Introduction
Mucus secretion functions as a guard and barrier for
the airway epithelium. An appropriate amount of mucus secretion
protects the airway epithelium from dust particles, smoke, bacteria
and viruses, and ciliary movement clears these irritants from the
airways (1,2). However, the chronic and
hypersecretion of mucus can induce severe airway obstruction and
repeated airway infection. It can contribute to the morbidity and
mortality of chronic inflammatory pulmonary diseases, particularly
chronic obstructive pulmonary disease (COPD) (3–5).
Mucins are the main secretory components of mucus (6,7).
To date, 20 mucin genes can be found to be expressed in the
airways. MUC5AC appears to be the most prominent phenotype,
particularly in the pathological state (6,7).
Cigarette smoke (CS) reportedly represents the first
risk of developing COPD, and the mortality rate for COPD has been
shown to be at least 7-fold higher in smokers than in non-smokers
(8,9). Repeated irritation from smoke
destroys mucociliary clearance, amplifies mucus production and
results in mucus hypersecretion, promoting the development of COPD
(10,11). Previous studies have implicated
the epidermal growth factor receptor (EGFR) in the response of
bronchial epithelial cells to cigarette smoke extract (CSE),
including the reduction in epithelial integrity and mucus
hypersecretion (12–14). The mechanisms responsible for the
secretion of MUC5AC induced by EGFR have been extensively
investigated. Studies have demonstrated that CS induces the
activation of EGFR, then triggers phosphatidylinostol-3-kinase
(PI3K)/Akt and mitogen-activated protein kinase (MAPK) signaling,
and finally increases MUC5AC gene expression and secretion
(15–17). However, the mechanisms responsible
for the activation of EGFR remain unclear.
Caveolae are 50–100 nm flask-shaped invaginations of
the plasma membrane (18).
Caveolae have been implicated in numerous biological functions,
including signal transduction, cellular metabolism, cholesterol
homeostasis and tumor suppression (19). The structural proteins required
for caveolae are caveolins, including caveolin-1, caveolin-2 and
caveolin-3 (20). As previously
demonstrated, caveolin-1 is a principal component of caveolar
membranes in many cell types, including airway epithelial cells,
and it plays physiological roles in regulating signaling molecules
within caveolar membranes (21).
These signaling molecules include heterotrimeric G proteins, the
Src-family, non-receptor tyrosine kinases, endothelial nitric oxide
synthase (eNOS) and p42/44 MAPK (21–23). Researchers have found that
caveolin-1 interacts with these signaling molecules through the
caveolin-1 scaffolding domain (CSD) (21). For example, EGFR mostly gathers in
the caveolae and caveolin-1 regulates the activation of EGFR and
signaling from EGFR to the nucleus, resulting in reduced cell
growth and increased apoptosis (24–26).
Previous studies have discovered the potential roles
of caveolin-1 in lung inflammation and injury (27,28). Caveolin-1 can reportedly regulate
the development of lung injury by modulating the acute inflammatory
response, capillary leakage and pulmonary edema. Researchers have
also found that the deletion of caveolin-1 increases the production
of the pro-inflammatory cytokines, interleukin-6 (IL-6) and tumor
necrosis factor-α (TNF-α), induced by lipopolysaccharides (28,29). These studies, combined with the
close association between MUC5AC secretion and airway inflammation,
led us to hypothesize that caveolin-1 may be an important regulator
involved in CS-induced MUC5AC production in lung epithelial cells.
To examine this hypothesis, we employed gain-and loss-function
approaches to assess the effects of caveolin-1 on the secretion of
MUC5AC stimulated by CSE, as well as the underlying mechanisms.
Materials and methods
Cells, reagents and antibodies
Human bronchial epithelial cells (16HBE cells) were
purchased from Fuxiang Biotechnology Co., Ltd. (Shanghai, China).
Fetal bovine serum (FBS), trypsin, Roswell Park Memorial Institute
(RPMI)-1640 medium, and Opti-MEM were obtained from Gibco
(Carpinteria, CA, USA). Lipofectamine 2000, primers for polymerase
chain reaction (PCR), TRIzol reagent and the PCR kit were purchased
from Invitrogen (Carlsbad, CA, USA). TransIT-TKO reagents were
purchased from Mirus Bio Corp. (Madison, WI, USA). Rabbit
anti-human caveolin-1 (#3238), rabbit anti-human EGFR (#2232),
rabbit anti-human Akt (#9272), rabbit anti-human phosphorylated Akt
(p-Akt; #9271) and mouse anti-human phosphorylated EGFR (p-EGFR;
#2236) antibodies, as well as LY294002 (a specific inhibitor of
PI3K) were purchased from Cell Signaling Technology (Beverly, MA,
USA). Mouse anti-human β-actin antibody, dimethyl sulfoxide (DMSO)
and cocktail were purchased from Sigma (St. Louis, MO, USA).
Horseradish peroxidase (HRP)-conjugated polyclonal anti-mouse and
anti-rabbit secondary antibodies were purchased from Jackson
Immunoresearch, Inc. (West Grove, PA, USA). AG1478, a specific
inhibitor of EGFR was purchased from Enzo Life Sciences
(Farmingdale, NY, USA). Caveolin-1-expressing plasmid and small
interfering RNA (siRNA) were designed by GeneChem Co. (Shanghai,
China). Phosphatase inhibitors were purchased from Roche (Basel,
Switzerland). The enzyme-linked immunosorbent assay (ELISA) for the
detection of MUC5AC was obtained from Cusabio Biotech Co. (Wuhan,
China).
Preparation of CSE
CSE was prepared as previously described (30–32). CSE (100%) was prepared by bubbling
smoke from 2 cigarettes in 10 ml of serum-free RPMI-1640 medium at
a rate of half a cigarette/min. The pH of the CSE was adjusted to
7.4 and CSE was sterile-filtered through a 0.22-μM filter.
The CSE was always freshly prepared on the day of the
experiment.
Cell culture and treatment
The 16HBE cells were propagated in RPMI-1640
supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml
streptomycin in a 37°C 5% CO2 incubator. The cells were
replenished with fresh medium every 2–3 days. Before additional
treatments, the cells were plated in 60×60 mm culture dishes at a
density of 2×106 cells/ml and were cultured in a 37°C 5%
CO2 incubator. Following serum starvation for 12 h, the
cells were treated with CSE at various concentrations (0, 2, 5, 10,
15 and 20%) for 24 h or 10% CSE for different periods of time (0,
6, 12, 24 and 48 h). Before the subsequent experiments, MTT assay
was used to evaluate the viability of the 16HBE cells. The
following experiments were performed using a culture of >80%
viable cells.
To investigate the signaling cascade from the
CSE-induced secretion of MUC5AC, the cells were pre-treated with 10
μM AG1478 (EGFR inhibitor) for 30 min or with 50 μM
LY294002 (PI3K inhibitor) for 1 h (33,34). Following 24 h of incubation in a
culture medium containing 10% CSE, the cells and culture
supernatants were harvested for further analysis.
In order to induce the overexpression or
downregulation of caveoln-1, the 16HBE cells were transiently
transfected with caveolin-1-expressing plasmid and empty vector
plasmid using Lipofectamine 2000 or caveolin-1 siRNA plasmid and
siRNA control plasmid using TransIT-TKO reagent. Following
incubation with the plasmids for 4–6 h, the cells were treated with
CSE (10%) for 24 h.
ELISA for MUC5AC in the cell
supernatant
The cell culture supernatants were collected and
used to assay the total protein concentration. MUC5AC protein
epxression was measured following the instructions provided with
the ELISA kit.
Reverse transcription-polymerase chain
reaction analysis (RT-PCR)
Total RNA was extracted from the 16HBE cells in each
group using TRIzol reagent. The extraction was verified by
electrophoresis on a 1.0% agarose gel and an absorbance (A260/280)
value of 1.8–2.0. Reverse transcription for complementary DNA was
performed using an RT-PCR kit. The PCR primers for MUC5AC were
5′-AACTGCAGCTGGACA GTGTG-3′ (forward) and 5′-TGCAGATCTGGGTCTC
ACAG-3′ (reverse); and those for β-actin were 5′-GGGCA
CGAAGGCTCATCATT-3′ (forward) and 5′-AGCGAGCATC CCCCAAAGTT-3′
(reverse). The PCR reactions were carried out as follows: a
pre-denaturing at 94°C for 5 min, followed by 28 cycles of
denaturation at 94°C for 45 sec, annealing at 58°C for 40 sec, and
extension at 72°C for 45 sec. PCR products were separated by
electrophoresis through 1% agarose gel containing ethidium bromide,
and the signal intensity was analyzed using Quantity One
software.
Western blot analysis
The cells were lysed in a RIPA lysis buffer with
protease inhibitor and phosphatase inhibitor. Equal amounts of cell
lysate from the protein samples were resolved by 10 and 12% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto polyvinylidene difluoride (PVDF) membranes. These
membranes were incubated with 5% skimmed milk or 5% BSA in PBST at
room temperature for 1 h and exposed to specific primary antibodies
against caveolin-1, EGFR, p-EGFR, Akt and p-Akt (at 1:1,000)
overnight at 4°C. This was then followed by incubation with
HRP-conjugated goat anti-mouse and anti-rabbit secondary antibodies
(at 1:10,000) for 1 h at room temperature. The blots were
visualized by enhanced chemiluminescence. The intensity of each
band was measured using Quantity One software. The relative protein
expression level was determined by normalization to that of
β-actin.
Statistical analysis
Data were analyzed using Pearson’s correlation
co-efficient with SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA)
and are presented as the means ± standard deviation. The data were
also analyzed using the Student’s t-test or one-way analysis of
variance followed by the Tukey’s test where appropriate. P-values
<0.05 were considered to indicate statistically significant
differences.
Results
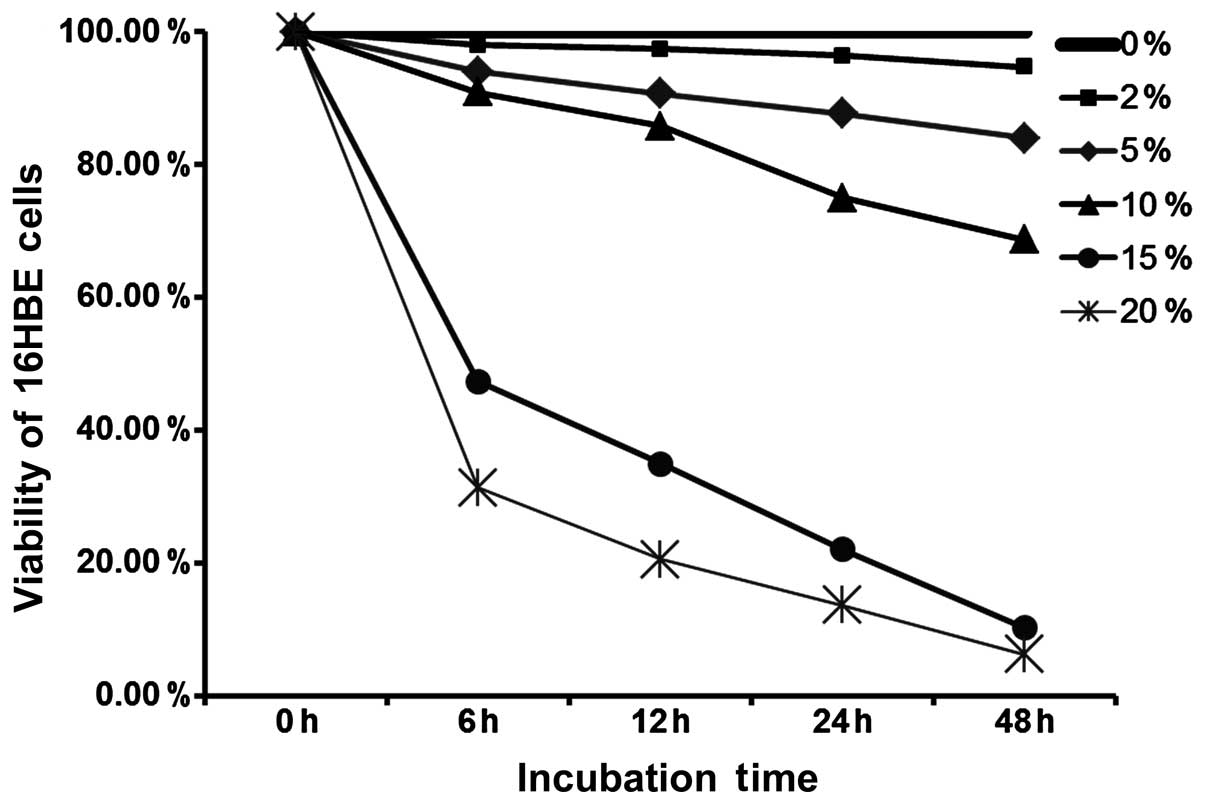
Cell cytotoxicity
Cell cytotoxicity was detected by MTT assay. CSE
exerted cytotoxic effects on the 16HBE cells in a time- and
concentration-dependent manner. A low concentration and short time
treatment of CSE had no effect on cell viability, and the viability
of the 16HBE cells decreased with the increasing CSE concentration
and the increase in the treatment duration (Fig. 1).
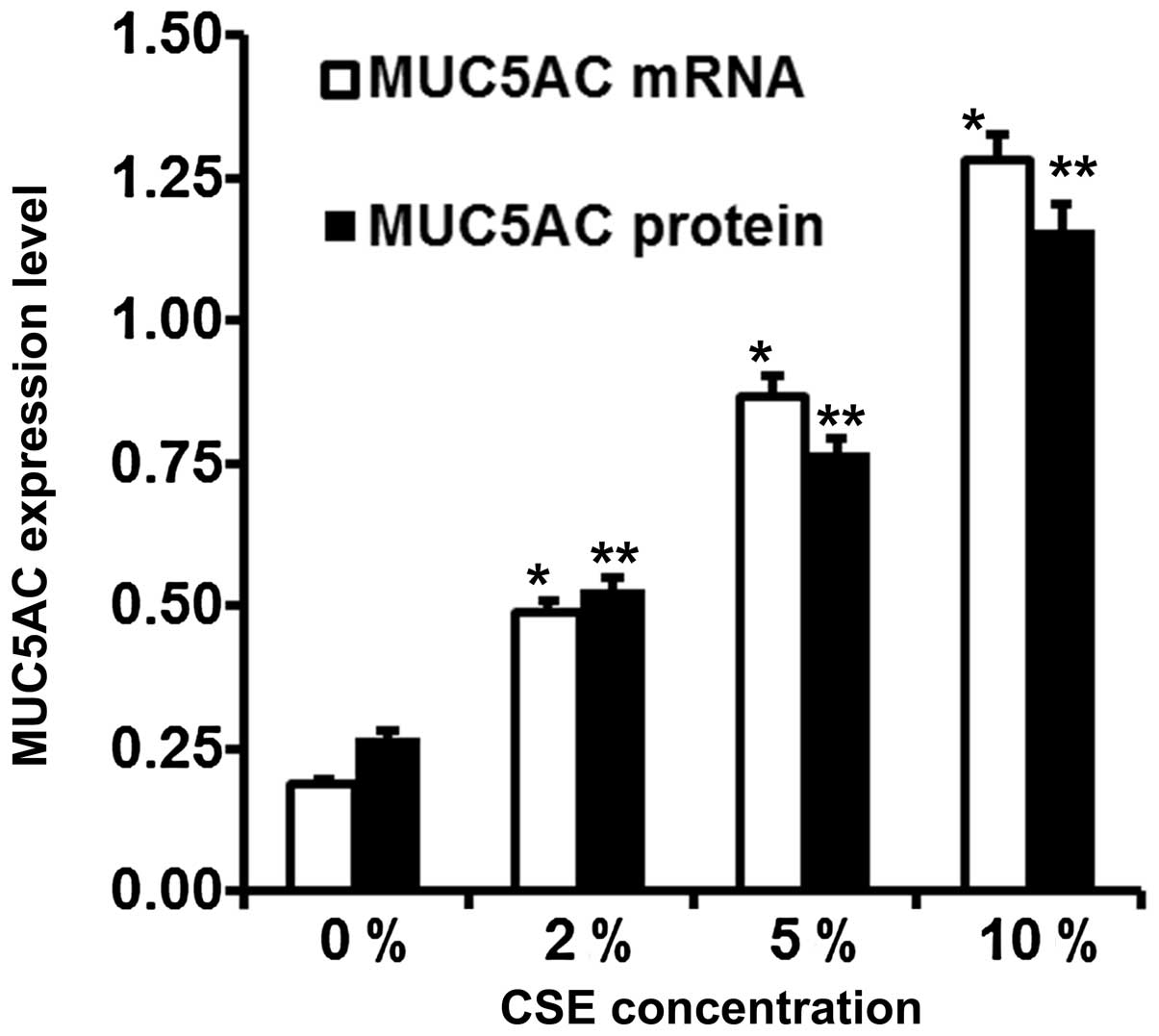
Effect of CSE on MUC5AC synthesis
We selected 24 h as the best incubation time due to
the MTT results and the results from previous studies (33,35). The synthesis of MUC5AC mRNA and
protein exhibited a dose-dependent increase in response to
treatment with CSE for 24 h. The mRNA expression level of MUC5AC
was increased by approximately 2.5-, 4.3- and 7.5-fold following
incubation with 2, 5 and 10% CSE, respectively (p<0.05).
Stimulation with 2, 5 and 10% CSE induced a 1.9-, 3.0- and 4.3-fold
increase, respectively in the protein expression of MUC5AC in the
culture supernatant (p<0.05). The cells exposed to CSE showed an
increase in the mRNA and protein expression of MUC5AC in a
concentration-dependent manner (Fig.
2).
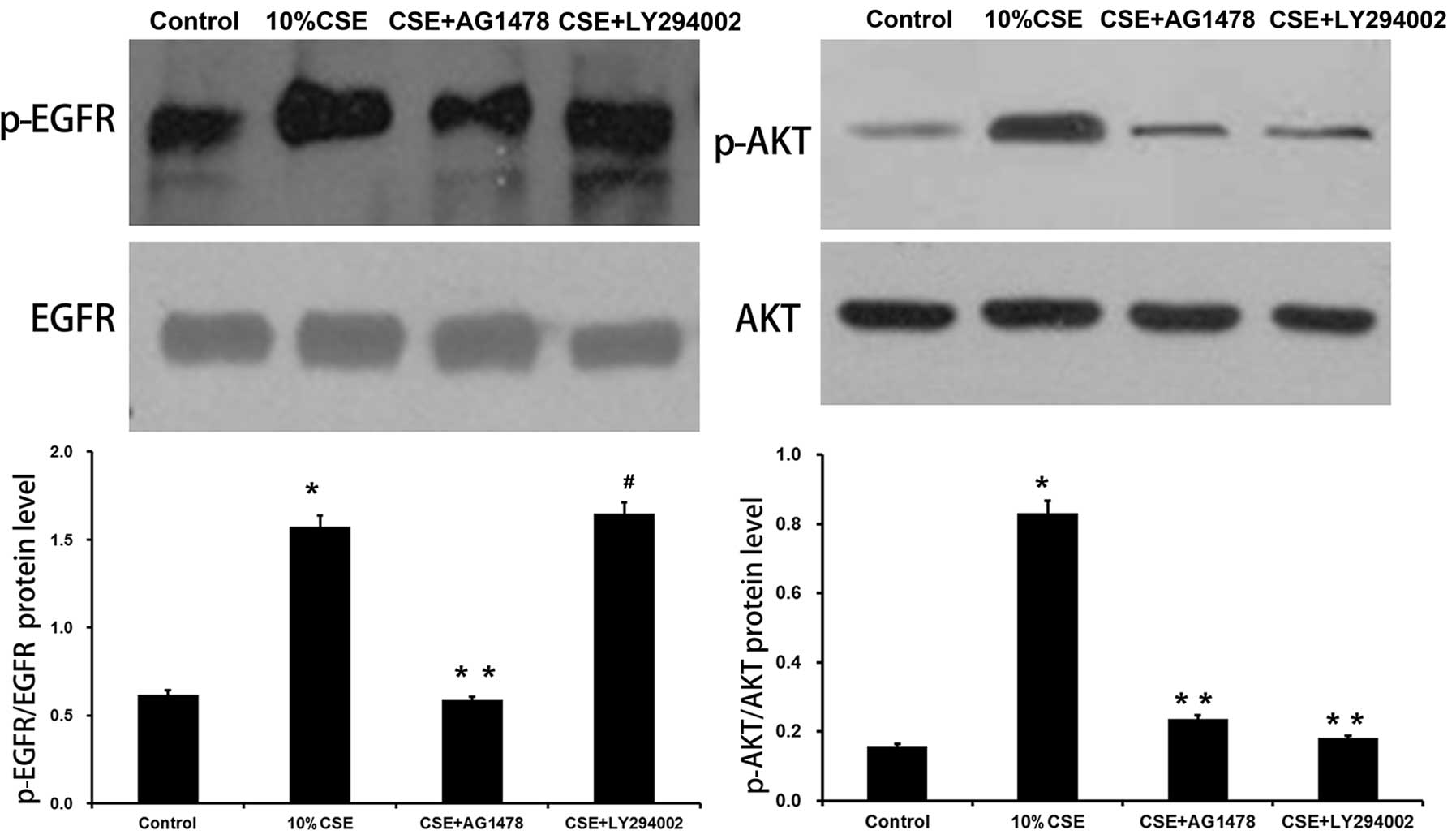
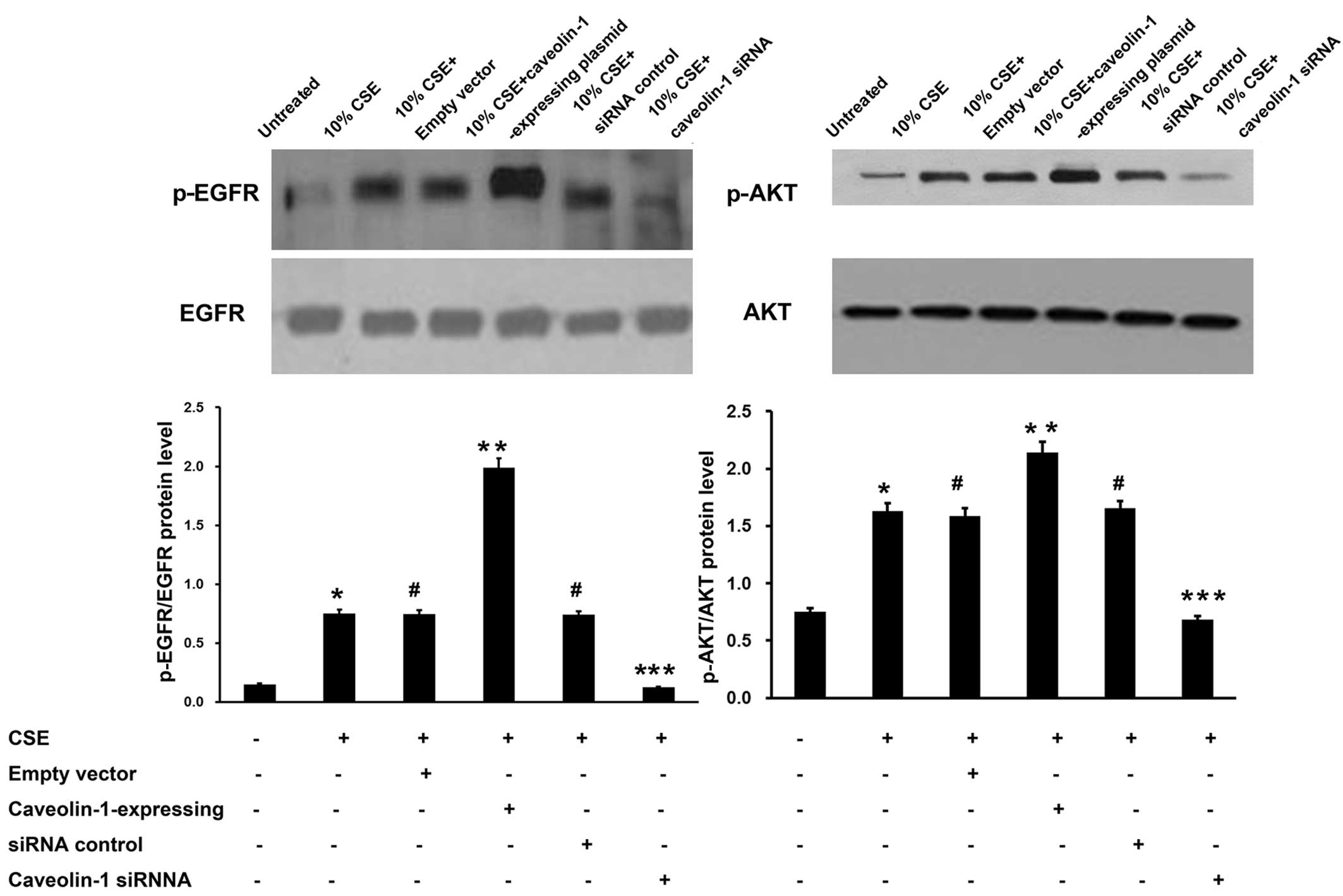
EGFR and PI3K/Akt signaling pathways
mediate CSE induced-MUC5AC expression
Previous studies have revealed a close association
between CSE and EGFR activation (12–14). Thus, we examined whether the EGFR
and PI3K/Akt signaling pathways are essential for the CSE-induced
secretion of MUC5AC. EGFR inhibitor (AG1478) and PI3K inhibitor
(LY294002) were used to treat the 16HBE cells prior to CSE
stimulation. Exposure of the 16HBE cells to CSE led to a
significant increase in the levels of p-EGFR (p<0.05 vs. control
group), and the activation of EGFR by CSE was partially inhibited
by AG1478 (p<0.05 vs. CSE group). The levels of p-Akt increased
following CSE stimulation (p<0.05 vs. control group), and
LY294002 markedly attenuated Akt phosphorylation, but did not
affect EGFR phosphorylation (p<0.05 vs CSE group, p>0.05 vs.
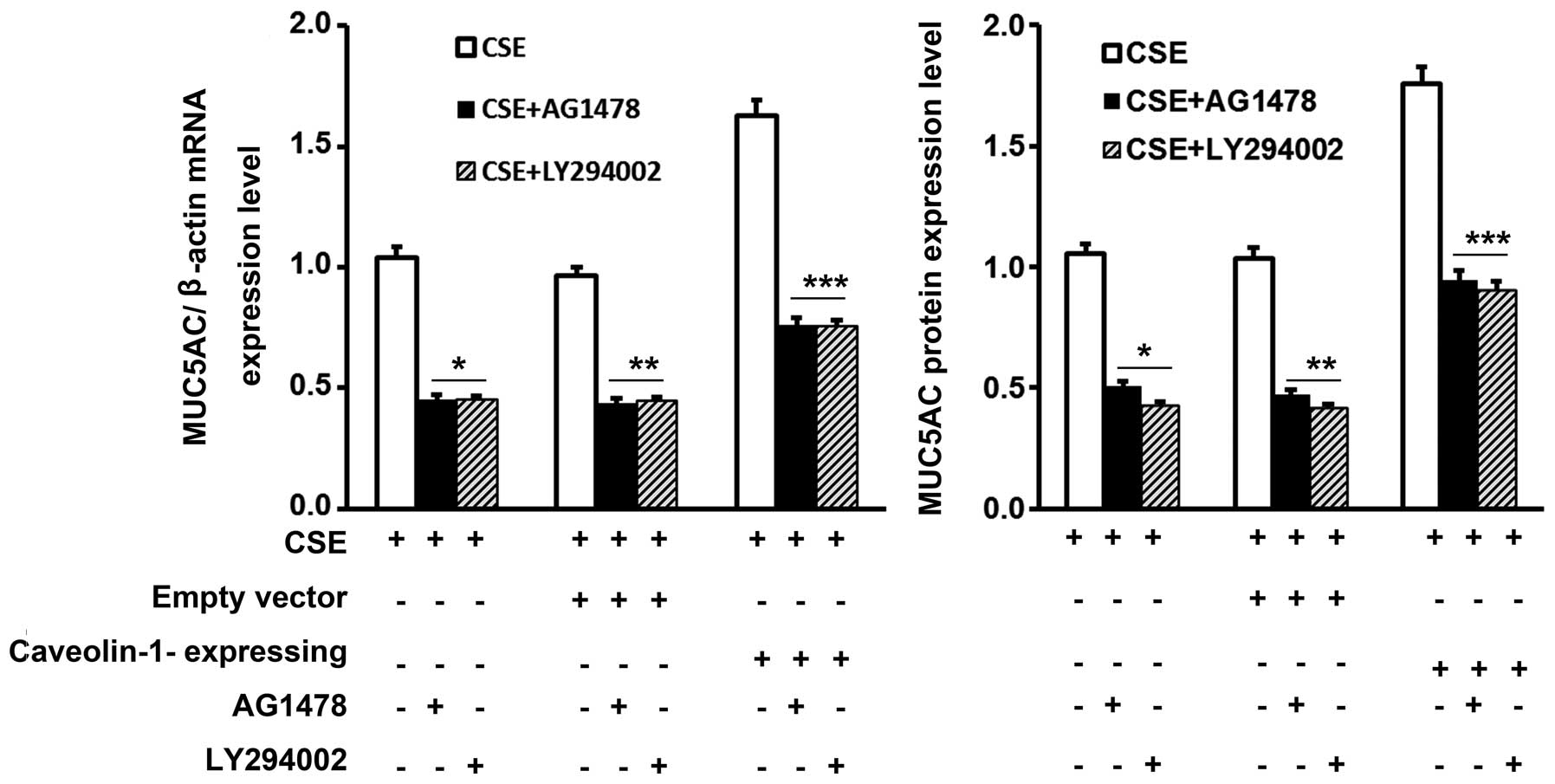
CSE group) (Fig. 3).
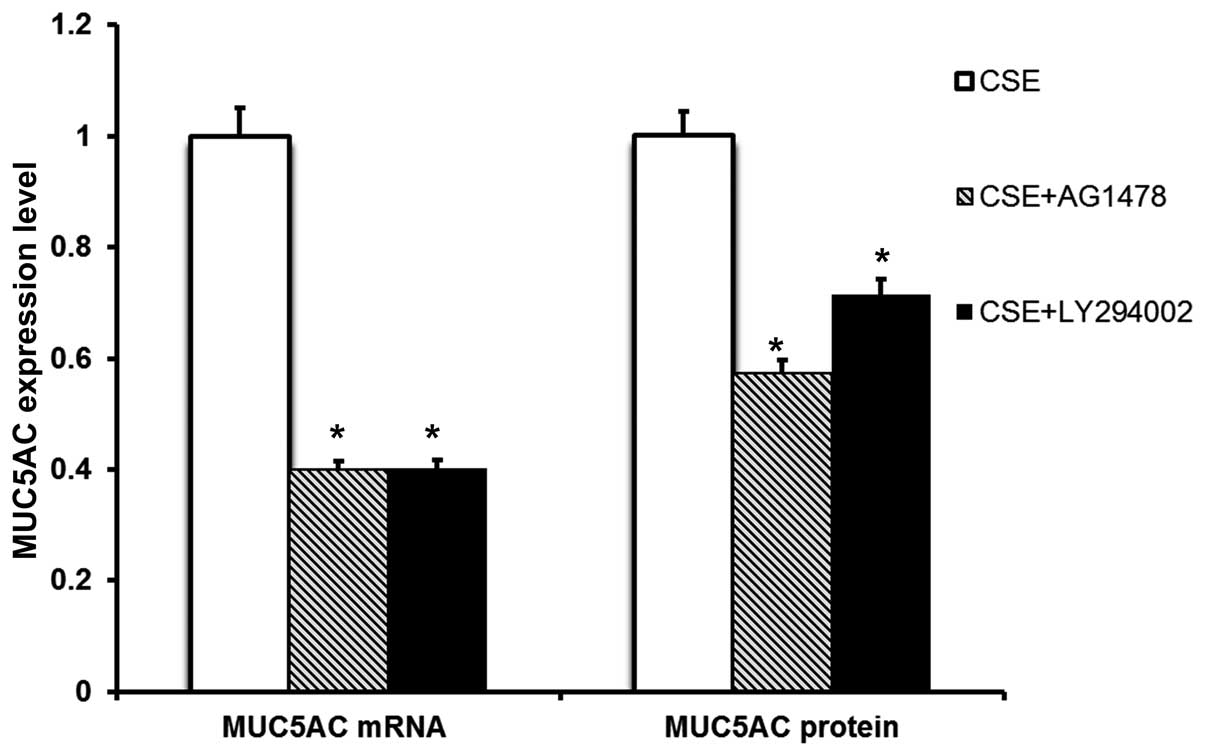
Pre-treatment with AG1478 or LY294002 markedly abrogated the
upregulation of MUC5AC mRNA expression and protein production
induced by CSE (Fig. 4).
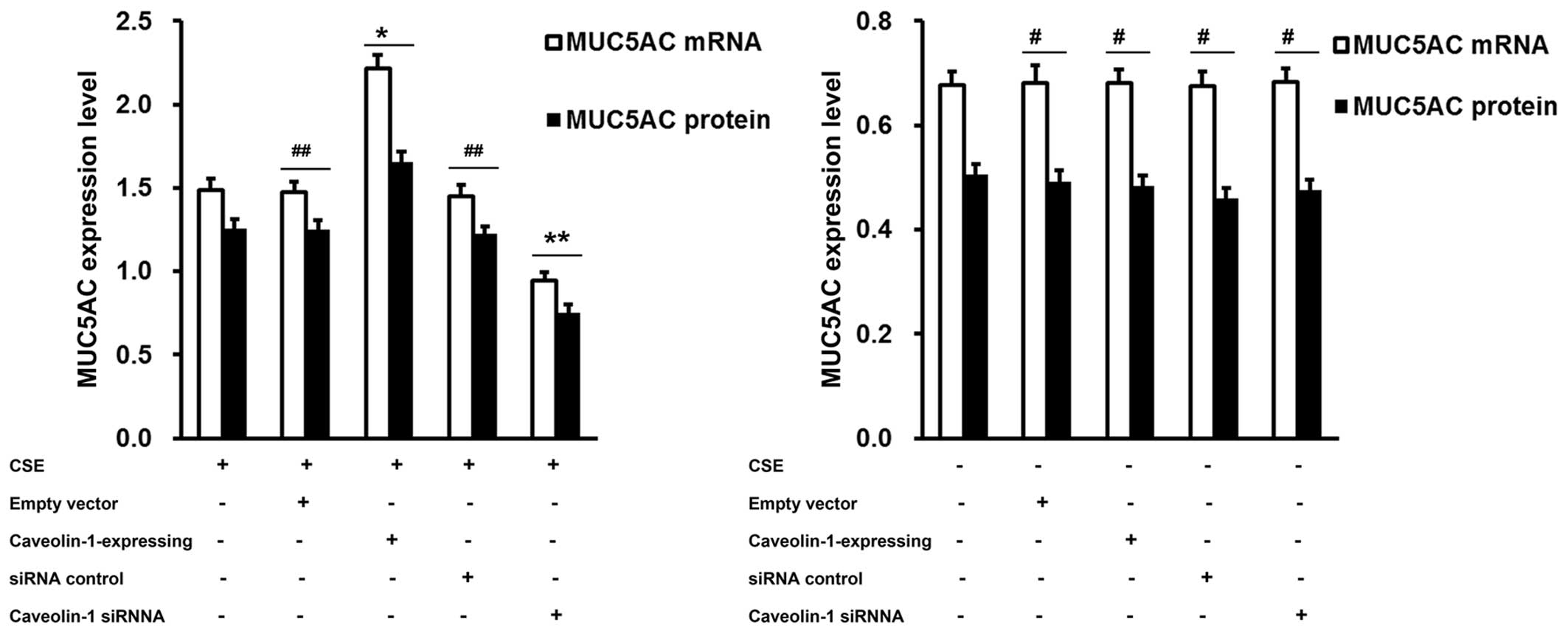
Regulation of CSE-mediated MUC5AC
expression by caveolin-1 in 16HBE cells
To determine whether the CSE-induced MUC5AC
expression is associated with caveolin-1, we either enforced the
expression of caveolin-1 in the 16HBE cells by transfection with
caveolin-1-expressing plasmid, or downregulated its expression by
transfection with caveolin-1 siRNA. An approximately 100% elevation
in MUC5AC mRNA and a 50% elevation in MUC5AC protein levels were
achieved by the delivery of caveolin-1-expression plasmids compared
to transfection with the control vector. Conversely, the knockdown
of caveolin-1 by siRNA resulted in an approximatley 1-fold
reduction in the MUC5AC mRNA and protein expression levels compared
to the cells treated with the siRNA control (Fig. 5). These results suggest that
caveolin-1 acts as a positive regulator of the hypersecretion of
MUC5AC by CSE; caveolin-1 had no effect on MUC5AC secretion in the
absence of CSE (Fig. 5).
EGFR/PI3K/Akt signaling pathway is
involved in the effects of caveolin-1 on CSE-induced MUC5AC
expression
As verified above, CSE induced MUC5AC expression
through the EGFR/PI3K/Akt signaling pathway and caveolin-1 enhanced
the CSE-mediated MUC5AC expression. Western blot analysis revealed
that the overexpression of caveolin-1 induced in the 16HBE cells by
caveolin-1-expressing plasmid caused a marked increase in EGFR and
Akt phosphorylation. Conversely, transfection with caveolin-1 siRNA
prior to incubation with CSE significantly decreased the levels of
p-EGFR and p-Akt (Fig. 6).
Furthermore, pre-treatment with AG1478 or LY294002 markedly
abrogated the upregulation of MUC5AC expression induced by CSE
through caveolin-1 in the 16HBE cells (Fig. 7).
Discussion
Caveolin-1, a 21–24 kDa cytomembrane protein, is the
major resident scaffolding protein constituent of caveolae that
participates in vesicular trafficking and signal transduction
(21,36). Caveolin-1 participates in several
biological processes, including cell growth, apoptosis and cell
proliferation (18). A recent
study indicated that caveolin-1 also plays a role in the
development of lung inflammation (28). Yuan et al found that
caveolin-1 knockout mice exhibited an increase in inflammation and
in the levels of superoxide in the lungs, and presented with
aggravated severe lung injury after Pseudomonas aeruginosa
infection (29). In alveolar and
peritoneal macrophages, researchers have shown that the
downregulation of caveolin-1 increases the LPS-induced production
of the pro-inflammatory cytokines, TNF-α and IL-6. By contrast, it
decreased anti-inflammatory cytokine IL-10 production (37). However, studies have produced
conflicting results on whether caveolin-1 attenuates lung
inflammation. The overexpression of caveolin-1 was shown to
aggravate the alveolar type-I cell injury induced by LPS in the
study by Lv et al (32).
In another study using caveolin-1 null mice, Hu et al
demonstrated that in polymorphonuclear neutrophils (PMN) from these
mice, PMN activation, adhesion, as well as PMN activation-induced
lung inflammation and vascular injury were reduced (38). These data indicate that caveolin-1
is involved in lung inflammation, although whether its role is
promotional or suppressive remains controversial. As is already
known, inflammatory reactions are essential processes in MUC5AC
secretion, and as mentioned above, caveolin-1 is an important
regulator of inflammation. It can thus be hypothesized that
caveolin-1 may be associated with MUC5AC secretion.
In this study, we established an in vitro
model of MUC5AC hypersecretion induced by CSE using HBE cells. We
used two independent methods to determine the role of caveolin-1 in
MUC5AC secretion. A gain-of-function experiment using a
caveolin-1-expressing plasmid transfection demonstrated that the
overexpression of caveolin-1 in the 16HBE cells increased the
CS-induced production of MUC5AC. A loss-of-function experiment
using transfection with caveolin-1 siRNA decreased the secretion of
MUC5AC induced by CS. Our results demonstrated that caveolin-1
promoted the CS-induced secretion of MUC5AC.
The biological significance of caveolin-1 is
dependent on its interaction with signaling molecules and
regulating their activation (18,23,36). Previous studies have indicated
that EGFR is concentrated in the caveolae, and that caveolin-1
modulates EGFR activation, leading to its complex involvement in
diseases (39,40).
CS is considered to be a significant etiology of
mucin hypersecretion. One way that CS exerts its biological effects
is by binding to EGFR, then leading to the activation of a cascade
of signaling pathways (41).
Several studies have raised the possibility that CS causes EGFR
activation by increasing the availability of soluble EGFR ligands
[e.g., transforming growth factor-bilamphiregulin], which then bind
to and activate EGFR in airway epithelial cells (42). In a previous study, exposure to CS
upregulated EGFR mRNA expression and induced EGFR-specific tyrosine
phosphorylation, resulting in the upregulation of MUC5AC mRNA and
protein expression. These effects were inhibited by selective EGFR
tyrosine kinase inhibitors (12).
The activation of EGFR promotes downstream signaling, such as
PIK/Akt, p38 MAPK and ERK1/2 (41). The EGFR/PI3K/Akt pathway is a key
step in MUC5AC production activated by a number of stimuli,
including CS (34,43,44). We thus hypothesized that
EGFR/PI3K/Akt signaling cascades are possible attractive target
candidates for caveolin-1.
In this study, we found that the overexpression of
caveolin-1 increased the levels of p-EGFR and p-Akt induced by CS
compared with the downregulation of caveolin-1 by siRNA. EGFR
inhibitor (AG1478) blocked the effects of CS on the levels of
p-EGFR and p-Akt, and MUC5AC production. PI3K inhibitor (LY294002)
attenuated the increase in the levels of p-Akt and MUC5AC
production, but did not affect p-EGFR expression. These results
indicate that caveolin-1 affects CS-induced MUC5AC production
through EGFR phosphorylation and the activation of the
EGFR/PI3K/Akt signaling pathway. Furthermore, we discovered that in
the absence of CSE, caveolin-1 did not regulate MUC5AC secretion,
which suggests that caveolin-1 has no effect on the basic secretion
of MUC5AC, but on CS-induced MUC5AC secretion.
Our data, as well as previously published data,
suggest that caveolin-1 interacts with EGFR to promote its
activation and downstream signaling. The study by Wang et al
asserted that the hypoxia-induced factor (HIF)-dependent
upregulation of caveolin-1 enhanced the phosphorylation of EGFR and
increased the proliferation, migration and invasion capacities of
renal cell carcinoma cells (45).
The disruption of caveolae by Filipin III and MβCD has been shown
to significantly attenuate the endothelin-1-induced phosphorylation
of EGFR in mesangial cells in the study by Hua et al
(46). Intriguingly, caveolin-1
was initially considered to be a negative regulator of signaling
molecules (21). The
overexpression of caveolin-1 in MCF-7 cells has been shown to
decrease the phosphorylation of EGFR in breast cancer (47). Another study demonstrated that
infection with adenovirus encoding caveolin-1 significantly
inhibited angiotensin II-induced EGFR activation, hypertrophy and
the migration of vascular smooth muscle cells (VSMCs).
Methyl-β-cyclodextrin (Mclod, a disrupter of caveolae structure,
stimulated EGFR activation in VSMCs (40). In addition, Mattson et al
demonstrated that the overexpression of caveolin-1 had no effect on
the phosphorylation of EGFR in fatty cells (48). To date, evidence suggests that the
multiple roles of caveolin-1 in EGFR activation may be dependent on
the types of cell and irritants (23,28,49). Futher studies are required to
clarify the exact mechanisms involved.
In conclusion, to the best of our knowledge, we
demonstrate for the first time that caveolin-1 plays a promoting
role in CS-induced MUC5AC secretion in 16HBE cells. We also provide
evidence that the effects of caveolin-1 involve the EGFR/PI3K/Akt
signaling pathway. It can thus be hypothesized that the
downregulation of caveolin-1 protects against the mucus
hypersecretion induced by CSE. Caveolin-1 may be a potential target
for the treatment of CS-induced mucus hypersecretion in COPD.
References
|
1
|
Voynow JA and Rubin BK: Mucins, mucus, and
sputum. Chest. 135:505–512. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adler KB, Tuvim MJ and Dickey BF:
Regulated mucin secretion from airway epithelial cells. Front
Endocrinol. 4:1292013. View Article : Google Scholar
|
|
3
|
Turner J and Jones CE: Regulation of mucin
expression in respiratory diseases. Biochem Soc Trans. 37:877–881.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cerveri I and Brusasco V: Revisited role
for mucus hypersecretion in the pathogenesis of COPD. Eur Respir
Rev. 19:109–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Curran DR and Cohn L: Advances in mucous
cell metaplasia: A plug for mucus as a therapeutic focus in chronic
airway disease. Am J Respir Cell Mol Biol. 42:268–275. 2010.
View Article : Google Scholar :
|
|
6
|
Rose MC and Voynow JA: Respiratory tract
mucin genes and mucin glycoproteins in health and disease. Physiol
Rev. 86:245–278. 2006. View Article : Google Scholar
|
|
7
|
Evans CM and Koo JS: Airway mucus: The
good, the bad, the sticky. Pharmacol Ther. 121:332–348. 2009.
View Article : Google Scholar
|
|
8
|
Halbert RJ, Natoli JL, Gano A, Badamgarav
E, Buist AS and Mannino DM: Global burden of COPD: Systematic
review and meta-analysis. Eur Respir J. 28:523–532. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kinnula VL, Vasankari T, Kontula E,
Sovijarvi A, Saynajakangas O and Pietinalho A: The 10-year COPD
Programme in Finland: Effects on quality of diagnosis, smoking,
prevalence, hospital admissions and mortality. Prim Care Respir J.
20:178–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoshida T and Tuder RM: Pathobiology of
cigarette smoke-induced chronic obstructive pulmonary disease.
Physiol Rev. 87:1047–1082. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tamimi A, Serdarevic D and Hanania NA: The
effects of cigarette smoke on airway inflammation in asthma and
COPD: Therapeutic implications. Respir Med. 106:319–328. 2012.
View Article : Google Scholar
|
|
12
|
Takeyama K, Jung B, Shim JJ, et al:
Activation of epidermal growth factor receptors is responsible for
mucin synthesis induced by cigarette smoke. Am J Physiol Lung Cell
Mol Physiol. 280:L165–L172. 2001.PubMed/NCBI
|
|
13
|
Basbaum C, Li D, Gensch E, Gallup M and
Lemjabbar H: Mechanisms by which gram-positive bacteria and tobacco
smoke stimulate mucin induction through the epidermal growth factor
receptor (EGFR). Novartis Found Symp. 248:171–182. 2002. View Article : Google Scholar
|
|
14
|
Heijink IH, Brandenburg SM, Postma DS and
van Oosterhout AJ: Cigarette smoke impairs airway epithelial
barrier function and cell-cell contact recovery. Eur Respir J.
39:419–428. 2012. View Article : Google Scholar
|
|
15
|
Deshmukh HS, Case LM, Wesselkamper SC, et
al: Metallo-proteinases mediate mucin 5AC expression by epidermal
growth factor receptor activation. Am J Respir Crit Care Med.
171:305–314. 2005. View Article : Google Scholar
|
|
16
|
Cortijo J, Mata M, Milara J, et al:
Aclidinium inhibits cholinergic and tobacco smoke-induced MUC5AC in
human airways. Eur Respir J. 37:244–254. 2011. View Article : Google Scholar
|
|
17
|
Yu H, Li Q, Kolosov VP, Perelman JM and
Zhou X: Regulation of cigarette smoke-mediated mucin expression by
hypoxia-inducible factor-1α via epidermal growth factor
receptor-mediated signaling pathways. J Appl Toxicol. 32:282–292.
2012. View
Article : Google Scholar
|
|
18
|
Thomas CM and Smart EJ: Caveolae structure
and function. J Cell Mol Med. 12:796–809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stan RV: Structure of caveolae. Biochim
Biophys Acta. 1746:334–348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hansen CG and Nichols BJ: Exploring the
caves: Cavins, caveolins and caveolae. Trends Cell Biol.
20:177–186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu P, Rudick M and Anderson RG: Multiple
functions of caveolin-1. J Biol Chem. 277:41295–41298. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schwencke C, Braun-Dullaeus RC, Wunderlich
C and Strasser RH: Caveolae and caveolin in transmembrane
signaling: Implications for human disease. Cardiovasc Res.
70:42–49. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boscher C and Nabi IR: Caveolin-1: Role in
cell signaling. Adv Exp Med Biol. 729:29–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Couet J, Sargiacomo M and Lisanti MP:
Interaction of a receptor tyrosine kinase, EGF-R, with caveolins.
Caveolin binding negatively regulates tyrosine and serine/threonine
kinase activities. J Biol Chem. 272:30429–30438. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gu D, Li H, Wang Z, Chen Q, Jiang J and
Zhu H: Caveolin-1 inhibits the growth of human laryngeal squamous
cell carcinoma and down regulates EGFR-MAPKs signaling pathway.
Laryngoscope. 117:1782–1789. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Han F, Gu D, Chen Q and Zhu H: Caveolin-1
acts as a tumor suppressor by down-regulating epidermal growth
factor receptor-mitogen-activated protein kinase signaling pathway
in pancreatic carcinoma cell lines. Pancreas. 38:766–774. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Garrean S, Gao XP, Brovkovych V, et al:
Caveolin-1 regulates NF-kappaB activation and lung inflammatory
response to sepsis induced by lipopolysaccharide. J Immunol.
177:4853–4860. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jin Y, Lee SJ, Minshall RD and Choi AM:
Caveolin-1: A critical regulator of lung injury. Am J Physiol Lung
Cell Mol Physiol. 300:L151–L160. 2011. View Article : Google Scholar
|
|
29
|
Yuan K, Huang C, Fox J, et al: Elevated
inflammatory response in caveolin-1-deficient mice with Pseudomonas
aeruginosa infection is mediated by STAT3 protein and nuclear
factor kappaB (NF-kappaB). J Biol Chem. 286:21814–21825. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kode A, Yang SR and Rahman I: Differential
effects of cigarette smoke on oxidative stress and proinflammatory
cytokine release in primary human airway epithelial cells and in a
variety of transformed alveolar epithelial cells. Respir Res.
7:1322006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang SR, Chida AS, Bauter MR, et al:
Cigarette smoke induces proinflammatory cytokine release by
activation of NF-kappaB and posttranslational modifications of
histone deacetylase in macrophages. Am J Physiol Lung Cell Mol
Physiol. 291:L46–57. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lv XJ, Li YY, Zhang YJ, Mao M and Qian GS:
Over-expression of caveolin-1 aggravate LPS-induced inflammatory
response in AT-1 cells via up-regulation of cPLA2/p38 MAPK. Inflamm
Res. 59:531–541. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shao MX, Nakanaga T and Nadel JA:
Cigarette smoke induces MUC5AC mucin overproduction via tumor
necrosis factor-alpha-converting enzyme in human airway epithelial
(NCI-H292) cells. Am J Physiol Lung Cell Mol Physiol.
287:L420–L427. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Binker MG, Binker-Cosen AA, Richards D,
Oliver B and Cosen-Binker LI: LPS-stimulated MUC5AC production
involves Rac1-dependent MMP-9 secretion and activation in NCI-H292
cells. Biochem Biophys Res Commun. 386:124–129. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu H, Li Q, Kolosov VP, Perelman JM and
Zhou X: Regulation of cigarette smoke-induced mucin expression by
neuregulin1β/ErbB3 signalling in human airway epithelial cells.
Basic Clin Pharmacol Toxicol. 109:63–72. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chidlow JH Jr and Sessa WC: Caveolae,
caveolins, and cavins: Complex control of cellular signalling and
inflammation. Cardiovasc Res. 86:219–225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang XM, Kim HP, Song R and Choi AM:
Caveolin-1 confers antiinflammatory effects in murine macrophages
via the MKK3/p38 MAPK pathway. Am J Respir Cell Mol Biol.
34:434–442. 2006. View Article : Google Scholar
|
|
38
|
Hu G, Ye RD, Dinauer MC, Malik AB and
Minshall RD: Neutrophil caveolin-1 expression contributes to
mechanism of lung inflammation and injury. Am J Physiol Lung Cell
Mol Physiol. 294:L178–186. 2008. View Article : Google Scholar
|
|
39
|
Park JH and Han HJ: Caveolin-1 plays
important role in EGF-induced migration and proliferation of mouse
embryonic stem cells: Involvement of PI3K/Akt and ERK. Am J Physiol
Cell Physiol. 297:C935–944. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Takaguri A, Shirai H, Kimura K, et al:
Caveolin-1 negatively regulates a metalloprotease-dependent
epidermal growth factor receptor transactivation by angiotensin II.
J Mol Cell Cardiol. 50:545–551. 2011. View Article : Google Scholar :
|
|
41
|
Yang CM, Lee IT, Lin CC, et al: Cigarette
smoke extract induces COX-2 expression via a PKCalpha/c-Src/EGFR,
PDGFR/PI3K/Akt/NF-kappaB pathway and p300 in tracheal smooth muscle
cells. Am J Physiol Lung Cell Mol Physiol. 297:L892–902. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hegab AE, Sakamoto T, Nomura A, et al:
Niflumic acid and AG-1478 reduce cigarette smoke-induced mucin
synthesis: The role of hCLCA1. Chest. 131:1149–1156. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li N, Li Q, Zhou XD, Kolosov VP and
Perelman JM: The effect of quercetin on human neutrophil
elastase-induced mucin5AC expression in human airway epithelial
cells. Int Immunopharmacol. 14:195–201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang J, Yu HM, Zhou XD, Kolosov VP and
Perelman JM: Study on TRPV1-mediated mechanism for the
hypersecretion of mucus in respiratory inflammation. Mol Immunol.
53:161–171. 2013. View Article : Google Scholar
|
|
45
|
Wang Y, Roche O, Xu C, et al: Hypoxia
promotes ligand-independent EGF receptor signaling via
hypoxia-inducible factor-mediated upregulation of caveolin-1. Proc
Natl Acad Sci USA. 109:4892–4897. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hua H, Munk S and Whiteside CI:
Endothelin-1 activates mesangial cell ERK1/2 via EGF-receptor
transactivation and caveolin-1 interaction. Am J Physiol Renal
Physiol. 284:F303–F312. 2003. View Article : Google Scholar
|
|
47
|
Agelaki S, Spiliotaki M, Markomanolaki H,
et al: Caveolin-1 regulates EGFR signaling in MCF-7 breast cancer
cells and enhances gefitinib-induced tumor cell inhibition. Cancer
Biol Ther. 8:1470–1477. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mattsson CL, Andersson ER and Nedergaard
J: Differential involvement of caveolin-1 in brown adipocyte
signaling: Impaired beta3-adrenergic, but unaffected LPA, PDGF and
EGF receptor signaling. Biochim Biophys Acta. 1803:983–989. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sedding DG and Braun-Dullaeus RC:
Caveolin-1: Dual role for proliferation of vascular smooth muscle
cells. Trends Cardiovasc Med. 16:50–55. 2006. View Article : Google Scholar : PubMed/NCBI
|