Introduction
Among the many types of cancer, it is universally
known that lung cancer has the highest morbidity and mortality
worldwide (1). Clinical trials
have demonstrated that approximately 80% of lung cancers are
non-small cell lung cancer (NSCLC) with a fairly low survival rate
(5-year survival rate <15%) (2,3).
Nowadays, in spite of the fact that substantial progress has been
made with traditional therapies, including combination regimens,
patients with advanced NSCLC still have a poor prognosis (4–6).
Therefore, the challenge in the treatment of NSCLC is to identify
novel targets that may complement therapies.
Recently, microRNAs (miRNAs or miRs) have been
implicated in various diseases (7). miRNAs with a length of 18–24
nucleotides modulate protein translation by directly binding the
3′-untranslated region (3′-UTR) of the target mRNA, thereby leading
to mRNA destabilization and degradation (8,9).
By modulating the protein expression of target genes, miRNAs are
involved in the regulation of numerous cellular processes,
including cell growth, proliferation, apoptosis, differentiation,
migration and metabolism (10,11). Therefore, the dysregulation of
miRNAs has been observed in a variety of cancer types (12,13). miRNAs not only play an important
role in tumorigenesis and cancer treatment, but are also useful
predictors for diagnosis and prognosis (14–16). Hence, cancer-related miRNAs may
represent a novel and potential target for the therapeutic
intervention of cancer.
It has been demonstrated that the miR-17-92 cluster
located on chromosome 13q31.3 comprises a subset of oncogenic
miRNAs that are extensively overexpressed in many types of cancer
(17). Compelling evidence has
indicated that miR-19a, belonging to the miR-17-92 cluster, is
associated with the pathogenesis and development of many types of
human cancer, including gastric cancer (18), cervical cancer (19) and colon cancer (20). The overexpression of miR-19a in
the serum and tumor tissue of patients with NSCLC has been found to
correlate with a worse prognosis (21). However, the role of miR-19a in
regulating NSCLC is largely unknown.
In the present study, we aimed to delineate the role
and the underlying mechanisms of action of miR-19a in the
development of NSCLC. We found that miR-19a was not only
overexpressed in tumor tissues of patients with NSCLC, but also in
A549 and NCI-H157 cells, which are 2 NSCLC cell lines. The enforced
expression of miR-19a increased cell growth and viability, cell
invasion and migration, and promoted the growth of xenograft
tumors, whereas the inhibition of miR-19a exerted the opposite
effects. More importantly, we found that miR-19a directly bound the
3′-UTR of the suppressor of cytokine signaling 1 (SOCS1) and
regulated its expression in NSCLC cells. By regulating SOCS1
expression, miR-19a activated the signal transducer and activator
of transcription (STAT)3, which is involved in various types of
cancer, including NSCLC (22).
Taken together, our data suggest a possible underlying mechanism of
action of miR-19a in the development of NSCLC and that miR-19a may
present a promising target for the therapeutic intervention of
NSCLC.
Materials and methods
Tissue samples, cell culture and
animals
Fifteen pairs of lung cancer tissue samples and
adjacent normal tissues were obtained from the Second Affiliated
Hospital of Zhengzhou University, Zhengzhou, China after obtaining
informed consent from the patients and approval from the Ethics
Committee of the Second Affiliated Hospital of Zhengzhou
University. The average age of the patients was 44.08±3.05 years.
Eleven of the specimens were lung squamous cell carcinoma and 4 of
the specimens were lung adenocarcinoma. The surgical removal of the
lung tumors was carried out prior to treatment with chemotherapy
and radiotherapy. The human NSCLC cell lines, A549 and NCI-H157,
were purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA) and grown in Dulbecco’s modified Eagle’s medium
(DMEM) supplemented with 10% fetal calf serum (FCS; Gibco-BRL,
Rockville, MD, USA) and 1% penicillin/streptomycin. The human
embryonic kidney cell line, HEK293T, and the immortalized
keratinocyte cell line, HaCaT, were obtained from the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China) and
maintained in DMEM supplemented with 10% FCS. All cells were
cultured under a humidified atmosphere containing 5% CO2
at 37°C in an incubator. Female, 6-week-old BALB/c nude mice (25–30
g) purchased from the Experimental Animal Center of Zhengzhou
University were raised under pathogen-free conditions with free
access to water and food. The animal experimental procedures were
approved and reviewed by the Institutional Animal Care and Use
Committee of Zhengzhou University.
Reverese transcription-polymerase chain
reaction (RT-PCR)
Total RNA from the tissue samples and cells was
isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and
small RNA was extracted using mirVana kits (Ambion, Inc., Austin,
TX, USA) according to the manufacturer’s instructions. A total of 5
μg RNA was reverse-transcribed into complementary DNA using
M-MLV reverse transcriptase (Clontech, Palo Alto, CA, USA) and the
TaqMan miRNA reverse transcription kit (Applied Biosystems, Foster
City, CA, USA). Gene expression was analyzed according to the
RT-PCR protocol. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH;
for SOCS1) and U6 snRNA (for miR-19a) were used as internal
references and the relative gene expression was quantified using
the 2−ΔΔCt method.
MTT assay
The cells were seeded in 96-well culture plates at
1×104 cells/well. When the cells reached 80% confluence,
50 nM of miR-19a mimics, miR-19a inhibitor or scrambled control
(GenePharma, Ltd., Shanghai, China) were transfected into the
cells. After 48 h of transfection, 20 μl/well MTT (5 mg/ml
in PBS) were added followed by continuous culture for 4 h.
Thereafter, 200 μl/well dimethylsulfoxide were added to
dissolve the formazan crystals for 15 min. The absorbance at 490 nm
was measured using an ELISA reader (BioTek Instruments, Inc.,
Winooski, VT, USA). The experiments were performed in quintuplicate
and repeated 3 times.
Cell invasion and migration assays
The potential of cell invasion and migration was
measured using Transwell inserts, as previously described (23). To determine the cell invasion
ability, 24-well plates with Transwell chambers and pre-coated
Matrigel membrane filter (Neuro Probe Inc., Gaithersburg, MD, USA)
were prepared. A total of 500 μl DMEM plus 10% FCS was added
to the lower chamber and 2×105 cells resuspended in 200
μl of serum-free DMEM were plated in the upper chamber of
chemotaxis chambers. After 48 h of incubation at 37°C, the inserts
were removed and were submerged in PBS to remove the unattached
cells. The cells were then fixed with paraformaldehyde (4%) and
stained with crystal violet (0.1%; Sigma-Aldrich, St. Louis, MO,
USA). The invading cells were photographed (×20) and 5 random
fields on each membrane were selected for cell number counting.
Cell migration ability was detected according to a protocol similar
to that for the cell invasion assay using a Matrigel-uncoated
24-well Transwell plate. These experiments were carried out in
triplicate.
Dual-luciferase reporter assay
The putative binding sequences of SOCS1 3′-UTR for
miR-19a were predicated by miRanda (http://www.microrna.org/), miRBase (http://www.mirbase.org/) and Targetscan (http://www.targetscan.org/). The 3′-UTR and mutated
3′-UTR constructs of SOCS1 were amplified and subcloned into a pGL3
luciferase promoter vector (Promega Corp., Madison, WI, USA) with
XbaI and NotI restriction sites. The HEK293T cells
were transfected with 50 ng of the pGL3 vector, pGL3-SOCS1-3′-UTR
or pGL3-SOCS1-mutated 3′-UTR (Mut-SOCS1-3′-UTR), and miR-19a
mimics, miR-19a inhibitor or scrambled control (50 nM) were then
added using lipofectamine transfection reagent (Invitrogen). After
48 h of incubation, the cells were harvested and the luciferase
activity was determined using the dual-luciferase reporter assay
kit (Promega Corp.). The relative quantification was normalized to
the luciferase activity in the control group.
Western blot analysis
A total of 20 μg proteins was isolated by
electrophoresis on 12% SDS-polyacrylamide gel and transferred to a
nitrocellulose membrane (Bio-Rad, Hercules, CA, USA). Non-specific
bindings in the membrane were blocked by skimmed milk (2.5%) for 1
h at 37°C. The membrane was then incubated with primary antibodies
in blocking buffer at 4°C overnight. The primary antibodies were as
follows: anti-SOCS1 (sc-7006), anti-STAT3 (sc-8019) and anti-GAPDH
(sc-20357) purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA); anti-p-STAT3 (#9145) was purchased from Cell
Signaling Technology, Inc., (Danvers, MA, USA). After washing with
Tris-buffered saline and Tween-20 (TBST) 3 times, secondary
antibody conjugated with horseradish peroxidase (bs-0293Gs; Bioss,
Beijing, China) was added followed by incubation for 4 h. Finally,
the protein band was detected using an enhanced chemiluminescence
(ECL) detection system (Amersham Pharmacia Biotech, Little
Chalfont, UK). The protein gray intensity was quantified using
Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville,
MD, USA). The fold changes in protein expression were presented
after normalization to the control group.
Mouse xenograft assay
For this experiment, 2×106 cells
suspended in 200 μl of PBS were injected subcutaneously into
the flanks of nude mice. Approximately 2×108
plaque-forming units of lentiviral vectors expressing miR-19a or
anti-miR-19a oligonucleotide (GenePharma, Ltd.) diluted in 50
μl of PBS were injected intratumorally twice per week. The
tumor volume was measured each day as presented by length ×
width2 × π/6. Approximately 40 days after cell
inoculation, the mice were injected subcutaneously with sodium
pentobarbital (40 mg/kg). The tumors were isolated and the total
RNA and protein was extracted for analysis.
Statistical analysis
Data are presented as the means ± standard deviation
(SD). Significant comparisons were carried out by the two-tailed
Student’s t-test or one-way ANOVA followed by the Bonferroni post
hoc test. A p-value <0.05 was considered to indicate a
statistically significant difference.
Results
miR-19a is overexpressed in lung cancer
tissues and NSCLC cells
To investigate the role of miR-19a in the
tumorigenesis of NSCLC, we firstly identified the expression
profile of miR-19a in tumor tissues and adjacent normal tissues by
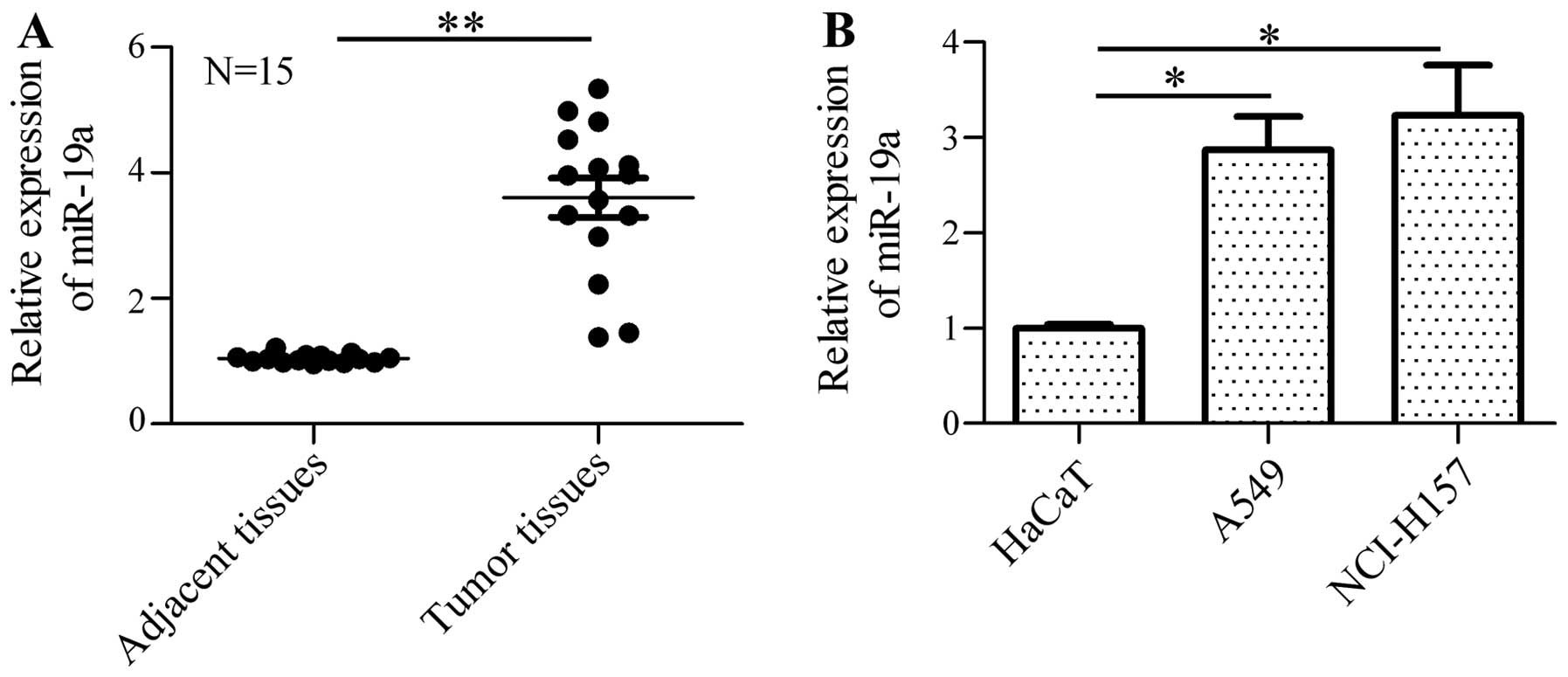
RT-PCR. The results revealed that the expression of miR-19a was
markedly increased in the human NSCLC samples in comparison with
the normal samples (Fig. 1A).
Furthermore, the expression of miR-19a in the NSCLC cell lines,
A549 and NCI-H157, was measured and the data indicated that miR-19a
was significantly upregulated in the A549 and NCI-H157 cells in
comparison with the control cells (HaCaT cells; Fig. 1B). These results suggest that
miR-19a may be involved in the mediation of tumorigenesis and the
development of NSCLC.
miR-19a enhances the cell growth and
viability of NSCLC cells
To determine the effects of miR-19a on the growth of
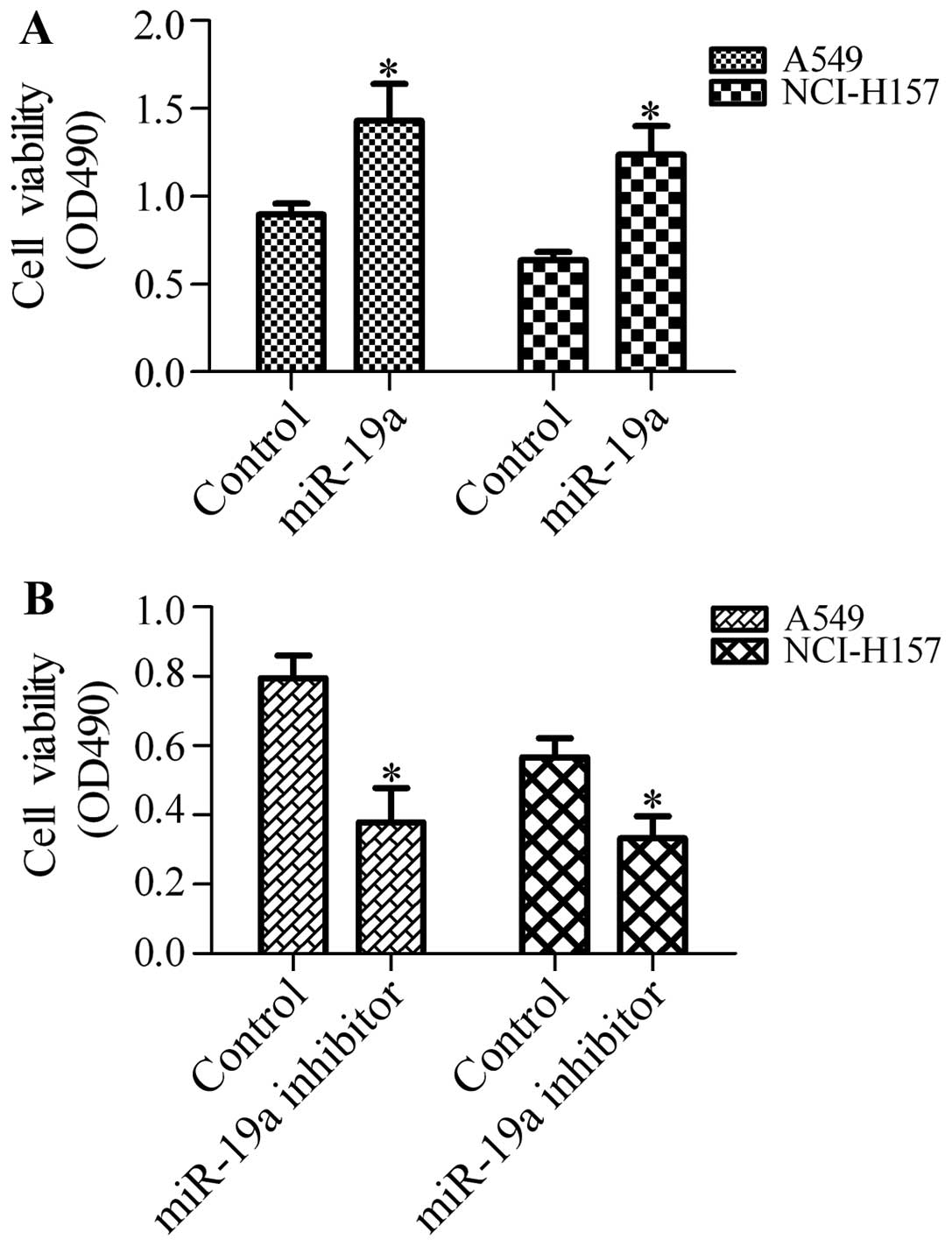
NSCLC cells, we transfected the A549 and NCI-H157 cells with
miR-19a mimics or miR-19a inhibitor and detected cell growth and
viability by MTT assay. The results revealed that transfection of
the cells with miR-19a mimics significantly enhanced the growth and
viability of the A549 and NCI-H157 cells (Fig. 2A). Conversely, transfection with
miR-19a inhibitor markedly inhibited the growth and viability of
the A549 and NCI-H157 cells (Fig
2B). These results suggest that miR-19a positively regulates
the growth and viability of NSCLC cells.
miR-19a promotes the invasion and
migration of NSCLC cells
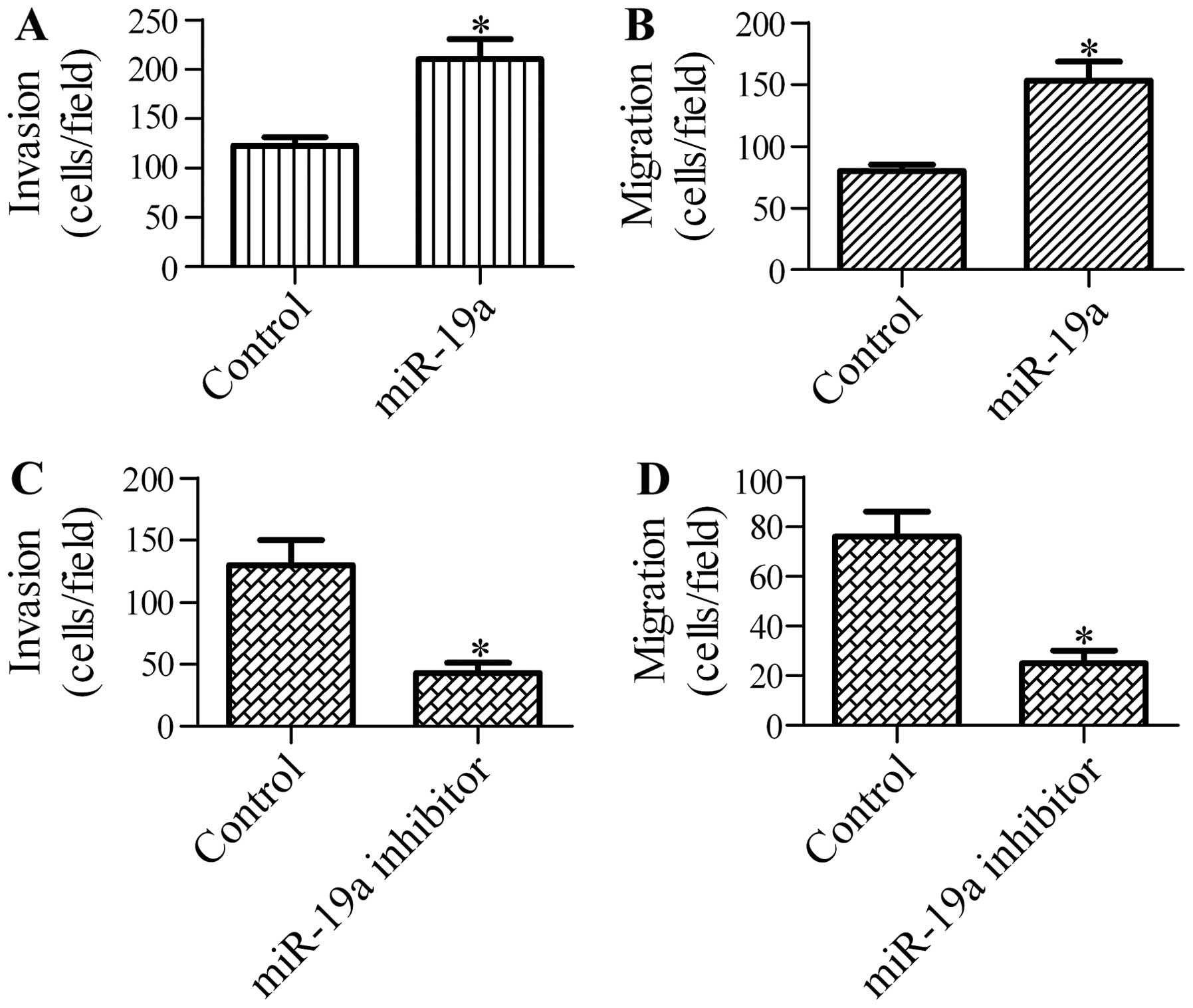
To further explore the function of miR-19a in NSCLC
cells, we determined the effects of miR-19a on the invasion and
migration ability of A549 cells. Transfection with miR-19a
significantly increased the cell invasion (Fig. 3A) and migration (Fig. 3B) ability of the A549 cells. By
contrast, cell invasion (Fig 3C)
and migration (Fig. 3D) were
markedly reduced by transfection of the A549 cells with miR-19a
inhibitor. These data indicate that miR-19a promotes the invasion
and migration of NSCLC cells.
SOCS1 is a direct target gene of
miR-19a
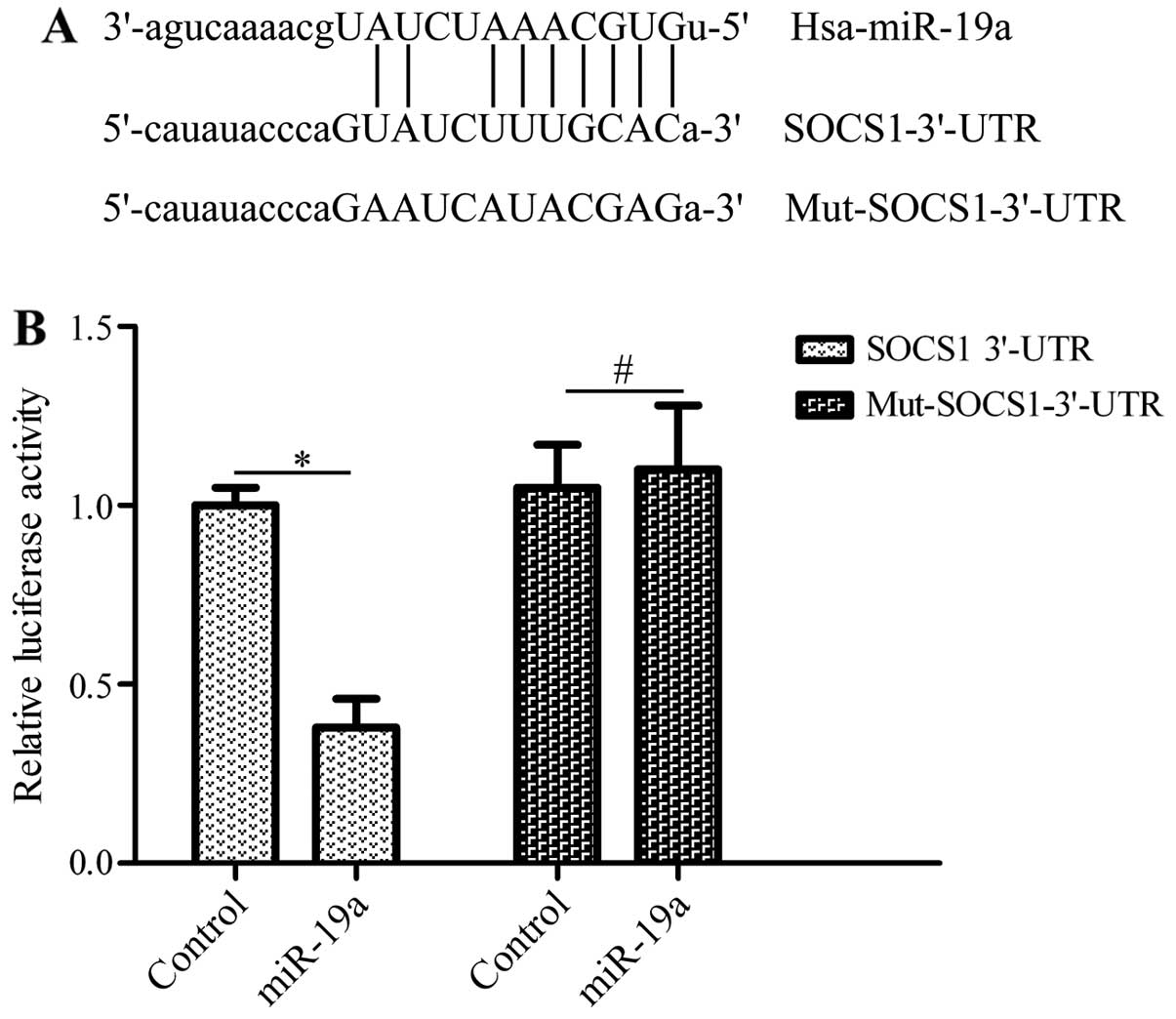
To elucidate the mechanisms of action of miR-19a in
regulating the development of NSCLC, the candidate target genes of
miR-19a were predicted by bioinformatics analysis. Of these genes,
SOCS1, a critical tumor-associated gene (24), was found to have putative bindings
sites within the 3′-UTR of miR-19a (Fig. 4A). To confirm the interaction of
miR-19a with the 3′-UTR of SOCS1, we subcloned the 3′-UTR or
mutated 3′-UTR of SOCS1 downstream of the luciferase reporter gene
in the pGL3 plasmid. These vectors were co-transfected with miR-19a
mimics into HEK293T cells. The results revealed that transfection
with miR-19a mimics and pGL3-SOCS1-3′-UTR significantly inhibited
luciferase activity compared with the controls, whereas the vector
bearing the mutated 3′-UTR of SOCS1 was not affected by
transfection with miR-19a mimics (Fig. 4B). These data suggest that miR-19a
directly binds to the 3′-UTR of SOCS1.
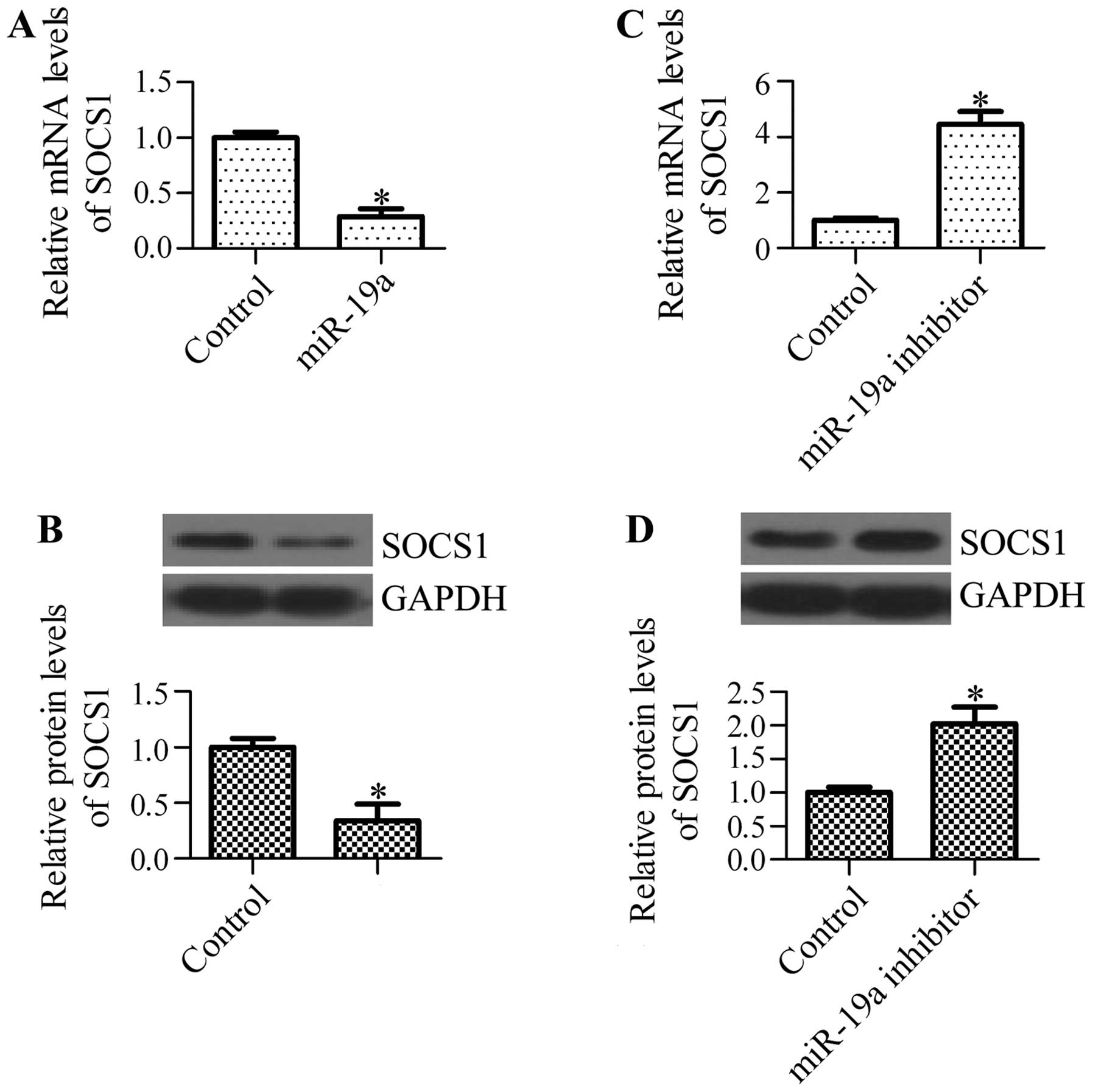
miR-19a regulates the mRNA and protein
expression levels of SOCS1
To determine whether miR-19a has an effect on
endogenous SOCS1 expression, we examined the mRNA and protein
expression levels of SOCS1 in A549 cells transfected with miR-19a
mimics or miR-19a inhibitor. We found that transfection with
miR-19a mimics significantly decreased the mRNA levels of SOCS1
(Fig. 5A) and decreased the
protein levels of SOCS1 (Fig.
5B). By contrast, the inhibition of miR-19a markedly
upregulated the mRNA (Fig. 5C)
and protein levels of SOCS1 (Fig.
5D). These results suggest that miR-19a regulates SOCS1
expression in NSCLC cells.
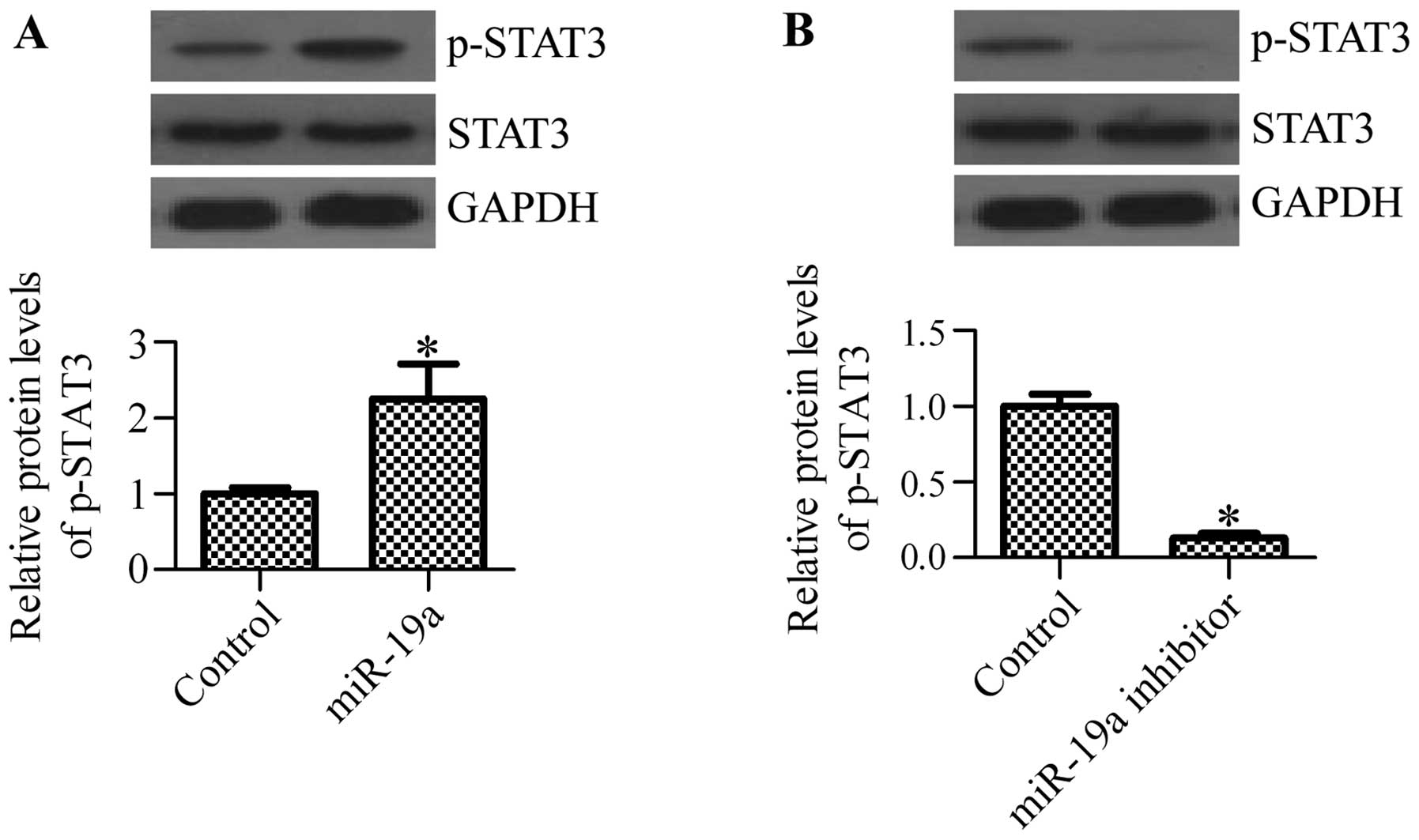
miR-19a mediates the activation of
STAT3
Previous studies have suggested that SOCS1
negatively regulates the activation of STAT3 (25–27). The constitutive activation of
STAT3 has been observed in various types of cancer, including NSCLC
(22). Therefore, considering the
regulatory effect of miR-19a on SOCS1, we hypothesized that miR-19a
may affect the activation of STAT3. To confirm this hypothesis, we
examined the activation state of STAT3 in cells transfected with
miR-19a mimics or miR-19a inhibitor. As expected, the
phosphorylation of STAT3 was significantly increased following
transfection with miR-19a mimics (Fig. 6A), whereas transfection with
miR-19a inhibitor markedly inhibited the phosphorylation of STAT3
(Fig. 6B). These results suggest
that the activation of STAT3 is regulated by miR-19a.
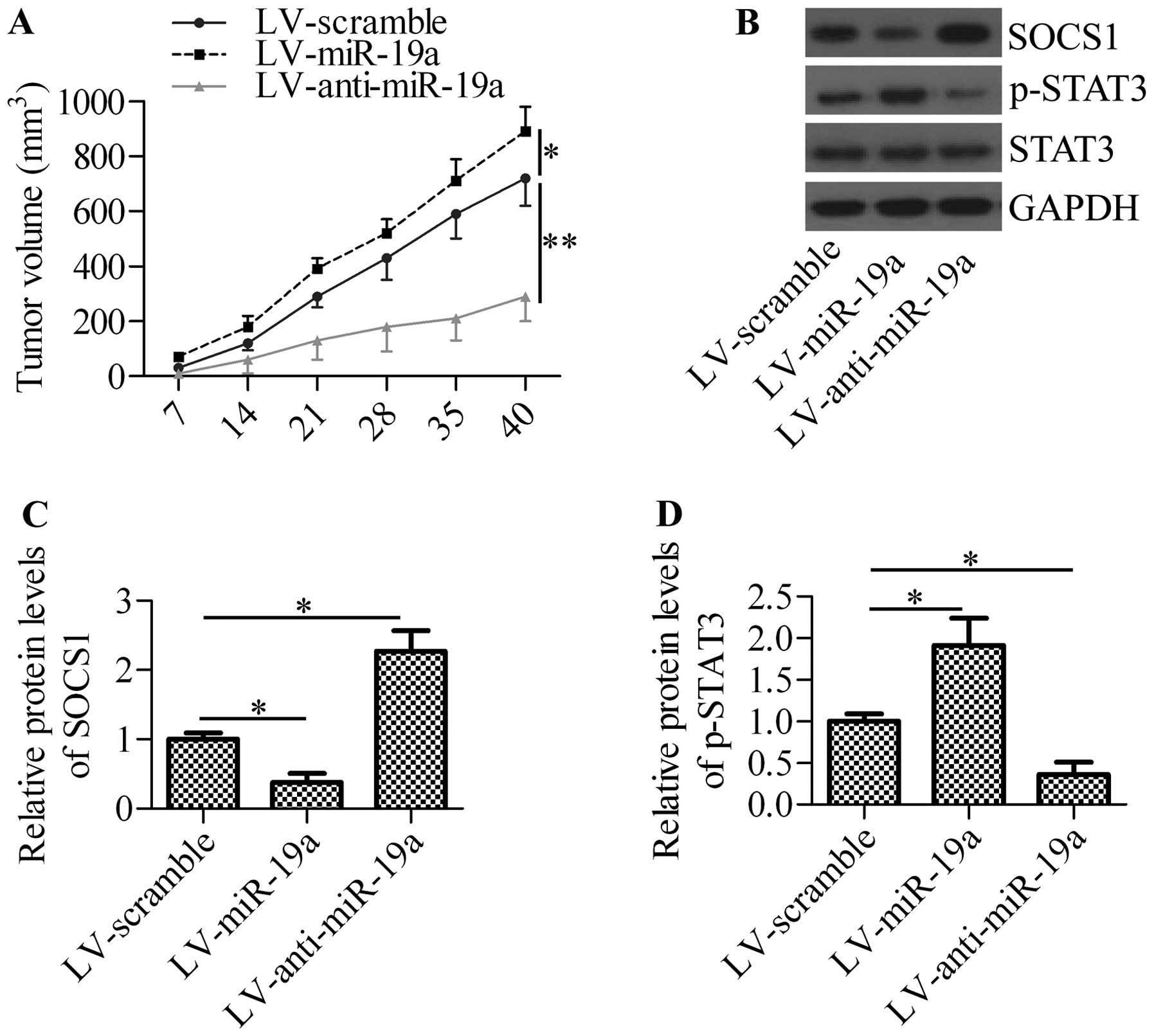
miR-19a promotes xenograft tumor
growth
To obtain further insight into the mechanisms of
action of miR-19a in NSCLC, we inoculated A549 cells into nude mice
and measured the effects of miR-19a expression on xenograft tumor
growth. The intratumoral injection of lentiviral vector expressing
miR-19a (LV-miR-19a) significantly increased tumor growth, whereas
the injection of lentiviral vector expressing anti-miR-19a
oligonucleotide (LV-anti-miR-19a) markedly decreased tumor growth
(Fig. 7A). By analyzing the
xenograft tumor tissues, we found that the injection of LV-miR-19a
significantly inhibited SOCS1 expression and increased the
phosphorylation of STAT3, whereas the injectoin of LV-anti-miR-19a
exerted the opposite effects (Fig.
7B–D). These results suggest that miR-19a regulates tumor
growth through the mediation of SOCS1 expression and STAT3
activation.
Discussion
miR-19a, as an oncogenic miRNA, has been found to be
dysregulated in various types of cancer. miR-19a has been found to
be overexpressed in tissue specimens of esophageal squamous cell
carcinoma, which is suggested as a potential unfavorable prognostic
biomarker (28). Recently, it has
been demonstrated that following serum therapy, miR-19a is
significantly decreased in early breast cancer and high-risk
patients are those with highly abundant serum levels of miR-19a
(29). In lung cancer
development, a high level of miR-19a has been observed in advanced
NSCLC (30). Accordingly, high
serum levels of miR-19a have been found to be an independent
prognostic factor for worse survival in patients with NSCLC
(21). In line with these
previous findings, in this study, we provide evidence that miR-19a
is overexpressed in both NSCLC tumor tissues and cell lines. The
aberrant high expression of serum miR-19a has been found to be a
predictor of resistance following chemotherapy (31). Our results further revealed that
miR-19a was an oncogenic gene, suggesting that miR-19a may not only
be a diagnostic and prognostic marker, but also a potential target
for the development of lung cancer therapeutics.
The dysregulated expression profile of miR-19a
indicates that miR-19a may participate in the regulation of
tumorigenesis. The inhibition of miR-19a has been shown to lead to
a decrease in tumor cell proliferation and brain allografts in
vivo of medulloblastoma and to prolong the survival of mice
(32). Li et al previously
demonstrated that curcumin is capable of downregulating the miR-19a
expression induced by bisphenol A in early breast cancer and
exhibits antitumor effects through the modulation of the
phosphatase and tensin homolog (PTEN)/AKT/p53 axis (33). miR-19a, along with miR-19b,
promotes the metastasis of gastric cancer cells by directly
targeting and inhibiting the tumor suppressor, MAX dimerization
protein 1 (MXD1) (18). In
laryngeal squamous cell carcinoma, miR-19a overexpression has been
suggested to correlate with reduced overall survival and the
inhibition of miR-19a has been shown to increase cell apoptosis and
decrease cell proliferation (34). By targeting PTEN, miR-19a promotes
multidrug resistance in gastric cancer cells (35). Therefore, miR-19a functions as an
oncogene in various types of cancer by regulating different target
genes. To the best of our knowledge, for the first time, in the
present study, we investigated the role of miR-19a in the
development of NSCLC. We found that transfection with miR-19a
mimics markedly enhanced the growth, cell invasion and migration of
NSCLC cell lines. Our findings are consistent with those of Xu
et al, who demonstrated that miR-19a regulates cell
proliferation and the invasion of cervical carcinoma cells by
targeting cullin 5 (19).
However, in this study, using dual-luciferase reporter assay, we
found that SOCS1, a critical tumor-associated gene (24), was a direct target gene of miR-19a
in NSCLC. In NSCLC cells, we further demonstrated that miR-19a
regulated the mRNA and protein expression of SOCS1. These data
suggest that miR-19a plays an important role in NSCLC by direct
targeting and regulating SOCS1 expression.
SOCS1 functions as a negative regulator of
inflammation (36,37) and plays critical roles in cells
and animals (38–40). The role of SOCS1 in the regulation
of cancer has been studied extensively (24,41). The inhibited expression profiles
of SOCS1 have been demonstrated in numerous types of cancer,
including liver cancer (42),
prostate cancer (43) and
pancreatic cancer (44). The
downregulation of SOCS1 has been found to be associated with
prolonged JAK/STAT signaling pathway activity (45,46). It has been suggested that SOCS1 is
able to interact with JAK through the SH2 domain, leading to STAT
inactivation. The constitutive activation of JAK2/STAT3 has been
shown in various solid tumors, including NSCLC (22,47,48). The enforced expression of SOCS1
exerts an anti-proliferative effect through the inactivation of
STAT3 and the p38 MAPK pathway in gastric cancer cells (49). The oncolytic adenovirus-mediated
SOCS1 expression has been shown to exert an antitumor effect
through the inhibition of STAT3 phosphorylation in hepatocellular
carcinoma cells (27). Shikonin
has been reported to inhibit the interleukin (IL)-17-induced
activation of STAT3 by upregulating SOCS1 (50). Huang et al demonstrated
that miR-155 promotes pancreatic cancer cell invasion and migration
by inhibiting SOCS1 expression and activating STAT3 signals
(25). In the present study, we
demonstrated that miR-19a suppressed the downstream target gene,
SOCS1, and increased the phosphorylation of STAT3. Therefore, the
miR-19a-induced increase in the activation of STAT3 may occur
through the inhibition of SOCS1 expression.
Shimada et al demonstrated that SOCS1
overexpression exerts a more potent inhibitory effect on STAT3
activation than JAK inhibitors in NSCLC cells (51), suggesting a significant antitumor
role of SOCS1 in NSCLC. In this study, we demonstrated that
miR-19a, which is highly overexpressed in NSCLC, directly targeted
and inhibited SOCS1 expression. The inhibition of miR-19a
significantly upregulated SOCS1 expression and exerted a marked
antitumor effect in vitro and in vivo. Taken
together, our data suggest that miR-19 functions as an oncogenic
miRNA and targeting miR-19a may provide novel strategies for the
development of anti-NSCLC therapeutics.
Acknowledgments
This study was supported by a grant from the
Crossing Research Projects of Zhengzhou University (no.
340700532007).
Abbreviations:
|
miRNAs
|
microRNAs
|
|
NSCLC
|
non-small cell lung cancer
|
|
SOCS1
|
suppressor of cytokine signaling 1
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
3′-UTR
|
3′-untranslated region
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Claassens L, van Meerbeeck J, Coens C, et
al: Health-related quality of life in non-small-cell lung cancer:
an update of a systematic review on methodologic issues in
randomized controlled trials. J Clin Oncol. 29:2104–2120. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu Z, Chen X, Zhao Y, et al: Serum
microRNA signatures identified in a genome-wide serum microRNA
expression profiling predict survival of non-small-cell lung
cancer. J Clin Oncol. 28:1721–1726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sandler A, Gray R, Perry MC, et al:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blumenschein GR Jr and Herbst RS:
Integration of targeted therapies in gemcitabine chemotherapy
regimens. Clin Lung Cancer. 4:217–223. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cabebe E and Wakelee H: Role of
anti-angiogenesis agents in treating NSCLC: focus on bevacizumab
and VEGFR tyrosine kinase inhibitors. Curr Treat Options Oncol.
8:15–27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ranganathan K and Sivasankar V: MicroRNAs
- Biology and clinical applications. J Oral Maxillofac Pathol.
18:229–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Winter J, Jung S, Keller S, Gregory RI and
Diederichs S: Many roads to maturity: microRNA biogenesis pathways
and their regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rottiers V, Najafi-Shoushtari SH, Kristo
F, et al: MicroRNAs in metabolism and metabolic diseases. Cold
Spring Harb Symp Quant Biol. 76:225–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aigner A: MicroRNAs (miRNAs) in cancer
invasion and metastasis: therapeutic approaches based on
metastasis-related miRNAs. J Mol Med (Berl). 89:445–457. 2011.
View Article : Google Scholar
|
|
12
|
Zimmerman AL and Wu S: MicroRNAs, cancer
and cancer stem cells. Cancer Lett. 300:10–19. 2011. View Article : Google Scholar
|
|
13
|
Esquela-Kerscher A and Slack FJ: Oncomirs
- microRNAs with a role in cancer. Nat Rev Cancer. 6:259–269. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang P, Chen L, Zhang J, et al:
Methylation-mediated silencing of the miR-124 genes facilitates
pancreatic cancer progression and metastasis by targeting Rac1.
Oncogene. 33:514–524. 2014. View Article : Google Scholar
|
|
15
|
Oh JS, Kim JJ, Byun JY and Kim IA:
Lin28-let7 modulates radiosensitivity of human cancer cells with
activation of K-Ras. Int J Radiat Oncol Biol Phys. 76:5–8. 2010.
View Article : Google Scholar
|
|
16
|
Wang P, Zhuang L, Zhang J, et al: The
serum miR-21 level serves as a predictor for the chemosensitivity
of advanced pancreatic cancer, and miR-21 expression confers
chemoresistance by targeting FasL. Mol Oncol. 7:334–345. 2013.
View Article : Google Scholar
|
|
17
|
Petrocca F, Visone R, Onelli MR, et al:
E2F1-regulated microRNAs impair TGFbeta-dependent cell-cycle arrest
and apoptosis in gastric cancer. Cancer Cell. 13:272–286. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu Q, Yang Z, An Y, et al: MiR-19a/b
modulate the metastasis of gastric cancer cells by targeting the
tumour suppressor MXD1. Cell Death Dis. 5:e11442014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu XM, Wang XB, Chen MM, et al:
MicroRNA-19a and -19b regulate cervical carcinoma cell
proliferation and invasion by targeting CUL5. Cancer Lett.
322:148–158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu G, Li H, Wang X, et al: MicroRNA-19a
targets tissue factor to inhibit colon cancer cells migration and
invasion. Mol Cell Biochem. 380:239–247. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin Q, Chen T, Lin Q, Lin G, et al: Serum
miR-19a expression correlates with worse prognosis of patients with
non-small cell lung cancer. J Surg Oncol. 107:767–771. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu H and Jove R: The STATs of cancer - new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tian M, Wan Y, Tang J, et al: Depletion of
tissue factor suppresses hepatic metastasis and tumor growth in
colorectal cancer via the downregulation of MMPs and the induction
of autophagy and apoptosis. Cancer Biol Ther. 12:896–907. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang J, Li H, Yu JP, Wang SE and Ren XB:
Role of SOCS1 in tumor progression and therapeutic application. Int
J Cancer. 130:1971–1980. 2012. View Article : Google Scholar
|
|
25
|
Huang C, Li H, Wu W, Jiang T and Qiu Z:
Regulation of miR-155 affects pancreatic cancer cell invasiveness
and migration by modulating the STAT3 signaling pathway through
SOCS1. Oncol Rep. 30:1223–1230. 2013.PubMed/NCBI
|
|
26
|
Cittadini A, Monti MG, Iaccarino G, et al:
SOCS1 gene transfer accelerates the transition to heart failure
through the inhibition of the gp130/JAK/STAT pathway. Cardiovasc
Res. 96:381–390. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu L, Li W, Wei X, et al: Potent
antitumor activity of oncolytic adenovirus-mediated SOCS1 for
hepatocellular carcinoma. Gene Ther. 20:84–92. 2013. View Article : Google Scholar
|
|
28
|
Xu XL, Jiang YH, Feng JG, et al:
MicroRNA-17, microRNA-18a, and microRNA-19a are prognostic
indicators in esophageal squamous cell carcinoma. Ann Thorac Surg.
97:1037–1045. 2014. View Article : Google Scholar
|
|
29
|
Sochor M, Basova P, Pesta M, et al:
Oncogenic MicroRNAs: miR-155, miR-19a, miR-181b, and miR-24 enable
monitoring of early breast cancer in serum. BMC Cancer. 14:4482014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Navarro A, Marrades RM, Vinolas N, et al:
MicroRNAs expressed during lung cancer development are expressed in
human pseudoglandular lung embryogenesis. Oncology. 76:162–169.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen Q, Xia HW, Ge XJ, et al: Serum
miR-19a predicts resistance to FOLFOX chemotherapy in advanced
colorectal cancer cases. Asian Pac J Cancer Prev. 14:7421–7426.
2013. View Article : Google Scholar
|
|
32
|
Murphy BL, Obad S, Bihannic L, et al:
Silencing of the miR-17~92 cluster family inhibits medulloblastoma
progression. Cancer Res. 73:7068–7078. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li X, Xie W, Xie C, et al: Curcumin
modulates miR-19/PTEN/AKT/p53 axis to suppress bisphenol A-induced
MCF-7 breast cancer cell proliferation. Phytother Res.
28:1553–1560. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu TY, Zhang TH, Qu LM, et al: MiR-19a is
correlated with prognosis and apoptosis of laryngeal squamous cell
carcinoma by regulating TIMP-2 expression. Int J Clin Exp Pathol.
7:56–63. 2013.
|
|
35
|
Wang F, Li T, Zhang B, et al:
MicroRNA-19a/b regulates multidrug resistance in human gastric
cancer cells by targeting PTEN. Biochem Biophys Res Commun.
434:688–694. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Krebs DL and Hilton DJ: SOCS proteins:
negative regulators of cytokine signaling. Stem Cells. 19:378–387.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yasukawa H, Sasaki A and Yoshimura A:
Negative regulation of cytokine signaling pathways. Annu Rev
Immunol. 18:143–164. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
He Y, Zhang W, Zhang R, Zhang H and Min W:
SOCS1 inhibits tumor necrosis factor-induced activation of ASK1-JNK
inflammatory signaling by mediating ASK1 degradation. J Biol Chem.
281:5559–5566. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hanada T, Yoshida H, Kato S, et al:
Suppressor of cytokine signaling-1 is essential for suppressing
dendritic cell activation and systemic autoimmunity. Immunity.
19:437–450. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chinen T, Kobayashi T, Ogata H, et al:
Suppressor of cytokine signaling-1 regulates inflammatory bowel
disease in which both IFNgamma and IL-4 are involved.
Gastroenterology. 130:373–388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sasi W, Sharma AK and Mokbel K: The role
of suppressors of cytokine signalling in human neoplasms. Mol Biol
Int. 2014:6307972014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chu PY, Yeh CM, Hsu NC, et al: Epigenetic
alteration of the SOCS1 gene in hepatocellular carcinoma. Swiss Med
Wkly. 140:w130652010.PubMed/NCBI
|
|
43
|
Neuwirt H, Puhr M, Santer FR, et al:
Suppressor of cytokine signaling (SOCS)-1 is expressed in human
prostate cancer and exerts growth-inhibitory function through
down-regulation of cyclins and cyclin-dependent kinases. Am J
Pathol. 174:1921–1930. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Komazaki T, Nagai H, Emi M, et al:
Hypermethylation-associated inactivation of the SOCS-1 gene, a
JAK/STAT inhibitor, in human pancreatic cancers. Jpn J Clin Oncol.
34:191–194. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lesinski GB, Zimmerer JM, Kreiner M, et
al: Modulation of SOCS protein expression influences the interferon
responsiveness of human melanoma cells. BMC Cancer. 10:1422010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zitzmann K, Brand S, De Toni EN, et al:
SOCS1 silencing enhances antitumor activity of type I IFNs by
regulating apoptosis in neuroendocrine tumor cells. Cancer Res.
67:5025–5032. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao M, Gao FH, Wang JY, et al: JAK2/STAT3
signaling pathway activation mediates tumor angiogenesis by
upregulation of VEGF and bFGF in non-small-cell lung cancer. Lung
Cancer. 73:366–374. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yu H, Pardoll D and Jove R: STATs in
cancer inflammation and immunity: a leading role for STAT3. Nat Rev
Cancer. 9:798–809. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Souma Y, Nishida T, Serada S, et al:
Antiproliferative effect of SOCS-1 through the suppression of STAT3
and p38 MAPK activation in gastric cancer cells. Int J Cancer.
131:1287–1296. 2012. View Article : Google Scholar
|
|
50
|
Xu Y, Xu X, Gao X, Chen H and Geng L:
Shikonin suppresses IL-17-induced VEGF expression via blockage of
JAK2/STAT3 pathway. Int Immunopharmacol. 19:327–333. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shimada K, Serada S, Fujimoto M, et al:
Molecular mechanism underlying the antiproliferative effect of
suppressor of cytokine signaling-1 in non-small-cell lung cancer
cells. Cancer Sci. 104:1483–1491. 2013. View Article : Google Scholar : PubMed/NCBI
|