Introduction
Periodontitis is a globally prevalent human
inflammatory disease that affects approximately 15% of the adult
human population and is characterized by periodontal tissue
destruction (1). Patients
afflicted with periodontitis commonly exhibit clinical symptoms,
such as the formation of deep periodontal pockets, extensive
alveolar bone loss, the development of a necrotic or exposed
cementum and inflammation of the connective tissues (2). Uncontrolled periodontal tissue
destruction eventually leads to tooth migration, increased tooth
mobility, tooth loss and impairments in chewing function, phonetics
and esthetics. Regeneration of destroyed alveolar bone is one of
the ultimate goals of periodontal therapy (1). Human periodontal ligament cells
(hPDLCs) have multipotential characteristics and have been regarded
as potentially useful sources for the regeneration of periodontal
tissue, including bone, cementum and the periodontal ligament
(2–5).
Calcium phosphate ceramics were introduced over 30
years ago as bone substitutes and have been used as bone-filling
materials to provide scaffolds for cell migration and rapid bone
formation due to their close chemical and crystal resemblance to
bone minerals (6). The most
widely used forms of calcium phosphate ceramics are hydroxyapatite
(HA, Ca10[PO4]6[OH]2)
and tricalcium phosphate (TCP, Ca3[PO4]). The
physicochemical properties of these two synthetic calcium
phosphates vary widely (7).
β-tricalcium phosphate (β-TCP) ceramics are typical representatives
that have been extensively investigated regarding their
applications for bone engineering due to their osteoconductivity
and bioresorbability (8–10). However, β-TCP ceramics have been
reported to have limited osteoinductivity when mixed with hPDLCs
(11–13). Additionally, it remains
controversial as to whether β-TCP possesses intrinsic
osteoinductive activity.
Demineralized bone matrix (DBM) is an
osteoconductive and osteoinductive commercial biomaterial and an
approved medical device for use in bone defects that has a long
track record of clinical uses in diverse forms (14). Unlike synthetic materials and
mineralized allografts, DBM is well known for being osteoinductive
and capable of stimulating bone formation in both heterotopic and
orthotopic implant sites due to its content of osteogenic factors,
including bone morphogenetic proteins (BMPs) and other osteogenic
non-collagenous proteins (15).
Numerous studies have demonstrated the potent effects of DBM on the
differentiation of osteoprogenitor cells into osteoblasts (14). However, only a few studies have
documented the effects of DBM on the proliferation and osteogenic
differentiation of hPDLCs in periodontal regeneration (16,17).
Thus, the objective of the present study was to
examine the effects of β-TCP ceramics and DBM on the proliferation
and osteogenic differentiation of hPDLCs.
Materials and methods
Cell culture
hPDLCs were obtained from healthy premolar teeth
that were extracted for orthodontic reasons from subjects under the
age of 20 years. The experimental protocol was approved by the
Ethics Committee of Sun Yat-sen University, Guangzhou, China and
informed consent was obtained from all subjects. The hPDLCs were
isolated from fresh periodontal ligament (PDL) tissues using the
protocols described in our previous study (18). Briefly, PDL tissue was removed
from the middle-third of the root using a sterile scalpel and then
dissected into small sections and soaked in fresh Dulbecco's
modified Eagle's medium (DMEM/high glucose; HyClone, Beijing,
China) containing 20% fetal bovine serum (FBS; BioInd, Kibbutz Beit
Haemek, Israel) and 2% (v/v) penicillin/streptomycin (Invitrogen,
Grand Island, NY, USA). The cells that migrated out of the tissue
samples were cultured at 37°C in a humidified atmosphere of 5%
CO2. Cells at passages 3–5 were used in the subsequent
experiments.
Scaffold processing and cell seeding
The porous β-TCP ceramics were obtained from the
National Engineering Research Center for Biomaterials (Chengdu,
China). The mean pore sizes ranged from 200 to 500 μm, and
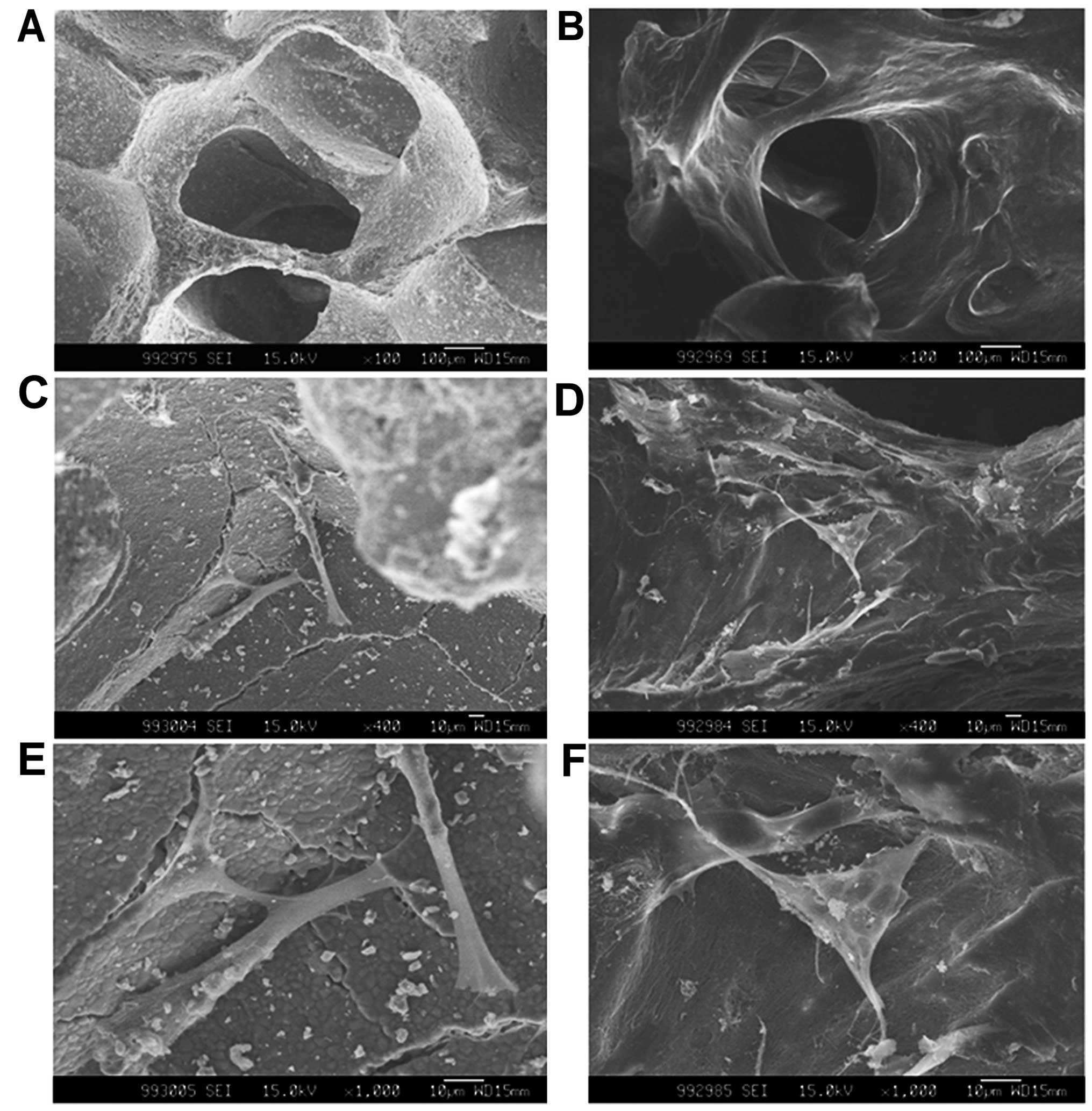
the total porosity was >60% (Fig.
1A). The ceramics were machined into cubes with dimensions of
5×5×5 mm. Prior to use, all scaffolds were sterilized for 15 min
(121°C, 15 bar pressure) by autoclaving.
DBM collagen scaffolds were prepared from the spongy
bone of humans. The spongy bone was provided by Osteolink
Biomaterials Inc. (Wuhan, China). The demineralization process was
similar to that described in the studies by Urist (19) and Reddi and Huggins (20), with the addition of several
washing steps to remove the residual chemicals. Briefly, the spongy
bone was separated and soaked in ethyl ether for 24 h to remove the
fatty components. Subsequently, 0.6 M HCl was used to demineralize
the spongy bone, and this step was followed by a complete washing
in phosphate-buffered saline (PBS) and subsequent freeze-drying.
Prior to use, all the samples were cut into 5×5×5-mm cube sizes and
sterilized by Co60 radiation (Fig.
1B).
Prior to cell seeding, all scaffolds were pre-wet
and incubated in DMEM supplemented with 10% FBS and 2%
penicillin/streptomycin for 4 h. Subsequently, the hPDLCs were
seeded on the scaffolds at two different densities. The
cell-scaffold complexes were transferred onto 24-well plates.
For the cell proliferation assay, 20 μl of
cell suspension (1×105 cells/ml) was used to seed the
cells on each scaffold. The cell-scaffold complexes were then
cultured in DMEM growth culture medium supplemented with 10% FBS
and 2% penicillin/streptomycin.
For the cell differentiation assay, 100 μl of
cell suspension (3×106 cells/ml) was seeded on each
scaffold. The cell-scaffold complexes were then cultured in growth
medium or osteogenic culture medium containing 10 mM
β-glycerophosphate (β-GP), 10−8 M dexamethasone (Dex),
50 μg/ml L-ascorbic acid and 50 μg/ml gentamycin. The
culture medium was refreshed every 3 days.
Scanning electron microscopy (SEM)
analysis
On day 3 after cell seeding, the cell-scaffold
complexes were washed in PBS twice and fixed in 2.5% glutaraldehyde
for 4 h. Then, the samples were dehydrated in a graded series of
ethanol and air-dried in tetramethylsilane (Merck, Darmstadt,
Germany). After gold sputtering, the specimens were examined under
a scanning electron microscope (JSM 5600; JEOL, Tokyo, Japan) at 15
kV.
Cell proliferation assay
The proliferation of the hPDLCs cultured on the
scaffolds was measured in the growth culture medium using the Cell
Counting kit-8 (CCK-8; Beyotime, Shanghai, China). In brief, the
hPDLCs on the scaffolds were cultured in growth medium for 1, 3, 5,
7, 9 or 11 days with 4 repeats for each group. The cell-scaffolds
complexes were cut into small sections, and the cells were removed
from the scaffolds by trypsinization. Ten microliters of CCK-8
solution were placed into each well. The absorbance of the
supernatant was read using an enzyme-labeled meter (Infinite 200;
Tecan, Grödig, Austria) at 490 nm. A standard curve relating cell
number to absorbance was established to determine the cell numbers.
The scaffolds with the cells in the same media were used as the
negative controls. The monolayer cultures of hPDLCs in the
microplates served as the positive controls.
Alkaline phosphatase (ALP) activity
Following 3, 7, 14, 21 and 28 days of culture, ALP
activity was measured using an ALP activity assay kit (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China) following the
manufacturer's instructions. All analyses were performed for 3
separate experiments. Briefly, the cell-scaffold complexes were cut
into small sections, and the proteins from the cell-scaffold
complexes were extracted by the addition of a cell lysis buffer
containing 0.1% Triton X-100 to the samples. Aliquots (50 μl
in each well) of these supernatants were placed into 24-well plates
containing 50 μl of an ALP substrate solution (2 mM
MgCl2 and 16 mM p-nitrophenyl phosphate). Following
incubation at 37°C for 30 min, the reaction was terminated by the
addition of 50 μl of 0.2 M NaOH, and the liberated
p-nitrophenol was measured on a plate reader at 520 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The expression levels of Runx2 and osteocalcin (OCN)
were confirmed by RT-qPCR on day 21. Total RNA from the cells was
prepared from the crushed sections of each scaffold (n=3) and then
harvested using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The
total RNA was used as a template for the synthesis of cDNA with
oligo(dT) and RevertAid™ M-MuLV reverse transcriptase
(MBI/Fermentas, Burlington, ON, Canada). The following PCR
amplification reaction employed Taq polymerase and specific
primers. Each PCR was duplicated with the same total RNA. The
relative gene expression levels of Runx2 and OCN were normalized to
the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase
(GAPDH). The primers for the selected genes are listed in Table I.
 | Table IPrimer sequences used for
RT-qPCR. |
Table I
Primer sequences used for
RT-qPCR.
| Genes | Primers | Size (bp) |
|---|
| Runx2 | F:
CCAACCCACGAATGCACTATC
R: TAGTGAGTGGTGGCGGACATAC | 91 |
| OCN | F:
CACTCCTCGCCCTATTGGC
R: GCCTGGGTCTCTTCACTACCT | 148 |
| GAPDH | F:
CATGTTCCAATATGATTCCACC
R: GATGGGATTTCCATTGATGAC | 88 |
Statistical analysis
All experiments were performed 3 or 4 times, and
each treatment was conducted in triplicate. The means (Ms) and
standard deviations (SDs) were calculated, and the statistical
significances of the differences between groups were examined with
one-way ANOVA followed by the least significant difference (LSD)
test. The SPSS 17.0 program was employed for all statistical
analyses, and differences were considered significant when the
P-value was <0.05.
Results
Scanning electron microscopy (SEM)
On day 3 after cell seeding, the hPDLCs had
successfully adhered to both scaffolds. The cell morphologies of
the hPDLCs on the β-TCP ceramics and DBM surfaces exhibited some
differences. The cells cultured on the β-TCP surfaces appeared
spindle-shaped and much more spread out than the cells cultured on
the DBM surfaces, and the hPDLCs within the DBM exhibited a
triangular shape (Fig. 1C–F).
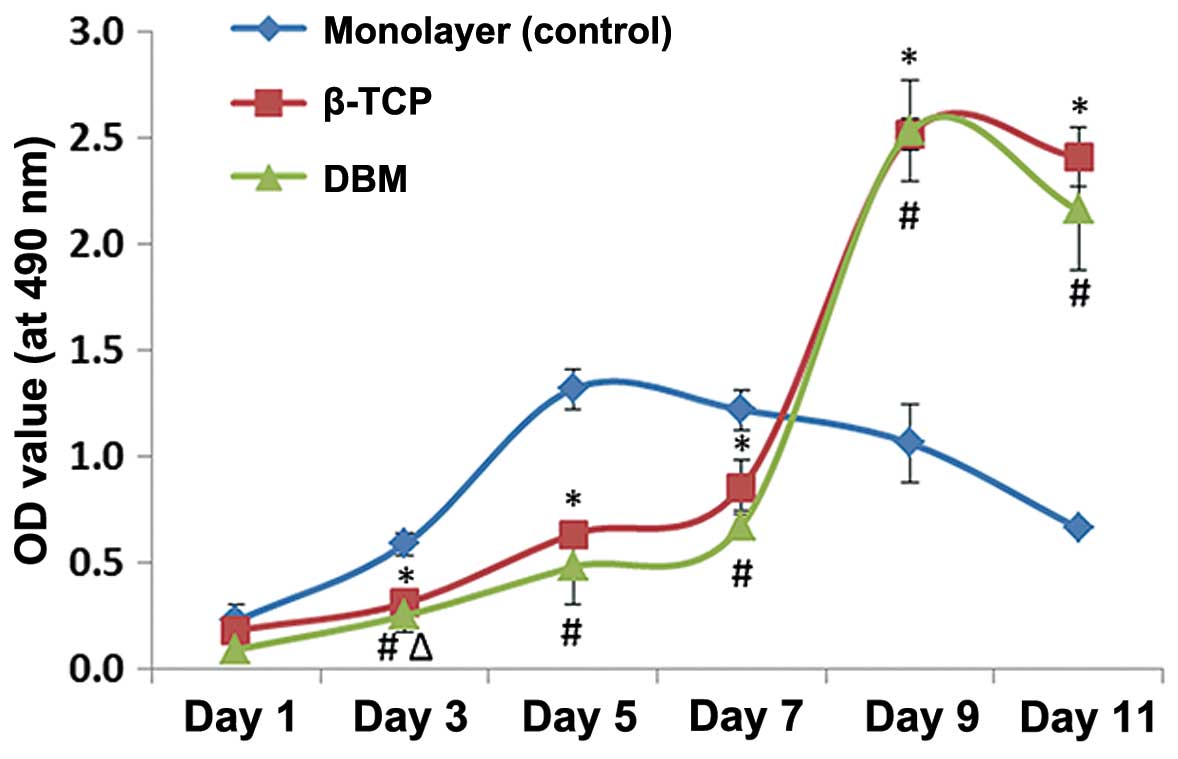
Cell proliferation
Compared with the monolayer group (positive
control), the cell proliferation levels in the scaffolds were
significantly inhibited from days 3 to 7, but significantly
elevated from days 9 to 11 (P<0.05). A comparison of the growth
of the cells in the scaffolds revealed that the cell numbers in the
DBM from days 1 to 11 were lower than those in the β-TCP ceramics.
However, the difference in cell proliferation between these 2
groups was not statistically significant (P>0.05) with the
exception of day 3 (Fig. 2).
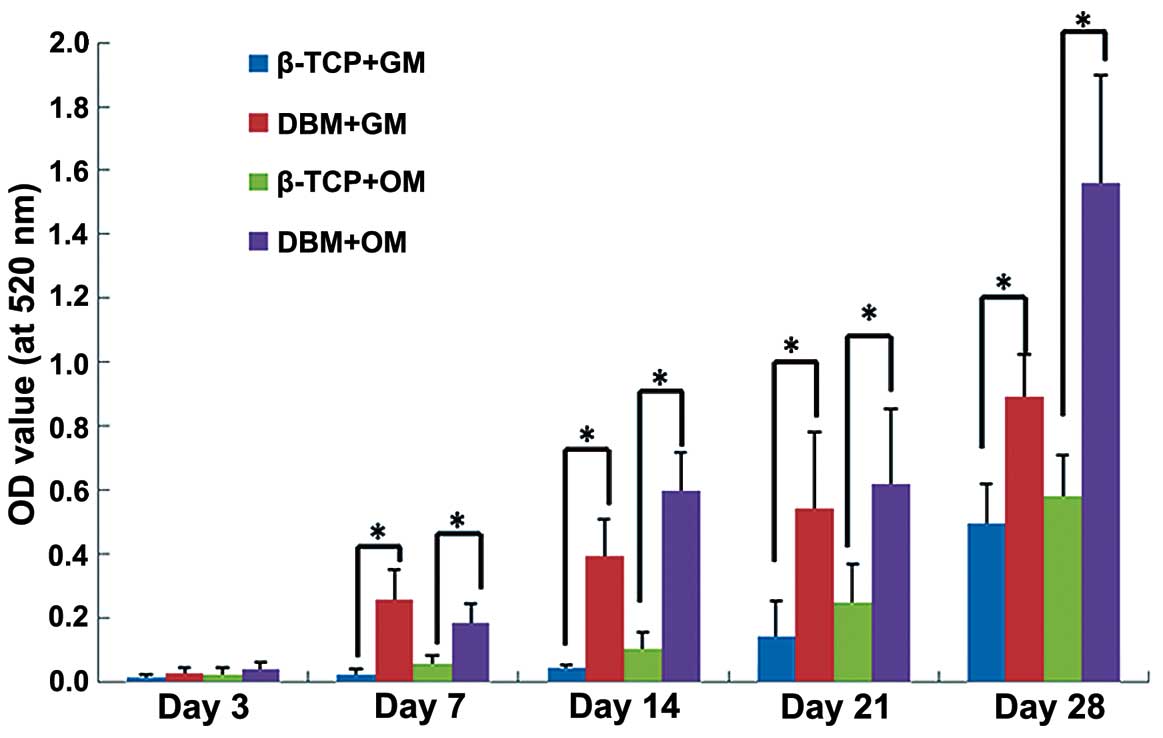
ALP activity
In both the growth culture medium and the osteogenic
culture medium, the ALP activity gradually increased from days 3 to
28. The hPDLCs cultured in the osteogenic medium exhibited greater
ALP activity than did those (same scaffoled different medium)
cultured in the growth medium. The ALP activity of the hPDLCs
cultured on the DBM was significantly greater than that of the
cells cultured on β-TCP at all time points in both the growth
medium and the osteogenic culture medium (Fig. 3).
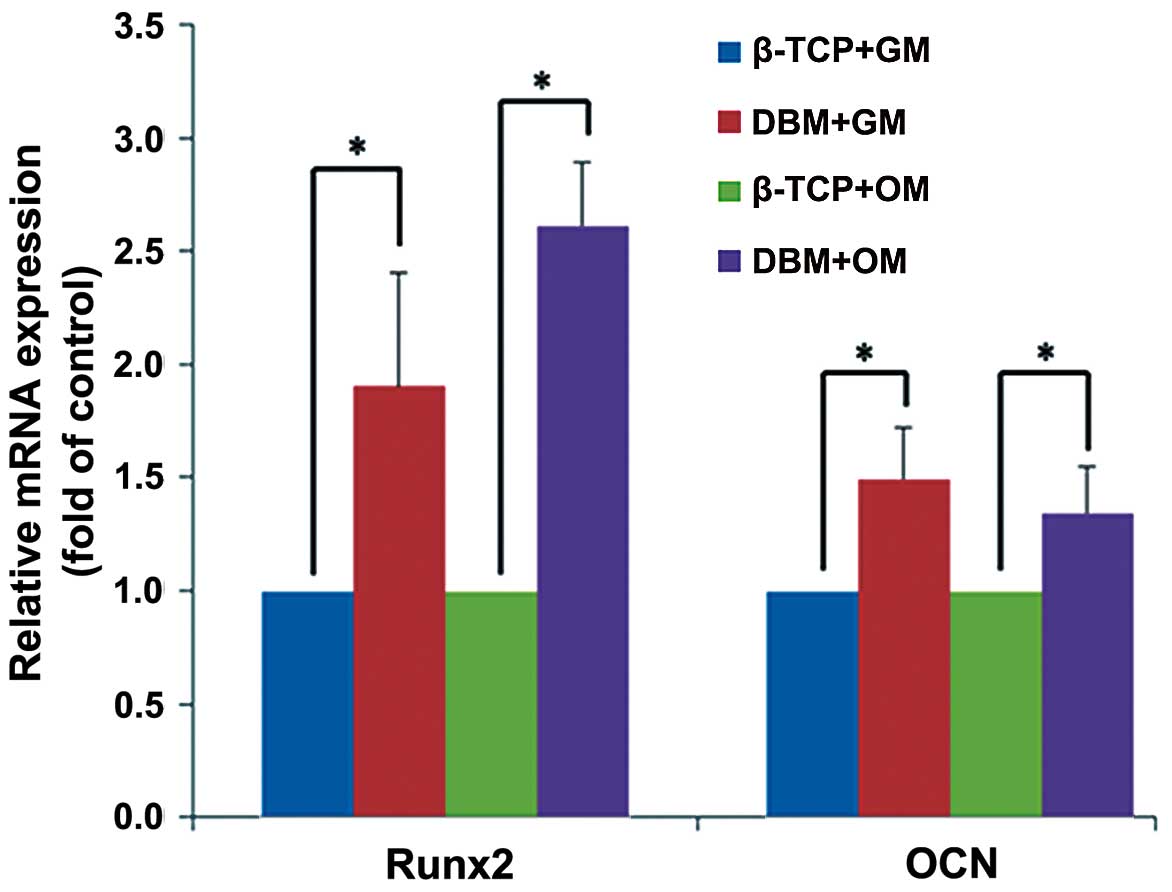
Expression of osteogenic differentiation
genes
After 21 days of culture, the hPDLCs cultured on the
DBM exhibited significantly greater levels of Runx2 and OCN both in
the growth medium and the osteogenic culture medium (P<0.05)
compared with those cultured on β-TCP. The mRNA expression of Runx2
was increased in the presence of the osteogenic inducers to a
greater extent than in the absence of the osteogenic inducers.
Runx2 expression was induced to a greater extent with osteogenic
inducers only with DBM. It was not as high with β-TCP with
osteogenic inducers. The mRNA expression of OCN exhibited no
detectable difference between the different culture media (Fig. 4).
Discussion
Scaffolding materials should be biocompatible and
bioactive to allow specific cells to attach, proliferate, and
differentiate on the scaffolding materials to enable tissue repair
and regeneration (4–8). β-TCP is a porous bioceramic material
with biological properties that include non-reactivity and
resorbability. β-TCP can serve as a scaffold for bone regeneration
as it is progressively degraded and replaced by bone. Due to its
osteoconductivity and bone replacement capability, β-TCP is highly
promising for use in numerous dental and craniofacial procedures,
including the reconstruction of frontal sinus cavities, the
augmentation of craniofacial skeletal defects and the repair of
periodontal tooth and bone defects (21–24). DBM products were clinically
introduced in 1991 and have since served as effective
osteoconductive scaffolds (25,26). The osteoinductivity of DBM can be
attributed to a series of low-molecular-weight glycoproteins that
include the BMPs. The decalcification of cortical bone exposes
these osteoinductive growth factors that are buried within the
mineralized matrix and thereby enhances the bone formation process
(27). DBM has a number of
additional advantages that make it an attractive bone graft
alternative. DBM is cost-effective and readily available from
tissue banks. The demineralization process destroys the antigenic
materials in the bone, which makes DBM less immunogenic than
mineralized allografts (25,28).
The present study investigated the effects of β-TCP
ceramics and DBM, which are commercially available bone graft
substitutes, on the proliferation and osteogenic differentiation of
hPDLCs. A CCK-8 assay revealed that both the β-TCP scaffolds and
DBM were able to promote the late, but not the early proliferation
of hPDLCs. SEM observations revealed that the hPDLCs attached to
the surfaces of the β-TCP scaffolds and the DBM by spreading
themselves out. Such responses are indicative of good
cytocompatibility and the close interactions of the scaffolds with
the hPDLCs. The cells on the β-TCP surfaces appeared spindle-shaped
and much more spread out than those on the DBM surfaces and the
hPDLCs within the DBM became triangle-shaped. These different
cellular morphologies may be associated with the differentiation of
the hPDLCs cultured on DBM toward the osteogenic lineage.
ALP is an early biomarker of osteogenic
differentiation and plays a key role in bone mineralization through
the initiation and/or promotion of the formation of hydroxyapatite
crystals in the matrix vesicles of osteoblasts (29). The present study revealed that the
hPDLCs cultured on the DBM exhibited greater ALP activity than did
the cells cultured on the β-TCP scaffolds. These results may be
related to the different chemical compositions of the scaffolds.
β-TCP is biodegradable and can release various concentrations of
calcium and phosphate ions into the culture media or body fluids
(30–32). In a previous study, the
concentrations of calcium and inorganic phosphate ions released
from β-TCP ceramics after 3 days of culture were 1.76 and 0.96
mmol/l, respectively (33). In a
previous study of ours, we demonstrated that this release of free
calcium and inorganic phosphate ions significantly reduces the ALP
activities of hPDLCs (18).
However, the DBM was prepared by acid extraction from human bone
sources and thus retained type I collagen, other proteins and BMPs.
BMPs are obviously able to stimulate ALP activity (34,35).
The differentiation of hPDLCs is one of the key
processes of bone regeneration. The mRNA expression levels of Runx2
and OCN were selected as markers of the osteogenic phenotype. β-TCP
ceramics possess osteoconductive properties, but they do not have
intrinsic osteoinductive capacities. DBM has been shown to have
osteoconductive and osteoinductive properties (36). In the present study, compared to
the β-TCP ceramics, the DBM scaffolds upregulated the expression
levels of these genes in both the growth and osteogenic culture
media on day 21. These results are consistent with those of a
previous study by Kasten et al (37) who reported that DBM increased OCN
gene expression in human bone marrow stromal cells. Runx2 has been
identified as a ‘master gene’ in the control of osteogenic
differentiation (38,39). Thus, the significant upregulation
of Runx2 gene expression led to elevated mRNA expression levels of
OCN in the hPDLCs within the DBM. Runx2 is an early-stage
transcription factor that activates osteoblastic differentiation
(36,37), and its gene expression was
significantly increased in the DBM substrates (1.9- and 2.6-fold in
the growth and osteogenic media, respectively). OCN is a late-stage
osteogenic differentiation marker and is synthesized only by mature
osteoblasts, odontoblasts and cementoblasts (38,39). The results from RT-qPCR revealed
that OCN gene expression was slightly increased in the DBM
substrates (1.5- and 1.3-fold in the growth and osteogenic media,
respectively). Thus, we conclude that DBM promoted the osteogenic
differentiation of hPDLCs.
β-TCP is a biodegradable inorganic bone substitute
that can release varying amounts of calcium and phosphate ions into
culture media or body fluids (30,31,40). These releases of free calcium or
phosphate ions significantly affect the proliferation and
osteogenic differentiation of hPDLCs (18). DBM is an allogenous material and
thus contains bone morphogenic and matrix proteins that ceramic
materials do not (14). The
results of the present study may be due to the sum of the effects
of the dissolved ions and matrix proteins and the direct effects of
the scaffold architecture and surface chemistry. Notably, the
chemical composition, surface topography, surface roughness and
other characteristics of scaffolds have been reported to affect
cell activities, such as adhesion, proliferation and
differentiation (41).
In conclusion, the results of the present study
revealed that in addition to the induction of cell proliferation in
the absence of an osteogenic inducer, DBM scaffolds exhibited
greater effects on the osteogenic differentiation of hPDLCs than
did porous β-TCP ceramics. The organic elements of bone have more
prominent effects on the osteogenic differentiation of hPDLCs than
do the inorganic bone elements. It may thus be possible to develop
novel periodontal tissue engineering scaffolds by combining the
organic (e.g., collagen, other proteins and BMPs) and inorganic
elements (e.g., calcium and phosphate ions) of bone in various
concentrations and ratios.
Acknowledgments
This study was supported by the Guangdong Natural
Science Foundation (grant no. S2012040007041).
References
|
1
|
Hughes FJ, Ghuman M and Talal A:
Periodontal regeneration: a challenge for the tissue engineer? Proc
Inst Mech Eng H. 224:1345–1358. 2010. View Article : Google Scholar
|
|
2
|
Ishikawa I, Iwata T, Washio K, Okano T,
Nagasawa T, Iwasaki K and Ando T: Cell sheet engineering and other
novel cell-based approaches to periodontal regeneration.
Periodontol 2000. 51:220–238. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin NH, Gronthos S and Mark Bartold P:
Stem cells and future periodontal regeneration. Periodontol 2000.
51:239–251. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartold PM, McCulloch CA, Narayanan AS and
Pitaru S: Tissue engineering: a new paradigm for periodontal
regeneration based on molecular and cell biology. Periodontol 2000.
24:253–269. 2000. View Article : Google Scholar
|
|
5
|
Taba M Jr, Jin Q, Sugai JV and Giannobile
WV: Current concepts in periodontal bioengineering. Orthod
Craniofac Res. 8:292–302. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wagoner Johnson AJ and Herschler BA: A
review of the mechanical behavior of CaP and CaP/polymer composites
for applications in bone replacement and repair. Acta Biomater.
7:16–30. 2011. View Article : Google Scholar
|
|
7
|
El-Ghannam A: Bone reconstruction: from
bioceramics to tissue engineering. Expert Rev Med Devices.
2:87–101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kamitakahara M, Ohtsuki C and Miyazaki T:
Review paper: behavior of ceramic biomaterials derived from
tricalcium phosphate in physiological condition. J Biomater Appl.
23:197–212. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
LeGeros RZ: Properties of osteoconductive
biomaterials: calcium phosphates. Clin Orthop Relat Res. 395:81–98.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nandi SK, Roy S, Mukherjee P, Kundu B, De
DK and Basu D: Orthopaedic applications of bone graft & graft
substitutes: a review. Indian J Med Res. 132:15–30. 2010.PubMed/NCBI
|
|
11
|
Iwata T, Yamato M, Tsuchioka H, Takagi R,
Mukobata S, Washio K, Okano T and Ishikawa I: Periodontal
regeneration with multi-layered periodontal ligament-derived cell
sheets in a canine model. Biomaterials. 30:2716–2723. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Anzai J, Kitamura M, Nozaki T, Nagayasu T,
Terashima A, Asano T and Murakami S: Effects of concomitant use of
fibroblast growth factor (FGF)-2 with beta-tricalcium phosphate
(β-TCP) on the beagle dog 1-wall periodontal defect model. Biochem
Biophys Res Commun. 403:345–350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xia L, Zhang Z, Chen L, Zhang W, Zeng D,
Zhang X, Chang J and Jiang X: Proliferation and osteogenic
differentiation of human periodontal ligament cells on akermanite
and β-TCP bioceramics. Eur Cell Mater. 22:68–82. 2011.
|
|
14
|
Gruskin E, Doll BA, Futrell FW, Schmitz JP
and Hollinger JO: Demineralized bone matrix in bone repair: history
and use. Adv Drug Deliv Rev. 64:1063–1077. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mauney JR, Jaquiery C, Volloch V, Heberer
M, Martin I and Kaplan DL: In vitro and in vivo evaluation of
differentially demineralized cancellous bone scaffolds combined
with human bone marrow stromal cells for tissue engineering.
Biomaterials. 26:3173–3185. 2005. View Article : Google Scholar
|
|
16
|
Miron RJ, Bosshardt DD, Laugisch O, Dard
M, Gemperli AC, Buser D, Gruber R and Sculean A: In vitro
evaluation of demineralized freeze-dried bone allograft in
combination with enamel matrix derivative. J Periodontol.
84:1646–1654. 2013.PubMed/NCBI
|
|
17
|
Papadopoulos CE, Dereka XE, Vavouraki EN
and Vrotsos IA: In vitro evaluation of the mitogenic effect of
platelet-derived growth factor-BB on human periodontal ligament
cells cultured with various bone allografts. J Periodontol.
74:451–457. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
An S, Ling J, Gao Y and Xiao Y: Effects of
varied ionic calcium and phosphate on the proliferation, osteogenic
differentiation and mineralization of human periodontal ligament
cells in vitro. J Periodont Res. 47:374–382. 2012. View Article : Google Scholar
|
|
19
|
Urist MR: Bone: formation by
autoinduction. 1965. Clin Orthop Relat Res. 395:4–10. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reddi AH and Huggins C: Biochemical
sequences in the transformation of normal fibroblasts in adolescent
rats. Proc Natl Acad Sci USA. 69:1601–1605. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shayegan A, Petein M and Vanden Abbeele A:
The use of beta-tricalcium phosphate, white MTA, white Portland
cement and calcium hydroxide for direct pulp capping of primary pig
teeth. Dent Traumatol. 25:413–419. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Elahi MM, Vanduzer S, Spears J, Gibson J
and Mitra A: Frontal sinus obliteration with beta-tricalcium
phosphate. J Craniofac Surg. 15:967–970. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mahr MA, Bartley GB, Bite U, Clay RP,
Kasperbauer JL and Holmes JM: Norian craniofacial repair system
bone cement for the repair of craniofacial skeletal defects.
Ophthal Plast Reconstr Surg. 16:393–398. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dori F, Arweiler N, Gera I and Sculean A:
Clinical evaluation of an enamel matrix protein derivative combined
with either a natural bone mineral or beta-tricalcium phosphate. J
Periodontol. 76:2236–2243. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sandhu HS, Khan SN, Suh DY and Boden SD:
Demineralized bone matrix, bone morphogenetic proteins, and animal
models of spine fusion: an overview. Eur Spine J. 10(Suppl 2):
S122–S131. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Martin GJ Jr, Boden SD, Titus L and
Scarborough NL: New formulations of demineralized bone matrix as a
more effective graft alternative in experimental posterolateral
lumbar spine arthrodesis. Spine. 24:637–645. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Urist MR, Mikulski A and Lietze A:
Solubilized and insolubilized bone morphogenetic protein. Proc Natl
Acad Sci USA. 76:1828–1832. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guizzardi S, Di Silvestre M, Scandroglio
R, Ruggeri A and Savini R: Implants of heterologous demineralized
bone matrix for induction of posterior spinal fusion in rats.
Spine. 17:701–707. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Orimo H and Shimada T: The role of
tissue-nonspecific alkaline phosphatase in the phosphate-induced
activation of alkaline phosphatase and mineralization in SaOS-2
human osteoblast-like cells. Mol Cell Biochem. 315:51–60. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Puleo DA and Nanci A: Understanding and
controlling the bone-implant interface (Review). Biomaterials.
20:2311–2321. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bernstein A, Nöbel D, Mayr HO, Berger G,
Gildenhaar R and Brandt J: Histological and histomorphometric
investigations on bone integration of rapidly resorbable calcium
phosphate ceramics. J Biomed Mater Res B Appl Biomater. 84:452–462.
2008. View Article : Google Scholar
|
|
32
|
Langstaff S, Sayer M, Smith TJ and Pugh
SM: Resorbable bioceramics based on stabilized calcium phosphates.
Part II: evaluation of biological response. Biomaterials.
22:135–150. 2001. View Article : Google Scholar
|
|
33
|
Ni S, Chang J, Chou L and Zhai W:
Comparison of osteoblast-like cell responses to calcium silicate
and tricalcium phosphate ceramics in vitro. J Biomed Mater Res B
Appl Biomater. 80:174–183. 2007. View Article : Google Scholar
|
|
34
|
Zimmermann G and Moghaddam A: Allograft
bone matrix versus synthetic bone graft substitutes. Injury.
42(Suppl 2): S16–S21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu Z, Peel SA, Lindholm TC, Sàndor GK,
Clokie CM and Su Y: Osteoinductivity of partially purified bovine,
ostrich and emu bone morphogenetic proteins in vitro. J Biomed
Mater Res A. 98:473–477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Katz JM, Nataraj C, Jaw R, Deigl E and
Bursac P: Demineralized bone matrix as an osteoinductive
biomaterial and in vitro predictors of its biological potential. J
Biomed Mater Res B Appl Biomater. 89:127–134. 2009. View Article : Google Scholar
|
|
37
|
Kasten P, Luginbühl R, van Griensven M,
Barkhausen T, Krettek C, Bohner M and Bosch U: Comparison of human
bone marrow stromal cells seeded on calcium-deficient
hydroxyapatite, beta-tricalcium phosphate and demineralized bone
matrix. Biomaterials. 24:2593–2603. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jensen ED, Gopalakrishnan R and Westendorf
JJ: Regulation of gene expression in osteoblasts. Biofactors.
36:25–32. 2010.PubMed/NCBI
|
|
39
|
Komori T: Regulation of bone development
and extracellular matrix protein genes by RUNX2. Cell Tissue Res.
339:189–195. 2010. View Article : Google Scholar
|
|
40
|
Burg KJ, Porter S and Kellam JF:
Biomaterial developments for bone tissue engineering. Biomaterials.
21:2347–2359. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee J, Cuddihy MJ and Kotov NA:
Three-dimensional cell culture matrices: state of the art. Tissue
Eng Part B Rev. 14:61–86. 2008. View Article : Google Scholar : PubMed/NCBI
|