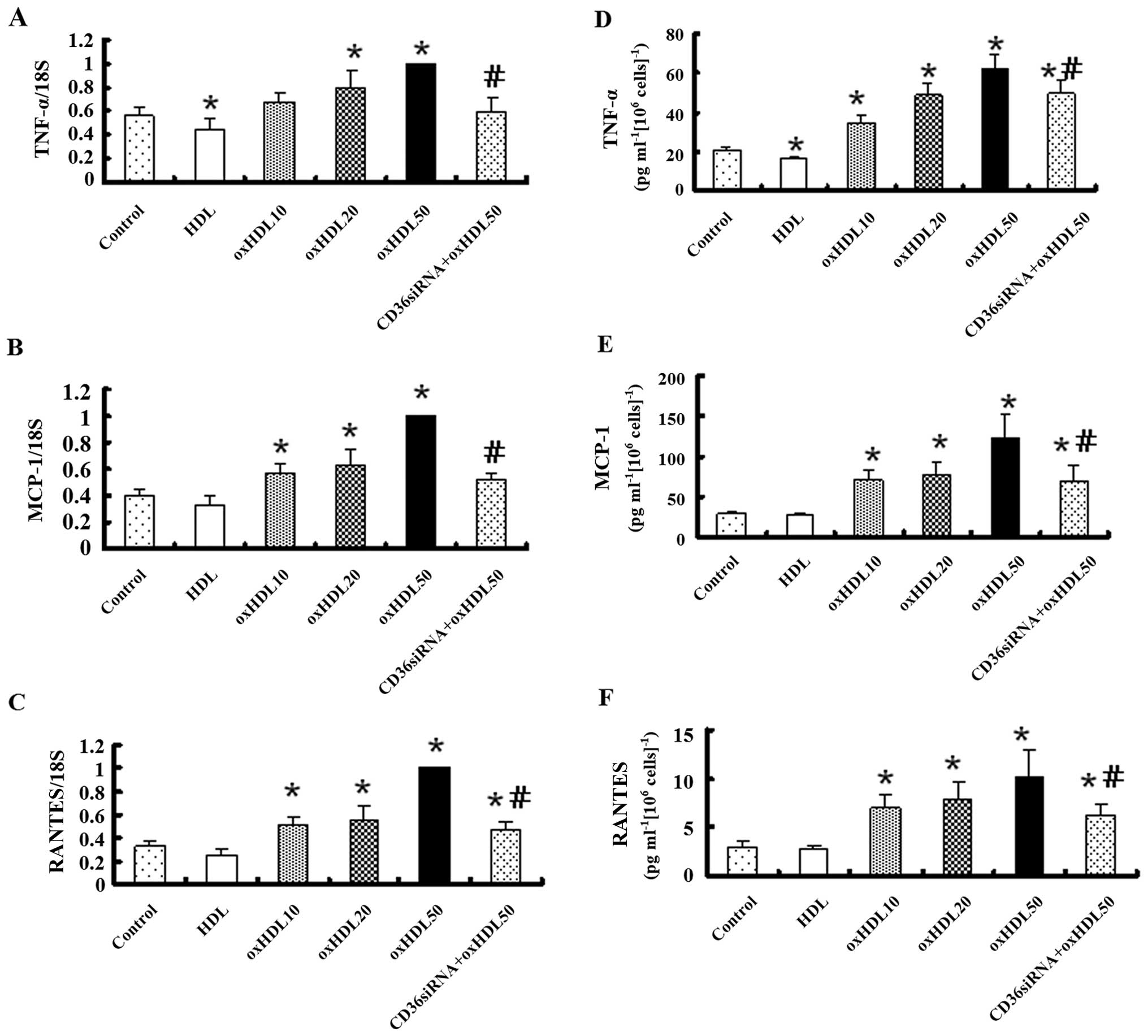
Oxidized HDL increases the mRNA and
protein expression of pro-inflammatory factors in HK-2 cells
Real-time reverse transcription PCR revealed that
the mRNA expression of TNF-α, MCP-1 and RANTES markedly increased
in a concentration-dependent manner following exposure to oxidized
HDL for 24 h, and this effect was markedly inhibited by treatment
with CD36 siRNA, while native HDL had no effect on the expression
levels of these inflammatory factors, apart from TNF-α (Fig. 3A–C). ELISA revealed that the
TNF-α, MCP-1 and RANTES protein levels in the conditioned culture
medium were altered in a pattern similar to that of the mRNA
expression (Fig. 3D–F).
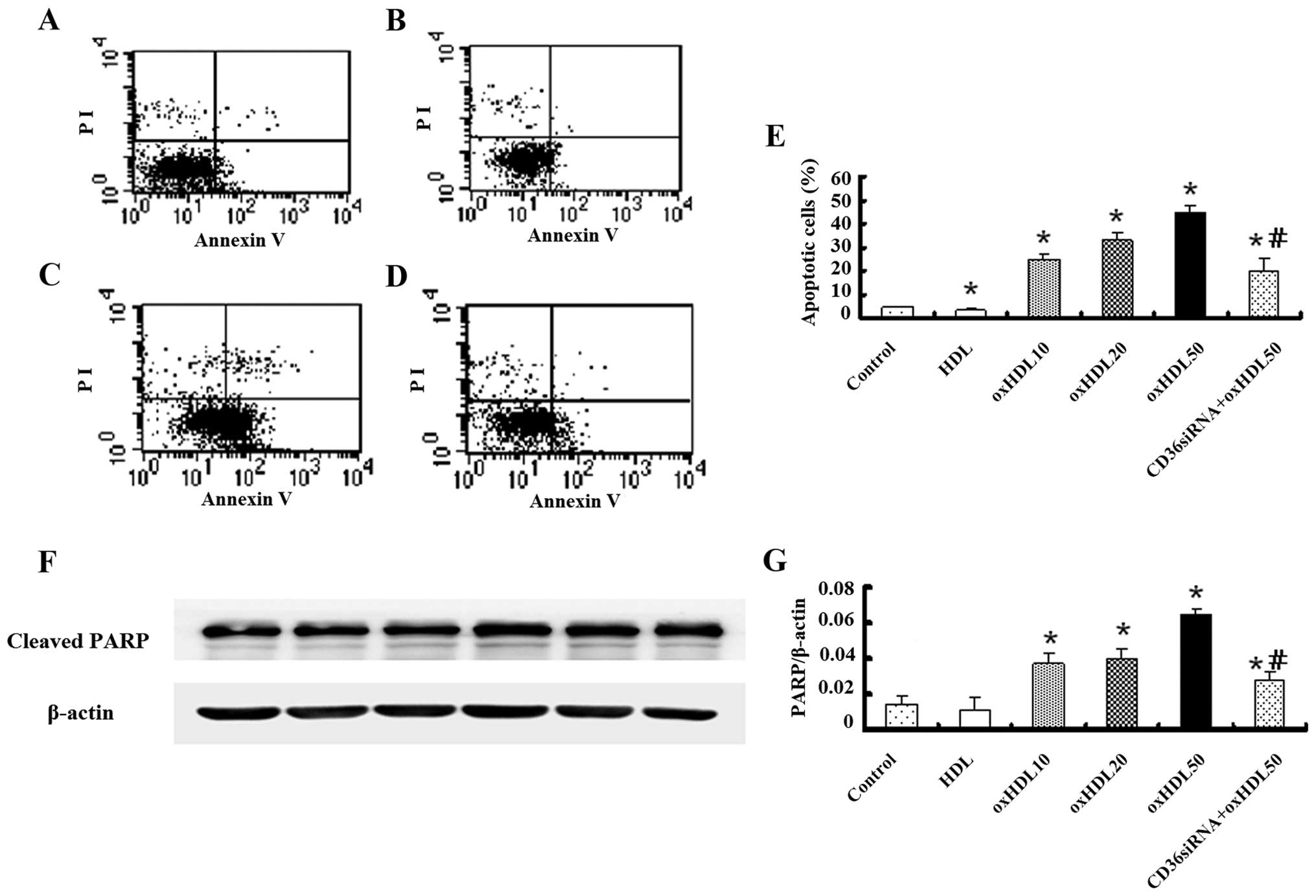
Oxidized HDL promotes HK-2 cell
apoptosis
Early apoptosis of the HK-2 cells was detected using
an Annexin V-FITC kit. To exclude dead cells, only the Annexin
V-positive and PI-negative cells were counted. As shown in Fig. 4A–E, oxidized HDL markedly promoted
the early apoptosis of the HK-2 cells compared with the control
group. This effect was markedly inhibited by treatment with CD36
siRNA; native HDL also attenuated the early apoptosis of the cells
compared with the control group.
In order to further confirm that the cell death
induced by oxidized HDL was primarily caused by apoptosis, the
cleavage of the caspase substrate, PARP, which is considered a
biochemical hallmark of apoptosis, was investigated by western blot
anaysis. The results revealed that the expression of cleaved PARP
increased as the concentration of oxidized HDL increased from 10 to
50 μg/ml, and this increment was reduced following the transfection
of the cells with CD36 siRNA (Fig. 4F
and G).
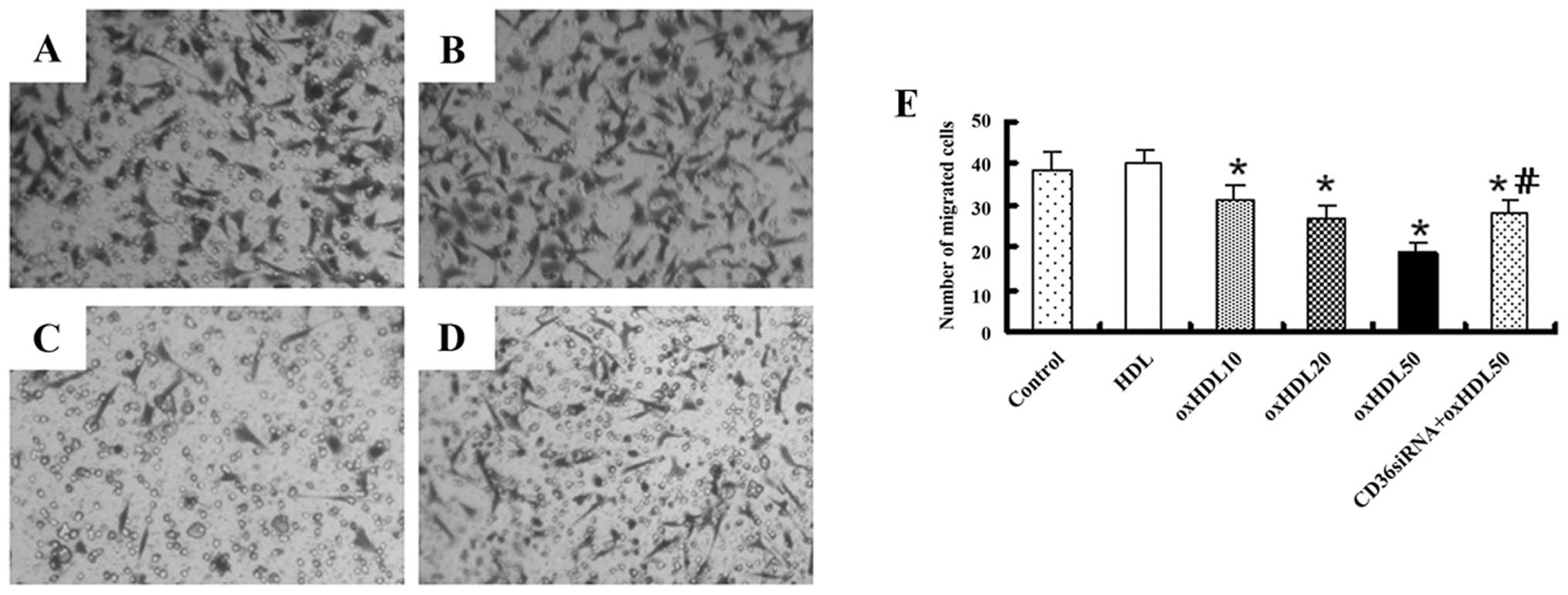
Oxidized HDL inhibits HK-2 cell migration
through CD36
The migration of the HK-2 cells was inhibited by
oxidized HDL in a dose-dependent manner, and this effect was
markedly attenuated by transfection with CD36 siRNA. There was no
significant difference between the control group and the native
HDL-treated group (Fig. 5).
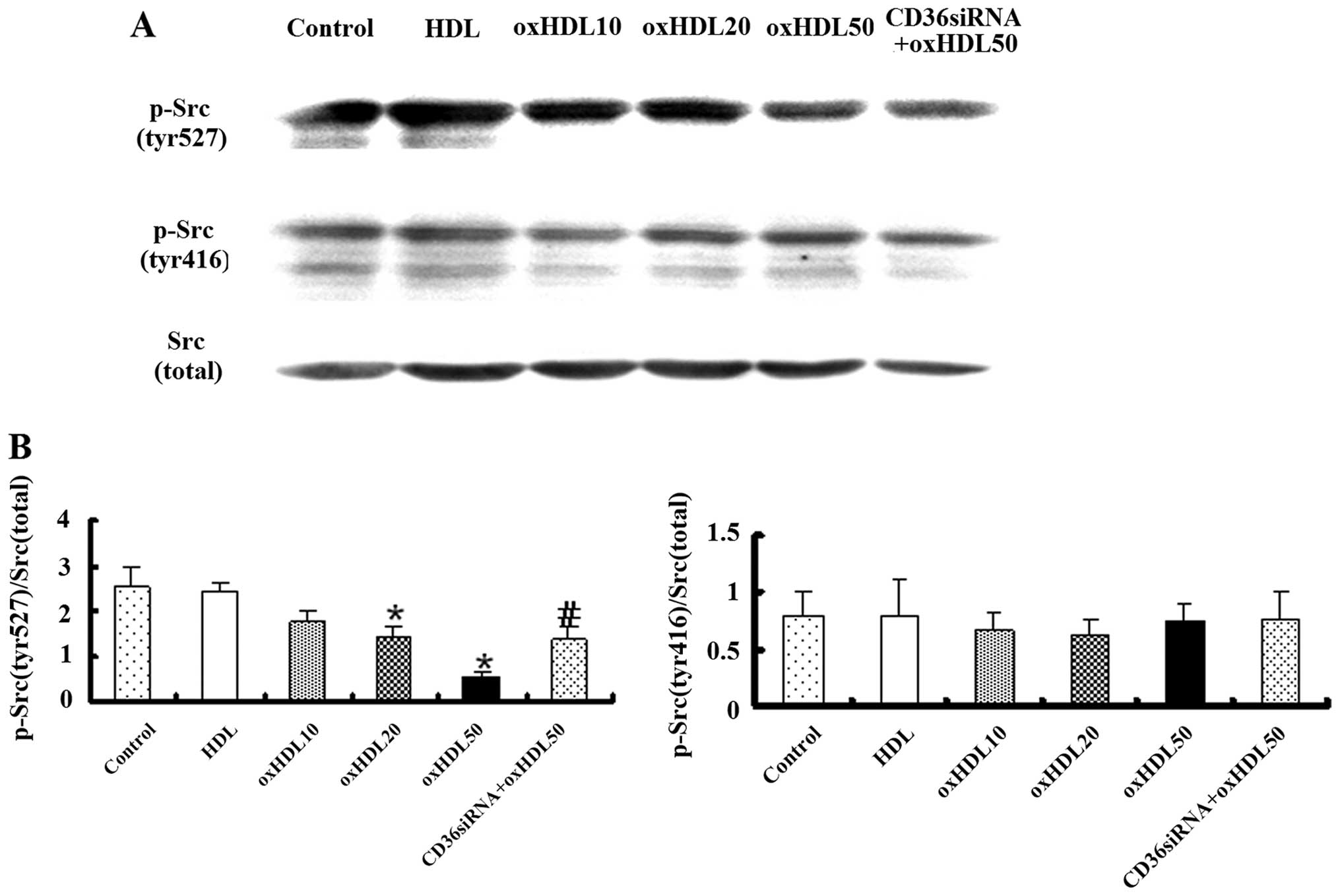
Oxidized HDL activates Src family
proteins in HK-2 cells
The Src family of protein tyrosine kinases is
important in the regulation of the growth and differentiation of
eukaryotic cells (19). Src
activity can be regulated by tyrosine phosphorylation at 2 sites,
with opposing effects. The phosphorylation of tyr416 in the
activation loop of the kinase domain upregulates enzyme activity,
while the phosphorylation of tyr527 in the carboxy-terminal tail
triggers enzyme inactivation (20). Our results revealed that the
phosphorylation of the Src-family kinase tyr527 was downregulated
by oxidized HDL in a dose-dependent manner, and this effect was
eliminated by treatment with CD36 siRNA, while the incubation of
the HK-2 cells with oxidized HDL did not affect the expression of
phospho-Src (tyr416) (Fig.
6).
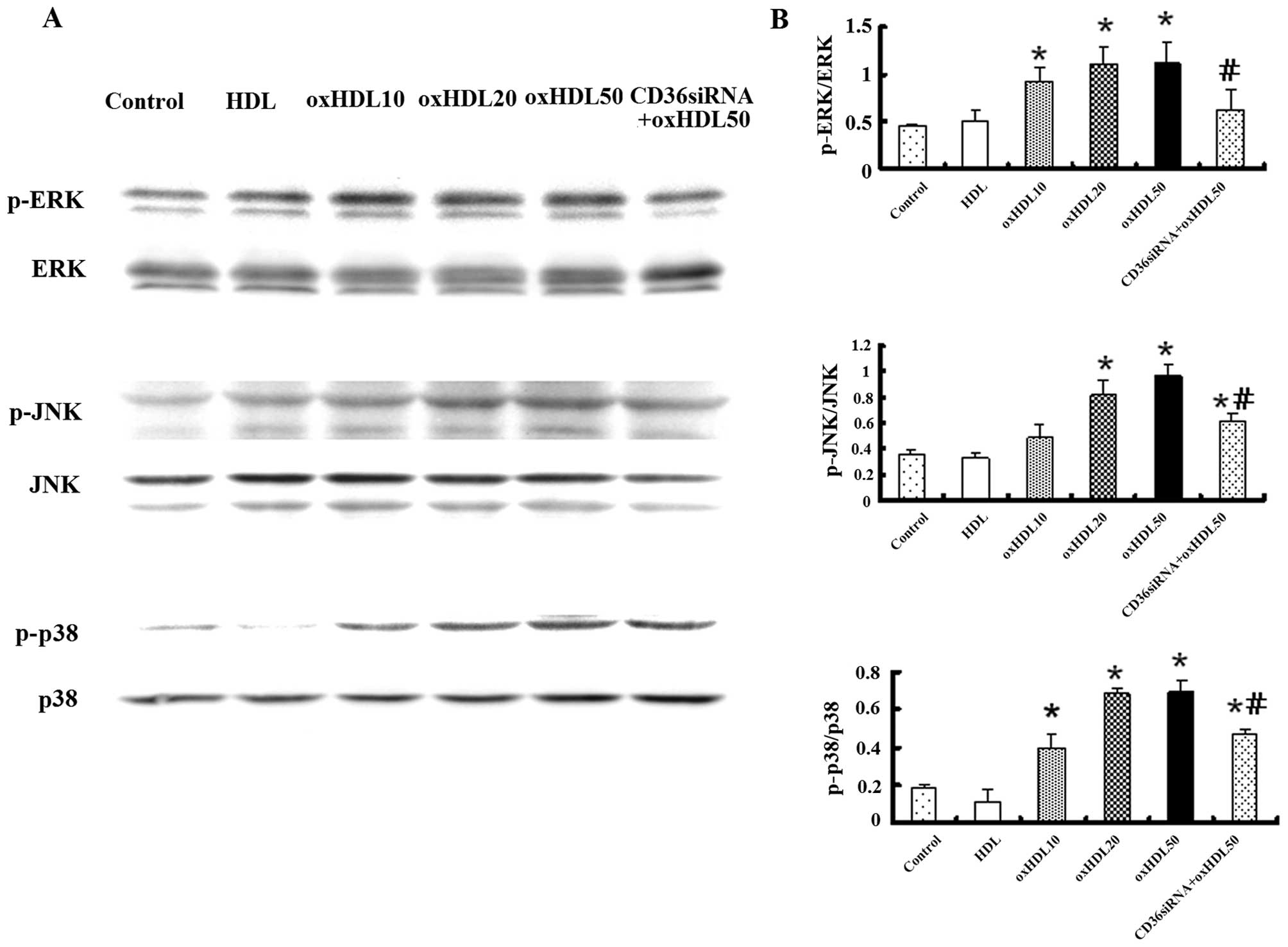
Oxidized HDL regulates mitogen-activated
protein kinase (MAPK) family proteins in HK-2 cells
MAPK mainly consists of 3 different pathways
(p38/MAPK, ERK/MAPK and JNK/MAPK) linking growth, differentiation,
proliferation and apoptotic signals with transcription in the
nucleus (21). In the present
study, the expression of MAPK family proteins was detected by
western blot analysis in order to determine whether it was affected
by oxidized HDL. The incubation of HK-2 cells with oxidized HDL
increased the phosphorylation of p38, JNK and ERK in a
dose-dependent manner. Pre-treatment with CD36 siRNA partially
attenuated the upregulation of phosphorylated p38, JNK and ERK by
oxidized HDL (Fig. 7).
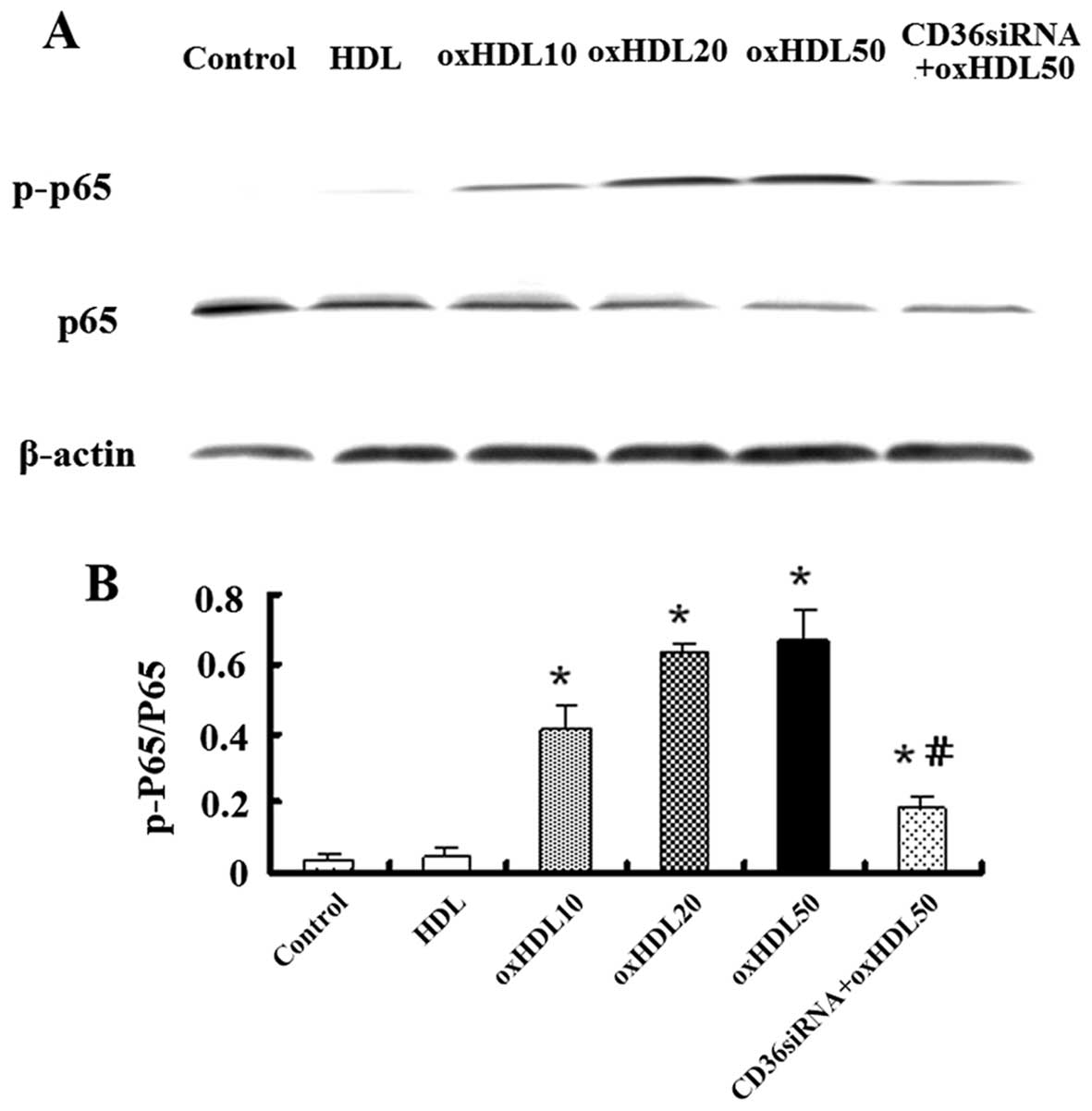
Oxidized HDL activates nuclear factor-κB
(NF-κB) proteins in HK-2 cells
The activation of the transcription factor, NF-κB,
is considered to be a vital signaling factor for apoptosis, ROS
generation, and in particular, inflammatory responses in HK-2 cells
(22). The phosphorylation of the
p65 unit of NF-κB has been reported to initiate its activation and
plays an important role in regulating the specificity of
NF-κB-dependent gene expression (23). In this study, to investigate
whether NF-κB activation is involved in the effects of oxidized
HDL, the HK-2 cells were pre-incubated with various concentrations
of oxidized HDL or HDL, as already mentioned in the previous
sections. The results of western blot analysis indicated that
oxidized HDL induced the phosphorylation of p65 and this effect was
attenuated by CD36 siRNA (Fig.
8).
Discussion
In the present study, we investigated the effects of
oxidized HDL on HK-2 cells, as well as the mechanisms involved. The
principal finding of this study was that oxidized HDL increased
intracellular the generation of ROS, promoted inflammation and
apoptosis, and inhibited the migration ability of HK-2 cells.
Oxidized HDL exerted negative effects on HK-2 cells which were
mediated through the scavenger receptor, CD36, inducing the
activation of the Src, MAPK and NF-κB pathways. These results
indicate that oxidized HDL, which negatively affects renal tubular
epithelial cell biology through CD36, may play an important role in
the pathogenesis of CKD.
Several factors are involved in the pathogenesis of
CKD, including oxidative stress, inflammation and dyslipidemia.
Lipid-mediated renal injury is an important component of CKD,
particularly in diabetic nephropathy (24,25). Under normal conditions, HDL plays
a protective role through reverse cholesterol transport and also
serves as a potent antioxidant, anti-inflammatory and
antithrombotic factor (6,7). However, HDL can lose its protective
capacity and even become a pro-inflammatory agent known as oxidized
HDL in the setting of systemic inflammation or under oxidative
damage (26). Recently, Vaziri
et al (13) reported that
a decrease in the HDL concentration was compounded by the severe
reduction in its antioxidant capacity in patients with CKD.
However, although renal tubulointerstitial inflammation plays a
central role in the progression of CKD, little is known about the
role of oxidized HDL in mediating renal tubular cell damage. In
this study, we found that oxidized HDL exerted several deleterious
effects on HK-2 cells. The exposure of renal tubular cells to
oxidized HDL potently increased the generation of ROS and
stimulated the production of pro-inflammatory factors, including
TNF-α, MCP-1 and RANTES. These factors may contribute to the
pathogenesis of cell injury, either by modulating the immune system
or by directly promoting renal damage, eventually resulting in cell
apoptosis and the inhibition of migration ability (3,27).
Other factors, including TGF-β, also play an important role in the
pathogenesis of inflammatory response during the course of chronic
kidney damage. Our preliminary experiments found that TGF-β
remained statistically unaltered (data not shown). We also measured
the release of lactate dehydrogenase (LDH) and no significant cell
lysis was detected up to a concentration of 100 μg/ml of oxidized
HDL (data not shown), indicating that oxidized HDL did not increase
the necrosis of renal tubular cells.
Scavenger receptors comprise a family of 9 classes
of structurally similar receptors that share oxidized lipoproteins
as their primary ligands (28).
CD36 has been identified as a class B transmembrane scavenger
receptor, which is known to be expressed by multiple cell types
(29). Previous studies have
shown that CD36 is predominantly expressed in tubular epithelial
cells with specific modulation of its expression patterns during
chronic renal injury (30–32).
Apart from binding to a variety of ligands, including oxidized
low-density lipoprotein, it has also been suggested to be a major
receptor for oxidized HDL (29–33). Our finding that the negative
effects exerted by oxidized HDL were blocked by transfection with
CD36 siRNA prior to treatment with oxidized HDL supports the
hypothesis that the oxidized HDL-induced effects on HK-2 cells are
largely mediated through the scavenger receptor, CD36.
Src family kinases are essential components of cell
growth and proliferation signaling at inflammatory sites (19,34). Previous studies have shown that
Src is activated in renal tubular injury and may be an important
regulator of tubular cell proliferation (19,35). The MAPK and NF-κB pathways are
both important signaling routes that are activated in response to a
variety of environmental stresses and inflammatory signals, and
promote apoptosis and growth inhibition (21,22). Downstream targets of Src, MAPK and
the transcription factor, NF-κB, regulate inflammation, and the
binding of oxidized HDL to CD36 may also activate these
intracellular signaling pathways that lead to pro-inflammatory
reactions (32,34). In accordance with these data, our
findings indicated that the incubation of HK-2 cells with oxidized
HDL inhibited the phosphorylation of Src-family kinase tyr527 in a
dose-dependent manner, mainly through CD36, without affecting the
expression of phospho-Src (tyr416). Since the phosphorylation of
tyr527 renders the enzyme less active, oxidized HDL upregulated Src
enzyme activity. Oxidized HDL also increased the phosphorylation of
p38, JNK and ERK in a dose-dependent manner. NF-κB activity was
altered with the level of Src and MAPK. These results further
confirm the involvement of the Src, MAPK and NF-κB pathways in the
negative effects induced by oxidized HDL on HK-2 cells.
In conclusion, our data are in support of the
hypothesis that oxidized HDL enhances oxidative stress and alters
the phenotype of proximal tubule epithelial cells to a more
dysfunctional pro-inflammatory state, ultimately succumbing to
apoptosis and the inhibition of migration. These effects are
largely mediated through the scavenger receptor, CD36, as well as
through the Src, MAPK and NF-κB signaling pathways. The ability of
oxidized HDL to negatively affect proximal tubule cell biology may
represent a novel pathological mechanism for the development and
progression of CKD.
Acknowledgements
We are grateful to Mr. Ye Lin for his technical
assistance with the experiments, and Professor Huimin Hu for
providing helpful discussions. This study was supported by the
Shanghai Leading Academic Discipline Project (B902) and the Major
Research Projects of Shanghai Science and Technology Commission
(Study of Diagnosis and Treatment of Chronic Kidney Diseases,
08dz1900600 to C.M., and by the National Natural Science Foundation
of China (81100526) to X.G.
References
|
1
|
Risdon RA, Sloper JC and De Wardener HE:
Relationship between renal function and histological changes found
in renal-biopsy specimens from patients with persistent glomerular
nephritis. Lancet. 2:363–366. 1968. View Article : Google Scholar
|
|
2
|
Rodriguez-Iturbe B and García García G:
The role of tubulointerstitial inflammation in the progression of
chronic renal failure. Nephron Clin Pract. 116:c81–c88. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gong R, Rifai A, Tolbert EM, Biswas P,
Centracchio JN and Dworkin LD: Hepatocyte growth factor ameliorates
renal interstitial inflammation in rat remnant kidney by modulating
tubular expression of macrophage chemoattractant protein-1 and
RANTES. J Am Soc Nephrol. 15:2868–2881. 2004. View Article : Google Scholar
|
|
4
|
Takase O, Minto AW, Puri TS, et al:
Inhibition of NF-kappaB-dependent Bcl-xL expression by clusterin
promotes albumin-induced tubular cell apoptosis. Kidney Int.
73:567–577. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bohle A, Wehrmann M, Bogenschütz O, et al:
The long-term prognosis of the primary glomerulonephritides. A
morphological and clinical analysis of 1747 cases. Pathol Res
Pract. 188:908–924. 1992.PubMed/NCBI
|
|
6
|
Davidson MH and Toth PP: High-density
lipoprotein metabolism: potential therapeutic targets. Am J
Cardiol. 100:n32–n40. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ansell BJ, Navab M, Hama S, et al:
Inflammatory/antiinflammatory properties of high-density
lipoprotein distinguish patients from control subjects better than
high-density lipoprotein cholesterol levels and are favorably
affected by simvastatin treatment. Circulation. 108:2751–2756.
2003. View Article : Google Scholar
|
|
8
|
Briel M, Ferreira-Gonzalez I, You JJ, et
al: Association between change in high density lipoprotein
cholesterol and cardiovascular disease morbidity and mortality:
systematic review and meta-regression analysis. BMJ. 338:b922009.
View Article : Google Scholar
|
|
9
|
Ferretti G, Bacchetti T, Nègre-Salvayre A,
Salvayre R, Dousset N and Curatola G: Structural modifications of
HDL and functional consequences. Atherosclerosis. 184:1–7. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu R, Yekta B, Vakili L, et al:
Proatherogenic high-density lipoprotein, vascular inflammation, and
mimetic peptides. Curr Atheroscler Rep. 10:171–176. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Valiyaveettil M, Kar N, Ashraf MZ, Byzova
TV, Febbraio M and Podrez EA: Oxidized high-density lipoprotein
inhibits platelet activation and aggregation via scavenger receptor
BI. Blood. 111:1962–1971. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsumura M, Kinouchi T, Ono S, Nakajima T
and Komoda T: Serum lipid metabolism abnormalities and change in
lipoprotein contents in patients with advanced-stage renal disease.
Clin Chim Acta. 314:27–37. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vaziri ND, Moradi H, Pahl MV, Fogelman AM
and Navab M: In vitro stimulation of HDL anti-inflammatory activity
and inhibition of LDL pro-inflammatory activity in the plasma of
patients with end-stage renal disease by an apoA-1 mimetic peptide.
Kidney Int. 76:437–444. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Norata GD, Banfi C, Pirillo A, et al:
Oxidised-HDL3 induces the expression of PAI-1 in human endothelial
cells. Role of p38MAPK activation and mRNA stabilization. Br J
Haematol. 127:97–104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu HT, Li WM, Xu G, et al: Chitosan
oligosaccharides attenuate hydrogen peroxide-induced stress injury
in human umbilical vein endothelial cells. Pharmacol Res.
59:167–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cirillo P, Gersch MS, Mu W, et al:
Ketohexokinase-dependent metabolism of fructose induces
proinflammatory mediators in proximal tubular cells. J Am Soc
Nephrol. 20:545–553. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao X, Huang L, Grosjean F, et al:
Low-protein diet supplemented with ketoacids reduces the severity
of renal disease in 5/6 nephrectomized rats: a role for KLF15.
Kidney Int. 79:987–996. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsunaga T, Hokari S, Koyama I, Harada T
and Komoda T: NF-kappa B activation in endothelial cells treated
with oxidized high-density lipoprotein. Biochem Biophys Res Commun.
303:313–319. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhuang S, Kinsey GR, Rasbach K and
Schnellmann RG: Heparin-binding epidermal growth factor and Src
family kinases in proliferation of renal epithelial cells. Am J
Physiol Renal Physiol. 294:F459–F468. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feistritzer C, Mosheimer BA, Tancevski I,
et al: Src tyrosine kinase-dependent migratory effects of
antithrombin in leukocytes. Exp Cell Res. 305:214–220. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Leung JC, Tang SC, Chan LY, Chan WL and
Lai KN: Synthesis of TNF-alpha by mesangial cells cultured with
polymeric anionic IgA - role of MAPK and NF-kappaB. Nephrol Dial
Transplant. 23:72–81. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bouma HR, Ploeg RJ and Schuurs TA: Signal
transduction pathways involved in brain death-induced renal injury.
Am J Transplant. 9:989–997. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sabatel H, Pirlot C, Piette J and Habraken
Y: Importance of PIKKs in NF-κB activation by genotoxic stress.
Biochem Pharmacol. 82:1371–1383. 2011.PubMed/NCBI
|
|
24
|
Moradi H, Pahl MV, Elahimehr R and Vaziri
ND: Impaired antioxidant activity of high-density lipoprotein in
chronic kidney disease. Transl Res. 153:77–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vaziri ND: Dyslipidemia of chronic renal
failure: the nature, mechanisms, and potential consequences. Am J
Physiol Renal Physiol. 290:F262–F272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ansell BJ, Fonarow GC and Fogelman AM: The
paradox of dysfunctional high-density lipoprotein. Curr Opin
Lipidol. 18:427–434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou W, Guan Q, Kwan CC, et al: Loss of
clusterin expression worsens renal ischemia-reperfusion injury. Am
J Physiol Renal Physiol. 298:F568–F578. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moore KJ and Freeman MW: Scavenger
receptors in atherosclerosis: beyond lipid uptake. Arterioscler
Thromb Vasc Biol. 26:1702–1711. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Febbraio M, Hajjar DP and Silverstein RL:
CD36: a class B scavenger receptor involved in angiogenesis,
atherosclerosis, inflammation, and lipid metabolism. J Clin Invest.
108:785–791. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Okamura DM, Lopez-Guisa JM, Koelsch K,
Collins S and Eddy AA: Atherogenic scavenger receptor modulation in
the tubulointerstitium in response to chronic renal injury. Am J
Physiol Renal Physiol. 293:F575–F585. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iwao Y, Nakajou K, Nagai R, et al: CD36 is
one of important receptors promoting renal tubular injury by
advanced oxidation protein products. Am J Physiol Renal Physiol.
295:F1871–F1880. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Okamura DM, Pennathur S, Pasichnyk K, et
al: CD36 regulates oxidative stress and inflammation in
hypercholesterolemic CKD. J Am Soc Nephrol. 20:495–505. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thorne RF, Mhaidat NM, Ralston KJ and
Burns GF: CD36 is a receptor for oxidized high density lipoprotein:
implications for the development of atherosclerosis. FEBS Lett.
581:1227–1232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Amin MA, Haas CS, Zhu K, et al: Migration
inhibitory factor upregulates vascular cell adhesion molecule-1 and
intercellular adhesion molecule-1 via Src, PI3 kinase, and
NFkappaB. Blood. 107:2252–2261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xing J, Zhang Z, Mao H, Schnellmann RG and
Zhuang S: Src regulates cell cycle protein expression and renal
epithelial cell proliferation via PI3K/Akt signaling-dependent and
-independent mechanisms. Am J Physiol Renal Physiol. 295:F145–F152.
2008. View Article : Google Scholar
|