Introduction
The epidermis is a stratified squamous epithelium in
which viable cells move outwardly from the basal layer to become
terminally differentiated keratinocytes that eventually constitute
the stratum corneum. Keratinocyte proliferation and
differentiation are closely regulated by cellular transcription
factors, including activator protein-1 family proteins (1), nuclear factor-κB family proteins
(2), cytidine-cytidine-adenosine-
adenosine-thymidine/enhancer-binding proteins (3), p53-related proteins (4), and POU transcription factors
(5). POU transcription factors
are characterized by a bipartite POU domain in which a homeodomain
is connected by a short linker region to an N-terminally located
POU-specific domain (6). The
POU-specific and POU homeodomain are DNA-binding domains of the
helix-turn-helix type.
Skn-1a, a member of the POU domain transcription
factor family, appears to be expressed predominantly in the
epidermal keratinocytes and is thought to play a critical role in
keratinocyte differentiation and proliferation (7–9).
Skn-1a transactivates the expression of the genes encoding K10 and
SPRP2A, which are expressed during keratinocyte differentiation,
suggesting that Skn-1a promotes keratinocyte differentiation.
Furthermore, we have previously reported that the mRNA expression
of Skn-1a increases in cultured normal human keratinocytes
subsequent to calcium-induced differentiation (9,10).
Similar to POU domain proteins characterized thus
far (2), Skn-1a exerts its
function in the nucleus. Nuclear proteins enter this cellular
compartment via the nuclear pore complex after being synthesized in
the cytoplasm, and usually, they are actively transported through
the nuclear pore (11). Such
active transportation requires energy, transport receptors, and an
endogenous nuclear localization signal (NLS) within the cargo
protein.
NLSs have been identified in a variety of nuclear
proteins ranging in size from <100 to >1,000 amino acids,
including polymerases, kinases, phosphatases, transcription
factors, histones, tumor suppressor molecules, and various viral
proteins. In the present study, we characterized the NLS of Skn-1a,
which was shown to be localized within its DNA-binding domain as a
motif highly conserved among the POU domain proteins. Its
identification enhances our understanding of the evolution of POU
domain proteins and the mechanisms involved in regulating the
access of POU domain proteins to the nucleus.
Materials and methods
Enhanced green fluorescent protein
(EGFP)-Skn-1a deletion and mutation reporter constructs
The EGFP-Skn-1a plasmid was constructed as described
below (7). First the Skn-1a
coding region, which contains the canonical NLS consensus sequence,
GRKRKKR (aa279–285), was amplified by performing PCR and subcloned
into the C-terminus of pEGFP (Clontech Laboratories, Palo Alto, CA,
USA). The primers were used for PCR, BsrGI-207; 5′-GGGTGTACA
AGTTCACACAGGGAGATGGGCTGGCGA-3′ and NotI-295;
5′-TATGCGGCCGCAGTCAGGCGGATGTTG GTCTC-3′ for the 207–295 fragment,
BsrGI-207 and NotI-287;
5′-GGGGCGGCCGCTTTAGCTGGTCCGTTTCTTTCT CTTCCTACCAAA-3′ for the
207–287 fragment, BsrGI-258; 5′-GGGTGTACCCTCTCCGTCAGA
CCCCTCAGTG-3′ and NotI-287 for the 258–287 fragment,
BsrGI-269; 5′-GGGTGT ACACCTCCTACCCCAGCCTCAGTGAA-3′ and
NotI-287 for the 269–287 fragment. The 273–287, 279–287 and
279–285 fragments were directly employed as the following linkers:
5′-GTACAGGCTCAGTGAAGTATTTGCTAGGAAGAG AAAGAAACGGACCAGCGC-3′ and
5′-GGCCGCGCT GGTCCGTTTCTTTCTCTTCGTACCAAATACTTCACT GAGCCT-3′ for the
273–287 fragment, 5′-GTACAC CGGTAGGAAGAGAAAGAAACGGACCAGCGC-3′ and
5′-GGCCGCGCTGGTCCGTTTCTTTCTCTTCCTAC CGGT-3′ for the 279–287
fragment, 5′-GTACACCGGTAG GAAGAGAAAGAAACGGACCAGCGC-3′ and 5′-GGCCG
CCCGTTTCTTTCTCTTCCTACCGGT-3′ for the 279–285 fragment. The
EGFP-Skn-1a deletion constructs pEGFP- Skn-1a 207–295, pEGFP-Skn-1a
207–287, pEGFP-Skn-1a 258–287, pEGFP- Skn-1a 269–287, pEGFP-Skn-1a
273–287, pEGFP-Skn-1a 279–287 and pEGFP-Skn-1a 279–285 were
generated by cloning the corresponding segments into pEGFP as
BsrGI-NotI fragments. We also constructed the
mutation constructs pEGFP-Skn-1a 279–285m in which both Arg282 and
Lys283 were replaced by alanines using the following linkers:
5′-GTACACCGGTAGGAAGGCTGCTAAACG GACCAGCGC-3′ and
5′-GGCCGCCCGTTTAGCAGCCTT CCTACCGGT-3′.
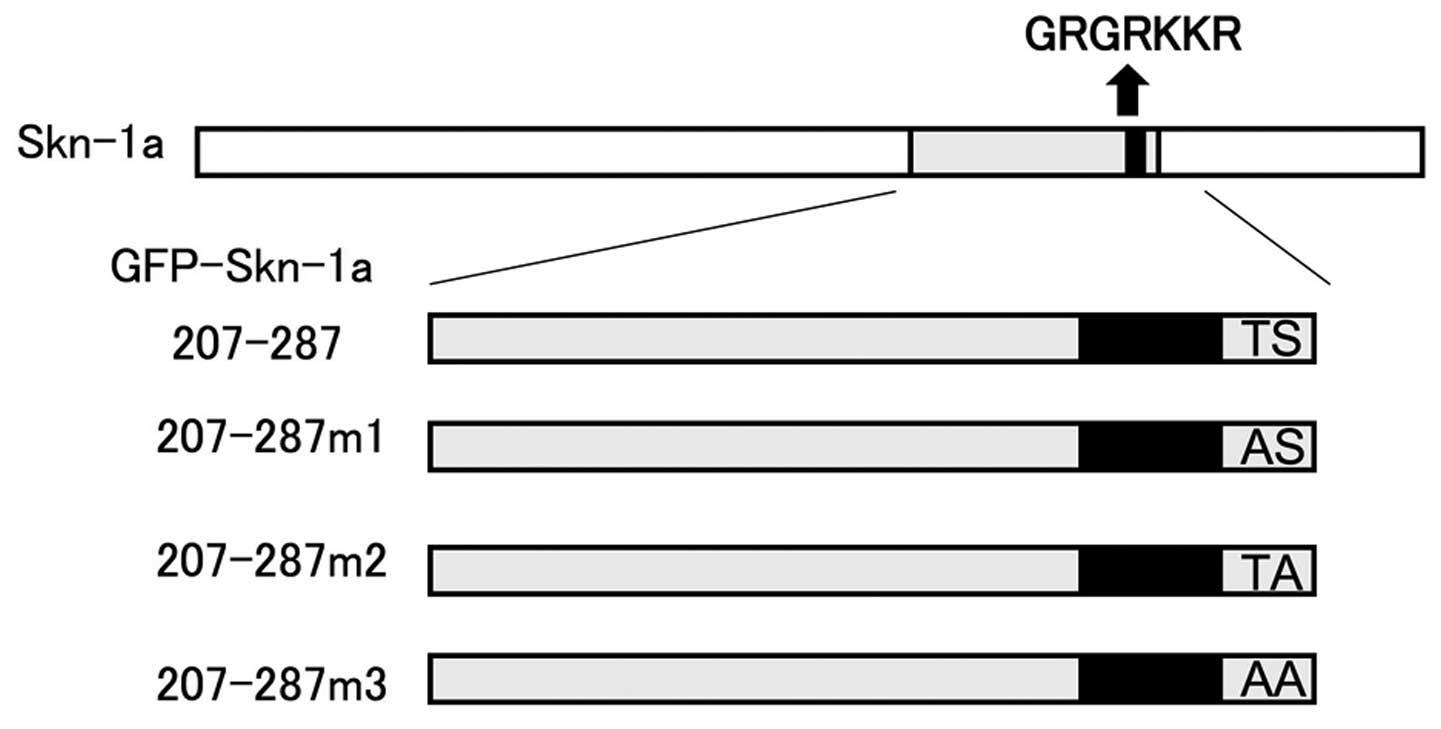
Plasmids for the EGFP-Skn-1a mutation reporter
constructs EGFP-Skn-1a 207–287m1 in which threonine (Thr) 286 was
replaced with alanine, EGFP-Skn-1a 207–287m2 in which serine (Ser)
287 was replaced with alanine, and EGFP- Skn-1a 207–287m3 in which
both Thr286 and Ser287 were replaced with alanine, were constructed
from pEGFP1- Skn-1a 258–287 by PCR with mutagenetic primers
(forward for all, BsrGI-207; 5′-GGGTGTACAAGTTCACACAGGGAG
ATGGGCTGGCGA-3′; reverse, 5′-GGGGCGGCCGCTT
TAGCCGGTCCGTTTCTTTCTCTTCCTACCAAA-3′ for m1,
5′-GGGGCGGCCGCTTTAGCTGCCCCGTTTCTTTC TCTTCCTACCAAA-3′ for m2, and
5′-GGGGCGGCCG CTTTAGCCGCCCCGTTTCTTTCTCTTCCTACCAAA-3′ for m3).
Cell culture
Normal human epidermal keratinocytes (NHEKs) from
neonatal foreskin were obtained commercially (Clonetics, San Diego,
CA, USA). Cultures were grown in a 60-mm culture dish in
keratinocyte growth medium containing human recombinant epidermal
growth factor (0.1 ng/ml), insulin (5 ng/ml), hydrocortisone (0.5
ng/ml), gentamicin (50 ng/ml), and amphotericin-B (50 ng/ml).
Phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich, St. Louis, MO,
USA) was used to induce keratinocyte differentiation.
Transfection and microscopy
The plasmid constructs described above were used in
transient transfection studies in cultured NHEKs (9,10).
After the cells had grown to ~70% confluence, they were transfected
with 5 ng of the EGFP-Skn-1a expression vector using the DOTAP
Liposomal Transfection Reagent (Roche Applied Science,
Indianapolis, NJ, USA). The pEGFP plasmid was used as the control.
The cells expressing EGFP were analyzed by fluorescence microscopy
of 10 randomly selected fields. The cells were considered positive
when the nuclear fluorescence signal was clearly stronger that the
cytoplasmic fluorescence signal. The total number of cells
expressing EGFP (A) and the number of the positive cells in which
EGFP was localized in the nucleus (B) were measured, and the rate
of B/A was calculated. The rate of nuclear translocation of each
construct was expressed as a percentage relative to that of
EGFP-Skn-1a 279–285 which contained the most minimum component of
the DNA fragment.
Western blot analysis
Western blot analysis was performed following a
routine method (9,10). Briefly, the transfected cells were
lysed by the sample buffer, electrophoresed on 15% polyacrylamide
gels, and then transferred onto a polyvinylidene difluoride
membrane. The membranes were incubated with monoclonal anti-GFP
antibodies (Roche Diagnostic Corp., Indianapolis, IN, USA),
followed by incubation with a horseradish peroxidase-labelled
secondary anti-mouse antibody. Immunocomplexes were visualized
using visualized using the Image Lab System (Bio-Rad, Hercules, CA,
USA). Anti-β-actin antibodies were used as a control.
Statistical analysis
The Student’s t-test was used to determine the level
of significance of differences in sample means. P<0.01 was
considered significant. Data were shown as mean ± SD.
Results
Nuclear translocation of the EGFP-Skn-1a
deletion proteins
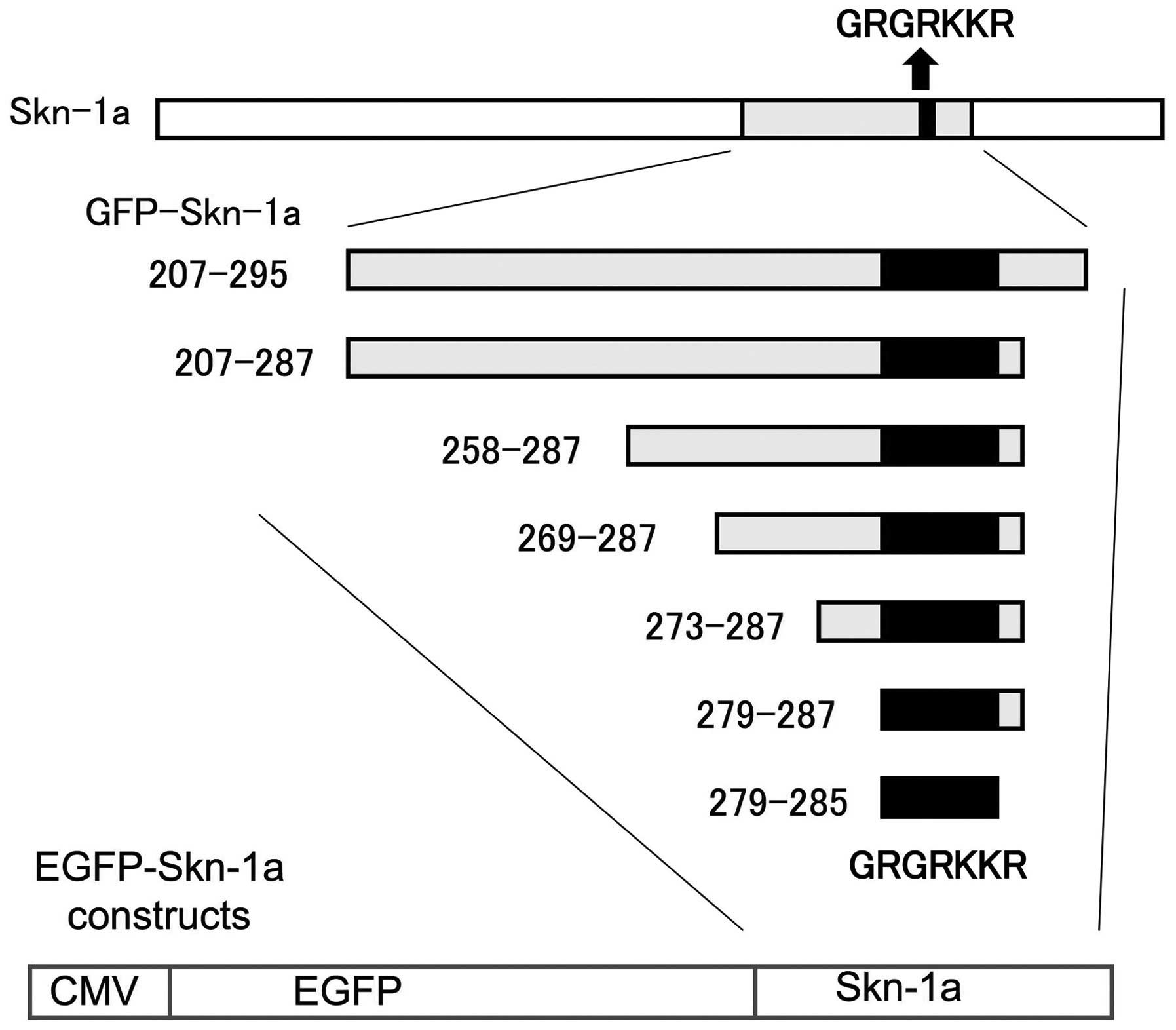
To identify the regions of the Skn-1a protein
required for its transport into the nucleus, we generated reporter
constructs for Skn-1a deletion proteins fused to EGFP at their
N-termini (Fig. 1). The putative
NLS consensus sequence, GRKRKKR is located between amino acids 279
and 285. We transfected the constructs into NHEKs and analyzed the
EGFP fusion protein localization 24 h later by fluorescence
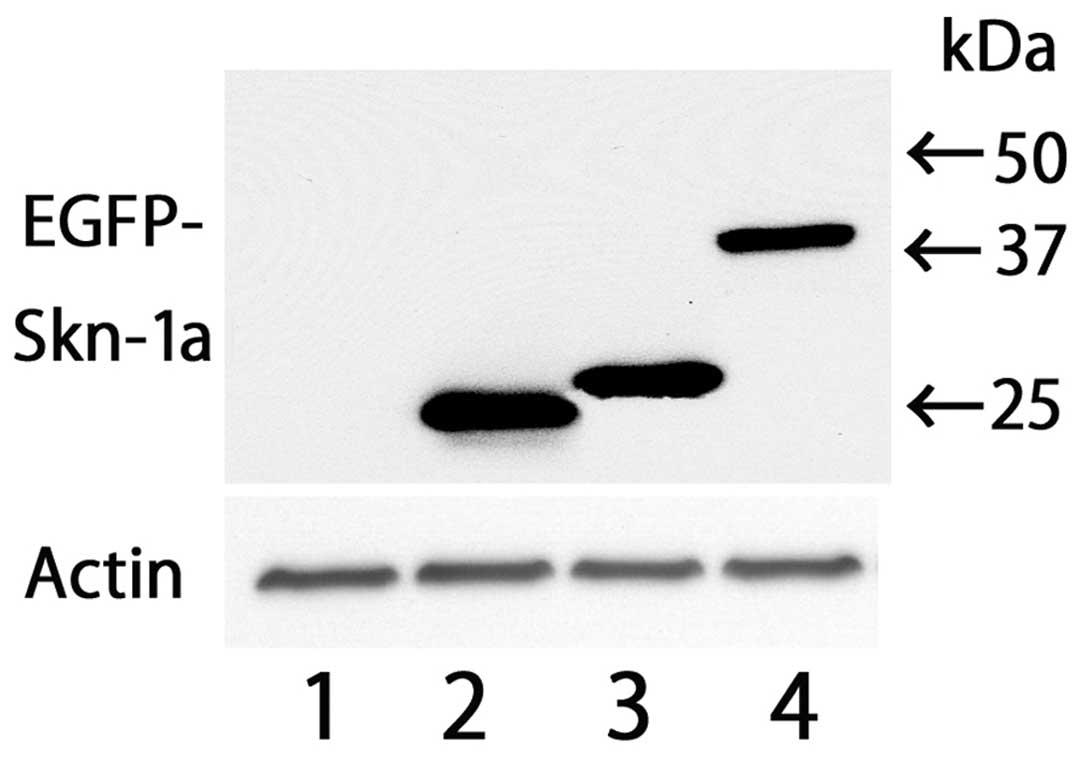
microscopy. Western blot analysis was also performed to confirm
production of the EGFP fusion protein. The result of western blot
analysis revealed that each construct exhibited a band with an
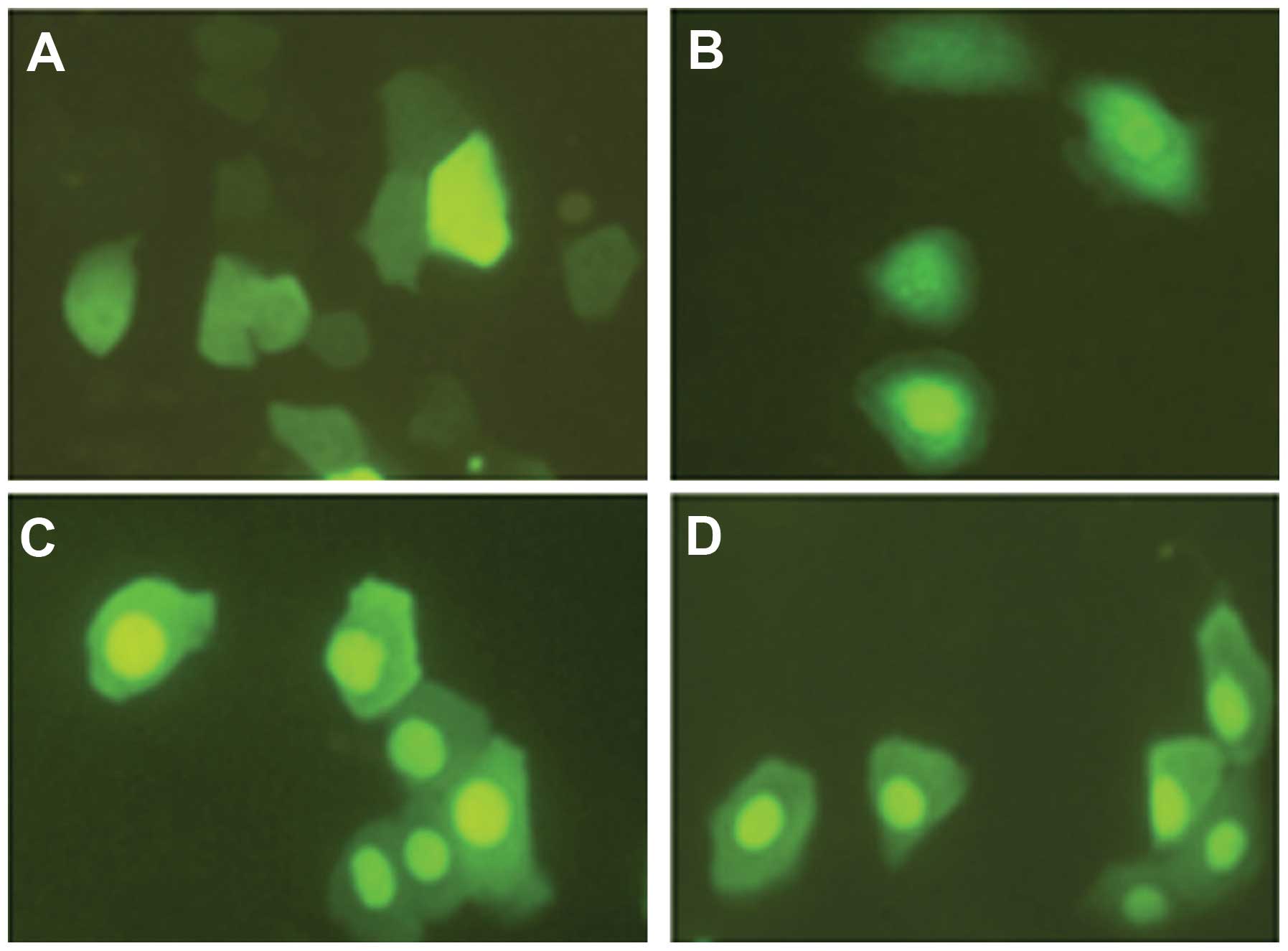
estimated size (Fig. 2). Typical
positive cells are shown in Fig.
3. The nuclear fluorescence signal was clearly stronger than
the cytoplasmic fluorescence signal. Results of the
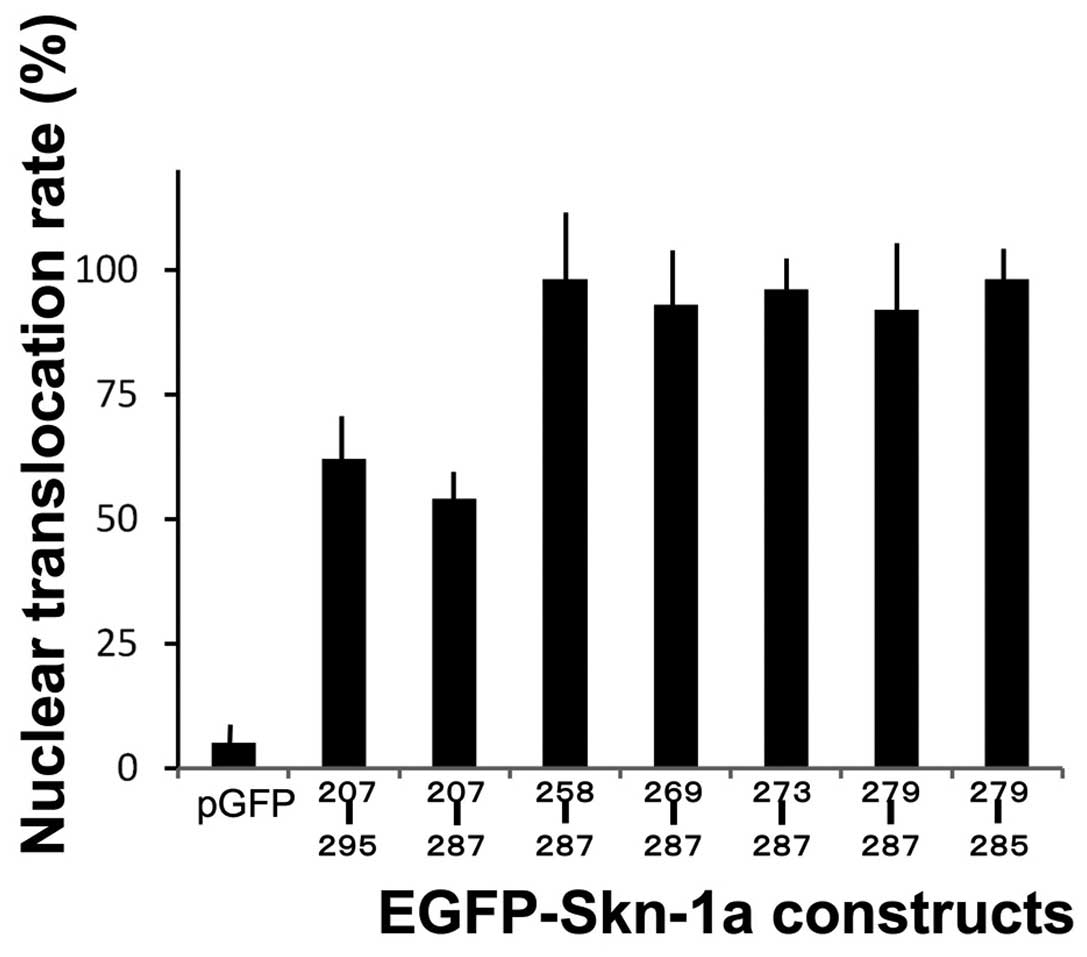
immunofluorescence experiments showed that the rates of nuclear
translocation of the fusion proteins from pEGFP-Skn-1a 258–287,
269–287, 273–287, 279–287 and 279–285 were almost identical
(Fig. 4). On the other hand,
rates for the fusion proteins from the constructs 207–295 and
207–287 were relatively low. The control plasmid pEGFP had little
or no ability to enter the nucleus. We also examined the mutation
constructs pEGFP-Skn-1a 279–285m in which both Arg282 and Lys283
were replaced by alanines. The result showed the rate to be
identical to that of control pEGFP (data not shown).
Nuclear translocation of the EGFP-Skn-1a
mutation proteins
The amino acids Thr286 and Ser287 reside adjacent to
the NLS on its carboxy-terminal side. We examined their role in
Skn-1a nuclear localization by generating three mutation reporter
constructs based on the pEGFP-Skn-1a 207–287 construct. Thr286 was
replaced with alanine to generate the construct pEGFP-Skn-1a
207–287m1, Ser287 with alanine for the construct pEGFP-Skn-1a
207–287m2, and both Thr286 and Ser287 residues were replaced with
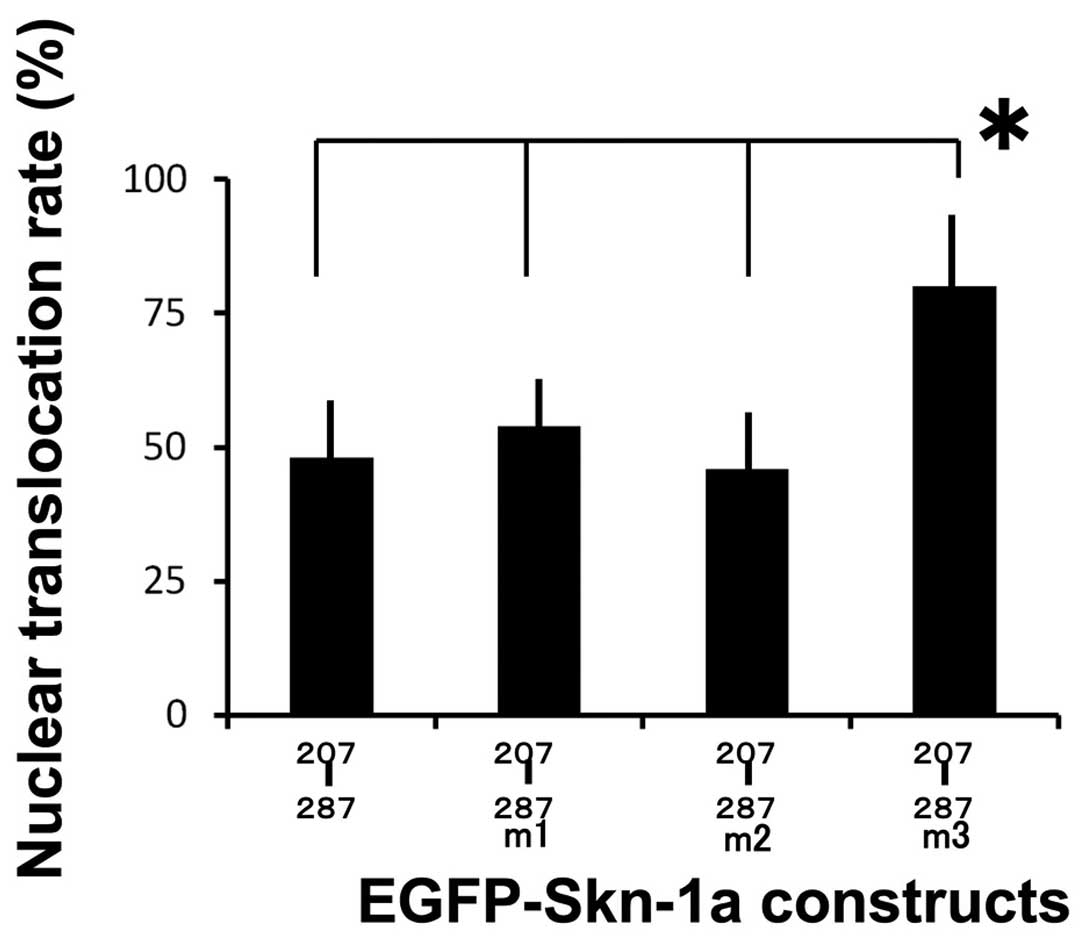
alanine for the construct pEGFP-Skn-1a 207–287m3 (Fig. 5). We then compared the nuclear
translocation rates of the pEGFP-Skn-1a mutation proteins. The
nuclear translocation rates of the pEGFP-Skn-1a 207–287m1 and
pEGFP-Skn-1a 207–287m2 mutation proteins were identical to that of
pEGFP-Skn-1a 207–287, whereas the pEGFP-Skn-1a 207–287m3 mutation
protein showed a significantly higher rate compared to the other
three constructs (Fig. 6).
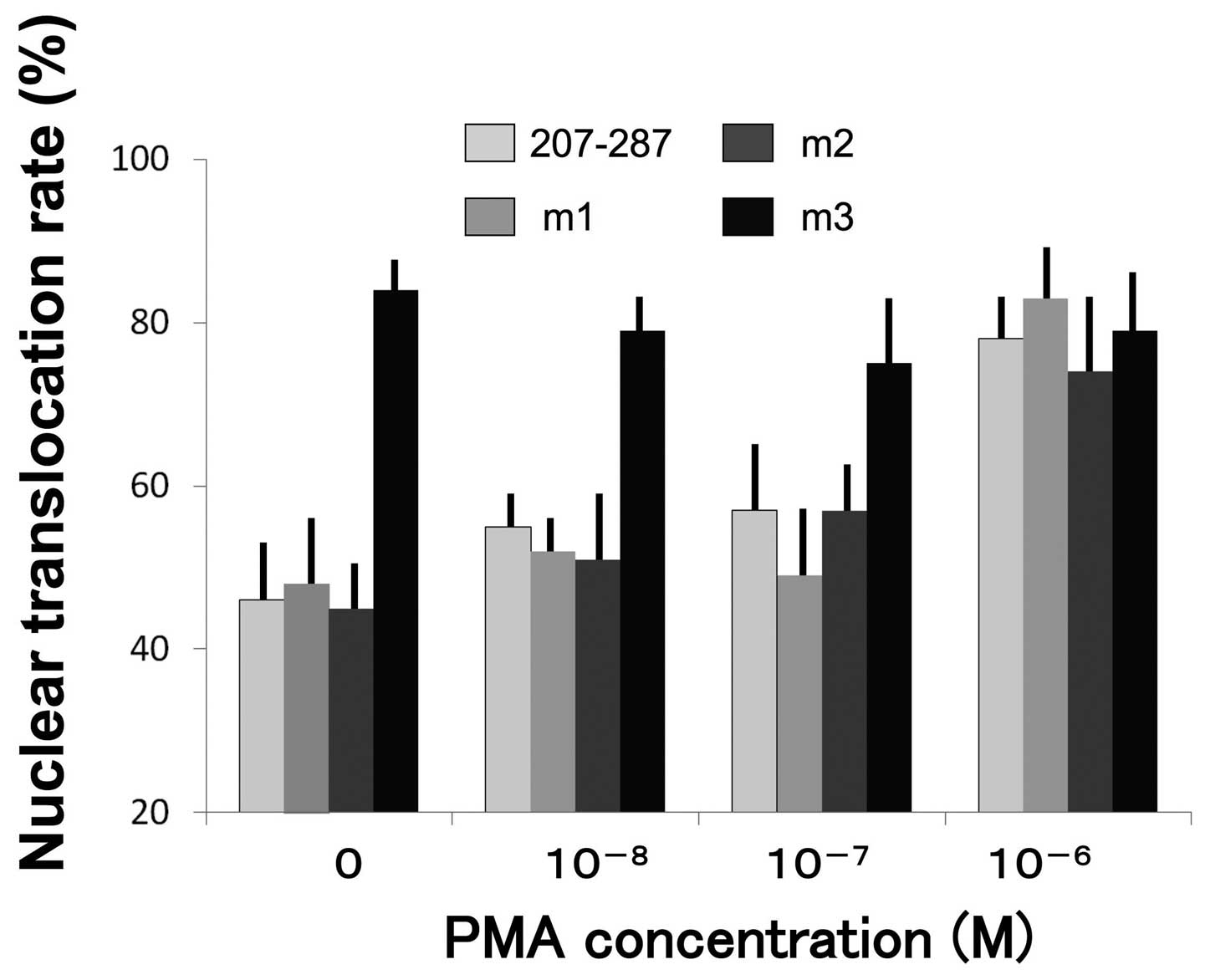
Effect of PMA treatment on nuclear
translocation
PMA has been well characterized as a protein kinase
C activator as well as an inducer of late differentiation marker
expression and cornified envelope assembly in vitro. In the
subsequent series of experiments we examined the rate of nuclear
translocation of the reporter proteins from pEGFP-Skn-1a 207–287,
pEGFP-Skn-1a 207–287m1, pEGFP-Skn-1a 207287m2, and pEGFP-Skn-1a
207–287m3 in NHEKs treated for 24 h with
10−8–10−6 M PMA. The nuclear translocation
rates of proteins from pEGFP-Skn-1a 207–287, pEGFP-Skn-1a
207–287m1, and pEGFP-Skn-1a 207–287m2 were enhanced by PMA.
However, the rate of the pEGFP-Skn-1a 207–287m3 mutation protein
was independent of PMA concentration, and following treatment with
10−8 M PMA all four proteins showed essentially the same
translocation rate (Fig. 7).
Discussion
The transport of proteins between the nucleus and
cytoplasm occurs primarily through the nuclear pore complex where
proteins can enter the nucleus either by diffusion or by
signal-mediated transport (11).
The structural constraints of the nuclear pore complex established
that only proteins with a molecular mass of <40 kDa are able to
enter the nucleus by passive diffusion. However, many proteins are
imported by signal-mediated transfer, among them Skn-1a, which is
~48 kDa in size (12). Based on
previous observations, we hypothesized that the regulation of
nuclear localization of Skn-1a serves as a molecular switch to
control the transcription of various epidermal genes.
The NLStradamus model predicts the sequence GRKRKKR
(aa279–285) to be the putative consensus NLS of Skn-1a (13). Therefore, we transiently expressed
in keratinocytes a number of EGFP fusion proteins containing the
putative NLS with deletions or mutations. The results clearly
demonstrate that Skn-1a contains a functional NLS domain, and that
the smallest domain necessary for Skn-1a transport is located
within amino acids 279–285 (Fig.
2), suggesting that this region of Skn-1a functions as an NLS.
Substitution of alanines for both Arg282 and Lys283 eliminated NLS
activity of the sequence GRKRKKR (aa279–285). We also found that
the deletion proteins from the constructs 207–295 and 207–287
showed a relatively low rate compared with the other shorter
constructs, suggesting the presence of a region spanning from
207–287 that potentially inhibits or counteracts the NLS function.
It is possible that Ser and Thr residues, prone to phosphorylation,
are present in the region 207–258. The computer program NetPhos 2.0
(14), which is utilized to
identify potential phospholylation sites, showed that the value of
Ser230 was relatively as compared to that of Ser287. Thus, studies
targeting Ser230 are to be conducted.
The three POU transcription factors Oct-1, Oct-6,
and Skn-1a are expressed in the epidermis. The corresponding NLS
regions of these human POU transcription factors Oct-1, Oct-6 and
Skn-1a are GLSRRRKKRTSIET, AQGRKRKKRTSIEV, VFGRKRKKRTSIET, respectively (the
NSL sequence is underlined) (7,15,16). Notably, all three POU factors
contain the identical TSIE amino acid sequence situated at the
C-terminus of the NLS. This binding suggests that this area may be
important in keratinocyte growth and differentiation. Findings of
previous studies have shown that the phosphorylation of particular
amino acids neighboring the NLS may regulate NLS activity (17), and that the amino acid residues
Thr and Ser are potential candidates for undergoing
phosphorylation. Consequently, we generated three mutation reporter
constructs derived from EGFP-Skn-1a 207–287, in which Thr286 was
replaced with alanine (EGFP-Skn-1a 207–287m1), Ser287 with alanine
(EGFP-Skn-1a 207–287m2), or both Thr286 and Ser287 with alanine
(EGFP-Skn-1a 207–287m3) and expressed them in keratinocytes.
Results of the present study demonstrate that the nuclear
translocation rate of construct m3 was higher than those of
EGFP-Skn-1a 207–287, and constructs m1 and m2, suggesting that
Thr286 and Ser287 play modulating negative roles in NLS function.
We also hypothesize that the nuclear accumulation of Skn-1a
correlates with the dephosphorylation of both Thr286 and
Ser287.
In the presence of low calcium concentration in the
medium, keratinocytes are maintained phenotypically as a basal
cell-like population of undifferentiated cells. Under these
conditions, the proteins from EGFP-Skn-1a 207–287, EGFP-Skn-1a
207–287m1 and EGFP-Skn-1a 207–287m2 translocate to the nucleus with
low efficiency, compared to EGFP-Skn-1a 207–287m3. PMA has been
well characterized as an activator of protein kinase C as well as
an inducer of late differentiation marker expression and cornified
envelope assembly in vitro. Therefore, we examined the
translocation rates of the mutation reporter proteins in
differentiated keratinocytes induced by PMA. As a result of PMA
treatment, the nuclear translocation rate of proteins from
EGFP-Skn-1a 207–287, EGFP-Skn-1a 207–287m1, and EGFP-Skn-1a
207–287m2 increased. However, the rate of EGFP-Skn-1a 207–287m3 was
independent of PMA concentration, and following 10–8 M
PMA treatment, all four proteins showed essentially the same
translocation rate. These data indicate that Thr286 and Ser287
residues play a role in keratinocyte differentiation, suggesting
that the epidermal differentiation signaling pathway, involving
kinase and phosphatase activation, regulates the NLS activity of
Skn-1a in the epidermal keratinocytes.
Results of the present study suggest that the
transcriptional regulation of various epidermal genes provides a
differentiation-specific phenotype of the epidermis. At present,
the precise mechanism of transcription factor nuclear translocation
in keratinocytes remains to be characterized. Further
characterization of the transportation mechanisms may facilitate an
understanding of signal transduction into the nucleus.
Acknowledgements
The authors thank Ms. Yuka Toyomaki, Mrs. Yukiko
Tamura, Mrs. Yuriko Takagi, and Ms. Nanako Seitoh for their
excellent technical assistance. This study was supported in part by
Grants-in-Aid from the Ministry of Education, Science, Sports, and
Culture of Japan.
Abbreviations:
|
EGFP
|
enhanced green fluorescent protein
|
|
NHEK
|
normal human epidermal
keratinocyte
|
|
NLS
|
nuclear localization signal
|
|
PMA
|
phorbol 12-myristate 13-acetate
|
References
|
1
|
Eckert RL, Crish JF and Robinson NA: The
epidermal keratinocyte as a model for the study of gene regulation
and cell differentiation. Physiol Rev. 77:397–424. 1997.PubMed/NCBI
|
|
2
|
Ryan AK and Rosenfeld MG: POU domain
family values: flexibility, partnerships, and developmental codes.
Genes Dev. 11:1207–1225. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seitz CS, Lin Q, Deng H and Khavari PA:
Alterations in NF-kappaB function in transgenic epithelial tissue
demonstrate a growth inhibitory role for NF-kappaB. Proc Natl Acad
Sci USA. 95:2307–2312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okuyama R, Tagami H and Aiba S: Notch
signaling: its role in epidermal homeostasis and in the
pathogenesis of skin diseases. J Dermatol Sci. 49:187–194. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maytin EV, Lin JC, Krishnamurthy R, et al:
Keratin 10 gene expression during differentiation of mouse
epidermis requires transcription factors C/EBP and AP-2. Dev Biol.
216:164–181. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Herr W, Sturm RA, Clerc RG, et al: The POU
domain: a large conserved region in the mammalian pit-1, oct-1,
oct-2, and Caenorhabditis elegans unc-86 gene products.
Genes Dev. 2:1513–1516. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goldsborough AS, Healy LE, Copeland NG, et
al: Cloning, chromosomal localization and expression pattern of the
POU domain gene Oct-11. Nucleic Acids Res. 21:127–134. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Andersen B, Weinberg WC, Rennekampff O, et
al: Functions of the POU domain genes Skn-1a/i and Tst-1/Oct-6/SCIP
in epidermal differentiation. Genes Dev. 11:1873–1884. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakajima K, Tamai K, Yamazaki T, et al:
Identification of Skn-1n, a splice variant induced by high calcium
concentration and specifically expressed in normal human
keratinocytes. J Invest Dermatol. 128:1336–1339. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takemoto H, Tamai K, Akasaka E, et al:
Relation between the expression levels of the POU transcription
factors Skn-1a and Skn-1n and keratinocyte differentiation. J
Dermatol Sci. 60:203–205. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McLane LM and Corbett AH: Nuclear
localization signals and human disease. IUBMB Life. 61:697–706.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hildesheim J, Foster RA, Chamberlin ME and
Vogel JC: Characterization of the regulatory domains of the human
SKN-1a/Epoc-1/Oct-11 POU transcription factor. J Biol Chem.
274:26399–26406. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nguyen Ba AN, Pogoutse A, Provart N and
Moses AM: NLStradamus: a simple hidden Markov model for nuclear
localization signal prediction. BMC Bioinformatics.
10:2022009.PubMed/NCBI
|
|
14
|
Blom N, Gammeltoft S and Brunak S:
Sequence and structure-based prediction of eukaryotic protein
phosphorylation sites. J Mol Biol. 294:1351–1362. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sturm RA, Das G and Herr W: The ubiquitous
octamer-binding protein Oct-1 contains a POU domain with a homeo
box subdomain. Genes Dev. 2:1582–1599. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suzuki N, Rohdewohld H, Neuman T, Gruss P
and Schöler HR: Oct-6: a POU transcription factor expressed in
embryonal stem cells and in the developing brain. EMBO J.
9:3723–3732. 1990.PubMed/NCBI
|
|
17
|
Hübner S, Xiao C and Jans DA: The protein
kinase CK2 site (Ser111/112) enhances recognition of the simian
virus 40 large T-antigen nuclear localization sequence by importin.
J Biol Chem. 272:17191–17195. 1997.
|