Introduction
Fungal metabolites are naturally occurring compounds
produced by fungi with known anticancer properties (1). This is supported by early studies on
cytochalasins, fungal metabolites that have been shown to inhibit
the growth and migration of prostate cancer cells (2), as a consequence of impairing the
biophysical function of actin filaments by binding and capping
their barbed ends, thus, preventing microfilament elongation.
Additionally, secondary metabolites (phenolic compounds, melanins
and lanostane-type triterpenoids) produced by the white rot fungus,
Inonotus obliquus, have also exhibited antitumorigenic
properties in breast, leukemia and gastric cancers (3–5).
Collectively, these studies provide experimental evidence for the
persistent exploration and utility of fungal metabolites as agents
for the treatment of human cancers.
In the present study, we evaluated the efficacy of
fusarochromanone (FC101), a fungal metabolite produced by
Fusarium equiseti (6), in
glioblastoma, the most common and aggressive type of malignant
primary brain tumor. To date, few studies have been performed that
have assessed the efficacy of FC101 as an anticancer agent.
However, experimental studies conducted by Furmanski et al
(7) and Dréau et al
(8) have demonstrated that FC101
inhibits the growth of breast cancer cells and the in vitro
and in vivo growth of melanomas. In addition a recent study
by our group [Mahdavian et al (unpublished data)] also
demonstrated that FC101 exerts antitumor affects in prostate and
bladder cancer, further establishing the anticancer activity of
this fungal metabolite. To this end, although the usage of fungal
metabolites as potential therapeutic agents for the treatment of
glioblastomas has received little attention, in this study, we
provide evidence of the antitumor effects of FC101 on glioblastoma
cell proliferation through the promotion of apoptotic cell death,
as well as its antagonizing affects on glioblastoma cell
migration.
Materials and methods
Cell conditions and reagents
U87, A172 and U251 glioblastoma cells were purchased
from the American Type Culture Collection (ATCC; Manassas, VA,
USA). All cell lines were maintained in Dulbecco’s modified Eagle’s
medium-DMEM (Invitrogen, Grand Island, NY, USA) containing 10%
fetal bovine serum (FBS; Invitrogen), 2 mM L-glutamine
(Invitrogen), 100 nM MEM non-essential amino acids (Invitrogen) and
penicillin-streptomycin (Invitrogen) at 37°C and 5% CO2.
FC101 was generously provided by Dr Elahe Mahdavian (Department of
Chemistry and Physics, LSU-Shreveport, Shreveport, LA, USA).
Crystal violet cell proliferation
assay
The cells were plated in 24-well plates, treated
with 10, 5, 2.5 and 1 μM FC101 and allowed to incubate for 48 h
(vehicle controls were treated with PBS) for dose-response
experiments. For time-course experiments, the cells were treated
with 1 μM FC101 and allowed to incubate for 24, 48, 72 and 96 h.
Subsequently, the tissue culture medium was removed, the cell
monolayer was fixed with 100% methanol for 5 min and stained with
0.5% crystal violet in 25% methanol for 10 min. The cells were then
washed 3 times for 5 min each with distilled water to remove excess
dye and allowed to dry overnight at room temperature. The
incorporated dye was then solubilized in 0.1 M sodium citrate
(Sigma-Aldrich, St. Louis, MO, USA) in 50% ethanol. Subsequently,
100 μl of treated and control samples were transferred to 96-well
plates and optical densities were read at 540 nm using an xMark
Microplate absorbance spectrophotometer (Bio-Rad Laboratories,
Hercules, CA, USA).
Cell motility
Motility assays were conducted according to the
manufacturer’s instructions (Cell Biolabs Inc., San Diego, CA,
USA). A cell suspension containing 0.5–1.0x106 cells/ml
was prepared in serum-free mediaum with the vehicle (PBS) or 1 μM
FC101, while 500 μl of medium containing 10% fetal bovine serum was
added to the lower chamber of the migration plate. Cell suspension
(300 μl) containing the vehicle or 1 μM FC101 was then added to the
inside of each insert and allowed to incubate for 24 h at 37°C and
5% CO2. Subsequently, the non-migratory cells were
removed from the plate inserts (as per the manufacturer’s
instructions), and the migratory cells were counterstained,
solubilized and the optical density densities were read at 560 nm
using an xMark Microplate absorbance spectrophotometer (Bio-Rad
Laboratories).
Measurement of reactive oxygen species
(ROS; H2O2) generation
ROS assays were conducted in accordance with the
manufacturer’s instructions (Cell Biolabs Inc.). A serial dilution
of H2O2 (0–100 μM) was prepared to generate a
standard curve, while the U87 and A172 cells (1x107)
were plated, exposed to 1 μM FC101 or the vehicle (PBS) for 24 h
and sonicated. Standards and samples were then mixed with an
aqueous working reagent (as per the manufacturer’s instructions),
incubated on a shaker for 30 min at room temperature, and the
optical densities were read at 595 nm with a xMark Microplate
absorbance spectrophotometer (Bio-Rad Laboratories).
ELISA
The cells were plated and treated with 1 μM FC101
for 24 h or the vehicle (PBS); the cells were then lysed with
CelLytic M Cell lysis reagent (Sigma-Aldrich) and protein
concentrations were determined using the Bradford method. Protein
extracts were diluted to a final concentration of 20 μg/ml in PBS,
coated to the wells of a PVC microtiter plate, and allowed to
incubate at 4°C overnight. Wells containing protein antigen were
then washed with PBS 3 times, blocked with 5% non-fat dry milk in
PBS for 2 h, and washed again with PBS 2 times. Subsequently,
incubation with α-actinin 1 (Abnova, Walnut, CA, USA) or α-actinin
4 (Abnova) antibodies was performed overnight at 4°C followed by
washing 4 times with PBS. Incubation with an HRP-conjugated
secondary antibody was performed for 3 h, followed by 4 washes with
PBS. TMB substrate solution (Thermo Fisher Scientific, Inc.,
Rockford, IL, USA) was added for 15 min followed by a stop reaction
with 0.2 M sulfuric acid. An absorbance reading at 450 nm was
performed with a xMark Microplate absorbance spectrophotometer
(Bio-Rad Laboratories). Absorbance readings were normalized to
FC101 and the vehicle-treated samples that were processed with
secondary antibody only.
Western blot analysis
The cells were plated and treated with 1 μM FC101
for 24 h or the vehicle (PBS), rinsed with PBS, and lysed with
CelLytic M Cell lysis reagent (Sigma-Aldrich). Protein
concentrations were subsequently determined using the Bradford
method. Proteins were separated by SDS-PAGE in 8% polyacrylamide
gels, then transferred to PVDF membranes. For immunoblotting, the
PVDF membranes were incubated with poly(ADP-ribose) polymerase
(PARP) and caspase-9 antibodies (Cell Signaling Technology,
Danvers, MA, USA) recognizing target proteins overnight at 4°C. The
membranes were then incubated with an HRP-conjugated secondary
antibody (1:2,000) for 1 h at room temperature, analyzed using the
enhanced chemiluminescence (ECL) detection system (Thermo Fisher
Scientific) and visualized by autoradiography. Tubulin (1:5,000)
was used as a loading control.
Results
Antiproliferative effects of FC101 on
glioblastomas
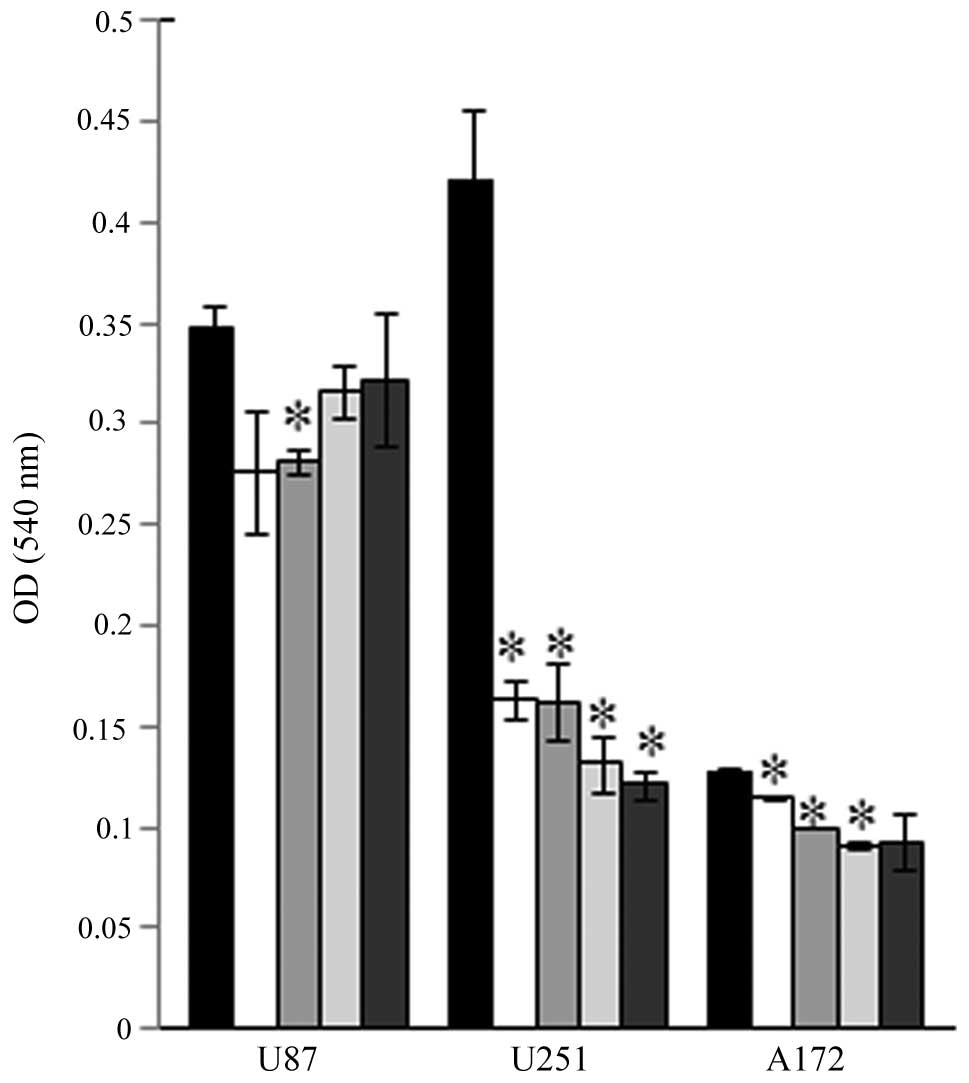
Our initial experiments evaluated the effects of
FC101 on glioblastoma cell proliferation in dose-response
experiments that showed that FC101 (10, 5, 2.5 and 1 μM) decreased
the proliferative capacity of the U87, A172 and U251 cells
(Fig. 1). The most demonstrative
effects of FC101 in dose-response experiments were observed in the
U251 cells which displayed a statistically significant decrease
(P<0.05) in cell proliferation, even when treated with the
lowest dose of 1 μM FC101, as compared to the vehicle-treated
control cells (Fig. 1). To
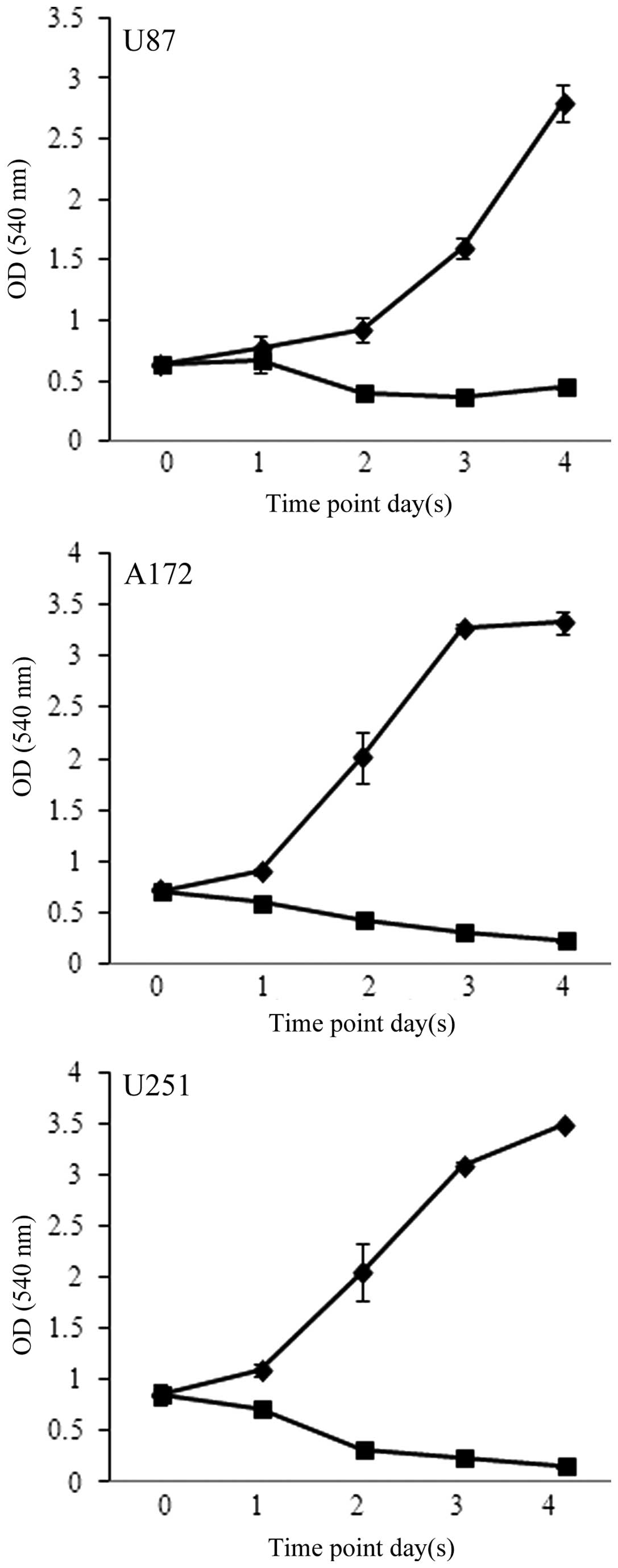
further assess the effects of FC101 on glioblastoma cell
proliferation, time-course experiments were performed in which the
cells were treated with 1 μM FC101 and examined over a 4-day period
(Fig. 2). Consistent with the
results from the dose-response experiments, time-course analysis
also revealed that FC101 reduced the proliferation of the
glioblastoma cells, as evidenced by a pronounced decrease in cell
proliferation 96 h post-FC101 exposure, as compared to the
vehicle-treated control cells examined at the same time point
(Fig. 2). Additionally, ANOVA
analysis of the time-course data revealed that the statistically
significant (P<0.05) decrease observed in glioblastoma cell
proliferation following treatment with FC101 was likely a
consequence of a cytotoxic cellular response. This was determined
by comparing glioblastoma cell viability at day 0 and 4 days
post-FC101 exposure, which showed a 31, 66 and 82% decrease in U87,
A172 and U251 cell proliferation, respectively (Fig. 2). These data are in accordance
with those of previous studies on FC101 in melanomas and breast
cancer (7,8), as well as with those of studies
conducted on glioblastomas with the fungal metabolites, ophiobolin
A (9) and fumonisin B1 (10–12) that also induced cytotoxic cellular
responses.
FC101 induces glioblastoma cell death via
apoptosis
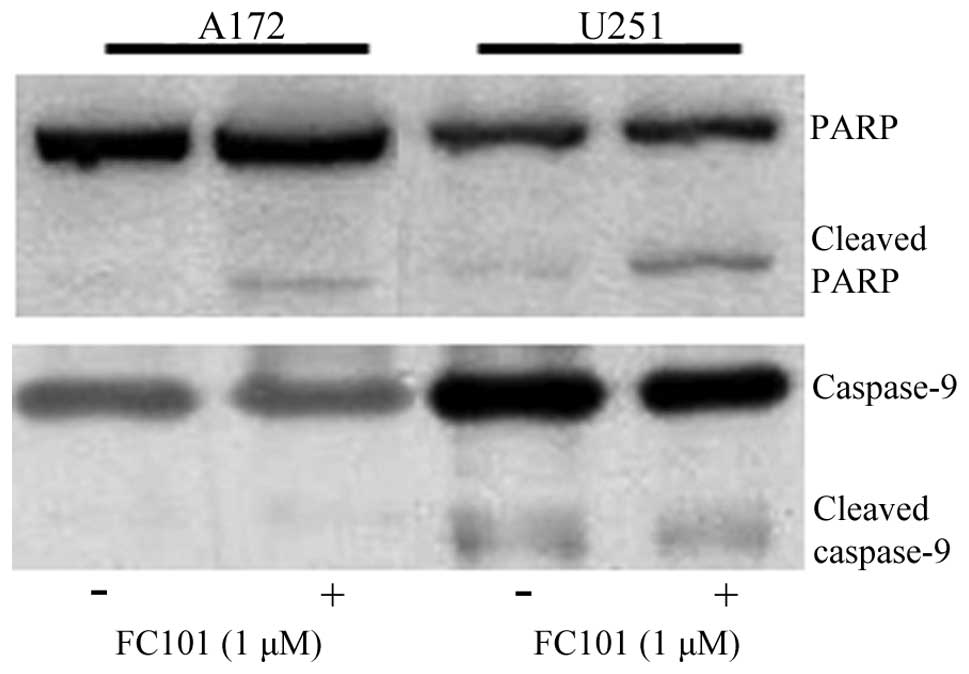
To determine the mechanisms underlying the cytotoxic
cellular response described above, we assessed the involvement of
programmed cell death-associated signaling proteins and oxidative
stress-related molecules in response to FC101. PARP, an enzymatic
protein that plays a role in DNA repair and a downstream target of
caspases, which mediate programmed cell death, was examined as an
indicator of apoptosis in glioblastoma cells treated with FC101.
Immunoblotting procedures revealed that the glioblastoma cells
exposed to 1 μM FC101 produced cleaved PARP protein expression, a
marker of cells undergoing apoptosis (Fig. 3), while no changes in caspase-9
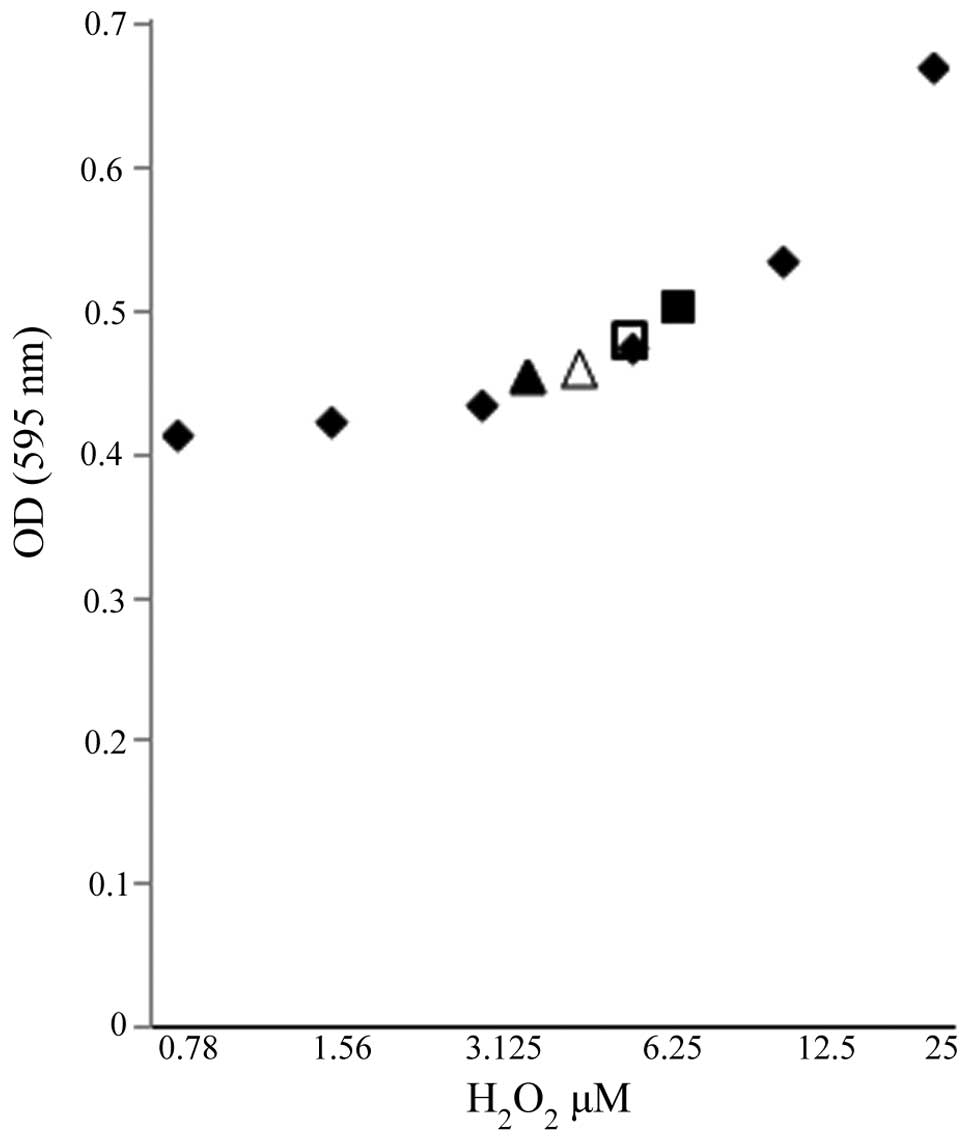
protein expression, an initiator caspase, were observed (Fig. 3). Additionally, ROS, known
inducers of death receptor and mitochondrial-mediated apoptosis in
cancer cells, were also examined as a mechanistic contributor to
FC101-induced glioblastoma cell death. To this end, we observed a
modest increase in the hydrogen peroxide concentration in the A172
cells treated with FC101 as compared to the vehicle-treated control
cells, while the U251 cells displayed a decrease in hydrogen
peroxide concentration following treatment with FC101 (Fig. 4). Taken together, these data
demonstrate that FC101 induces a differential oxidative stress
response that is cell type-dependent and that this fungal
metabolite promotes apoptotic cell death in glioblastoma cells via
caspase signaling.
Inhibition of glioblastoma cell
migration
Glioblastomas are highly invasive tumors that
ultimately result in high rates of disease recurrence. Therefore,
in this study, we analyzed the ability of FC101 to affect
glioblastoma cell migration and structural proteins involved in
this cellular process. α-actinin 1 and 4, actin-binding proteins
with an established role in cell migration (13,14) and shown to be expressed at high
levels in glioblastomas as compared to normal brain tissue
(14), were examined in the
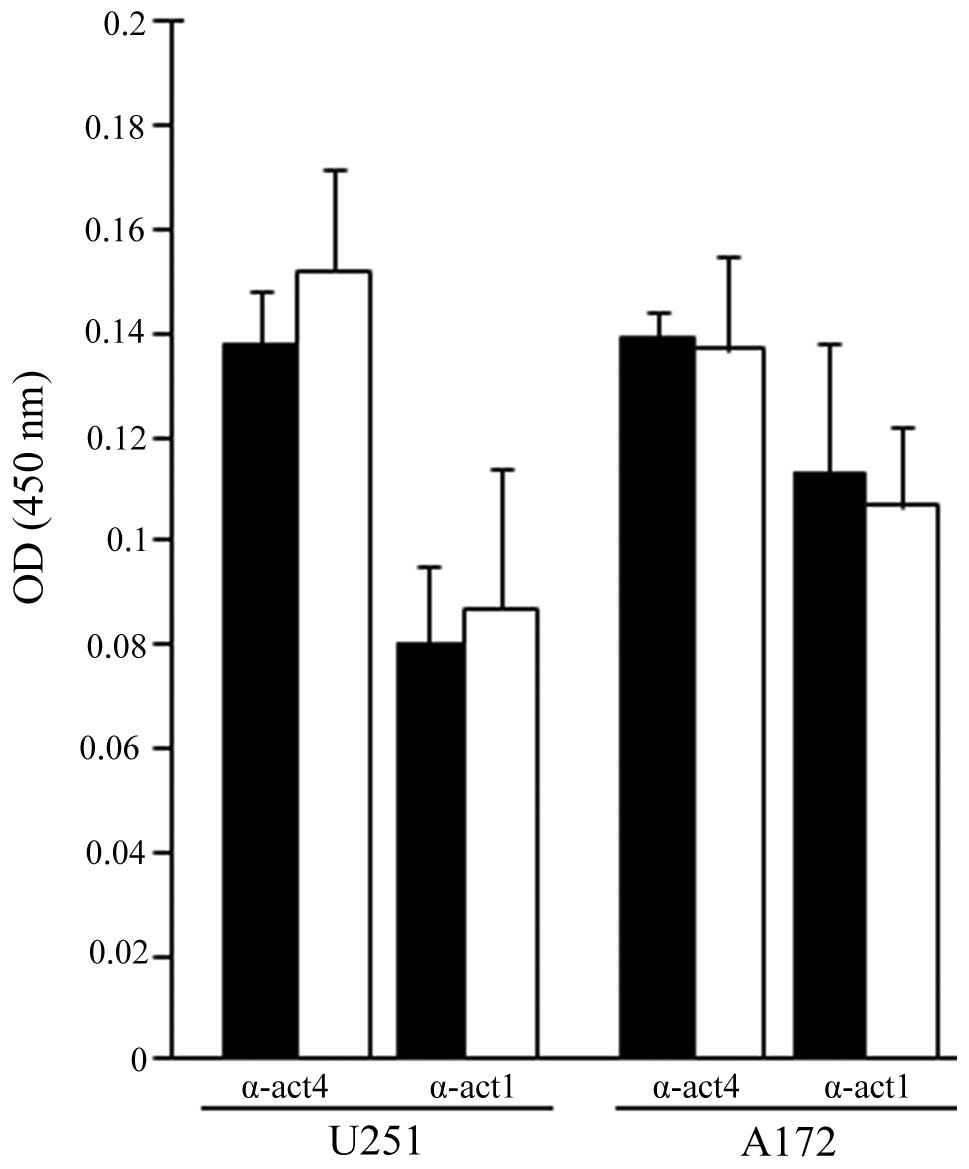
FC101-treated glioblastoma cells. Using immunoenzyme linked assays,
a moderate increase in α-actinin 1 and 4 expression was observed in
the U251 cells exposed to FC101 when compared to the
vehicle-treated control cells, while a diminutive decrease in
α-actinin 1 and 4 expression was observed in the A172 cells treated
with FC101 (Fig. 5).
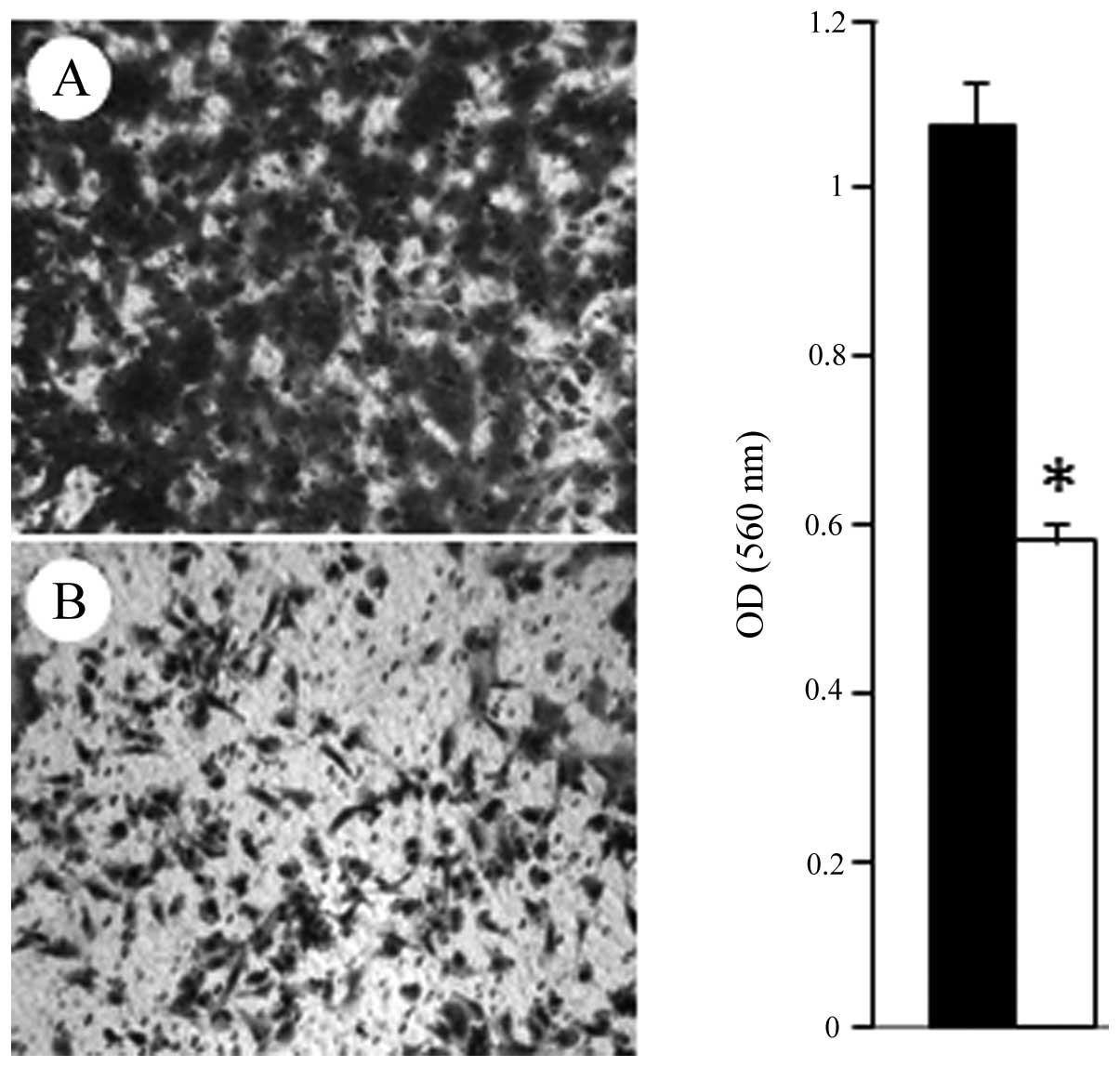
Subsequently, cell migration assays revealed that 1 μM FC101
induced a 46% decrease (P<0.05) in glioblastoma cell migration
as compared to the vehicle-treated control cells (Fig. 6). These results are in accordance
with the findings of a recent study by Bury et al (15), that also showed that the fungal
metabolite, fusicoccin A, impaired the migratory and invasion
ability of glioblastoma cells.
Discussion
Fungal metabolites are emerging as promising
anticancer agents for the treatment of human cancers, in part,
since they are naturally derived products. However, the utility of
metabolic products from fungi as adjuvant or neoadjuvant agents for
the treatment of brain tumors, specifically glioblastomas, has
received little attention. To date, the most well characterized
fungal metabolite exhibiting cytotoxic anticancer activity in
glioblastomas is the fumonisin, fumonisin B1, which exerts its
biological effects by interfering with sphingolipid metabolism
(10–12). Additionally, studies on the fungal
metabolites, brefeldin A and ophiobolin A, which impair the
structure and functions of the Golgi apparatus and calmodulin,
respectively, have also shown that they promote glioblastoma cell
death (9,16). Collectively, these studies
establish the cytotoxic antitumor effects of fungi-produced
metabolites in glioblastomas by affecting a range of biological
processes.
In this study, we demonstrated that the fungal
metabolite, FC101, exhibited antitumorigenic affects in
glioblastomas as a consequence of inducing cell death. This was
supported by cell proliferation data from the present study that
displayed an IC90 in glioblastomas treated with 1 μM
FC101 and examined 4 days later. Our findings are in accordance
with those from other studies on breast, melanoma and bladder
cancers, that also showed that therapeutic applicable
concentrations of FC101 were effective at promoting tumor cell
killing in these human cancers (7,8).
In this study, it was also determined that FC101 induced
caspase-dependent apoptotic glioblastoma cell death, as indicated
by PARP cleavage, a caspase target. This is consistent with data
from other studies on melanomas and breast cancer, that also
demonstrated caspase-dependent apoptosis as the underlying
mechanism of tumor cell death observed in these cancers in response
to FC101 [(8) and study by
Mahdavian et al (unpublished data)]. Although PARP cleavage
provided substantial evidence that FC101 promoted caspase-dependent
apoptotic glioblastoma cell death, changes in caspase-9 protein
expression were not detected. The lack of an affect on caspase-9
expression supports the notion that FC101 does not induce apoptotic
cell death in glioblastomas via an intrinsic mechanism, as
suggested in in another study of ours [Mahdavian et al
(unpublished data)], which showed that FC101 had no effect on
intrinsic apoptotic pathway proteins (BAD, BAK and BAX) in breast
cancer cells. In addition to caspase signaling, oxidative stress
has also been shown to act as a mechanistic contributor of fungal
metabolite-induced apoptotic cell death (10,11,17,18). Stockmann-Juvala et al
(10) and Chaudhari et al
(17) demonstrated that fumonisin
B1 and the trichothecene, T-2 toxin, elicited apoptotic cell death
as a consequence of increased ROS production and reduced
glutathione levels in cervical cancer and glioblastomas,
respectively. However, the assessment of oxidative stress in our
study revealed differential ROS levels in the A172 and U251
glioblastoma cells treated with FC101 as compared to the
control-treated cells, suggesting that variations in the genetic
backgrounds of these cells likely contribute to the divergent
responses observed and the role of oxidative stress in
FC101-induced apoptotic glioblastoma cell death.
In this study, we further demonstrated that FC101
also exhibits antitumor properties in glioblastomas by abrogating
cell migration, a cell behavior that contributes to the metastatic
invasiveness of human cancers, ultimately leading to disease
recurrence. This observation is consistent with recent studies on
the fungal metabolites, fusicoccin A and chaetoglobosin A that also
had antimigratory effects on glioblastoma and leukemia cells which
were attributed to the impairment of the actin cytoskeleton
(15,19). However, in this study, the
analysis here of α-actinin 1 and 4, structural elements of the
actin cytoskeleton with well-characterized roles in cell migration
were minimally affected by exposure to FC101 (13,14). It should be stated that although
FC101 appeared to have little affect on α-actinin 1 and 4, the
disruption of subcellular actin filament structures (leading edge,
trailing edge) important for cell migration may underlie the
affects of FC101 on glioblastosma cell migration, even in the
absence of significant changes in protein expression. Additionally,
the antimigratory response of glioblastomas to FC101 may be
causally related to the down-regulation of metalloproteinases, as
observed in osteosarcomas and mesothelioma cells treated with the
fungal metabolites, 3-O-methylfunicone and
fucoxanthin(20,21). Furthermore, to the best of our
knowledge, this is the first study that illustrates the
antimigratory effects of FC101 on tumor cells.
In the present study, we demonstrate the
effectiveness of FC101 as an anticancer agent in glioblastomas,
expanding its potential therapeutic utility for the treatment of
human cancers. The prospective application of FC101 for the
treatment of cancer is furthered by experimental studies that
demonstrate that cancer cells are more sensitive to this fungal
metabolite than normal tissue (7,8),
suggesting that FC101 circumvents normal tissue toxicity. This
therapeutic characteristic of FC101 is particularly advantageous
when considering neurotoxicity is often a limiting factor for
chemotherapeutic drugs used for the treatment of glioblastomas.
Additional caveats to the clinical treatment of these tumors is
their therapeutic resistance attributed in part to high recurrence
rates that have made combinatorial therapy approaches essential for
the management and treatment of this disease. FC101 represents a
novel compound that can be explored for its therapeutic
applications as a combinatorial agent that augments surgical and
radiotherapy approaches for the treatment of glioblastomas.
Acknowledgements
The present study was supported in part by the
Department of Biological Sciences at Tennessee State University.
The project described herein was also supported by the National
Center for Research Resources (award no. 5P20RR016456-11) and the
National Institute of General Medical Sciences (award no. 8 P20
GM103424-11) from the National Institutes of Health.
Abbreviations:
|
FC101
|
fusarochromanone
|
|
ROS
|
reactive oxygen species
|
|
PARP
|
poly(ADP-ribose) polymerase
|
References
|
1
|
Wang LW, Zhang YL, Lin FC, Hu YZ and Zhang
CL: Natural products with antitumor activity from endophytic fungi.
Mini Rev Med Chem. 11:1056–1074. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Duncan MD, Harmon JW and Duncan LK: Actin
disruption inhibits bombesin stimulation of focal adhesion kinase
(pp125FAK) in prostate carcinoma. J Surg Res. 63:359–363. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nomura M, Takahashi T, Uesugi A, Tanaka R
and Kobayashi S: Inotodiol, a lanostane triterpenoid, from Inonotus
obliquus inhibits cell proliferation through caspase-3-dependent
apoptosis. Anticancer Res. 28:2691–2696. 2008.PubMed/NCBI
|
|
4
|
Handa N, Yamada T and Tanaka R: An unusual
lanostane-type triterpenoid, spiroinonotsuoxodiol, and other
triterpenoids from Inonotus obliquus. Phytochemistry.
14–15:1774–1779. 2010.PubMed/NCBI
|
|
5
|
Ma L, Chen H, Dong P and Lu X:
Anti-inflammatory and anticancer activities of extracts and
compounds from the mushroom Inonotus obliquus. Food Chem.
139:503–508. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Krogh P, Christensen DH, Hald B, Harlou B,
Larsen C, Pedersen EJ and Thrane U: Natural occurrence of the
mycotoxin fusarochromanone, a metabolite of Fusarium
equiseti, in cereal feed associated with tibial
dyschondroplasia. Appl Environ Microbiol. 55:3184–3188.
1989.PubMed/NCBI
|
|
7
|
Furmanski BD, Dréau D, Wuthier RE and
Fuseler JW: Differential uptake and selective permeability of
fusarochromanone (FC101), a novel membrane permeable anticancer
naturally fluorescent compound in tumor and normal cells. Microsc
Microanal. 15:545–557. 2009. View Article : Google Scholar
|
|
8
|
Dréau D, Foster M, Hogg M, Culberson C,
Nunes P and Wuthier RE: Inhibitory effects of fusarochromanone on
melanoma growth. Anticancer Drugs. 18:897–904. 2007.PubMed/NCBI
|
|
9
|
Bury M, Girault A, Mégalizzi V, et al:
Ophiobolin A induces paraptosis-like cell death in human
glioblastoma cells by decreasing BKCa channel activity. Cell Death
Dis. 4:e5612013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stockmann-Juvala H, Mikkola J, Naarala J,
Loikkanen J, Elovaara E and Savolainen K: Fumonisin B1-induced
toxicity and oxidative damage in U-118MG glioblastoma cells.
Toxicology. 202:173–183. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stockmann-Juvala H, Mikkola J, Naarala J,
Loikkanen J, Elovaara E and Savolainen K: Oxidative stress induced
by fumonisin B1 in continuous human and rodent neural cell
cultures. Free Radic Res. 9:933–942. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stockmann-Juvala H, Naarala J, Loikkanen
J, Vähäkangas K and Savolainen K: Fumonisin B1-induced apoptosis in
neuroblastoma, glioblastoma and hypothalamic cell lines.
Toxicology. 225:234–241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sen S, Dong M and Kumar S:
Isoform-specific contributions of alpha-actinin to glioma cell
mechanobiology. PLoS One. 4:e84272009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Quick Q and Skalli O: Alpha-actinin 1 and
alpha-actinin 4: contrasting roles in the survival, motility, and
RhoA signaling of astrocytoma cells. Exp Cell Res. 316:1137–1147.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bury M, Andolfi A, Rogister B, et al:
Fusicoccin a, a phytotoxic carbotricyclic diterpene glucoside of
fungal origin, reduces proliferation and invasion of glioblastoma
cells by targeting multiple tyrosine kinases. Transl Oncol.
6:112–123. 2013. View Article : Google Scholar
|
|
16
|
Pommepuy I, Terro F, Petit B, et al:
Brefeldin A induces apoptosis and cell cycle blockade in
glioblastoma cell lines. Oncology. 4:459–467. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chaudhari M, Jayaraj R, Bhaskar AS and
Lakshmana Rao PV: Oxidative stress induction by T-2 toxin causes
DNA damage and triggers apoptosis via caspase pathway in human
cervical cancer cells. Toxicology. 262:153–161. 2009. View Article : Google Scholar
|
|
18
|
Acuña UM, Wittwer J, Ayers S, Pearce CJ,
Oberlies NH and De Blanco EJ: Effects of (5Z)-7-oxozeaenol on
MDA-MB-231 breast cancer cells. Anticancer Res. 32:2415–2421.
2012.PubMed/NCBI
|
|
19
|
Knudsen PB, Hanna B, Ohl S, et al:
Chaetoglobosin A preferentially induces apoptosis in chronic
lymphocytic leukemia cells by targeting the cytoskeleton. Leukemia.
28:1289–1298. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Buommino E, De Filippis A, Nicoletti R, et
al: Cell-growth and migration inhibition of human mesothelioma
cells induced by 3-O-methylfunicone from Penicillium
pinophilum and cisplatin. Invest New Drugs. 30:1343–1351. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rokkaku T, Kimura R, Ishikawa C, Yasumoto
T, Senba M, Kanaya F and Mori N: Anticancer effects of marine
carotenoids, fucoxanthin and its deacetylated product,
fucoxanthinol, on osteosarcoma. Int J Oncol. 43:1176–1186.
2013.PubMed/NCBI
|