Introduction
As the aging population continuously increases, the
age-related dermatological concerns are continuously addressed by
the cosmetic and medical industries (1). Skin aging is a multisystem
degenerative process, which is characterized by the loss of
structural integrity and physiological function by both intrinsic
and extrinsic determinants (2).
Intrinsic skin aging is caused by genetic determinants; therefore,
this mechanism is an inevitable natural consequence of
physiological changes in the absence of altered genetic information
(3). Extrinsic skin aging, on the
other hand, is caused by extracellular factors, such as ultraviolet
radiation, pollutants and nicotine; therefore, this type of aging
is controllable as individuals can avoid these age-inducing factors
(3). Among the signs of skin
aging, wrinkling is the primary characteristic, which is induced by
decreasing collagen and increasing collagenase production (4). Collagen, which is the most abundant
protein in connective tissue in mammals, contributes to firmness
and is thus vital for skin elasticity. The most common type of
collagen in the skin is type 1 collagen, which comprises >90% of
the collagen in the body (5).
Type 1 collagen is composed of two α1(I) chains and one α2(I)
chain, which are encoded by the COL1A1 and COL1A2
genes, respectively (5). The
synthesis of type 1 collagen is transcriptionally regulated in skin
fibroblast cells, and UV-induced photoaging involves the
downregulation of COL1A1 and COL1A2 and the
consequent loss of collagen that induces fine wrinkles. In
addition, the level of collagen is regulated by its proteolytic
enzymes, matrix metalloproteinases (MMPs). Studies have
demonstrated that skin aging induced by UV exposure is mediated by
the upregulation of MMP1 expression (6,24).
Therefore, several cosmetic products, including
epigallocatechin-3-gallate (EGCG), exert their anti-aging effects
through the upregulation of collagen synthesis and the
downregulation of MMPs in skin fibroblasts (7).
In addition to the application of phytochemicals as
potential anti-aging agents, several light sources have been
investigated for their anti-aging effects on skin cells and
tissues. Intense pulsed light (IPL) at wavelengths between 570 and
615 nm have proven to be an effective treatment for refractory
melasma in Asian patients with minimal side-effects (8). In addition Moreover, 530-nm IPL may
improve inflammatory acne through the downregulation of tumor
necrosis factor-α (TNF-α) (9).
Furthermore, studies have demonstrated that light-emitting diode
(LED) irradiation at wavelengths of 590, 630, 633 and 660 nm is
effective in photorejuvenation in vitro and in clinical
studies in vivo (10–15). Following irradiation with a 590-nm
LED, type 1 collagen levels have been shown to increase, while MMP1
levels have been shown to decrease (12). In a previous study, irradiation
with a 660-nm LED was found to modulate the levels of type 1
procollagen and MMP1 by 31 and -18%, respectively (10). In another study, the irradiation
of fibroblasts with a combination of a 630- and 830-nm LED or a
660- and 850-nm LED also increased collagen levels (14). These clinical and in vitro
studies, however, examined the effects of single or interval LED
irradiation, as opposed to continuous LED irradiation. Of note, to
the best of our knowledge, the effects of continuous LED
irradiation on skin cells and tissues have not yet been thoroughly
investigated. To overcome experimental limitations, we manufactured
a new cell culture incubator containing a 633-nm LED device and
used this equipment to investigate the effects of continuous LED
irradiation on skin fibroblasts and keratinocytes. Furthermore, for
confirmation of the effects of continuous LED irradiation on human
skin tissue, we cultured human skin explants in the LED incubator.
We also investigated the safety of continuous LED irradiation at
633 nm by examining the levels of inflammatory genes in
vitro and the migration rate of Langerhans cells to the dermis
in tissues.
Materials and methods
Cell lines and chemicals
CCD-986sk normal human skin fibroblasts and HaCaT
keratinocytes were purchased from the American Type Culture
Collection (ATCC; Manassas, VA, USA) and Cell Lines Service (DKFZ,
Eppelheim, Germany), respectively. The CCD-986sk and HaCaT cells
were grown in Dulbecco’s modified Eagle’s medium (DMEM supplemented
with 10% fetal bovine serum, 100 U/ml penicillin and 100
μg/ml streptomycin (all from Gibco Life Technologies, Grand
Island, NY, USA). The 1-chloro-2,4-dinitrobenzene (DNCB) and the
Dermatophagoides farinae body extract, which are agents used
to induce an allergic reaction, were purchased from Sigma-Aldrich
(St. Louis, MO, USA) and Biostir Inc. (Kobe, Japan),
respectively.
Light source
The LED device, which emits a 633-nm wavelength, was
provided by Samsung Electronics Co., Ltd. (Seoul, Korea). The
spectral characteristics of the LED device were as follows: the
peak wavelength was 633±3 nm and the full width at half maximum was
approximately 20 nm. The effective irradiance values on the cell
plates were adjusted to be 5 and 47.5 μW/cm2. For
comparison, lighting devices with organic LEDs with the same
spectral characteristics were prepared. Due to the high humidity
(almost 100%) in the cell culture incubator, we sprayed a silicone
varnish on the lighting devices to provide waterproofing and
insulation. Cells subjected to no irradiation were used as
controls.
Cell viability assay
The cytotoxic effects of the LED lights on the
CCD-986sk and HaCaT cells were evaluated using a water-soluble
tetrazolium salt (WST-1) assay (EZ-Cytox Cell Viability Assay kit;
Itsbio, Seoul, Korea). WST-1 solution (1/10 culture medium volume)
was added to the cultures, and the cells were incubated at 37°C for
0.5 h. Cell viability was determined according to the absorbance at
450 nm using an iMark™ microplate reader (Bio-Rad, Hercules, CA,
USA). All results are presented as the mean percentage ± standard
deviation (SD) of 3 independent experiments.
Enzyme-linked immunosorbent assay (ELISA)
for procollagen type I
The ELISA assay for procollagen type 1 was performed
using the Procollagen Type I C-Peptide (PIP) EIA kit (Clontech
Laboratories Inc./Takara Bio Inc., Otsu, Japan) according to the
manufacturer’s instructions. Briefly, the CCD-986sk cells were
irradiated with a low (5 μW/cm2) and high (47.5
μW/cm2) intensity LED for 24 and 72 h. Following
irradiation, the cell supernatants were collected. The
antibody-peroxidase (POD) conjugate solution (Clontech Laboratories
Inc./Takara Bio Inc.) was transferred to a well of an anti-PIP
monoclonal antibody-coated plate (Clontech Laboratories Inc./Takara
Bio Inc.), and the supernatant was subsequently added. The mixed
samples were incubated. After discarding the well contents, the
wells were washed with wash buffer (Clontech Laboratories
Inc./Takara Bio Inc.). Substrate solution (Clontech Laboratories
Inc./Takara Bio Inc.) was then added to each well, and the plates
were incubated at room temperature for 15 min. Stop solution
(Clontech Laboratories Inc./Takara Bio Inc.) was added, and the
absorbance at 450 nm was measured using an iMark microplate reader.
All results are presented as the mean percentage ± standard
deviation (SD) of 3 independent experiments. A p-value <0.01, as
determined by the Student’s t-test, was considered to indicate a
statistically significant difference.
Isolation of total RNA and quantitative
reverse transcription PCR (RT-qPCR)
After 24 and 72 h of irradiation, total RNA was
isolated using TRIzol reagent (Life Technologies) according to the
manufacturer’s instructions. The purity and concentration of the
RNA was evaluated based on the optical density (OD) at 260 nm and
the OD 260/230 and 260/280 ratios using MaestroNano®, a
micro-volume spectrophotometer (Maestrogen, Las Vegas, NV, USA).
cDNA was then synthesized using a SuperScript® III
First-Strand Synthesis System for RT-PCR (Life Technologies).
Quantitative (real-time) PCR was performed using
SYBR®-Green PCR Master Mix and the
SYBR®-Green RT-PCR Reagents kit (Life Technologies) and
analyzed using Line-Gene K software (Bioer Technology Co. Ltd.,
Hangzhou, China). The CT value for each gene was
normalized to GAPDH. The 2−ΔΔCT method was used
to calculate the relative expression levels of each gene. The
primers used for RT-qPCR are presented in Table I.
 | Table ISequences of the specific primers
used in this study. |
Table I
Sequences of the specific primers
used in this study.
| Gene | Direction | Primer
sequences |
|---|
| COL1A1 | F |
5′-AGCCAGCAGATCGAGAACAT-3′ |
| R |
5′-TCTTGTCCTTGGGGTTCTTG-3′ |
| MMP1 | F |
5′-GATGTGGAGTGCCTGATGTG-3′ |
| R |
5′-TGCTTGACCCTCAGAGACCT-3′ |
| MMP2 | F |
5′-GTGCTGAAGGACACACTAAAGAAGA-3′ |
| R |
5′-TTGCCATCCTTCTCAAAGTTGTAGG-3′ |
| TNF-α | F |
5′-GACCTCTCTCTAATCAGCCC-3′ |
| R |
5′-CAAAGTAGACCTGCCCAGACC-5′ |
| COX-2 | F |
5′-TTCAAATGAGATTGTGGAAAAATTGCT-3′ |
| R |
5′-AGATCATCTCTGCCTGAGTATCTT-3′ |
| IL-1a | F |
5′-GTCTCTGAATCAGAAATCCTTCTATC-3′ |
| R |
5′-CATGTCAAATTTCACTGCTTCATCC-3′ |
| GAPDH | F |
5′-CGCTCTCTGCTCCTCCTGTT-3′ |
| R |
5′-CCATGGTGTCTGAGCGATGT-3′ |
Human skin explant-based assay
For analysis of the safety of low (5
μW/cm2) and high (47.5 μW/cm2)
intensity LED irradiation on human skin, we utilized the expertise
of BIO-EC Laboratory (Longjumeau, France). Prior to this study, the
BIO-EC Laboratory submitted these procedures to the Ethics
Committee for the appropriate French geographic sector (Hôpital du
Kremelin-Bicêtre, Le Kremelin Bicêtre, France) via the website of
the French Ministry of Health. Furthermore, BIO-EC had previously
developed a professional relationship with clinics and hospitals
and was thus allowed to use human skin explants that were not
specifically excised for experimentation in accordance with all
applicable ethical principles.
A total of 33 human skin explants obtained from an
abdominoplasty from a 45-year-old female were used. The diameter of
each explant was ~10 mm. The explants were preserved in BEM medium
(BIO-EC’s Explant Medium batch 060208) at 37°C in a moist
atmosphere containing 5% CO2 for 12 h before the study
was initiated. The DNCB solution (0.0062%) and the
Dermatophagoides farinae body extract (10 mg/cm2)
were applied topically to each explant for 1 or 2 days. Other skin
explants were irradiated with low (5 μW/cm2) and
high (47.5 μW/cm2) intensity LED for 1 or 2 days.
After the 1- or 2-day applications, the skin explants were fixed in
Bouin’s solution (30% formaldehyde, 5% acetic acid, 4%
methyl-alcohol and 1% picric acid; BIO-EC Laboratory).
Subsequently, a dehydration and impregnation process was used for
the processing of the explants. During this process, water was
progressively removed and replaced with ethanol using a 70% ethanol
solution followed by a 95% ethanol solution. Ethanol was then
replaced by butanol, and the explants were finally immersed in a
bath containing paraffin at 56°C. This 3-day process included 3
baths of 70% ethanol, 3 baths of 95% ethanol, 5 baths of butanol
and 2 baths of paraffin. After the preparation, the explants were
placed in blocks with a Leica EG 1160 coating station (Leica
Microsystems, Nussloch, Germany), and 5-μm-thick slices were
prepared using a Minot-type microtome (Leica 2125; Leica
Microsystems) and pressed onto superfrosted silanized glass slides.
Microscopic observations were performed using an optical microscope
(Leica-type DLMB microscope; Leica Microsystems) with a x20
objective. Photomicrographs were performed captured with a CCD Sony
DXC-390P camera (Sony, Tokio Tokyo, Japan) and stored with Leica
IM1000 data archiving software (Leica Microsystems). The
observations of general morphology were performed on paraffin
slices dyed with Masson’s trichrome, Goldner variant. As this study
resulted in only observational and descriptive data, no statistical
analysis was appropriate or feasible.
Analysis of CD1a-positive Langerhans
cells
The DNCB solution (0.0062%), the Dermatophagoides
farinae body extract (10 mg/cm2), and low (5
μW/cm2) and high (47.5 μW/cm2)
intensity LED irradiation were applied topically to each explant
for 1 or 2 days as described above. After the samples were fixed
and paraffinized, the sections were immunostained using anti-CD1a
1gG antibody (Cat, no. sc-5265; Santa Cruz Biotechnology, Santa
Cruz, CA, USA). The CD1a-positive cells were enumerated lengthways
for each explant, and the number was extrapolated to the epidermic
length in centimeters.
Results
Characterization of LED-induced
cytotoxicity in human skin fibroblasts and keratinocytes
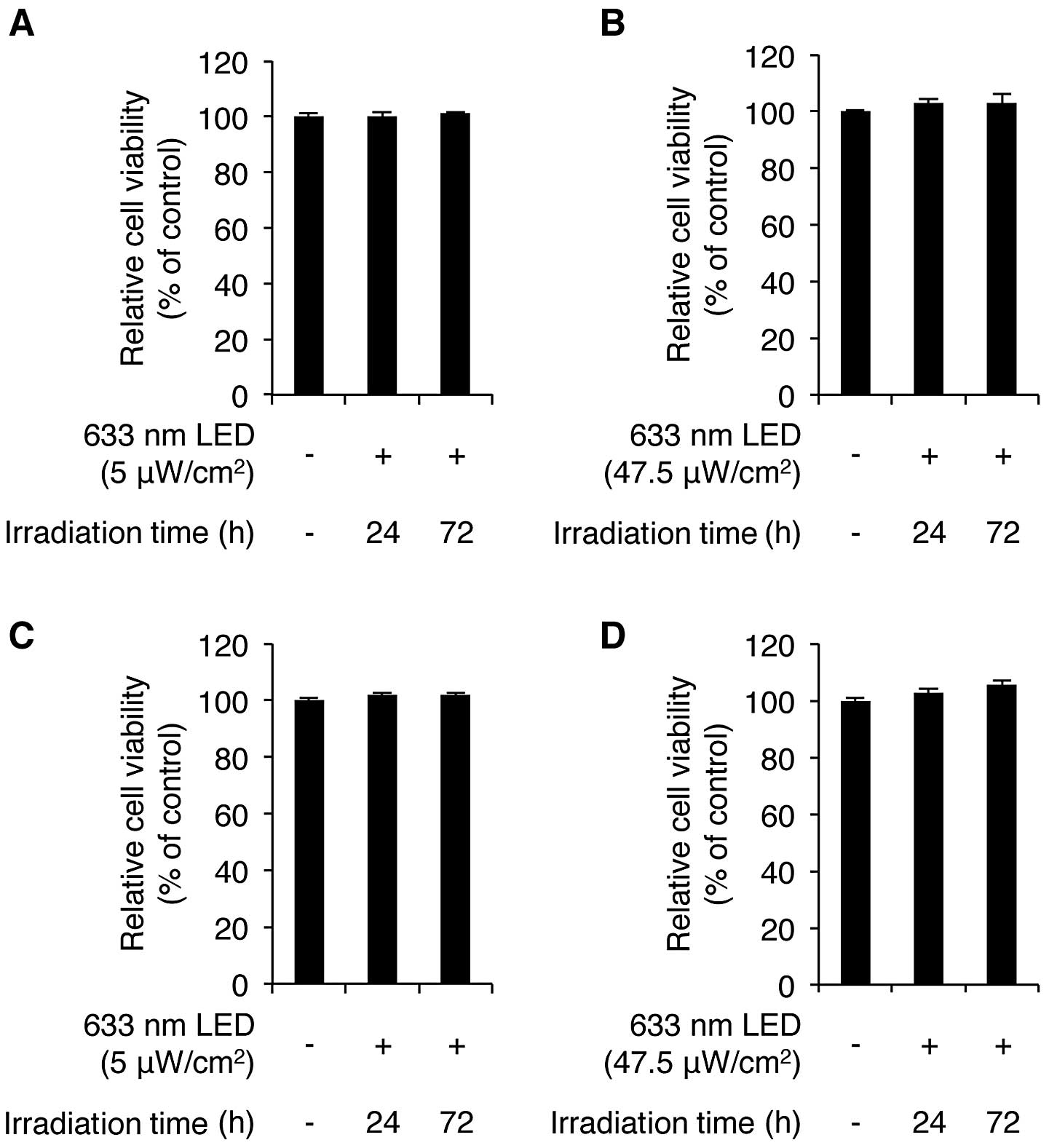
We first sought to determine whether LED irradiation
at a wavelength of 633 nm affects the viability of human skin
cells. CCD-986sk normal human skin fibroblasts and HaCaT
keratinocytes were irradiated with low (5 μW/cm2)
and high (47.5 μW/cm2) intensity LED at 633 nm
for 24 and 72 h. As shown in Fig.
1A, the exposure of the CCD-986sk cells to low intensity LED
irradiation for 24 and 72 h induced no cytotoxicity, according to
the results of the WST-1 assay. We also found that high intensity
LED irradiation did not cause any cytotoxicity to these cells
(Fig. 1B). We then examined the
LED-mediated cytotoxic effects on human HaCaT keratinocytes. The
HaCaT cells were irradiated with low and high intensity LED at 633
nm for 24 and 72 h. LED irradiation-mediated cytotoxicity was not
observed in the HaCaT cells irradiated with low and high intensity
LED (Fig. 1C and D). Of note,
both low and high intensity LED irradiation slightly increased the
viability of the HaCaT cells. More specifically, high intensity LED
irradiation for 24 and 72 h increased the viability of the cells to
102.9±1.2% and 105.7±1.3% of the control value (n=3), respectively.
These results demonstrate that 633-nm LED irradiation does not
induce any cytotoxic effects on CCD-986sk human skin fibroblasts
and HaCaT keratinocytes.
Characterization of the LED
irradiation-induced production of procollagen in human skin
fibroblasts
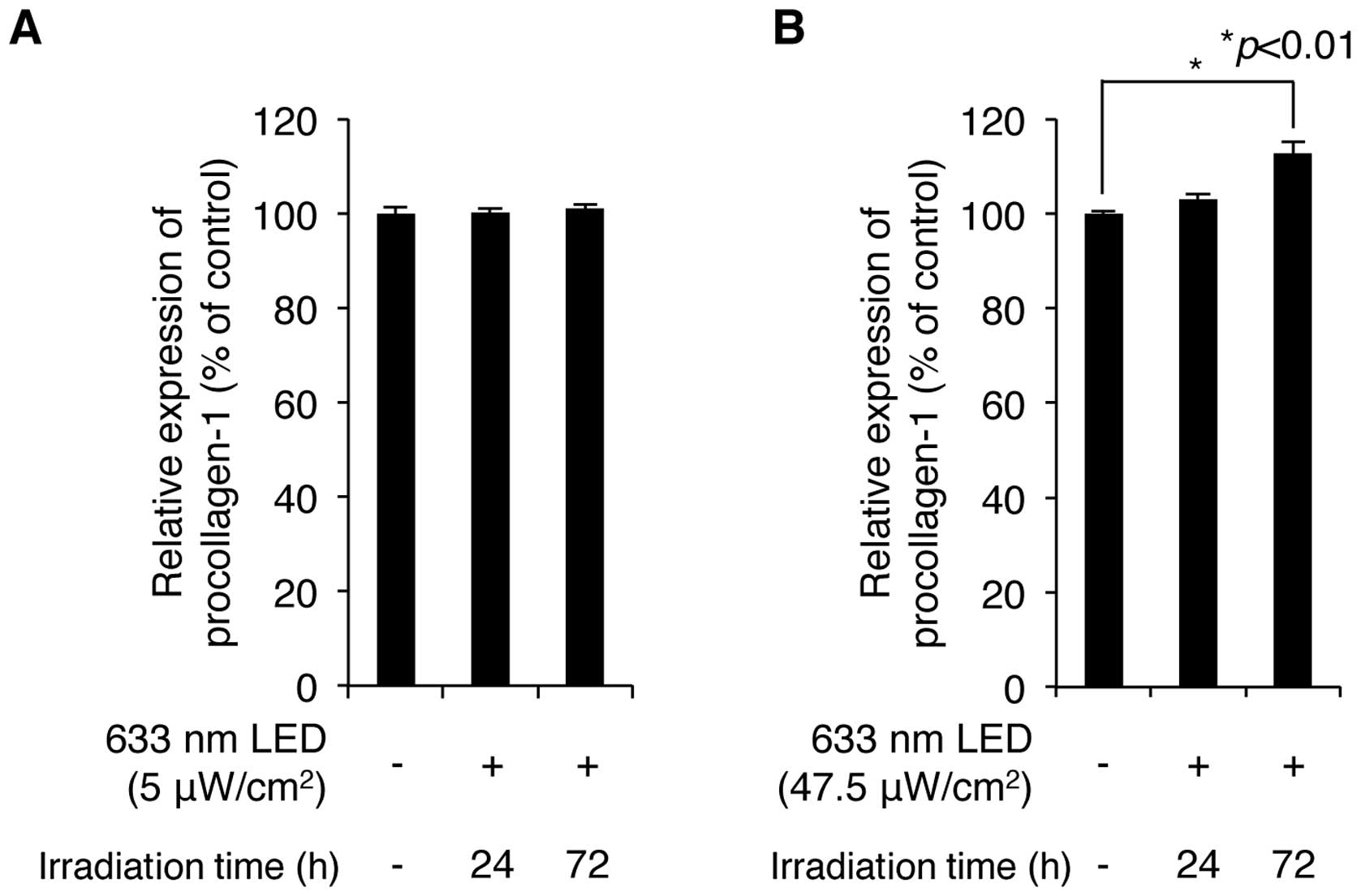
We then examined whether irradiation with a 633-nm
LED increases the production of procollagen type 1, which is
synthesized and secreted by skin fibroblasts and provides strength
and elasticity in skin tissue. CCD-986sk normal human skin
fibroblasts were exposed to low and high intensity 633-nm LED
irradiation for 24 and 72 h. The levels of procollagen type 1 in
the cultured cell supernatants were analyzed by ELISA using a
procollagen type 1-specific antibody. The exposure of the cell
cultures to LED irradiation ranging from 5 to 47.5
μW/cm2 for 24 and 72 h increased the production
of procollagen type 1 in an intensity-and exposure time-dependent
manner (Fig. 2A and B). Although
LED irradiation at 5 μW/cm2 slightly increased
the production of procollagen type 1, maximal production was
obtained at an intensity of 47.5 μW/cm2 for 72 h.
These conditions increased the levels of procollagen type 1 to
112.8±2.3% of the control value (n=3). These results demonstrate
that a relatively high intensity of irradiation (633-nm LED)
positively regulates the production of procollagen type 1 in human
skin fibroblasts.
LED irradiation downregulates the
expression levels of MMP1 and MMP2 in human skin fibroblasts
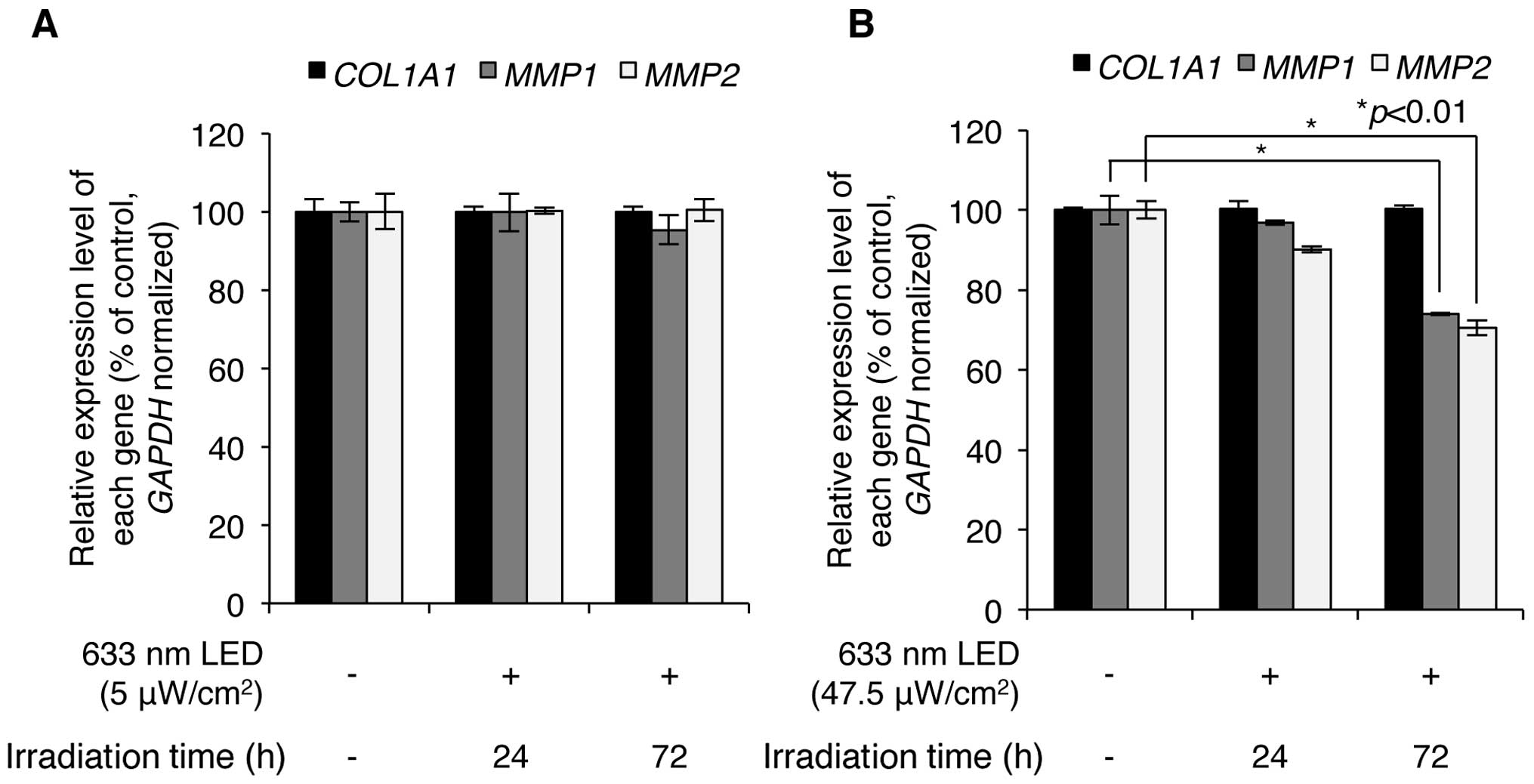
Subsequently, we wished to determine whether LED
irradiation regulates the expression levels of COL1A1,
MMP1 and MMP2. Following exposure of the CCD-986sk
human skin fibroblasts to low (5 μW/cm2) and high
(47.5 μW/cm2) intensity 633-nm LED irradiation,
the expression of COL1A1, MMP1 and MMP2 was
analyzed using RT-qPCR with specific primers for each mRNA
sequence. As shown in Fig. 3A,
exposure of the cells to low intensity LED irradiation at 633 nm
for 24 and 72 h did not result in the altered mRNA expression of
COL1A1, MMP1 and MMP2; however, high intensity
LED irradiation at 633 nm significantly downregulated the
expression of MMP1 and MMP2, but not that of
COL1A1 (Fig. 3B). Notably,
following 633-nm LED irradiation at 47.5 μW/cm2
for 24 h, the expression levels of MMP1 decreased to
96.9±0.6% of the control and those for MMP2 decreased to
90.1±0.7% of the control; following LED irradiation for 72 h, the
expression levels of MMP1 decreased to 74.0±0.3% of the
control and those for MMP2 decreased to 70.6±1.9% of the
control (Fig. 3B). However, the
expression level of COL1A1 was not altered by low and high
intensity LED irradiation in CCD-986sk human skin fibroblasts.
These results indicate that 633-nm LED irradiation induces the
downregulation of MMP1 and MMP2, but does not affect
COL1A1 expression in human skin fibroblasts.
LED irradiation downregulates the
expression of genes related to the inflammatory response in human
keratinocytes
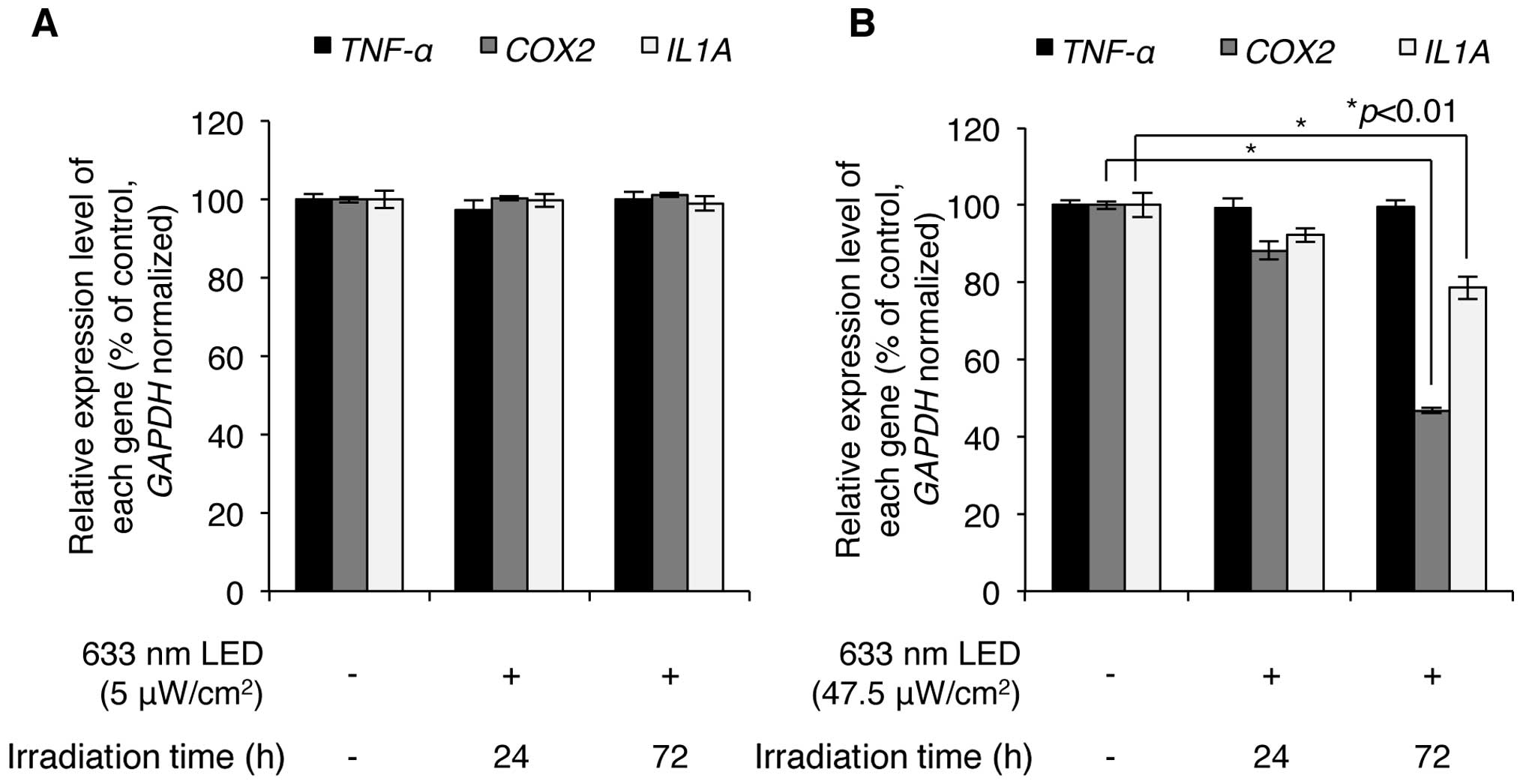
We then investigated whether LED irradiation affects
skin inflammation. HaCaT normal human keratinocytes were irradiated
with low (5 μW/cm2) and high (47.5
μW/cm2) intensity LED at 633 nm, and the effects
of this irradiation on skin inflammation were analyzed by
determining the expression levels of inflammation-related genes,
such as TNF-α, cyclooxygenase-2 (COX-2) and
interleukin-1-α (IL-1α). Low-intensity 633-nm LED
irradiation resulted in very minor changes in the expression levels
of these genes in the HaCaT cells (Fig. 4A), indicating that low intensity
LED irradiation may not induce a skin inflammatory effect. High
intensity 633-nm LED irradiation, however, significantly
downregulated the levels of COX-2 and IL-1α (Fig. 4B). Notably, following 47.5
μW/cm2 LED (633 nm) irradiation for 24 h, the
COX-2 expression levels decreased to of 88.2±2.4% of the
controls and the IL-1α expression levels decreased to
92.3±1.8% of the control; following 72 h of irradiation, the
expression levels of COX-2 decreased to 46.8±0.8% of the
controls and the IL-1α expression levels decreased to
78.6±2.9% of the controls. Of note, the expression of TNF-α
was not altered by LED irradiation, indicating that the expression
of TNF-α was not regulated under these LED-irradiating
conditions. Overall, these results suggest that 633-nm LED
irradiation has a potential anti-inflammatory effect on the
skin.
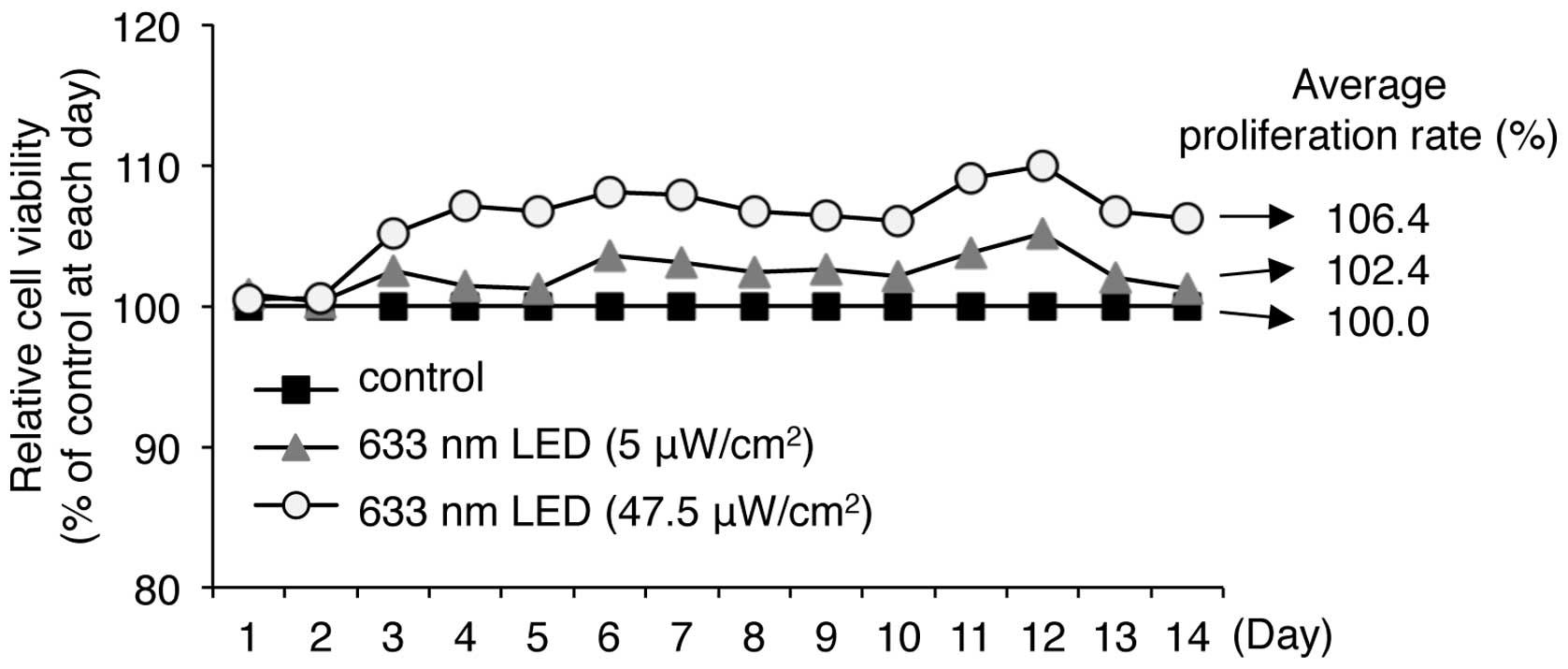
LED irradiation promotes the
proliferation of human keratinocytes
As described above, we found that 633-nm LED
irradiation was not cytotoxic and slightly increased the viability
of HaCaT human keratinocytes. We therefore evaluated the long-term
effects of LED irradiation on the proliferation of HaCaT cells in
culture for up to 14 days. Long-term irradiation with low (5
μW/cm2) and high (47.5 μW/cm2)
intensity 633 nm LED for 14 days did not induce cytotoxicity, but
continuously and moderately promoted cell viability (Fig. 5). Irradiation with 5 and 47.5
μW/cm2 LED at 633 nm resulted in cell
proliferation rates of 102.4 and 106.4% of the controls,
respectively, indicating that long-term LED irradiation at 633 nm
does not induce any cytotoxic effects on keratinocytes.
Safety of LED irradiation on human skin
tissue
We sought to confirm the safety of 633-nm LED
irradiation with respect to the skin allergic reaction using human
skin explants. The skin explants were obtained as full-thickness
human skin biopsies from an abdominoplasty of a 45-year-old
Caucasian woman. The skin explants were subjected to 1 of 3
experimental conditions. The first group of samples served as the
control explants (n=9), which were not treated with any agents or
exposed toLED irradiation. The second group of samples served as
the positive control explants (n=12), which were treated with the
allergic response-inducing agents, DNCB and the Dermatophagoides
farinae body extract. The third group of samples served as the
experimental explants (n=12), which were irradiated with low (5
μW/cm2) and high (47.5 μW/cm2)
intensity LED at 633 nm. The agents and LED irradiation were
applied for 0, 1 or 2 days. We first analyzed the general
morphology of the explants by staining with Masson’s trichrome,
Goldner variant. As shown in Fig.
6, the batch irradiated with the low and high intensity 633-nm
LED did not exhibit any changes in morphology on days 1 and 2,
indicating that irradiation with a 633-nm LED does not induce any
notable changes in skin tissue. Subsequently, we determined whether
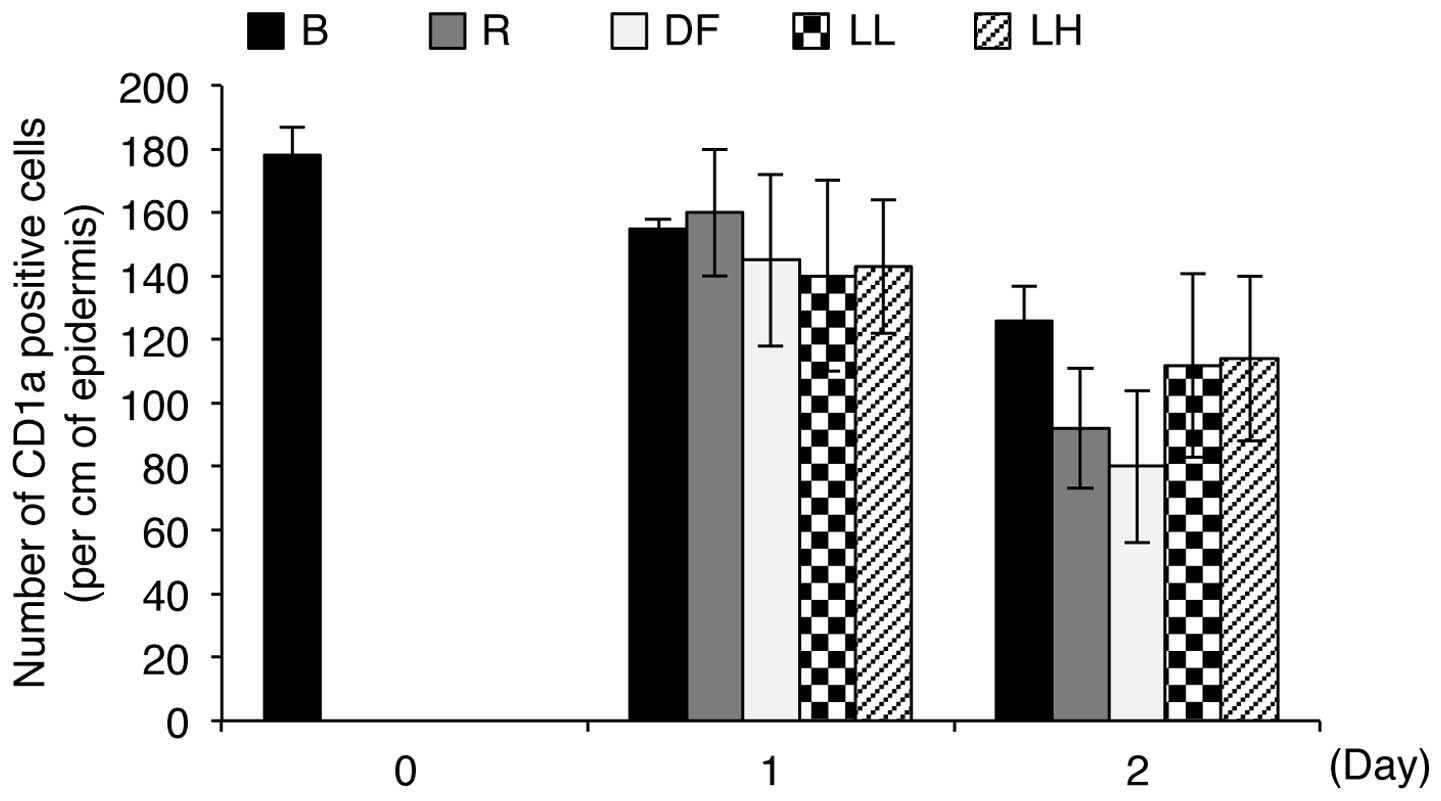
LED irradiation induces an allergic reaction in human skin by
counting the number of CD1a-positive Langerhans cells in the
epidermis in each batch sample. Under allergy-inducing conditions,
Langerhans cells migrate from the epidermis to the dermis,
resulting in a decrease in the number of positive cells in the
epidermis (16). As shown in
Fig. 7, the positive control
samples, which were treated with DNCB solution and
Dermatophagoides farinae body extract for 1 or 2 days,
showed a decrease in the number of CD1a-positive Langerhans cells
in the epidermis compared to the control batches, indicating that
these agents induce an allergic reaction. However, the experimental
batches, which were exposed to low (5 μW/cm2) and
high (47.5 μW/cm2) intensity 633-nm LED
irradiation for 1 or 2 days, showed only slight differences in the
number of CD1a-positive Langerhans cells in the epidermis compared
to the control batches (Fig. 7).
In addition, the number of CD1a-positive Langerhans cells in the
epidermis in the irradiated samples was not altered in an
intensity-dependent manner (Fig.
7). Notably, the numbers of CD1a-positive Langerhans cells in
the epidermis in the control batches and the batches irradiated
with low and high intensity LED for 2 days were 126±11, 112±29 and
114±26, respectively, whereas the numbers of the cells in the
epidermis in the positive control batches were 92±19 and 80±24,
respectively (Fig. 7). These
results indicate that irradiation with a 633-nm LED does not induce
allergic reactions in human skin explants.
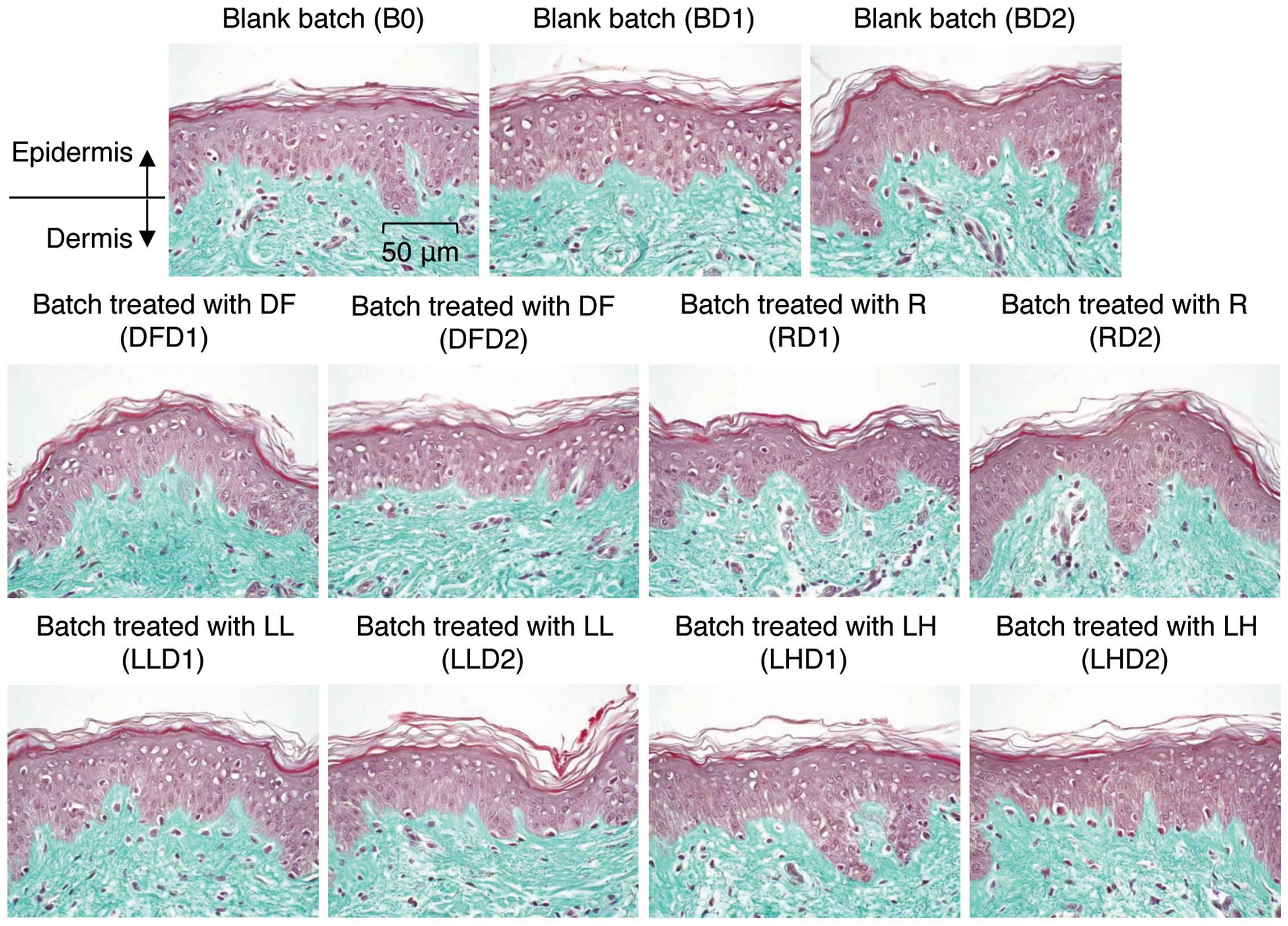 | Figure 6Tissue morphologies for the batches
of human skin explants. One group of human tissue explants was
treated with topical 1-chloro-2,4-dinitrobenzene (DNCB) solution or
Dermatophagoides farinae body extract for 1 and 2 days.
Another group of explants was irradiated with low (5
μW/cm2) or high (47.5 μW/cm2)
intensity LED for 1 and 2 days. After treatment, the explants were
fixed and sliced. General morphology was analyzed microscopically
in paraffinized sections dyed with Masson’s trichrome. B, blank
batch; 0, control; D1, 1 day; D2, 2 days; DF, Dermatophagoides
farinae body extract-treated batch; R, DCNB-treated batch; LL,
low-intensity LED; LH, high-intensity LED. |
Discussion
The present study examined the anti-aging effects of
LED irradiation at a wavelength of 633 nm on human skin cells.
Exposure to 5 μW/cm2 and 47.5
μW/cm2 irradiation from a 633-nm LED was
non-cytotoxic and exerted a positive effect on skin strength and
elasticity through the upregulation of procollagen type 1 molecules
in human skin fibroblasts. The synthesis of collagen in the skin is
regulated by transcriptional and post-translational mechanisms in
fibroblasts (17,18). The upregulation of COL1A1,
which encodes the α1 chain of type 1 collagen, directly increases
the synthesis of type 1 procollagen (17). In this study, we found that
irradiation with a 633-nm LED did not increase the transcription of
COL1A1, indicating that the observed LED
irradiation-mediated increase in type 1 procollagen production is
not a COL1A1 transcription-dependent mechanism. Rather, this
effect occurs through another mechanism. The efficiency in
chaperone-mediated procollagen folding is also directly engaged in
the level of procollagen synthesis in fibroblasts (19,20). In addition, studies have
identified the heat shock protein (HSP)47 as a specific chaperone
of procollagen and have demonstrated that the level of HSP47 is
decreased in in old donor and senescent cells (20,21). Furthermore, a previous study found
that irradiation with a toxic light source, such as ultraviolet B
(UVB), significantly decreased the level of HSP47 (21). Therefore, the LED
irradiation-mediated increase in procollagen production may be
induced by an alteration in the levels of a procollagen-specific
chaperone protein directly or indirectly.
The atrophy of collagen fibers in skin aging
predominantly results from the increased expression of the
degradative enzymes, MMP1 and MMP2 (22,23). Therefore, MMP1 and
MMP2 are considered skin aging-related genes, and the
over-expression of MMP1 and MMP2 genes has been found
in aged/photoaged skin in vivo (24). Thus, in this study, we examined
the LED-mediated anti-aging effects by analyzing the expression
levels of MMP1 and MMP2. We found that 633-nm LED
irradiation decreased the expression of both of these genes. While
low intensity LED did not markedly decrease the expression of these
genes, exposure to 47.5 μW/cm2 of 633-nm LED
irradiation for 2 days (maximum dose used in this study) decreased
the levels of MMP1 and MMP2 by 24 and 29%,
respectively. Indeed, MMPs are essential to epidermal
(keratinocyte) differentiation and the prevention of wound scars,
indicating that the excessive loss of MMPs yields rather harmful
effects on skin integrity (25).
In addition, such a loss in MMP expression induces keratinocyte
hyperproliferation, which is one of the main characteristics of
aged skin (26,27). We also confirmed that long-term
exposure to the maximum dose of 633-nm LED irradiation resulted in
a moderate increase in the proliferation rate of keratinocytes for
up to 14 days. Therefore, our data indicate that a decrease in
MMP1 and MMP2 expression at such a moderate level may
not be harmful to skin health.
Protection of the skin from inflammatory reaction is
paramount. UV irradiation and allergen exposure may lead to acute
and chronic inflammation of the skin. Accumulating evidence
suggests that inflammation is causally linked to carcinogenesis and
acts as a driving force in the premalignant and malignant
transformation of cells. Indeed, in a mouse skin model, the topical
application of the inflammation-inducing agent,
12-O-tetradecanoylphorbol-13-acetate (TPA), was shown to
lead to papilloma formation in mice through the enhancement of the
expression of COX-2, which is a key enzyme in the
inflammatory response (28).
Based on this evidence, in this study, we examined the effects of
633-nm LED irradiation on skin inflammation by analyzing the
expression levels of inflammation-related genes using an in
vitro cell culture model and by examining the general
morphological changes and allergic effects in an ex vivo
human skin model. Our data indicated that the maximum dose of
633-nm LED radiation decreased the expression levels of
COX-2 and IL-1α by 53.2 and 21.4%, respectively,
compared to the controls. COX-2 is a highly inducible enzyme
that is involved in the production of a secondary mediator known to
be triggered in many inflammatory states (29). IL-1α, which has been
identified as a useful screening tool for skin irritation,
initiates a signaling cascade involved in the inflammatory response
(30,31). Studies have demonstrated that both
IL-1α and TNF-α are key molecules that induce the
expression of several cytokines, as well as COX-2 (31,32). However, our study revealed that
neither the minimum nor the maximum dose of 633-nm LED irradiation
regulated the expression of TNF-α, indicating that, although
the level of COX-2 and IL-1α was downregulated by
this irradiation, the TNF-α-mediated inflammatory response
was not regulated by this exposure. Notably, TNF-α
expression is observed at very low levels in cultured keratinocytes
and appears to play a lesser role in inflammation in vivo as
compared to IL-1α (33,34). Therefore, it is noteworthy that
irradiation with a 633-nm LED does not induce a skin inflammatory
response, but rather exerts an anti-inflammatory effect in
vitro.
Since extrapolation from the in vitro
experiments to the more relevant in vivo situation is
difficult, we investigated the effects of a 633-nm LED irradiation
on skin inflammation in human skin explant in ex vivo
experiments. A total of 33 skin explants from a Caucasian woman
were obtained, and the possibility of a skin allergic reaction
following LED irradiation was examined. We found that both the
minimum and the maximum dose of LED irradiation resulted in
indistinguishable changes in the level of allergic response
compared to the control human skin explants. Therefore, our data
indicate that irradiation with a 633-nm LED does not induce skin
inflammation in vitro or in vivo.
In conclusion, we demonstrated that 633-nm LED
irradiation exerts an anti-aging and anti-wrinkle effect on human
skin through the upregulation of type 1 procollagen and the
downregulation of MMP1 and MMP2. We also confirmed
that this irradiation is not cytotoxic and does not induce
inflammatory effects in human skin using both an in vitro
skin cell culture system and an ex vivo human skin explant
system. In addition, this irradiation did not induce keratinocyte
hyperproliferation. Taken together, these findings suggest that the
irradiation of human skin using a 633-nm LED may prove useful for
the prevention and treatment of skin aging and photodamage-induced
aging, although the clinical significance of 633-nm LED irradiation
in vivo requires further investigation.
References
|
1
|
Farage MA, Miller KW, Elsner P and Maibach
HI: Intrinsic and extrinsic factors in skin ageing: a review. Int J
Cosmet Sci. 30:87–95. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Friedman O: Changes associated with the
aging face. Facial Plast Surg Clin North Am. 13:371–380. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bergfeld WF: The aging skin. Int J Fertil
Womens Med. 42:57–66. 1997.PubMed/NCBI
|
|
4
|
Sjerobabski-Masnec I and Situm M: Skin
aging. Acta Clin Croat. 49:515–518. 2010.
|
|
5
|
Gelse K, Poschl E and Aigner T: Collagens
- structure, function, and biosynthesis. Adv Drug Deliv Rev.
55:1531–1546. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yin L, Morita A and Tsuji T: Skin aging
induced by ultraviolet exposure and tobacco smoking: evidence from
epidemiological and molecular studies. Photodermatol Photoimmunol
Photomed. 17:178–183. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee JH, Chung JH and Cho KH: The effects
of epigallocatechin-3-gallate on extracellular matrix metabolism. J
Dermatol Sci. 40:195–204. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang CC, Hui CY, Sue YM, Wong WR and Hong
HS: Intense pulsed light for the treatment of refractory melasma in
Asian persons. Dermatol Surg. 30:1196–1200. 2004.PubMed/NCBI
|
|
9
|
Taylor M, Porter R and Gonzalez M: Intense
pulsed light may improve inflammatory acne through TNF-alpha
down-regulation. J Cosmet Laser Ther. 16:96–103. 2014. View Article : Google Scholar
|
|
10
|
Barolet D, Roberge CJ, Auger FA, Boucher A
and Germain L: Regulation of skin collagen metabolism in vitro
using a pulsed 660 nm LED light source: clinical correlation with a
single-blinded study. J Invest Dermatol. 129:2751–2759. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee SY, Park KH, Choi JW, et al: A
prospective, randomized, placebo-controlled, double-blinded, and
split-face clinical study on LED phototherapy for skin
rejuvenation: clinical, profilometric, histologic, ultrastructural,
and biochemical evaluations and comparison of three different
treatment settings. J Photochem Photobiol B. 88:51–67. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weiss RA, McDaniel DH, Geronemus RG and
Weiss MA: Clinical trial of a novel non-thermal LED array for
reversal of photoaging: clinical, histologic, and surface
profilometric results. Lasers Surg Med. 36:85–91. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Russell BA, Kellett N and Reilly LR: A
study to determine the efficacy of combination LED light therapy
(633 nm and 830 nm) in facial skin rejuvenation. J Cosmet Laser
Ther. 7:196–200. 2005. View Article : Google Scholar
|
|
14
|
Tian YS, Kim NH and Lee AY: Antiphotoaging
effects of light-emitting diode irradiation on narrow-band
ultraviolet B-exposed cultured human skin cells. Dermatol Surg.
38:1695–1703. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goldberg DJ, Amin S, Russell BA, Phelps R,
Kellett N and Reilly LA: Combined 633-nm and 830-nm led treatment
of photoaging skin. J Drugs Dermatol. 5:748–753. 2006.PubMed/NCBI
|
|
16
|
Ouwehand K, Scheper RJ, de Gruijl TD and
Gibbs S: Epidermis-to-dermis migration of immature Langerhans cells
upon topical irritant exposure is dependent on CCL2 and CCL5. Eur J
Immunol. 40:2026–2034. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grant ME and Ayad S: The collagens of
skin: structure and assembly. Biochem Soc Trans. 16:663–666.
1988.PubMed/NCBI
|
|
18
|
Cutroneo KR: How is type I procollagen
synthesis regulated at the gene level during tissue fibrosis. J
Cell Biochem. 90:1–5. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lamande SR and Bateman JF: Procollagen
folding and assembly: the role of endoplasmic reticulum enzymes and
molecular chaperones. Semin Cell Dev Biol. 10:455–464. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ishida Y and Nagata K: Hsp47 as a
collagen-specific molecular chaperone. Methods Enzymol.
499:167–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nizard C, Noblesse E, Boisde C, et al:
Heat shock protein 47 expression in aged normal human fibroblasts:
modulation by Salix alba extract. Ann NY Acad Sci. 1019:223–227.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khorramizadeh MR, Tredget EE, Telasky C,
Shen Q and Ghahary A: Aging differentially modulates the expression
of collagen and collagenase in dermal fibroblasts. Mol Cell
Biochem. 194:99–108. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Millis AJ, Hoyle M, McCue HM and Martini
H: Differential expression of metalloproteinase and tissue
inhibitor of metalloproteinase genes in aged human fibroblasts. Exp
Cell Res. 201:373–379. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ
and Fisher GJ: Matrix-degrading metalloproteinases in photoaging. J
Investig Dermatol Symp Proc. 14:20–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Philips N, Auler S, Hugo R and Gonzalez S:
Beneficial regulation of matrix metalloproteinases for skin health.
Enzyme Res. 2011:4272852011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Philips N, Tuason M, Chang T, Lin Y, Tahir
M and Rodriguez SG: Differential effects of ceramide on cell
viability and extracellular matrix remodeling in keratinocytes and
fibroblasts. Skin Pharmacol Physiol. 22:151–157. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Buisson-Legendre N, Emonard H, Bernard P
and Hornebeck W: Relationship between cell-associated matrix
metalloproteinase 9 and psoriatic keratinocyte growth. J Invest
Dermatol. 115:213–218. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Seo HJ, Park KK, Han SS, et al: Inhibitory
effects of the standardized extract (DA-9601) of Artemisia asiatica
Nakai on phorbol ester-induced ornithine decarboxylase activity,
papilloma formation, cyclooxygenase-2 expression, inducible nitric
oxide synthase expression and nuclear transcription factor kappa B
activation in mouse skin. Int J Cancer. 100:456–462. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dubois RN, Abramson SB, Crofford L, et al:
Cyclooxygenase in biology and disease. FASEB J. 12:1063–1073.
1998.PubMed/NCBI
|
|
30
|
Wilmer JL, Burleson FG, Kayama F, Kanno J
and Luster MI: Cytokine induction in human epidermal keratinocytes
exposed to contact irritants and its relation to chemical-induced
inflammation in mouse skin. J Invest Dermatol. 102:915–922. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Galley HF and Webster NR: The
immuno-inflammatory cascade. Br J Anaesth. 77:11–16. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Williams IR and Kupper TS: Immunity at the
surface: homeostatic mechanisms of the skin immune system. Life
Sci. 58:1485–1507. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kock A, Schwarz T, Kirnbauer R, et al:
Human keratinocytes are a source for tumor necrosis factor alpha:
evidence for synthesis and release upon stimulation with endotoxin
or ultraviolet light. J Exp Med. 172:1609–1614. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Newby CS, Barr RM, Greaves MW and Mallet
AI: Cytokine release and cytotoxicity in human keratinocytes and
fibroblasts induced by phenols and sodium dodecyl sulfate. J Invest
Dermatol. 115:292–298. 2000. View Article : Google Scholar : PubMed/NCBI
|