Introduction
Vascular diseases involving atherosclerosis are the
major chronic complications of diabetes mellitus (DM) and the
primary prognostic determinants of diabetic patients. It has been
estimated that 75% of all deaths among diabetic patients are caused
by cardiovascular complications (1). The mechanisms underlying diabetic
vascular injuries, however, remain unclear.
Endothelial dysfunction, defined as an imbalance of
endothelium-derived vasoconstrictor and vasodilator substances,
precedes and dominates the pathogenesis and progression of both
macro- and microvascular complications associated with diabetes
(2). Nitric oxide (NO) is one of
the most well characterized vasodilators, and is also the first
gaseous molecule identified as a smooth muscle relaxer (3). Following the discovery of NO, carbon
monoxide (CO) was found to have similar functions (4,5).
Hydrogen sulfide (H2S) was the third endogeneous
gasotransmitter idendified following NO and CO (6). H2S was first discovered
in the brain as an endogenous neuromodulator (7,8).
Shortly after, it was found that H2S is also present in
the endothelium and plays an important role in the regulation of
vascular tone (9). To date, 3
enzymes that produce H2S have been identified:
cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE) and
3-mercaptopyruvate sulfur transferase (3MST). Both CBS and 3MST are
predominantly expressed in the brain, whereas CSE is primarily
localized in the vascular system (6,10).
Numerous studies have demonstrated that H2S is able to
relax blood vessels and lower blood pressure by opening adenosine
triphosphate (ATP)-sensitive potassium (K+) channels in
vascular smooth muscle (9,11–13).
In a previous study, the targeted deletion of the CSE gene
in mice markedly reduced H2S levels in serum and these
mice had elevated blood pressure and reduced endothelium-dependent
vasorelaxation (14). Moreover,
H2S has been shown to exert cytoprotective effects
against ischemic injury in various animal models of acute ischemia
(15–19).
Recently, there has been evidence suggesting that
H2S metabolism is dysregulated in diabetes. In non-obese
diabetic (NOD) mice, it has been reported that endogenous
H2S production in the vascular system is significantly
impaired, and that this is associated with marked endothelial
dysfunction (20). Similarly, it
has been shown that plasma H2S levels are markedly
reduced in diabetic patients (21). Hyperglycemia is the hallmark of
diabetes and has been recognized as an initiator of diabetic
endothelial dysfunction. In the present study, the effects of high
glucose on the production of H2S in human umbilical vein
endothelial cells (HUVECs) were investigated. Furthermore, the
effects of H2S on the secretion of endothelin-1 (ET-1)
by HUVECs were also determined. The aim of the present study was to
address the role and mechanisms of action of H2S in
endothelial dysfunction and vascular complications associated with
diabetes.
Materials and methods
Cell culture
Human umbilical cords were collected from healthy
full-term pregnant mothers during delivery, following the approval
of the Shandong University Research Ethics Committee (Jinan,
China). Signed informed consent was provided by all donors. The
umbilical cords were collected from the Department of Obstetrics,
Shandong Provincial Hospital from March to December 2010.
HUVECs were isolated from human umbilical veins and
were identified by their cobblestone morphology under a microscope
(Zeiss Axioplan; Zeiss, Weimar, Germany) and the strong positive
immunoreactivity to von Willebrand factor (data not shown). The
cells were grown in 5% CO2 at 37°C in M199 medium
(HyClone, Logan, UT, USA) containing 10% fetal bovine serum (FBS;
Gibco, Waltham, MA, USA), penicillin (100 U/ml), streptomycin (100
U/ml), L-glutamine and 20 ng/ml vascular endothelial growth factor
(VEGF). For all the experiments, cells of passage 2–3 were used.
When the cells were 80% confluent, they were grown in medium
containing 2% FBS and treated with various concentrations of
glucose. To maintain an equal osmotic pressure, 24.5 mmol/l
D-mannitol was used to adjust the osmotic concentration.
Measurement of H2S
The levels of H2S were measured as
previously described (22,23).
Briefly, the cells were collected in 500 μl ice-cold 100
mmol/l potassium phosphate buffer (pH 7.4) and homogenized. The
assay mixture containing homogenized cell lysates (430 μl),
L-cysteine (10 mmol/l; 20 μl), pyridoxal 5′-phosphate (2
mmol/l, 20 μl) and phosphate-buffered saline (PBS; 30
μl) was incubated at 37°C for 30 min in tightly sealed
Eppendorf vials. Zinc acetate [1% (w/v), 250 μl] was then
injected to trap the generated H2S followed by
trichloroacetic acid (TCA) [10% (w/v), 250 μl] to
precipitate the protein and thus terminate the reaction.
Subsequently, N,N-dimethyl-p-phenylenediamine sulfate
(NNDPD) (20 mmol/l; 133 μl) in 7.2 mol/l hydrochloric acid
(HCl) was added followed by FeCl3 (30 mmol/l; 133
μl) in 1.2 mol/l HCl and the absorbance (670 nm) of the
aliquots of the resulting solution was determined. The
H2S concentration of each sample was calculated against
a calibration curve of sodium hydrosulfide (NaHS; 0–250
μmol/l) and expressed as nanomoles of H2S per
milligram soluble protein.
Assay for the secretion of ET-1
The HUVECs were seeded in 60-mm cell dishes at
3×105 cells/ml, followed by treatment with various
concentrations of glucose. Cell supernatants were collected and the
content of ET-1 was detected by ELISA (R&D Systems,
Minneapolis, MN, USA). The results were normalized to the cellular
protein content in all experiments.
Real-time RT-PCR for CSE
Total RNA was isolated using TRIzol reagent (Takara
Bio, Beijing, China) from the treated cells. For real-time RT-PCR,
160 ng template was used in a 10-μl reaction containing 8
pmol of each primer pair and 10 μl of SYBR-Green Premix Ex
TaqII (Takara Bio). Reactions were performed using the following
cycling conditions: 95°C for 15 sec, followed by 40 cycles of 95°C
for 10 sec, 60°C for 20 sec and 72°C for 20 sec. The value of each
sample was calculated and expressed as the cycle threshold. The
amount of gene expression for each sample was calculated as the
difference (ΔCT) between the CT value of the target gene and the CT
value of the endogenous control (β-actin). Relative expression was
calculated as the difference (ΔΔCT) between the ΔCT values of the
test and the control samples for the target gene. The relative
level of expression was measured as 2−ΔΔCT. The human
primers used were as follows: CSE, forward, CAC TGTCCACCACGTTCAAG
and reverse, GTGGCTGCTAA ACCTGAAGC; β-actin, forward,
ACAGAGCCTCGCCTT TGCCG and reverse, ACATGCCGGAGCCGTTGTCG.
Western blot analysis for CSE
The cells were homogenized in RIPA lysis buffer with
1% proteinase inhibitor, phenylmethylsulfonyl fluoride (PMSF
solution). The protein concentration was measured using the
Bradford method. A total of 40 μg of total protein was
separated on 10% SDS-PAGE and transferred onto nitrocellulose
membranes. The blots were blocked in 5% BSA in TBST solution (10 mM
Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% Tween-20) for 30 min at room
temperature, followed by incubation for 1 h at room temperature in
1% BSA in TBST solution containing monoclonal anti-CSE antibody
(1:4,000, Cat. no. H00001434-M01; Abnova, Taipei, Taiwan).
Following incubation with horseradish peroxidase-conjugated
secondary antibodies (1:5,000, Cat. no. DkxMu-003-DHRPX), the
membranes were washed and developed using an enhanced
chemiluminescence kit. Anti-β-actin was routinely blotted and used
as a protein loading control. The quantification of band intensity
upon western blot analysis was conducted using NIH Image software
(ProteinSimple, Santa Clara, CA, USA).
Statistical analysis
All data are presented as the means ± standard
deviation (SD). Statistical analysis was performed using one-way
ANOVA. The Student-Newman-Keuls test was used for comparisons
between groups. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
High glucose reduces the production of
H2S in HUVECs
The effects of high glucose on the production of
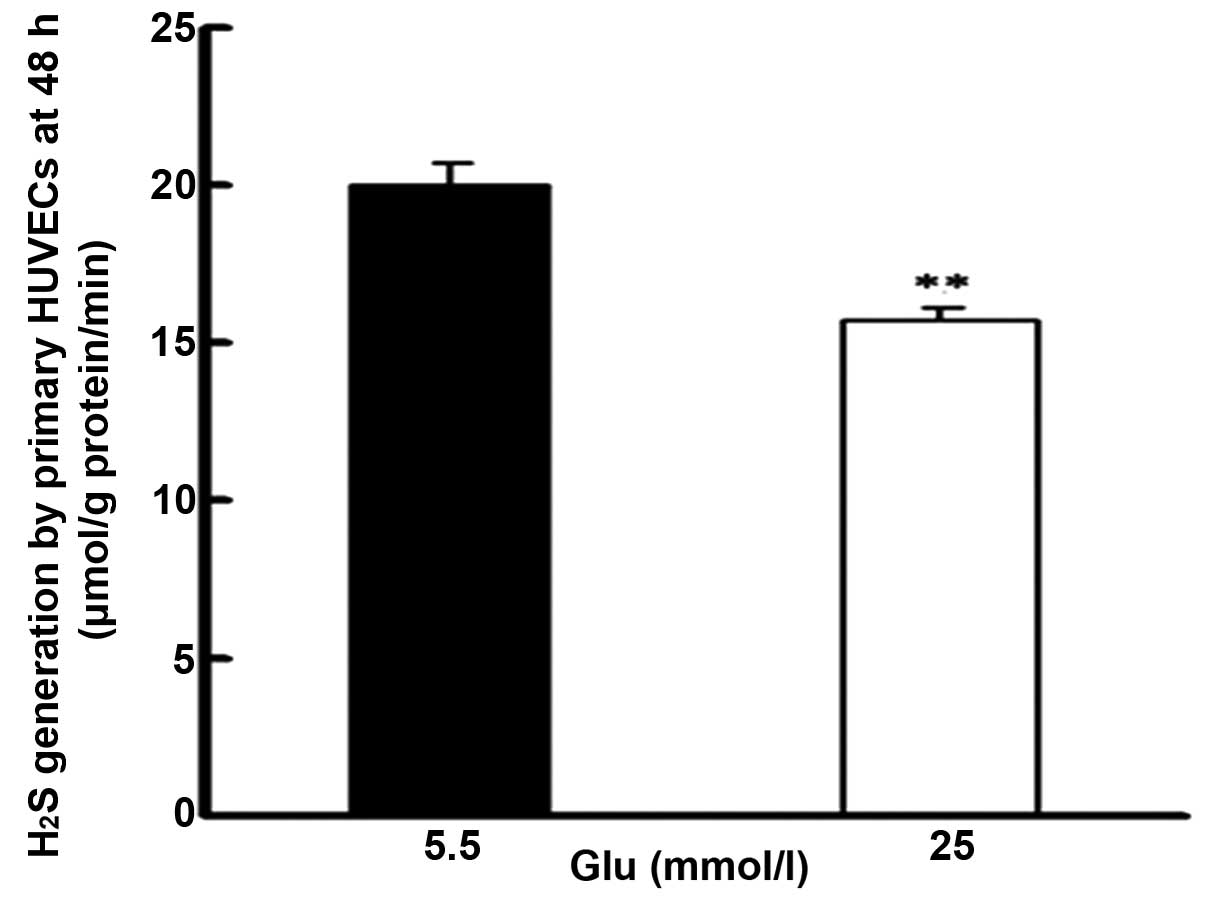
H2S in HUVECs were investigated. As shown in Fig. 1, treatment with high glucose
concentrations (25 mmol/l) for 48 h significantly reduced the
production of H2S compared with treatment with low
glucose (5.5 mmol/l).
High glucose suppresses the expression of
CSE in HUVECs
To elucidate the mechanisms responsible for the
inhibition of H2S production by high glucose, further
experiments were conducted to determine the effects of high glucose
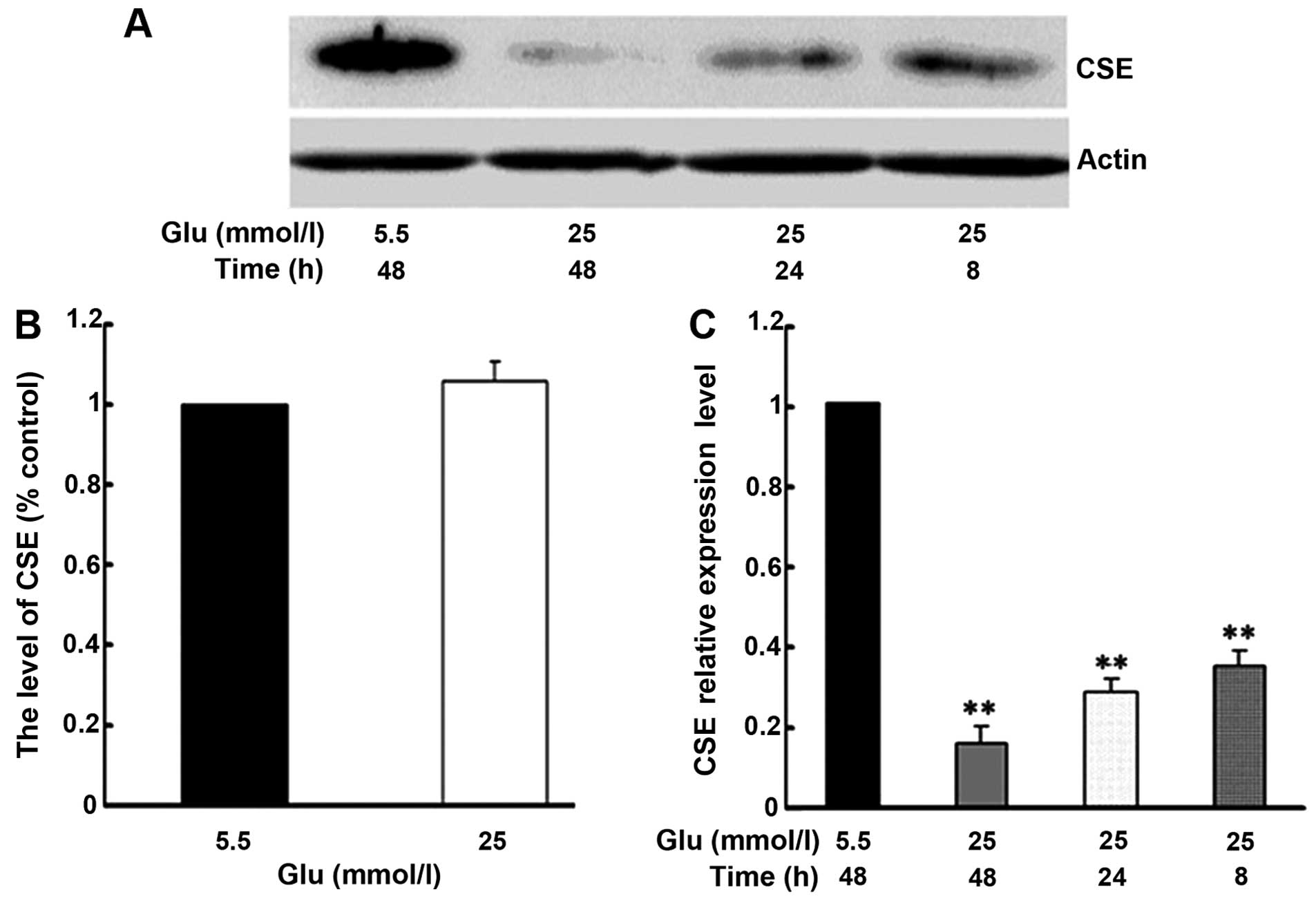
on the expression of CSE. As shown in Fig. 2B, treatment of the HUVECs with
high glucose (25 mmol/l) did not significantly alter the CSE mRNA
levels compared to treatment with low glucose (5 mmol/l). By
contrast, compared to treatment with 5.5 mmol/l glucose, treatment
with 25 mmol/l glucose significantly inhibited the CSE protein
expression in a time-dependent manner (Fig. 2A and C).
Exogenous H2S inhibits the
high glucose-induced secretion of ET-1 by HUVECs
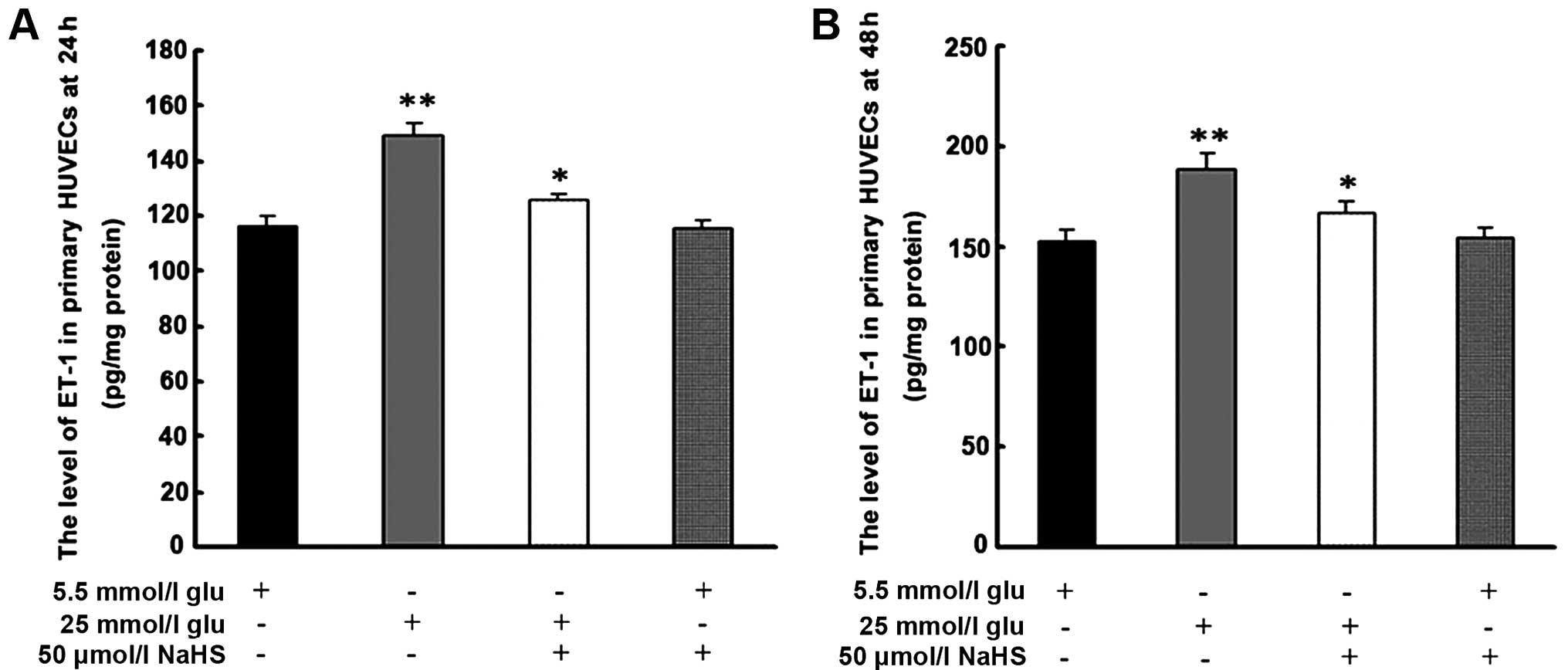
As shown in Fig.
3, treatment with 25 mmol/l glucose significantly increased the
level of ET-1 following 24 and 48 h of treatment. Pre-treatment for
30 min with 50 μmol/l NaHS inhibited the high
glucose-induced secretion of ET-1 at each time point.
Effects of NaHS on CSE protein expression
in HUVECs
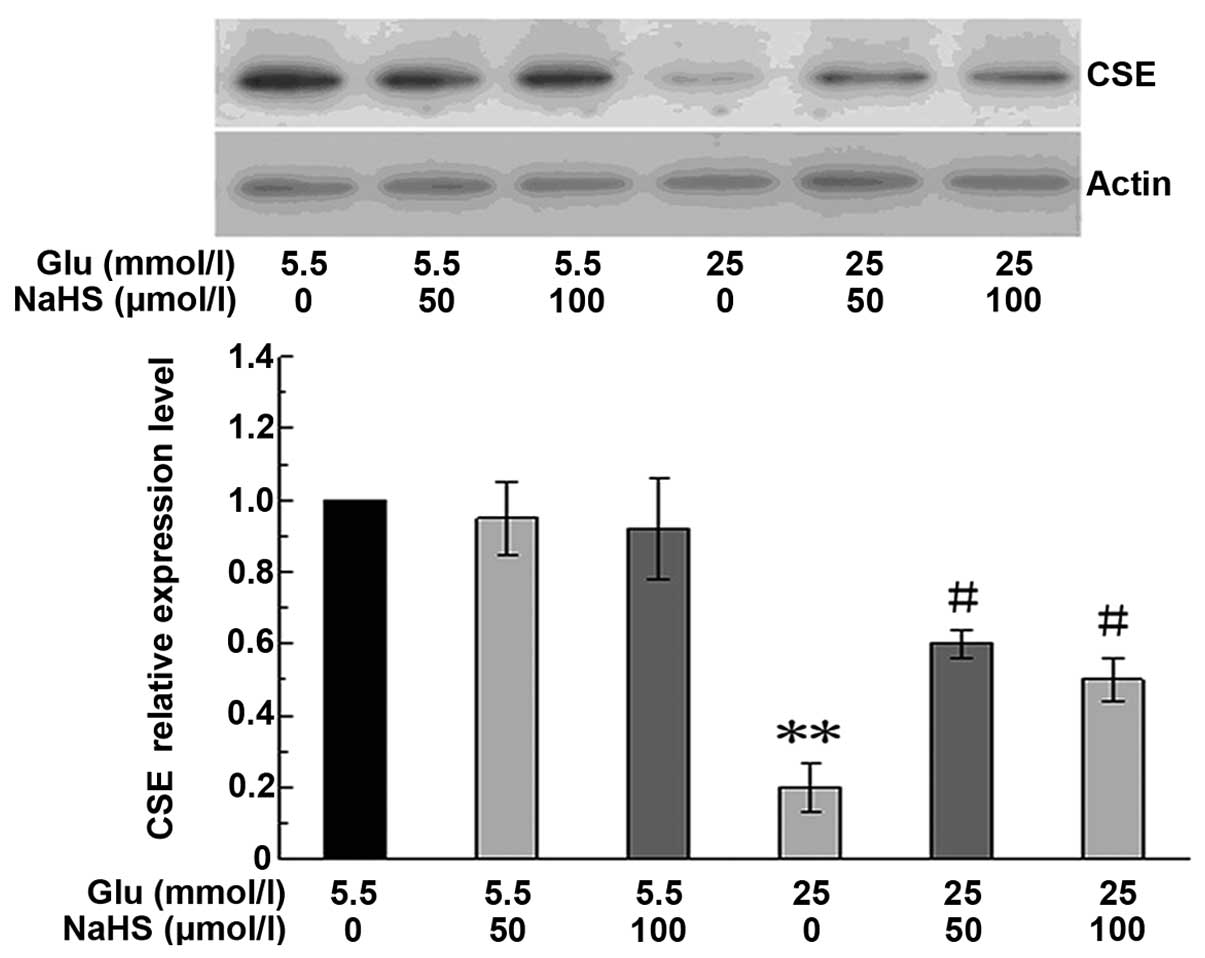
To determine whether NaHS has an effect on CSE
expression, the HUVECs were cultured in 5.5 mmol/l or 25 mmol/l
glucose, and treated with 50 or 100 μmol/l NaHS for 48 h.
The CSE protein levels were then determined by western blot
analysis. As shown in Fig. 4,
treatment with 25 mmol/l glucose for 48 h significantly reduced CSE
protein expression. This response was significantly attenuated by
NaHS at both concentrations examined (50 and 100 μmol/l).
However, NaHS at these concentrations showed no evident effect on
the basal CSE protein expression in the cells cultured under normal
glucose conditions (5.5 mmol/l).
Discussion
As a novel gasotransmitter, H2S has been
demonstrated to play important roles in the pathophysiology of
several biological systems. In particular, H2S has been
investigated extensively in the cardiovascular system. The
association between H2S and hypertension was firstly
investigated in a study on hypertensive rats (24). In that study, the authors
demonstrated that in the hypertensive rats, the level of endogenous
H2S was reduced and that exogenous H2S
effectively prevented the development of hypertension (24). In the nervous system,
H2S has been demonstrated to protect neurons from
apoptosis by increasing the production of the antioxidant,
glutathione, thus reducing the toxic effects induced by glutamic
acid (25). In addition,
H2S has been shown to play important roles in the
digestive system (26,27) and the respiratory system (28–30). To date, little is known however
about the role of H2S in diabetes-associated vascular
complications. In the present study, the effects of high glucose on
the production of H2S in HUVECs were investigated, as
well as the effects of exogenous H2S on the secretion of
ET-1.
A previous study demonstrated that a decrease in the
plasma levels of H2S correlated with coronary heart
disease (CHD) risk factors, such as smoking, hypertension and high
blood glucose levels (31). The
direct association between H2S and high blood glucose
levels, however, remains unclear. In the present study, treatment
with high glucose reduced the production of H2S in the
HUVECs. In the vascular system, CSE is the primary enzyme
responsible for H2S production (6). To investigate the mechanisms through
which high glucose reduces the production of H2S, CSE
protein expression and mRNA levels were analyzed by western blot
analysis and real-time RT-PCR, respectively in the current model.
The results revealed that high glucose significantly reduced CSE
protein expression in the HUVECs, whereas the CSE mRNA expression
was not affected. The precise mechanisms involved however are
unclear and require further investigation.
ET-1 is one of the most potent endogenous
vasoconstrictors released by endothelial cells (32). The balance between NO and ET-1
plays an essential role in the maintenance of vascular tone. It is
known that the effects of ET-1 are mediated by a G-protein coupled
receptor (33). In response to
various endothelial injuries, the release of ET-1 is increased.
Consequently, the level of ET-1 is recognized as an indicator of
endothelial dysfunction (34). In
addition, it has been shown that ET-1 expression is upregulated by
high blood glucose levels (35,36). In accordance with the above
observations, the present study demonstrated that high glucose
increased thee secretion of ET-1 from primary HUVECs.
In the present study, the high glucose-induced
secretion of ET-1 coincided with the reduced CSE protein expression
and the reduced generation of H2S. Therefore, the
effects of H2S on the release of ET-1 in HUVEcs were
investigated. NaHS is a widely used source of exogenous
H2S. When dissolved in solution, NaHS rapidly
dissociates to Na+ and HS−. Following this,
HS− associates with H+ to produce
H2S (37). Our results
demonstrated that NaHS, as a donor of exogenous H2S,
significantly inhibited the high glucose-induced release of ET-1 by
HUVECs, consistent with the results of previous studies (38,39). Taken together, these data suggest
that H2S may be an upstream regulator of ET-1. In
addition to direct conversion into H2S, it has been
suggested that NaHS may also indirectly influence the endogenous
generation of H2S. In guinea pigs with allergic rhinitis
(AR), NaHS was shown to increase CSE expression and H2S
production (40). In cultured rat
vascular smooth muscle cells, methylglyoxal (MG), an intermediate
of glucose metabolism, decreased cellular H2S levels by
downregulating CSE protein expression (41). At the same time, NaHS prevented
the reduction in CSE protein expression by reducing the cellular MG
levels (41). In the present
study, NaHS prevented the high glucose-induced downregulation of
CSE expression, but did not affect CSE protein expression under
normal glucose conditions. These results suggest that NaHS may
indirectly influence the production of H2S by regulating
CSE protein expression in a high glucose environment.
In conclusion, the results of the present study
suggest that the induction of ET-1 secretion by high glucose may be
partially mediated through the downregulation of CSE protein
expression and thereby, the reduction of the production of
H2S. This study also raises the possibility of the use
of NaHS as a potential therapeutic agent for diabetic vascular
complications. Additional studies are required to confirm these
findings in vivo.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (30971408).
References
|
1
|
Grundy SM, Howard B, Smith S Jr, Eckel R,
Redberg R and Bonow RO: Prevention Conference VI: Diabetes and
Cardiovascular Disease: executive summary: conference proceeding
for healthcare professionals from a special writing group of the
American Heart Association. Circulation. 105:2231–2239. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guangda X and Yuhua W: Apolipoprotein e4
allele and endothelium-dependent arterial dilation in Type 2
diabetes mellitus without angiopathy. Diabetologia. 46:514–519.
2003.PubMed/NCBI
|
|
3
|
Lüscher TF and Vanhoutte PM: The
Endothelium: Modulator of Cardiovascular Function. CRC Press; Boca
Raton, FL: pp. 1–228. 1990
|
|
4
|
Kharitonov VG, Sharma VS, Pilz RB, Magde D
and Koesling D: Basis of guanylate cyclase activation by carbon
monoxide. Proc Natl Acad Sci USA. 92:2568–2571. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yet SF, Tian R, Layne MD, et al:
Cardiac-specific expression of heme oxygenase-1 protects against
ischemia and reperfusion injury in transgenic mice. Circ Res.
89:168–173. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang R: Two’s company, three’s a crowd:
can H S be the third endogenous gaseous transmitter? FASEB J.
16:17922–1798. 2002. View Article : Google Scholar
|
|
7
|
Warenycia MW, Goodwin LR, Benishin CG, et
al: Acute hydrogen sulfide poisoning. Demonstration of selective
uptake of sulfide by the brainstem by measurement of brain sulfide
levels. Biochem Pharmacol. 38:973–981. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abe K and Kimura H: The possible role of
hydrogen sulfide as an endogenous neuromodulator. J Neurosci.
16:1066–1071. 1996.PubMed/NCBI
|
|
9
|
Hosoki R, Matsuki N and Kimura H: The
possible role of hydrogen sulfide as an endogenous smooth muscle
relaxant in synergy with nitric oxide. Biochem Biophys Res Commun.
237:527–531. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shibuya N, Tanaka M, Yoshida M, et al:
3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and
bound sulfane sulfur in the brain. Antioxid Redox Signal.
11:703–714. 2009. View Article : Google Scholar
|
|
11
|
Zhao W, Zhang J, Lu Y and Wang R: The
vasorelaxant effect of H2S as a novel endogenous gaseous
KATP channel opener. EMBO J. 20:6008–6016. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cheng Y, Ndisang JF, Tang G, Cao K and
Wang R: Hydrogen sulfide-induced relaxation of resistance
mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol.
287:H2316–H2323. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao W and Wang R: H2S-induced
vasorelaxation and underlying cellular and molecular mechanisms. Am
J Physiol Heart Circ Physiol. 283:H474–H480. 2002.PubMed/NCBI
|
|
14
|
Yang G, Wu L, Jiang B, et al:
H2S as a physiologic vasorelaxant: hypertension in mice
with deletion of cystathionine gammalyase. Science. 322:587–590.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Johansen D, Ytrehus K and Baxter GF:
Exogenous hydrogen sulfide (H2S) protects against
regional myocardial ischemia-reperfusion injury - Evidence for a
role of K ATP channels. Basic Res Cardiol. 101:53–60. 2006.
View Article : Google Scholar
|
|
16
|
Kimura H, Nagai Y, Umemura K and Kimura Y:
Physiological roles of hydrogen sulfide: synaptic modulation,
neuroprotection, and smooth muscle relaxation. Antioxid Redox
Signal. 7:795–803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jha S, Calvert JW, Duranski MR,
Ramachandran A and Lefer DJ: Hydrogen sulfide attenuates hepatic
ischemia-reperfusion injury: role of antioxidant and antiapoptotic
signaling. Am J Physiol Heart Circ Physiol. 295:H801–H806. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tripatara P, Patel NS, Collino M, et al:
Generation of endogenous hydrogen sulfide by cystathionine
gamma-lyase limits renal ischemia/reperfusion injury and
dysfunction. Lab Invest. 88:1038–1048. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fu Z, Liu X, Geng B, Fang L and Tang C:
Hydrogen sulfide protects rat lung from ischemia-reperfusion
injury. Life Sci. 82:1196–1202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brancaleone V, Roviezzo F, Vellecco V, De
Gruttola L, Bucci M and Cirino G: Biosynthesis of H2S is
impaired in non-obese diabetic (NOD) mice. Br J Pharmacol.
155:673–680. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Whiteman M, Gooding KM, Whatmore JL, et
al: Adiposity is a major determinant of plasma levels of the novel
vasodilator hydrogen sulphide. Diabetologia. 53:1722–1726. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li L, Bhatia M, Zhu YZ, et al: Hydrogen
sulfide is a novel mediator of lipopolysaccharide-induced
inflammation in the mouse. FASEB. 19:1196–1198. 2005.
|
|
23
|
Lee M, Schwab C, Yu S, McGeer E and McGeer
PL: Astrocytes produce the antiinflammatory and neuroprotective
agent hydrogen sulfide. Neurobiol Aging. 30:1523–1534. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhong G, Chen F, Cheng Y, Tang C and Du J:
The role of hydrogen sulfide generation in the pathogenesis of
hypertension in rats induced by inhibition of nitric oxide
synthase. J Hypertens. 21:1879–1885. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kimura Y and Kimura H: Hydrogen sulfide
protects neurons from oxidative stress. FASEB J. 18:1165–1167.
2004.PubMed/NCBI
|
|
26
|
Perini R, Fiorucci S and Wallace JL:
Mechanisms of nonsteroidal anti-inflammatory drug-induced
gastrointestinal injury and repair: a window of opportunity for
cyclooxygenase-inhibiting nitric oxide donors. Can J Gastroenterol.
18:229–236. 2004.PubMed/NCBI
|
|
27
|
Fiorucci S, Antonelli E, Distrutti E, et
al: Inhibition of hydrogen sulfide generation contributes to
gastric injury caused by anti-inflammatory nonsteroidal drugs.
Gastroenterology. 129:1210–1224. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li T, Zhao B, Wang C, et al: Regulatory
effects of hydrogen sulfide on IL-6, IL-8 and IL-10 levels in the
plasma and pulmonary tissue of rats with acute lung injury. Exp
Biol Med. 233:1081–1087. 2008. View Article : Google Scholar
|
|
29
|
Fang L, Li H, Tang C, Geng B, Qi Y and Liu
X: Hydrogen sulfide attenuates the pathogenesis of pulmonary
fibrosis induced by bleomycin in rats. Can J Physiol Pharmacol.
87:531–538. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen YH, Yao WZ, Geng B, et al: Endogenous
hydrogen sulfide in patients with COPD. Chest. 128:3205–3211. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang HL, Wu HC, Li ZL, Geng B and Tang
CS: Changes of the new gaseous transmitter H S in patients with
coronary heart disease. Di Yi Jun Yi Da Xue Xue Bao. 25:951–954.
2005.In Chinese. PubMed/NCBI
|
|
32
|
Chester AH: Endothelin-1 and the aortic
valve. Curr Vasc Pharmacol. 3:353–357. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yanagisawa M, Kurihara H, Kimura S, et al:
A novel potent vasoconstrictor peptide produced by vascular
endothelial cells. Nature. 332:411–415. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Quiñones MJ and Nicholas SB: Insulin
resistance and the endothelium. Curr Diab Rep. 5:246–253. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
DeLoach S, Huan Y, Daskalakis C and
Falkner B: Endothelin-1 response to glucose and insulin among
African Americans. J Am Soc Hypertens. 4:227–235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu J, Wei S, Tian L, Yan L, Guo Q and Ma
X: Effects of endomorphins on human umbilical vein endothelial
cells under high glucose. Peptides. 32:86–92. 2011. View Article : Google Scholar
|
|
37
|
Beauchamp RO Jr, Bus JS, Popp JA, Boreiko
CJ and Andjelkovich DA: A critical review of the literature on
hydrogen sulfide toxicity. Crit Rev Toxicol. 13:25–97. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li XH, Du JB and Tang CS: Impact of
hydrogen sulfide donor on pulmonary vascular structure and
vasoactive peptides in rats with pulmonary hypertension induced by
high pulmonary blood flow. Zhongguo Yi Xue Ke Xue Yuan Xue Bao.
28:159–163. 2006.In Chinese. PubMed/NCBI
|
|
39
|
Li X, Du J, Jin H, Geng B and Tang C:
Sodium hydrosulfide alleviates pulmonary artery collagen remodeling
in rats with high pulmonary blood flow. Heart Vessels. 23:409–419.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shaoqing Y, Ruxin Z, Yinjian C, Jianqiu C,
Zhiqiang Y and Genhong L: Down-regulation of endogenous hydrogen
sulphide pathway in nasal mucosa of allergic rhinitis in guinea
pigs. Allergol Immunopathol. 37:180–187. 2009. View Article : Google Scholar
|
|
41
|
Chang T, Untereiner A, Liu J and Wu L:
Interaction of methylglyoxal and hydrogen sulfide in rat vascular
smooth muscle cells. Antioxid Redox Signal. 12:1093–1100. 2010.
View Article : Google Scholar
|