Introduction
Liver fibrosis is the response of the liver to
diverse chronic insults such as chronic viral infection (mostly
hepatitis B and C virus), parasitic disease and toxic damage
(1). Liver fibrosis is
characterized by the activation of hepatic stellate cells (HSCs),
which are then involved in the synthesis of matrix proteins and the
regulation of matrix degradation (2). The advanced stage of fibrosis is
cirrhosis. Liver cirrhosis, the irreversible terminal stage of
chronic liver disease, is a major cause of morbidity and mortality
worldwide, with no effective therapy (3). Many factors are involved in the
development of fibrosis, including profibrotic cytokines,
chemokines, eicosanoids, fibrinolytic/fibrinogenic factors, matrix
metalloproteinases and their inhibitors, and oxidative stress
(4).
Interleukin-10 (IL-10) is a cytokine that
downregulates the proinflammatory response and has a modulatory
effect on hepatic fibrogenesis (5–7).
IL-10 has a direct effect on the production of collagen and
collagenases and modulates remodeling of the extracellular matrix
(ECM) (8). Results of previous
studies have shown that IL-10 may be important in antifibrogenesis
during CCl4-induced hepatic fibrogenesis (9–11).
However, the half-life of IL-10 is extremely short, at
approximately 2–3 h. Maintaining a stable expression in the serum
is difficult. Gene therapy is expected to resolve this problem and
may be a useful method for the early stage of liver cirrhosis. Gene
therapy may prove successful following the development of effective
gene delivery systems. An ideal vector would be able to deliver
genetic material efficiently, and would result in a high-level,
properly-regulated and prolonged expression. Additionally, this
vector would be non-toxic, non-immunogenic, and have a broad host
range (12). It has been reported
that high levels of foreign gene expression in murine hepatocytes
can be achieved by the rapid injection of a large volume of a naked
DNA solution into the tail vein. This process is known as
‘hydrodynamics-based transfection (HBT)’ (13,14). The aim of the present study was to
evaluate the anti-fibrotic effects of the rat interleukin-10
(rIL-10) gene by HBT gene delivery system on experimental
liver fibrosis in rats and its possible mechanism.
Materials and methods
Rats
Clean male Sprague-Dawley rats weighing 100–120 g
were provided by the Shanghai Experimental Animal Center (Shanghai,
China). The rats were bred at a room temperature of 22±2°C,
humidity of 55±5%, with a 12-h alternating light/dark cycle and
access to water and food ad libitum. The feed was provided
by the BK Company (Shanghai, China). Animal procedures were
performed under the control of the animal care committee of Fujian
Medical University in accordance with the Guidelines on Animal
Experiments in Fujian Medical University.
Plasmid DNA preparation
Large-scale plasmid DNA preparation was produced
using the alkaline lysis method (Qiagen, Beijing, China; no. 12362,
USA). The plasmid preparation for in vivo injections was
suspended in sterile deionized water.
Intravenous injection of plasmid DNA
Injection of plasmid DNA was performed as described
by Liu et al and Zhang et al (13,14). Briefly, plasmid DNA (1.4 μg/g) in
lactated Ringer’s solution (0.1 ml/g body weight) was injected into
the tail vein. The DNA injection was completed in 10–15 sec. With
this delivery system, the plasmid was trapped in the liver where it
produced cytokine which was then transported into the bloodstream
and perfused the organs (15,16).
Animal model and experimental
protocols
Twenty-seven Sprague-Dawley rats with a body weight
of 100–120 g were used, and experiments were performed in
accordance with the institutional ethics guidelines of the Fujian
Medical University Union Hospital. Hepatic fibrosis was induced by
intraperitoneal injections of 0.5 ml porcine serum (PS) (PAA
Laboratories, Linz, Austria) twice a week for 8 weeks. Control rats
(CTRL) were injected 0.5 ml physiological saline twice a week by
intraperitoneal injection. From the 5th week, fibrotic rats were
randomly divided into 3 groups: fibrotic rats injected weekly with
Ringer’s solution through the caudal vein (PS), or rIL-10
recombinant plasmid DNA (PS-pcDNA3-rIL-10), or PcDNA3 empty vector
(PS-pcDNA3). At the end of the 8th week, the experimental rats were
sacrificed under anesthesia of 10% chloral hydrate.
Histopathological examination
Liver of rats receiving a rIL-10 plasmid DNA
(pcDNA3-rIL-10) injection on days 1, 7 and 14 following the gene
transfer and liver of fibrotic rats at the end of the 8th week were
harvested. The samples were fixed in 10% formalin and embedded with
paraffin. Sections were stained with hematoxylin and eosin
(H&E) and evaluated by two pathologists.
Sirius red staining and collagen
measurement
The sections were deparaffinized with xylene and
rehydrated with graded ethanol. After rinsing the sections with
distilled water 3 times, the sections were stained in 0.1% Sirius
red in saturated picric acid solution for 30 min, and placed in
ethanol for differentiation for 2 min. The sections were then
rinsed in phosphate-buffered saline once and water twice for 30 sec
each to remove any unbound dye. After drying for 2 h, the slides
were mounted. The quantitative analysis of collagen type I and III
was carried out using the Olympus-BX41 image analyzing system in
five microscopic fields (magnification, x40) per section. The
average of the five fields was calculated for assessment of the
degree of fibrosis in each case. All the sections were examined by
the same pathologist who was blind to the experimental design. The
liver tissue was distinguished from the background according to a
difference in light density. This allowed the measurement of the
total liver tissue area. The amount of connective tissue, stained
red, was then measured. Subsequently, the percentage of collagen on
the section was measured.
Liver function assays
Serum levels of aspartate aminotransferase (AST) and
alanine aminotransferase (ALT) were measured by routine methods in
the clinical laboratory of our institution.
ELISA assay
Serum samples and culture supernatant were assayed
for rIL-10 using an ELISA kit according to the manufacturer’s
instructions (Biosource International, Inc., Camarillo, CA,
USA).
RT-PCR assay
Total RNA was extracted using TRIzol reagent (Gentra
Systems, Inc., Minneapolis, MN, USA), and then reverse transcribed
to cDNA according to the instructions of the MMLV reverse
transcription kit (Promega, Madison, WI, USA). Using 2 μl RT
products as the template, the PCR reaction contained 10 pmol for
each primer (rIL-10, sense: 3′-cgaagcttgccaccatgcttggctcagcac-5′
and antisense: 3′-cgtcta gatcaatttttcattttgagtg-5′, product, 559
bp; β-actin, sense: 3′-ccaaccgtgaaaagatgacc-5′ and antisense
3′-caggaggagcaa tgatcttg-5′, product, 660 bp to detect the rIL-10
mRNA expression in rat liver, lung, heart, kidney and spleen
tissue. The samples were placed in a thermocycler with the
incubation program at 95°C for 5 min, then 30 cycles at 94°C for 45
sec, at 61°C for 30 sec, and an extension at 72°C for 1 min.
Products of RT-PCR were electrophoresed on a 16 g/l agarase gel to
reveal the amplified bands.
Immunohistochemistry
Rat liver tissues were sectioned at a thickness of 4
μm. Following deparaffinization with xylene and dehydration with
graded ethanol, the sections were incubated in PBS containing 30
ml/l H2O2 to remove endogenous peroxidase and
in PBS containing 0.1 mol/l citrate to retrieve microwave antigens
and then incubated with normal goat serums to block the
non-specific binding sites. Following incubation with primary
antibodies against rIL-10 (Biosource International, Inc.), α-SMA
(Abcam, Hong Kong, China), the sections were treated with instant
S-P immunohistochemical reagents (Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China) and incubated in a buffer
solution containing 3,3′-diaminobenzidine tetrahydrochloride (DAB)
and H2O2 to produce a brown reaction product.
The sections were then dehydrated and coverslipped. Microscopic
examination of the sections was performed and the results were
assessed as previously described (9).
BRL cell culture and transfection
BRL cells, an immortalized normal rat hepatocyte
line obtained from the cell bank of Academia Sinica, Shanghai,
China, were seeded in 30-ml plastic flasks at 1x106
cells/well and cultivated in DMEM supplemented with 10% fetal
bovine serum (FBS) in a humidified incubator containing 5%
CO2 at 37°C. When cell density reached 60–70%
confluence, transfection was performed according to the
manufacturer’s instructions (Polyplus Transfection, New York, NY,
USA). BRL cells were separately transfected with plasmid
pcDNA3-rIL-10, pcDNA3 and normal saline for 24 h using
JetPEI™-Hepatocyte DNA transfection reagent (Polyplus Transfection,
New York, NY, USA). The transfection system contained 5 μg DNA and
16 μl JetPEI-Hepatocyte per flask.
HSC culture
HSCs, an immortalized normal rat HSCs line obtained
from the cell bank of Academia Sinica, were seeded in 6-wells at
3x105 cells/well in 2 ml DMEM containing 10% FBS the day
prior to BRL cell transfection.
Co-culture of HSC and BRL cells
Following transfection for 24 h, BRL cells were
digested and seeded in 30-mm filter inserts (Millipore, Billerica,
MA, USA) and placed in 6-well culture plates at 2x105
cells/insert in 3 ml DMEM containing 10% FBS for 3 h. Inserts
planted with BRL were placed in the 6-well plastic tissue culture
plates which had been planted with HSCs and the culture medium was
then refreshed. The co-culture cells were divided into HSCs
co-cultured with BRL transfected with i) normal saline (HSCs/BRL);
ii) pcDNA3.0 (HSCs/BRL-rIL-10−); and iii) pcDNA3.0-IL-10
(HSCs/BRL-rIL-10+). Co-culture was terminated after 48
h.
Western blotting
After 48-h co-culture, HSCs were washed with PBS
twice and lysed with cell lytic buffer containing 50 mM Tris pH
8.0, 150 mM NaCl, 0.2 mg/ml NaN3, 1 mg/ml SDS, 0.1 mg/ml aprotinin,
10 mg/ml NP-40, and 5 mg/ml sodium deoxycholate and 0.1 mg/ml
phenyl-methylsulfonyl fluoride. The supernatants were obtained
following centrifugation at 1,500 x g for 10 min. The protein
concentrations of the cytosolic extracts were determined by the
Bradford protein assay. Equal amounts of protein were separated on
a 12% SDS-polyacrylamide gel and transferred onto nitrocellulose
membranes. Monoclonal antibodies of procollagen type I, α-SMA
(Abcam) and β-tubulin (Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA), respectively, were used at a dilution of 1:50, 1:400 and
1:150, respectively, followed by incubation with the homologous
secondary antibody labeled with HRP (Santa Cruz Biotechnology,
Inc.). The signals were visualized using an ECL kit.
Statistical analysis
Data are expressed as the means ± SD. The
significance for the difference between the groups was studied
using one-way ANOVA with SPSS 13.0 software. P<0.05 was
considered to indicate statistical significance.
Results
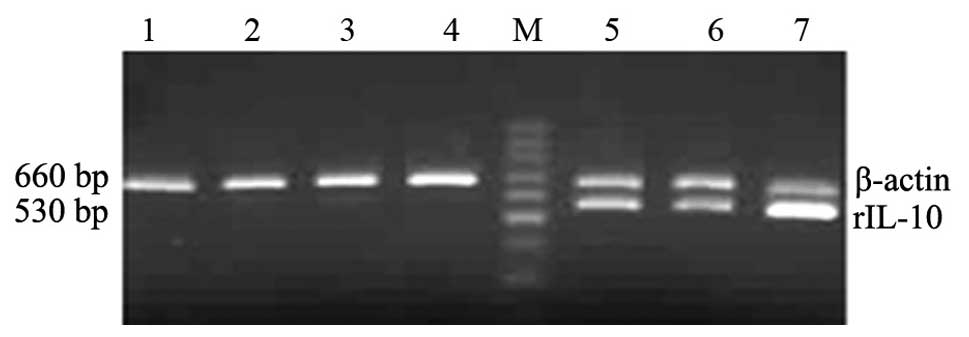
Distribution of rIL-10 mRNA following
rIL-10 gene by HBT
An effective liver-targeted gene delivery system is
crucial for a successful gene therapy in liver disease. Therefore,
in this study, the rIL-10 gene was used as a reporter
gene. RT-PCR was used to detect the expression of rIL-10 and
control β-actin mRNA in liver, spleen, kidney, heart and lung after
PcDNA3-rIL-10 plasmid DNA was transferred by HBT. The results
showed a positive expression of rIL-10 mRNA was observed mainly in
the liver on day 1 after transfection and the expression of rIL-10
mRNA in the liver was sustained for two weeks after single
hydrodynamic injection (Fig.
1).
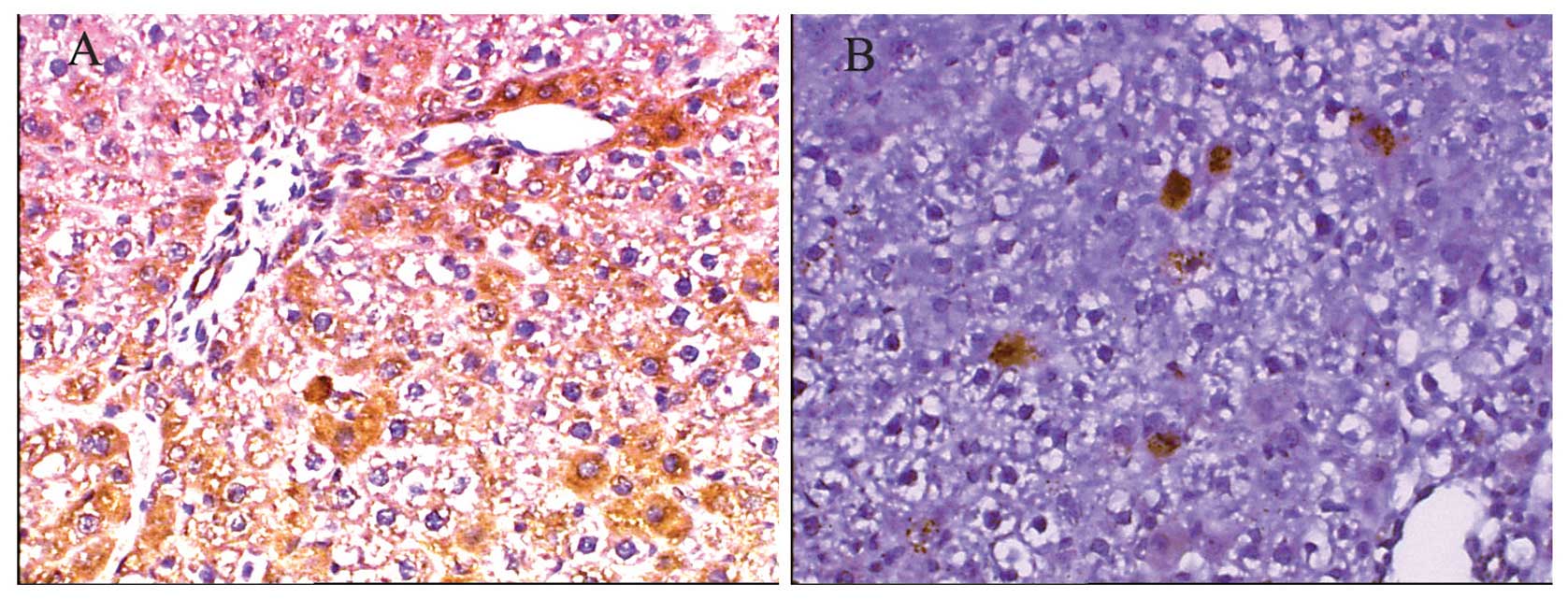
Protein expression of rIL-10 in the liver
after rIL-10 gene by HBT
To confirm rIL-10 expression mainly in the liver
after gene transfer, we detected the protein expression of rIL-10
in the liver, spleen, kidney, heart and lung after
rIL-10 gene via HBT on days 1 and 7 by
immunohistochemical analysis. The results showed that a positive
expression of rIL-10 was detected mainly in hepatocytes, with ~70%
hepatocytes expressing rIL-10 protein on day 1 (Fig. 2A), while only some positive
expression was detected in kidney. However, no positive expression
after rIL-10 gene was transferred for 24 h (data not
shown) was detected in the spleen, heart and lung tissue. The
number of positively stained cells was decreased on day 7 after
rIL-10 gene transfer (Fig. 2B). Results of the
semi-quantitative analysis revealed that the expression of rIL-10
on day 1 was 9-fold higher (9165.98±2031.71) than that on day 7
(1047.94±69.00).
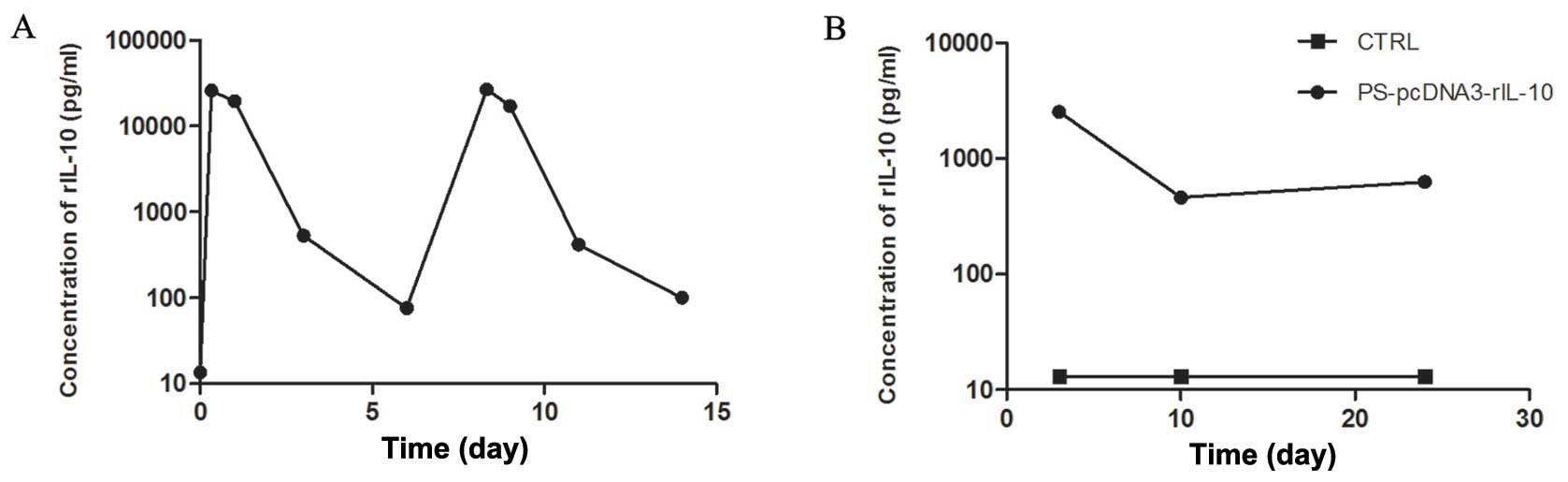
Levels of rIL-10 in the serum after
rIL-10 gene by HBT
Maintaining a stable gene expression in vivo
for the gene therapy of liver fibrosis was crucial. Therefore,
ELISA was assayed to detect the levels of rIL-10 in serum. As shown
in Fig. 3A, the time-response
curve showed levels of rIL-10 in the serum reached a peak level ~8
h after rIL-10 gene transfer and subsequently
decreased. However, the peak level of rIL-10 expression was
regained by repeated injection of rIL-10 plasmid DNA.
To examine the effect of rIL-10 gene
treatment on liver fibrosis induced by PS in rats, we detected the
rIL-10 expression in serum of PS-induced fibrotic rats on day 3
after rIL-10 gene treatment. The results showed that
levels of rIL-10 maintained high levels in the rIL-10
gene-treated rats (Fig. 3B).
Effect of plasmid transfer by HBT on
hepatic function
To determine whether the gene transfer procedure
caused any adverse effects in rats, we detected the serum
concentration of AST and ALT. Rats were assigned to two groups: one
group of rats received an injection of Ringer’s solution containing
pcDNA3 null plasmid, while the other group of rats only received an
injection of Ringer’s solution. The ALT serum of pcDNA3 and
Ringer’s solution rats was significantly increased on day 1 after
the injection, and subsequently returned to normal levels by day 3,
and maintained to day 7. Similar results regarding AST serum levels
were observed in the two groups (Table I).
 | Table IEffect of plasmid transfer by HBT on
hepatic function. |
Table I
Effect of plasmid transfer by HBT on
hepatic function.
| ALT | AST |
|---|
|
|
|
|---|
| Groups (day) | Ringer’s
solution | Plasmid DNA
solution | Ringer’s
solution | Plasmid DNA
solution |
|---|
| −1 | 44.5±8.83 | | 68.33±11.55 | |
| 1 |
202.33±59.33b | 228.5±30.34b |
428.5±121.30b |
451.33±118.19b |
| 3 | 45.17±17.22 | 40.83±11.41 | 77.17±13.21 | 94.00±30.29a |
| 7 | 34.83±11.03 | 38.50±13.52 | 65.00±15.05 | 72.50±21.22 |
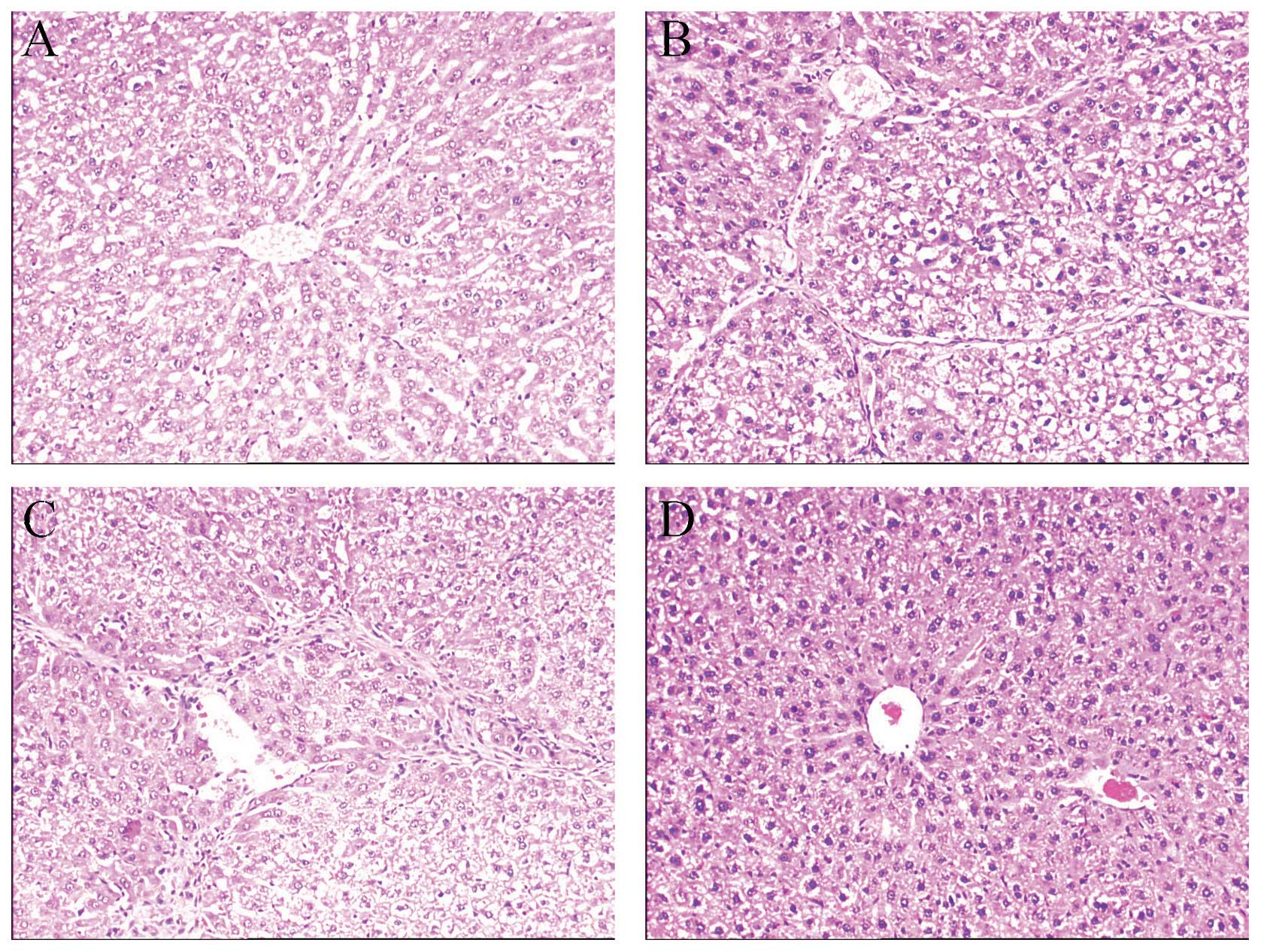
The therapeutic effects of rIL-10 gene
treatment by HBT on liver fibrosis in rats
The results of our experiment showed that high
levels of rIL-10 gene expression in rat hepatocytes
can be achieved by HBT. To confirm the therapeutic effect of liver
targeting rIL-10 expression on liver fibrosis, we performed a
second experiment. The liver fibrosis model was induced by PS, with
rats being treated with rIL-10 gene by HBT from the 4th
week. Liver biopsy is the gold-standard method for detecting
changes in liver fibrosis. Fig. 4
shows representative histological changes in the liver of different
groups. The H&E staining showed that advanced fibrosis was
established after 8-week administration of PS. Rats in group CTRL
(Fig. 4A) showed normal lobular
architecture with central veins and radiating hepatic cords. Rats
in group PS (Fig. 4B) and group
PS-pcDNA3 (Fig. 4C) showed a
slight chronic inflammatory infiltrate in the portal area,
scattered necrotic and regenerative hepatocytes, and marked
increase in ECM content, which resulted in large fibrous septa and
distorted tissue architecture, and formed abnormal hepatic lobules.
Rats in group PS-pcDNA3-rIL-10 (Fig.
4D) showed a marked reduction of inflammation, hepatocyte
damage and deposition of collagen fibers.
To determine the degree of necro-inflammatory liver
injury and fibrosis, histological grading and quantification were
blindly performed by the pathologist. The grade of inflammation and
stage of fibrosis in liver were markedly decreased in group
PS-pcDNA3-rIL-10 compared to groups PS and PS-pcDNA3 (p<0.01).
No significant differences were observed in the grade of
inflammation and stage of fibrosis between groups PS and PS-pcDNA3
(p>0.05; Table II).
 | Table IIThe grading and staging of HAI. |
Table II
The grading and staging of HAI.
| | Grading of
inflammation | Staging of
fibrosis |
|---|
| |
|
|
|---|
| Groups | Nos. | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 |
|---|
| CTRL | 6 | 6 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 |
| PSa,c | 7 | 0 | 2 | 5 | 0 | 0 | 0 | 0 | 0 | 2 | 5 |
| PS + pcDNA3a,c | 6 | 0 | 1 | 5 | 0 | 0 | 0 | 0 | 0 | 2 | 4 |
| PS +
pcDNA3-rIL-10b,d | 7 | 4 | 3 | 0 | 0 | 0 | 0 | 6 | 1 | 0 | 0 |
Effects of rIL-10 gene treatment on the
hepatic function in fibrotic rats
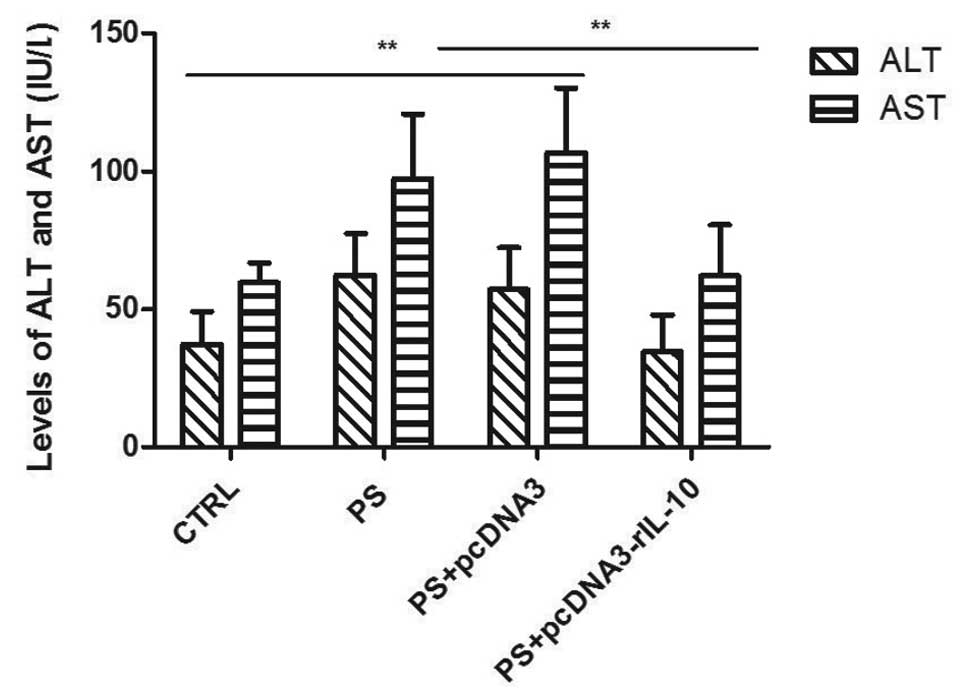
At the end of the experiment, an analysis of ALT and
AST serum was carried out to evaluate the amount of liver injury in
fibrotic rats. The results showed that levels of ALT and AST in
group PS and PS-pcDNA3 were significantly higher than those in
group CTRL (p<0.01). Compared to group PS and PS-pcDNA3, levels
of ALT and AST evaluated in group PS-pcDNA3-rIL-10 were
significantly decreased (p<0.01). No significant differences
were observed in ALT and AST between group CTRL and group
PS-pcDNA3-rIL-10 (p>0.05; Fig.
5).
The effects of rIL-10 gene treatment on
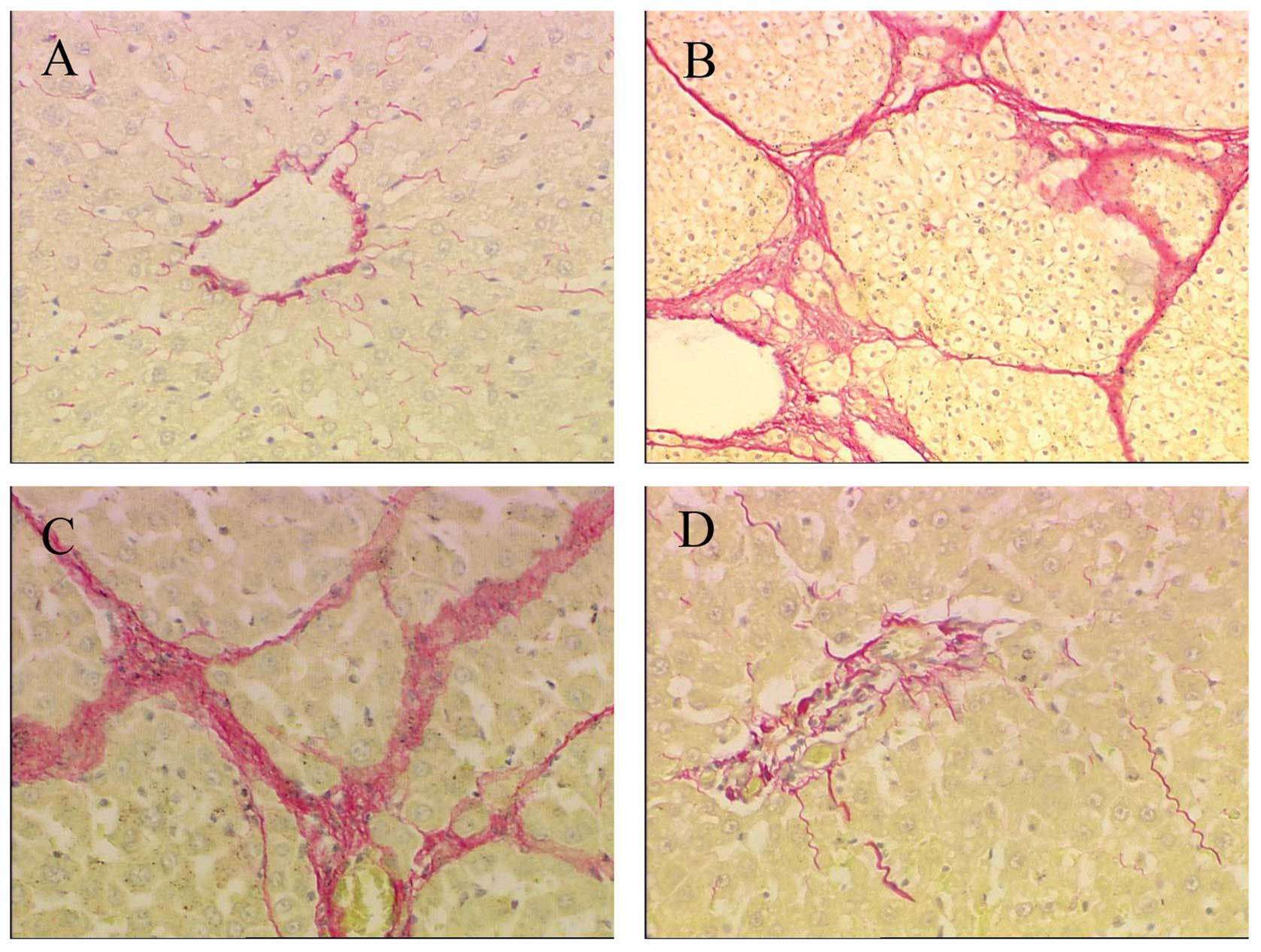
the deposition of collagen in fibrotic rats
One of the key characteristics of liver fibrosis is
the excessive deposition of collagen in the liver, especially
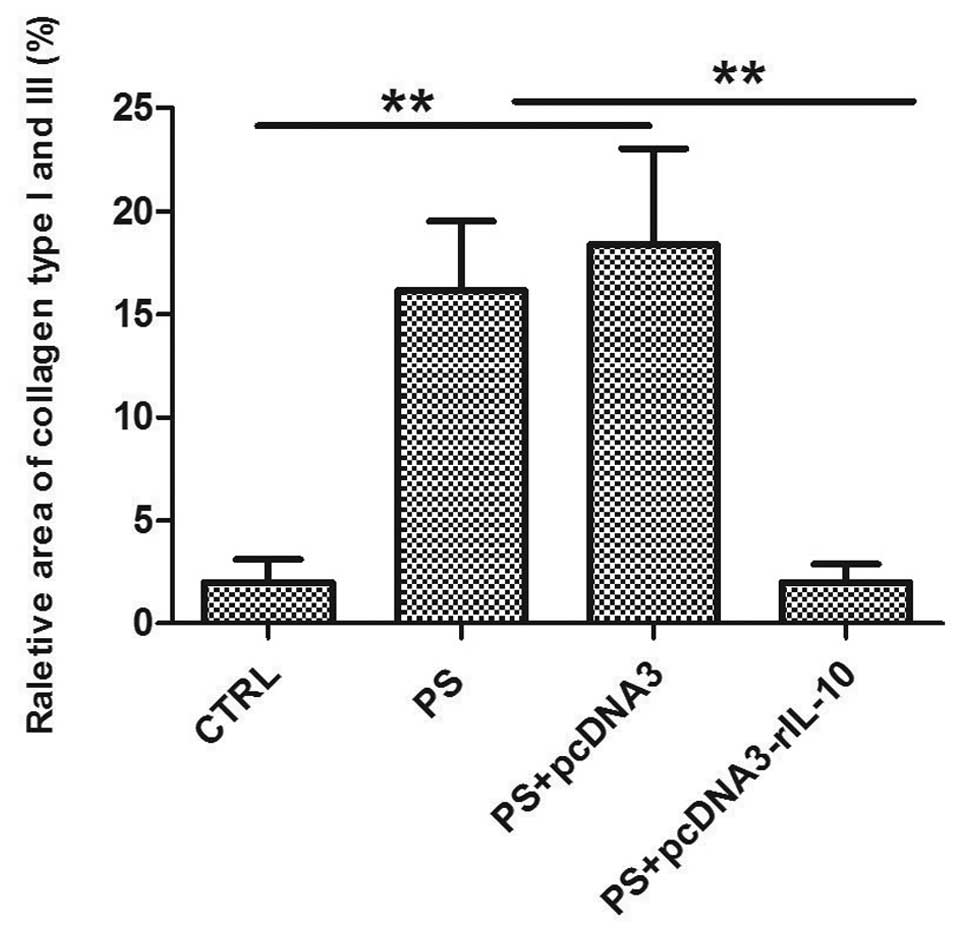
collagen types I and III. Sirius red staining was used to detect
the deposition of collagen. Collagen types I and III were stained
intensely red with the Sirius red staining, while the non-collagen
tissue was stained yellow. PS treatment for 8 weeks induced a
significant deposition of collagen types I and III (Fig. 6B), which developed a severe
fibrosis, compared with the group CTRL (Fig. 6A). Similar results were observed
in the group PS-pcDNA3 (Fig. 6C).
Following IL-10 gene treatment for 4 weeks, the
deposition of collagen types I and III was markedly reduced
(Fig. 6D). Results of the
semi-quantification analysis of the area of collagen types I and
III revealed that the collagen content in group PS increased 9-fold
compared with group CTRL (p<0.01). No significant differences
were detected in the collagen content between group PS and
PS-pcDNA3 (p>0.05), while the areas of collagen were markedly
decreased in the group PS-pcDNA3-rIL-10 (p<0.01; Fig. 7).
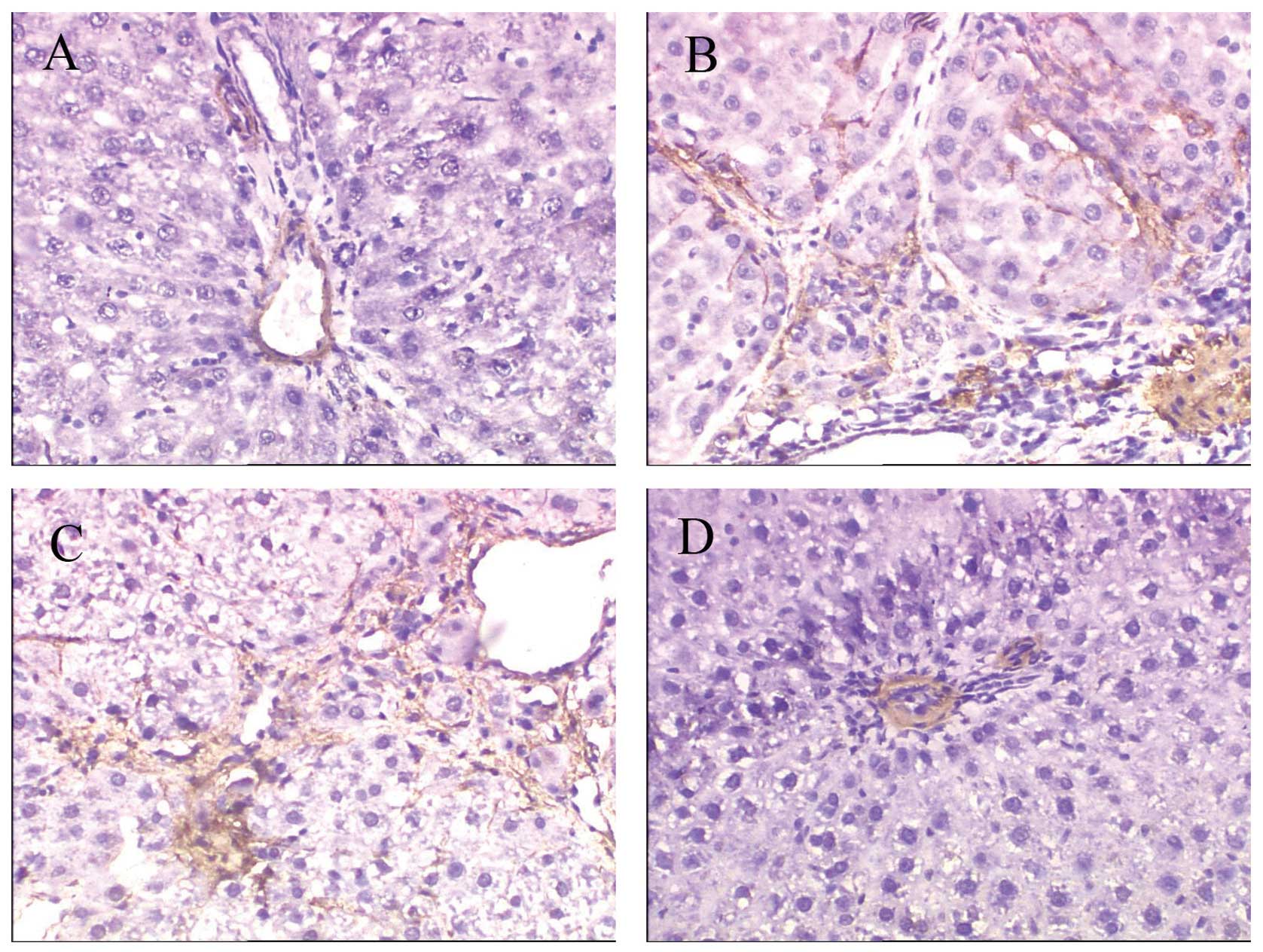
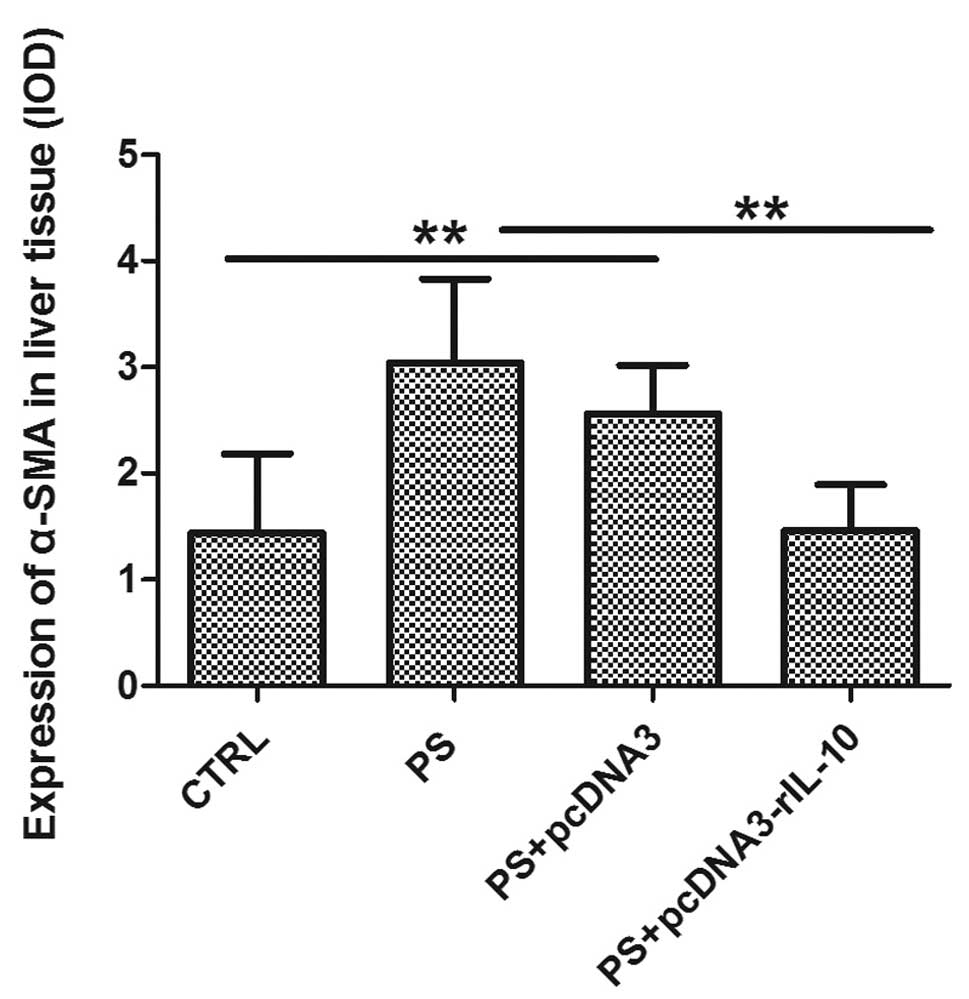
Effects of rIL-10 gene treatment on the
HSC activation in fibrotic rats
The activated HSCs are a rich source of ECM proteins
such as collagen type I and fibronectin (17). To determine the effect of
rIL-10 gene treatment on liver fibrosis in rats by
inhibiting the activation of HSC, we measured the expression of the
HSC activation marker α-SMA by immunohistochemical analysis. The
results revealed that a positive expression of α-SMA was localized
only in vascular smooth muscle cells in CTRL rats (Fig. 8A), while the distribution of
α-SMA-positive cells in group PS was similar to that of deposition
of collagen. α-SMA-positive cells were significantly increased in
the liver of PS and PS-pcDNA3 rats compared with CTRL rats
(Fig. 8B–C), while the
rIL-10 gene treatment reduced the number of
α-SMA-positive cells in PS-induced rats (Fig. 8D). Semi-quantitative analysis
revealed that the expression of α-SMA was markedly higher in groups
PS and PS-pcDNA3 as compared to group CTRL (p<0.01), while the
expression of α-SMA in group PS-pcDNA3-rIL-10 was significantly
reduced compared to group PS (p<0.01; Fig. 9).
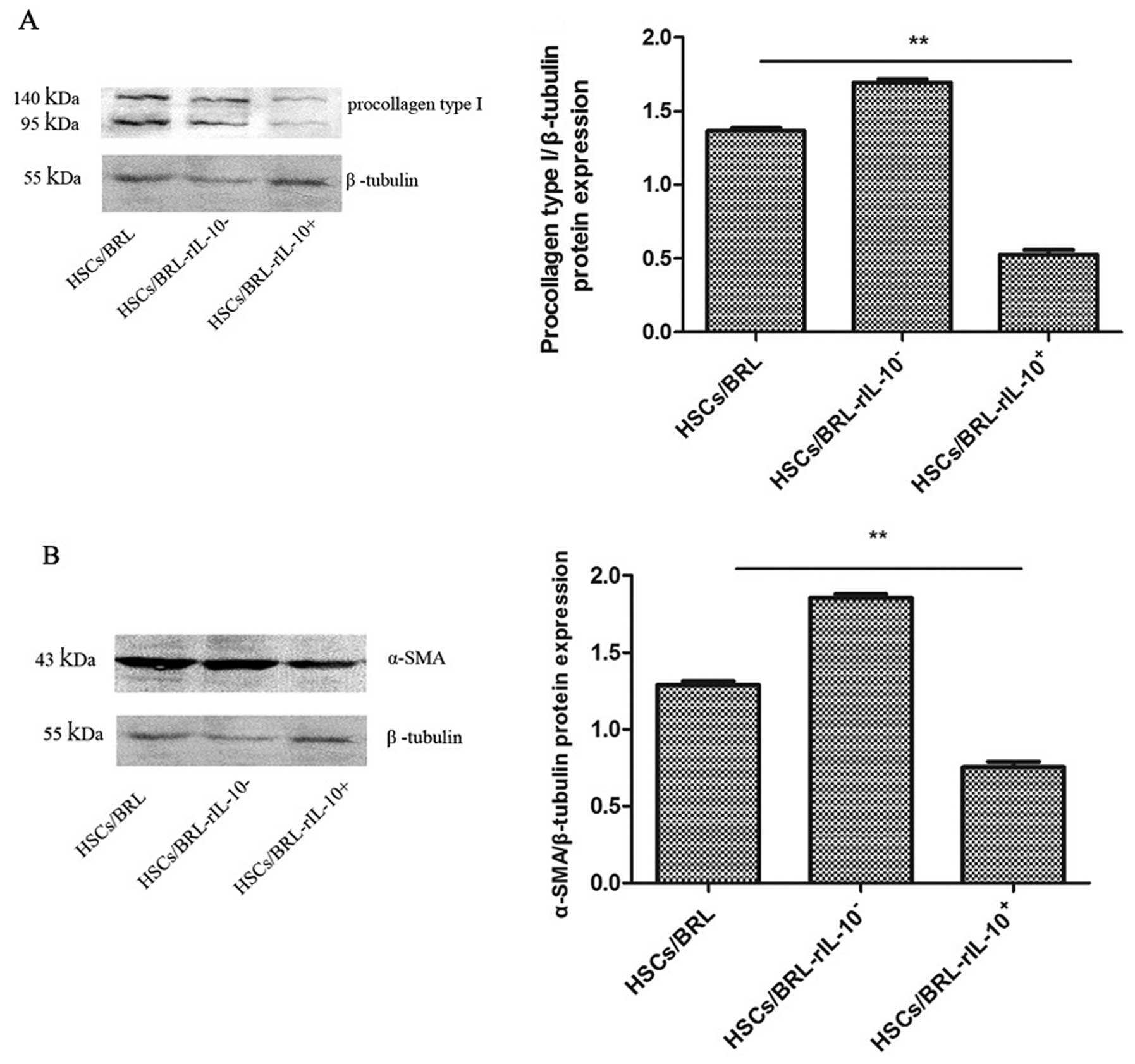
The expression of procollagen type I and
α-SMA in HSCs co-cultured with BRL cells transfected with rIL-10
gene
To confirm in vivo the therapeutic effects of
hepatocyte targeting expression rIL-10 on liver fibrosis, in
vitro we examined the expression of procollagen type I and
α-SMA in HSCs co-cultured with BRL cells transfected with rIL-10
expression plasmid pcDNA3-rIL-10, null plasmid pcDNA3 or saline.
Results of the western blot analysis revealed that the expression
of procollagen type I was significantly decreased in group
HSCs/BRL-rIL-10+ compared with the control group
HSCs/BRL-rIL-10− and HSCs/BRL (p<0.01; Fig. 10A). No significant differences
were detected in the levels of procollagen type I between groups
HSCs/BRL-rIL-10− and HSCs/BRL (p>0.05). The
expression of α-SMA in HSCs was detected in the three groups by
western blotting, while the expression of α-SMA in group
HSCs/BRL-rIL-10+ was significantly decreased compared to
the control group HSCs/BRL-rIL-10− and HSCs/BRL
(p<0.01; Fig. 10B).
Discussion
The present study has demonstrated that
rIL-10 gene transfer by HBT attenuates PS-induced
liver fibrosis in rats, while the mechanism was associated with the
hepatocytes targeting the expression of cytokine rIL-10 via
paracrine action, thereby inhibiting the activation of HSCs and
promoting degeneration of collagen type I .
IL-10 was initially discovered in 1989, as a
cytokine synthesis inhibitory factor for T lymphocytes (18). IL-10 is a pleiotropic cytokine,
and one of the most important properties of IL-10 is its
anti-inflammatory inhibitory action which restrains the immune
response under various stimuli (8). Results of previous studies have
shown that IL-10 exerts antifibrotic effects on fibrosis in rats
(19,20). The study by Wang et al has
shown that IL-10 mRNA and protein levels were increased in early
liver fibrosis and disappeared in advanced liver fibrosis (6). Previously, we showed that exogenous
cytokine IL-10 suppresses liver fibrosis progression induced by
CCL4 in rats (9). However,
recombinant cytokine IL-10 has short half-lives, necessitating
frequent administration (21). To
overcome this problem, a gene therapy system providing a continuous
delivery may be more effective than an intermittent one. The most
important factor for successful gene therapy of liver disease is to
express the relevant gene effectively in the living organisms.
Among the various gene delivery systems, the virus vector is
laborious and expensive and may induce an immune response and lead
to side effects that restrict repeated administration thereof
(22). However, gene delivery
based on plasmid DNA seems to be the simplest and safest strategy.
It has been reported that high levels of foreign gene expression in
murine hepatocytes can be achieved by the rapid injection of a
large volume of naked DNA solution into the tail vein (22). This is known as ‘HBT’ (15), however, whether this gene delivery
system is suitable for rats remains to be determined. Results of a
previous study have confirmed that death of rats was markedly
increased when the volume of Ringer’s solution reached 100
ml.kg−1 (data not shown). Thus, in this study, a volume
of 80 ml.kg−1 of Ringer’s solution containing rIL-10
expression plasmid (pcDNA3-rIL-10) was rapidly injected into rat by
HBT. The results showed that the liver is the major organ and
hepatocytes are the major cells involved in rIL-10
gene expression, while rIL-10 serum levels markedly increased
following the transfer for 8 h. High levels of rIL-10 were
sustained for at least 1 week in the serum and hepatocyte, while
the rIL-10 mRNA expression in liver was sustained for 2 weeks
following a single injection, which is in agreement with a previous
study (23). AST and ALT serum is
excreted from liver tissue into the circulation in proportion to
the degree of hepatocyte damage, and the levels are thought to be
one of the most sensitive markers of liver injury and disease
progression (24). Therefore, we
detected the serum concentration of AST and ALT to assess the
security of gene transfer by HBT. The data showed that AST and ALT
levels retained normal level after injection for 3 days. These
results demonstrate that rIL-10 transfer into the liver using the
HBT system maintained a sustained high-level expression of rIL-10
in rats. Thus this technique has the potential to be applicable to
the treatment of liver disease, especially liver fibrosis.
Most hepatic fibrosis models are established by
inducing so-called post-necrotic hepatic fibrosis. However, the
PS-induced hepatic fibrosis model is characterized by minor
hepatocyte damage but intense immune response. Given the chronic
administration of the heterogeneous serum (25), the mechanisms of fibrogenesis are
similar to those of hepatic disease in human (26), especially viral hepatitis.
Therefore we used PS-induced liver fibrosis to mimic immune
pathogenesis in human. The fibrotic rats received the rIL-10
gene treatment by HBT starting from the 4th week. Liver biopsy is
the gold-standard method for detecting changes in liver fibrosis
(27). H&E staining results
showed that PS-induced fibrotic rats exhibited a marked increase in
ECM content, which resulted in large fibrous septa and distorted
tissue architecture, and formed abnormal hepatic lobules, although
hepatocyte damage was slight, as observed in findings of a recent
study (28). Hepatocyte necrosis,
deposition of collagen, the score of fibrosis and AST and ALT serum
levels were markedly reduced in the rIL-10 gene
treatment for 4 weeks. The results suggest that liver targeting
expression of rIL-10 gene showed significant therapeutic
effects for PS-induced liver fibrosis in rats.
During fibrotic progression, inflammation and liver
injury trigger complex cell events that result in collagen
deposition and the disruption of normal liver architecture.
Activated HSCs are considered the most important cell type for the
production of collagens (29).
The PS-induced rat liver fibrosis is also caused by activated HSCs
(27). During activation, HSCs
transit into myofibro-blast-like cells that express α-SMA and these
activated HSCs excrete ECM proteins, especially collagen types I
and III, in hepatic fibrosis (30). α-SMA and collagen type I are
useful biomarkers for the assessment of the therapeutic efficacy of
the rIL-10 gene treatment. The present results show
that rIL-10 gene treatment by HBT potently inhibited
the α-SMA expression and significantly decreased the deposition of
collagen types I and III in fibrotic rats liver. The present study
confirmed that hepatocytes are the main cell involved in rIL-10
expression after gene transfer by HBT. We suggest that the
therapeutic effects of rIL-10 gene treatment by HBT
are possible through rIL-10 paracrine hepatocytes to affect the
activation of HSCs and the deposition of ECM. To confirm that the
rIL-10 gene in hepatocyte targeting expression
inhibits the activation of HSCs and reduces the deposition of ECM,
in vitro HSCs co-cultured with BRL cells were used to
transfect the rIL-10 gene or control null plasmid or
saline. The results showed that the expression of procollagen type
I and α-SMA in HSCs co-cultured with BRL-transfected
rIL-10 gene was significantly lower than the
expression of the saline and null plasmid control groups. These
data in vitro suggest that rIL-10 gene in
hepatocyte targeting expression can inhibit the activation of HSCs
and promote the degeneration of collagen and confirm that
hepatocyte targeting gene delivery may be an ideal technique for
the IL-10 gene therapy of liver fibrosis.
In conclusion, the study in vivo and in
vitro suggests that HBT of the rIL-10 gene
ameliorates the degree of PS-induced liver fibrosis in rats, while
its mechanisms may involve inhibition of the activation of HSCs and
promotion of the degeneration of collagen.
Acknowledgements
We thank Xiao-Ling Zhou for providing technical
support and Xiu-Fang Lin and Huan-Xin Yang for providing technical
support in hepatic histology examination. This research was
supported by grants from Fujian Province Nature Scientific
Foundation of China (no. 2010J05062), the Young Scientific Research
Fund of Department of Public Health of Fujian Province, China
(2010-1-10) and the key clinical specialty discipline construction
program of Fujian, P.R.C.
References
|
1
|
Luo YJ, Yu JP, Shi ZH and Wang L: Ginkgo
biloba extract reverses CCl4-induced liver fibrosis in rats. World
J Gastroenterol. 10:1037–1042. 2004.PubMed/NCBI
|
|
2
|
Reeves HL and Friedman SL: Activation of
hepatic stellate cells - a key issue in liver fibrosis. Front
Biosci. 7:808–826. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schuppan D and Pinzani M: Anti-fibrotic
therapy: lost in translation? J Hepatol. 56(Suppl 1): S66–S74.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Friedman SL: Molecular regulation of
hepatic fibrosis, an integrated cellular response to tissue injury.
J Biol Chem. 275:2247–2250. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hung KS, Lee TH, Chou WY, et al:
Interleukin-10 gene therapy reverses thioacetamide-induced liver
fibrosis in mice. Biochem Biophys Res Commun. 336:324–331. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang SC, Ohata M, Schrum L, Rippe RA and
Tsukamoto H: Expression of interleukin-10 by in vitro and in vivo
activated hepatic stellate cells. J Biol Chem. 273:302–308. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nelson DR, Lauwers GY, Lau JY and Davis
GL: Interleukin 10 treatment reduces fibrosis in patients with
chronic hepatitis C: a pilot trial of interferon nonresponders.
Gastroenterology. 118:655–660. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Louis H, Le Moine O, Goldman M and Deviere
J: Modulation of liver injury by interleukin-10. Acta Gastroenterol
Belg. 66:7–14. 2003.PubMed/NCBI
|
|
9
|
Huang YH, Shi MN, Zheng WD, Zhang LJ, Chen
ZX and Wang XZ: Therapeutic effect of interleukin-10 on
CCl4-induced hepatic fibrosis in rats. World J Gastroenterol.
12:1386–1391. 2006.PubMed/NCBI
|
|
10
|
Zhang LJ, Zheng WD, Chen YX, et al:
Antifibrotic effects of interleukin-10 on experimental hepatic
fibrosis. Hepatogastroenterology. 54:2092–2098. 2007.PubMed/NCBI
|
|
11
|
Wang XZ, Zhang SJ, Chen YX, Chen ZX, Huang
YH and Zhang LJ: Effects of platelet-derived growth factor and
interleukin-10 on Fas/Fas-ligand and Bcl-2/Bax mRNA expression in
rat hepatic stellate cells in vitro. World J Gastroenterol.
10:2706–2710. 2004.PubMed/NCBI
|
|
12
|
Shetty K, Wu GY and Wu CH: Gene therapy of
hepatic diseases: prospects for the new millennium. Gut.
46:136–139. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu F, Song Y and Liu D:
Hydrodynamics-based transfection in animals by systemic
administration of plasmid DNA. Gene Ther. 6:1258–1266. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang GF, Budker V and Wolff JA: High
levels of foreign gene expression in hepatocytes after tail vain
injection of naked DNA. Hum Gene Ther. 1735–1737. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sebestyen MG, Budker VG, Budker T, et al:
Mechanism of plasmid delivery by hydrodynamic tail vein injection.
I. Hepatocyte uptake of various molecules. J Gene Med. 8:852–873.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Budker VG, Subbotin VM, Budker T,
Sebestyen MG, Zhang G and Wolff JA: Mechanism of plasmid delivery
by hydrodynamic tail vein injection. II. Morphological studies. J
Gene Med. 8:874–888. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim BH, Yoon JH, Yang JI, et al:
Guggulsterone attenuates activation and survival of hepatic
stellate cell by inhibiting NF-kappaB activation and inducing
apoptosis. J Gastroenterol Hepatol. 28:1859–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fiorentino DF, Bond MW and Mosmann TR: Two
types of mouse T helper cell. IV. Th2 clones secrete a factor that
inhibits cytokine production by Th1 clones. J Exp Med.
170:2081–2095. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chou WY, Lu CN, Lee TH, et al:
Electroporative interleukin-10 gene transfer ameliorates carbon
tetrachloride-induced murine liver fibrosis by MMP and TIMP
modulation. Acta Pharmacol Sin. 27:469–476. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakagome K, Dohi M, Okunishi K, Tanaka R,
Miyazaki J and Yamamoto K: In vivo IL-10 gene delivery attenuates
bleomycin induced pulmonary fibrosis by inhibiting the production
and activation of TGF-beta in the lung. Thorax. 61:886–894. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Asadullah K, Sterry W and Volk HD:
Interleukin-10 therapy--review of a new approach. Pharmacol Rev.
55:241–269. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang J, Yamato E and Miyazaki J:
Intravenous delivery of naked plasmid DNA for in vivo cytokine
expression. Biochem Biophys Res Commun. 289:1088–1092. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sung CM, Yeh CT, Shiau SS, Liang CK and
Chang ML: Hydrodynamics-based transfection of the combination of
betacellulin and neurogenic differentiation 1 DNA ameliorates
hyperglycemia in mice with streptozotocin-induced diabetes.
Diabetes Technol Ther. 13:519–525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu T, Wang X, Karsdal MA, Leeming DJ and
Genovese F: Molecular serum markers of liver fibrosis. Biomark
Insights. 105–117. 2012.
|
|
25
|
Osuna-Martinez U, Reyes-Esparza JA,
Petricevich VL, Hernandez-Pando R and Rodriguez-Fragoso L:
Protective effect of thymic humoral factor on porcine serum-induced
hepatic fibrosis and liver damage in Wistar rats. Ann Hepatol.
10:540–551. 2011.PubMed/NCBI
|
|
26
|
Baba Y, Saeki K, Onodera T and Doi K:
Serological and immunohistochemical studies on
porcine-serum-induced hepatic fibrosis in rats. Exp Mol Pathol.
79:229–235. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Y, Peng J and Hou J: Non-invasive
assessment of liver fibrosis in patients with chronic hepatitis B.
Hepatol Int. 356–368. 2013. View Article : Google Scholar
|
|
28
|
Ping J, Gao AM, Xu D, Li RW and Wang H:
Therapeutic effect of indole-3-carbinol on pig serum-induced
hepatic fibrosis in rats. Yao Xue Xue Bao. 46:915–921. 2011.(In
Chinese).
|
|
29
|
Kong X, Horiguchi N, Mori M and Gao B:
Cytokines and STATs in liver fibrosis. Front Physiol. 3:1–7. 2012.
View Article : Google Scholar
|
|
30
|
Friedman SL: Hepatic stellate cells:
protean, multifunctional, and enigmatic cells of the liver. Physiol
Rev. 88:125–172. 2008. View Article : Google Scholar : PubMed/NCBI
|