Introduction
Breast cancer is the most common type of cancer in
women worldwide. It has become the second most common cause of
cancer-related mortality in women according to the statistical data
supplied by Siegel et al in 2013 (1). Although the mortality rate due to
breast cancer has declined by approximately 30% over the past 20
years, metastatic breast cancer remains incurable (1). A low oxygen status (hypoxia or
anoxia) is a characteristic of most solid tumors and is associated
with malignant progression in several types of cancer, including
breast cancer (2). It also
severely affects the efficacy of radiotherapy or chemotherapy, as
hypoxia usually protects tumor cells from being damaged through
several pathways (3–6): i) severe structural and functional
abnormalities of tumor microvessels (perfusion-limited
O2 delivery), ii) deterioration of diffusion geometry
(diffusion-limited O2 delivery) and iii)
tumor-associated and/or therapy-induced anemia leading to a reduced
O2 transport capacity of the blood (anemic hypoxia)
(7). The hypoxia-inducible factor
(HIF) transcription factors mediate the primary transcriptional
responses to hypoxic stress in normal and transformed cells. An
O2-labile α-subunit (HIF-1α, HIF-2α and HIF-3α) and a
stable β-subunit (Arnt1, Arnt2 and Arnt3) constitute the
heterodimeric HIF proteins. The difference between HIF-1α and
HIF-2α is mainly observed in the N-terminal transactivation domain
(N-TAD), which contributes to target gene specificity, while the
C-terminal transactivation domain (C-TAD) is homologous between the
isoforms and promotes the expression of their common target genes
(8). Recent studies have reported
interesting data on distinguishing the roles of HIF-1α and HIF-2α.
Although the two isoforms share some similar properties, they
exhibit unique and even opposite characteristics when expressed in
the same cell type (9,10). These effects are partly mediated
through the regulation of distinct targets, as well as through
direct and indirect interactions with complexes that contain
important oncoproteins and tumor suppressors.
As one of the significant oncoproteins, c-Myc has
been reported to participate in multiple aspects of cellular
function, such as replication, growth, metabolism, differentiation,
apoptosis and carcinogenesis (11–16). It activates and represses the
transcription of target genes by binding to the E-box (CACGTG)
motif after forming a heterodimer with another basic
helix-loop-helix/leucine zipper (bHLH/Zip) protein MAX. The
aberrant expression of c-Myc is closely related to various types of
cancer.
Human differentiated embryonic chondrocyte expressed
genes (DECs), mouse stimulated by retinoic acid (STRA), and rat
split- and hairy-related protein (SHARP) constitute a structurally
distinct class of bHLH proteins. DEC2 is an important factor in the
regulation of apoptosis, cell differentiation, tumor progression,
circadian rhythms and the response to hypoxia. However, the roles
of DEC2 under hypoxic conditions have not yet been clarified. The
present study demonstrates that DEC2 promotes the proliferation of
MCF-7 breast cancer cells by regulating the expression of the c-Myc
oncoprotein under hypoxic conditions and through the activation of
the PI3K/Akt signaling pathway.
Materials and methods
Cell culture and treatment
MCF-7 human breast cancer cells were cultured as
previously described (17).
Hypoxia (3% O2) was induced by the culture of cells for
various periods of time (2, 8 and 24 h) inside an air-tight chamber
with inflow and outflow valves that was infused with a mixture of
3% O2, 5% CO2, 92% N2 (BNP-110;
ESPEC Corp., Osaka, Japan). In oder to block PI3K, the cells were
incubated with the PI3K/Akt inhibitor, LY294002 (Calbiochem, San
Diego, CA, USA) at 10 μM for 1 h, followed by incubation at
3% O2 for 24 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Three independent RNA samples were prepared from the
above cells for RT-qPCR. Total RNA was isolated using an RNeasy RNA
Isolation kit (Qiagen GmbH, Hilden, Germany). First-strand cDNA was
synthesized from 1 μg of total RNA using ReverTra Ace
(Toyobo Co., Ltd., Osaka, Japan). Quantitative (real-time) PCR was
performed using SYBR-Green Master Mix (Life Technologies, Carlsbad,
CA, USA). The sequences and product sizes of the DEC1
(BHLHE40/STRA13/SHARP2) and DEC2 (BHLHE41/STRA13/SHARP1) primer
sets were described in a previous study (18).
DEC1 and DEC2 overexpression
Human DEC1 and DEC2 cDNA were subcloned into
pcDNA/zeo as previously described (18). MCF-7 cells were seeded at
5×104 cells/35-mm well. DEC1 or DEC2 pcDNA was
transfected into the cells using the lipofectamine LTX (Invitrogen,
Carlsbad, CA, USA) 24 h later. Following transfection, the cells
were incubated for an additional 24 h and subjected to western blot
analysis.
Short interference RNA (siRNA)
siRNA oligos against human HIF-2α were obtained from
Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). For the siRNA
transfection experiments, the cells were seeded at 5×104
cells/35-mm well. The siRNA was transfected into the cells 24 h
later using the lipofectamine RNAiMAX reagent (Invitrogen)
according to the manufacturer’s instructions. Following
transfection, the cells were incubated under normoxic
(O2 21%) or hypoxic (O2 3%) conditions for a
further 24 h and subjected to western blot analysis.
Western blot analysis
The cells were lysed using M-PER lysis buffer
(Thermo Fisher Scientific, Rockford, IL, USA) and the protein
concentration was determined using the bicinchoninic acid (BCA)
assay. Cell lysates were subjected to SDS-PAGE, and the proteins
were transferred onto PVDF membranes (Immobilion P; Millipore,
Tokyo, Japan), which were then incubated with antibodies. Bound
antibodies were visualized by chemiluminescence using the ECL or
ECL Prime Western Blotting Detection system (Amersham Biosciences,
Uppsala, Sweden). The experiment was repeated 3 times.
Antibodies
The membranes for western blot analysis were
incubated with antibodies specific to DEC1 (1:10,000; Novus
Biologicals, Inc. Littleton, CO, USA), DEC2 (1:20,000; H-72X; Santa
Cruz Biotechnology, Inc.), HIF-1α (1:3,000; H-206X; Santa Cruz
Biotechnology, Inc.), HIF-2α (1:3,000; Santa Cruz Biotechnology,
Inc.), c-Myc (1:3,000; Epitomics, Inc., Burlingame, CA, USA),
phospho-Akt (1:6,000; Epitomics Inc.), Akt (1:10,000; Epitomics
Inc.) and actin (1:20,000; Sigma, St. Louis, MO, USA), followed by
horseradish peroxidase-conjugated secondary antibody (IBL, Fujioka,
Gunma, Japan). Can Get Signal Immunoreactions Enhancer Solution
(Toyobo Co., Ltd.) or Immunoshot Immunoreaction Enhancer Solution
(Cosmobio Co., Ltd., Tokyo, Japan) was used to dilute the primary
antibodies.
Cell proliferation assay
Cell proliferation was measured by
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) assay. The MCF-7 cells were seeded in 96-well plates. The
cells were transfected with an empty plasmid (pcDNA) or the DEC1 or
DEC2 expression plasmid. After 18 h of transfection, the cells were
cultured under hypoxic conditions for a further 24 h. Subsequently,
the cells were added to each well along with the CellTiter
96® AQueous One Solution Reagent (Promega
Corp., Madison, WI, USA) and were incubated at 37°C for an
additional 1 h. Absorbance (OD490 nm) was measured using
a 96-well plate reader.
Statistical analysis
The results are presented as the means ± standard
error of the mean (SEM) of the number of experiments indicated in
the figure legends. Statistical analysis was performed using the
Student’s t-test. The level of statistically significant
differences was set at P<0.05, and the level of highly
significant differences at P<0.001.
Results
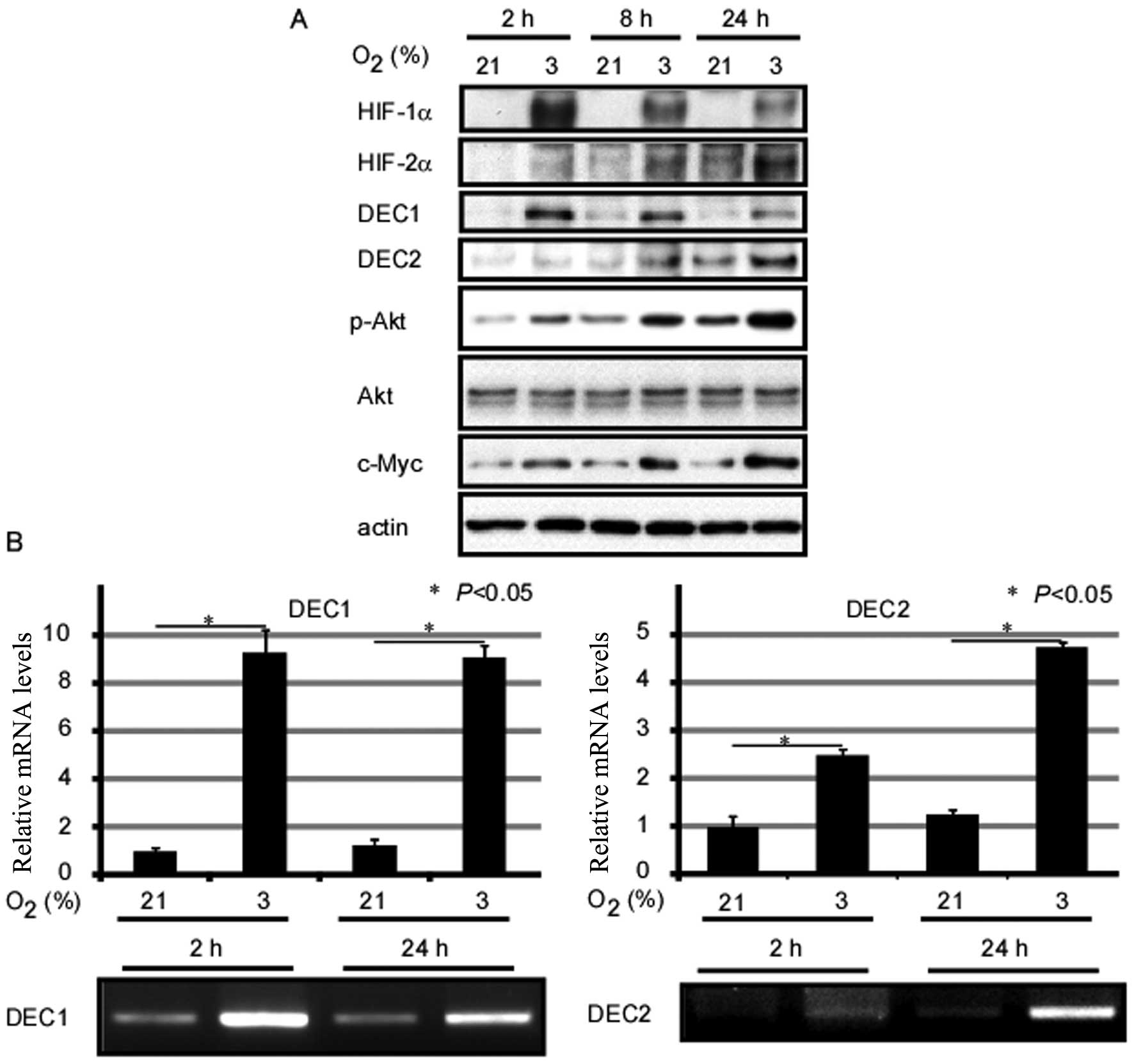
Effects of exposure to hypoxia on the
expression of DEC1 and DEC2 in MCF-7 cells
Firstly, we analyzed the effects of exposure to
hypoxia on gene expression in MCF-7 human breast cancer cells. As
shown in Fig. 1, exposure of the
cells to O2 (3%) for 2, 8 and 24 h induced the protein
expression of HIF-1α and HIF-2α when compared with the control
cells exposed to normal oxygen conditions. In addition, HIF-1α
expression levels reached a peak at 2 h, whereas HIF-2α expression
levels reached a peak at 24 h. Although the endogenous expression
of DEC1 and DEC2 was weak in the MCF-7 cells, their mRNA and
protein expression levels markedly increased under hypoxic
conditions. Furthermore, the DEC1 protein level reached its peak
when the cells were incubated under hypoxic conditions for 2 h,
whereas the DEC2 protein expression level reached its peak
following the exposure of the cells to hypoxia for 24 h. However,
the relative mRNA expression of DEC1 showed no significant change
between the 2- and 24-h time points following exposure to hypoxia.
Moreover, exposure to hypoxia induced the expression of c-Myc and
the phosphorylation of Akt in the MCF-7 cells (Fig. 1).
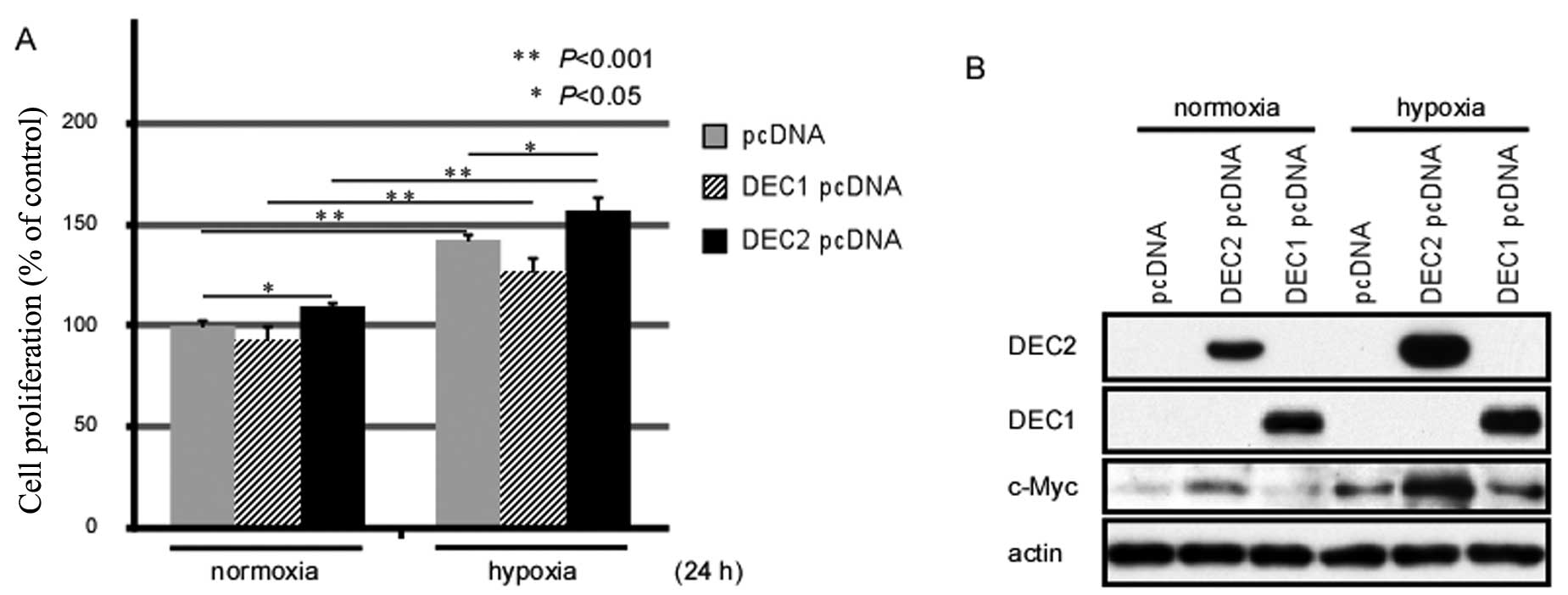
Exposure to hypoxia for 24 h enhances the
proliferation of MCF-7 cells
Both Akt phosphorylation and c-Myc upregulation are
closely associated with the proliferation of various types of
cancer cells (14,19). Thus, we examined whether exposure
to hypoxia affects the proliferation of MCF-7 human breast cancer
cells by MTS assay (Fig. 2A).
Exposure to hypoxia for 24 h induced the proliferation of the MCF-7
cells, and the DEC2-overexpressing MCF-7 cells showed an increased
proliferation rate when compared with either the pcDNA-transfected-
or the DEC1 pcDNA-transfected cells. Moreover, DEC2 overexpression
enhanced the proliferation of the MCF-7 cells under both normoxic
and hypoxic conditions.
Overexpression of DEC2 induces c-Myc
expression in MCF-7 cells
To clarify the biological functions of DEC1 and DEC2
in breast cancer cells, we transiently transfected the expression
vectors for DEC1 or DEC2 into the MCF-7 cells and analyzed whether
DEC1 or DEC2 affects the expression of c-Myc. Under normoxic
conditions, c-Myc expression was slightly induced by the
overexpression of DEC2; however, the c-Myc protein level was
sharply elevated when DEC2 was overexpressed under hypoxic
conditions. By contrast, DEC1 overexpression under both normoxic
and hypoxic conditions did not affect the expression levels of
c-Myc when compared with the empty vector-transfected cells
(Fig. 2B). The above data suggest
that DEC2, but not DEC1, regulates the expression of c-Myc in MCF-7
cells.
Knockdown of HIF-2α inhibits Akt
phosphorylation, as well as the expression of DEC2 and c-Myc
It has been reported that HIF-2α induces the
proliferation of renal cell carcinoma cells (20). We thus hypothesized that HIF-2α
may contribute to the increased proliferation of MCF-7 cells under
hypoxic conditions. In order to characterize the function of HIF-2α
protein, the MCF-7 cells were transiently transfected with siRNA
against HIF-2α for 24 h, followed by another 24 h of exposure to
hypoxia or normoxia; western blot analysis was then performed. The
silencing of HIF-2α suppressed Akt phosphorylation, as well as DEC2
and c-Myc protein expression, regardless of the oxygen
concentration (Fig. 3A). However,
the expression of DEC1 was not affected by HIF-2α siRNA (data not
shown).
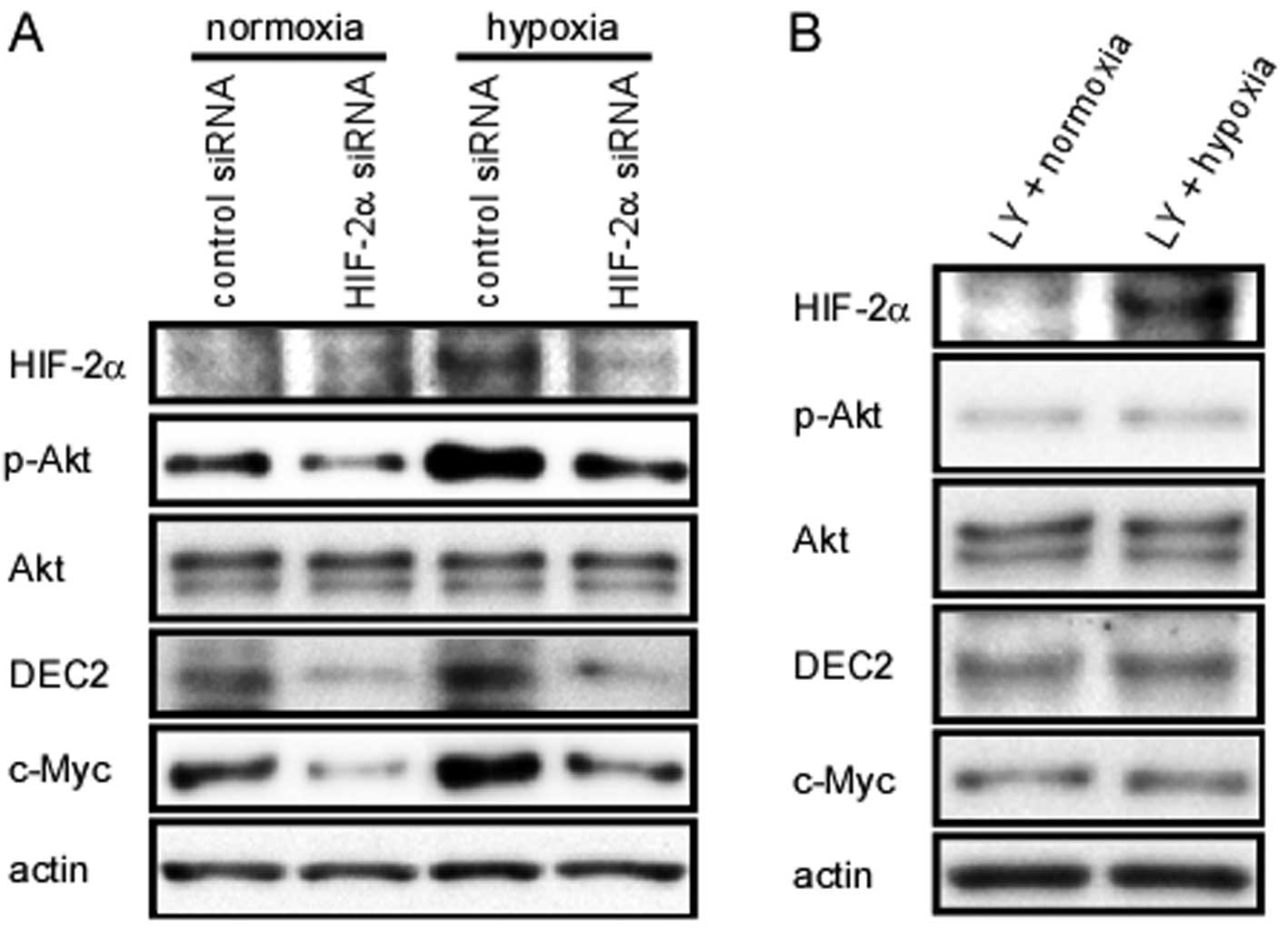 | Figure 3Hypoxia-inducible factor (HIF)-2α
regulates differentiated embryonic chondrocyte expressed gene
(DEC)2 and c-Myc expression by activating Akt. (A) MCF-7 cells were
transfected with HIF-2α short interference RNA (siRNA) and
incubated for 24 h. Subsequently, the cells were incubated under
hypoxic or normoxic conditions for an additional 24 h, before being
lysed. The lysates were subjected to western blot analysis for the
expression of HIF-2α, pAkt, Akt, DEC2, c-Myc and actin. (B) MCF-7
cells were incubated with LY294002 (PI3K/Akt inhibitor; 10
μM) for 1 h before being exposed to hypoxia for 24 h. The
lysates were prepared and subjected to western blot analysis for
the expression of HIF-2α, pAkt, Akt, DEC2, c-Myc and actin. |
Inhibition of Akt phosphorylation
decreases the induction of DEC2 by exposure to hypoxia
DEC1 has been reported to be involved in the
phosphorylation of Akt (21).
Therefore, we examined the association between Akt and DEC2.
Pre-treatment of the MCF-7 cells with LY294002, an inhibitor of the
PI3K/Akt signaling pathway, abrogated the hypoxia-induced Akt
phosphorylation, as well as DEC2 expression (Fig. 3B). In addition, exposure to
hypoxia failed to upregulate the protein level of c-Myc when the
PI3K/Akt signaling was blocked.
Discussion
In the present study, we demonstrate that DEC2 plays
a role, at least in part, in the proliferation of MCF-7 breast
cancer cells induced by HIF-2α under low oxygen conditions.
Silencing the expression of HIF-2α inhibited the phosphorylation of
Akt, as well as the expression of DEC2 and c-Myc. It seems that
DEC2 functions as an effector of the PI3K/Akt signaling pathway,
since the interruption of this pathway blocked the induction of
DEC2 expression following exposure to hypoxia. In addition, our
results demonstrated that DEC2, but not DEC1, upregulated the
expression of c-Myc. DEC2 and DEC1 are both transcription factors
and have been shown to transrepress their target genes by binding
to the E-box elements (22–24). Thus, it is suggested that the
regulation of c-Myc by DEC2 may not be mediated through binding to
the E-boxes. Future studies are required to clarify the details of
the mechanisms through which DEC2 regulates c-Myc expression, and
the differences between DEC2 and DEC1 in regulating their target
genes.
Although no significant changes in the mRNA level of
DEC1 were observed between the cells exposed to hypoxia for 2 and
those exposed for 24 h, the protein expression of DEC1 was
significantly reduced in the cells cultured under hypoxic
conditions for 24 h compared with that in the cells cultured under
hypoxic conditions for 2 and 8 h. DEC1 has been reported as a
marker of tumor hypoxia in the A549 lung adenocarcinoma cell line
(25). In that study, the mRNA
and protein expression levels of DEC1, as well as those of HIF-1α,
were upregulated by hypoxia in a time-dependent manner, and the
expression levels reached their peak at the time point of 48 h
(25). However, in our study, the
protein expression of DEC1 decreased after the cells were cultured
under hypoxic conditions for 24 h, when compared with those
cultured for 2 h; however, the mRNA expression level of DEC1
remained stable at all time points. We hypothesized that the
differences may arise from the specificity of the cell line and the
different methods used for detecting the expression of the protein.
In the other studies mentioned above, the expression of DEC1 and
HIF-1α was detected by immunocytochemical staining. Moreover,
previous studies have demonstrated that DEC2 and DEC1 inhibit each
other by binding the E-box on their promoters (23). Therefore, the reverse expression
patterns observed under hypoxic conditions may be partly caused by
the inhibitory effects exerted on each other.
DEC expression can be induced by a variety of other
stimuli, such as transforming growth factor (TGF)-β, cytokines,
retinoic acid, insulin and light (26–30). In the present study, we found that
exposure of the cells to hypoxia increased the expression of DEC1
and DEC2 in a different manner. More specifically, DEC1 expression
reached its peak at 2 h following exposure to hypoxia, whereas DEC2
expression reached its peak at 24 h following exposure to hypoxia.
We also observed that DEC1 had an expression pattern similar to
HIF-1α protein, whereas DEC2 had an expression pattern similar to
that of HIF-2α protein. Although DEC1 and DEC2 exhibit a similar
structure in their N-terminal, the C-terminal sequences are quite
different. That is, the proline-rich region of DEC1 is replaced by
the alanine/glycine-rich domain in DEC2 protein (31). Existing evidence suggests that
DEC1 and DEC2 are expressed and function in a different manner
during physiological and pathological processes (31). In the present study, we proved
that DEC2, but not DEC1, induced cell proliferation through the
upregulation of c-Myc expression. Further studies are required to
elucidate the function of the C-terminal of DEC1 and DEC2.
As an adverse prognostic factor, independent of
standard prognostic factors, such as tumor stage and nodal status,
hypoxia has been observed in a wide range of malignancies,
including cancer of the breast, the uterine cervix, the rectum, the
pancreas, the lungs, the brain, as well as soft tissue sarcomas,
non-Hodgkin’s lymphomas, malignant melanomas, metastatic liver
tumors and renal cell cancer (7).
The hypoxic microenvironment of tumors contributes to malignant
progression, poor prognosis and resistance to chemotherapy and
radiation. HIFs are crucial regulators of the response to low
oxygen conditions (7). To date,
HIF-1α and HIF-2α are the most extensively studied among the HIF
isoforms. While the two isoforms share similar domain structures
and overlapping target genes, it is now clear that they also have
unique target genes. Moreover, HIF-1α and HIF-2α usually have
divergent roles even in regulating the same genes (32). Recently, it has been reported that
HIF-1α is activated during the initial response to hypoxia, whereas
HIF-2α plays a major role during exposure to chronic hypoxia
(33). The similar expression
patterns of DEC2 and HIF-2α demonstrated in our study suggests that
DEC2 is an important mediator during exposure to chronic
hypoxia.
The c-Myc gene, which was first described by
Bishop et al in 1982, is a bHLH/Zip-type transcription
factor (34). c-Myc forms a
heterodimer with the bHLH/Zip protein MAX. Following dimerization,
this complex binds to specific DNA sites, at CACGTG sequences known
as the E-box motif, to activate and repress the transcription of
target genes, as well as to modulate chromatin (35). As one of the major human
oncogenes, c-Myc is frequently altered in many forms of cancer
(35–38). It modulates the cell cycle and
cell proliferation, increases cell metabolism and stimulates
differentiation, among its many other biological functions. Studies
have produced conflicting results as to how HIF-2α affects c-Myc
expression; some researchers have demonstrated that HIF-2α inhibits
c-Myc through direct interaction under physiological conditions
(20,39,40). However, others have suggested that
HIF-2α enhances c-Myc activity by binding and stabilizing c-Myc/Max
complexes (32). Our results are
in agreement with the latter, as we demonstrated that DEC2
regulated c-Myc expression through a yet undetermined
mechanism.
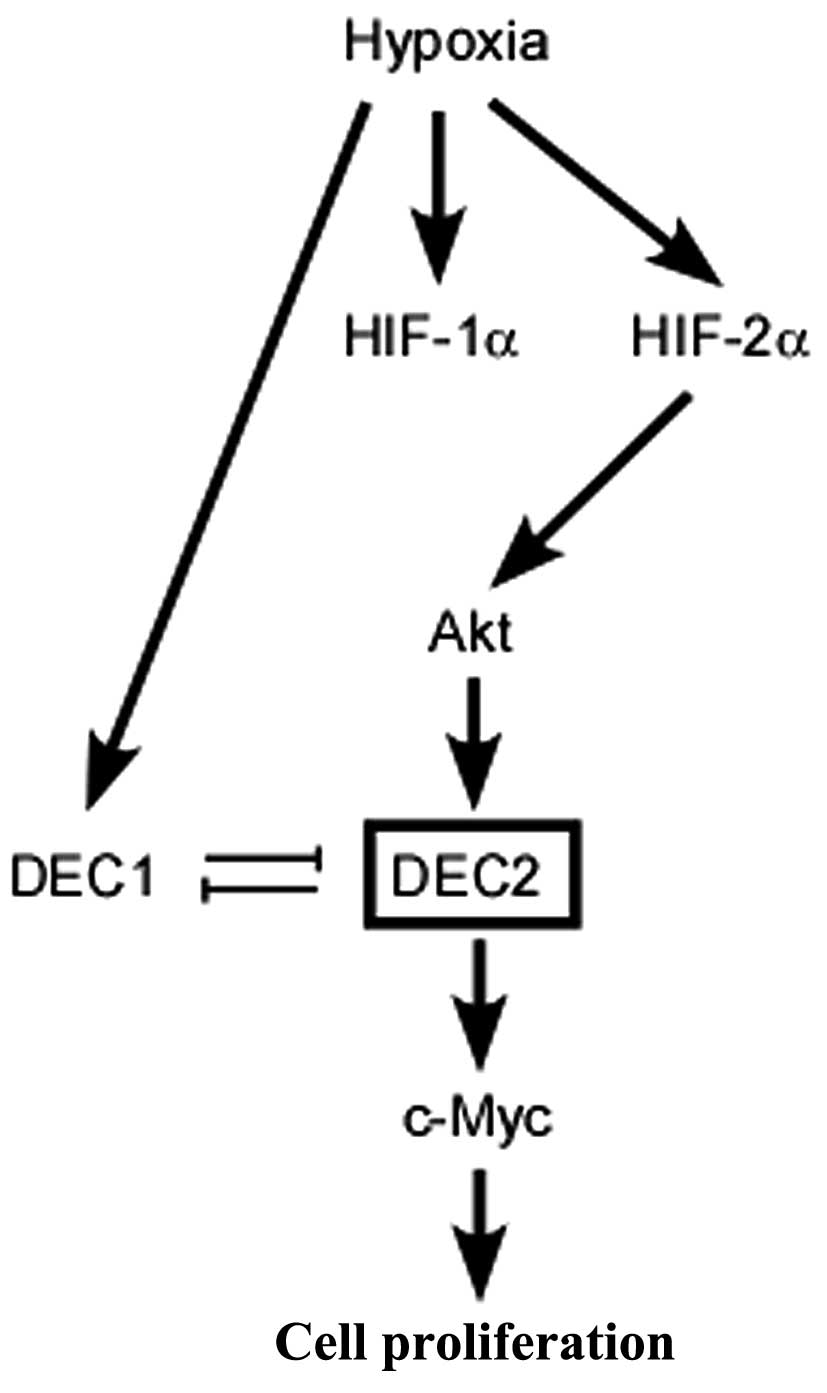
Fig. 4 summarizes
the possible pathways responsible for the proliferation of MCF-7
breast cancer cells induced by exposure to hypoxia. Although the
amino acid sequences of DEC1 and DEC2 share an approximately 40%
homology, they have different effects on the cell proliferation
induced by hypoxia and on apoptosis, as previously reported
(17). In the present study, we
found the c-Myc expression was increased by DEC2 overexpression in
the MCF-7 cells. In general, DEC2 is thought to function as a
transcriptional repressor by binding to the E-box in the promoters
of its target genes (31). In our
study, however, DEC2 was shown to function as a positive inducer by
enhancing the expression of c-Myc. In our previous study, we
demonstrated that siRNA against DEC2 decreased the mRNA level of
c-Myc in MCF-7 cells (18). We
deduced that DEC2 regulated the expression of c-Myc, not only at
the transcriptional level, but also at the post-transcriptional
level. DEC1 and DEC2 have also been reported to play distinct roles
in the epithelial-mesenchymal transition of pancreatic carcinoma
cells (41). The differential
protein structure and subcellular localization of DEC1 and DEC2 may
contribute to the divergent roles in regulating target genes.
Undoubtedly, further investigation is warranted to determine the
detailed mechanisms through which DEC2 regulates c-Myc
expression.
Acknowledgments
The present study was supported by Grants-in-Aid for
Science from the Ministry of Education, Culture, Sports, Science,
and Technology of Japan, a Grant for Hirosaki University
Institutional Research and the Fund for the Promotion of
International Scientific Research.
Abbreviations:
|
DEC1
|
differentiated embryonic chondrocyte
expressed gene 1
|
|
DEC2
|
differentiated embryonic chondrocyte
expressed gene 2
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Favaro E, Lord S, Harris AL and Buffa FM:
Gene expression and hypoxia in breast cancer. Genome Med. 3:552011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fyles A, Milosevic M, Hedley D, et al:
Tumor hypoxia has independent predictor impact only in patients
with node-negative cervix cancer. J Clin Oncol. 20:680–687. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hockel M, Schlenger K, Aral B, Mitze M,
Schaffer U and Vaupel P: Association between tumor hypoxia and
malignant progression in advanced cancer of the uterine cervix.
Cancer Res. 56:4509–4515. 1996.PubMed/NCBI
|
|
5
|
Jubb AM, Buffa FM and Harris AL:
Assessment of tumour hypoxia for prediction of response to therapy
and cancer prognosis. J Cell Mol Med. 14:18–29. 2010. View Article : Google Scholar
|
|
6
|
Nordsmark M, Bentzen SM, Rudat V, et al:
Prognostic value of tumor oxygenation in 397 head and neck tumors
after primary radiation therapy. An international multi-center
study. Radiother Oncol. 77:18–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vaupel P and Mayer A: Hypoxia in cancer:
significance and impact on clinical outcome. Cancer Metastasis Rev.
26:225–239. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu CJ, Sataur A, Wang L, Chen H and Simon
MC: The N-terminal transactivation domain confers target gene
specificity of hypoxia-inducible factors HIF-1alpha and HIF-2alpha.
Mol Biol Cell. 18:4528–4542. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Keith B, Johnson RS and Simon MC: HIF1α
and HIF2α: sibling rivalry in hypoxic tumour growth and
progression. Nat Rev Cancer. 12:9–22. 2012.
|
|
10
|
Loboda A, Jozkowicz A and Dulak J: HIF-1
versus HIF-2–is one more important than the other? Vascul
Pharmacol. 56:245–251. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Packham G and Cleveland JL: c-Myc and
apoptosis. Biochim Biophys Acta. 1242:11–28. 1995.PubMed/NCBI
|
|
12
|
Hoffman B and Liebermann DA: The
proto-oncogene c-myc and apoptosis. Oncogene. 17:3351–3357. 1998.
View Article : Google Scholar
|
|
13
|
Dang CV: c-Myc target genes involved in
cell growth, apoptosis, and metabolism. Mol Cell Biol. 19:1–11.
1999.
|
|
14
|
Dang CV, Resar LM, Emison E, et al:
Function of the c-Myc oncogenic transcription factor. Exp Cell Res.
253:63–77. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elend M and Eilers M: Cell growth:
downstream of Myc - to grow or to cycle? Curr Biol. 9:R936–R938.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Prendergast GC: Mechanisms of apoptosis by
c-Myc. Oncogene. 18:2967–2987. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu Y, Sato F, Bhawal UK, et al: Basic
helix-loop-helix transcription factors DEC1 and DEC2 regulate the
paclitaxel-induced apoptotic pathway of MCF-7 human breast cancer
cells. Int J Mol Med. 27:491–495. 2011.PubMed/NCBI
|
|
18
|
Liu Y, Sato F, Kawamoto T, et al:
Anti-apoptotic effect of the basic helix-loop-helix (bHLH)
transcription factor DEC2 in human breast cancer cells. Genes
Cells. 15:315–325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang J, Zubovitz J, Petrocelli T, et al:
PKB/Akt phosphorylates p27, impairs nuclear import of p27 and
opposes p27-mediated G1 arrest. Nat Med. 8:1153–1160. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gordan JD, Thompson CB and Simon MC: HIF
and c-Myc: sibling rivals for control of cancer cell metabolism and
proliferation. Cancer Cell. 12:108–113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bhawal UK, Sato F, Arakawa Y, et al: Basic
helix-loop-helix transcription factor DEC1 negatively regulates
cyclin D1. J Pathol. 224:420–429. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hamaguchi H, Fujimoto K, Kawamoto T, et
al: Expression of the gene for Dec2, a basic helix-loop-helix
transcription factor, is regulated by a molecular clock system.
Biochem J. 382:43–50. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fujimoto K, Hamaguchi H, Hashiba T, et al:
Transcriptional repression by the basic helix-loop-helix protein
Dec2: multiple mechanisms through E-box elements. Int J Mol Med.
19:925–932. 2007.PubMed/NCBI
|
|
24
|
Nakashima A, Kawamoto T, Honda KK, et al:
DEC1 modulates the circadian phase of clock gene expression. Mol
Cell Biol. 28:4080–4092. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang L and Li QQ: Embryo-chondrocyte
expressed gene 1, downregulating hypoxia-inducible factor 1alpha,
is another marker of lung tumor hypoxia. Acta Pharmacol Sin.
28:549–558. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zawel L, Yu J, Torrance CJ, et al: DEC1 is
a downstream target of TGF-beta with sequence-specific
transcriptional repressor activities. Proc Natl Acad Sci USA.
99:2848–2853. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ivanova AV, Ivanov SV, Zhang X, Ivanov VN,
Timofeeva OA and Lerman MI: STRA13 interacts with STAT3 and
modulates transcription of STAT3-dependent targets. J Mol Biol.
340:641–653. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boudjelal M, Taneja R, Matsubara S,
Bouillet P, Dolle P and Chambon P: Overexpression of Stra13, a
novel retinoic acid-inducible gene of the basic helix-loop-helix
family, inhibits mesodermal and promotes neuronal differentiation
of P19 cells. Genes Dev. 11:2052–2065. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamada K, Kawata H, Shou Z, Mizutani T,
Noguchi T and Miyamoto K: Insulin induces the expression of the
SHARP-2/Stra13/DEC1 gene via a phosphoinositide 3-kinase pathway. J
Biol Chem. 278:30719–30724. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Honma S, Kawamoto T, Takagi Y, et al: Dec1
and Dec2 are regulators of the mammalian molecular clock. Nature.
419:841–844. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamada K and Miyamoto K: Basic
helix-loop-helix transcription factors, BHLHB2 and BHLHB3; their
gene expressions are regulated by multiple extracellular stimuli.
Front Biosci. 10:3151–3171. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Loboda A, Jozkowicz A and Dulak J: HIF-1
and HIF-2 transcription factors - similar but not identical. Mol
Cells. 29:435–442. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koh MY and Powis G: Passing the baton: the
HIF switch. Trends Biochem Sci. 37:364–372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bishop JM: Retroviruses and cancer genes.
Adv Cancer Res. 37:1–32. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liao DJ and Dickson RB: c-Myc in breast
cancer. Endocr Relat Cancer. 7:143–164. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Weng W, Yang Q, Huang M, et al: c-Myc
inhibits TP53INP1 expression via promoter methylation in esophageal
carcinoma. Biochem Biophys Res Commun. 405:278–284. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dang CV, O’Donnell KA, Zeller KI, Nguyen
T, Osthus RC and Li F: The c-Myc target gene network. Semin Cancer
Biol. 16:253–264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Adhikary S and Eilers M: Transcriptional
regulation and transformation by Myc proteins. Nat Rev Mol Cell
Biol. 6:635–645. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang H, Gao P, Fukuda R, et al: HIF-1
inhibits mitochondrial biogenesis and cellular respiration in
VHL-deficient renal cell carcinoma by repression of C-MYC activity.
Cancer Cell. 11:407–420. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Corn PG, Ricci MS, Scata KA, et al: Mxi1
is induced by hypoxia in a HIF-1-dependent manner and protects
cells from c-Myc-induced apoptosis. Cancer Biol Ther. 4:1285–1294.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu Y, Sato F, Yamada T, et al: The BHLH
transcription factor DEC1 plays an important role in the
epithelial-mesenchymal transition of pancreatic cancer. Int J
Oncol. 41:1337–1346. 2012.PubMed/NCBI
|