Introduction
Tissue-specific stem cells have differentiation
potential and regenerate missing tissue continuously (1,2).
At present, tissue regeneration using stem cells is considered
effective with good therapeutic value. However, there are some
technical issues, such as where stem cells can be obtained from,
how to induce differentiation into target tissue cells and how long
the cells can maintain their healthy status in the body. Adult stem
cells, such as hematopoietic, epithelial and neural stem cells, are
found in some parts of the human body (3–6).
Dental pulp cells (DPCs), which have the capacity to produce dentin
matrix, can be differentiated into osteoblasts, chondrocytes,
adipocytes, endothelial cells and neurons (7–10).
The undifferentiated progenitor pulp cells, residing in the
perivascular niche of the dental pulp, the so-called
subodontoblastic cell-rich zone, may migrate to the site of injury
for reparative dentinogenesis (11–13). Human dental pulp contains a
stromal stem cell population with high proliferative potential that
induces mineralized dentinogenesis and is positive for cell surface
markers, such as STRO-1, CD44 and CD146 (14–16). Although the expression levels of
these factors differ among species, including pigs, rats and
rodents, some markers provide the possibility of detecting specific
stemness in human DPCs (hDPCs) (17–20). In addition to DPCs, human
periodontal ligament stem cells (hPDLSCs) are found near the
periodontal ligament of the teeth and are involved in the
regeneration of the periodontal ligament, alveolar bone and
cementum (12,21–23). The morphology and mineralization
of hPDLSCs isolated from periodontal ligament tissue is similar to
that of hDPCs (21,24). Gingival fibroblasts (GFs) organize
the oral tissue attached to the alveolar bone of tooth sockets and
function as a biological mucosal barrier, producing inflammatory
cytokines such as interleukin (IL)-6 and IL-8 (25). Mesenchymal cells from the oral
mucosa contain stem cell factors that are excreted from human GF
(hGF)-like cells (26,27). Although it has been previously
reported that GFs contribute to oral wound healing with
phenotypically different features from other fibroblasts and
contain a mesenchymal stem cell population (28), their stemness remains unclear. The
present study focused on a comparison of the in vitro
osteo/dentinogenic differentiation potential of hPDLSCs, hGFs and
hDPCs together during extended culture. In addition, we
investigated whether hPDLSCs and/or hGFs affect the
osteo/dentinogenic differentiation of hDPCs in a co-culture
experiment.
Materials and methods
Isolation and culture of hDPCs, hPDLSCs
and hGFs
Five human third molars were collected from patients
aged 20–25 years under guidelines approved by the Institutional
Review Board (IRB) of Dankook Dental Hospital, Cheonan-si, Korea.
Following extraction, the periodontal ligament and attached
gingival tissue were scraped from the surface of the teeth. The
dental crown was fractured and the dental pulp was recovered. The
tissue was chopped with a scalpel into small sections under
sterilized conditions, and the suspension was incubated in α-MEM
(Gibco/Life Technologies, Carslbad, CA, USA) containing 20% fetal
bovine serum (FBS; HyClone, Logan, UT, USA) and antibiotics at 37°C
in a humid atmosphere containing 5% CO2 for 3–5 days.
The cells stretched out from the tissue sections were re-plated,
and 80% confluent cultures were used as passage number 0. Adherent
cells grown to 70% confluency were subcultured at a 1/5 dilution
for later passage. The media were replaced every 3 days until the
cells had grown to the appropriate confluency. The primary cultures
of pulp, periodontal ligament and gingival tissue were performed
separately with the cells of each tooth, and each culture was
analyzed separately. For differentiation, 5 mM β-glycerophosphate,
100 nM dexamethasone and 100 μM ascorbic acid were added to the
culture media as additives and the cells were incubated for 8–14
days, as previously described (15). For the indirect co-culture of the
cells, 2×105 cells of human DPCs at passage number 2
were cultured in a 6-well plate, and the same numbers of hPDLSCs
and hGFs were seeded into a 40-μm cell strainer (BD Falcon™; BD
Biosciences, San Jose, CA, USA), was placed on a 6-well plate
containing pulp cells for indirect co-culture. For culture on
polycaprolactone (PCL) membrane (kind gift from Dr H.W. Kim,
Dankook University), the membranes were sterilized in 70% alcohol
overnight, rinsed thoroughly with PBS, and treated with ultraviolet
(UV) light overnight. After sterilization, PCL membranes were
placed in tissue culture plates. The cell suspension at a density
of 80,000 cells/ml was seeded into each well and maintained in a
humidified atmosphere with 5% CO2 at 37°C.
Flow cytometry and immunophenotyping
At each passage, the hDPCs, hPDLs and hGFs were
harvested using trypsin-free dissociation buffer (Millipore,
Billerica, MA, USA) and suspended in PBS containing 5% fetal bovine
serum at 1×106 cells/ml of concentration. To
immunophenotype the primary cells, the cells were treated with
antibodies against the following human cell surface antigens: CD24
(555428), CD44 (550989), CD73 (550257), CD90 (555596), CD106
(555647), CD146 (550315) and CD166 (559263). Anti-human IgG was
used as a negative control. All the antibodies were labeled with
phycoerythrin (PE) and purchased from BD Biosciences. PE-conjugated
STRO-1 antibody was purchased from Santa Cruz Biotechnology
(sc-47733; Santa Cruz, CA, USA). The cells were analyzed by flow
cytometry using a FACSCalibur flow cytometer (BD Biosciences). The
FACS data were analyzed and plotted by measuring the mean
fluorescence using CellQuest software and the WinMDI program.
Alizarin red S staining
The hDPCs, hPDLs and hGFs were cultured and
stimulated for differentiation as previously described (15). Culture dishes were washed with
Ca2+-free PBS twice. The cells were fixed with 70%
ethanol for 5 min at room temperature, and were then allowed to dry
completely. For staining, the cells were treated with 2% Alizarin
red S (pH 4.5) (Sigma-Aldrich, St. Louis, MO, USA) for 1 min and
then washed with distilled water. For quantification, the deposits
bound to Alizarin red were extracted with 10% acetic acid.
Following neutralization with 10% ammonium hydroxide and the
optical density was analyzed at a wavelength of 405 nm.
Western blot analysis
Total protein was extracted from the cells using
cell lysis buffer (10 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA and
0.5% NP-40), and the protein concentration was estimated using
Bradford reagent (Bio-Rad Laboratories, Hercules, MA, USA). Forty
micrograms of total protein were resolved on SDS-PAGE,
electrotransferred onto a PVDF membrane, and probed with the
appropriate antibodies in TBS containing 0.05% Tween-20 and 5% skim
milk. Protein signals were visualized using an enhanced
chemiluminescence (ECL) detection system (Amersham Biosciences,
Piscataway, NJ, USA). Antibodies for dentin sialophosphoprotein
(DSPP; SC-73633) and dentin matrix protein-1 (DMP-1; M176) were
obtained from Santa Cruz Biotechnology and Takara Bio Inc. (Tokyo,
Japan), respectively. The primary antibodies and secondary
antibody, goat anti-rabbit IgG (Millipore), were used at a dilution
of 1:200–1:500 and 1:2,000, respectively.
Reverse transcription quantitative
(real-time) polymerase chain reaction (RT-qPCR)
Total RNA was isolated from
5×105–1×106 cells using the
NucleoSpin® RNA XS RNA prep kit (Macherey-Nagel Inc.,
Bethlehem, PA, USA) and 2 μg of total RNA were reverse-transcribed
into cDNA using the iScript cDNA Synthesis kit (Bio-Rad
Laboratories) with oligo primer in 25 μl of reaction mixture
consisting of RNA, dNTP, primer, RNasin and AMV reverse
transcriptase. Following a denaturation step, synthesis reaction
was performed for 60 min at 42°C. Equal amounts of cDNA were used
for the real-time amplification of the target genes in triplication
using the SsoFast EvaGreen Supermix/iQ SYBR®-Green
Supermix kit (Bio-Rad Laboratories) according to the instructions
for the CFX96 Real-Time PCR detection system (Bio-Rad
Laboratories). The relative expression levels of GAPDH, DMP-1 and
DSPP were determined. The primers used for real-time PCR were as
follows: GAPDH, forward, 5′-GGAGTCCACTGGCGTCTTCAC-3′ and reverse,
5′-GCTG ATGATCTTGAGGCTGTTGTC-3′; DMP-1, forward, 5′-CAG
GAAGAGGTGGTGAGTGAGTC-3′ and reverse, 5′-CTGGA TTCGCTGTCTGCTTGC-3′;
DSPP, forward, 5′-CAGTAC AGGATGAGTTAAATGCCAGTG-3′ and reverse,
5′-CCATT CCCTTCTCCCTTGTGACC-3′. The expression of GAPDH was used as
a reference. CFX Manager™ software version 1.5 was used in the
analysis of the results.
Results
hDPC, hPDLSC and hGF primary cultures
contain stem cell populations
To investigate passage-dependence of stemness from
the oral cell population, we obtained pulp, periodontal ligament
and attached gingival tissue from human adult third molars, and
performed primary cultures. Due to the fact that specific hDPSC and
hPDLSC surface markers have not been identified to date, general
mesenchymal stem cell markers were used to detect the stem cell
population within the primary cultures. We used CD44, CD73, CD90,
CD106, CD146, CD166 and STRO-1 to identify the stem cell
populations. CD44, CD73, CD90, CD146 and STRO-1 are general surface
proteins for identifying mesenchymal and hematopoietic stem cells
(29–36). In addition, both CD106 (VCAM-1)
and CD166 are markers for bone marrow mesenchymal stem cells
(37,38). The cells from each primary culture
were pooled and treated with surface antibody conjugated with PE,
and the fluorescence intensity of the cells was analyzed by flow
cytometry. The hDPCs and hPDLSCs during early passage strongly
expressed CD44, CD73, CD90, CD146 and CD166 (Fig. 1A and B, panels b-d, f and g).
Although CD24 and CD106 are dental apical papilla stem cell markers
(9,39), they were not expressed in the
hDPCs and hPDLSCs used in the present study (Fig. 1A and B, panels a and e). In
addition, STRO-1 was not a proper surface marker to define dental
stem cells from pulp and the periodontal ligament (data not shown),
although it has been used as a general mesenchymal stem cell
marker. The expression of CD44, CD73, CD90 and CD166 increased
during hDPC passage numbers 4–6 and decreased at passage number 8
(Fig. 1D, circle in panels a-c
and e). CD146 expression increased during early passage (Fig. 1D, circle in panel d). The levels
of CD146 and CD166 peaked during the initial passages of hPDLSCs
(Fig. 1D, square in panels d and
e), whereas CD73 and CD90 were highly expressed during passage
numbers 4–6 (Fig. 1D, square in
panels b and c). The expression of CD44 in the hPDLSCs was
consistent during the extended culture (Fig. 1D, square in panel a). When the
cells from the attached gingival tissue were cultured, the very
early passage numbers expressed the general mesenchymal stem cell
markers, CD44, CD73, CD90, CD146 and CD166 [Fig. 1C and D (triangle in panels a-e)].
The expression of these markers decreased rapidly with the increase
in passage number during extended culture. These findings indicate
that hGFs, as well as hDPCs and hPDLSCs, contain a stem cell
population during early passage in primary culture.
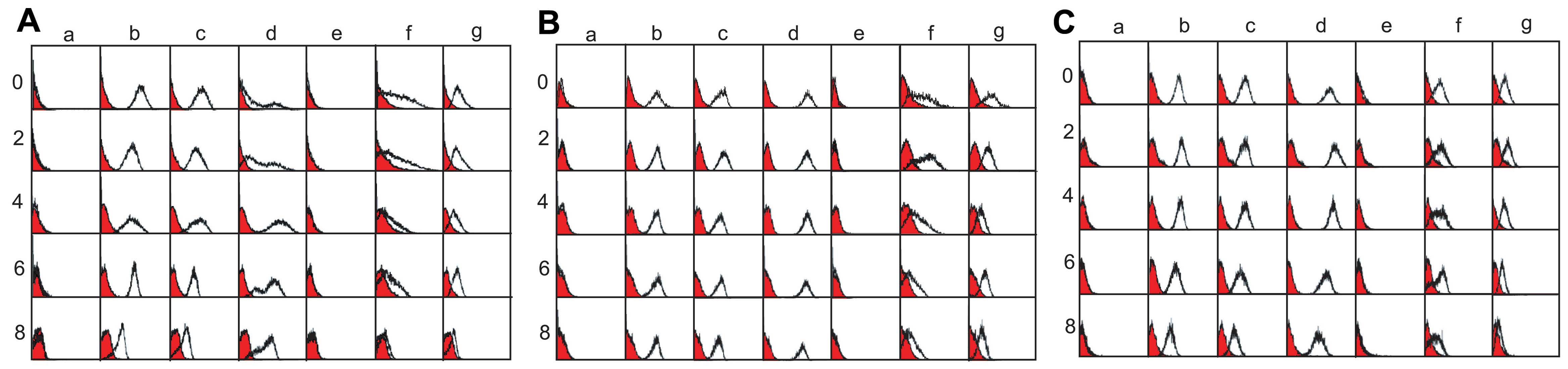 | Figure 1Immunophenotyping of (A) human dental
pulp cells (hDPCs), (B) periodontal ligament stem cells (hPDLSCs)
and (C) gingival fibroblasts (hGFs). Cells were treated with
phycoerythrin (PE)-conjugated antibodies against the cell surface
antigens. Anti-human IgG was used as a negative control (filled
curves), and the fluorescence intensity of the specific antibody
binding is indicated by open curves. Cells at passages 0–8 are
shown. Panels a, CD24; b, CD44; c, CD73; d, CD90; e, CD106; f,
CD146; and g, CD166. (D) The antibody binding affinity was
quantified and re-estimated as the mean fluorescence intensity
using CellQuest software and the WinMDI 2.9 program. panels a,
CD44; b, CD73; c, CD90; d, CD146; and e, CD166. |
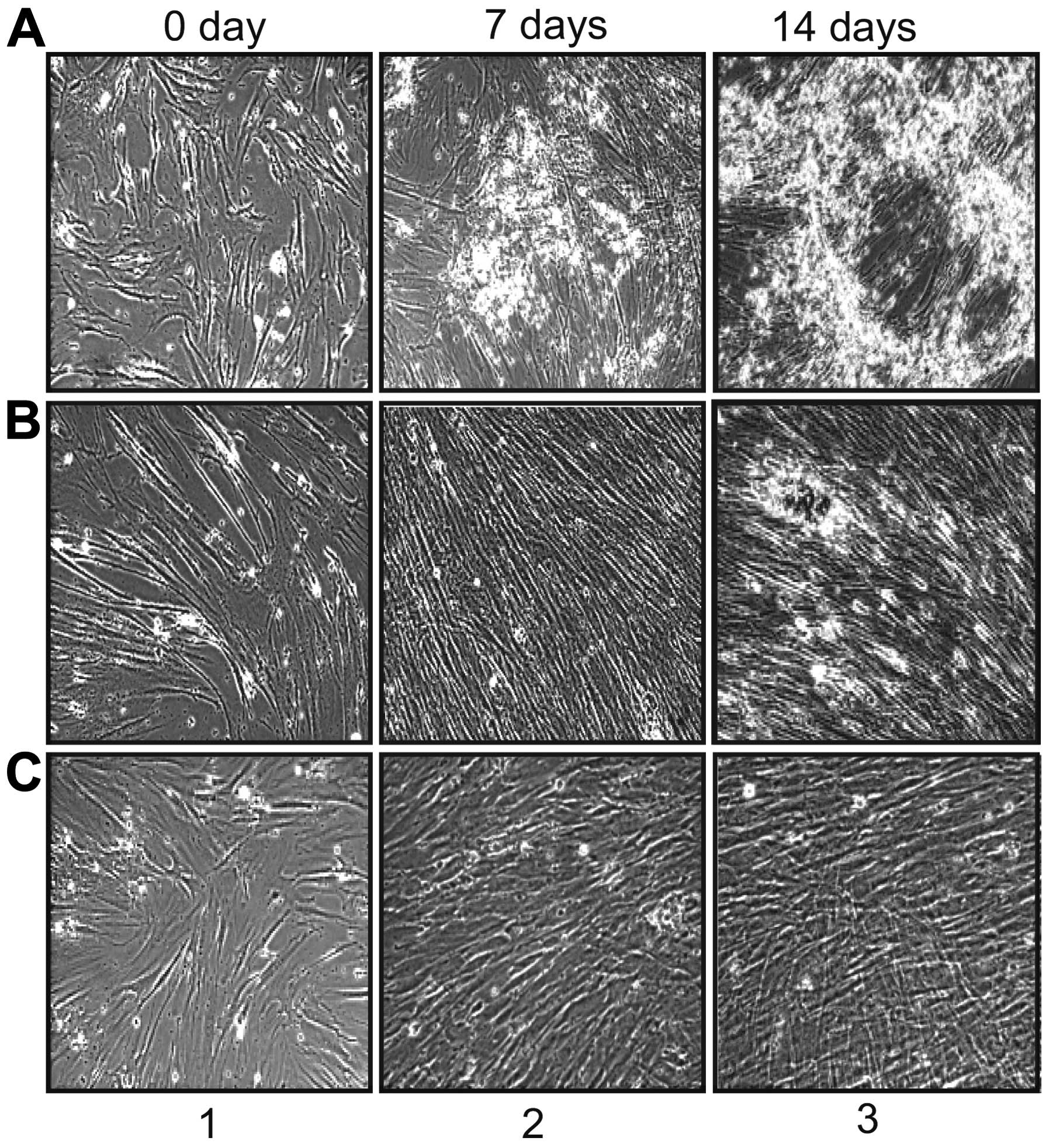
Mineralization efficiency of hDPCs,
hPDLSCs and hGFs
When we cultured hDPCs, hPDLSCs and hGFs devided
from tissue sections of third molar teeth, cells from individual
cultures had a fibroblast-like, spindle-shaped and elongated
morphology without treatment with differentiation additives
(Fig. 2, panel 1). To investigate
the osteo/dentinogenic potential of these dental cells, cells from
passage number 2 were treated with differentiation additives
containing β-glycerophosphate, ascorbic acid and dexamethasone.
After 7 days of treatment with differentiation additives, the hDPCs
and hPDLSCs formed sporadic aggregates (Fig. 2Aand B, panel 2). These aggregates
developed into a layer covering the cell surface after 2 weeks
(Fig. 2A and B, panel 3). During
the induction of differentiation, the depository layer produced on
the hDPCs was more extensive than that on the hPDLSCs and covered
the entire adherent layer on the surface of the hDPCs. Although
these aggregative structures were also formed without additive
treatment during early passage in the hDPCs and hPDLSCs, the
efficiency of aggregate formation was very low (data not shown), as
shown in a previous study (15).
The hGFs also showed a fibroblast-like and elongated morphology
without treatment with differentiation additives, similar to the
hDPCs and hPDLSCs (Fig. 2C, panel
1). However, following the induction of differentiation, the
population of hGFs appeared as a fine mesh-like structure without
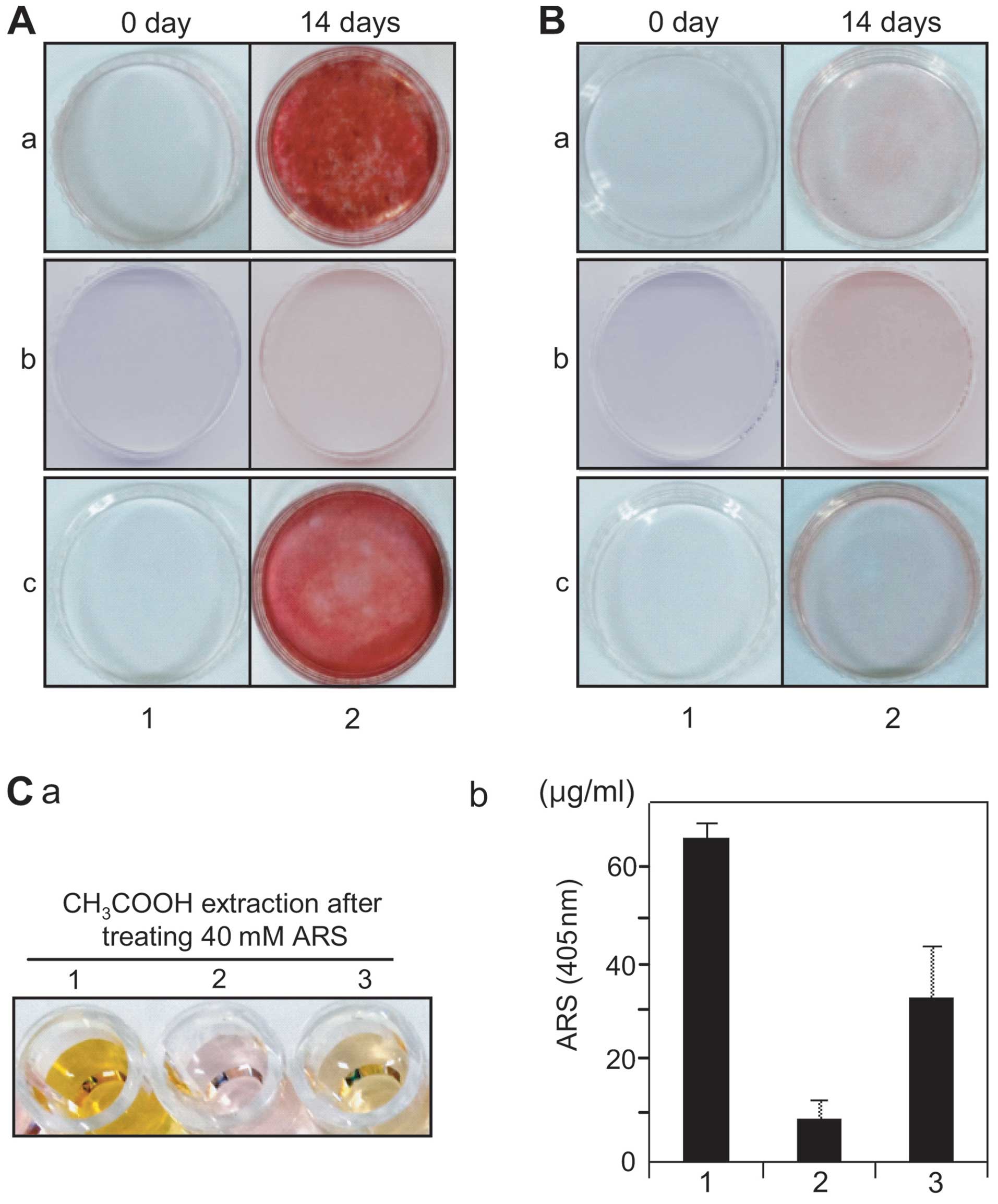
the depository layer overspreading the entire culture (Fig. 2C, panel 3). To investigate whether
these aggregates or the sheath-like structure included calcium
deposits by mineralization, the cells were stained with Alizarin
red S solution. Alizarin red S-positive deposits were observed over
the whole cell layer evenly in both the hDPCs and hPDLSCs from
early passage (Fig. 3A, panel 2
parts a and c), whereas these deposits were not detected in the
hGFs (Fig. 3A, panel 2 part b).
The hDPCs and hPDLSCs in extended passage did not form calcium
deposits under differentiation conditions (Fig. 3B, panel 2 parts a and c). Due to
the fact that the efficiency of cell adhesion and
osteo/dentinogenic differentiation of hDPCs, hPDLSCs and hGFs may
be affected on biomaterial surface mimicking the mechanical
environment of the extracellular matrix, these cells were cultured
on poly(ɛ-caprolactone) (PCL) fibrous membranes, as previously
described (40,41). After 2 weeks of differentiation,
the amount of mineral deposits from the hDPCs cultured on the PCL
membranes was greater than that from the hPDLSCs and hGFs (Fig. 3C, parts a and b, lanes 1–3). Under
the same conditions, the hGFs did not form mineral deposits during
culture on biomaterial (Fig. 3C,
parts a and b, lane 2).
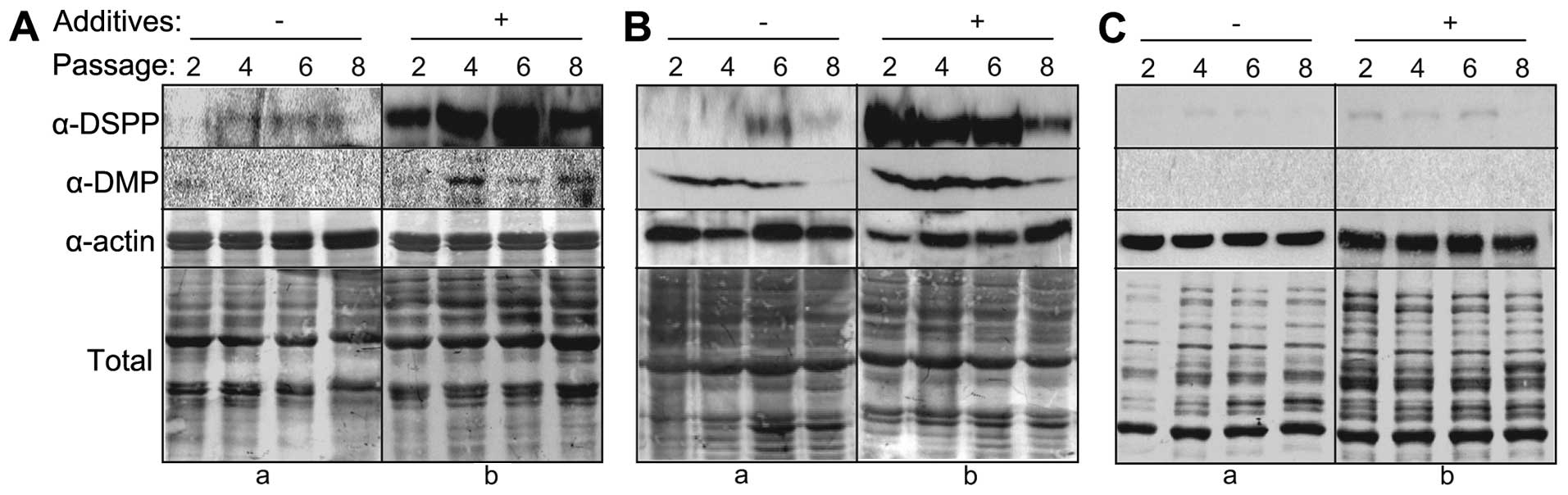
Expression of dentin markers in hDPCs,
hPDLSCs and hGFs under differentiation conditions
To investigate the dentinogenic potential of hDPCs,
hPDLSCs and hGFs during differentiation, the endogenous protein
levels of dentinogenic markers were analyzed during extended cell
culture. Under differentiation conditions, the expression of DSPP
in the hDPCs and hPDLSCs markedly increased (Fig. 4A and B, α-DSPP in panel b). The
expression levels of DMP-1 also increased in both cell types under
the same conditions, although at lower levels than those of DSPP
(Fig. 4A and B, α-DMP in panel
b). Of note, dentin marker expression decreased with the increasing
passage number during extended culture. In both cases, dentin
marker expression peaked during early passage (passage numbers
4–6), and diminished at passage 8 (Fig. 4A and B, lane 8). By contrast,
dentin marker expression did not increase in the hGFs during
extended culture (Fig. 4C, α-DSPP
and α-DMP in panel b).
Dentinogenic potential of hDPCs during
co-culture with hPDLSCs and/or hGFs
We performed an indirect co-culture experiment with
the hPDLSCs and/or hGFs to determine whether any soluble factors
from these cells can provide a dentinogenic niche for dental pulp
stem cells. The cell culture inserts were placed in wells seeded
with hDPCs and the PDLSCs and/or hGFs were seeded within the
inserts. This condition created a cell-cell barrier between the
hDPCs and the hPDLSCs and/or hGFs, thus allowing the diffusion of
medium and proteins. After 7 days of differentiation, the
transcriptional expression of dentinogenic markers from the total
RNA purified from the hDPCs was analyzed by RT-qPCR. The
transcriptional expression of DSPP and DMP-1 increased when the
cells were stimulated with the additives (Fig. 5). When the differentiation of the
hDPCs was induced without co-culture, the expression of
dentinogenic markers increased with the increasing duration of
induction. The DSPP transcript level increased ≥3-fold compared to
the control (at 0 days of differentiation) after 7 and 14 days of
incubation (Fig. 5A, bar graph
a). The DMP-1 transcript level increased 2- and 5-fold compared to
the control after 7 and 14 days of incubation, respectively
(Fig. 5B, bar graph a). When the
hDPCs were co-cultured with hGFs, the transcript levels of these
markers also increased during differentiation to similar levels in
comparison with those of the hDPCs cultured alone (Fig. 5A and B, bar graph b). When the
hDPCs were co-cultured with hPDLSCs, the transcript levels of DSPP
and DMP-1 were increased by >2-fold compared to the hDPCs
cultured alone, even when the cells were not treated with
differentiation additives (Fig. 5A
and B, bar graphs a and c, black bars). Although the expression
levels of these genes were observed to be slightly increased after
7 and 14 days of differentiation in comparison with those of the
hDPCs cultured alone, the rate of increment from the expression
levels at day 0 was not significant under the co-culture conditions
during differentiation (Fig. 5A and
B, bar graph c, grey and dark grey bars). These data suggested
that dentinogenic gene expression in hDPCs may be induced by
indirect co-culture with hPDLSCs without inducing differentiation;
however, the effects of indirect co-culture with hPDLSCs and those
of treatment with differentiation additives on the mineralization
of hDPCs were not synergistic. Additionally, when the hDPCs were
co-cultured with hPDLSCs and hGFs in a co-culture insert, no
significant synergistic effect was observed on the transcriptional
expression of dentinogenic markers in hDPCs after 7 and 14 days of
differentiation (Fig. 5A and B,
graph d).
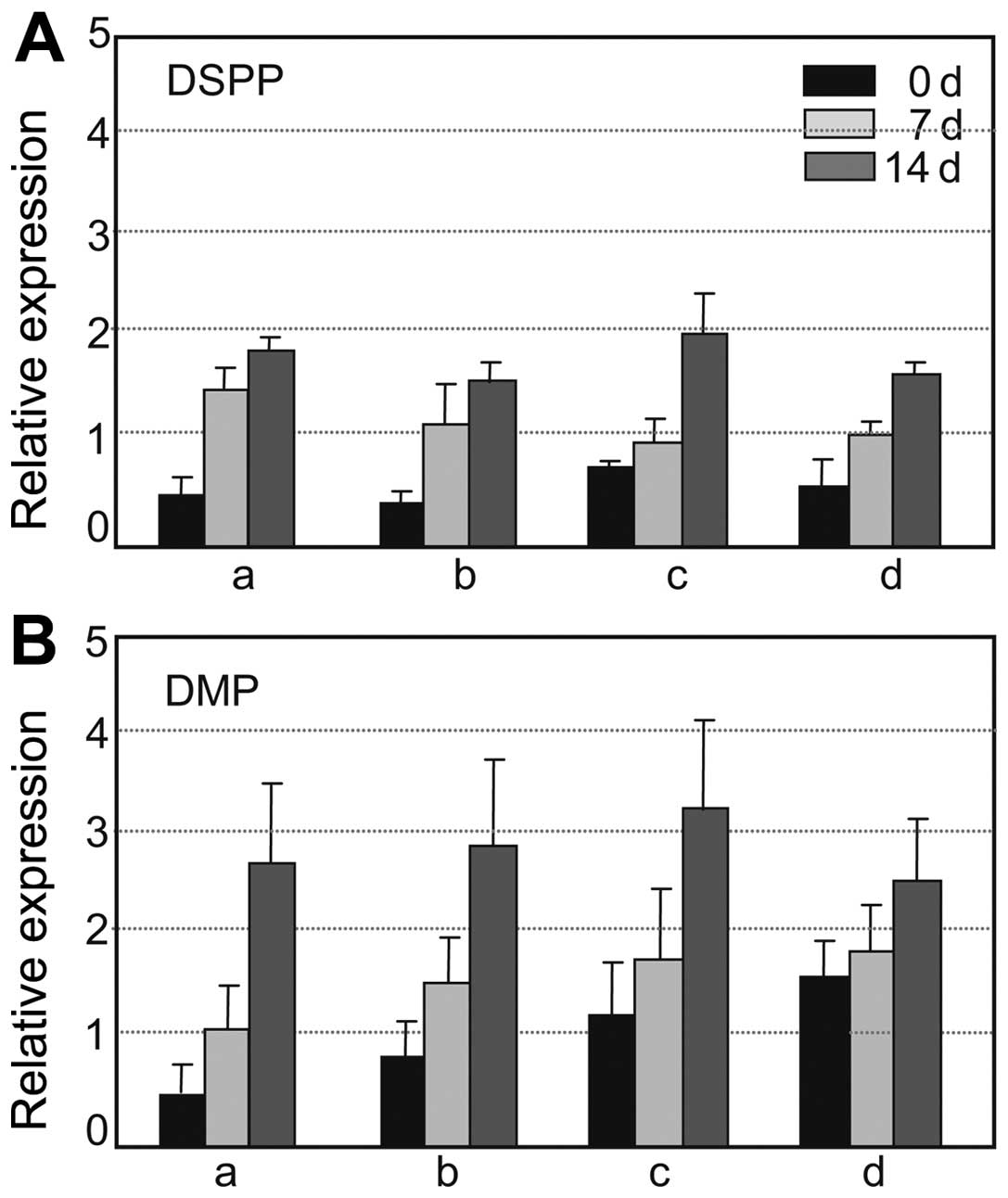 | Figure 5Dentinogenic differentiation
potential of human dental pulp cells (hDPCs) following indirect
co-culture with periodontal ligament stem cells (hPDLSCs) and/or
gingival fibroblasts (hGFs). Following culture or co-culture for
the indicated periods of time (0 d, 0 days; 7 d, 7 days; 14 d, 14
days) with differentiation additives, the hDPCs were harvested for
RNA preparation: black bars, without treatment with additives;
light gray bars, 7-days of incubation with additives; dark gray
bars, 14 days of incubation with additives. Transcript levels of
the dentin markers, (A) DSPP and (B) DMP were estimated by RT-qPCR
as described in the ‘Materials and methods’. (A and B) Bar graph a,
hDPCs cultured alone; bar graph b, co-culture of hDPCs with hGFs;
bar graph c, co-culture of DPCs with hPDLSCs; bar graph d,
co-culture of hDPCs with both hGFs and hPDLSCs. |
Discussion
Previous studies have investigated the
differentiation potential of DPCs, PDLs and GFs isolated from the
dental tissue of various species. Although it has been suggested
PDLSCs, and GFs, as well as DPCs have stemness and differentiation
potential (17,21,42), systematic and comparative studies
on the stemness of these oral cells are limited. In the present
study, we confirmed the presence of a stem cell-like population in
hDPCs, PDLSCs and hGFs comparatively by the systematic study of the
osteo/dentinogenic differentiation of these cells. The hDPCs and
hPDLSCs during early passage strongly expressed representative
mesenchymal stem cell markers (Fig.
1A and B, panels b-d, f and g), whereas the already known
dental apical papilla stem cell markers, CD24 and CD106, were not
expressed in the hDPCs and hPDLSCs used in the present study
(Fig. 1A and B, panels a and e).
These findings strongly suggest that hDPCs and hPDLSCs are
different lineages from dental apical papilla cells, although they
contain a mesenchymal stem cell-like population (21,24,26). Under osteogenic conditions, the
hPDLSCs, as well as the hDPCs, highly mineralized and expressed
dentinogenic markers (Figs. 3 and
4). During extended culture, the
differentiation potential of the hPDLSCs peaked during early
passage and decreased gradually after 8–9 passages (Fig. 4B, panels a and b), indicating that
the hPDLSCs may have differentiated and/or lost their stemness
potential during extended culture in a similar manner with the
hDPCs, as desribed in a previous study (15). Moreover, we demonstrated that the
hPDLSCs have dentinogenic potential (Fig. 4B). As previously reported, PDLSCs
are also involved in the regeneration of the periodontal ligament,
alveolar bone and cementum (21).
These data indicate that hPDLSCs, as well as hDPCs may be a adult
stem cell source for the regeneration of teeth and their periphery.
It is disputed whether GFs contain stem cells that have the
potential to regenerate multiple tissue cells. Mitrano et al
(28) reported that primary cells
from gingival connective tissue contain mesenchymal stem cells and
have adipogenic, chondrogenic and osteogenic differentiation
potential. In the present study, when hGFs from the attached
gingival tissue were cultured, the cells from very early passages
expressed the general mesenchymal stem cell markers, CD44, CD73,
CD90, CD146 and CD166 [Fig. 1C and
D (triangle in panels a-e)]. However, these cells did not show
mineralization potential (Fig.
3), suggesting that there are few dentinogenic progenitors in
primary cell populations. Previously, Carmona-Rodriguez et
al (42) reported that the
mineralization potential of hGFs can be controversial, due to
variations in the external environment, such as the composition of
extracellular matrix and the existence of functional osteoblasts.
In addition, the association, if any exits, between the expression
of mesenchymal stem cell markers and dentinogenic potential remains
to be elucidated. Indeed, although CD44, CD73 and CD90 were
strongly expressed on the cell surface of the hGFs in our
immunotyping experiment (Fig. 1C,
panels b-d), dentinogenic markers, such as DSPP and DMP-1, were not
expressed during differentiation (Fig. 4C, panel b). These results also
indicate that mesenchymal stem cell markers, such as CD44 and CD90,
are not proper markers for discriminating dentinogenic progenitors,
and that more specific surface markers are required to detect
dentinogenic stem/progenitor cells.
The stem cell niche refers to an in vivo or
in vitro stem cell microenvironment that interacts with stem
cells to regulate cell fate. In adult stem cells, stem cell niches
maintain stem cells in a quiescent state; however, following tissue
injury, the surrounding microenvironment signals stem cells to
either promote self renewal or differentiation for tissue
regeneration. Cell-cell interactions between stem cells, as well as
extracellular matrix components, growth factors, cytokines and the
physiochemical nature of the environment, including pH,
Ca2+ and metabolites, such as adenosine triphosphate
(ATP), are important factors regulating stem cell characteristics
within the niche (43). In this
study, to investigate whether hPDLSCs and/or hGFs provide a
dentinogenic niche of dental pulp stem cells during the
developmental stage, we performed indirect co-culture experiments.
Using culture inserts, soluble factors from the cultures of hPDLSCs
and hGFs diffused into the hDPC culture at the bottom of the well.
After 7 and 14 days of co-culture under differentiation conditions,
the transcript levels of DSPP and DMP-1 did not increase in
comparison with those in the hDPCs cultured alone (Fig. 5). In future studies, we aim to
perform a co-culture experiment in order to investigate the direct
effect on dentinogenesis by cell-to-cell interaction, which would
address the issue of whether a microenvironment from the
extracellular matrix of hPDLSCs and/or hGFs can be a dentinogenic
niche for the differentiation of hDPCs in vitro.
Acknowledgements
The present study was supported by the Priority
Research Center Program (2013-0093829) funded by the National
Research Foundation of Korea, and by the research fund of Dankook
University (BK21 PLUS) in 2013.
References
|
1
|
Reynolds SD, Giangreco A, Power JH and
Stripp BR: Neuroepithelial bodies of pulmonary airways serve as a
reservoir of progenitor cells capable of epithelial regeneration.
Am J Pathol. 156:269–278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
LaBarge MA and Blau HM: Biological
progression from adult bone marrow to mononucleate muscle stem cell
to multinucleate muscle fiber in response to injury. Cell.
111:589–601. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morshead CM, Craig CG and van der Kooy D:
In vivo clonal analyses reveal the properties of endogenous neural
stem cell proliferation in the adult mammalian forebrain.
Development. 125:2251–2261. 1998.PubMed/NCBI
|
|
4
|
Otsuka H, Kusumi T, Kanai S, Koyama M,
Kuno Y and Takizawa R: Stem cell factor mRNA expression and
production in human nasal epithelial cells: contribution to the
accumulation of mast cells in the nasal epithelium of allergy. J
Allergy Clin Immunol. 102:757–764. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seigel GM, Sun W, Salvi R, Campbell LM,
Sullivan S and Reidy JJ: Human corneal stem cells display
functional neuronal properties. Mol Vis. 9:159–163. 2003.PubMed/NCBI
|
|
6
|
Lemischka IR, Raulet DH and Mulligan RC:
Developmental potential and dynamic behavior of hematopoietic stem
cells. Cell. 45:917–927. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Wang X, Sun Z, Yang H, Shi S and
Wang S: Stem cells from human-exfoliated deciduous teeth can
differentiate into dopaminergic neuron-like cells. Stem Cells Dev.
19:1375–1383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miura M, Gronthos S, Zhao M, Lu B, Fisher
LW, Robey PG and Shi S: SHED: stem cells from human exfoliated
deciduous teeth. Proc Natl Acad Sci USA. 100:5807–5812. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gronthos S, Mankani M, Brahim J, Robey PG
and Shi S: Postnatal human dental pulp stem cells (DPSCs) in vitro
and in vivo. Proc Natl Acad Sci USA. 97:13625–13630. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arthur A, Rychkov G, Shi S, Koblar SA and
Gronthos S: Adult human dental pulp stem cells differentiate toward
functionally active neurons under appropriate environmental cues.
Stem Cells. 26:1787–1795. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gronthos S, Brahim J, Li W, et al: Stem
cell properties of human dental pulp stem cells. J Dent Res.
81:531–535. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi S, Robey PG and Gronthos S: Comparison
of human dental pulp and bone marrow stromal stem cells by cDNA
microarray analysis. Bone. 29:532–539. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tziafas D: Basic mechanisms of
cytodifferentiation and dentinogenesis during dental pulp repair.
Int J Dev Biol. 39:281–290. 1995.PubMed/NCBI
|
|
14
|
Kuznetsov SA, Krebsbach PH, Satomura K,
Kerr J, Riminucci M, Benayahu D and Robey PG: Single-colony derived
strains of human marrow stromal fibroblasts form bone after
transplantation in vivo. J Bone Miner Res. 12:1335–1347. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Min JH, Ko SY, Cho YB, Ryu CJ and Jang YJ:
Dentinogenic potential of human adult dental pulp cells during the
extended primary culture. Hum Cell. 24:43–50. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi S and Gronthos S: Perivascular niche
of postnatal mesenchymal stem cells in human bone marrow and dental
pulp. J Bone Miner Res. 18:696–704. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mensing N, Gasse H, Hambruch N, Haeger JD,
Pfarrer C and Staszyk C: Isolation and characterization of
multipotent mesenchymal stromal cells from the gingiva and the
periodontal ligament of the horse. BMC Vet Res. 7:422011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hayashi Y, Imai M, Goto Y and Murakami N:
Pathological mineralization in a serially passaged cell line from
rat pulp. J Oral Pathol Med. 22:175–179. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iwata T, Yamakoshi Y, Simmer JP, Ishikawa
I and Hu JC: Establishment of porcine pulp-derived cell lines and
expression of recombinant dentin sialoprotein and recombinant
dentin matrix protein-1. Eur J Oral Sci. 115:48–56. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakashima M: Establishment of primary
cultures of pulp cells from bovine permanent incisors. Arch Oral
Biol. 36:655–663. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seo BM, Miura M, Gronthos S, et al:
Investigation of multipotent postnatal stem cells from human
periodontal ligament. Lancet. 364:149–155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ivanovski S, Gronthos S, Shi S and Bartold
PM: Stem cells in the periodontal ligament. Oral Dis. 12:358–363.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Y, Zheng Y, Ding G, et al: Periodontal
ligament stem cell-mediated treatment for periodontitis in
miniature swine. Stem Cells. 26:1065–1073. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mrozik K, Gronthos S, Shi S and Bartold
PM: A method to isolate, purify, and characterize human periodontal
ligament stem cells. Methods Mol Biol. 666:269–284. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takada H, Mihara J, Morisaki I and Hamada
S: Induction of interleukin-1 and -6 in human gingival fibroblast
cultures stimulated with Bacteroides lipopolysaccharides. Infect
Immun. 59:295–301. 1991.PubMed/NCBI
|
|
26
|
Gagari E, Rand MK, Tayari L, Vastardis H,
Sharma P, Hauschka PV and Damoulis PD: Expression of stem cell
factor and its receptor, c-kit, in human oral mesenchymal cells.
Eur J Oral Sci. 114:409–415. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Okazaki M, Yoshimura K, Uchida G and Harii
K: Elevated expression of hepatocyte and keratinocyte growth factor
in cultured buccal-mucosa-derived fibroblasts compared with
normal-skin-derived fibroblasts. J Dermatol Sci. 30:108–115. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mitrano TI, Grob MS, Carrion F, et al:
Culture and characterization of mesenchymal stem cells from human
gingival tissue. J Periodontol. 81:917–925. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Covas DT, Panepucci RA, Fontes AM, et al:
Multipotent mesenchymal stromal cells obtained from diverse human
tissues share functional properties and gene-expression profile
with CD146+ perivascular cells and fibroblasts. Exp
Hematol. 36:642–654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Majeti R, Park CY and Weissman IL:
Identification of a hierarchy of multipotent hematopoietic
progenitors in human cord blood. Cell Stem Cell. 1:635–645. 2007.
View Article : Google Scholar
|
|
31
|
Zhu H, Mitsuhashi N, Klein A, et al: The
role of the hyaluronan receptor CD44 in mesenchymal stem cell
migration in the extracellular matrix. Stem Cells. 24:928–935.
2006. View Article : Google Scholar
|
|
32
|
Dennis JE, Carbillet JP, Caplan AI and
Charbord P: The STRO-1+ marrow cell population is
multipotential. Cells Tissues Organs. 170:73–82. 2002. View Article : Google Scholar
|
|
33
|
Barry F, Boynton R, Murphy M, Haynesworth
S and Zaia J: The SH-3 and SH-4 antibodies recognize distinct
epitopes on CD73 from human mesenchymal stem cells. Biochem Biophys
Res Commun. 289:519–524. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Craig W, Kay R, Cutler RL and Lansdorp PM:
Expression of Thy-1 on human hematopoietic progenitor cells. J Exp
Med. 177:1331–1341. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Simmons PJ and Torok-Storb B:
Identification of stromal cell precursors in human bone marrow by a
novel monoclonal antibody, STRO-1. Blood. 78:55–62. 1991.PubMed/NCBI
|
|
36
|
Russell KC, Phinney DG, Lacey MR,
Barrilleaux BL, Meyertholen KE and O’Connor KC: In vitro
high-capacity assay to quantify the clonal heterogeneity in
trilineage potential of mesenchymal stem cells reveals a complex
hierarchy of lineage commitment. Stem Cells. 28:788–798. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Barreiro O, Yanez-Mo M, Serrador JM, et
al: Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin
in a novel endothelial docking structure for adherent leukocytes. J
Cell Biol. 157:1233–1245. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Majumdar MK, Banks V, Peluso DP and Morris
EA: Isolation, characterization, and chondrogenic potential of
human bone marrow-derived multipotential stromal cells. J Cell
Physiol. 185:98–106. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Morsczeck C, Schmalz G, Reichert TE,
Vollner F, Galler K and Driemel O: Somatic stem cells for
regenerative dentistry. Clin Oral Investig. 12:113–118. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Binulal NS, Deepthy M, Selvamurugan N, et
al: Role of nanofibrous poly(caprolactone) scaffolds in human
mesenchymal stem cell attachment and spreading for in vitro bone
tissue engineering-response to osteogenic regulators. Tissue Eng
Part A. 16:393–404. 2010. View Article : Google Scholar
|
|
41
|
Yang X, Yang F, Walboomers XF, Bian Z, Fan
M and Jansen JA: The performance of dental pulp stem cells on
nanofibrous PCL/gelatin/nHA scaffolds. J Biomed Mater Res Part A.
93:247–257. 2010.
|
|
42
|
Carmona-Rodriguez B, Alvarez-Perez MA,
Narayanan AS, et al: Human Cementum Protein 1 induces expression of
bone and cementum proteins by human gingival fibroblasts. Biochem
Biophys Res Commun. 358:763–769. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li L and Xie T: Stem cell niche: structure
and function. Annu Rev Cell Dev Biol. 21:605–613. 2005. View Article : Google Scholar : PubMed/NCBI
|