Introduction
The placenta, a unique endocrine organ during
pregnancy, plays a critical role in embryonic and fetal
development. The main functions of the placenta are executed by
trophoblasts, which originate from the trophectoderm layer of the
blastocyst. During morula-to-blastocyst transition, the surface
cells become trophoblasts and give rise to extraembryonic
structures, including the placenta (1). The trophoblast, which behaves like a
‘pseudotumor’, proliferates, differentiates, migrates and invades
the endometrium in a controlled manner (2). However, implantation and
placentation are strictly regulated in time and space; they are
intimately linked to and enable the embryo to be anchored to the
uterine wall, and thus ensure a normal pregnancy and fetal growth
through the placenta. Any abnormal factors promoting or inhibiting
this process may cause pregnancy-related diseases. One of these
diseases is preeclampsia (PE).
As one of the first imprinted genes, the H19
gene was initially isolated in 1984 (3) and further cloning and sequence
determination was carried out in 1988 (4). This gene is composed of 5 exons and
4 small introns (4). H19
RNA is transcribed by RNA polymerase II, then spliced and exported
into the cytoplasm. Due to the lack of a long conserved open
reading frame between the murine and human genome, the product of
H19 appears to be a non-coding RNA (5). The allele-specific expression
profile of the H19 gene in human placental tissue may result
from dynamic alternations during fetal development (6). The H19 gene is highly
expressed during mammalian embryonic development (7).
In the murine placenta, the H19 gene is the
second most abundant transcript (7), which is activated in the cells of
the trophectoderm at the time of implantation (8) and accumulates to extremely high
levels in the cells. These cells exclusively ontribute to the
formation of extraembryonic tissue. Moreover, the H19
transcript from human placental tissue during the first and third
trimester may play a regulatory role in trophoblast differentiation
(9). In a previous study of ours,
we indicated that the knockdown of H19 inhibits the
proliferation and apoptosis of human trophoblast-derived
choriocarcinoma cells (10).
Besides, the lack of H19 gives rise to placental overgrowth
(7). Thus, the pattern of
H19 expression suggests that it may regulate the function of
trophoblasts during the stage of embryogenesis and placental
development. We thus hypothesized that exon 1 is the longest exon
and functional region of the H19 gene, which may contribute
to the modulation and function of the placenta via
trophoblasts.
The methylation patterns of specific genes have been
found to undergo dynamic changes in the germ line and early embryo
(11). Variations in DNA
methylation are involved in the developmental process occurring in
normal and abnormal human placentas (12). The demethylating agent,
5-azacytidine, has been shown to change methylation profiles in
pregnant rats and to induce a marked reduction in placental growth
(13). Using
methylation-sensitive high resolution melting, Gao et al
found that the promoter region of the H19 gene was
hypermethylated in early-onset PE placentas compared with
third-trimester normal controls (14). Moreover, the H19 promoter
and exon 1 were differentially methylated in embryonic and somatic
tissues in mice (15).
Although our understanding of the intricate role of
the H19 gene in the regulation of the placental function in
mice has improved, the methylation status of H19 exon 1 in
human placental development and trophoblast function remains
unclear. Furthermore, little is known about the temporal
methylation variation of H19 exon 1 across gestation, and
whether the methylation status in the region is stable throughout
the first trimester and during the later stages of gestation. Thus,
in the present study, we aimed to assess the DNA methylation
pattern of H19 exon 1 in trophoblasts and human placental
tissues obtained from women undergoing normal pregnancy and in
pregnant women withPE.
Materials and methods
Subjects and sample collection
The experimental protocols in this study were
reviewed and approved by the Institutional Review Boards of the
corresponding hospitals and written informed consent was obtained
from all participants. A total of 37 subjects (21–39 years old) at
different stages of gestation were recruited from outpatient and
inpatient services at the Department of Obstetrics and Gynecology,
Daping Hospital, Xinan Hospital and Xinqiao Hospital of the Third
Military Medical University, the First Affiliated Hospital of
Chongqing Medical University and the Reproductive Centre of the
District of Banan, Chongqing, China, from August 2007 to March
2008. Six subjects in the first trimester of pregnancy (FTP), 16
subjects in the third trimester of pregnancy (TTP) and 15 women
with severe PE (sPE) were included in the present study. The
placental villous tissues from women undergoing normal pregnancy
between 6 and 9 weeks of gestation were collected during the
procedure of induced abortion, while the placental tissues from
women undergoing normal full-term pregnancy or from women with sPE
in the third trimester were obtained by cesarean section. Patients
with sPE were diagnosed according to the criteria stated in the
American College of Obstetricians and Gynecologists (ACOG) Practice
Bulletin (2002), which included blood pressure of ≥160/110 mmHg on
2 occasions at least 6 h apart after 20 weeks of gestation and
proteinuria (≥5 g/24 h or ≥3+ on 2 random urine samples collected
at least 4 h apart). Patients with a history of chronic
hypertension, diabetes mellitus, nephropathy or a recent urinary
tract infection were excluded from this study. All the specimens
were quickly dissected, snap-frozen in liquid nitrogen and stored
at −80°C until further analyses.
Cell culture
The human choriocarcinoma cell line, JEG-3, was
obtained from the American Type Culture Collection (ATCC, Mannasas,
VA, USA). The cells were cultured in Dulbecco’s modified Eagle’s
medium/nutrient mixture F-12 medium (DMEM/F-12; Gibco-BRL,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS;
Gibco-BRL) at 37°C in a humidified atmosphere containing 5%
CO2. To assess the effects of methylation inhibition,
the cells were treated with 5-aza-2′-deoxycytidine (5-Aza-Dc;
Sigma-Aldrich, St. Louis, MO, USA).
DNA methylation assay
The cells were pelleted following treatment with
5-Aza-Dc at concentrations of 1, 10, or 100 μmol/l for 24, 48 and
72 h. Cells treated with 0.05% DMSO were used as controls. Genomic
DNA was extracted from the cells or frozen tissues using the
phenol-chloroform method. The bisulphite conversion of DNA was
performed using a EZ DNA Methylation-Gold™ kit (Zymo Research,
Orange, CA, USA) following the manufacturer’s instructions. DNA
methylation analysis was conducted following bisulfite sequencing
PCR (BSP). The bisulfite sequence of the primers was as follows:
5′-TTGGAGTTTGGTAGGAGTGATG-3′ (forward) and
5′-CCCAAACCCTAAAATCAAACCCT-3′ (reverse). PCR amplification was
conducted under the following conditions: 94°C for 5 min, then 34
cycles of 94°C for 30 sec, 61°C for 30 sec, 72°C for 1 min,
followed by 72°C for 10 min for the final extension. All products
were confirmed to be single bands by 2% agarose gel
electrophoresis. PCR products were sequenced by Shanghai Invitrogen
Biotechnology Co., Ltd. (Shanghai, China).
RNA extraction and real-time reverse
transcription PCR (RT-qPCR)
The cells were treated with 5-Aza-Dc at
concentrations of 0.1, 1, 10 or 100 μmol/l for 24, 48 or 72 h and
pelleted. Cells treated with 0.05% DMSO were used as controls.
Total RNA from the tissues and cells was extracted using TRIzol
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) and
reverse transcribed into cDNA using the RT kit (BioBRK, Chengdu,
China) according to the manufacturer’s instructions. Quantitative
(real-time) PCR was performed using the SYBR-Green PCR kit (Takara
Bio Inc., Shiga, Japan). The primer sequences were as follows: H19
forward, 5′-GGCAAGAAGCGGGTCTGT-3′ and reverse,
5′-GCTGCTGTTCCGATGGTGT-3′; and GAPDH forward,
5′-ACCCATCACCATCTTCCAGGAG-3′ and reverse,
5′-GAAGGGGCGGAGATGATGAC-3′.
The reactions in triplicate were first denatured at
94°C for 5 min and then subjected to 35 cycles of 94°C for 30 sec,
59°C for 30 sec and 72°C for 30 sec. Data were normalized to those
of GAPDH. The relative mRNA level was calculated using the
2−ΔΔCt method.
MTT assay
The effects of 5-Aza-Dc on cell proliferation were
determined by MTT assay. The cells (3.5×103 cells/well)
were seeded in 96-well plates with 100 μl DMEM/F-12 with 10% FBS in
each well in sextuplicate for 24 h, and then treated without or
with 5-Aza-Dc at concentrations of 0.01, 0.1, 1, 10 or 100 μmol/l
for 24, 48 or 72 h. Cells treated with 0.05% DMSO were used as
controls. On the day of the assay, the growth medium was replaced
with serum-free medium containing 5 mg/ml MTT (Sigma-Aldrich) and
incubated at 37°C for 4 h. At the end of the incubation period, the
cells were solubilized in 150 μl of DMSO, and colorimetric
determination was performed at 570 nm absorbance with a plate
reader. The data are presented as the mean values from 3
independent experiments.
Cell migration and invasion assays
A cell migration assay were was performed using
Transwell inserts (6.5 mm diameter; 8 μm pore size polycarbonate
membrane; BD Biosciences, Franklin Lakes, NJ, USA). For cell
invasion assay, Transwell plates were coated with 50 μl Matrigel
(1:10 dilution; BD Biosciences) solution per well and dried for 30
min at 37°C with 5% CO2. Cells (1×105
cells/well) in 0.2 ml serum-free medium with or without 5-Aza-Dc
(100 μmol/l) were placed in the upper chamber, whereas the lower
chamber was loaded with 0.8 ml medium containing 10% FBS. The cells
were allowed to migrate or invade for 24 h at 37°C with 5%
CO2. After removing the upper Transwell, the
non-migrated or non-invaded cells on the inner surface of the
Transwell were carefully removed using a cotton swab. The cells
that had penetrated to the bottom side of the membrane were then
fixed in buffered formalin and stained using haematoxylin. The
number of stained cells/well was counted using an inverted
microscope (Olympus, Tokyo, Japan).
Statistical analysis
Analysis of variance (ANOVA) or Fisher’s exact test
was used for statistical analysis. A probability (P)-value <0.05
was considered to indicate a statistically significant difference.
All statistical analyses were performed using SPSS software version
13.0 or GraphPad Prism version 5.01.
Results
The methylation status at the H19 exon 1
region in human placental tissue
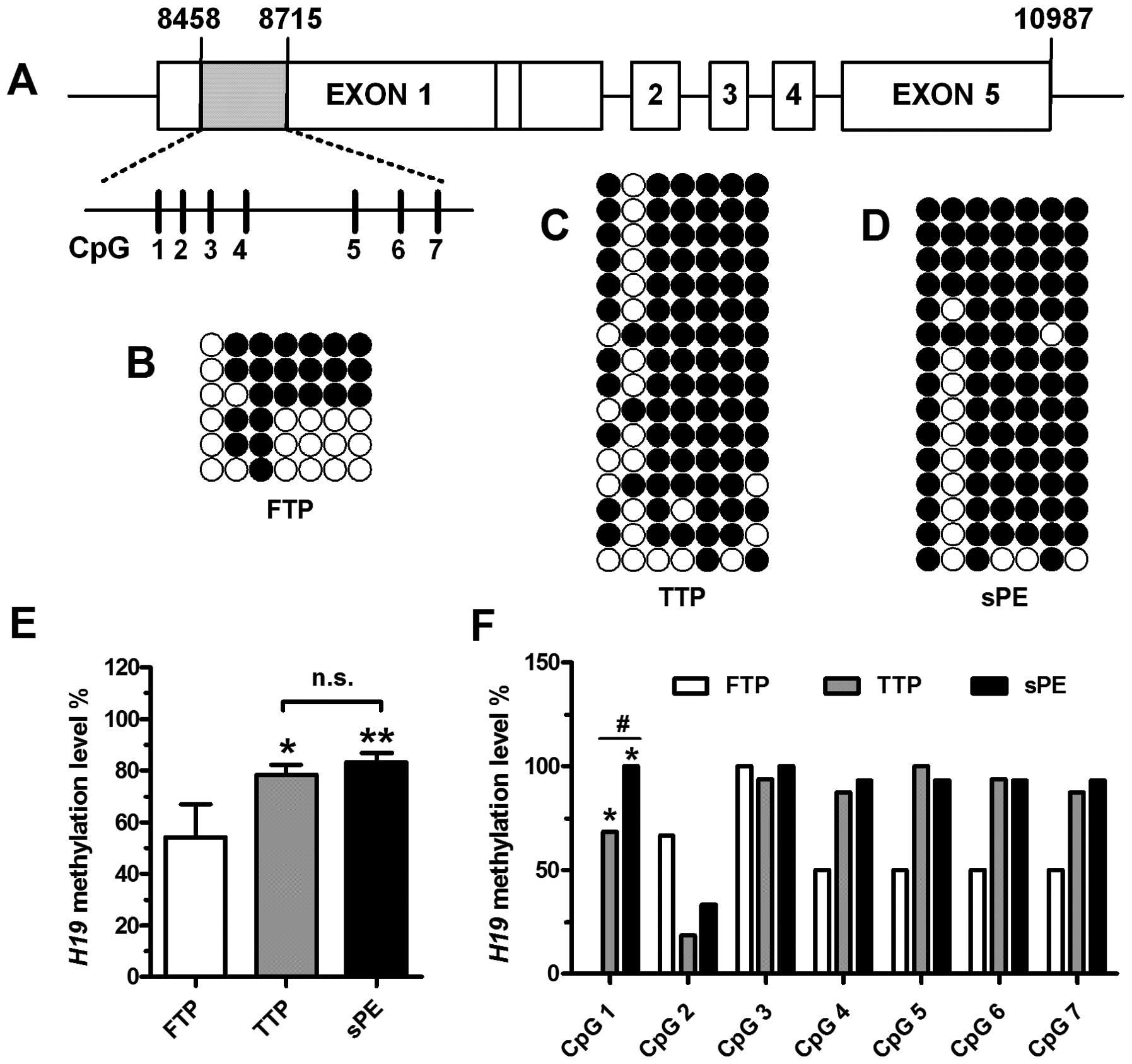
As shown in Fig.
1A, we used BSP assay to quantitatively measure the levels of
methylation at 7 CpG sites (CpG 1, 8,547 bp; CpG 2, 8,558 bp; CpG
3, 8,571 bp; CpG 4, 8,587 bp; CpG 5, 8,637 bp; CpG 6, 8,658 bp; CpG
7, 8,675 bp) in H19 exon 1 (GenBank accession no. AF087017)
in human placental tissues obtained from women at different stages
of gestation. Methylation levels in the first exon of the
H19 gene in placental tissue from women in FTP (Fig. 1B), TTP (Fig. 1C) and from women with sPE
(Fig. 1D) were 54.17±12.81%,
78.44±15.63% and 83.21±14.33%, respectively. The region showed
significant hypomethylation in the placental tissue from women in
FTP compared with that from women in TTP or women with sPE
(P<0.05) (Fig. 1E). When each
CpG site was analyzed independently, the methylation level at CpG 1
of H19 exon 1 displayed significant demethylation in the
placental tissue from women in FTP in comparison to that from women
in TTP (P<0.01) (Fig. 1F). The
methylation levels at this site of H19 were significantly
increased in the placental tissue from women with sPE compared with
the levels in the placental tissue from women in TTP
(P<0.01).
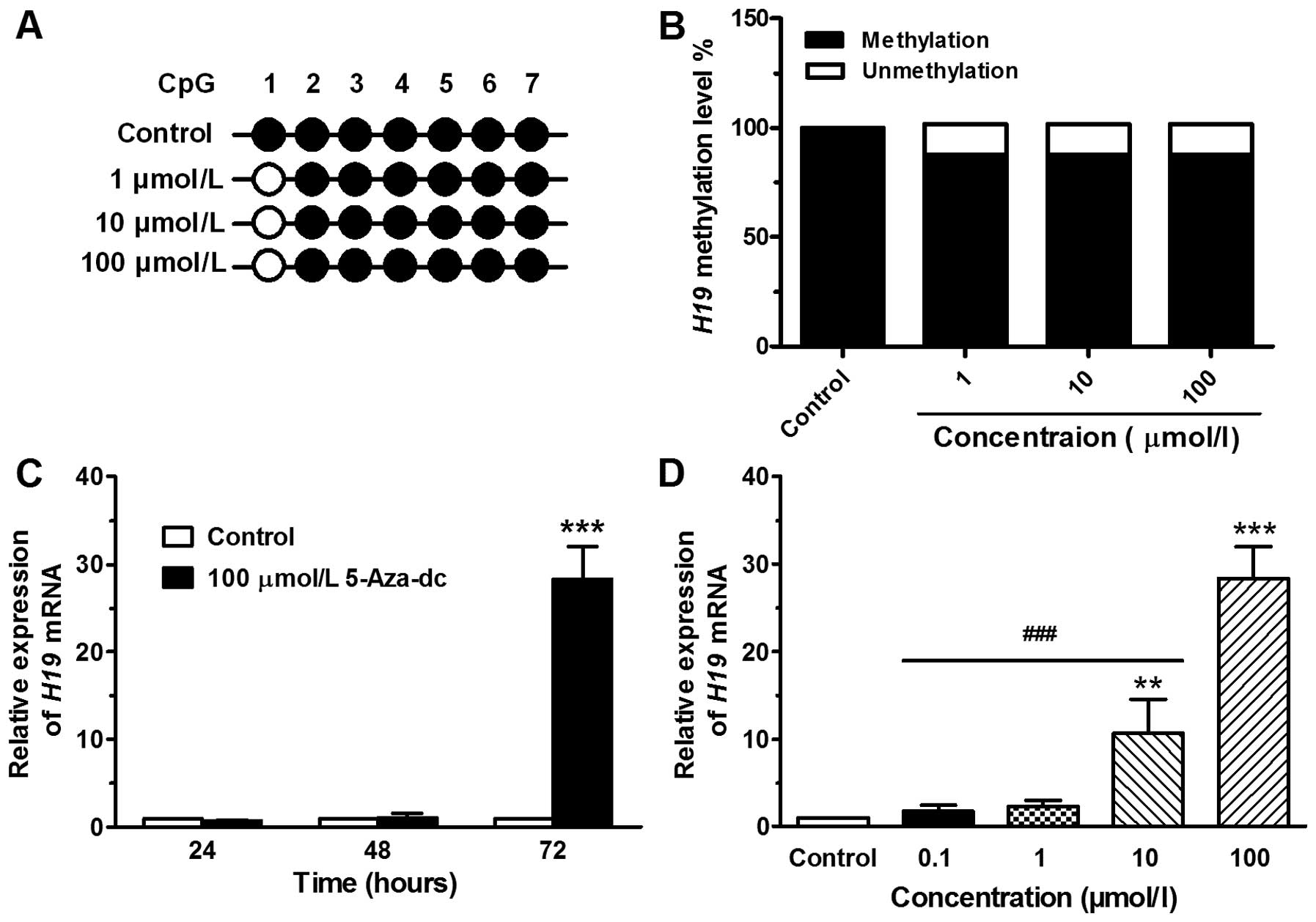
Methylation level of the H19 exon 1
region in JEG-3 cells treated with 5-Aza-Dc
The chemical agent, 5-Aza-Dc, a cytidine analog, has
been reported to effectively result in the demethylation of DNA
(16). In this study, the region
(H19 exon 1) containing 7 CpG sites, spanning 258 bp, was analyzed
(Fig. 2A). DNA from the JEG-3
cells treated without or with 5-Aza-Dc at concentrations of 1, 10
or 100 μmol/l was extracted at 72 h and then analyzed by BSP assay.
The methylation level of the exon 1 region was signaficantly
demethylated in the cells treated with 5-Aza-Dc compared with the
control (DMSO-treated) cells, and the effect was dose-independent
(Fig. 2B). However, the
demethylation effect of 5-Aza-Dc was only observed at the CpG 1
site and not at the other 6 CpG sites (Fig. 2A).
Expression level of H19 in JEG-3 cells
treated with 5-Aza-Dc
Subsequently, we examined whether H19 exon 1
demethylation leads to an increase in the H19 mRNA level in
JEG-3 cells. Following treatment with 5-Aza-Dc at 100 μmol/l for
24, 48 or 72 h, the H19 mRNA expression was 0.71±0.07,
1.05±0.49 and 28.35±3.72, respectively (Fig. 2C). Following treatment for 72 h
with 5-Aza-Dc at concentrations of 0.1, 1, 10 or 100 μmol/l, the
H19 mRNA expression was 1.82±0.64, 2.32±0.69, 10.70±3.89 and
28.35±3.72, respectively (Fig.
2D). As the treatment time or concentration increased, a marked
increase in H19 expression was observed.
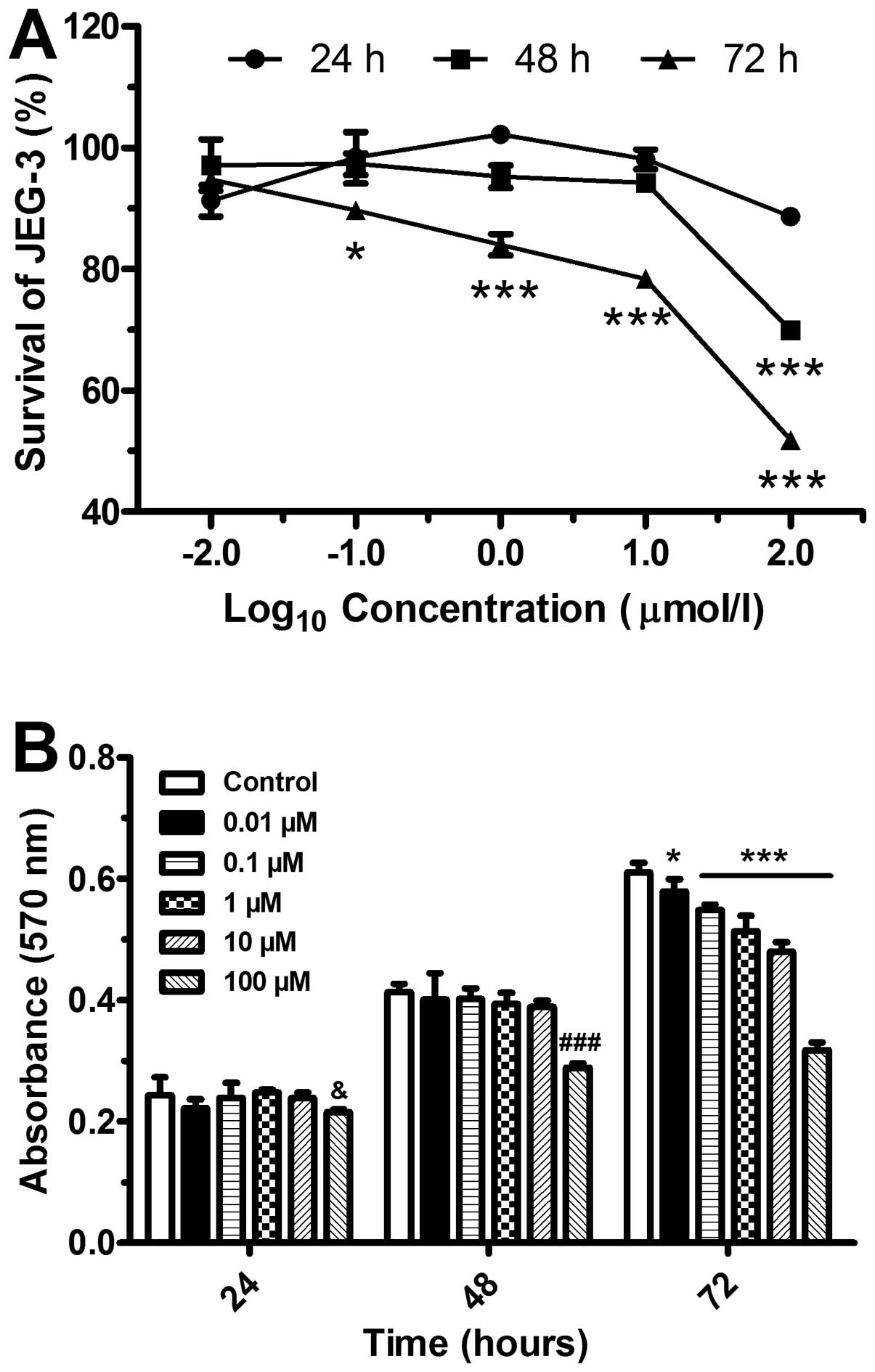
Inhibition of cell proliferation by
5-Aza-Dc
We then investigated JEG-3 cell viability following
treatment with 5-Aza-Dc at various concentrations and different
periods of time points. Cell proliferation was suppressed in a
concentration-dependent manner (Fig.
3A). In comparison to the control (DMSO-treated) group, the
cells treated for 24 and 48 h displayed a significant inhibition of
proliferation at the concentration of 100 μmol/l (P<0.001)
(Fig. 3B). By contrast, the
proliferation of the cells treated for 72 h with various
concentrations of 5-Aza-Dc was significantly inhibited.
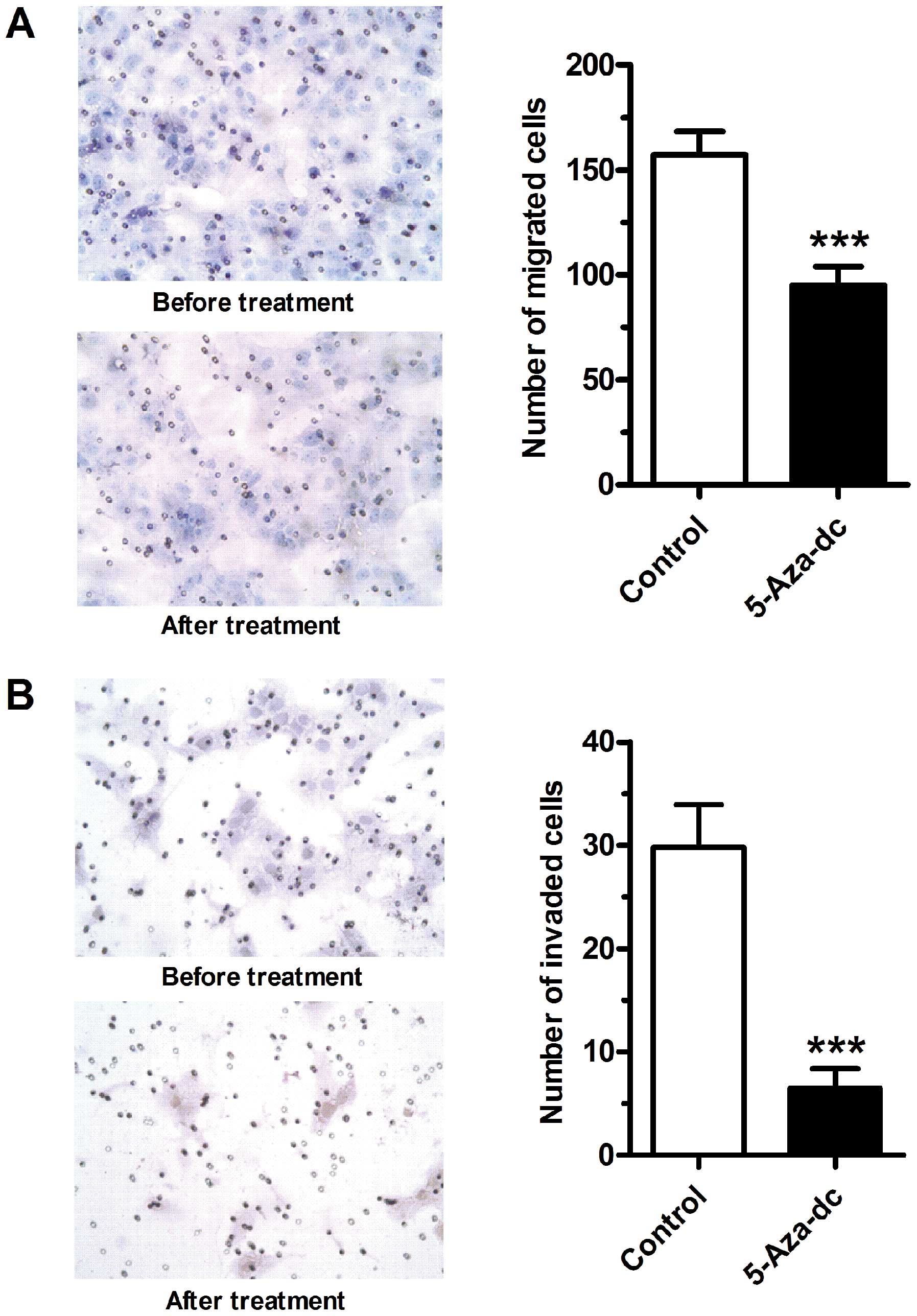
Demethylation by 5-Aza-Dc inhibits
trophoblast-derived cell migration and invasion
The effects of 5-Aza-Dc on the migratory and
invasive properties of JEG-3 cells were then examined. Following
treatment with 5-Aza-Dc at 100 μmol/l for 24 h, both the migration
(Fig. 4A) and invasion (Fig. 4B) abilities of the JEG-3 cells
were markedly inhibited (P<0.001).
Discussion
The placenta is a unique endocrine organ during
pregnancy that, although it is only transiently required, plays a
critical role in protecting and nourishing the growing fetus
(17). Trophoblasts developing
into placental tissue are exclusive to this type of tissue. During
normal gestation, these trophoblasts act as tumor-like cells, with
enhanced proliferative and invasive activities, but behave in a
moderate and balanced way (2).
Once this balance is broken, corresponding diseases may occur.
Excessive invasion may lead to invasive moles or choriocarcinoma
(18). On the other hand, poor
invasion may cause obstetric complications, including miscarriage,
intrauterine growth restriction and PE (19). PE is a life-threatening,
pregnancy-specific disorder characterized by hypertension and
proteinuria (20). It is
generally agreed that the presence of the placenta, and more
specifically the presence of trophoblasts, is a major cause of this
disorder (17). Elucidating the
modulation of trophoblast development is key to understanding the
pathogenesis of PE.
Previous studies on DNA methylation of the
H19 gene have mainly focused on the imprinting control
region (ICR), the H19 promoter region, or the H19
transcription start site (21–23). In the present study, we
investigated the methylation status of H19 exon 1 in
placental tissue from women undergoing normal pregnancy during the
first and third trimesters, as well as pregnant women with PE. As
one of the first imprinted genes (3), the H19 transcript is
abundantly expressed in mouse placental tissue and human
intermediate trophoblasts and cytotrophoblasts. Mice that carry a
deletion of H19 display placental overgrowth (7). In a previous study of ours, we
demonstrated that the H19 gene imprinting status in human
placental tissue is markedly altered during normal pregnancy, but
imprinting is lost in the placental tissue of PE patients (24). These differences suggest that
epigenetic changes in the H19 gene may be relevant to the
pathogenesis of PE. DNA methylation is fundamental for epigenetic
modulation in mammalian development (25). It typically occurs at the C5
position of cytosine residues in a CpG dinucleotide context.
CG-rich regions, known as CpG islands, most often localize at the
promoter and exon 1 regions (26). H19 exon 1 is highly
conserved in both marsupials and eutherians (27), suggesting that this region may
play some unknown important role in mammalian development.
In this study, we assessed the methylation status in
H19 exon 1 in placental tissue from women in FTP, TTP, as
well as in pregnant women with sPE by BSP. We found that the DNA
methylation level of H19 exon 1 was significantly increased
in placental tissue from women in TTP in comparison to that in
placental tissue from women in FTP. It was observed that, compared
with the placental tissue from women in TTP, the methylation levels
in placental tisssue from women with sPE were only slightly
enhanced in this region (P>0.05) (Fig. 1E). We further analyzed the
methylation status of each CpG site independently. The methylation
levels at the CpG 1 site were significantly increased in the
placental tissue from women in TTP in comparison to those in
placental tissue from women in FTP. More importantly,
hypermethylation at the CpG 1 site was markedly increased in the
placental tissue from women with sPE when compared with the
placental tissue from women in TTP (Fig. 1F). These results indicate that the
methylation levels of H19 exon 1 in the human placenta are
dynamically altered during gestation. Furthermore, there was marked
hypermethylation at individual CpG sites of H19 exon 1 in
the placental tissue from women with PE. Hence, the alteration of
the methylation status in individual CpG sites may be associated
with abnormal placentation and the pathogenesis of PE.
In this study, to investigate the effects of
demethylation on trophoblasts, 5-Aza-Dc, a cytidine analog, we used
as a DNA methylation inhibitor. The CpG 1 site rather than the
other sites in this region showed marked demethylation in the
treated cells (Fig. 2A and B).
These results indicated that the demethylation agent, 5-Aza-Dc,
does not act on each CpG site but on some individual positions in
DNA sequences. Besides, the relative expression of H19 mRNA
increased following treatment of the trophoblasts with 5-Aza-Dc
(Fig. 2C and D), indicating that
demethylation at some individual CpG sites may correlate with the
enhanced mRNA expression of H19.
Originating from trophoblasts, the hydatidiform mole
has a propensity to malignancy. The expression of H19 has
been shown to be decreased during the transition from a complete
hydatidiform mole to choriocarcinoma (28), which indicates an inhibitory role
for H19 during the malignant transformation of trophoblastic
diseases. This is consistent with our present results that the
proliferation (Fig. 3), migratory
and invasive (Fig. 4) abilities
of the choriocarcinoma cells were suppressed following
demethylation treatment, which induced the upregulation of
H19 expression.
In conclusion, in this study, we demonstrate that
the CpG 1 site in H19 exon 1 is hypermethylated in placental
tissue from pregnant women with PE. Following treatment of the
cells with the methylation inhibitor, 5-Aza-Dc, the methylation
levels at this site in the trophoblasts were markedly decreased and
the mRNA levels of the H19 gene were increased. Furthermore,
the proliferative, migratory and invasive abilities of the
trophoblasts were significantly inhibited. Therefore, our data
suggest that hypermethylation at the CpG 1 site of H19 exon
1 may be associated with the overproliferation of trophoblasts and
may contribute to the pathogenesis of PE.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81070505 and
81070496).
References
|
1
|
Red-Horse K, Zhou Y, Genbacev O, et al:
Trophoblast differentiation during embryo implantation and
formation of the maternal-fetal interface. J Clin Invest.
114:744–754. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Merviel P, Carbillon L, Challier JC,
Rabreau M, Beaufils M and Uzan S: Pathophysiology of preeclampsia:
links with implantation disorders. Eur J Obstet Gynecol Reprod
Biol. 115:134–147. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pachnis V, Belayew A and Tilghman SM:
Locus unlinked to alpha-fetoprotein under the control of the murine
raf and Rif genes. Proc Natl Acad Sci USA. 81:5523–5527. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pachnis V, Brannan CI and Tilghman SM: The
structure and expression of a novel gene activated in early mouse
embryogenesis. The EMBO J. 7:673–681. 1988.PubMed/NCBI
|
|
5
|
Brannan CI, Dees EC, Ingram RS and
Tilghman SM: The product of the H19 gene may function as an RNA.
Mol Cell Biol. 10:28–36. 1990.PubMed/NCBI
|
|
6
|
Szabo PE and Mann JR: Allele-specific
expression and total expression levels of imprinted genes during
early mouse development: implications for imprinting mechanisms.
Genes Dev. 9:3097–3108. 1995. View Article : Google Scholar
|
|
7
|
Keniry A, Oxley D, Monnier P, et al: The
H19 lincRNA is a developmental reservoir of miR-675 that suppresses
growth and Igf1r. Nat Cell Biol. 14:659–665. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Poirier F, Chan CT, Timmons PM, Robertson
EJ, Evans MJ and Rigby PW: The murine H19 gene is activated during
embryonic stem cell differentiation in vitro and at the time of
implantation in the developing embryo. Development. 113:1105–1114.
1991.PubMed/NCBI
|
|
9
|
Rachmilewitz J, Gileadi O, Eldar-Geva T,
Schneider T, de-Groot N and Hochberg A: Transcription of the H19
gene in differentiating cytotrophoblasts from human placenta. Mol
Reprod Dev. 32:196–202. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu LL, Chang K, Lu LS, et al:
Lentivirus-mediated RNA interference targeting the H19 gene
inhibits cell proliferation and apoptosis in human choriocarcinoma
cell line JAR. BMC Cell Biol. 14:262013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kafri T, Ariel M, Brandeis M, et al:
Developmental pattern of gene-specific DNA methylation in the mouse
embryo and germ line. Genes Dev. 6:705–714. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Avila L, Yuen RK, Diego-Alvarez D,
Penaherrera MS, Jiang R and Robinson WP: Evaluating DNA methylation
and gene expression variability in the human term placenta.
Placenta. 31:1070–1077. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Serman L, Vlahovic M, Sijan M, et al: The
impact of 5-azacytidine on placental weight, glycoprotein pattern
and proliferating cell nuclear antigen expression in rat placenta.
Placenta. 28:803–811. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao WL, Li D, Xiao ZX, et al: Detection of
global DNA methylation and paternally imprinted H19 gene
methylation in preeclamptic placentas. Hypertens Res. 34:655–661.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bartolomei MS, Webber AL, Brunkow ME and
Tilghman SM: Epigenetic mechanisms underlying the imprinting of the
mouse H19 gene. Genes Dev. 7:1663–1673. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Creusot F, Acs G and Christman JK:
Inhibition of DNA methyltransferase and induction of Friend
erythroleukemia cell differentiation by 5-azacytidine and
5-aza-2′-deoxycytidine. J Biol Chem. 257:2041–2048. 1982.PubMed/NCBI
|
|
17
|
Ji L, Brkic J, Liu M, Fu G, Peng C and
Wang YL: Placental trophoblast cell differentiation: physiological
regulation and pathological relevance to preeclampsia. Mol Aspects
Med. 34:981–1023. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Altieri A, Franceschi S, Ferlay J, Smith J
and La Vecchia C: Epidemiology and aetiology of gestational
trophoblastic diseases. Lancet Oncol. 4:670–678. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anin SA, Vince G and Quenby S: Trophoblast
invasion. Hum Fertil (Camb). 7:169–174. 2004. View Article : Google Scholar
|
|
20
|
Sibai BM: Diagnosis and management of
gestational hypertension and preeclampsia. Obstet Gynecol.
102:181–192. 2003. View Article : Google Scholar
|
|
21
|
Bourque DK, Avila L, Penaherrera M, von
Dadelszen P and Robinson WP: Decreased placental methylation at the
H19/IGF2 imprinting control region is associated with normotensive
intrauterine growth restriction but not preeclampsia. Placenta.
31:197–202. 2010. View Article : Google Scholar
|
|
22
|
Buckberry S, Bianco-Miotto T, Hiendleder S
and Roberts CT: Quantitative allele-specific expression and DNA
methylation analysis of H19, IGF2 and IGF2R in the human placenta
across gestation reveals H19 imprinting plasticity. PLoS One.
7:e512102012. View Article : Google Scholar
|
|
23
|
Yuen RK, Penaherrera MS, von Dadelszen P,
McFadden DE and Robinson WP: DNA methylation profiling of human
placentas reveals promoter hypomethylation of multiple genes in
early-onset preeclampsia. Eur J Hum Genet. 18:1006–1012. 2010.
View Article : Google Scholar
|
|
24
|
Yu L, Chen M, Zhao D, et al: The H19 gene
imprinting in normal pregnancy and pre-eclampsia. Placenta.
30:443–447. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jones PA and Takai D: The role of DNA
methylation in mammalian epigenetics. Science. 293:1068–1070. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Larsen F, Gundersen G, Lopez R and Prydz
H: CpG islands as gene markers in the human genome. Genomics.
13:1095–1107. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Smits G, Mungall AJ, Griffiths-Jones S, et
al: Conservation of the H19 noncoding RNA and H19-IGF2 imprinting
mechanism in therians. Nat Genet. 40:971–976. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Walsh C, Miller SJ, Flam F, Fisher RA and
Ohlsson R: Paternally derived H19 is differentially expressed in
malignant and nonmalignant trophoblast. Cancer Res. 55:1111–1116.
1995.PubMed/NCBI
|