Introduction
Tanshinone IIA (Tan-IIA;
C19H18O3), a phenanthrenequinone
derivative extracted from Danshen, Salviae Miltiorrhizae
Radix (1,2), is a natural anti-cancer agent, which
possesses antitumor activity in a variety of human cancer cells,
such as lung (3), colon (4) and breast cancer (5). It has been well documented that
Tan-IIA can induce apoptosis and reverse the malignant phenotype of
SGC7901 gastric cancer cells. Tan-IIA also exerted powerful
inhibitory effects in the gastric cancer cells, SGC7901, in a time-
and dose-dependent manner, and arrested gastric cancer cells in the
G0/G1 phase (6,7).
Tan-IIA induced growth inhibition and apoptosis in gastric cancer
in vitro and in vivo. Tan-IIA not only arrested
gastric cancer MKN-45 cells in G2/M phase, but also
triggered the intrinsic apoptotic-signaling pathway, through
upregulating expression of the p53 gene and downregulating the
expression of the B-cell lymphoma (Bcl) 2 gene (8,9).
These studies indicate that Tan-IIA may serve as an effective
adjunctive reagent in the treatment of gastric cancer. However, the
molecular mechanisms of Tan-IIA in gastric cancer cells remain
unclear. In previous studies by my group, it was shown that Tan-IIA
inhibited the growth of pancreatic cancer BxPC-3 cells by
decreasing the protein expression of translationally-controlled
tumor protein (TCTP), myeloid cell leukemia 1 protein (Mcl-1) and
Bcl-extra large (Bcl-xL) (10).
Tan-IIA inhibited the growth of breast cancer cells, BT-20, through
increasing the protein expression of caspase-12, GADD153 and
phospho-p38 (11). Tan-IIA also
inhibited the growth of hepatocellular carcinoma Hep-J5 cells by
increasing calreticulin, caspase-12 and GADD153 protein expression
(12). However, the molecular
mechanisms that cause Tan-IIA to induce apoptosis in human gastric
cancer via interaction of endoplasmic reticulum stress (ER stress)
and intrinsic apoptotic signaling pathway have not been clarified.
In the present study, the effects of Tan-IIA in human gastric
cancer AGS cells were investigated.
Materials and methods
Materials
The AGS human gastric adenocarcinoma cell line (BCRC
no.: 60102) was obtained from the Food Industry Research and
Development Institute (Hsinchu, Taiwan). Tan-IIA (CAS-no.:
568-72-9), 3-(4,5-dimethylthiazol-2-y1)-2,5-diphenyltetrazolium
bromide (MTT), sodium deoxycholate, leupeptin, Triton X-100,
Tris-HCl, ribonuclease-A, sodium pyruvate,
4-(2-hydroxethyl)-1-piperazineethanesulphonic acid,
dimethylsulfoxide (DMSO) and Tween-20, mouse anti-β-actin were
obtained from Sigma-Aldrich (St. Louis, MO, USA). Potassium
phosphate and 0.2 mm polyvinylidene fluoride membranes were
purchased from Merck KGaA (Darmstadt, Germany). F-12K medium, fetal
bovine serum (FBS), penicillin-streptomycin and glutamine were
obtained from Gibco-BRL (Carlsbad, CA, USA). BioMax film was
obtained from Kodak. The Bcl-2-associated X protein (Bax) [no.:
2774; molecular weight (MW) 20 kDa], Bcl-xL (no.: 2764; MW 30 kDa),
Mcl-1 (no.: 5453; MW 40 kDa), TCTP (no.: 8441; MW 23 kDa), binding
immunoglobulin protein (BiP) (no.: 3177; MW 78 kDa), calnexin (no.:
2679; MW 90 kDa), protein kinase-like endoplasmic reticulum kinase
(PERK) (no.: 5683; MW 140 kDa), eIF2α (no.: 9722; MW 38 kDa),
inositol-requiring enzyme 1α (IRE1α) (no.: 3294; MW 130 kDa),
caspase-12 (no.: 2202; MW 42 kDa), caspase-9 (no.: 9502; MW 35
kDa), caspase-3 (no.: 9661; MW 17 kDa), ERK (no.: 4370; MW 44/42
kDa), and p38 (no.: 4511; MW 40 kDa) antibodies were all obtained
from Cell Signaling Technology, Inc. (Beverly, MA, USA).
C/EBP-homologous protein (CHOP) (NB600-1335; MW 29 kDa), p53
(NB100-92601; MW 43 kDa) and c-Jun N-terminal kinase (NB100-192, MW
42 kDa) antibodies were obtained from Novus Biologicals (Littleton,
CO, USA). Activating transcription factor 4 (ATF4) (ab1371; MW 38
kDa) and ATF6 (ab11909; MW 75 kDa) antibodies were obtained from
Abcam (Cambridge, MA, USA).
Cell culture
The human gastric adenocarcinoma AGS cells were
obtained from the Food Industry Research and Development Institute.
The AGS cells were placed into 75-cm2 tissue culture
flasks and maintained in F-12K with 10% heat-inactivated FBS, 100
U/ml penicillin and 100 μg/ml streptomycin. Cells were grown at
37°C in a humidified atmosphere of 5% CO2. All the data
presented are from at least three independent experiments.
Cytotoxicity assay
The cytotoxicity of Tan-IIA for AGS cells was
evaluated by the MTT assay in triplicate as previously described
(13). Briefly, the AGS cells
were plated in 96-well plates at a density of 2×104
cells/well for 16–20 h. After this the cells were treated with
various concentrations (0, 1, 3, 9, 15, 30 and 60 μg/ml) of Tan-IIA
for 24, 48 and 72 h. Subsequently, the cells were incubated with 1
mg/ml of MTT in fresh complete F-12K medium for 1 h. The surviving
cells converted MTT to formazan by forming a blue-purple color when
dissolved in DMSO. The intensity of formazan was measured at 590 nm
using a microplate reader. The relative percentage of cell
viability was calculated by dividing the absorbance of treated
cells by that of the control in each experiment, using the
following formula: Proliferation rate (%) = (OD test - OD blank) ×
100, where OD test and OD blank are the optical density of the test
substances and the blank control, respectively.
Western blot analysis
The western blot analysis procedures are as
previously described (14,15).
Briefly, AGS cells were treated with various concentrations of
Tan-IIA for different durations, and the cells were lysed in the
ice-cold whole cell extract buffer containing the protease
inhibitors. The lysate was agitated for 30 min at 4°C and
centrifuged at 12,281 × g for 10 min. Protein concentration was
measured by the bicinchoninic acid protein assay kit (Pierce
Biotechnology, Inc., Rockford, IL, USA). Equal amounts of proteins
were subjected to electrophoresis using 12% sodium dodecyl
sulfate-polyacrylamide gels. To verify equal protein loading and
transfer, proteins were transferred to polyvinylidene difluoride
membranes and the membranes were blocked for 1 h at 4°C using
blocking buffer (5% skimmed dried milk in solution containing 50 mM
Tris-HCl (pH 8.0), 2 mM CaCl2, 80 mM sodium chloride,
0.05% Tween-20 and 0.02% sodium azide). The membranes were
subsequently incubated for 2 h at room temperature with the
specific primary antibody followed by anti-rabbit or anti-mouse
immunoglobulin G-horseradish peroxidase-conjugated secondary
antibodies. The membranes were washed three times for 10 min with
washing solution. Finally, the protein bands were visualized on the
X-ray film using the enhanced chemiluminescence detection system
(PerkinElmer Life and Analytical Sciences, Inc., Boston, MA,
USA).
Statistical analysis
Values are presented as the means ± standard
deviation. The Student’s t-test was used to analyze statistical
significance. P<0.05 was considered to indicate a statistically
significant difference for all the tests.
Results
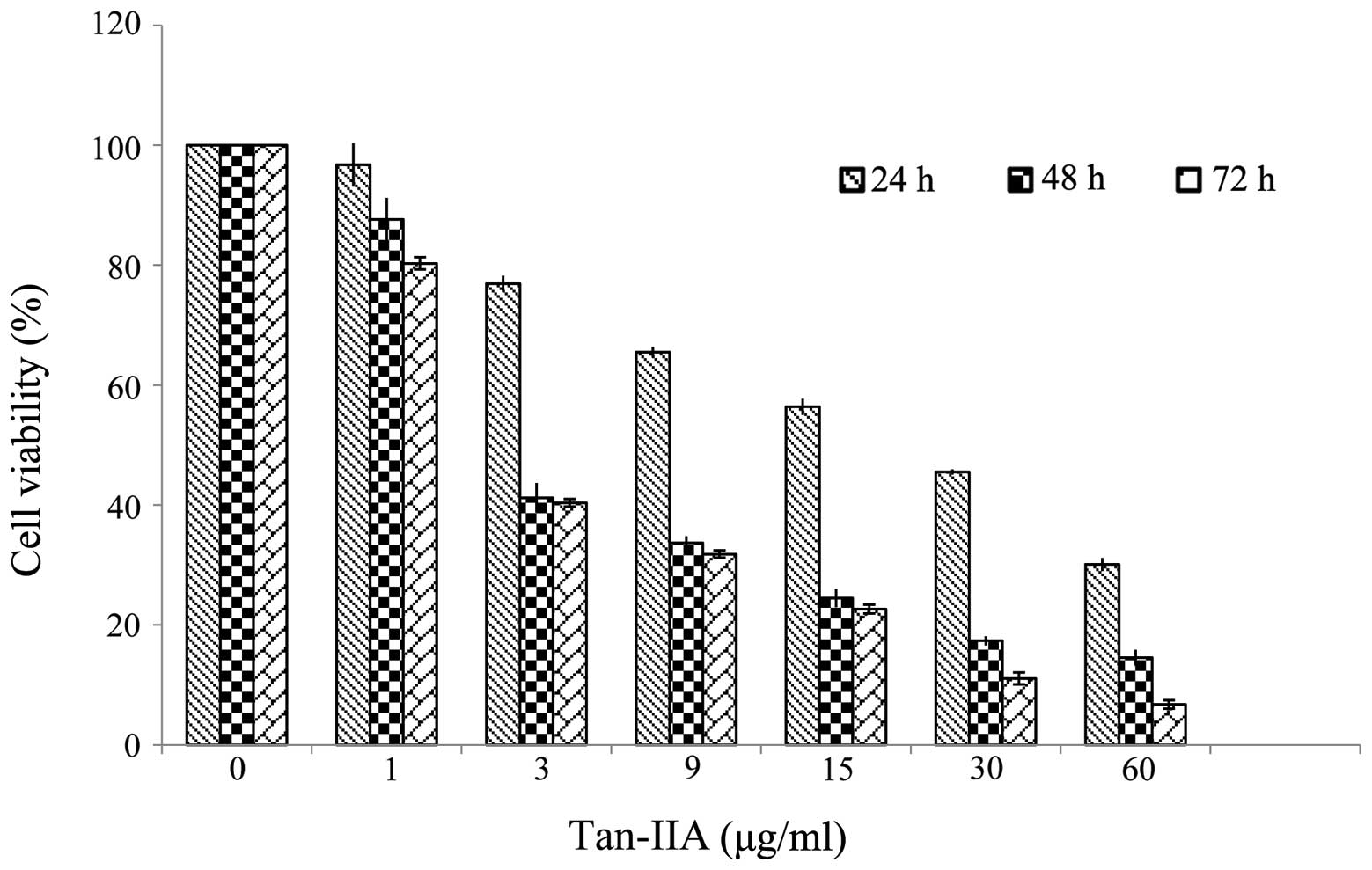
Effects of Tan-IIA in the viability of
AGS cells
The results revealed that Tan-IIA inhibited the
proliferation of AGS cells in a time- and dose-dependent manner.
The half maximal inhibitory concentration (IC50) was
5.5, 3.7 and 3.5 μg/ml at 24, 48 and 72 h, respectively (Fig. 1).
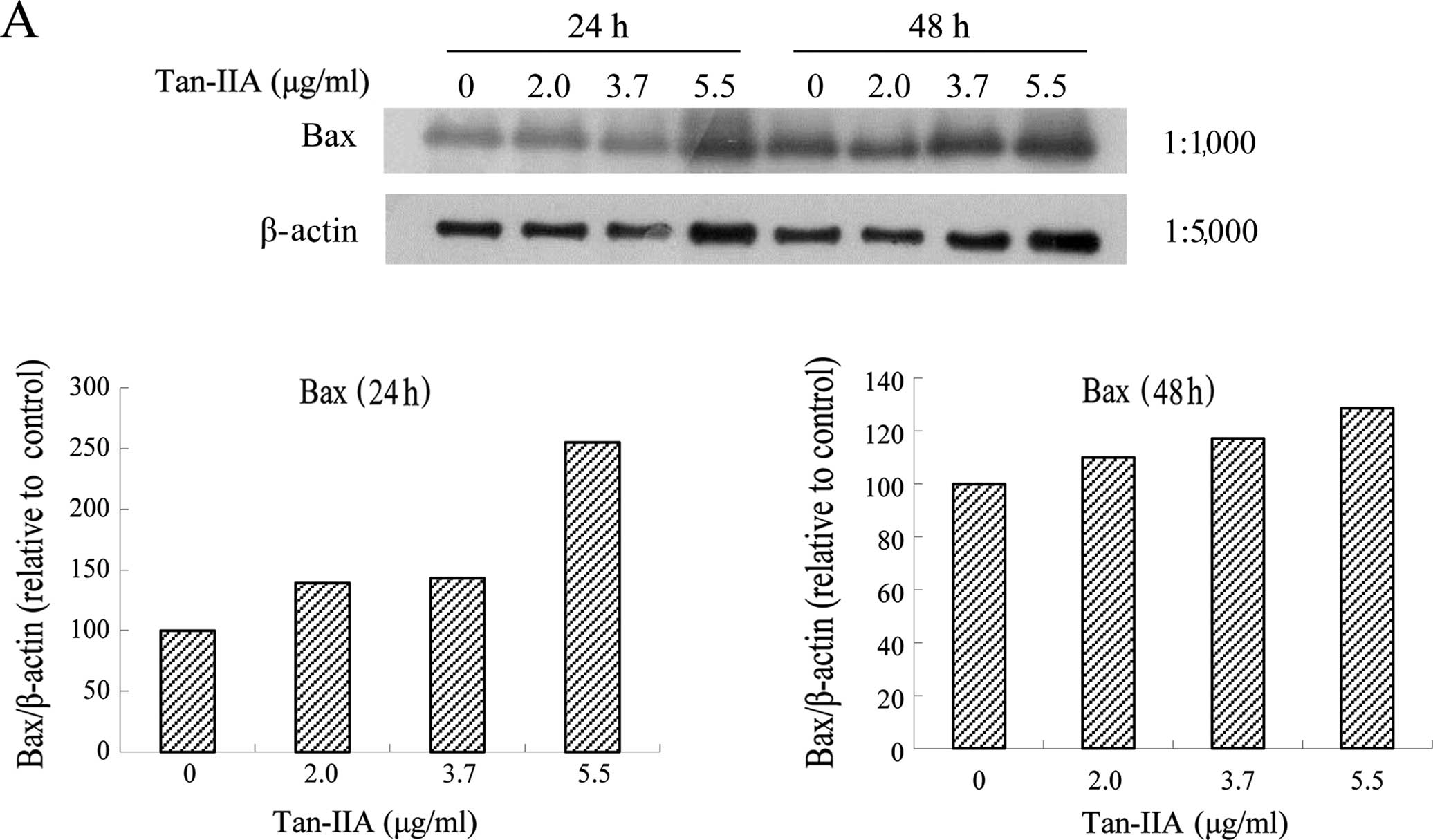
Effects of various concentrations of
Tan-IIA on the protein expression of Bax, Bcl-xL, Mcl-1, TCTP and
β-actin in AGS cells
The AGS cells were treated with various
concentrations of Tan-IIA (0, 2.0, 3.7 and 5.5 μg/ml) for 24 or 48
h and the protein expression levels of Bax, Bcl-xL, Mcl-1, TCTP and
β-actin were evaluated by western blot analysis. The results
revealed that Tan-IIA increased the protein expression levels of
Bax (Fig. 2A), but significantly
decreased Bcl-xL (Fig. 2B), Mcl-1
(Fig. 2C) and TCTP (Fig. 2D) levels.
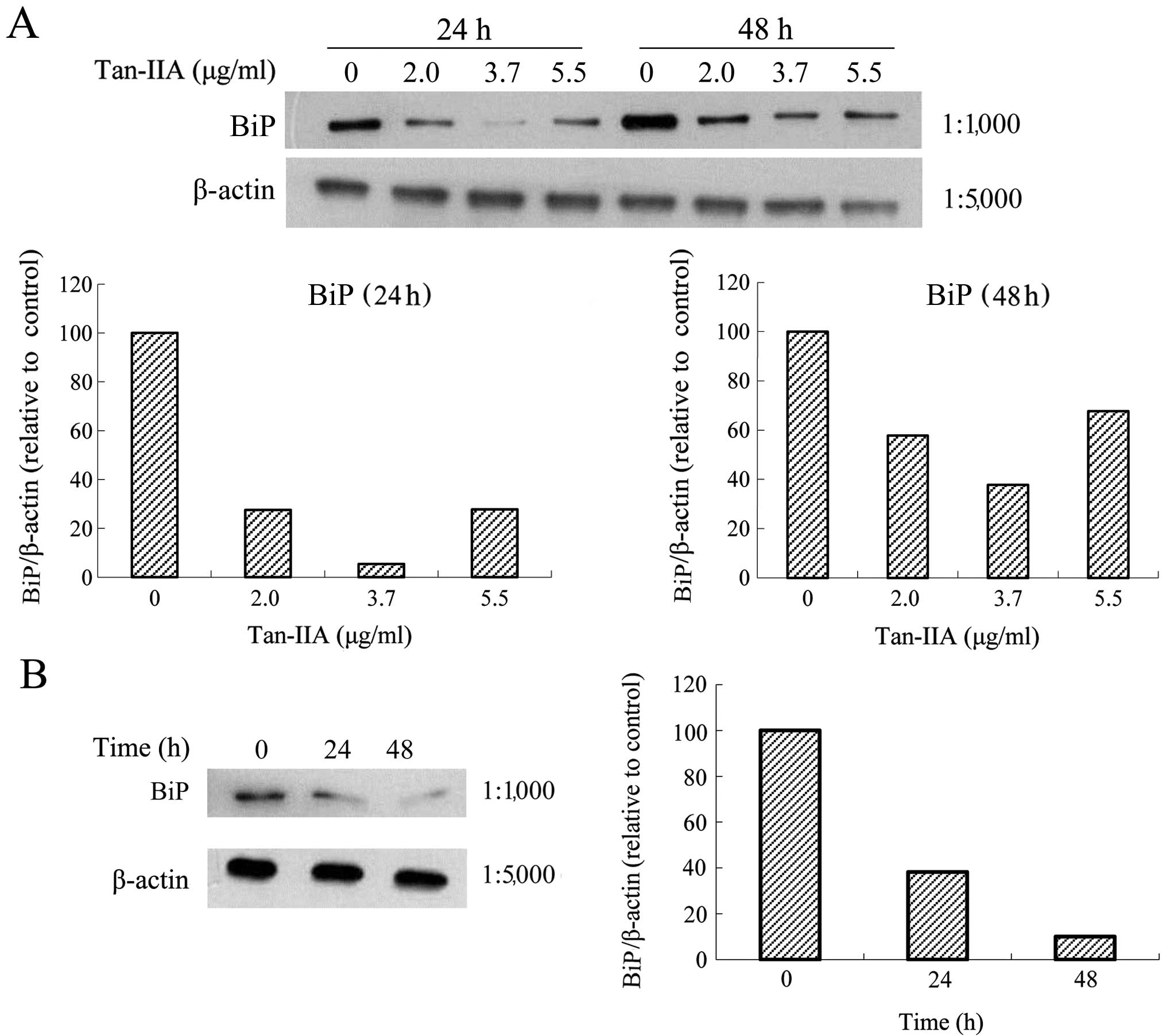
Effects of various concentrations of
Tan-IIA on the protein expression of BiP, calnexin, PERK, eIF2α,
ATF4, IRE1α, ATF6, caspase-12, caspase-9, caspase-3, CHOP and
β-actin in AGS cells
The AGS cells were treated with various
concentrations of Tan-IIA (0, 2.0, 3.7 and 5.5 μg/ml) for 24 or 48
h and the protein expression levels of BiP, calnexin, PERK, eIF2α,
ATF4, IRE1α, ATF6, caspase-12, caspase-9, caspase-3, CHOP and
β-actin were evaluated by western blot analysis. The results
revealed that Tan-IIA can decrease the protein expression level of
BiP (Fig. 3A) but increased
caspase-12 (Fig. 4A), caspase-9
(Fig. 4B), caspase-3 (Fig. 4C), and CHOP (Fig. 4D) levels significantly. The
protein expression levels of calnexin, PERK, eIF2α, ATF4, IRE1α and
ATF6 did not change significantly (data not shown).
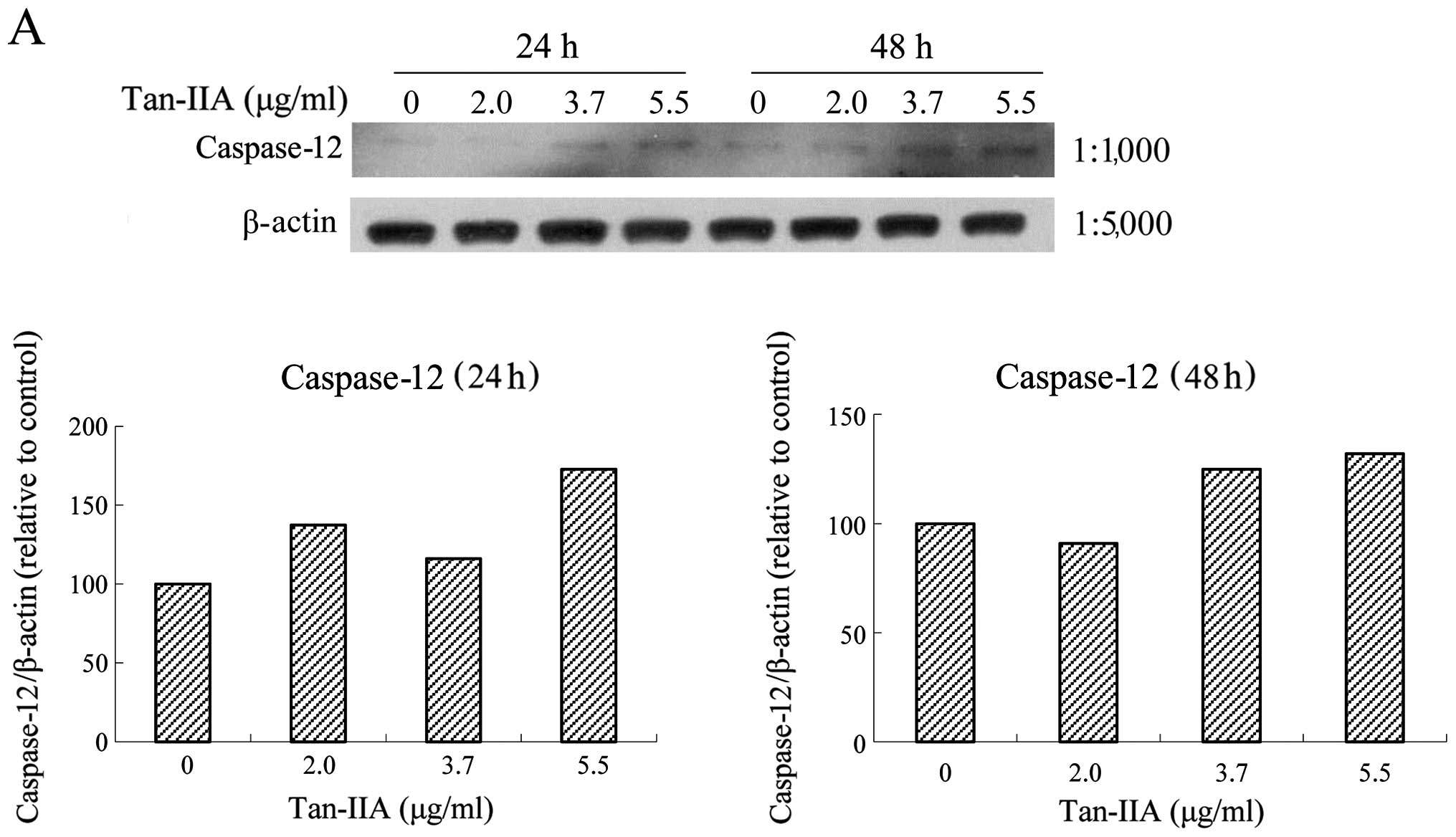 | Figure 4Protein expressions of binding
immunoglobulin protein (BiP), calnexin, protein kinase-like
endoplasmic reticulum kinase (PERK), eIF2α, activating
transcription factor 4 (ATF4), inositol-requiring enzyme 1α
(IRE1α), ATF6, caspase-12, caspase-9, caspase-3, C/EBP-homologous
protein (CHOP) and β-actin in AGS cells. The AGS cells were treated
with various concentrations of tanshinone IIA (Tan-IIA) (0, 2.0,
3.7 and 5.5 μg/ml) for 24 or 48 h and the protein expression levels
were evaluated by western blot analysis as described in ‘Materials
and methods’. The results revealed that Tan-IIA significantly
increased (A) caspase-12, (B) caspase-9, (C) caspase-3 and (D) CHOP
levels in a dose-dependent manner. |
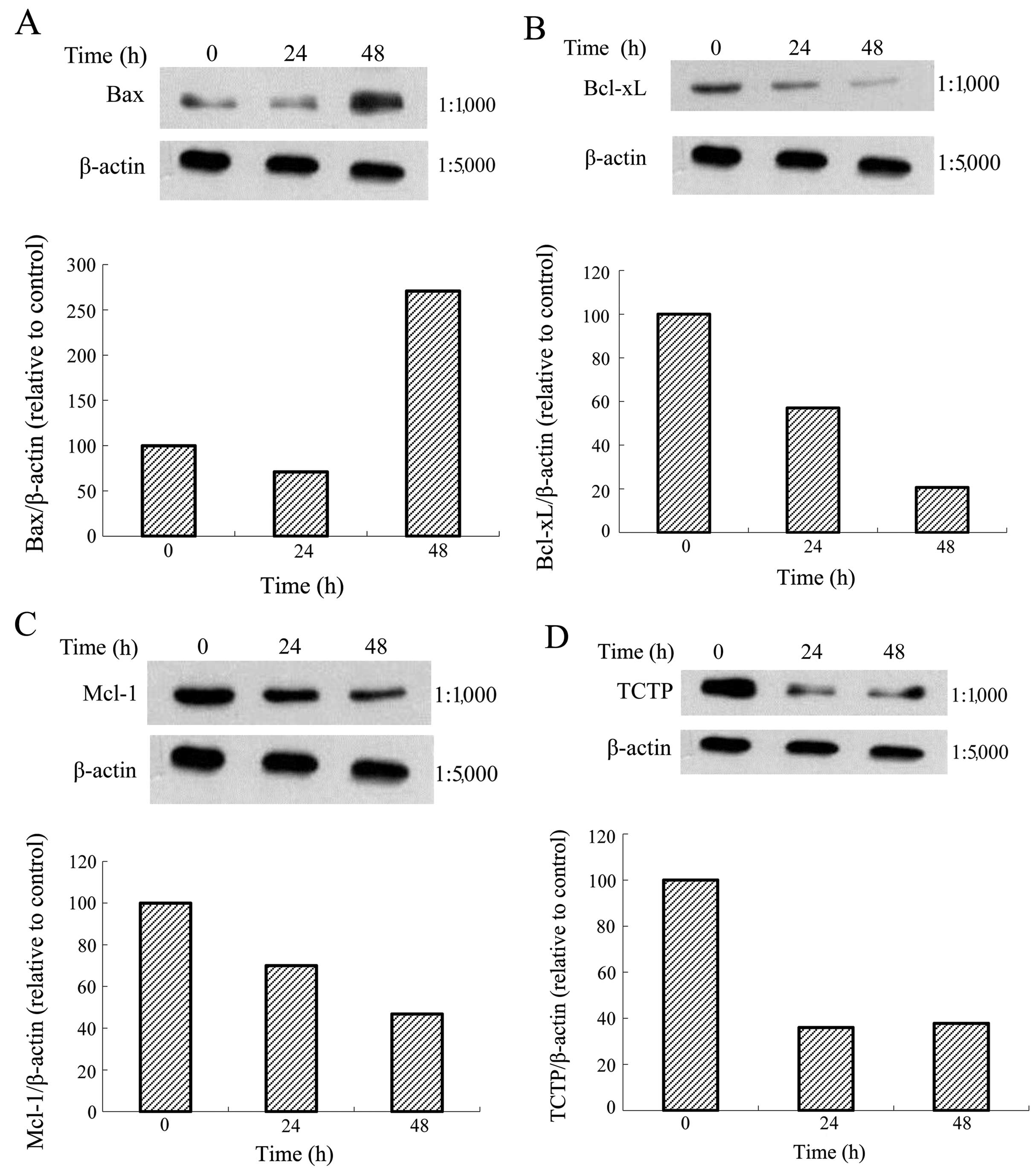
Effects of one Tan-IIA concentration on
the protein expression of Bax, Bcl-xL, Mcl-1, TCTP and β-actin in
AGS cells
The AGS cells were treated with Tan-IIA (3.7 μg/ml)
for different durations (0, 24 and 48 h) and subsequently the
protein expression levels of Bax, Bcl-xL, Mcl-1, TCTP and β-actin
were evaluated by western blot analysis. The results revealed that
Tan-IIA increased the protein expression levels of Bax (Fig. 5A) but decreased Bcl-xL (Fig. 5B), Mcl-1 (Fig. 5C) and TCTP (Fig. 5D) levels significantly.
Effects of one Tan-IIA concentration on
the protein expression of BiP, calnexin, PERK, eIF2α, ATF4, IRE1α,
ATF6, caspase-12, caspase-9, caspase-3, CHOP and β-actin in AGS
cells
The AGS cells were treated with Tan-IIA (3.7 μg/ml)
for different durations (0, 24 and 48 h) and the protein expression
levels of BiP, calnexin, PERK, eIF2α, ATF4, IRE1α, ATF6,
caspase-12, caspase-9, caspase-3, CHOP and β-actin were evaluated
by western blot analysis. The results revealed that Tan-IIA can
decrease the protein expression level of BiP (Fig. 3B) but increased caspase-12
(Fig. 6A), caspase-9 (Fig. 6B), caspase-3 (Fig. 6C), and CHOP (Fig. 6D) levels significantly.
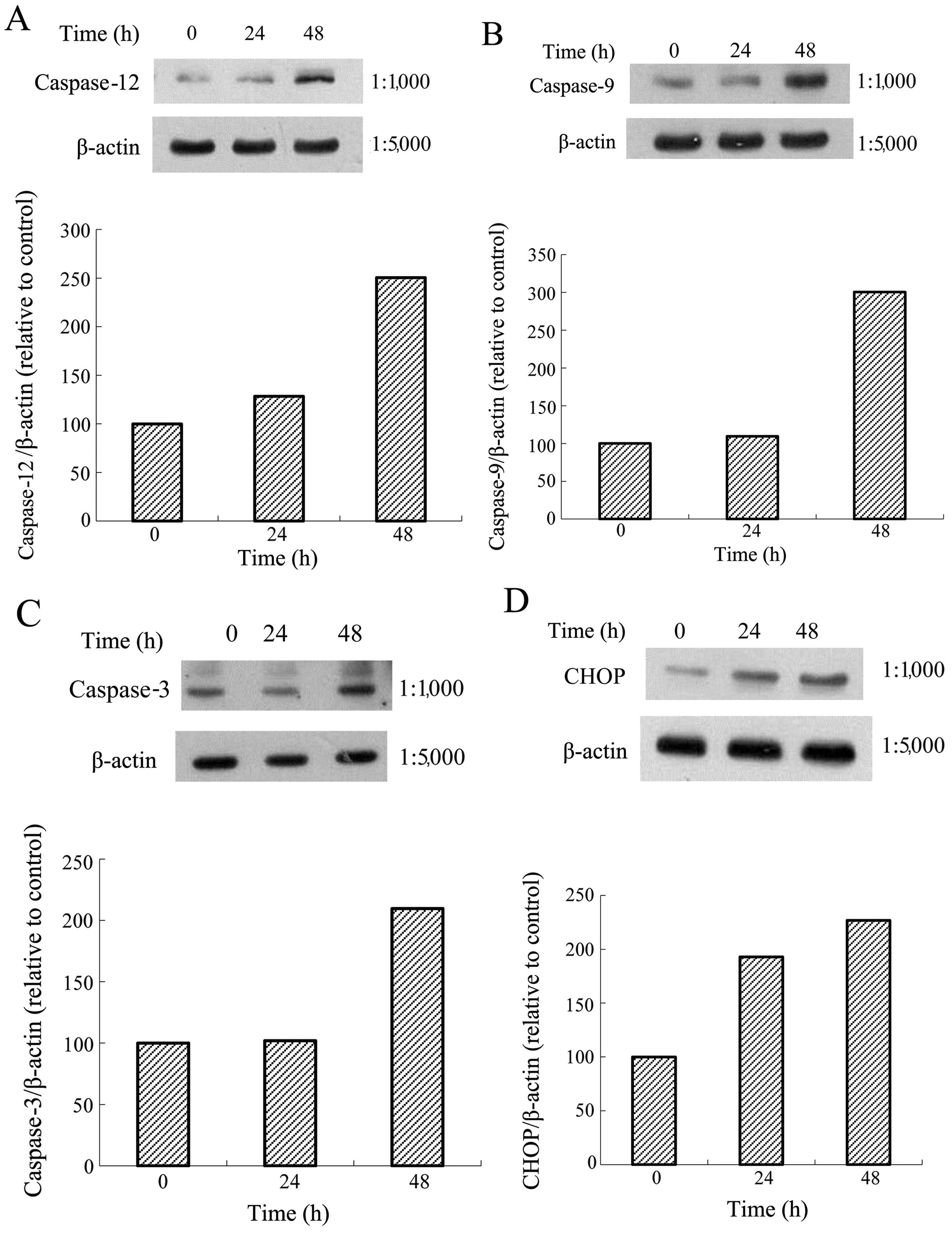 | Figure 6Protein expressions of calnexin,
protein kinase-like endoplasmic reticulum kinase (PERK), eIF2α,
activating transcription factor 4 (ATF4), inositol-requiring enzyme
1α (IRE1α), ATF6, caspase-12, caspase-9, caspase-3,
C/EBP-homologous protein (CHOP) and β-actin in AGS cells. The AGS
cells were treated with tanshinone IIA (Tan-IIA) (3.7 μg/ml) for
different durations (0, 24 and 48h) and the protein expression
levels were evaluated by western blot analysis as described in
‘Materials and methods’. The results revealed that Tan-IIA
significantly increased (A) caspase-12, (B) caspase-9, (C)
caspase-3 and (D) CHOP levels in a time-dependent manner. |
Discussion
The results of the present study revealed that
Tan-IIA inhibited the proliferation of human gastric cancer AGS
cells in a time- and dose-dependent manner. This is in accordance
with previous studies (6–9). TCTP was discovered in Ehrlich
ascites tumor cells (16), is
conserved in all eukaryotes and encodes for a hydrophilic protein
of 18–23 kDa (17). TCTP has been
implicated in the protection of cells against apoptosis (18). Susini et al (19) showed that TCTP protects from
apoptotic cell death by antagonizing Bax function. Liu et al
(20) also documented that TCTP
stabilized and enhanced the antiapoptotic activity of Mcl-1. These
results indicate that TCTP binds to Mcl-1, antagonizing Bax, and
thus inhibiting the induction of apoptosis. The present results
demonstrated that the treatment of AGS cells with Tan-IIA decreased
the protein expression levels of Mcl-1, Bcl-xL and TCTP, but
increased Bax, caspase-9 and caspase-3 levels with a time- and
dose-dependent manner. Therefore, one of the molecular mechanisms
of Tan-IIA involved in the inhibition of human gastric cancer AGS
cell proliferation may be through decreasing the protein expression
of Mcl-1, Bcl-xL and TCTP, but increasing Bax, caspase-9 and
caspase-3, thus inducing apoptosis. Following endoplasmic reticulum
response over loading, ER stress is activated, and the upstream
element, caspase-12, is activated to increase the target protein,
CHOP (also known as GADD153) (21). Our previous study showed that
Tan-IIA induced ER stress to inhibit human breast cancer BT-20
cells (11) and human
hepatocellular cancer Hep-J5 cells (12). In the present study, the results
showed that Tan-IIA increased the protein expression levels of
caspase-12, caspase-9, caspase-3 and CHOP. These results indicate
that Tan-IIA induced ER stress to inhibit the proliferation of AGS
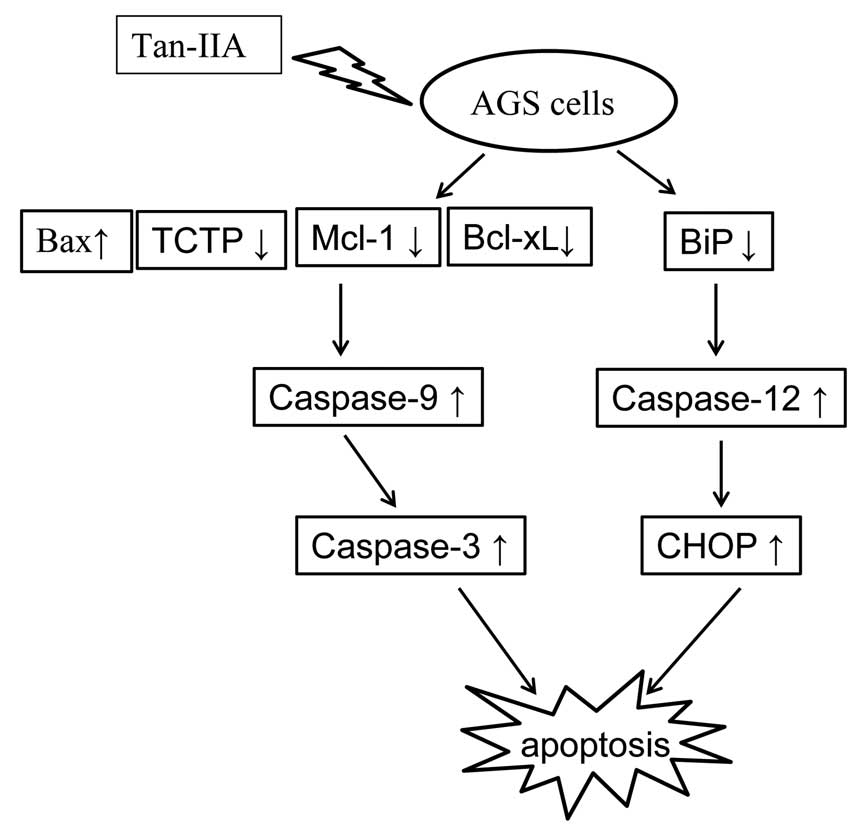
cells. The proposed model of the interactions between Bax, TCTP,
Mcl-1, Bcl-xL and the ER stress pathway in AGS cells treated with
Tan-IIA is shown in Fig. 7.
To the best of our knowledge, this is the first
study to demonstrate that Tan-IIA inhibited human gastric cancer
AGS cells. One of the molecular mechanisms may be through
decreasing the protein expression of BiP to induce the activation
of ER stress, followed by increasing the protein expression of
caspase-12 to upregulate CHOP expression. The other may be through
decreasing the protein expression of Mcl-1, Bcl-xL and TCTP, but
increasing Bax, caspase-9 and caspase-3.
Acknowledgements
The present study was supported by grant no.
102-CCH-IRP-066 from the Research Section of the Changhua Christian
Hospital, Changhua, Taiwan, R.O.C.
References
|
1
|
Che AJ, Zhang JY, Li CH, Chen XF, Hu ZD
and Chen XG: Separation and determination of active components in
Radix Salviae miltiorrhizae and its medicinal preparations
by nonaqueous capillary electrophoresis. J Sep Sci. 27:569–575.
2004.PubMed/NCBI
|
|
2
|
Zhou L, Zuo Z and Chow MS: Danshen: an
overview of its chemistry, pharmacology, pharmacokinetics, and
clinical use. J Clin Pharmacol. 45:1345–1359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chiu TL and Su CC: Tanshinone IIA induces
apoptosis in human lung cancer A549 cells through the induction of
reactive oxygen species and decreasing the mitochondrial membrane
potential. Int J Mol Med. 25:231–236. 2010.PubMed/NCBI
|
|
4
|
Su CC, Chen GW, Kang JC and Chan MH:
Growth inhibition and apoptosis induction by tanshinone IIA in
human colon adenocarcinoma cells. Planta Med. 74:1357–1362. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Su CC and Lin YH: Tanshinone IIA inhibits
human breast cancer cells through increased Bax to Bcl-xL ratios.
Int J Mol Med. 22:357–361. 2008.PubMed/NCBI
|
|
6
|
Hou J, He J, Jin X, Hu T and Zhang Y:
Study on optimisation of extraction process of tanshinone IIA and
its mechanism of induction of gastric cancer SGC7901 cell
apoptosis. Afr J Tradit Complement Altern Med. 10:456–458. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu M, Cao FL, Li NY, Liu YQ, Li YP and Lv
CL: Tanshinone IIA reverses the malignant phenotype of SGC7901
gastric cancer cells. Asian Pac J Cancer Prev. 14:173–177. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen J, Shi DY, Liu SL and Zhong L:
Tanshinone IIA induces growth inhibition and apoptosis in gastric
cancer in vitro and in vivo. Oncol Rep. 27:523–528.
2012.PubMed/NCBI
|
|
9
|
Dong X, Dong J and Peng G:
Growth-inhibiting and apoptosis-inducing effects of Tanshinone II A
on human gastric carcinoma cells. J Huazhong Univ Sci Technolog Med
Sci. 27:706–709. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang CY, Chiu TL, Kuo SJ, Chien SY, Chen
DR and Su CC: Tanshinone IIA inhibits the growth of pancreatic
cancer BxPC-3 cells by decreasing protein expression of TCTP, MCL-1
and Bcl-xL. Mol Med Rep. 7:1045–1049. 2013.PubMed/NCBI
|
|
11
|
Yan MY, Chien SY, Kuo SJ, Chen DR and Su
CC: Tanshinone IIA inhibits BT-20 human breast cancer cell
proliferation through increasing caspase 12, GADD153 and
phospho-p38 protein expression. Int J Mol Med. 29:855–863.
2012.PubMed/NCBI
|
|
12
|
Cheng CY and Su CC: Tanshinone IIA
inhibits Hep-J5 cells by increasing calreticulin, caspase 12 and
GADD153 protein expression. Int J Mol Med. 26:379–385.
2010.PubMed/NCBI
|
|
13
|
Mossman T: Rapid colorimetric assay for
cellular growth and survival: application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen HC, Hsieh WT, Chang WC and Chung JG:
Aloe-emodin induced in vitro G2/M arrest of cell cycle in human
promyelocytic leukemia HL-60 cells. Food Chem Toxicol.
42:1251–1257. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yenofsky R, Cereghini S, Krowczynska A and
Brawerman G: Regulation of mRNA utilization in mouse
erythroleukemia cells induced to differentiate by exposure to
dimethyl sulfoxide. Mol Cell Biol. 3:1197–1203. 1983.PubMed/NCBI
|
|
17
|
Bommer UA, Lazaris-Karatzas A, De
Benedetti A, et al: Translational regulation of the mammalian
growth-related protein P23: involvement of eIF-4E. Cell Mol Biol
Res. 40:633–641. 1994.PubMed/NCBI
|
|
18
|
Bommer UA and Thiele BJ: The
translationally controlled tumour protein (TCTP). Int J Biochem
Cell Biol. 36:379–385. 2004. View Article : Google Scholar
|
|
19
|
Susini L, Besse S, Duflaut D, et al: TCTP
protects from apoptotic cell death by antagonizing bax function.
Cell Death Differ. 15:1211–1220. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu H, Peng HW, Cheng YS, Yuan HS and
Yang-Yen HF: Stabilization and enhancement of the antiapoptotic
activity of mcl-1 by TCTP. Mol Cell Biol. 25:3117–3126. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma Y and Hendershot LM: The role of the
unfolded protein response in tumour development: friend or foe? Nat
Rev Cancer. 4:966–977. 2004. View
Article : Google Scholar : PubMed/NCBI
|