Introduction
Thymosin β4 (Tβ4), an
oligopeptide consisting of 43 amino acids, is known to sequester
G-actin monomers. Upregulation of Tβ4 by cDNA-mediated
transfection was found to cause actin depolymerization in
fibroblast cells (1), which
demonstrated that the modulation of Tβ4 affected the
polymerization state of actin cytoskeleton, as well as other cell
processes associated with the organization of actin cytoskeleton,
such as cell migration. Ito et al (2) showed that overexpression of
Tβ4 enhanced the formation of actin-based pseudopodia
and cell motility in prostate cancer cells, providing direct
evidence that Tβ4 plays a role in cell migration. Colon
cancer cells overexpressing Tβ4 also exhibited enhanced
cell migration, due in part to the activation of the Rac1 signaling
pathway (3). In addition,
extracellular, administered Tβ4 promoted the cell
migration of various types of cells including cardiomyocytes, human
umbilical vein endothelial cells and conjunctival epithelial cells
(4–6). The mechanism by which exogenous
Tβ4 influences cell migration remains to be elucidated.
However, results of a recent study showed that Tβ4 was
rapidly internalized by cells, suggesting the involvement of an
intracellular receptor in the effects of the peptide (7). Besides the effect on cell migration,
exogenous Tβ4 was found to have multifunctional
activities such as angiogenesis, anti-apoptosis, anti-oxidative
stress and anti-inflammation (8–11),
which emphasize its therapeutic potential in the repair of damaged
tissues or wound healing.
Tβ4 is distributed ubiquitously in most
tissues and cells, and is also known to concentrate highly at blood
platelets (12). These findings
suggest that endogenous Tβ4 likely promotes the healing
of damaged tissues. Exogenous Tβ4 was also reported to
accelerate the tissue repair of damaged cardiac, corneal and dermal
tissues (4,6,13),
which demonstrates potential for clinical applications in wound
healing.
Generally, wound healing in the oral cavity is known
to occur more quickly and scar less than dermal tissue, which may
be due to the elements in saliva and unique phenotype of oral
fibroblasts (14–16). Despite the relatively rapid wound
healing, however, tissues damaged during periodontal and implant
surgery are continuously challenged by bacterial infection in the
oral cavity, necessitating meticulous maintenance of oral hygiene
and additional plaque control. Prevention of bacterial
contamination is even more important in the case of gingival graft
surgery because a significant amount of tissue is lost at a palatal
donor site. Autogenous gingival grafts are often accompanied by
discomfort, pain and retarded tissue repair depending on a
patient’s condition. To avoid these post-operative problems,
topical application of an antimicrobial treatment is recommended
(17). Furthermore, several
dressing materials, which are supposed to aid in tissue repair, are
commonly applied to palatal wounds during the healing process
(18,19). Accelerated regeneration of palatal
mucosa was reported to occur following treatment with a basic
fibroblast growth factor impregnated in collagen-gelatin scaffold
(20). The aforementioned studies
showed that dressing materials or chemicals employed for the
treatment of dermal wound healing can also be effective for the
regeneration of oral mucosa. Therefore, Tβ4, which is
known to enhance the regeneration of different types of tissue, is
also expected to accelerate mucosal wound healing. In a previous
study, Tβ4 was documented to be a natural component of
saliva. The concentrations in human saliva ranged from 0.2 to 3.6
μg/ml, varying with age and state of disease (21). The Tβ4 levels in
gingival crevicular fluid from patients with periodontal disease
were higher than those from healthy patients in the control group
(22). Considering the various
functions involved in wound healing, Tβ4 in saliva or
gingival crevicular fluid is thought to promote the repair of
damaged oral tissues. The aim of this study was to evaluate the
effect of Tβ4 on palatal wound closure in a rat model.
As Tβ4 already existed in saliva, we applied relatively
high concentrations of Tβ4 with carboxymethyl cellulose
(CMC) ointment. We also evaluated the effects of Tβ4 on
the growth, adhesion and migration of rat palatal (RP) cells.
Furthermore, the mRNA and protein expression of matrix
metalloproteinase 2 (MMP2) and vascular endothelial growth factor
(VEGF) were analyzed in Tβ4-treated RP cells.
Materials and methods
Chemical reagents and cell culture
Cell culture medium and reagents were purchased from
Gibco-BRL (Grand Island, NY, USA). Other experimental reagents were
obtained from Sigma-Aldrich Co. (St. Louis, MO, USA), unless
otherwise specified.
RP cells were obtained from the palatal tissues of
5-week-old male Sprague-Dawley (SD) rats. Isolated palatal tissues
were washed with phosphate-buffered saline (PBS), minced into
sections, and cultured in Dulbecco’s modified Eagle’s medium (DMEM)
containing 10% fetal bovine serum (FBS) and antibiotic solution
(100 U/ml of penicillin-G and 100 μg/ml of streptomycin) at 37°C in
a 5% CO2 humidified incubator. After 20 days of culture
with medium changes every 3 days, RP cells were collected and
subcultured under the same conditions. Passages 5–8 were used for
the present study.
Cell adhesion and proliferation
assay
Adhesion and proliferation of RP cells on the
polystyrene surface of culture plates were observed in the presence
of Tβ4. For the adhesion assay, 1×104 palatal
cells were incubated in the wells of a 96-well plate with
Tβ4 at various concentrations of 1–1,000 ng/ml for 4 h.
The wells were gently washed three times with PBS, and the number
of attached cells was quantified using
2-(2-methoxy-4-nitropenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium
(WST-8; Dojindo Laboratories, Kumamoto, Japan). The cells were
incubated in 100 μl of WST-8 solution for 1 h at 37°C in a
humidified atmosphere (5% CO2/95% air). The absorbance
was measured at a wavelength of 450 nm using a plate reader
(Sunrise; Tecan Austria GmbH, Salzburg, Austria). To observe
proliferation, palatal cells were treated with Tβ4 for
24 h, and the number of cells was measured by using WST-8.
In vitro wound closure assay
For the in vitro wound closure assay, a
culture insert (ibidi GmbH, Martinsried, Germany) was employed to
create a wound in cell culture. The culture insert was placed on a
culture dish, and 70 μl of RP cell suspension (5×105
cells/ml) was added into the two wells of the insert. The RP cells
were incubated at 37°C for 18 h, and were serum-starved for 24 h.
Following serum starvation, the culture insert was carefully
removed, and the cells were exposed to Tβ4 at various
concentrations of 0–1,000 ng/ml. The wound closure was observed and
recorded at intervals under a phase contrast microscope (Olympus,
Tokyo, Japan). This experiment was replicated three times.
mRNA expression analysis of MMP2 and
VEGF
To investigate the effect of Tβ4 on the
mRNA expression of genes related to cell migration and
angiogenesis, mRNA expression of MMP2 and VEGF was analyzed by
quantitative polymerase chain reaction (RT-qPCR) assay. Following
serum starvation for 24 h, the palatal cells were treated with
Tβ4 for 6 and 24 h and the total RNA was isolated using
RNA extraction reagent (WelPrep Total RNA isolation reagent;
Welgene Inc., Daegu, Korea). From the total RNA, cDNA was prepared
using a cDNA synthesis kit (Power cDNA Synthesis kit; Intron
Biotechnology, Seongnam, Korea) and RT-qPCR was performed in an ABI
PRISM 7500 Sequence Detection System Thermal Cycler (Applied
Biosystems, Foster City, CA, USA) with 20 μl reaction volumes
containing 10 μl SYBR Premix Ex Taq (Takara Bio, Otsu, Japan), 0.4
μl ROX reference dye II (Takara Bio), cDNA, and primers. The
primers for gene amplification were: VEGF, sense:
5′-GAGTATATCTTCAAGCCGTCCTGT-3′ and antisense:
5′-ATCTGCATAGTGACGTTGCTCTC-3′; MMP2, sense:
5′-CAGGGAATGAGTACTGGGTCTATT-3′ and antisense:
5′-ACTCCAGTTAAAGGCAGCATCTAC-3′; GAPDH, sense:
5′-TGTGTCCGTCGTGGATCTGA-3′ and antisense:
5′-CCTGCTTCACCACCTTCTTGAT-3′. The PCR conditions were 95°C for 30
sec, followed by 40 cycles of denaturation at 95°C for 5 sec and
annealing at 60°C (34 sec) for MMP2 and 63°C (34 sec) for VEGF. The
reactions were run in triplicate. Gene expression was evaluated
based on the threshold cycle (Ct value) and normalized to the
amount of GAPDH transcript.
Western blot analysis
Western blot analysis was performed to examine the
protein expression of MMP2 and VEGF in Tβ4-treated
palatal cells. After treatment with Tβ4 for 6 h, the
cells were centrifuged and re-suspended in an extraction buffer
containing 50 mM Tris base-HCl (pH 8.0), 150 mM NaCl, 0.5% Triton
X-100, and 1 tablet of protease inhibitor cocktail (1 tablet/10 ml;
Roche Applied Science, Mannheim, Germany) for 45 min on ice.
Extracts containing equal amounts of protein were run on 10% sodium
dodecyl sulfate polyacrylamide gels and transferred to
polyvinylidene difluoride membranes. The blots were incubated with
rabbit polyclonal antibodies against VEGF, MMP2 or GAPDH in PBST
(PBS containing 0.1% Tween-20) for 1.5 h, washed three times with
PBST, and then probed with goat anti-rabbit secondary antibodies
conjugated to horseradish peroxidase. The antibodies were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The
blots were developed using a chemiluminescence kit (West-Zol plus
western blot analysis detection system; Intron Biotechnology).
Chemiluminescence was detected using the LAS 1000 Plus Luminescent
Image Analyzer (Fuji Photo Film Co., Ltd., Tokyo, Japan).
RP wound healing assay
The effect of Tβ4 on the wound healing of
palatal tissue was investigated in a RP wound model.
Thirteen-week-old SD male rats, weighing 300–350 g, were used in
this study. Animal experiments were performed under the control of
the animal welfare committee of Seoul National University
Institutional Animal Care and Use Committee. Under general
anesthesia, punch wounds were made on a central area of hard palate
with a disposable 3-mm diameter biopsy punch (Kai Industries Co.,
Ltd., Gifu, Japan), exposing a circular area of bare bone. The
wound area was covered with 30% CMC ointment containing 0 or 1
mg/ml Tβ4. Six rats were used for each group. After the
surgery, the animals were fed a standard diet of pellets and water
with enrofloxacin. The agents were re-applied on day 2 and 4 to
reduce stress by anesthesia, and the rats were sacrificed on day 7.
The maxillae were separated, and wound area was observed by
stereoscopic microscope (Nikon, Tokyo, Japan) and by histological
analysis. The wound areas in the microscopic images were calculated
using CellSense Dimension 1.6 software (Olympus).
The palatal specimens were fixed in 10% formalin for
at least 24 h, decalcified in Calci-Clear Rapid solution (National
Diagnostics, Atlanta, GA, USA) for 35 h, and processed for
histological analysis. Serial sections, 5 μm apart, were cut across
the wound, perpendicular to the palatal midline at the widest
diameter of the wound, and stained with hematoxylin and eosin
(H&E). The sections were examined under a light microscope
(Olympus), and the distance of wound margins in each section was
measured with a calibrated ocular micrometer (Olympus).
Statistical analysis
Each experiment was performed in triplicate unless
otherwise specified. Data were presented as the mean ± SD.
Statistical analyses were performed by the Student’s t-test.
P<0.05 was considered statistically significant.
Results
Effects of Tβ4 on the
adhesion, proliferation and migration of palatal cells
To investigate the effects of Tβ4 on the
adhesion and proliferation of palatal cells, the cells were
incubated for 4 and 24 h in the presence of Tβ4. At
concentrations of 1–1,000 ng/ml, Tβ4 did not exert any
significant effects on the adhesion and proliferation of palatal
cells (data not shown), whereas cell migration was affected
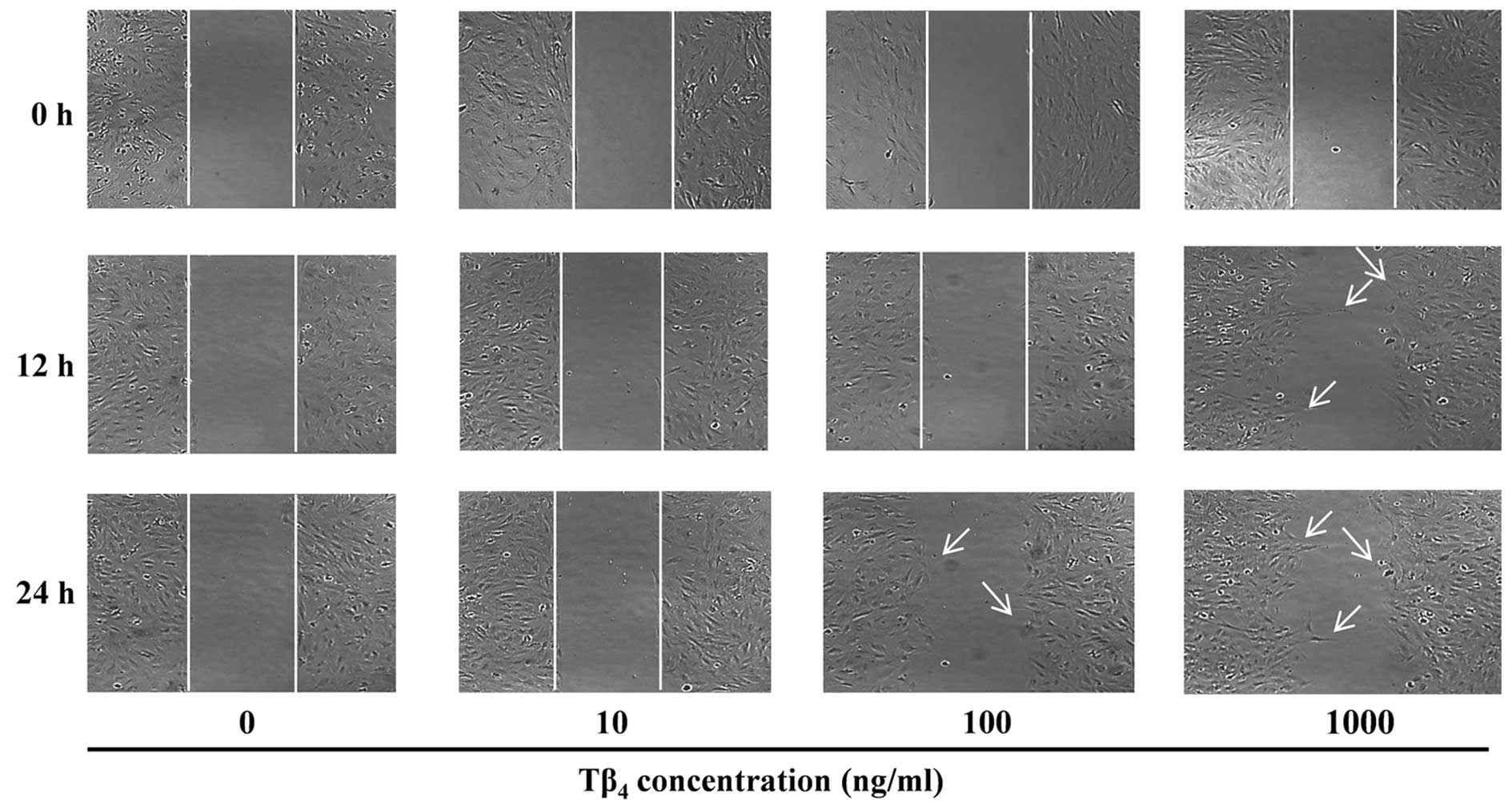
(Fig. 1). Untreated control cells
did not exhibit any movement during 24 h, possibly due to the
serum-starved test conditions. However, cell movement was observed
when Tβ4 was present at concentrations >100 ng/ml.
The migration effect of Tβ4 was more rapid at the higher
concentrations and cell motility was observed at 12 h when
incubated with 1,000 ng/ml Tβ4.
Effects of Tβ4 on mRNA
expression of MMP2 and VEGF genes
The mRNA expression of MMP2 and VEGF was quantified
to investigate the effects of Tβ4 on cell migration and
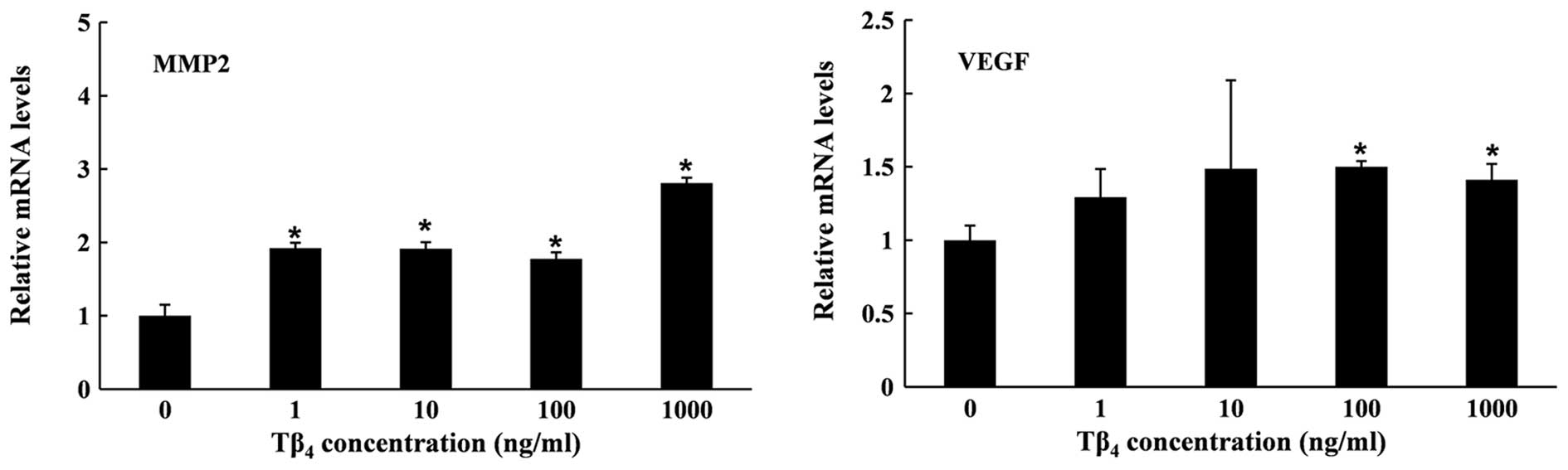
angiogenesis at molecular levels. As shown in Fig. 2, Tβ4 enhanced the gene
expression of MMP2 and VEGF after 6 h. The expression of MMP2 in
the Tβ4-treated group was significantly higher than that
in the untreated cells. Although the expression did not increase in
a dose-dependent manner, the highest concentration of
Tβ4 (1,000 ng/ml) led to the strongest induction of
MMP2. Tβ4 also enhanced mRNA expression of the VEGF gene
in RP cells, inducing an ~1.4-fold increase at 1,000 ng/ml at 6 h.
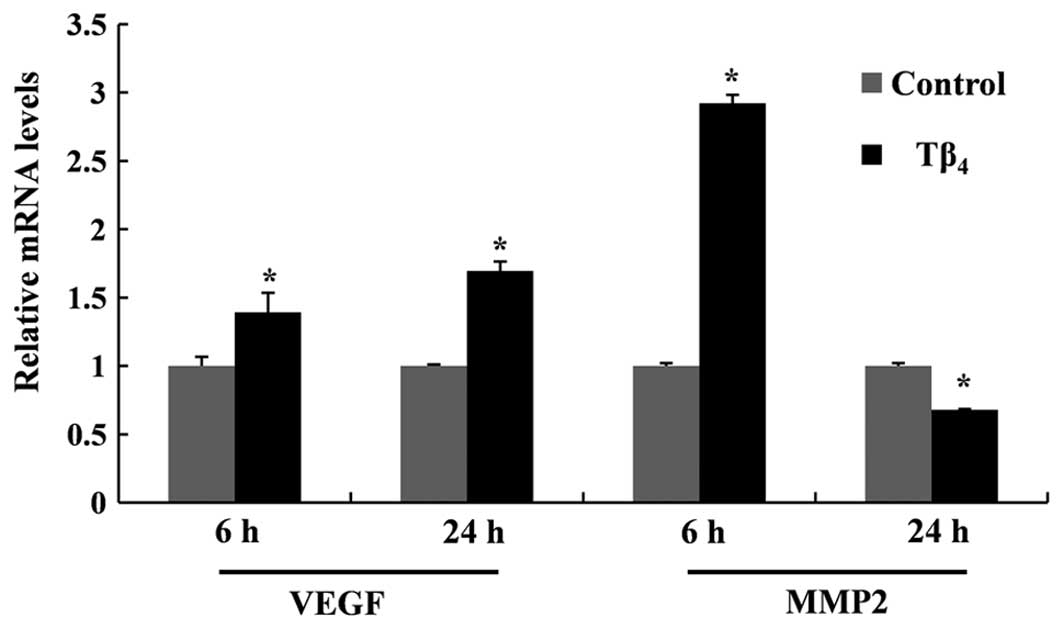
A further increase of VEGF mRNA was obtained when exposed for 24 h.
However, the expression level of the MMP2 gene was not maintained
over the 24-h period, with expression levels decreasing below those
of the untreated control (Fig.
3).
Effect of Tβ4 on VEGF and MMP2
protein level
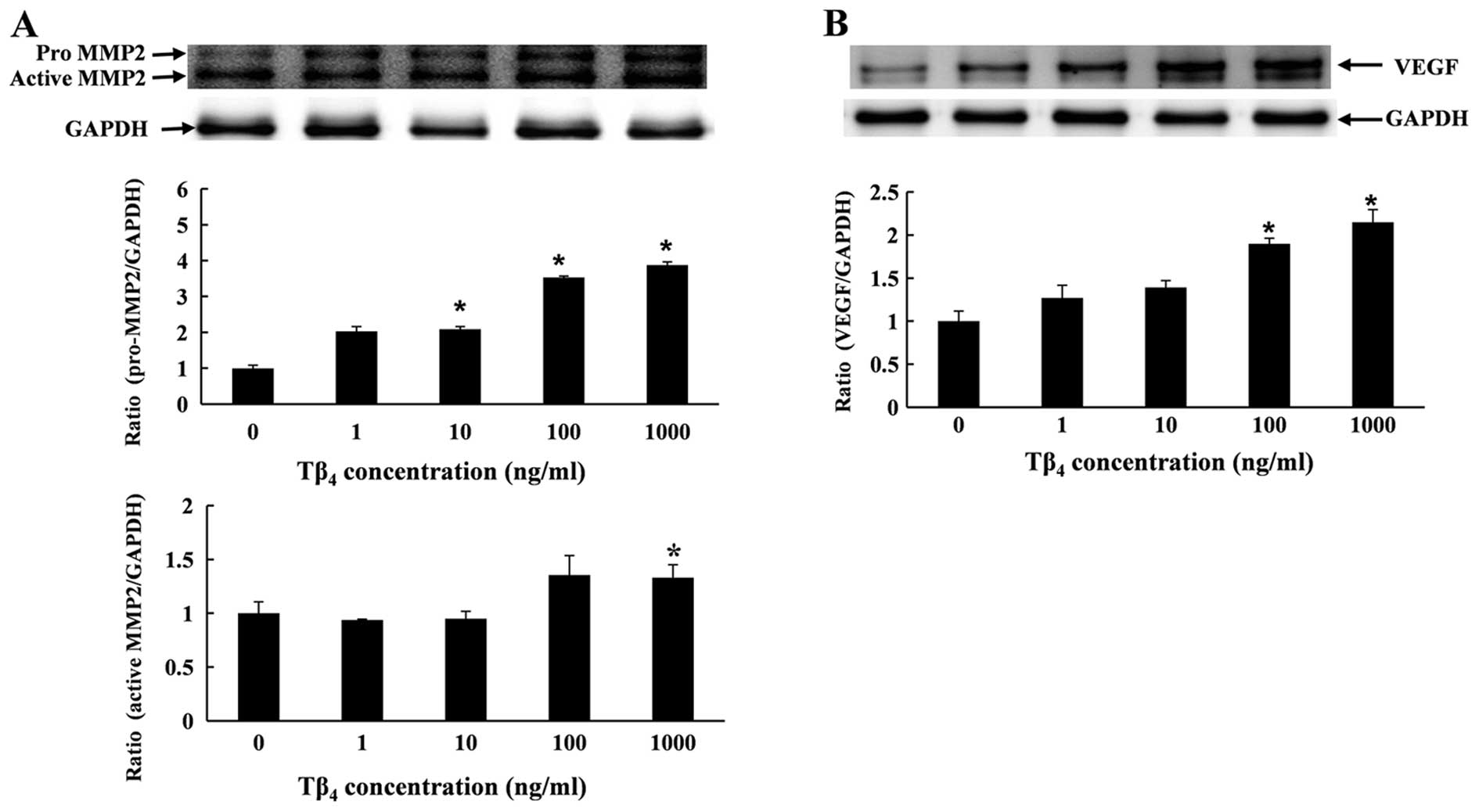
The protein expression of MMP2 and VEGF was
determined by western blot analysis. RP cells were treated with
Tβ4 for 6 h at various concentrations. The results
showed that Tβ4 upregulated the expression of VEGF and
MMP2 proteins in a dose-dependent manner (Fig. 4). The quantitative measurement of
VEGF and MMP2 protein showed that treatment with Tβ4
(1,000 ng/ml) significantly enhanced the level of VEGF protein by
2.1-fold, and enhanced the level of pro-MMP2 and active MMP2 by
3.8- and 1.3-fold, respectively, when compared to the control
(Fig. 4).
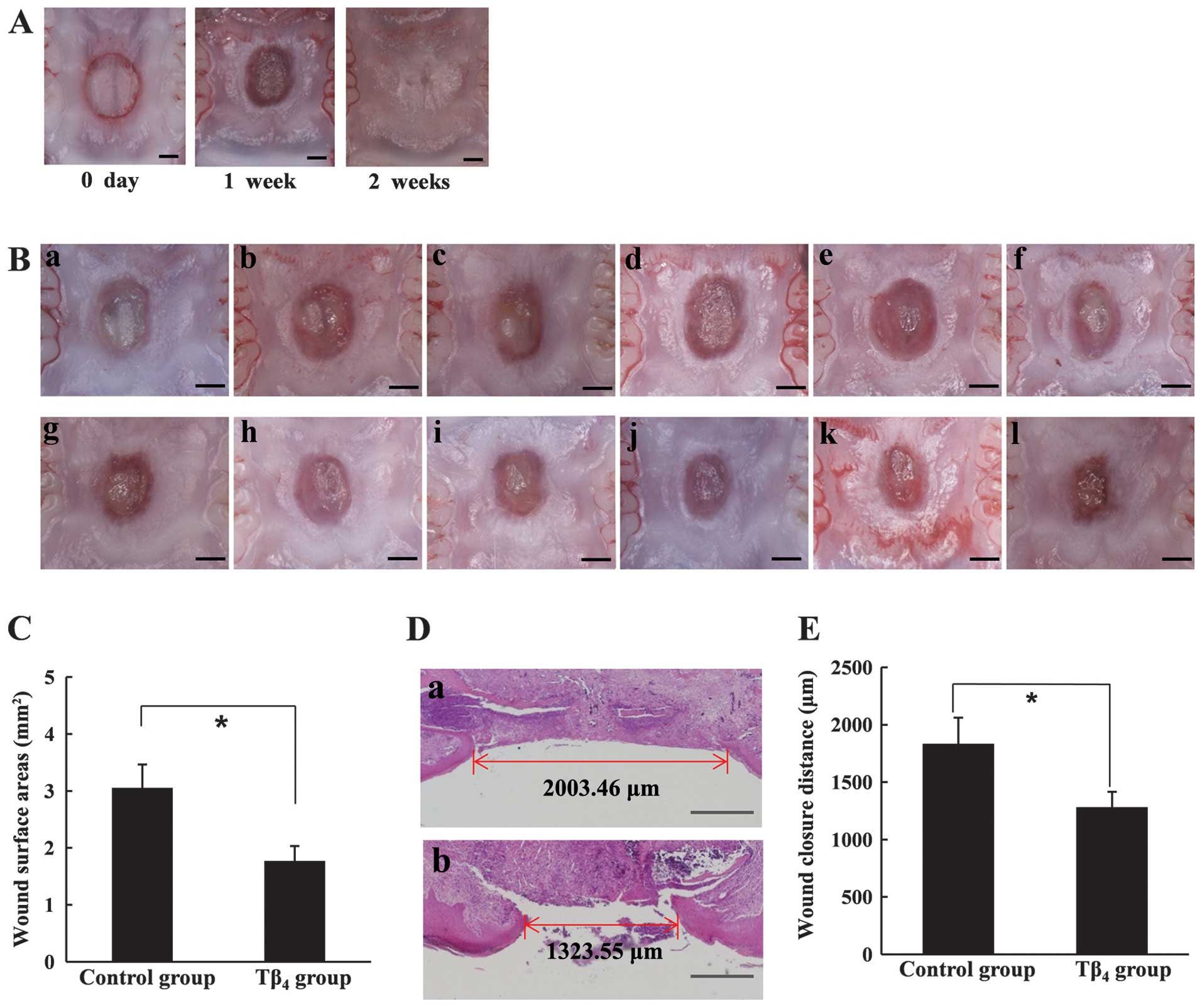
Effects of Tβ4 on the palatal
wound healing of rats
The wound healing effect of Tβ4 was
observed seven days after surgery, whereas the palatal wound gap
was completely closed <2 weeks in the untreated rats (Fig. 5A). As shown in Fig. 5B, the wound area was not
completely epithelized in either the control or the test groups.
However, microscopically smaller wound areas were observed in the
Tβ4-treated test group, indicating that more advanced
epithelization at the wound margin had occurred in the
Tβ4-treated rats (Fig.
5B). The mean area of unepithelized surface in the
Tβ4-treated rats was significantly smaller than that in
the control group (Fig. 5C). The
histological examination also demonstrated an accelerated
epithelization by Tβ4 (Fig. 5D). The mean distance between the
epithelized margin at the center of a palatal wound was ~1,280 μm
in Tβ4-treated rats, while 1,830 μm of the wound
remained unepithelized, on average, in the control group (Fig. 5E). Therefore, we have clearly
demonstrated that Tβ4 significantly accelerated the
epithelization of RP wounds.
Discussion
Wound healing is a series of events including
inflammation, angiogenesis and re-epithelization (23). Previous studies (8–11)
reported that Tβ4 played a multi-functional role in
wound repair processes, demonstrating a potential for the clinical
use of Tβ4 in wound care. In this study, we aimed to
investigate the feasibility of using Tβ4 for oral wound
repair. As shown in Fig. 1,
Tβ4 promoted palatal cell migration. Previously, MMPs
were reported to be upregulated during dermal wound repair in
Tβ4-treated rats (24), and cell migration, promoted by
Tβ4, was blocked by a MMP inhibitor (25). The aforementioned studies
demonstrated a critical role of MMPs in Tβ4-aided cell
migration and wound healing. In this study, we investigated the
expression of MMP2 as mucosal fibroblasts are known to mainly
express MMP2 in the MMP family, which is associated with wound
healing (26,27). As shown in Fig. 2, the mRNA and protein expression
of MMP2 was enhanced by Tβ4, suggesting that the
promotion of palatal cell migration by Tβ4 was mediated
by MMP2. The precise mechanism underlying the induction of MMP
expression by Tβ4 is not yet fully understood. However,
previous studies suggesting Tβ4 as a hypoxia-responsible
regulator (28,29) lead us to speculate that
hypoxia-inducible factor (HIF)-1α may be involved in the
Tβ4-derived modulation of MMPs, because MMPs are known
to be regulated by hypoxia or HIF-1α in other cell types (30,31). Together with MMP2, VEGF, another
main factor related to wound healing, is also a well-known target
of HIF-1α. In this study, we have shown that VEGF was upregulated
by Tβ4 (Fig. 4).
Considering the ability of Tβ4 to induce HIF-1α
stabilization, it is assumed that HIF-1α was also involved in the
enhanced expression of VEGF in the palatal cells.
In the animal model, Tβ4 significantly
accelerated the closure or re-epithelization of palatal wounds
(Fig. 5). The multi-functional
effects of Tβ4 on cell migration, angiogenesis and
inflammation are all thought to contribute to wound repair.
Although Tβ4 is an endogenous component of saliva, our
results showed that exogenous administration of Tβ4 was
effective for the rapid healing of palatal wounds. In contrast to
the applications of dermal wound healing, the administered
Tβ4 can be easily washed away by saliva in the oral
environment. Therefore, we expect that a more appropriate delivery
system, that supplies Tβ4 continuously, would accelerate
healing processes. This study employed normal healthy rats to
investigate the effects of Tβ4. Further experiments
elucidating the efficacy of Tβ4 in regeneration-impaired
animals due to diabetes or advanced age would be highly beneficial
for determining the clinical potential of this treatment.
In conclusion, the cell migration of RP cells was
stimulated by Tβ4. The protein and mRNA expression of
MMP2 and VEGF were also enhanced by Tβ4. The topical
application of Tβ4 greatly enhanced palatal wound
healing in rats. Our results suggest that Tβ4 can be
used for the promotion of oral wound healing.
Acknowledgements
This study was supported by a grant of the Korea
Health Technology R&D project, Ministry of Health and Welfare,
Korea (A120822).
References
|
1
|
Sanders MC, Goldstein AL and Wang YL:
Thymosin beta 4 (Fx peptide) is a potent regulator of actin
polymerization in living cells. Proc Natl Acad Sci USA.
89:4678–4682. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ito M, Iguchi K, Usui S and Hirano K:
Overexpression of thymosin beta4 increases pseudopodia formation in
LNCaP prostate cancer cells. Biol Pharm Bul. 32:1101–1104. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang MC, Chan LC, Yeh YC, Chen CY, Chou
TY, Wang WS and Su Y: Thymosin beta 4 induces colon cancer cell
migration and clinical metastasis via enhancing ILK/IQGAP1/Rac1
signal transduction pathway. Cancer Lett. 308:162–171. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bock-Marquette I, Saxena A, White MD,
Dimaio JM and Srivastava D: Thymosin beta4 activates
integrin-linked kinase and promotes cardiac cell migration,
survival and cardiac repair. Nature. 432:466–472. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Malinda KM, Goldstein AL and Kleinman HK:
Thymosin beta 4 stimulates directional migration of human umbilical
vein endothelial cells. FASEB J. 11:474–481. 1997.PubMed/NCBI
|
|
6
|
Sosne G, Hafeez S, Greenberry AL II and
Kurpakus-Wheater M: Thymosin beta4 promotes human conjunctival
epithelial cell migration. Curr Eye Res. 24:268–273. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cierniewski CS, Sobierajska K, Selmi A,
Kryczka J and Bednarek R: Thymosin β4 is rapidly internalized by
cells and does not induce intracellular Ca2+ elevation. Ann N Y
Acad Sci. 1269:44–52. 2012.
|
|
8
|
Grant DS, Rose W, Yaen C, Goldstein A,
Martinez J and Kleinman H: Thymosin beta4 enhances endothelial cell
differentiation and angiogenesis. Angiogenesis. 3:125–135. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reti R, Kwon E, Qiu P, Wheater M and Sosne
G: Thymosin beta4 is cytoprotective in human gingival fibroblasts.
Eur J Oral Sci. 116:424–430. 2008. View Article : Google Scholar
|
|
10
|
Kumar S and Gupta S: Thymosin beta 4
prevents oxidative stress by targeting antioxidant and
anti-apoptotic genes in cardiac fibroblasts. Plos One.
6:e269122011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sosne G, Qiu P, Christopherson PL and
Wheater MK: Thymosin beta 4 suppression of corneal NFkappaB: A
potential anti-inflammatory pathway. Exp Eye Res. 84:663–669. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huff T, Otto AM, Müller CS, Meier M and
Hannappel E: Thymosin beta4 is released from human blood platelets
and attached by factor XIIIa (transglutaminase) to fibrin and
collagen. FASEB J. 16:691–696. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Philp D, Badamchian M, Scheremeta B,
Nguyen M, Goldstein AL and Kleinman HK: Thymosin beta 4 and a
synthetic peptide containing its actin-binding domain promote
dermal wound repair in db/db diabetic mice and in aged mice. Wound
Repair Regen. 11:19–24. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee HG and Eun HC: Differences between
fibroblasts cultured from oral mucosa and normal skin: implication
to wound healing. J Dermatol Sci. 21:176–182. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Häkkinen L, Uitto VJ and Larjava H: Cell
biology of gingival wound healing. Periodontology 2000. 24:127–152.
2000.
|
|
16
|
Lygoe KA, Norman JT, Marshall JF and Lewis
MP: alphaV integrins play an important role in myofibroblast
differentiation. Wound Repair Regen. 12:461–470. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kozlovsky A, Artzi Z, Hirshberg A,
Israeli-Tobias C and Reich L: Effect of local antimicrobial agents
on excisional palatal wound healing: a clinical and
histomorphometric study in rats. J Clin Periodontol. 34:164–171.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hammad HM, Hammad MM, Abdelhadi IN and
Khalifeh MS: Effects of topically applied agents on intra-oral
wound healing in a rat model: a clinical and histomorphometric
study. Int J Dent Hyg. 9:9–16. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shanmugam M, Kumar TS, Arun KV, Arun R and
Karthik SJ: Clinical and histological evaluation of two dressing
materials in the healing of palatal wounds. J Indian Soc
Periodontol. 14:241–244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ayvazyan A, Morimoto N, Kanda N, Takemoto
S, Kawai K, Sakamoto Y, Taira T and Suzuki S: Collagen-gelatin
scaffold impregnated with bFGF accelerates palatal wound healing of
palatal mucosa in dogs. J Surg Res. 171:e247–e257. 2011. View Article : Google Scholar
|
|
21
|
Badamchian M, Damavandy AA, Damavandy H,
Wadhwa SD, Katz B and Goldstein AL: Identification and
quantification of thymosin beta4 in human saliva and tears. Ann N Y
Acad Sci. 1112:458–465. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kwon E, Jacobs LC and Wheater M: Gingival
crevicular fluid levels of thymosin beta4 in periodontal health and
disease. J Adv Oral Res. 4:1–6. 2013.
|
|
23
|
Singer AJ and Clark RA: Cutaneous wound
healing. N Engl J Med. 341:738–746. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Philp D, Scheremeta B, Sibliss K, Zhou M,
Fine EL, Nguyen M, Wahl L, Hoffman MP and Kleinman HK: Thymosin
beta4 promotes matrix metalloproteinase expression during wound
repair. J Cell Physiol. 208:195–200. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qiu P, Kurpakus-Wheater M and Sosne G:
Matrix metalloproteinase activity is necessary for thymosin beta 4
promotion of epithelial cell migration. J Cell Physiol.
212:165–173. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mäkelä M, Salo T, Uitto VJ and Larjava H:
Matrix metalloproteinases (MMP-2 and MMP-9) of the oral cavity:
cellular origin and relationship to periodontal status. J Dent Res.
73:1397–1406. 1994.PubMed/NCBI
|
|
27
|
Salo T, Mäkelä M, Kylmäniemi M,
Autio-Harmainen H and Larjava H: Expression of matrix
metalloproteinase-2 and -9 during early human wound healing. Lab
Invest. 70:176–182. 1994.PubMed/NCBI
|
|
28
|
Moon EY, Im YS, Ryu YK and Kang JH:
Actin-sequestering protein, thymosin beta-4, is a novel hypoxia
responsive regulator. Clin Exp Metastasis. 27:601–609. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oh JM, Ryoo IJ, Yang Y, Kim HS, Yang KH
and Moon EY: Hypoxia-inducible transcription factor (HIF)-1 alpha
stabilization by actin-sequestering protein, thymosin beta-4 (TB4)
in HeLa cervical tumor cells. Cancer Lett. 264:29–35. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ahn JK, Koh EM, Cha HS, Lee YS, Kim J, Bae
EK and Ahn KS: Role of hypoxia-inducible factor-1 alpha in
hypoxia-induced expressions of IL-8, MMP-1 and MMP-3 in rheumatoid
fibroblast-like synoviocytes. Rheumatology (Oxford). 47:834–839.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ben-Yosef Y, Miller A, Shapiro S and Lahat
N: Hypoxia of endothelial cells leads to MMP-2-dependent survival
and death. Am J Physiol Cell Physiol. 289:C1321–C1331. 2005.
View Article : Google Scholar : PubMed/NCBI
|