Introduction
Hearing loss is the most common sensory impairment
in humans, affecting 5% of individuals in industrialized nations
(1,2). Evidence strongly suggests that
hearing loss is a serious health issue in the elderly and 40% of
the population aged >65 years have a hearing loss severe enough
to impair communication (3,4).
It has been suggested that hearing loss is induced by multiple
causes, including infection/inflammation, noise and ototoxic drugs
(4–7).
Cyclooxygenase (COX), also known as prostaglandin
(PG) H synthase, is the rate-limiting enzyme in the biosynthesis of
PGs from arachidonic acid (8).
Physiologically, PGs are involved in inflammatory responses, bone
development, wound healing and reproductive function. However, the
excessive production of PGs is associated with many diseases,
including inflammation, atherosclerosis, cardiovascular diseases
and cancer (8–10). COX exists in two isoforms, COX-1
and COX-2 (11). COX-1 is
constitutively expressed in most cell types and is thought to be
involved in the maintenance of physiological functions, including
the cytoprotection of the stomach, platelet aggregation and the
regulation of renal blood flow. By contrast, COX-2 is inducible by
inflammatory stimuli, such as interleukin (IL)-1β, tumor necrosis
factor-α (TNF-α) and lipopolysaccharide (LPS) (8–12).
The overexpression of COX-2 is closely associated with a number of
inflammatory diseases (13). Of
note, previous studies have indicated that COX-1 and COX-2 are
expressed in the cochlea of humans and guinea pigs (14,15). However, the regulatory mechanism
of COX-2 expression in the cochlea remains unclear.
Glucocorticoids are the most effective
anti-inflammatory drugs used in the treatment of
inflammation-related diseases, including rheumatoid arthritis and
asthma (16,17). It has been demonstrated that
glucocorticoids bind to the glucocorticoid receptor (GR) and
negatively regulate the expression of several inflammatory
mediators, including COX-2 and cytokines by inhibiting
transcription factors, such as activator protein (AP)-1, nuclear
factor-κB (NF-κB), signal transducers and activators of
transcription (STATs) and nuclear factor of activated T cells
(NF-AT) (18–21). Among the glucocorticoids,
prednisone (PDN) is currently the most effective regimen clinically
used in the treatment of hearing loss. However, little is known
about the association between PDN and the regulation of COX-2
expression in the cochlea.
House Ear Institute-Organ of Corti 1 (HEI-OC1) cell
is a murine auditory cell line that is widely used as an in
vitro system for screening and/or evaluating the potential
ototoxicity or otoprotective properties of pharmacological drugs
(22). In the present study, we
investigated the inhibitory effects and mechanisms of action of PDN
on COX-2 expression in IL-1β-stimulated HEI-OC1 cells.
Materials and methods
Cell lines and culture
The HEI-OC1 cells were purchased from the House Ear
Institute (Los Angeles, CA, USA) and grown in DMEM without sodium
pyruvate (Gibco, Carlsbad, CA, USA) supplemented with 10%
heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin and
100 mg/ml streptomycin (all from Wellgene, Daegu, Korea) at 37°C in
a humidified 5% CO2 atmosphere.
Materials
Antibodies to phosphorylated (p-) extracellular
signal-regulated kinase (ERK)-1/2, total (t-)ERK-1/2, p-c-Jun
N-terminal kinase (JNK)-1/2, t-JNK-1/2, p-p38 mitogen-activated
protein kinase (MAPK), t-p38 MAPK, p-protein kinase B (PKB), t-PKB
and IκB-α were purchased from Cell Signaling Technology, Inc.
(Beverly, MA, USA). An anti-COX-2 polyclonal antibody was purchased
from Cayman Chemical Co. (Ann Arbor, MI, USA). An anti-actin
antibody was obtained from Sigma-Aldrich (St. Louis, MO, USA).
Anti-rabbit or mouse secondary horseradish peroxidase antibodies
were obtained from Amersham Biosciences (Amersham, UK). Actinomycin
D (Act D) and cycloheximide (CHX) were purchased from
Sigma-Aldrich.
Preparation of whole cell lysates
Whole cell lysate was prepared in a modified RIPA
buffer [50 mM Tris-Cl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1%
sodium deoxycholate, 1% Nonidet P-40, 1 mM
Na3VO4, 1 mM NaF, 1 mM EDTA, 200 nM
aprotinin, 20 μM leupeptin, 50 μM phenanthroline and 280 μM
benzamidine-HCl]. For the detection of phosphorylated proteins, the
cells were twice washed with ice-cold phosphate-buffered saline
(PBS) supplemented with 1 mM Na3VO4 and 1 mM
NaF, and lysed in the modified RIPA buffer mentioned above.
Following centrifugation at the lysed cell suspension at 12,000 rpm
for 20 min at 4°C, the following supernatant was saved as whole
cell lysate. The protein concentration of the whole cell lysate was
measured using Bradford reagent (Bio-Rad Laboratories, Mississauga,
ON, USA) using bovine serum albumin as the standard.
Western blot analysis
Equal amounts of protein (50 μg/lane) were resolved
by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
and transferred onto a nitrocellulose membrane (Millipore Co.,
Bedford, MA, USA). The membrane was then washed with Tris-buffered
saline (TBS) (10 mM Tris, 150 mM NaCl) containing 0.05% Tween-20
(TBST) and blocked in TBST supplemented with 5% non-fat dried milk.
The membrane was further incubated with the respective primary
antibodies, p-ERK-1/2 (1:2,000), t-ERK-1/2 (1:2,000), p-JNK-1/2
(1:2,000), t-JNK-1/2 (1:2,000), p-p38 MAPK (1:2,000), t-p38 MAPK
(1:2,000), p-PKB (1:2,000), t-PKB (1:2,000), IκB-α (1:2,000), COX-2
(1:2,000) or β-actin (1:10,000). The membrane was subsequently
incubated with appropriate secondary antibodies coupled to
horseradish peroxidase and developed in enzyme-linked
chemiluminescence (ECL) western blotting detection reagents
(Amersham Pharmacia Biotech, Oakville, ON, USA).
Analysis of COX-2 protein stability
The HEI-OC1 cells were initially grown in the
absence or presence of IL-1β for 8 h to highly induce endogenous
COX-2 protein. The cells were then treated with IL-1β alone or
IL-1β plus PDN for an additional 8, 16 or 24 h in the presence of
CHX, a translational inhibitor. At each time point, whole cell
lysate was prepared and subjected to western blot analysis for
COX-2 or actin to determine the amount of each protein remaining in
the cells.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) according to the instructions
provided by the manufacturer. Five micrograms of total RNA were
reverse transcribed using 8 μl of M-MLV reverse transcription 5×
buffer, 3 μl of 10 mM dNTPs, 0.45 μl of 10,000 units (U) RNase
inhibitor, 0.3 μl of 50,000 U M-MLV reverse transcriptase (Promega,
Madison, WI, USA) and 1.5 μl of 50 pM oligo(dT) (Bioneer, Chungbuk,
Korea) in a 40 μl volume. Single-stranded cDNA was then amplified
by PCR using 4 μl of 5× green GoTaq® flexi buffer, 0.4
μl of 10 mM dNTPs, 0.1 μl of 500 U Taq polymerase, 1.2 μl of 25 mM
MgCl2 (Promega) and 0.4 μl of each 20 pM of specific
sense and antisense primer of COX-2 or glyceraldehyde-3-phosphate
dehydrogenase (GAPDH). The primer sequences used for PCR were as
follows: COX-2 forward, 5′-CTG TAC TAC GCC GAG ATT CCT GA-3′ and
reverse, 5′-GTC CTC GCT TCT GAT CTG TCT TG-3′; GAPDH forward,
5′-GGT GAA GGT CGG TGT GAA CG-3′ and reverse, 5′-GGT AGG AAC ACG
GAA GGC CA-3′. The PCR products were then analyzed on a 1.2%
agarose gel.
Analysis of COX-2 mRNA stability
The HEI-OC1 cells were initially grown in the
absence or presence of IL-1β for 8 h to highly induce endogenous
COX-2 mRNA expression. The cells were then treated with IL-1β alone
or IL-1β plus PDN for an additional 2, 4 or 8 h in the presence of
Act D, a transcriptional inhibitor, to block ongoing transcription
for an additional 1, 2, 4 or 8 h. At each time point, total RNA was
isolated and subjected to RT-PCR for COX-2 or GAPDH to determine
the amount of mRNA remaining in the cells.
Transfection and luciferase assay
The HEI-OC1 cells were seeded in 6-well plates the
day prior to transfection at a concentration of 2×105
cells/plate in a 2 ml volume. The cells were transfected with 1 μg
of the luciferase DNA construct containing the COX-2 promoter or 20
ng of the Renilla luciferase expression vector control
pRL-TK DNA (Promega) using Lipofectamine 2000 reagent (Invitrogen)
according to the manufacturer’s instructions. At 24 h
post-transfection, the cells were grown for an additional 8 h in
the absence or presence of PDN. The cells were then washed and
lysed, followed by the measurement of luciferase activity using a
Dual-Luciferase assay kit (Promega) and a luminometer Victor3
(product no: 1420-011; Perkin Elmer Medical Imaging, Santa Clara,
CA, USA). The luciferase activity was normalized with expression of
control pRL-TK.
Measurement of prostaglandin
E2 (PGE2) production
PGE2 production was measured using a
PGE2 enzyme-linked immunosorbent assay (ELISA) kit
(Amersham Biosciences). Briefly, 6×105 cells plated were
initially serum starved for 24 h and were then treated with IL-1β
alone or with IL-1β plus PDN at the indicated concentrations for 8
h. The conditioned medium was collected and subjected to
PGE2 ELISA assay in a 96-well plate according to the
manufacturer’s instructions. In principal, the product of this
enzymatic reaction has a blue color that is absorbed at 450 nm. The
extent of the color is inversely proportional to the amount of free
PGE2 present in the well during incubation.
Statistical analysis
The results are expressed as the means ± standard
error (SE), and the significance of differences between groups was
determined by one-way ANOVA. Differences were considered
statistically significant when the P-value was <0.05.
Results
IL-1β induces the expression of COX-2
through transcriptional upregulation in HEI-OC1 cells
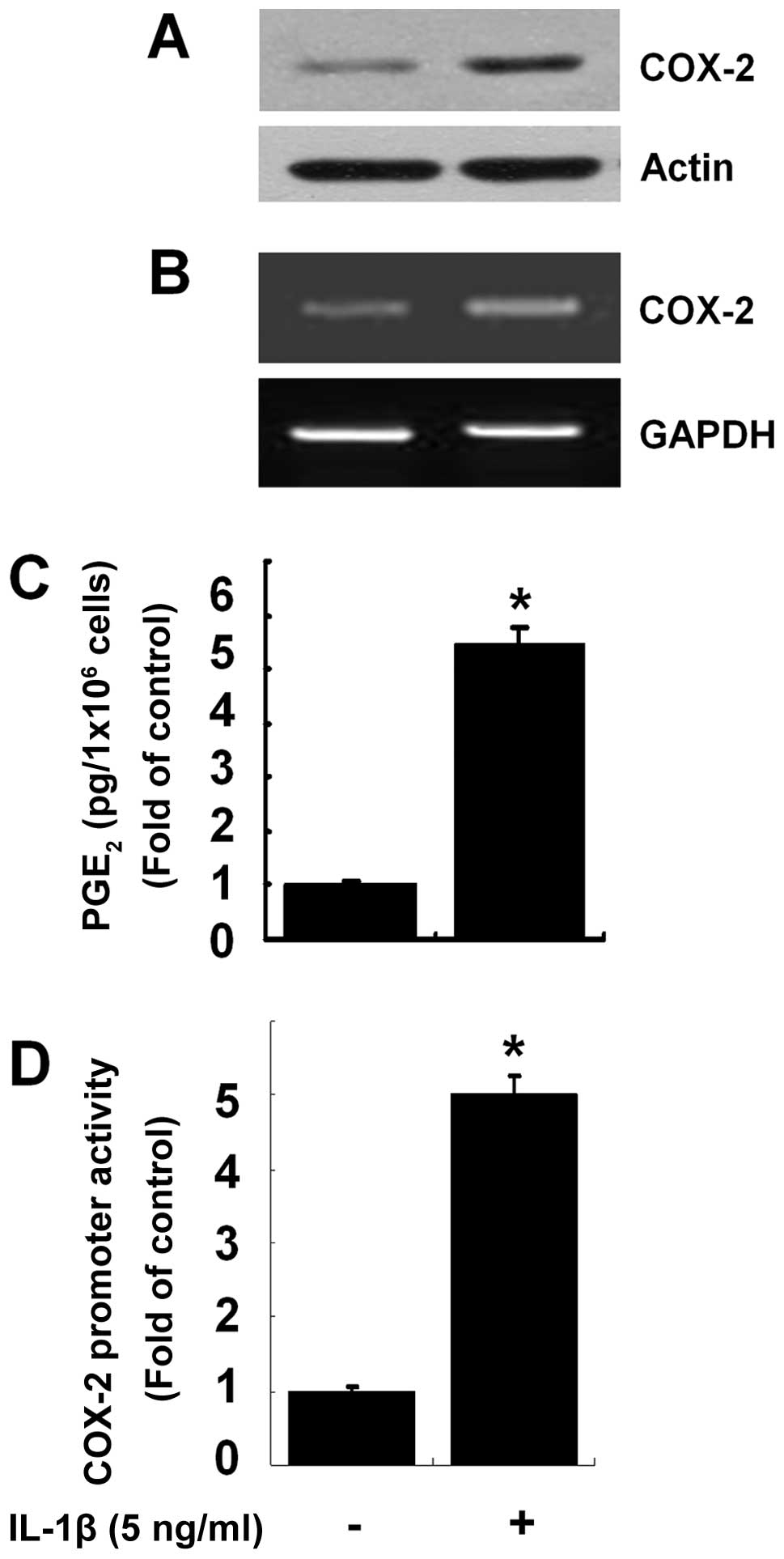
Initially, we investigated the effects of IL-1β on
the protein expression of COX-2 in the HEI-OC1 cells. Treatment
with IL-1β at 5 ng/ml for 8 h increased COX-2 protein expression in
the HEI-OC1 cells compared with the control (no IL-1β treatment)
(Fig. 1A). RT-PCR was then
performed to determine whether the protein expression of COX-2
induced by IL-1β was due to the increased transcription of COX-2.
Treatment with IL-1β at 5 ng/ml for 8 h also increased the mRNA
levels of COX-2 in the HEI-OC1 cells compared with the control
(Fig. 1B). To determine the
induction of COX-2 protein expression by IL-1β at the functional
level, the effects of IL-1β on the production of PGE2, a
major and stable COX-2 metabolite, in the IL-1β-treated HEI-OC1
cells was also determined. Compared with the control, treatment
with IL-1β at 5 ng/ml for 8 h led to marked increase in the
production of PGE2, indicating that COX-2 was
enzymatically active. Luciferase transfection experiments were then
performed to determine the effects of IL-1β on COX-2 promoter
activity in the HEI-OC1 cells. Treatment with IL-1β stimulated the
COX-2 promoter-driven luciferase expression in the HEI-OC1 cells
(Fig. 1D).
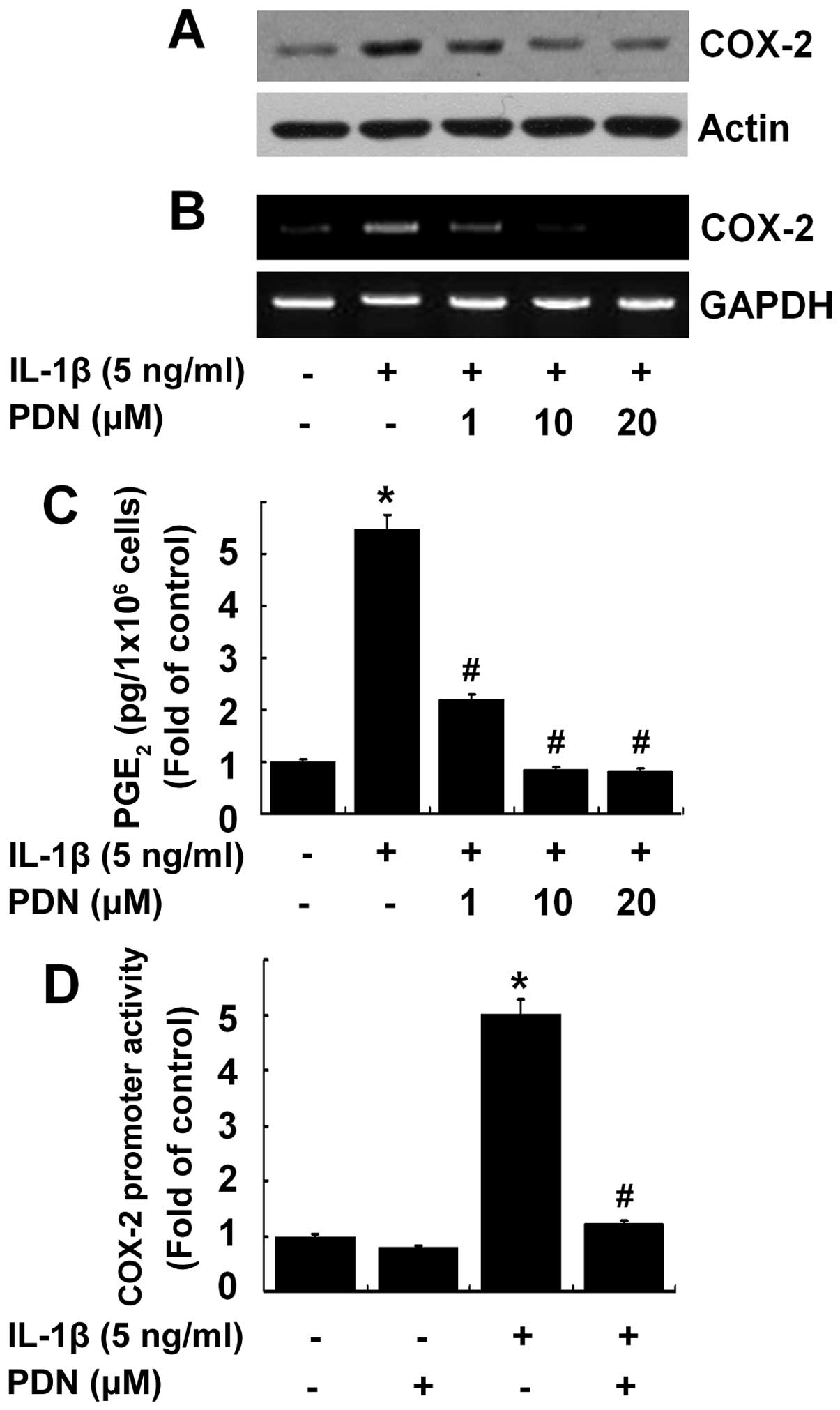
PDN inhibits IL-1β-induced COX-2
expression and PGE2 production in HEI-OC1 cells
We then determined whether PDN inhibits the
IL-1β-induced COX-2 expression and activity in the HEI-OC1 cells.
Treatment with PDN suppressed the protein and mRNA expression of
COX-2 induced by IL-1β in a concentration-dependent manner
(Fig. 2A and B), as well as the
production of PGE2 (Fig.
2C) in the HEI-OC1 cells. Treatment with PDN at the dose of 10
μM was sufficient to suppress the IL-1β-induced expression and
activity of COX-2. Treatment with PDN at 10 μM also markedly
suppressed the COX-2 promoter-driven luciferase expression induced
by IL-1β in the HEI-OC1 cells (Fig.
2D).
PDN does not affect COX-2 protein and
mRNA stability in IL-1β-treated HEI-OC1 cells
COX-2 expression is also regulated by protein
turnover (35). This promptly led
us to investigate whether PDN affects the stability of COX-2
protein in the IL-1β-treated HEI-OC1 cells. As shown in Fig. 3A, the HEI-OC1 cells were initially
treated with IL-1β (lanes 2–8) or without IL-1β (lane 1) for 6 h to
induce the protein expression of COX-2. After 6 h, the cells (lanes
1 and 2) were subjected to total cell lysate extraction (indicated
as the 0 h time point). The remaining cells (lanes 3–8) were then
exposed to IL-1β with or without PDN in the presence of CHX, a
translational inhibitor, for an additional 8, 16 or 24 h, following
the preparation of total cell lysates at each time point. When
ongoing translation was blocked by CHX, there was no change in the
protein levels of COX-2 in the IL-1β-treated HEI-OC1 cells in the
absence or presence of PDN, suggesting that PDN does not affect
COX-2 protein stability in the IL-1β-treated HEI-OC1 cells. The
protein expression of actin was not affected in the HEI-OC1 cells
treated with or without IL-1β in the absence or presence of PDN at
the indicated doses and for the indicated periods of time (Fig. 3A and B, lanes 1–8). COX-2
expression is also controlled by the COX-2 mRNA turnover (35,42,43). When ongoing transcription was
blocked by Act D, a transcriptional inhibitor, there was no
difference in the COX-2 mRNA levels in the IL-1β-treated HEI-OC1
cells either in the absence or presence of PDN (Fig. 3B), indicating that PDN had no
effect on COX-2 mRNA stability in the IL-1β-treated cells. The mRNA
expression of GAPDH remained constant in the HEI-OC1 cells treated
without or with IL-1β in the absence or presence of PDN at the
indicated doses and for the indicated periods of time (Fig. 3B, lanes 1–10).
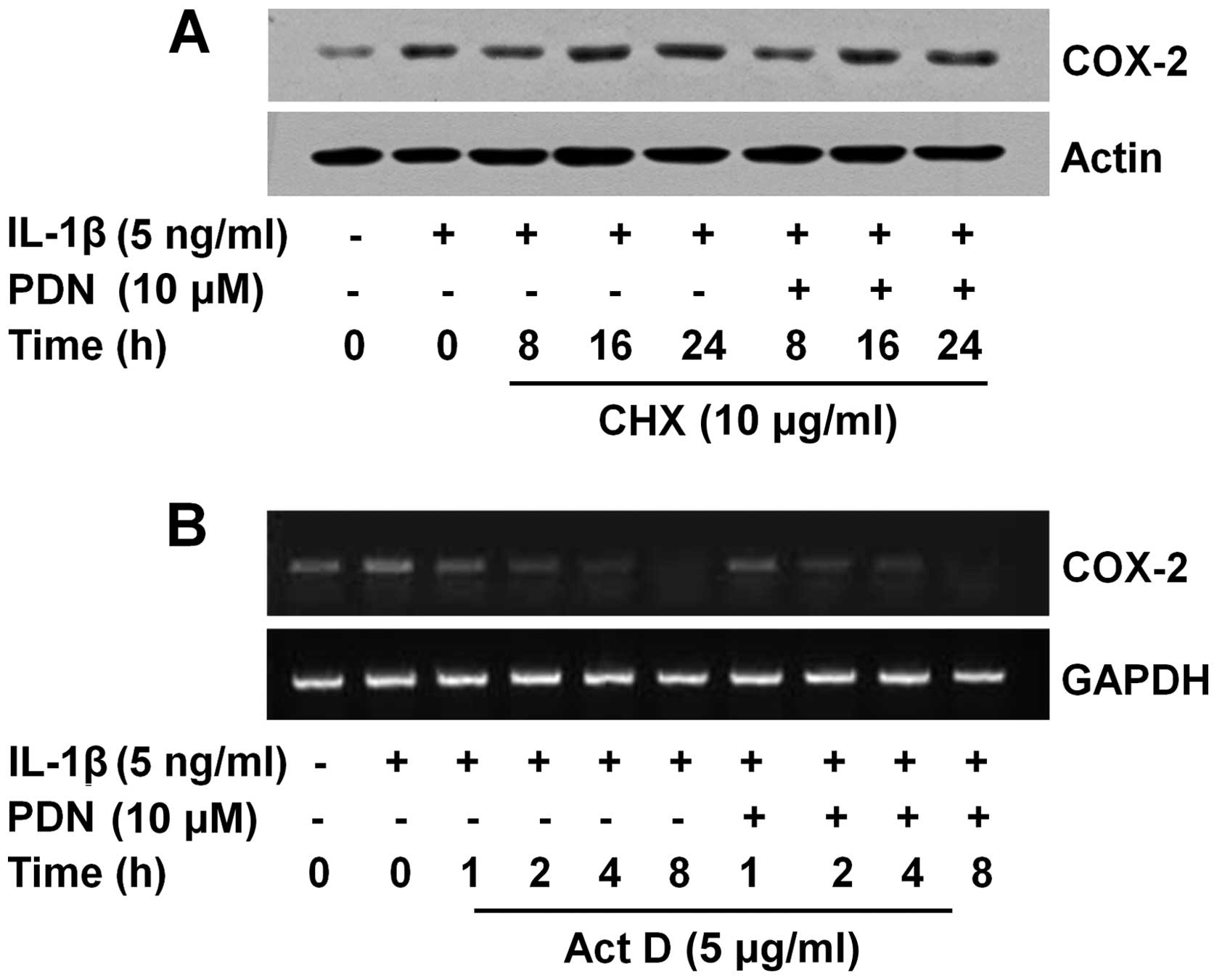 | Figure 3Effects of prednisone (PDN) on the
stability of cyclooxygenase (COX)-2 protein and mRNA in interleukin
(IL)-1β-treated House Ear Institute-Organ of Corti 1 (HEI-OC1)
cells. (A) HEI-OC1 cells were serum-starved for 24 h, and then
treated with (lanes 2–8) or without (lane 1) IL-1β for 8 h to
highly induce COX-2 protein expression. HEI-OC1 cells were further
exposed to IL-1β with (lanes 6–8) or without (lanes 3–5) or with
PDN in the presence of cycloheximide (CHX), a translational
inhibitor, for an additional 8, 16 or 24 h. At each time point,
whole cell lysates were prepared and analyzed by western blot
analysis. Each blot is representative of 3 independent experiments.
(B) HEI-OC1 cells were serum-starved for 24 h and were then treated
with (lanes 2–10) or without (lane 1) IL-1β for 8 h to highly
induce COX-2 mRNA expression. HEI-OC1 cells were further exposed to
IL-1β with (lanes 7–10) or without (lanes 3–6) PDN in the presence
of actinomycin D (Act D), a transcriptional inhibitor, for an
additional 1, 2, 4 or 8 h. At each time point, total RNA was
prepared and analyzed by RT-PCR. Each blot is representative of 3
independent experiments. |
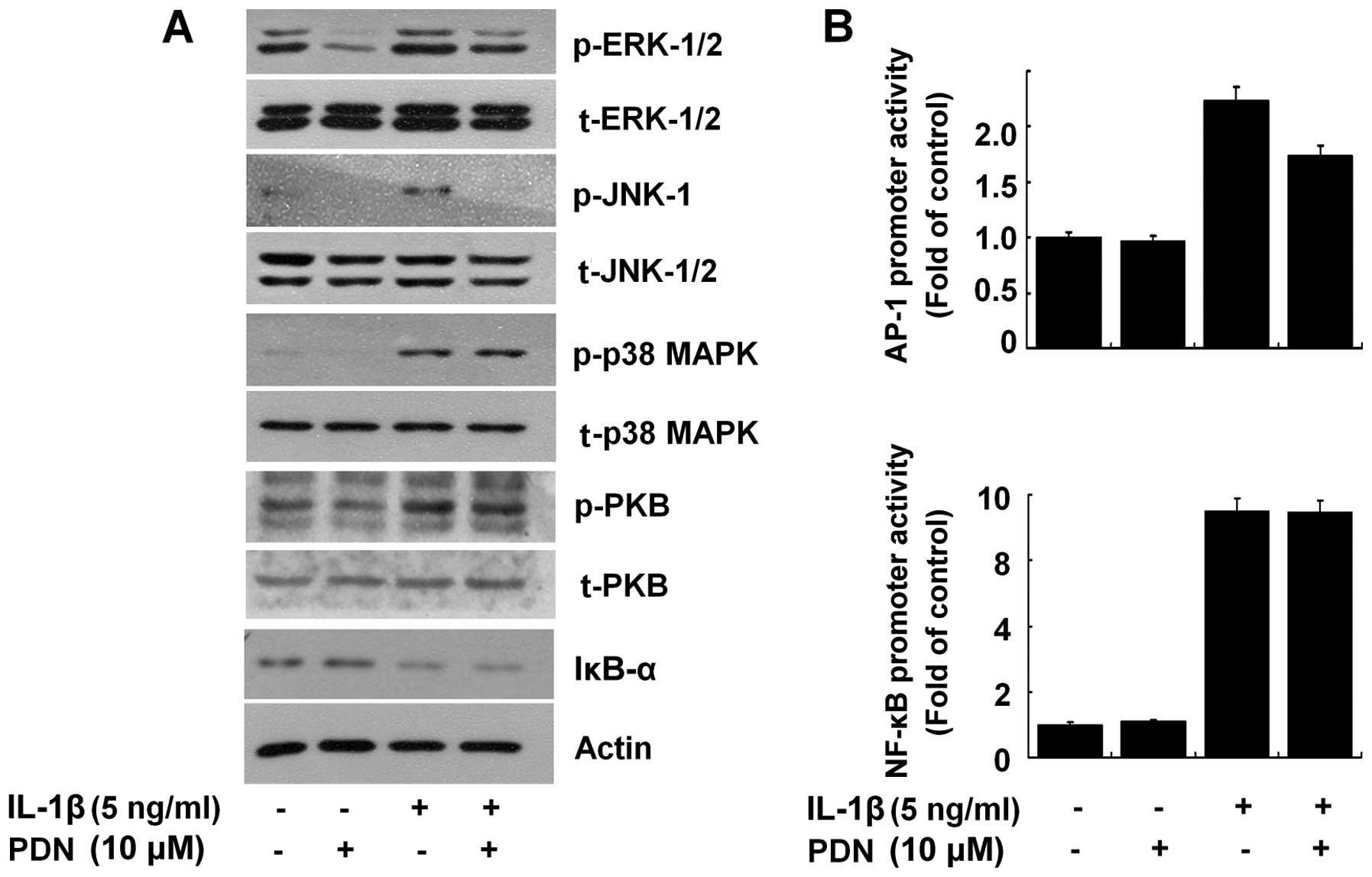
PDN selectively blocks the IL-1β-induced
activation of ERK-1/2, JNK-1 and AP-1 in HEI-OC1 cells
We then determined whether PDN affects the
activation of intracellular signaling proteins, herein the family
of MAPKs (ERK-1/2, JNK-1/2 and p38 MAPK), PKB and the NF-κB
transcription factor in the IL-1β-treated HEI-OC1 cells. The
activation of the MAPK family, PKB and NF-κB by IL-1β was assessed
by measuring the phosphorylation levels of ERK-1/2, JNK-1/2, p38
MAPK or PKB and the proteolysis of IκB-α (an inhibitor of NF-κB
activation). As shown in Fig. 4A,
compared with the control (no treatment; lane 1), the exposure of
HEI-OC1 cells to IL-1β increased the phosphorylation levels of
ERK-1/2, JNK-1, p38 MAPK and PKB, and induced the proteolysis of
IκB-α (lane 3). Notably, PDN inhibited the IL-1β-induced
phosphorylation of ERK-1/2 and JNK-1, but did not affect the
IL-1β-induced phosphorylation of PKB and the proteolysis of IκB-α
in the HEI-OC1 cells (Fig. 4A,
lane 4). The total expression levels of ERK-1/2, JNK-1/2, p38 MAPK,
PKB and actin remained largely unaffected in the HEI-OC1 cells
treated without or with IL-1β in the absence or presence of PDN.
Subsequent luciferase experiments revealed that PDN partially
suppressed the AP-1 promoter-driven luciferase expression, but not
the NF-κB promoter-driven luciferase expression induced by IL-1β in
the HEI-OC1 cells (Fig. 4B).
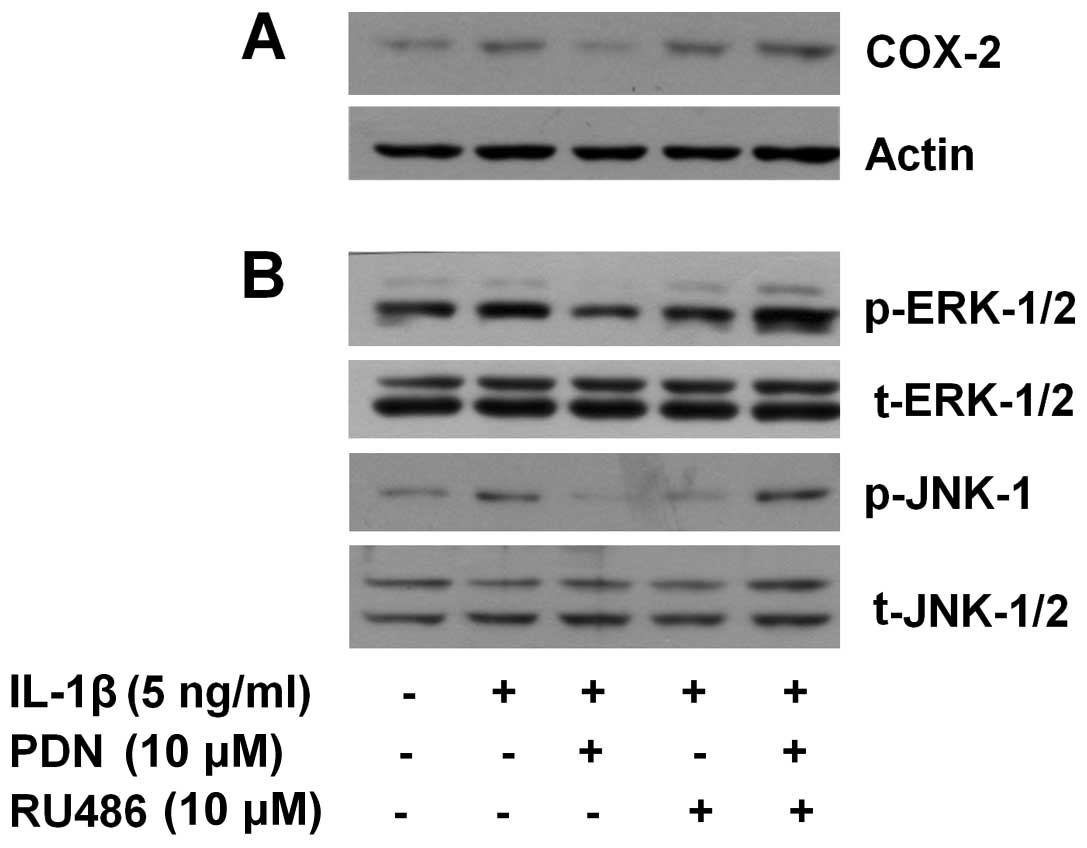
RU486 attenuates the inhibitory effects
of PDN on the IL-1β-induced expression of COX-2 and activation of
ERK-1/2 and JNK-1 in HEI-OC1 cells
Glucocorticoids bind to and act through the GR.
RU486 is an antagonist of GR (37). Using RU486, we then determined
whether PDN exerts its inhibitory effects on the IL-1β-induced
expression of COX-2 and the activation of ERK-1/2 and JNK-1 in the
IL-1β-treated HEI-OC1 cells through GR. As anticipated, the
IL-1β-induced expression of COX-2 and the activation of ERK-1/2 and
JNK-1 in the HEI-OC1 cells (Fig.
5, lane 2) was largely suppressed by PDN (Fig. 5, lane 3). In the absence of PDN,
pre-treatment with RU486 did not affect the IL-1β-induced
expression of COX-2 and the activation of ERK-1/2 and JNK-1 in the
HEI-OC1 cells (Fig. 5, lane 4).
However, the suppressive effects of PDN on the IL-1β-induced
expression of COX-2 and the activation of ERK-1/2 and JNK-1 in the
HEI-OC1 cells were not evident following pre-treatment with RU486
(Fig. 5, lane 5). The total
expression levels of ERK-1/2, JNK-1/2 and actin remained constant
in the HEI-OC1 cells treated with or without IL-1β in the absence
or presence of PDN and/or RU486.
Discussion
Existing evidence suggests a causative role of
cochlear inflammation in the development of hearing loss. Little is
known about the regulation of COX-2 gene expression by IL-1β, a
pro-inflammatory cytokine, and/or PDN, a well known steroid
clinically used in the treatment of hearing loss (5,6).
In the present study, we investigated the effects of IL-1β and/or
PDN on COX-2 expression in HEI-OC1 murine cochlear cells. To the
best of our knowledge, this is the first study to demonstrate that
IL-1β induces COX-2 expression through transcriptional
upregulation, while PDN inhibits the IL-1β-induced COX-2 expression
through GR by transcriptional downregulation. Moreover, we provide
evidence that PDN selectively blocks IL-1β signaling to activate
ERK-1/2, JNK-1 and AP-1, which contributes to the transcriptional
downregulation of COX-2.
It has been reported that an inflammatory response
occurs in the inner ear under ear-damaging conditions, such as
bacterial infection or noise overstimulation (23). In a previous study, it was
demonstrated that the expression of pro-inflammatory cytokines,
including IL-1, IL-6 and TNF-α, is increased in Haemophilus
Influenzae-injected mice with acute otitis media (24), suggesting that these cytokines
play a role in cochlear inflammation. In this study, we
demonstrated that the exposure of HEI-OC1 cells to IL-1β
upregulated the protein expression of COX-2 (Fig. 1A), which was attributed to
increased COX-2 transcription (Fig.
1B) and promoter activity (Fig.
1D); we also demonstrated the IL-1β-induced COX-2 protein at
the functional level (Fig. 1C),
as assessed by the high production of PGE2 in the
HEI-OC1 cells. It is likely that IL-1β plays a role in cochlear
inflammation by producing inflammatory mediators, such as COX-2 and
PGE2, while PDN may reduce the cochlear inflammatory
response and/or the mediators triggered by the cytokine.
The regulation of COX-2 gene expression is primarily
controlled at the transcriptional level. Earlier studies have shown
that the COX-2 transcriptional induction is largely dependent on
the activities of several transcription factors, which cognately
bind to cis-acting elements, including NF-κB, AP-1, cyclic
AMP response element (CRE) or NF-IL6, within the COX-2 promoter
(25–27). Among these, NF-κB is a
redox-sensitive transcription factor and its activation is closely
linked to the rapid proteolytic degradation of IκB-α, a NF-κB
inhibitory protein (28). The
rapid degradation of IκB-α unmasks the nuclear localization signals
of NF-κB, which then translocates to the nucleus and activates the
transcription of multiple genes, including COX-2. Ample evidence
indicates that NF-κB activation is important for COX-2
transcriptional upregulation in response to extracellular stimuli
(27,29,30). AP-1 is another transcription
factor that binds to AP-1 cis-acting element and stimulates
COX-2 transcription (31), and
its activation is largely dependent on the activity of upstream
signaling effectors, such as ERK-1/2 and JNK-1/2 (32,33). It has been demonstrated that
treatment with IL-1β induces the phosphorylation of MAPKs (ERK-1/2,
JNK-1/2 and p38 MAPK) and the IκB-α proteolysis-dependent
activation of NF-κB, and their activation is critical for the
cytokine-mediated induction of COX-2 expression in various types of
cells (29–31,34,35). Of note, has also been demonstrated
that treatment with IL-1β (1 ng/ml) for 15 min increases the
phosphorylation of ERK-1/2 and JNK-1/2, but not that of p38 MAPK in
HEI-OC1 cells (36). In this
study, we demonstrated that treatment with IL-1β (5 ng/ml) for 30
min induced not only the phosphorylation of ERK-1/2, JNK-1/2 and
p38 MAPK but also the proteolysis of IκB-α in the HEI-OC1 cells
(Fig. 4A). Furthermore, it was
demonstrated that treatment with IL-1β induced the AP-1 or NF-κB
promoter-driven luciferase expression in the HEI-OC1 cells
(Fig. 4B). These results
therefore suggest that treatment with IL-1β rapidly triggers the
ERK-1/2- and JNK-1-dependent activation of AP-1, as well as IκB-α
proteolysis-dependent NF-κB activation in the HEI-OC1 cells, which
contribute to COX-2 transcriptional upregulation.
Glucocorticoids are among the most widely used drugs
worldwide and are effective in a number of inflammatory and immune
diseases (37). A major factor
contributing to the anti-inflammatory action of glucocorticoids is
the suppression of the expression and/or production of inflammatory
mediators (38). PDN, a
glucocorticoid agonist, has been shown to be an effective treatment
for several inner ear disorders, including sudden idiopathic
sensorineural hearing loss (39).
At present, however, the PDN regulation of the cytokine-induced
inflammatory response and signal transduction in cochlear cells is
not well understood. In the present study, we demonstrated the
ability of PDN to interfere with IL-1β signaling to induce the
activation of ERK-1/2, JNK-1 and AP-1 without affecting the
phosphorylation of p38 MAPK, the proteolysis of IκB-α and the
activation of NF-κB in the HEI-OC1 cells (Fig. 4), suggesting that PDN selectively
blocks these signaling components activated by IL-1β in the cells.
Considering that the activity of ERK-1/2, JNK-1/2 and AP-1 is
important for COX-2 expression (30,31,34), it is likely that suppressive
effects of PDN on the IL-1β-induced expression of COX-2 in HEI-OC1
cells is through the inhibition of ERK-1/2, JNK-1 and AP-1
activity. Glucocorticoids act by binding to GR and subsequently
translocating to the nucleus where they either increase
(transactivate) or decrease (transrepress) target gene expression
(18,40,41). In this study, we demonstrated that
the inhibitory effects of PDN on the IL-1β-induced COX-2 expression
and the activation of ERK-1/2 and JNK-1 in HEI-OC1 cells were
largely diminished by pre-treatment with RU486, a GR antagonist
(Fig. 5B). These results strongly
suggest that PDN, through GR, abrogates IL-1β signal transduction
leading to COX-2 expression and the activation of ERK-1/2, JNK-1
and AP-1 in the HEI-OC1 cells.
We, as well as others have previously reported that
COX-2 expression is regulated at the post-transcriptional (mRNA
stability) and/or translational (protein turnover) levels (35,42–44). In the present study, however,
experiments using Act D and CHX revealed that PDN did not affect
the mRNA and protein stability of COX-2 in the IL-1β-treated
HEI-OC1 cells (Fig. 3), further
stressing that the inhibitory effects of PDN on IL-1β-induced COX-2
expression occur through transcriptional downregulation.
In conclusion, to the best of our knowledge, this is
the first study to demonstrate that IL-1β induces the high
expression of functional COX-2 through COX-2 transcriptional
upregulation and the activation of ERK-1/2, JNK-1, p38 MAPK and
NF-κB, whereas PDN inhibits the cytokine-induced COX-2 expression
through GR-dependent COX-2 transcriptional downregulation and the
inhibition of ERK-1/2, JNK-1 and AP-1 activity in HEI-OC1 cells.
The findings presented herein provide insight into the molecular
basis of the beneficial effects of PDN against cochlear
inflammation-related hearing loss.
Acknowledgements
This study was supported by the research promotion
grant from the Keimyung University Dongsan Medical Center in
2008.
References
|
1
|
World Health Organization. Epidemic
meningococcal disease. WHO fact sheet. (105)1998.
|
|
2
|
Schuchat A, Robinson K, Wenger JD, et al:
Bacterial meningitis in the United States in 1995. Active
Surveillance Team. N Engl J Med. 337:970–976. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davis AC: Epidemiological profile of
hearing impairments: the scale and nature of the problem with
special reference to the elderly. Acta Otolaryngol Suppl.
476:23–31. 1990.PubMed/NCBI
|
|
4
|
Ries PW: Prevalence and characteristics of
persons with hearing trouble: United States, 1990–91. Vital Health
Stat. 188:1–75. 1994.PubMed/NCBI
|
|
5
|
Satoh H, Firestein GS, Billings PB, Harris
JP and Keithley EM: Proinflammatory cytokine expression in the
endolymphatic sac during inner ear inflammation. J Assoc Res
Otolaryngol. 4:139–147. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wakabayashi K, Fujioka M, Kanzaki S, et
al: Blockade of interleukin-6 signaling suppressed cochlear
inflammatory response and improved hearing impairment in
noise-damaged mice cochlea. Neurosci Res. 66:345–352. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matz G: Aminoglycoside cochlear
ototoxicity. Otolaryngol Clin North Am. 26:705–712. 1993.PubMed/NCBI
|
|
8
|
Smith WL, DeWitt DL and Garavito RM:
Cyclooxygenases: structural, cellular, and molecular biology. Annu
Rev Biochem. 69:145–182. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smith WL and DeWitt DL: Prostaglandin
endoperoxide H synthases-1 and -2. Adv Immunol. 62:167–215. 1996.
View Article : Google Scholar
|
|
10
|
Vane JR, Bakhle YS and Botting RM:
Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 38:97–120.
1998. View Article : Google Scholar
|
|
11
|
Feng L, Sun W, Xia Y, et al: Cloning two
isoforms of rat cyclooxygenase: differential regulation of their
expression. Arch Biochem Biophys. 307:361–368. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cho JW, Park K, Kweon GR, et al: Curcumin
inhibits the expression of COX-2 in UVB-irradiated human
keratinocytes (HaCaT) by inhibiting activation of AP-1: p38 MAP
kinase and JNK as potential upstream targets. Exp Mol Med.
37:186–192. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dubois RN, Abramson SB, Crofford L, Gupta
RA, Simon LS, Van De Putte LB and Lipsky P: Cyclooxygenase in
biology and disease. FASEB J. 12:1063–1073. 1998.PubMed/NCBI
|
|
14
|
Stjernschantz J, Wentzel P and
Rask-Andersen H: Localization of prostanoid receptors and
cyclo-oxygenase enzymes in guinea pig and human cochlea. Hear Res.
197:65–73. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ziegler EA, Brieger J, Heinrich UR and
Mann WJ: Immuno-histochemical localization of cyclooxygenase
isoforms in the organ of Corti and the spiral ganglion cells of
guinea pig cochlea. ORL J Otorhinolaryngol Relat Spec. 66:297–301.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Malysheva OA, Wahle M, Wagner U, Pierer M,
Arnold S, Häntzschel H and Baerwald CG: Low-dose prednisolone in
rheumatoid arthritis: adverse effects of various disease modifying
antirheumatic drugs. J Rheumatol. 35:979–985. 2008.PubMed/NCBI
|
|
17
|
Barnes PJ and Adcock IM: How do
corticosteroids work in asthma? Ann Intern Med. 139:359–370. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scheinman RI, Gualberto A, Jewell CM,
Cidlowski JA and Baldwin AS Jr: Characterization of mechanisms
involved in transrepression of NF-kappa B by activated
glucocorticoid receptors. Mol Cell Biol. 15:943–953.
1995.PubMed/NCBI
|
|
19
|
De Bosscher K, Beck IM, Dejager L, et al:
Selective modulation of the glucocorticoid receptor can distinguish
between transrepression of NF-κB and AP-1. Cell Mol Life Sci.
71:143–163. 2014.PubMed/NCBI
|
|
20
|
Langlais D, Couture C, Balsalobre A and
Drouin J: The Stat3/GR interaction code: predictive value of
direct/indirect DNA recruitment for transcription outcome. Mol
Cell. 47:38–49. 2012.PubMed/NCBI
|
|
21
|
Adcock IM: Glucocorticoid-regulated
transcription factors. Pulm Pharmacol Ther. 14:211–219. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kalinec GM, Webster P, Lim DJ and Kalinec
F: A cochlear cell line as an in vitro system for drug ototoxicity
screening. Audiol Neurootol. 8:177–189. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fujioka M, Kanzaki S, Okano HJ, Masuda M,
Ogawa K and Okano H: Proinflammatory cytokines expression in
noise-induced damaged cochlea. J Neurosci Res. 83:575–583. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ghaheri BA, Kempton JB, Pillers DA and
Trune DR: Cochlear cytokine gene expression in murine acute otitis
media. Laryngoscope. 117:22–29. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Herschman HR, Reddy ST and Xie W: Function
and regulation of prostaglandin synthase-2. Adv Exp Med Biol.
407:61–66. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Inoue H, Yokoyama C, Hara S, Tone Y and
Tanabe T: Transcriptional regulation of human
prostaglandin-endoperoxide synthase-2 gene by lipopolysaccharide
and phorbol ester in vascular endothelial cells. Involvement of
both nuclear factor for interleukin-6 expression site and cAMP
response element. J Biol Chem. 270:24965–24971. 1995. View Article : Google Scholar
|
|
27
|
Inoue H and Tanabe T: Transcriptional role
of the nuclear factor kappa B site in the induction by
lipopolysaccharide and suppression by dexamethasone of
cyclooxygenase-2 in U937 cells. Biochem Biophys Res Commun.
244:143–148. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ghosh S and Baltimore D: Activation in
vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B.
Nature. 344:678–682. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Newton R, Kuitert LM, Bergmann M, Adcock
IM and Barnes PJ: Evidence for involvement of NF-kappaB in the
transcriptional control of COX-2 gene expression by IL-1beta.
Biochem and Biophys Res Commun. 237:28–32. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jang BC, Paik JH, Kim SP, et al: Catalase
induced expression of inflammatory mediators via activation of
NF-κB, PI3K/AKT, p70S6K, and JNKs in BV2 microglia. Cell Signal.
17:625–633. 2005.
|
|
31
|
Allport VC, Slater DM, Newton R and
Bennett PR: NF-kappaB and AP-1 are required for cyclo-oxygenase 2
gene expression in amnion epithelial cell line (WISH). Mol Hum
Reprod. 6:561–565. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Benkoussa M, Brand C, Delmotte MH,
Formstecher P and Lefebvre P: Retinoic acid receptors inhibit AP1
activation by regulating extracellular signalregulated kinase and
CBP recruitment to an AP1-responsive promoter. Mol Cell Biol.
22:4522–4534. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Caelles C, Gonzalez-Sancho JM and Munoz A:
Nuclear hormone receptor antagonism with AP-1 by inhibition of the
JNK pathway. Genes Dev. 11:3351–3364. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Newton R, Stevens DA, Hart LA, Lindsay M,
Adcock IM and Barnes PJ: Super-induction of COX-2 mRNA by
cyclohexamide and interleukin-1beta involves increased
transcription and correlates with increased NF-kappaB and JNK
activation. FEBS Lett. 418:135–138. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jang BC, Sung SH, Park JG, et al:
Glucosamine hydrochloride specifically inhibits COX-2 by preventing
COX-2 N-glycosylation and by increasing COX-2 protein turnover in a
proteasome-dependent manner. J Biol Chem. 282:27622–27632. 2007.
View Article : Google Scholar
|
|
36
|
Nam SI: Interleukin-1beta up-regulates
inducible nitric oxide by way of phosphoinositide
3-kinase-dependent in a cochlear cell model. Laryngoscope.
116:2166–2170. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Barnes PJ: How corticosteroids control
inflammation: Quintiles Prize Lecture 2005. Br J Pharmacol.
148:245–254. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bailey JM: New mechanisms for effects of
anti-inflammatory glucocorticoids. Biofactors. 3:97–102.
1991.PubMed/NCBI
|
|
39
|
Herr BD and Marzo SJ: Intratympanic
steroid perfusion for refractory sudden sensorineural hearing loss.
Otolaryngol Head Neck Surg. 132:527–531. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
De Bosscher K, Van Craenenbroeck K, Meijer
OC and Haegeman G: Selective transrepression versus transactivation
mechanisms by glucocorticoid receptor modulators in stress and
immune systems. Eur J Pharmacol. 583:290–302. 2008.PubMed/NCBI
|
|
41
|
Newton R and Holden NS: Separating
transrepression and transactivation: a distressing divorce for the
glucocorticoid receptor? Mol Pharmacol. 72:799–809. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ristimaki A, Garfinkel S, Wessendorf J,
Maciag T and Hla T: Induction of cyclooxygenase-2 by interleukin-1
alpha. Evidence for post-transcriptional regulation. J Biol Chem.
269:11769–11775. 1994.PubMed/NCBI
|
|
43
|
Jang BC, Sanchez T, Schaefers HJ, et al:
Serum withdrawal-induced post-transcriptional stabilization of
cyclooxygenase-2 mRNA in MDA-MB-231 mammary carcinoma cells
requires the activity of the p38 stress-activated protein kinase. J
Biol Chem. 275:39507–39515. 2000. View Article : Google Scholar
|
|
44
|
Park YK, Hong H and Jang BC:
Transcriptional and translational regulation of COX-2 expression by
cadmium in C6 glioma cells. Int J Mol Med. 30:960–966.
2012.PubMed/NCBI
|