Introduction
Hypercholesterolemia is a major underlying cause of
ischemic stroke (1) and
therapeutics targeting hypercholesterolemia decrease the risk of
stroke in high-risk individuals. Chronic systemic inflammatory
conditions, such as atherosclerosis, diabetes and obesity are
associated with increased risk of stroke (2,3).
Stroke is also characterized by massive inflammation in areas
surrounding the injury that magnifies damage to the brain (4). Therefore, modulation of inflammation
may be an effective therapeutic strategy for reducing the impact of
stroke in hypercholesterolemic subjects.
Probucol is a cholesterol-lowering drug with
antioxidant, anti-inflammatory and anti-atherosclerogenic
properties (5). Cilostazol is a
type 3 phosphodiesterase inhibitor that exhibits antiplatelet and
vasodilating activity by suppressing the degradation of cAMP
(6). Cilostazol has also been
shown to exert in vivo neuroprotective effects against
cerebral ischemic injury via anti-inflammatory effects (7). A previous in vitro study
using cultured human coronary artery endothelial cells (8), as well as in vivo studies in
rats, low-density lipoprotein (LDL) receptor-deficient mice and
apolipoprotein E (ApoE) knockout (KO) mice revealed the synergistic
effects of probucol plus cilostazol against atherosclerotic lesions
and ischemic brain injury (9–11).
The aforementioned studies suggested that combined administration
of these drugs may result in synergistic effects in mice with
stroke and hypercholesterolemia. In addition, probucol and
cilostazol have anti-inflammatory effects and may act
synergistically to accelerate the outcome process via the
inhibition of inflammation.
Chemokines, which are well-known regulators of
peripheral immune cell recruitment and trafficking under
physiological and pathological conditions, can cause secondary
damage during inflammation and affect cerebral ischemia. MCP-1, a
pro-inflammatory chemokine, is a potent chemoattractant capable of
promoting monocyte recruitment into an inflammatory or pathological
site. Once activated near the site of pathology, the recruited
cells can produce more pro-inflammatory mediators, inducing
inflammation (12,13). Therefore, understanding the
modulation of chemokines may result in the development of
treatments specific for cerebral ischemia with
hypercholesterolemia.
In the present study, we investigated whether
hypercholesterolemia exacerbates ischemic outcomes and any
exacerbation of stroke was associated with neuroinflammation in the
brain by coupling hypercholesterolemia in an experimental stroke
model using mice. In addition, we focused on the effect of probucol
and cilostazol administered in combination with conventional
therapy due to their anti-inflammatory properties. The tissue
outcome and functional outcome were determined following transient
middle cerebral artery occlusion (MCAO) in ApoE KO mice fed the
high-fat diet (HFD) for 10 weeks using combinatorial treatment of
probucol plus cilostazol. Microglia and astrocyte activation in
accordance with chemokine levels were determined to be the action
mechanisms. The current study presented evidence with regard to the
anti-inflammatory effects of combinatorial therapy with probucol
and cilostazol during the management of stroke patients undergoing
hypercholesterolemia.
Materials and methods
General surgical preparation
Male ApoE KO mice (Japan SLC, Shizuoka, Japan) with
a C57BL/6J genetic background were housed under diurnal lighting
conditions and allowed food and tap water ad libitum. The
study was carried out in strict accordance with the recommendations
in the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health. In addition, the protocol was
approved by the Pusan National University Institutional Animal Care
and Use Committee (permit no.: PNU-2011-000419). Four-week-old ApoE
KO mice were fed a Western-type HFD (42% of total calories from
fat, 0.15% cholesterol; Research Diets, Inc., New Brunswick, NJ,
USA) containing 0.3% (wt/wt) probucol, 0.2% (wt/wt) cilostazol, or
0.3% (wt/wt) probucol and 0.2% (wt/wt) cilostazol for 10 weeks.
Anesthesia was achieved by isoflurane (2% induction and 1.5%
maintenance, in 80% N2O and 20% O2)
administered via a face mask. The femoral artery was catheterized
for measurement of the mean arterial blood pressure using a model
MLT844 physiological pressure transducer (AD Instruments, Medford,
MA, USA). The data were continuously recorded using a Power Lab
data acquisition and analysis system (AD Instruments) and stored in
a computer. The depth of anesthesia was checked by the absence of
cardiovascular changes in response to tail pinch. Rectal
temperature was kept at 36.5–37.5°C using a PanlabTM
thermostatically controlled heating mat (Harvard Apparatus,
Holliston, MA, USA). Arterial blood gases and pH were measured
prior to ischemia using i-Stat System (Abbott, Abbott Park, IL,
USA).
Focal cerebral ischemia
A fiber-optic probe was affixed to the skull over
the MCA for measurement of the regional CBF (rCBF) by a PeriFlux
Laser Doppler System 5000 (Perimed, Stockholm, Sweden). Baseline
values were measured prior to internal carotid artery ligation
(considered to be 100% flow). Focal cerebral ischemia was induced
by occluding the MCA using a previously described intraluminal
filament technique (14). MCAO
was induced by a silicon-coated 7-0 monofilament in the internal
carotid artery and the monofilament was advanced to occlude the
MCA. In all the animals, rCBF was measured to confirm the
achievement of consistent and similar levels of ischemic induction.
The filament was withdrawn 40 min after occlusion and reperfusion
was confirmed using laser Doppler. The surgical wound was sutured
and mice were allowed to recover from anesthesia. The brains were
removed at 48 h after MCAO. Cerebral infarct size was determined on
2,3,5-triphenyltetrazolium chloride (TTC)-stained, 2-mm-thick brain
sections (n=10–11). Infarction areas were quantified with iSolution
full image analysis software (Image & Microscope Technology,
Vancouver, BC, Canada). To account for and eliminate the effects of
swelling/edema, infarction volume was calculated by indirect
measurement by summing the volumes of each section according to the
formula: contralateral hemisphere (mm3) - undamaged
ipsilateral hemisphere (mm3) (15).
Neurological score
Neurological deficit was scored in each mouse at 24
and 48 h after ischemic insult in a blinded manner according to the
following graded scoring system (n=12–14): 0, no deficit; 1,
forelimb weakness and torso turning to the ipsilateral side when
held by tail; 2, circling to the affected side; 3, unable to bear
weight on the affected side; and 4, no spontaneous locomotor
activity or barrel rolling (16).
Wire-grip test
Vestibule-motor function was assessed using a
wire-grip test 24 and 48 h after cerebral ischemia (17) (n=12–14). Briefly, mice were placed
on a metal wire (45 cm long) suspended 45 cm above protective
padding and allowed to traverse the wire for 60 sec. The latency
for which a mouse remained on the wire within a 60-sec interval was
measured, and the wire grip score was quantified using the
following 5-point scale: unable to remain on the wire for 30 sec,
0; failure to hold on to the wire with both sets of fore paws and
hind paws together, 1; holding on to the wire with both forepaws
and hind paws but not the tail, 2; holding on to the wire using the
tail along with both forepaws and both hind paws, 3; moving along
the wire on all four paws plus tail, 4; a score of 4 points in
addition to the mouse ambulating down one of the posts used to
support the wire, 5. Tests were administered in triplicate and the
average value was calculated for each mouse on each test day.
Cylinder test
The cylinder test was modified for mice in order to
determine forelimb use and rotation asymmetry (18) (n=12–14). Briefly, the mouse was
placed in a transparent cylinder (diameter, 9-cm and height, 15
cm). After the mouse was placed into the cylinder, forelimb use of
the first contact against the wall following rearing and during
lateral exploration was recorded. The final score was calculated
as: (non-impaired forelimb movement - impaired forelimb
movement)/(non-impaired forelimb movement + impaired forelimb
movement + both movement). This test evaluates forelimb use
asymmetry for weight shifting during vertical exploration and
provides high inter-rater reliability, even when inexperienced
raters are included.
Isolation of total RNA and RT-PCR
Total RNA was isolated from the ischemic area in the
brain using TRIzol (Invitrogen Life Technologies, Carlsbad, CA,
USA) according to the manufacturer’s insructions (n=5). The total
RNA was reverse-transcribed for 1 h at 42°C with Moloney Murine
Leukemia Virus reverse transcriptase (Promega, Madison, WI, USA) to
produce cDNA. RT-generated cDNA encoding the MCP-1,
VCAM, iNOS, COX-2, TNF-α, IL-1β
and GAPDH genes was amplified by PCR using the primers:
MCP-1 (forward 5′-TGGCTCAGCCAGA TGCAGTTAA-3′ and reverse
5′-CTAGTTCACTGTCACAC TGGTC-3′), VCAM (forward 5′-GAACCTGACCTGCTCAA
GTGAT-3′ and reverse 5′-TACCAAGGAAGATGCGCAG TAG-3′), iNOS (forward
5′-CACTTGGATCAGGAACCTG AAG-3′ and reverse
5′-CCAGCTTCTTCAATGTGGTAGC-3′), COX-2 (forward
5′-CTTGGTCTACAAGACGCCACAT-3′ and reverse
5′-GCCATAGAATAATCCTGGTCGG-3′), TNF-α (forward
5′-CATCTTCTCAAAATTCGAGTGACAA-3′ and reverse
5′-TGGGAGTAGACAAGGTACACCCC-3′), IL-1β (forward
5′-AAGGGCTGCTTCCAAACCTTTGAC-3′ and reverse
5′-TGCCTGAAGCTCTTGTTGATGTGC-3′) and mGAPDH (forward
5′-ATGACCACAGTCCATGCCATCA-3′ and reverse
5′-TTACTCCTTGGAGGCCATGTAG-3′). Products were size-separated by
electrophoresis on 2% agarose gels and visualized after staining
with ethidium bromide. Thermal cycling conditions consisted of 94°C
for 5 min and 30 cycles of 94°C for 30 sec, 55°C for 30 sec and
72°C for 30 sec.
Immunohistochemistry
Forty-eight hours after MCAO, the mice were deeply
anesthetized with thiopental sodium and subsequently perfused
transcardially with cold phosphate buffered saline (PBS), after
which they were perfused for fixing using 4% paraformaldehyde
(n=3). The brain of each mouse was then removed and fixed for 48 h
in 4% paraformaldehyde at 4°C. Subsequently, it was subjected to
cryoprotection in 30% sucrose for 24 h at 4°C. The isolated brains
were then frozen and stored at −80°C until further processing. The
frozen brains were cut into sections (14 μm) using a model CM 3050
cryostat (Leica Microsystems, Wetzlar, Germany). The sections were
immunostained with antibodies against MCP-1 (Abcam, Cambridge, UK),
CD11b (Serotec, Oxford, UK) and GFAP (Dako, Glostrup, Denmark).
After additional incubation with biotinylated secondary antibody,
the samples were incubated in ABC reagent (Vector Laboratories,
Burlingame, CA, USA). Reactions were visualized by development in
3,3′-diaminobenzidine substrates (Vector Laboratories). The samples
were then visualized using a light microscope (Carl Zeiss, Jena,
Germany).
Drugs
Probucol
[4,4′-(isopropylidenedithio)bis(2,6-di-t-buty- lophenol)] and
cilostazol [OPC-13013, 6-[4-(1-cycl ohexyl-1H -tetrazol-5-yl)
butoxy]-3,4-dihydro-2-(1H)-quinolinone] were donated by Otsuka
Pharmaceutical (Tokushima, Japan) and were added to the HFD.
Data analysis
Data are expressed as the means ± standard error of
the mean (SEM). The control vs. vehicle group and vehicle vs.
probucol-treated group were compared by the unpaired t-test, while
vehicle vs. each concentration of probucol was compared by one-way
ANOVA followed by Dunnett’s test. Differences were considered
statistically significant, when the two-tailed P-values were
<0.05. Statistical analyses were performed using the SAS
software (SAS Institute Japan, R9.1).
Results
Physiological parameters
The body weights of ApoE KO mice fed a HFD for 10
weeks were higher than those of mice fed a normal diet (control)
(32.71±1.05 vs. 25.90±0.73 g, respectively, P<0.01; n=15–16).
Treatment with 0.3% probucol and 0.2% cilostazol did not affect the
body weight or blood pressure of HFD-fed mice (Table I). After 10 weeks of HFD, large
increases in total cholesterol and LDL-cholesterol plasma were
observed in ApoE KO mice (P<0.01 vs. control) (Table I). Probucol alone and probucol
plus cilostazol in combination led to a significant decrease in the
total- and LDL-cholesterol levels in ApoE KO mice fed the HFD
(P<0.01 vs. vehicle).
 | Table IPhysiological parameters. |
Table I
Physiological parameters.
| Variables | Control (n=15) | Vehicle (n=16) | Probucol
(n=15) | Cilostazol
(n=15) | Probucol +
cilostazol (n=16) |
|---|
| Body weight | 25.90±0.73 | 32.71±1.05b | 33.77±0.51 | 33.99±1.25 | 33.09±0.63 |
| MABP | 82.48±2.36 | 89.38±2.13a | 85.17±3.09 | 87.34±1.77 | 85.34±2.12 |
| pH | 7.34±0.01 | 7.33±0.01 | 7.32±0.02 | 7.32±0.01 | 7.32±0.01 |
| pO2 | 111.13±4.11 | 105.75±3.56 | 107.33±3.68 | 111.20±3.34 | 112.94±3.82 |
|
pCO2 | 41.56±1.62 | 38.76±1.21 | 41.05±1.68 | 33.38±1.40 | 39.67±0.20 |
| Total
cholesterol | 261.67±5.24 |
546.33±33.05b |
245.00±14.47c | 531.67±16.29 |
242.00±13.05c |
|
LDL-cholesterol | 211.67±3.53 |
420.67±28.72b |
185.67±18.77c | 409.33±20.85 | 179.00±5.57c |
Effect of probucol plus cilostazol in
combination on tissue and functional outcome in focal cerebral
ischemia
To determine whether probucol plus cilostazol
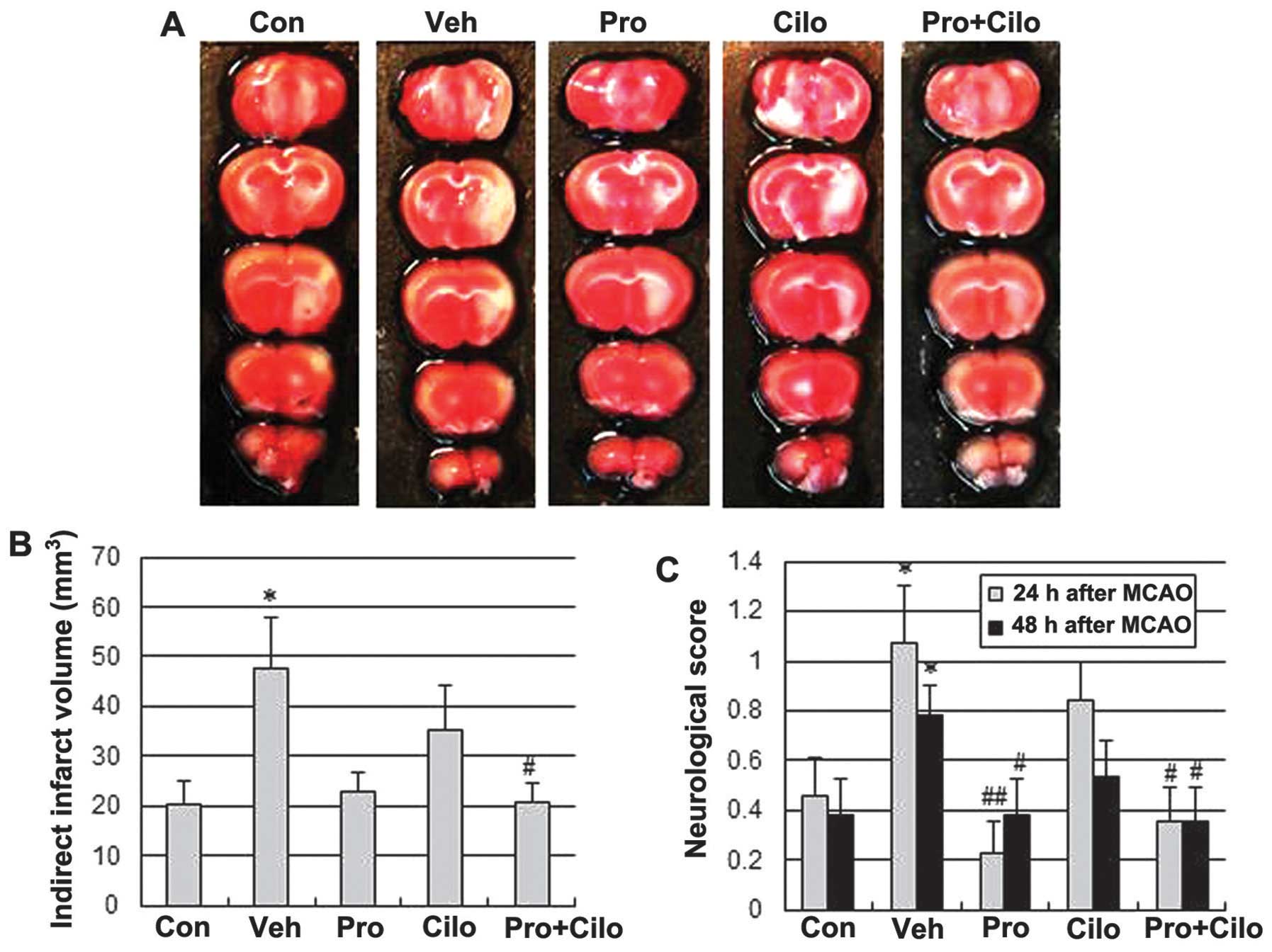
improved the tissue outcome after cerebral ischemia in
hypercholesterolemic mice, the infarct size was measured 48 h after
a 40-min transient MCAO. MCAO resulted in 137% larger infarct
volumes in ApoE KO fed the HFD for 10 weeks when compared to those
fed the regular diet (47.66±10.15 mm3 vs. 20.13±4.76
mm3; P<0.05) (Fig. 1A
and B); however, this increase was significantly reduced by
treatment with probucol plus cilostazol (20.90±3.71 mm3;
P<0.05 vs. vehicle group). The group that received probucol
alone also tended to have a smaller infarct volume (22.79±3.93
mm3; P=0.055 vs. vehicle group). To assess the effects
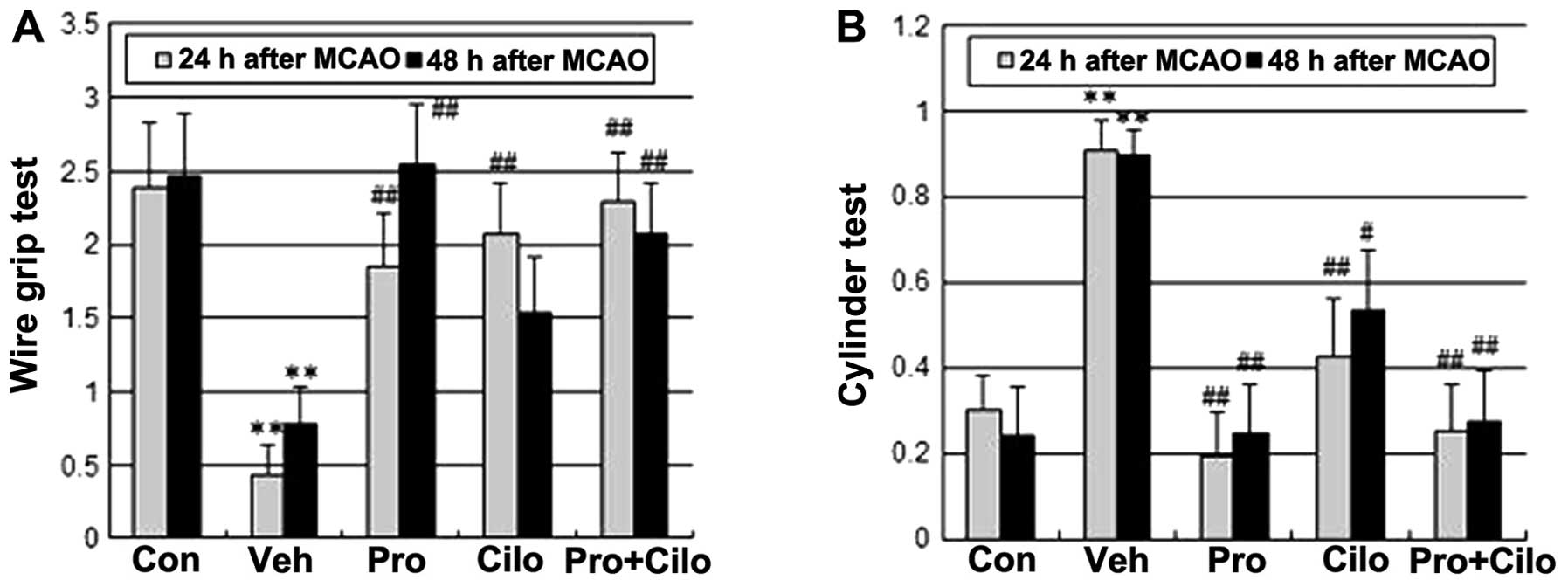
of probucol plus cilostazol on functional recovery after cerebral
ischemia in hypercholesterolemic mice, the neurological score,
wire-grip test and cylinder test were assessed 24 and 48 h after a
40-min transient MCA occlusion. Consistent with a smaller infarct
size, probucol alone and combined treatment with cilostazol led to
prominent improvement of neurological and motor function at both 24
and 48 h after ischemic injury (Figs.
1C and 2).
Effect of probucol plus cilostazol in
combination on inflammatory mediator mRNA levels in the ischemic
brain
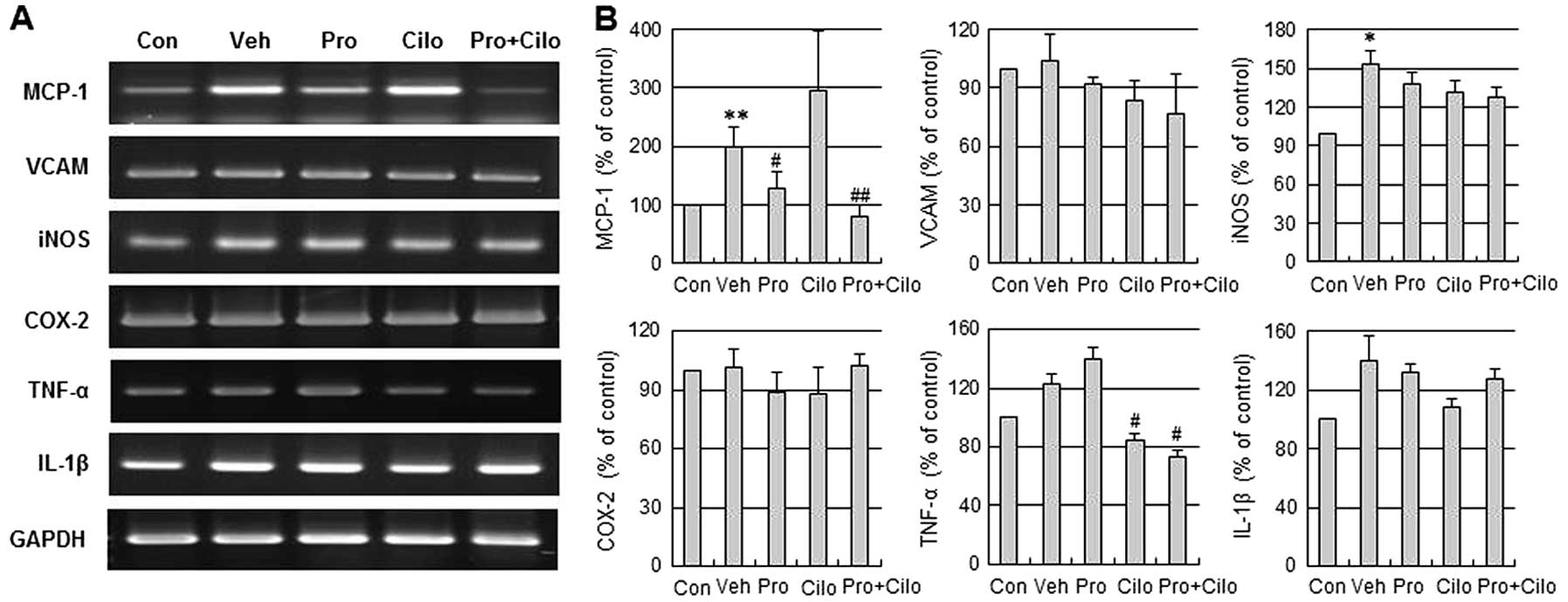
To examine the anti-neuroinflammatory effects of
probucol plus cilostazol on focal cerebral ischemia with
hypercholesterolemia, we assessed the mRNA levels of MCP-1, VCAM,
iNOS, COX-2, TNF-α and IL-1β in the ischemic brain. MCP-1, iNOS,
TNF-α and IL-1β mRNA levels were increased in the ischemic brain of
hypercholesterolemic mice, but VCAM and COX-2 mRNA levels were not
increased. Moreover, this increase of MCP-1 and TNF-α mRNA was
significantly decreased by probucol plus cilostazol in combination
compared with the vehicle group (Fig.
3).
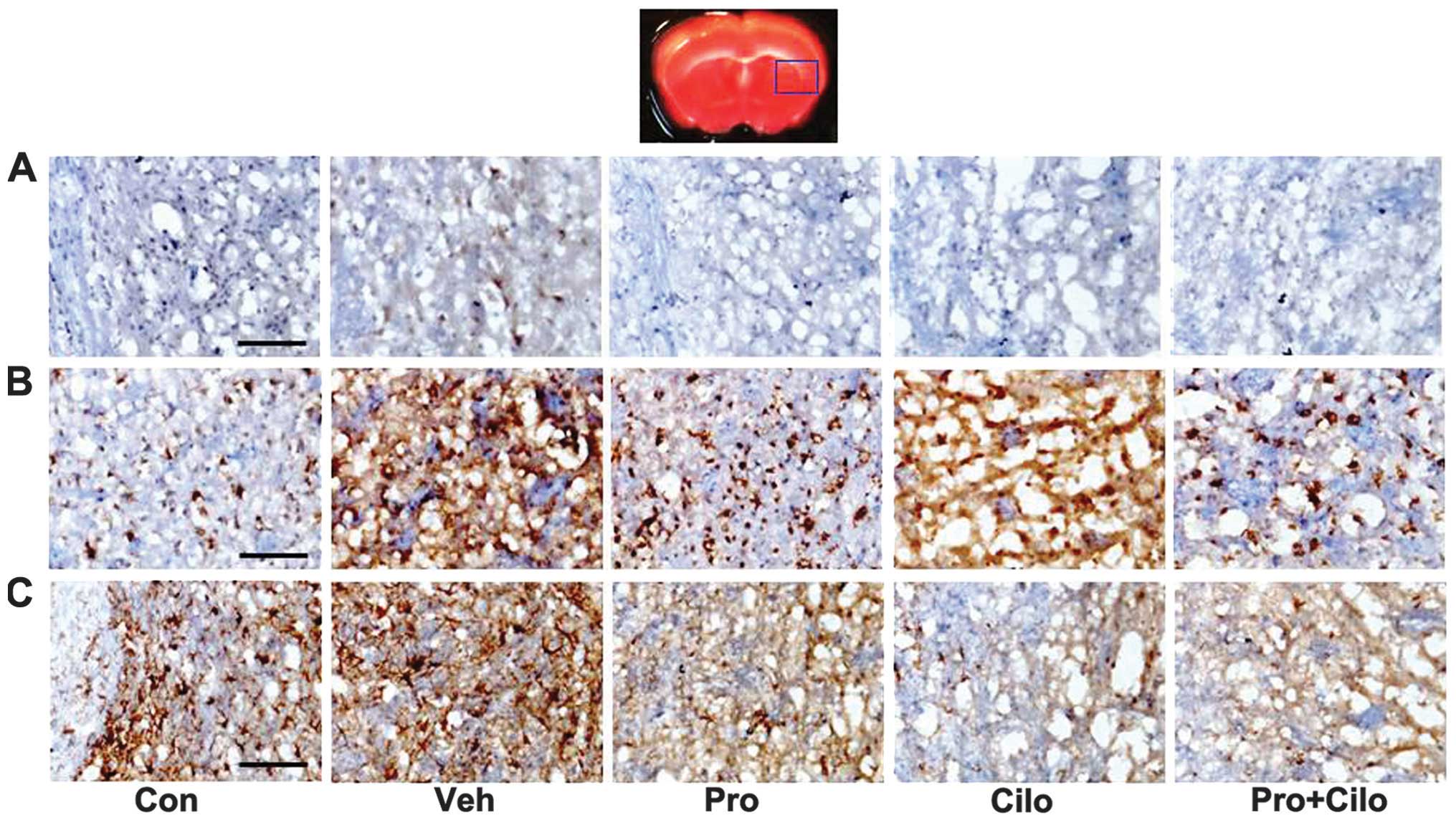
Effect of probucol plus cilostazol in
combination on MCP-1, CD11b and GFAP expression in the ischemic
cortex
MCP-1 expression in the ischemic brain was assessed
by immunohistochemistry. MCP-1 expression increased in the ischemic
brain of hypercholesterolemic mice, which was decreased by probucol
alone, cilostazol alone or probucol plus cilostazol (Fig. 4A). CD11b (also known as
αmβ2 integrin and Mac1) and GFAP are
sensitive markers of microglial and astrocyte activation,
respectively. CD11b immunoreactivity was increased in the vehicle
group, but this increase was attenuated by probucol alone and
probucol plus cilostazol (Fig.
4B). Immunostaining of GFAP was evident in the ischemic brain
of the control and hypercholesterolemic mice and mild GFAP
immunostaining appeared in mice that received probucol alone,
cilostazol alone and probucol plus cilostazol (Fig. 4C).
Discussion
The present study was conducted to elucidate the
protective effects of probucol plus cilostazol in combination
against cerebral ischemic injury with hypercholesterolemia and the
mechanism by which these effects occur. MCAO (40 min) and
reperfusion (48 h) resulted in significantly larger infarct volumes
in ApoE KO mice fed HFD for 10 weeks when compared to ApoE KO mice
fed a regular diet, although these increased volumes were
significantly reduced in the probucol plus cilostazol group.
Consistent with the smaller infarct size, probucol alone and
combined treatment with cilostazol greatly improved the
neurological and motor function. In addition, probucol alone and
probucol and cilostazol in combination decreased MCP-1 mRNA
expression, as well as MCP-1, CD11b and GFAP immunoreactivity in
the ischemic cortex. These findings suggest that the inhibitory
effect of probucol and cilostazol in combination through an
inflammatory mediator, MCP-1 expression, in the ischemic brain with
hypercholesterolemia allowed the identification of one of the
mechanisms responsible for its anti-inflammatory action.
The lack of successful translation from the
laboratory to clinical settings may be the result of prevailing
clinical conditions, such as hypercholesterolemia, hypertension and
insulin intolerance, which are correlated with a higher incidence
of stroke (2,19,20), which are not included in animal
models used in the study of stroke. Animal stroke studies conducted
using mostly young animals without risk factors (21) do not accuratley reflect the
pathophysiological conditions in humans. The ApoE KO mouse model is
the classical model of atherosclerosis. These mice start to develop
severe hypercholesterolemia, induce inflammation and
atherosclerotic lesions in the aorta and pulmonary, coronary and
carotid arteries at the age of 8 or 10 weeks, depending on the type
of diet administered (22). By
coupling hypercholesterolemia in an experimental mouse stroke
model, the present study investigated whether hypercholesterolemia
exacerbates ischemic outcomes and any exacerbation of stroke was
connected with inflammation in the brain. In addition, we addressed
whether probucol and cilostazol administered in combination
attenuate hypercholesterolemia-induced exacerbation in ischemic
brain injury via anti-inflammatory effects.
Hypercholesterolemia is a risk factor for ischemic
stroke. In large clinical trials or patient registries, ~45–60% of
patients exhibit elevated serum cholesterol levels (23,24). It is well known that cholesterol
induces inflammation in the brain (25–27), and that oxidized metabolites of
cholesterol may be involved in the upregulation of inflammatory
markers (28,29). There is also considerable evidence
that hypercholesterolemia and derivations of cholesterol contribute
to a breakdown of the blood brain barrier (30,31), thereby making hypercholesterolemic
patients more susceptible to stroke. Consistent with the
aforementioned studies, our results showed that ischemic cerebral
infarcts induced by 40 min MCAO and 48 h reperfusion resulted in
significantly larger infarct volumes, neurological deficits and
motor deficits in ApoE KO mice fed HFD for 10 weeks when compared
to ApoE KO mice fed a regular diet. These results are similar to
those of a previous study (11).
Moreover, MCP-1 mRNA levels and MCP-1, CD11b and GFAP
immunoreactivity, which are thought to be involved in
neuroinflammation, increased in the ischemic brain of
hypercholesterolemic mice.
Obesity is also a well-established major risk factor
for stroke (32). Clinical
studies suggest that the prevalence of ischemic stroke has markedly
increased in children and young adults, which positively correlated
with an increase in risk factors including obesity, lipid disorders
and diabetes (33). In addition,
experimental studies in genetic or diet-induced obesity models have
shown increased cerebral infarct size and poor outcomes of stroke
(34,35). In the present study, we found that
the body weights of ApoE KO mice fed a HFD for 10 weeks were
significantly higher than those of mice fed a regular diet.
However, although probucol alone and probucol plus cilostazol in
combination did not affect the body weight, they improved tissue
and functional outcome after ischemic brain injury with
hypercholesterolemia. On the other hand, probucol alone and
probucol plus cilostazol in combination led to a significant
decrease in total- and LDL-cholesterol levels in ApoE KO mice fed
the HFD, suggesting that the beneficial effects of probucol and
cilostazol in stroke with hypercholesterolemia may be due, not to
lowering body weight, but only in part to lipid-lowering properties
of the drugs.
Inflammation in the brain caused by activated
microglia is a prominent pathological feature associated with
hypercholesterolemia or ischemic stroke. Under pathological
conditions, activated microglia release a variety of neurotoxic
compounds and proinflammatory mediators, which are thought to be
responsible for the pathological conditions. MCP-1 is a
particularly important chemokine that is primarily responsible for
the initiation and progression of inflammatory responses by
promoting the migration and recruitment of inflammatory cells
(36). MCP-1 has been found to
regulate the migration of activated microglia cells to sites of
inflammation in the CNS (37).
Overexpression of MCP-1 exacerbates ischemic injury and enhances
recruitment of inflammatory cells to the sites of injury (38), whereas absence of the mcp-1
gene has been shown to reduce ischemia-reperfusion injury (39). We found increased MCP-1, CD11b and
GFAP immunoreactivity in hypercholesterolemic ischemic brains,
suggesting that MCP-1 production and microglia and astrocyte
activation are involved in the promotion of hyperlipidemia-induced
inflammation and injury in the ischemic brain. Profound shifts to
the reduced MCP-1 and microglia and astrocyte activation in
probucol plus cilostazol-treated mice suggest that probucol and
cilostazol in combination exert neuroprotective effects via
anti-inflammation in hypercholesterolemic ischemic brains.
Probucol, a lipid-lowering agent with antioxidant
properties, is involved in the protection against atherogenesis
(40). Probucol is capable of
inhibiting the expression of oxidation-sensitive inflammatory
factors, such as VCAM-1 (41),
MCP-1 (42) and IL-1 (43), and attenuating inflammation and
increasing stability of vulnerable atherosclerotic plaques in
rabbits (44). Serum
concentrations of inflammatory cytokines and matrix
metalloproteinases, and expression levels of the Toll-like receptor
(TLR)-2, TLR-4, MCP-1, ICAM-1, scavenger receptor A, CD36 and
oxidized LDL receptor 1 within the lesions were markedly decreased
in probucol treatment groups as compared to the control group
(44). Cilostazol, another drug
in the combination treatment in this study, is an inhibitor of type
3 phosphodiesterase that exerts antiplatelet activity through the
suppression of cAMP degradation (6) and also has pleiotropic effects
against inflammation on vascular function and atherosclerosis
(45). Furthermore, cilostazol
has demonstrated in vivo neuroprotective effects against
cerebral ischemic injury via anti-inflammatory effects (7). Therefore, the combination of
probucol and cilostazol may have a synergistic effect, as the two
agents inhibit inflammation. A previous in vitro study of
cultured human coronary artery endothelial cells (8), in vivo studies in rats, LDL
receptor-deficient mice and ApoE KO mice revealed a synergistic
effect of probucol plus cilostazol against atherosclerotic lesions
and ischemic brain injury (9–11).
Park et al showed that probucol and cilostazol combination
therapy inhibited the expression of VCAM-1 and MCP-1 in cultured
human coronary artery endothelial cells with MCP-1 being more
effectively inhibited than when treated with probucol or cilostazol
monotreatment (8).
Recently, authors of this study demonstrated a
cerebrovascular protective effect of probucol plus cilostazol in
combination in focal ischemic mice with hypercholesterolemia that
occurred via the upregulation of endothelial nitric oxide synthase
and adiponectin in acute ischemic injury (11). In the present study, we focused on
the inflammatory properties of probucol and cilostazol administered
in combination with conventional therapy in the subacute phase
after cerebral ischemia. In addition, we reduced the probucol dose
from 0.5 to 0.3% to observe the synergistic effects of probucol and
cilostazol against cerebral ischemic brain injury with
hypercholesterolemia in this study. Treatment with 0.3% probucol
plus 0.2% cilostazol significantly reduced the infarct volume with,
not only neurological deficits, but also motor deficits in ApoE KO
mice fed a HFD. Furthermore, probucol and cilostazol in combination
reduced hyperlipidemia-induced inflammation via the inhibition of
MCP-1 and microglia and astrocyte activation. However, 0.3%
probucol is likely too high a dose for the synergistic effects of
probucol and cilostazol to become evident. Accordingly, more
studies are required to determine the clinically relevant probucol
dose for the synergistic effect of probucol and cilostazol in
ischemic brain injury with hypercholesterolemia. Collectively, the
beneficial effects of probucol and cilostazol in stroke with
hypercholesterolemia may be due only in part to lipid-lowering
properties, with the primary benefit derived from improved
endothelial function and the anti-inflammatory actions of the
drugs.
In summary, the present study has demonstrated that
probucol and cilostazol in combination exerted inhibitory effects
against the expression of the inflammatory chemokine, MCP-1, in
ischemic brains with hypercholesterolemia, which allowed
identification of one of the mechanisms responsible for its
anti-inflammatory action. These findings suggest that probucol plus
cilostazol is a potential therapeutic strategy for reducing the
impact of stroke in hypercholesterolemic subjects.
Acknowledgements
This study was supported by Basic Science Research
Program through the National Research Foundation of Korea (NRF)
funded by the Ministry of Science, ICT and Future Planning
(NRF-2013R1A2A2A03067417) and Otsuka Pharmaceutical.
References
|
1
|
Amarenco P: Hypercholesterolemia,
lipid-lowering agents, and the risk for brain infarction.
Neurology. 57:S35–S44. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Engstrom G, Lind P, Hedblad B, Stavenow L,
Janzon L and Lindgarde F: Effects of cholesterol and
inflammation-sensitive plasma proteins on incidence of myocardial
infarction and stroke in men. Circulation. 105:2632–2637. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Macrez R, Ali C, Toutirais O, et al:
Stroke and the immune system: from pathophysiology to new
therapeutic strategies. Lancet Neurol. 10:471–480. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dirnagl U, Iadecola C and Moskowitz MA:
Pathobiology of ischaemic stroke: an integrated view. Trends
Neurosci. 22:391–397. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamashita S and Matsuzawa Y: Where are we
with probucol: a new life for an old drug? Atherosclerosis.
207:16–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weintraub WS: The vascular effects of
cilostazol. Can J Cardiol. 22(Suppl B): 56B–60B. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee JH, Park SY, Shin YW, et al:
Neuroprotection by cilostazol, a phosphodiesterase type 3
inhibitor, against apoptotic white matter changes in rat after
chronic cerebral hypoperfusion. Brain Res. 1082:182–191. 2006.
View Article : Google Scholar
|
|
8
|
Park SY, Lee JH, Shin HK, et al:
Synergistic efficacy of concurrent treatment with cilostazol and
probucol on the suppression of reactive oxygen species and
inflammatory markers in cultured human coronary artery endothelial
cells. Korean J Physiol Pharmacol. 12:165–170. 2008. View Article : Google Scholar
|
|
9
|
Park SY, Lee JH, Kim CD, Rhim BY, Hong KW
and Lee WS: Beneficial synergistic effects of concurrent treatment
with cilostazol and probucol against focal cerebral ischemic injury
in rats. Brain Res. 1157:112–120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoshikawa T, Mitani K, Kotosai K, Nozako
M, Miyakoda G and Yabuuchi Y: Antiatherogenic effects of cilostazol
and probucol alone, and in combination in low density lipoprotein
receptor-deficient mice fed with a high fat diet. Horm Metab Res.
40:473–478. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim JH, Park SH, Bae SS, et al:
Combinatorial effect of probucol and cilostazol in focal ischemic
mice with hypercholesterolemia. J Pharmacol Exp Ther. 338:451–457.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zisman DA, Kunkel SL, Strieter RM, et al:
MCP-1 protects mice in lethal endotoxemia. J Clin Invest.
99:2832–2836. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rankine EL, Hughes PM, Botham MS, Perry VH
and Felton LM: Brain cytokine synthesis induced by an
intraparenchymal injection of LPS is reduced in MCP-1-deficient
mice prior to leucocyte recruitment. Eur J Neurosci. 24:77–86.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang Z, Huang PL, Panahian N, Dalkara T,
Fishman MC and Moskowitz MA: Effects of cerebral ischemia in mice
deficient in neuronal nitric oxide synthase. Science.
265:1883–1885. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin TN, He YY, Wu G, Khan M and Hsu CY:
Effect of brain edema on infarct volume in a focal cerebral
ischemia model in rats. Stroke. 24:117–121. 1993.PubMed/NCBI
|
|
16
|
Li X, Blizzard KK, Zeng Z, DeVries AC,
Hurn PD and McCullough LD: Chronic behavioral testing after focal
ischemia in the mouse: functional recovery and the effects of
gender. Exp Neurol. 187:94–104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen T, Liu W, Chao X, et al: Salvianolic
acid B attenuates brain damage and inflammation after traumatic
brain injury in mice. Brain Res Bull. 84:163–168. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hua Y, Schallert T, Keep RF, Wu J, Hoff JT
and Xi G: Behavioral tests after intracerebral hemorrhage in the
rat. Stroke. 33:2478–2484. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Strandgaard S: Hypertension and stroke. J
Hypertens Suppl. 14:S23–S27. 1996. View Article : Google Scholar
|
|
20
|
Kernan WN and Inzucchi SE: Type 2 diabetes
mellitus and insulin resistance: stroke prevention and management.
Curr Treat Options Neurol. 6:443–450. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fisher M, Feuerstein G, Howells DW, et al:
Update of the stroke therapy academic industry roundtable
preclinical recommendations. Stroke. 40:2244–2250. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakashima Y, Plump AS, Raines EW, Breslow
JL and Ross R: ApoE-deficient mice develop lesions of all phases of
atherosclerosis throughout the arterial tree. Arterioscler Thromb.
14:133–140. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sacco RL, Diener HC, Yusuf S, et al:
Aspirin and extended-release dipyridamole versus clopidogrel for
recurrent stroke. N Engl J Med. 359:1238–1251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rother J, Alberts MJ, Touze E, et al: Risk
factor profile and management of cerebrovascular patients in the
REACH Registry. Cerebrovasc Dis. 25:366–374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rahman SM, Van Dam AM, Schultzberg M and
Crisby M: High cholesterol diet results in increased expression of
interleukin-6 and caspase-1 in the brain of apolipoprotein E
knockout and wild type mice. J Neuroimmunol. 169:59–67. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thirumangalakudi L, Prakasam A, Zhang R,
et al: High cholesterol-induced neuroinflammation and amyloid
precursor protein processing correlate with loss of working memory
in mice. J Neurochem. 106:475–485. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xue QS, Sparks DL and Streit WJ:
Microglial activation in the hippocampus of hypercholesterolemic
rabbits occurs independent of increased amyloid production. J
Neuroinflammation. 4:202007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Morello F, Saglio E, Noghero A, et al:
LXR-activating oxysterols induce the expression of inflammatory
markers in endothelial cells through LXR-independent mechanisms.
Atherosclerosis. 207:38–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sottero B, Gamba P, Gargiulo S,
Leonarduzzi G and Poli G: Cholesterol oxidation products and
disease: an emerging topic of interest in medicinal chemistry. Curr
Med Chem. 16:685–705. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jeitner TM, Voloshyna I and Reiss AB:
Oxysterol derivatives of cholesterol in neurodegenerative
disorders. Curr Med Chem. 18:1515–1525. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kalayci R, Kaya M, Uzun H, et al:
Influence of hypercholesterolemia and hypertension on the integrity
of the blood-brain barrier in rats. Int J Neurosci. 119:1881–1904.
2009. View Article : Google Scholar
|
|
32
|
Campos P, Saguy A, Ernsberger P, Oliver E
and Gaesser G: The epidemiology of overweight and obesity: public
health crisis or moral panic? Int J Epidemiol. 35:55–60. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
George MG, Tong X, Kuklina EV and Labarthe
DR: Trends in stroke hospitalizations and associated risk factors
among children and young adults, 1995–2008. Ann Neurol. 70:713–721.
2011.PubMed/NCBI
|
|
34
|
Deutsch C, Portik-Dobos V, Smith AD, Ergul
A and Dorrance AM: Diet-induced obesity causes cerebral vessel
remodeling and increases the damage caused by ischemic stroke.
Microvasc Res. 78:100–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Osmond JM, Mintz JD, Dalton B and Stepp
DW: Obesity increases blood pressure, cerebral vascular remodeling,
and severity of stroke in the Zucker rat. Hypertension. 53:381–386.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang D, Han Y, Rani MR, et al: Chemokines
and chemokine receptors in inflammation of the nervous system:
manifold roles and exquisite regulation. Immunol Rev. 177:52–67.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
McManus CM, Liu JS, Hahn MT, et al:
Differential induction of chemokines in human microglia by type I
and II interferons. Glia. 29:273–280. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Y, Hallenbeck JM, Ruetzler C, et al:
Overexpression of monocyte chemoattractant protein 1 in the brain
exacerbates ischemic brain injury and is associated with
recruitment of inflammatory cells. J Cereb Blood Flow Metab.
23:748–755. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hughes PM, Allegrini PR, Rudin M, Perry
VH, Mir AK and Wiessner C: Monocyte chemoattractant protein-1
deficiency is protective in a murine stroke model. J Cereb Blood
Flow Metab. 22:308–317. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kuzuya M and Kuzuya F: Probucol as an
antioxidant and antiatherogenic drug. Free Radic Biol Med.
14:67–77. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu BJ, Di Girolamo N, Beck K, et al:
Probucol
[4,4′-[(1-methylethylidene)bis(thio)]bis-[2,6-bis(1,1-dimethylethyl)phenol]]
inhibits compensatory remodeling and promotes lumen loss associated
with atherosclerosis in apolipoprotein E-deficient mice. J
Pharmacol Exp Ther. 321:477–484. 2007.
|
|
42
|
Chang MY, Sasahara M, Chait A, Raines EW
and Ross R: Inhibition of hypercholesterolemia-induced
atherosclerosis in the nonhuman primate by probucol. II. Cellular
composition and proliferation. Arterioscler Thromb Vasc Biol.
15:1631–1640. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ku G, Doherty NS, Schmidt LF, Jackson RL
and Dinerstein RJ: Ex vivo lipopolysaccharide-induced interleukin-1
secretion from murine peritoneal macrophages inhibited by probucol,
a hypocholesterolemic agent with antioxidant properties. FASEB J.
4:1645–1653. 1990.
|
|
44
|
Li T, Chen W, An F, et al: Probucol
attenuates inflammation and increases stability of vulnerable
atherosclerotic plaques in rabbits. Tohoku J Exp Med. 225:23–34.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee JH, Oh GT, Park SY, et al: Cilostazol
reduces atherosclerosis by inhibition of superoxide and tumor
necrosis factor-alpha formation in low-density lipoprotein
receptor-null mice fed high cholesterol. J Pharmacol Exp Ther.
313:502–509. 2005.PubMed/NCBI
|