Introduction
MicroRNAs (miRNAs or miRs) are short non-coding RNAs
that regulate the target mRNA by binding mostly to the 3′
untranslated region (3′ UTR), inducing either translational
repression or the degradation of the target (1–3).
The aberrant expression of miRNAs has been reported in multiple
human cancer types and miRNAs are known to play an oncogenic or
tumor suppressor role. They are also known to play key roles in
cell survival, proliferation, apoptosis, migration, invasion, as
well as in other processes that are associated with human cancers
(4,5). More than 50% of the known miRNAs
have been shown to participate in human tumorigenesis and/or
metastasis by directly targeting oncogenes or tumor suppressor
genes (6,7). miR-96 is markedly downregulated in
pancreatic cancer compared to normal tissue and it suppresses KRAS
and functions as a tumor suppressor gene (8). However, the mechanisms of action of
miR-96 as a tumor suppressor in pancreatic cancer have not yet been
fully elucidated.
Novel (nua) kinase family 1 (NUAK1), also known as
KIAA0537/ARK5, and is identified as the fifth member of the
adenosine monophosphate (AMP)-activated protein kinase
(AMPK)-related kinase (ARK) family (9). Akt phosphorylates NUAK1 at Ser600, a
C-terminal site outside the catalytic domain, which leads to the
activation of this 74-kDa kinase. During glucose deprivation or
response to adenosine monophosphate, NUAK1 supports the survival of
cells in an Akt dependent manner (9). NUAK1 suppresses cell death induced
by nutrient starvation and the activation of death receptors
through the inhibition of caspase-8, as well as through the
negative regulation of pro-caspase-6 (10,11). NUAK1 is strongly associated with
tumor invasion and metastasis, and is a factor associated with
tumor survival and progression (12–14). Recently, it has been reported that
a high NUAK1 expression correlates with a poor prognosis and plays
an important role in human non-small cell lung cancer (NSCLC) cell
migration and invasion (15). The
inhibition of miR-211 has been shown to increase NUAK1 expression
and decreases melanoma cell adhesion, whereas the upregulation of
miR-211 restores cell adhesion through the suppression of NUAK1
expression (16). NUAK1 has been
shown to promote glioma cell invasion, and its elevated expression
correlates with a poor clinical outcome (17). NUAK1 has also been shown to be
associated with a more invasive phenotype and metastatic potential
in human breast cancer dependent on Akt (18). In addition, the overexpression of
NUAK1 is associated with a poor prognosis in hepatocellular
carcinoma (19). NUAK1 has been
shown to stimulate the invasion, metastasis, tumorigenesis and to
suppress the necrosis of PANC-1 pancreatic cancer cells (14).
In this study, firstly, we demonstrate that NUAK1
expression is specifically upregulated in pancreatic cancer and
that it promotes the proliferation, migration and invasion of MIA
PaCa-2 pancreatic cancer cells. Secondly, we performed an analysis
of potential miRNA target sites using three commonly used
prediction algorithms: miRanda, TargetScan and PicTar. All three
algorithms predicted that miR-96 targets the 3′ UTR of NUAK1.
Further experiments confirmed this prediction, namely that miR-96
suppresses the expression of NUAK1 by targeting its 3′ UTR.
Finally, we demonstrate that the introduction of NUAK1 cDNA lacking
predicted sites of the 3′ UTR abrogates miR-96 cellular
function.
Materials and methods
Human tissue samples
Ten pairs of human pancreatic tissue samples were
obtained from patients who underwent surgical resection at the
Second Artillery General Hospital of PLA (Beijing, China) or
Tianyou Hospital Affiliated to Wuhan University of Science and
Technology (Wuhan, China) between 2013 and 2014 and were diagnosed
with pancreatic cancer based on a histopathological evaluation. The
matched non-tumor adjacent tissue was obtained from a segment of
the resected specimens that was the farthest from the tumor. The
samples were snap-frozen in liquid nitrogen and stored at −80°C
until use. No local or systemic treatment was conducted on these
patients prior to surgery.
The use of human tissue samples followed
internationally recognised guidelines, as well as local and
national regulations. All research carried out on human
participants followed international and national regulations.
Ethics approval for this study was obtained from the Medical Ethics
Committee of Tianyou Hospital Affiliated to Wuhan University of
Science and Technology and all participants provided written
informed consent prior to enrollment.
Cell culture, plasmids and
transfection
The pancreatic cancer cell lines, MIA PaCa-2 and
PANC-1, were obtained from the American Type Culture Collection
(ATCC, Manassas, VA, USA) and cultured in DMEM (Sigma, St. Louis,
MO, USA) supplemented with 10% fetal bovine serum (Gibco, Grand
Island, NY, USA) at 37°C with 5% CO2. NUAK1-expressing
plasmids and empty vector (pcDNA3.1; mock) were purchased from
R&D Systems (Abingdon, UK). Pre-miR-96 and control-miR
(scramble) were purchased from Ambion (Austin, TX, USA).
Anti-miR-96 and control-anti-miR (scramble) also were purchased
from Ambion (Austin, TX, USA). For the transfection experiments,
the cells were cultured in serum-free medium without antibiotics at
60% confluence for 24 h, and then transfected with Lipofectamine
2000 transfection reagent (Invitrogen, Carlsbad, CA, USA) according
to the manufacturer’s instructions. Following incubation for 6 h,
the medium was removed and replaced with normal culture medium for
48 h.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
The effects of treatments on cell proliferation were
assessed by MTT assay (Sigma) which was carried out as previously
described (20). Briefly, MTT
(Sigma) was added to a final concentration of 1 mg/ml, the reaction
mixture was incubated for 3 h at 37°C, and the absorbance was
measured at 570 nm. The absorbance was directly proportional to the
number of surviving cells.
BrdU cell proliferation assay
Cell proliferation was also assessed using a
colorimetric BrdU proliferation kit according to the manufacturer’s
instructions (Cat. no. 11647229001; Roche, Indianapolis, IN, USA).
The cells transfected with NUAK1-expressing plasmids or the empty
vector (pcDNA3.1; mock) were labeled with BrdU for 3–4 h. The
genomic DNA was fixed and denatured, and then incubated with
peroxidase-conjugated anti-BrdU antibody for 90 min. A substrate
for the conjugated peroxidase was then added and the reaction
product was quantified by measuring the absorbance. The results
were then normalized to the number of total viable cells.
Western blot analysis
Western blot analysis was performed as previously
described (20). Briefly,
following incubation with rabbit anti-NUAK1, anti-Ki67,
anti-proliferating cell nuclear antigen (PCNA), anti-p27, anti-p21,
c-myc, anti-CDK1, anti-CDK2, anti-p53 or anti-β-actin antibodies
(all from Abcam, Cambridge, MA,USA) overnight at 4°C,
IRDye™-800-conjugated anti-rabbit secondary antibodies (LI-COR
Biosciences, Lincoln, NE, USA) were used for 30 min at room
temperature. The specific proteins were visualized using the
Odyssey™ Infrared Imaging System (Gene Company, Ltd., Hong Kong,
China).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from the cultured cells, with efficient
recovery of small RNA, was isolated using the mirVana miRNA
Isolation kit (Cat. no. AM1561, Ambion). Detection of the mature
form of miRNAs was performed using the mirVana qRT-PCR miRNA
Detection kit and qRT-PCR Primer Sets, according to the
manufacturer’s instructions (Ambion). The U6 small nuclear RNA was
used as an internal control.
Bioinformatics analysis
The analysis of potential miRNA target sites was
carried out using three commonly used prediction algorithms:
miRanda (http://www.microrna.org/), TargetScan
(http://www.targetscan.org) and PicTar
(http://pictar.mdc-berlin.de/).
Luciferase reporter assay
The luciferase reporter plasmid of the 3′ UTR of
NUAK1 (NUAK1-WT-luc) was donated by Dr Jun Zhou, Department of
Gastroenterology and Hepatology, University Medical Center,
Utrecht, the Netherlands. Site-directed mutagenesis of the miR-96
target-sites in the 3′ UTR of NUAK1 was carried out using the
QuikChange Site-Directed Mutagenesis kit (Cat. no. 200519;
Stratagene, Heidelberg, Germany), with NUAK1-WT-luc as a template.
For reporter assays, the MIA PaCa-2 cells were transiently
transfected with the wild-type (WT) or mutant reporter plasmid and
miR or anti-miR (as indicated in Fig.
3G) using Lipofectamine 2000 (Invitrogen). Reporter assays were
performed 36 h post-transfection using the
Dual-Luciferase® Reporter Assay System (Promega),
normalized for transfection efficiency by co-transfecting with
Renilla-luciferase.
Migration and invasion assay
For the Transwell migration assays,
2.5×104–5×104 cells were plated in the top
chamber with a non-coated membrane (24-well insert; pore size, 8
mm; BD Biosciences, Franklin Lakes, NJ, USA). For the invasion
assays, 1.25×105 cells were plated in the top chamber
with a Matrigel-coated membrane (24-well insert; pore size, 8 mm;
BD Biosciences). In both assays, the cells were plated in medium
without serum, and medium supplemented with serum was used as a
chemoattractant in the lower chamber. The cells were incubated for
24 h and the cells that did not migrate or invade through the pores
were removed using a cotton swab. The cells on the lower surface of
the membrane were stained with the Diff-Quick Stain Set (Dade
Behring, Inc., Deerfield, IL, USA) and counted.
Statistical analysis
Data are expressed as the means ± SE, and were
derived from three independent experiments. The data were analyzed
using the Student’s t test. A value of P<0.05 was considered to
indicate a statistically significant difference.
Results
Aberrant NUAK1 expression in pancreatic
cancer
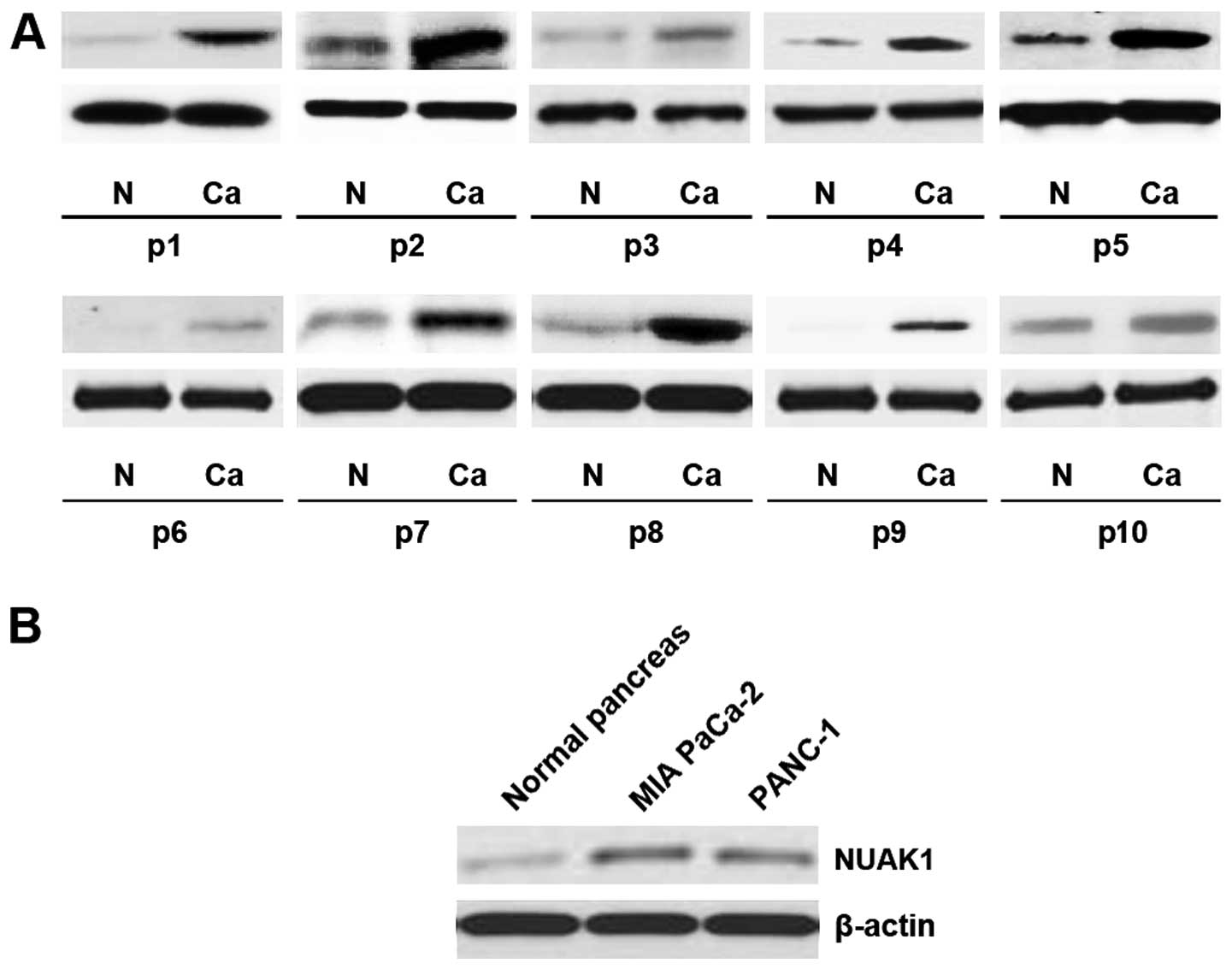
In order to determine the expression levels of NUAK1
in the pancreatic cancer tissue samples, western blot analysis was
conducted in 10 pairs of pancreatic cancer tissue and matched
adjacent normal tissue samples. The expression of NUAK1 was
consistently higher in the pancreatic cancer tissue samples than in
the normal tissue samples (Fig.
1A). Moreover, the analysis of NUAK1 expression in two
pancreatic cancer cell lines (MIA PaCa-2 and PANC-1) revealed that
NUAK1 was upregulated in the tumor cell lines as well (Fig. 1B). These data support the notion
that NUAK1 functions as an oncogene in pancreatic cancer.
The overexpression of NUAK1 in MIA PaCa-2
pancreatic cancer cells promotes cell proliferation, migration and
invasion
In an attempt to identify the role of NUAK1 in
regulating the proliferation of MIA PaCa-2 cells, the cells were
transfected with NUAK1 plasmids. Following stable transfection,
NUAK1 expression was detected by western blot analysis and the
expression of eight proliferation-associated markers of MIA PaCa-2
cells (Ki67, PCNA, p27, p21, c-myc, CDK1, CDK2 and p53) was also
determined by western blot analysis. The results revealed that
NUAK1 plasmids evidently increased NUAK1 protein expression, and
suppressed p53, p21 and p27 expression and promoted CDK1, CDK2 and
Ki67 expression in the MIA PaCa-2 cells (Fig. 2A). Moreover, the proliferation
rates of the MIA PaCa-2 cells were examined by MTT assay. The
results revealed that overexpression of NUAK1 significantly
increased the proliferation rate of the MIA PaCa-2 cells and that
this increase in cell proliferation occurred in a dose-dependent
manner (Fig. 2B). This was
further revealed by BrdU incorporation assay, showing that
transfection with NUAK1 resulted in increased DNA synthesis
activity per viable cell in the MIA PaCa-2 cells also in a
dose-dependent manner (Fig.
2C).
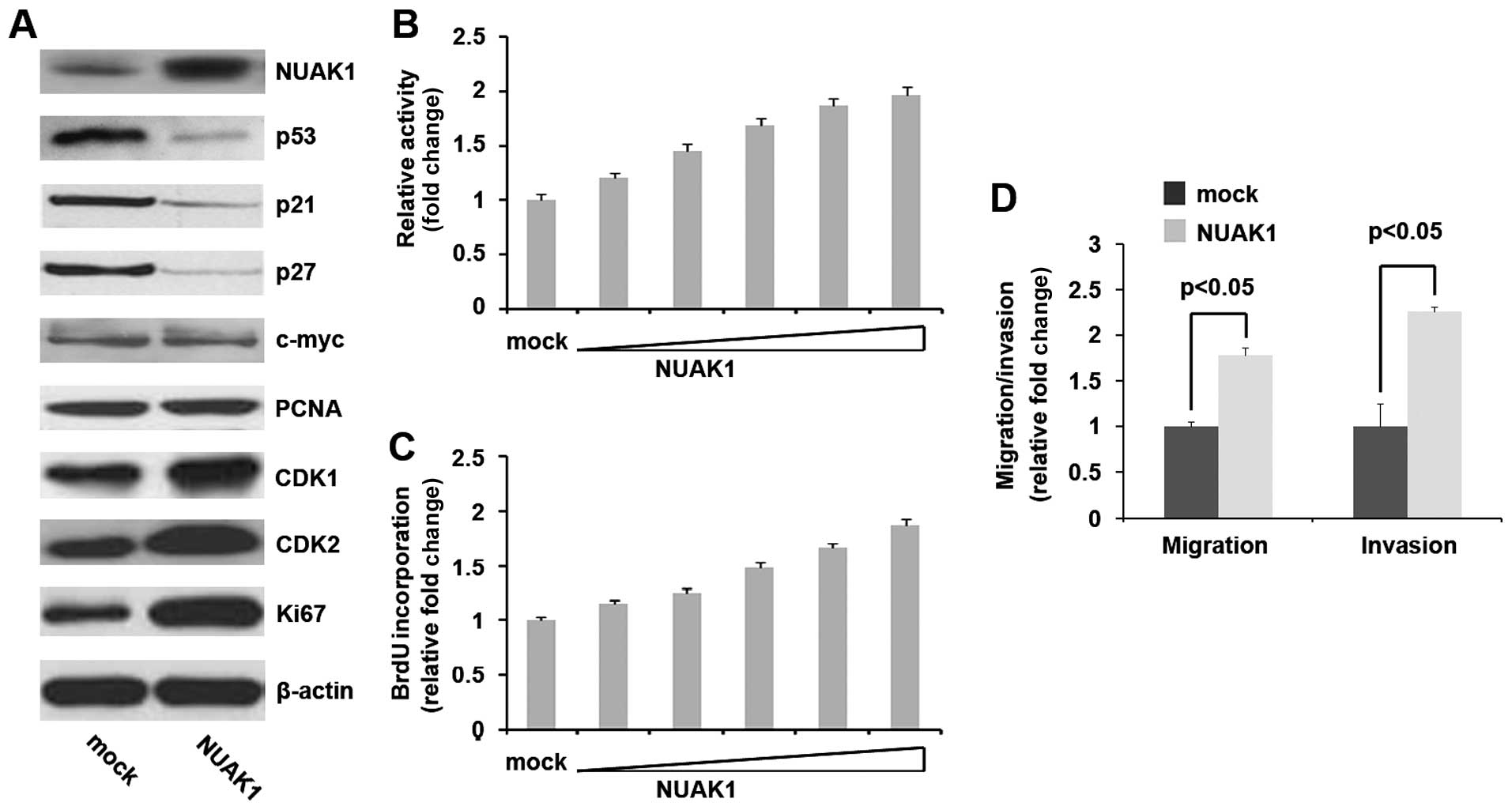 | Figure 2Novel (nua) kinase family 1 (NUAK1)
promotes the proliferation, migration and invasion of MIA PaCa-2
pancreatic cancer cells. (A) Western blot analysis of NUAK1, p53,
p21, p27, c-myc, PCNA, CDK1, CDK2 and Ki67 protein expression in
MIA PaCa-2 cells. The cells were transfected with NUAK1-expressing
plasmids (1, 2, 3, 4 and 5 μg) or pcDNA3.1 (mock). β-actin was used
as a loading control (n=3). (B) MTT assay of MIA PaCa-2 cells
transfected with NUAK1-expressing plasmids or pcDNA3.1 (mock)
(n=3). (C) BrdU incorporation assay of MIA PaCa-2 cells transfected
with NUAK1-expressing plasmids or pcDNA3.1 (mock) (n=3). (D)
Invasion and migration assays using MIA PaCa-2 cells infected with
NUAK1- expressing plasmids (1, 2, 3, 4 and 5 μg) or pcDNA3.1 (mock)
(n=3). |
Given that NUAK1 markedly promoted MIA PaCa-2 cell
proliferation, we then wished to determine whether NUAK1 has an
effect on the migration and invasion of MIA PaCa-2 cells. The cell
migration and invasion assay of MIA PaCa-2 cells revealed that the
overexpression of NUAK1 not only induced the migration of the MIA
PaCa-2 cells, but also promoted the invasion of these cells
(Fig. 2D).
NUAK1 is a target of miR-96 in MIA PaCa-2
pancreatic cancer cells
Having demonstrated that NUAK1 expression is
specifically upregulated and that it promotes the proliferation,
migration and invasion of MIA PaCa-2 cells, then investigated the
mechanisms responsible for promoting NUAK1 expression. miRNAs are a
new class of small (~22 nucleotide) non-coding RNAs that negatively
regulate protein-coding gene expression by targeting mRNA
degradation or translation inhibition (1–3).
We hypothesized that NUAK1 expression was upregulated due to a
defect in a specific miRNA in pancreatic cancer.
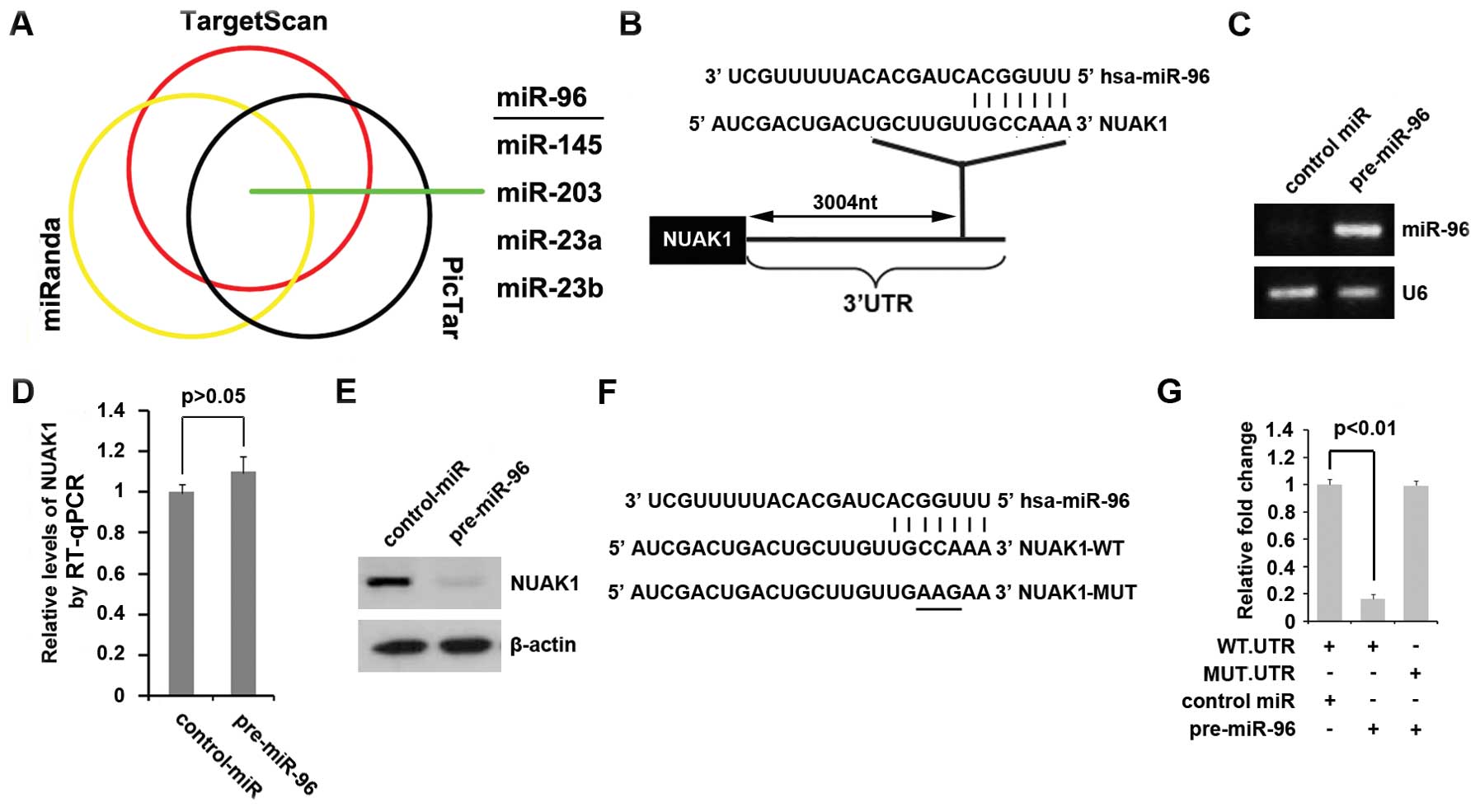
To further confirm this hypothesis, on the one hand,
we used three commonly used prediction algorithms: miRanda
(http://www.microrna.org/), TargetScan (http://www.targetscan.org) and PicTar (http://pictar.mdc-berlin.de/) to analyze the 3′ UTR of
NUAK1. All three algorithms predicted that miR-96, miR-145,
miR-203, miR-23a and miR-23b target the 3′ UTR of NUAK1 (Fig. 3A). miR-96 is downregulated in
pancreatic cancer and the overexpression of miR-96 in pancreatic
cancer has been shown to suppress cell proliferation, migration and
invasion (8). Thus, we
hypothesized that the upregulation of NUAK1 in pancreatic cancer
results from a defect in miR-96. The predicted target sites of
miR-96 are shown in Fig. 3B.
To determine whether NUAK1 can be downregulated by
miR-96, we transfected the MIA PaCa-2 cells with pre-miR-96 and
then RT-qPCR was performed to detect the mRNA expression of miR-96
and NUAK1 in the cells. The results revealed that transfection with
pre-miR-96 markedly increased miR-96 expression (Fig. 3C), but it did not affect NUAK1
mRNA expression in the MIA PaCa-2 cells (Fig. 3D). Since miRNAs can suppress mRNA
translation without degrading the mRNA, we also performed western
blot analysis to determine whether miR-96 affects NUAK1 protein
expression. The results of western blot analysis demonstrated that
NUAK1 protein expression was markedly downregulated following
transfection with pre-miR-96 in the MIA PaCa-2 cells (Fig. 3E).
To further demonstrate the direct regulation of
NUAK1 by miR-96 through its 3′ UTR, we constructed luciferase
reporters with the targeting sequences of wild-type (NUAK1-WT-luc)
and mutated 3′ UTRs of NUAK1 (NUAK1-MUT-luc) (Fig. 3F). To determine whether miR-96
targets the 3′ UTR of NUAK1, a luciferase assay was performed. Our
results revealed that pre-miR-96 inhibited the effects of the
NUAK1-WT-luc plasmids, but not those of the NUAK1-MUT-luc plasmids
(Fig. 3G). Our data confirm that
miR-96 negatively regulates the expression of the protein-coding
gene, NUAK1, by targeting its 3′ UTR; thus, this suggests that the
upregulation of NUAK1 is associated with the low expression of
miR-96 in pancreatic cancer.
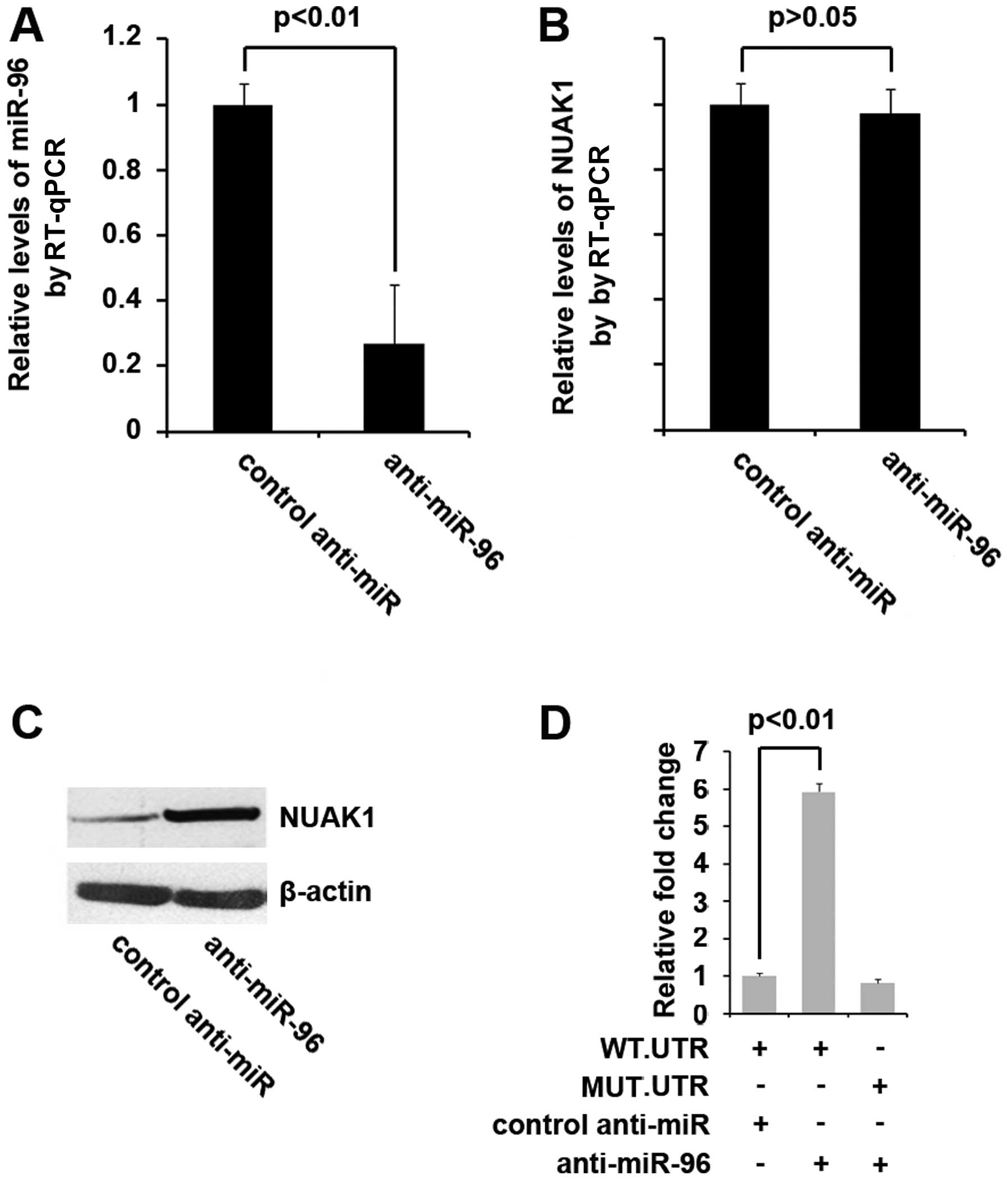
Silencing miR-96 promotes the expression
of NUAK1
To determine whether NUAK1 is indeed regulated by
miR-96 and that the upregulation of NUAK1 is associated with the
low expression of miR-96, we transfected the MIA PaCa-2 cells with
anti-miR-96 or control anti-miR and RT-qPCR was performed to
determine the expression levels. Our results revealed that
anti-miR-96 effectively inhibited miR-96 expression in the cells
(Fig. 4A). We then performed
RT-qPCR and western blot analysis to determine NUAK1 expression
levels in the cells transfected with anti-miR-96. The results
revealed that transfection with anti-miR-96 did not inhibit NUAK1
mRNA expression (Fig. 4B),
although it inhibited NUAK1 protein expression (Fig. 4C). To further demonstrate the
direct regulation of NUAK1 by anti-miR-96, both the wild-type and
mutant reporter contrstucts were introduced into the MIA PaCa-2
cells. The luciferase activity induced by NUAK1-WT-luc, but not
that induced by NUAK1-MUT-luc was significantly promoted by
transfection with anti-miR-96 in the MIA PaCa-2 cells (Fig. 4D).
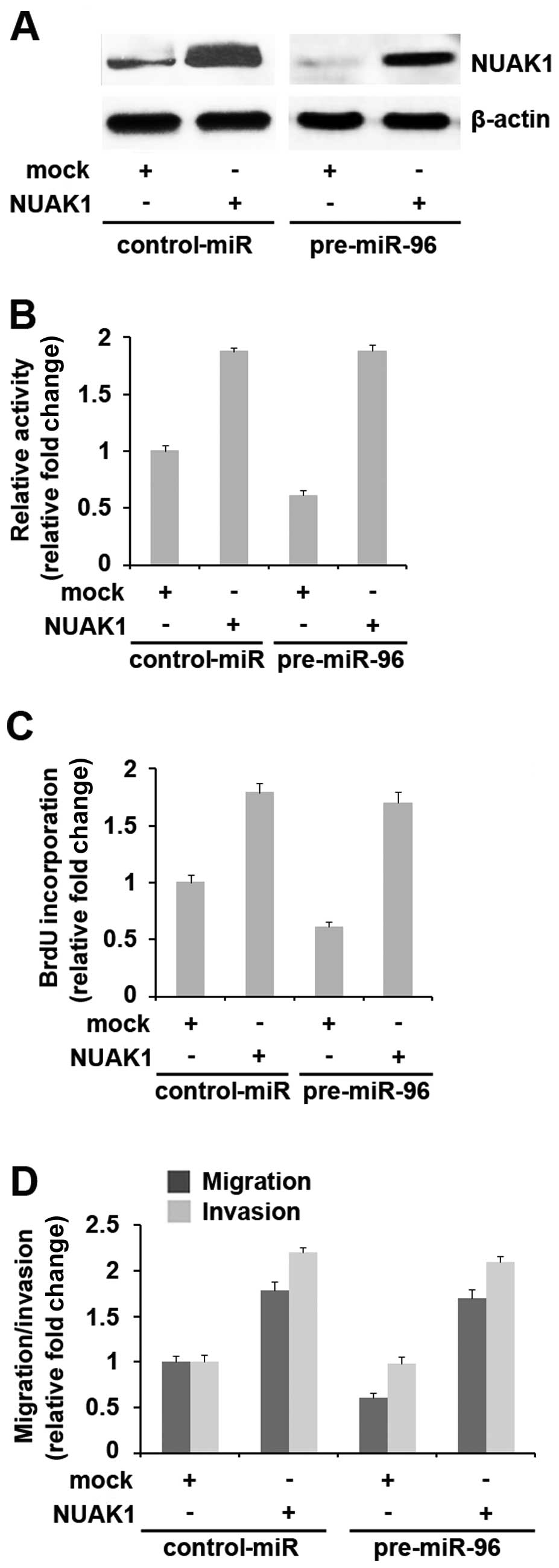
Introduction of NUAK1 cDNA lacking
predicted sites of 3′ UTR abrogates miR-96 cellular function
Since miR-96 directly targets NUAK1 through its 3′
UTR, we reasoned that the ectopic expression of NUAK1 by
transfection with cDNA that did not contain the predicted target of
3′ UTR (in this study, the NUAK1-expressing plasmids did not
contain the target of miR-96 in its 3′ UTR predicted by
bioinformatics analysis) would allow NUAK1 to evade regulation by
miR-96 and would thus attenuate or decrease miR-96 function. To
this end, we transfected NUAK1-expressing plasmids or pcDNA3.1 into
control-miR- or pre-miR-96-treated-MIA PaCa-2 cells. Western blot
analysis revealed that transfection with NUAK1 plasmids eliminated
the effects of pre-miR-96 on NUAK1 protein expression (Fig. 5A).
As the overexpression of miR-96 in pancreatic cancer
inhibits proliferation, migration and invasion (8), in order to determine whether NUAK1
abrogates the roles of miR-96 in cell proliferation, control-miR-
or pre-miR-96-transfected MIA PaCa-2 cells were treated with either
NUAK1-expressing plasmids or pcDNA3.1. We then performed MTT and
BrdU incorporation assays and found that the pre-miR-96-treated MIA
PaCa-2 cells displayed a 30–40% decrease in proliferation compared
with the control-miR-treated cells (Fig. 5B) and in DNA synthesis (Fig. 5C). The overexpression of NUAK1
reversed the loss in proliferation observed in the
pre-miR-96-treated cells.
We then treated the contro- miR- or
miR-96-transfected MIA PaCa-2 cells with either NUAK1-expressing
plasmids or pcDNA3.1 and performed migration and invasion assays.
The results revealed that the overexpression of NUAK1 reversed the
loss in migration and invasion observed in the pre-miR-96-treated
cells (Fig. 5D). Hence, the
suppression of NUAK1 expression may account for the reduced cell
migration and invasion following treatment with pre-miR-96.
Discussion
The prognosis of patients with pancreatic cancer
remains dismal (21,22). The disease is extremely aggressive
and is profoundly resistant to all forms of therapy (23). Given the frequent failure of
conventional treatment strategies, many cancer-related molecules
have been characterized toward the goal of developing novel
anti-cancer therapies, such as molecular-targeted drugs and
antibodies or cancer vaccines (24,25). Tumor malignancy, including
invasion and metastasis, accelerated by Akt activation has been
well documented for breast cancer, ovarian cancer, squamous cell
carcinoma, colorectal cancer and pancreatic cancer (26–29). Suzuki et al suggested that
NUAK1 overexpression is involved in the tumor progression of colon
cancer (14). However, the role
of NUAK1, as a tumor-associated factor downstream of Akt signaling
(14), in pancreatic cancer has
not yet been elucidated. In the present study, we demonstrated that
NUAK1 expression was specifically upregulated in pancreatic cancer
and that it promoted the proliferation, migration and invasion of
MIA PaCa-2 pancreatic cancer cells by targeting NUAK1.
miRNAs are a new class of small (~22 nucleotide)
non-coding RNAs that negatively regulate protein-coding gene
expression by targeting mRNA degradation or translation inhibition
(1–3). Profiling studies have revealed the
contribution of aberrant miRNA expression to pancreatic initiation
and progression by perturbing the function of target genes
(30–32). It has been previously demonstrated
that miR-96 is poorly expressed in human pancreatic cancer and that
miR-96 deregulates KRAS by targeting its 3′ UTR, ultimately
functioning as a tumor suppressor gene in pancreatic cancer
(8). Although miR-96 is
downregulated and suppresses cell proliferation, migration and
invasion, and targets KRAS in human pancreatic cancer, the
mechanisms of action of miR-96 functioning as a tumor suppressor
gene have not yet been fully clarified. Consistent with a previous
study (8), we found that miR-96
inhibited the proliferation, migration and invasion of MIA PaCa-2
pancreatic cancer cells by targeting NUAK1; other mechanisms of
action of miR-96 in regulating proliferation, migration and
invasion are emerging.
The miR-96/NUAK1-mediated regulation of the
proliferation, migration and invasion of MIA PaCa-2 pancreatic
cancer cells demonstrated in this study has potential basic and
clinical implications. On the one hand, miR-96 is a powerful tumor
suppressor by exerting anti-proliferative, anti-migratory and
anti-invasive effects in human pancreatic cancer and the
pharmacological restoration of miR-96 expression may represent a
promising therapeutic strategy in pancreatic cancer. On another
hand, NUAK1, as an oncogene, may be a therapeutic target in
patients with pancreatic cancer. However, further studies are
required to fully elucidate the comprehensive roles of miR-96 and
NUAK1 in pancreatic cancer.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. C0704).
References
|
1
|
Lee RC, Feinbaum RL and Ambros V: The
C. elegans heterochronic gene lin-4 encodes small RNAs with
antisense complementarity to lin-14. Cell. 75:843–854. 1993.
|
|
2
|
Pasquinelli AE, Reinhart BJ, Slack F, et
al: Conservation of the sequence and temporal expression of let-7
heterochronic regulatory RNA. Nature. 408:86–89. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reinhart BJ, Slack FJ, Basson M, et al:
The 21-nucleotide let-7 RNA regulates developmental timing in
Caenorhabditis elegans. Nature. 403:901–906. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar
|
|
5
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar
|
|
6
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in cancer. Annu Rev Med. 60:167–179. 2009. View Article : Google Scholar
|
|
7
|
Slack FJ and Weidhaas JB: MicroRNA in
cancer prognosis. N Engl J Med. 359:2720–2722. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu S, Lu Z, Liu C, et al: miRNA-96
suppresses KRAS and functions as a tumor suppressor gene in
pancreatic cancer. Cancer Res. 70:6015–6025. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suzuki A, Kusakai G, Kishimoto A, et al:
Identification of a novel protein kinase mediating Akt survival
signaling to the ATM protein. J Biol Chem. 278:48–53. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suzuki A, Kusakai G, Kishimoto A, et al:
ARK5 suppresses the cell death induced by nutrient starvation and
death receptors via inhibition of caspase 8 activation, but not by
chemotherapeutic agents or UV irradiation. Oncogene. 22:6177–6182.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suzuki A, Kusakai G, Kishimoto A, et al:
Regulation of caspase-6 and FLIP by the AMPK family member ARK5.
Oncogene. 23:7067–7075. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kusakai G, Suzuki A, Ogura T, Kaminishi M
and Esumi H: Strong association of ARK5 with tumor invasion and
metastasis. J Exp Clin Cancer Res. 23:263–268. 2004.PubMed/NCBI
|
|
13
|
Kusakai G, Suzuki A, Ogura T, et al: ARK5
expression in colorectal cancer and its implications for tumor
progression. Am J Pathol. 164:987–995. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suzuki A, Lu J, Kusakai G, Kishimoto A,
Ogura T and Esumi H: ARK5 is a tumor invasion-associated factor
downstream of Akt signaling. Mol Cell Biol. 24:3526–3535. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen P, Li K, Liang Y, Li L and Zhu X:
High NUAK1 expression correlates with poor prognosis and involved
in NSCLC cells migration and invasion. Exp Lung Res. 39:9–17. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bell RE, Khaled M, Netanely D, et al:
Transcription factor/microRNA axis blocks melanoma invasion program
by miR-211 targeting NUAK1. J Invest Dermatol. 134:441–451. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu S, Niu N, Guo H, et al: ARK5 promotes
glioma cell invasion, and its elevated expression is correlated
with poor clinical outcome. Eur J Cancer. 49:752–763. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang XZ, Yu J, Liu HY, Dong RH and Cao
XC: ARK5 is associated with the invasive and metastatic potential
of human breast cancer cells. J Cancer Res Clin Oncol. 138:247–254.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cui J, Yu Y, Lu GF, et al: Overexpression
of ARK5 is associated with poor prognosis in hepatocellular
carcinoma. Tumour Biol. 34:1913–1918. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo XG, Zou JN, Wang SZ, Zhang TC and Xi
T: Novobiocin decreases SMYD3 expression and inhibits the migration
of MDA-MB-231 human breast cancer cells. IUBMB Life. 62:194–199.
2010.PubMed/NCBI
|
|
21
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
22
|
Sant M, Allemani C, Santaquilani M, et al:
EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999
Results and commentary. Eur J Cancer. 45:931–991. 2009.
|
|
23
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar
|
|
24
|
Kelly K, Crowley J, Bunn PA Jr, et al:
Randomized phase III trial of paclitaxel plus carboplatin versus
vinorelbine plus cisplatin in the treatment of patients with
advanced non-small-cell lung cancer: a Southwest Oncology Group
trial. J Clin Oncol. 19:3210–3218. 2001.PubMed/NCBI
|
|
25
|
Hennessy BT, Hanrahan EO and Daly PA:
Non-Hodgkin lymphoma: an update. Lancet Oncol. 5:341–353. 2004.
View Article : Google Scholar
|
|
26
|
Ekstrand AI, Jönsson M, Lindblom A, Borg A
and Nilbert M: Frequent alterations of the PI3K/AKT/mTOR pathways
in hereditary nonpolyposis colorectal cancer. Fam Cancer.
9:125–129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li B, Tsao SW, Li YY, et al: Id-1 promotes
tumorigenicity and metastasis of human esophageal cancer cells
through activation of PI3K/AKT signaling pathway. Int J Cancer.
125:2576–2585. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ohta T, Isobe M, Takahashi T,
Saitoh-Sekiguchi M, Motoyama T and Kurachi H: The Akt and ERK
activation by platinum-based chemotherapy in ovarian cancer is
associated with favorable patient outcome. Anticancer Res.
29:4639–4647. 2009.PubMed/NCBI
|
|
29
|
Simon PO Jr, McDunn JE, Kashiwagi H, et
al: Targeting AKT with the proapoptotic peptide, TAT-CTMP: a novel
strategy for the treatment of human pancreatic adenocarcinoma. Int
J Cancer. 125:942–951. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee EJ, Gusev Y, Jiang J, et al:
Expression profiling identifies microRNA signature in pancreatic
cancer. Int J Cancer. 120:1046–1054. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dillhoff M, Liu J, Frankel W, Croce C and
Bloomston M: MicroRNA-21 is overexpressed in pancreatic cancer and
a potential predictor of survival. J Gastrointest Surg.
12:2171–2176. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ji Q, Hao X, Zhang M, et al: MicroRNA
miR-34 inhibits human pancreatic cancer tumor-initiating cells.
PLoS One. 4:e68162009. View Article : Google Scholar : PubMed/NCBI
|