Introduction
Pancreatic cancer is one of the most aggressive
types of cancer. It is estimated that this disease caused over
38,000 deaths in the United States in 2013 (1). Pancreatic cancer arises from the
morphologically and genetically defined precursor lesions through a
step-wise accumulation of genetic alterations. In the majority of
patients diagnosed with the disease, symptoms do not develop until
the tumor is either unresectable or metastatic, rendering it
difficult to cure (2–4). Despite great advances in the
treatment of cancer, pancreatic cancer is still the fourth most
frequent cuase of cancer-related mortality in the Western world
(1,2). The 5-year survival for individuals
with pancreatic cancer is <5%, and conventional treatment
approaches, such as surgery, radiation, chemotherapy, or
combinations of these, have had little effect on the course of this
aggressive neoplasm (3–7). The low survival rate of patients
points towards an increased need for the development of novel
anticancer agents and effective combination therapies for the
treatment of pancreatic cancer.
The phorbol ester,
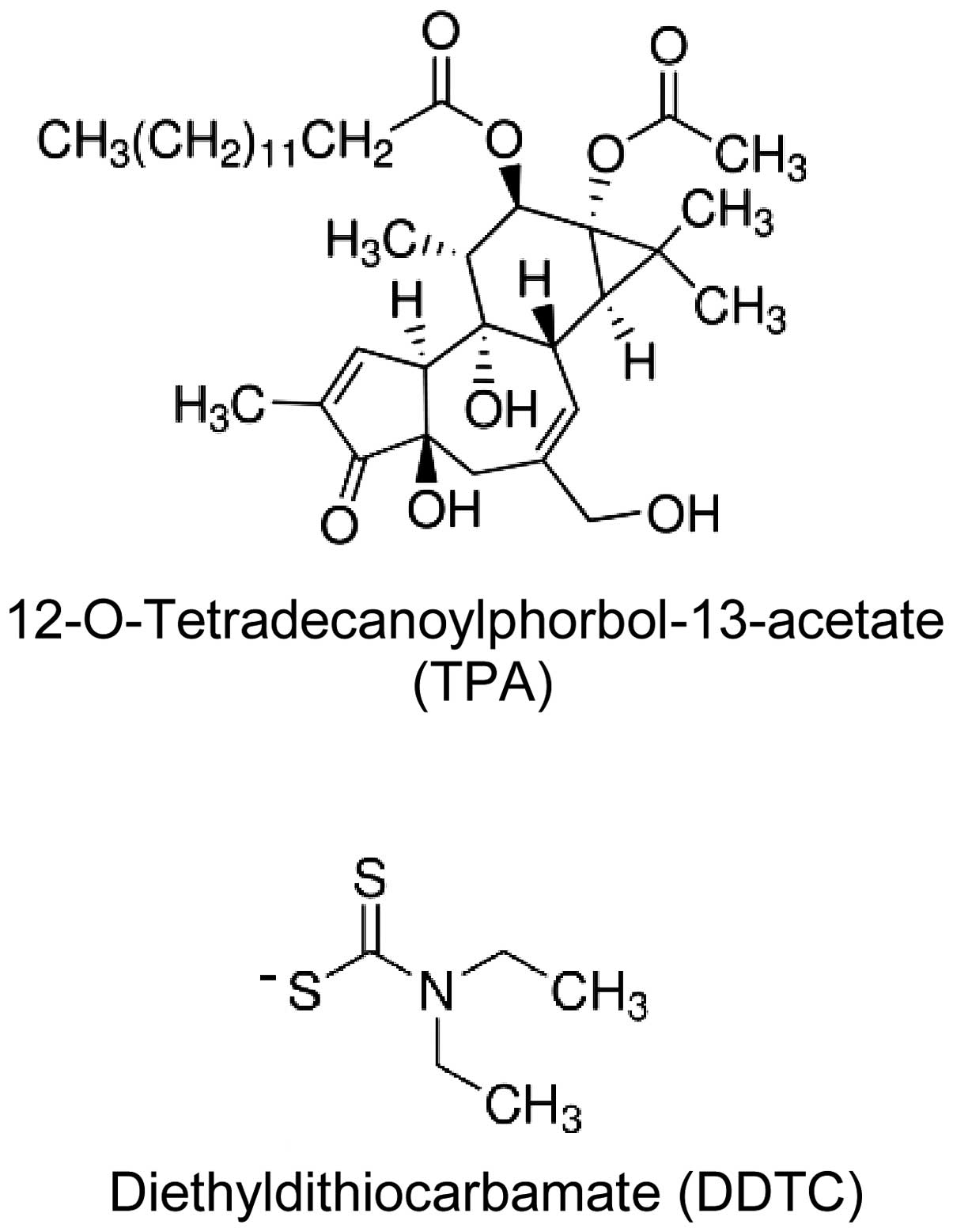
12-O-tetradecanoylphorbol-13-acetate (TPA) (Fig. 1), is a major active constituent of
the seed oil of Croton tiglium L., a leafy shrub of the
Euphorbiaceae family that is native to Southeastern Asia. In
previous studies by our group, we demonstrated the pharmacological
activity of TPA in myeloid leukemia patients with an acceptable
toxicity profile (8–10). Combinations with other agents have
been shown to enhance the anticancer effects of TPA in myeloid
leukemia and prostate cancer cells (11,12). Studies that have been carried out
by our laboratory team, as well as other investigators have
demonstrated that TPA inhibits the growth and induces the apoptosis
of cultured pancreatic cancer cells (13–17). The nuclear factor-κB (NF-κB)
transcription factor is constitutively activated in the majority of
pancreatic cancers and is involved in the regulation of many
aspects of tumor development and progression (18). Previous studies by our research
team have indicated that the inhibition of NF-κB enhances the
effects of TPA on leukemia and prostate cancer cells (19,20). Combination with pharmacological
inhibitors of NF-κB may thus be an effective approach with which to
increase the inhibitory effects of TPA on pancreatic cells.
Diethyldithiocarbamate (DDTC) (Fig. 1), a member of the dithiocarbamate
family, is a potent inhibitor of NF-κB (21,22). DDTC is a major metabolite of
disulfiram, an agent used in the treatment of alcoholism (23–25). Many clinical aspects of DDTC, such
as the treatment of metal toxicity and cancer, have been
investigated (26,27). Disulfiram, DDTC and pyrrolidine
dithiocarbamate (PDTC) are well-known inhibitors of NF-κB. These
compounds inhibit IκB phosphorylation, NF-κB nuclear translocation
and proteasome degradation (21,22,28,29). DDTC has also been shown to induce
the apoptosis of cancer cells (21,22,26). In addition, recent studies have
demonstrated that a complex constituted by DDTC and copper inhibits
the proliferation ofpancreatic cancer cells (30), and that DDTC synergistically
enhances the effects of gemcitabine on pancreatic cells (31). Based on this evidence, we thus
hypothesized that DDTC may inhibit the activation of NF-κB and may
thus enhance the anticancer activity of TPA in pancreatic cancer
cells.
The present study was undertaken to examine our
hypothesis that DDTC enhances the growth inhibitory and
apoptosis-promoting effects of TPA on pancreatic cancer cells. For
this purpose, we determined the effects of DDTC and TPA alone or in
combination on pancreatic cancer cells in conventional monolayer
cultures, as well as in 3 dimensional (3D) cultures. In addition,
the effects of TPA alone or in combination with DDTC on the growth
of PANC-1 xenograft tumors in NCr nude mice were determined. To the
best of our knowledge, the present study provides the first
evidence that DDTC inhibits NF-κB activity, decreases the
expression of Bcl-2 and enhances the inhibitory effects of TPA on
pancreatic cancer cell growth in vitro and in
vivo.
Materials and methods
Cells and reagents
The human pancreatic cancer cell lines, PANC-1, MIA
PaCa-2 and BxPC-3, were obtained from the American Type Culture
Collection (ATCC, Rockville, MD, USA). TPA was obtained from Alexis
Co. (San Diego, CA, USA) and DDTC was from Sigma-Aldrich (St.
Louis, MO, USA). The cells were maintained in Dulbecco’s modified
Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) that
was supplemented with penicillin (100 U/ml)-streptomycin (100
μg/ml) and L-glutamine (300 μg/ml). The cultured
cells were grown at 37°C in a humidified atmosphere of 5%
CO2 and were passaged twice a week.
Determination of the number of viable
cells
The number of viable cells after each treatment was
determined using a hemocytometer under a light microscope (Nikon
Optiphot, Tokyo, Japan). Cell viability was determined by the
trypan blue exclusion assay, which was performed by mixing 80
μl of cell suspension and 20 μl of 0.4% trypan blue
solution for 2 min. Blue cells were counted as dead cells and the
cells that did not absorb the dye were counted as live cells.
Assessment of apoptotic cells by
morphological analysis and by the activation of caspase-3
Apoptosis was determined by the morphological
assessment of the cells stained with propidium iodide using a
fluorescence microscope (Nikon Eclipse TE200; Nikon, Tokyo, Japan).
Apoptotic cells were identified by classical morphological
characteristics, including nuclear condensation, cell shrinkage and
the formation of apoptotic bodies (20). The activation of caspase-3 was
measured using an EnzoLyte AMC Caspase-3 Assay Fluorimetric kit
(AnaSpec, Fremont, CA, USA) following the instructions of the
manufacturer (32). Fluorescence
intensity was measured using a Tecan Infinite M200 plate reader
(Tecan US Inc., Durham, NC, USA).
NF-κB-dependent reporter gene expression
assay
NF-κB transcriptional activity was measured by
NF-κB-luciferase reporter gene expression assay. The
NF-κB-responsive luciferase construct was transiently transfected
into the PANC-1 cells by using Lipofectamine™ 2000 (Invitrogen Life
Technologies, Grand Island, NY, USA) following the manufacturer’s
instructions. The cells were then treated with DDTC or TPA alone or
in combination for 24 h, and the NF-κB-luciferase activities were
measured using the luciferase assay kits (E1500; Promega Madison,
WI, USA) according to the manufacturer’s instructions.
Western blot analysis
Following treatment with TPA, DDTC or a combination
of both, the cell lysates were prepared as described in a previous
study (12). Proteins were
subjected to sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and subsequently transferred onto
nitrocellulose membranes. After blocking the non-specific binding
sites with blocking buffer, the membranes were incubated overnight
at 4°C with Bcl-2 primary antibody (05-729; Millipore Corp.,
Billerica, MA, USA). β-actin was used as a loading control.
Following the removal of the primary antibody, the membranes were
washed 3 times with TBS (PBS containing 0.05% Tween-20) buffer at
room temperature and subsequently incubated with
fluorochrome-conjugated secondary antibody (sc-3738; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). Final detection was
performed using an Odyssey Infrared Imaging system (LI-COR
Biosciences, Lincoln, NE, USA).
3D cell culture
The PANC-1 cells were mixed with Matrigel
(Collaborative Research Inc., Bedford, MA, USA) on ice at a density
of 0.5×105 cells/ml. The Matrigel containing the PANC-1
cells was placed on a 12-well plate (1 ml/well) and incubated at
37°C for 2 h to allow the Matrigel to solidify. Subsequently, DMEM
was added to each well on top of the gel. The cells were incubated
for 24 h and then treated with DDTC or TPA alone or in combination
once every other day. On day 10, the 3D cultures were examined
under a microscope (Nikon Optiphot; Nikon) for the determination of
the formation of tissue-like structures.
Xenograft tumors in NCr nude mice
Male NCr nude mice (6–7 weeks old) were obtained
from Taconic Farms Inc. (Germantown, NY, USA). The animals were
housed in sterile filter-capped microisolator cages and provided
with sterilized food and water. The PANC-1 cells (2×106
cells/mouse) suspended in 50% Matrigel (Collaborative Research
Inc.) in DMEM were injected subcutaneously into the right flank of
the mice. When the tumors reached a moderate size (0.6–1.0 cm in
width and 0.6–1.0 cm in length), the mice received daily
intraperitoneal (i.p.) injections with solvent (controls) which
consisted of propylene glycol, polysorbate 80, benzyl alcohol,
ethanol and water (40:0.5:1:10:48.5; control), TPA (50 ng/g body
weight/day), DDTC (30 μg/g body weight/day) or a combination
of TPA (50 ng/g/day) and DDTC (30 μg/g/day) for 28 days.
Tumor size (length × width) and body weight were measured 3 times a
week. The animal experiments were carried out under an
Institutional Animal Care and Use Committee (IACUC)-approved
protocol (Rutgers University).
Tumor cell proliferation
The proliferation of the PANC-1 tumor cells was
determined by the expression of proliferating cell nuclear antigen
(PCNA) using immunohistochemical staining. In brief, tumors were
excised from each mouse and weighed at the end of the experiment.
Tumor tissues were fixed in buffered formalin for 24 h and then
with ethanol for 48 h. Paraffin blocks of tumor tissues were
prepared and paraffin sections of tumor tissues were processed for
immunohistochemical staining. The sections were incubated with PCNA
antibody (MAB424; Millipore Corp.) for 1 h at room temperature. The
sections were then incubated with a biotinylated secondary antibody
for 30 min followed by incubation with horseradish peroxidase
conjugated-avidin solution for 30 min using the Elite ABC kit
(PK-6100; Vector Laboratories, Burlingame, CA, USA). PCNA staining
in the tumor cells (brown color in nucleus) was examined under a
microscope (Nikon Optiphot; Nikon). At least 1,000 cells were
counted for each section.
Statistical analysis
The analysis of variance (ANOVA) method and the
Tukey-Kramer test were used for the comparison of viable cells,
apoptosis and NF-κB luciferase activity in the cultured pancreatic
cancer cells. These statistical methods were also used for the
comparison of tumor size and body weight among the different
treatment groups in the in vivo experiments. A P-value
<0.05 was considered to indicate a statistically signficant
difference.
Results
Effects of TPA and DDTC on the growth and
apoptosis of pancreatic cancer cells
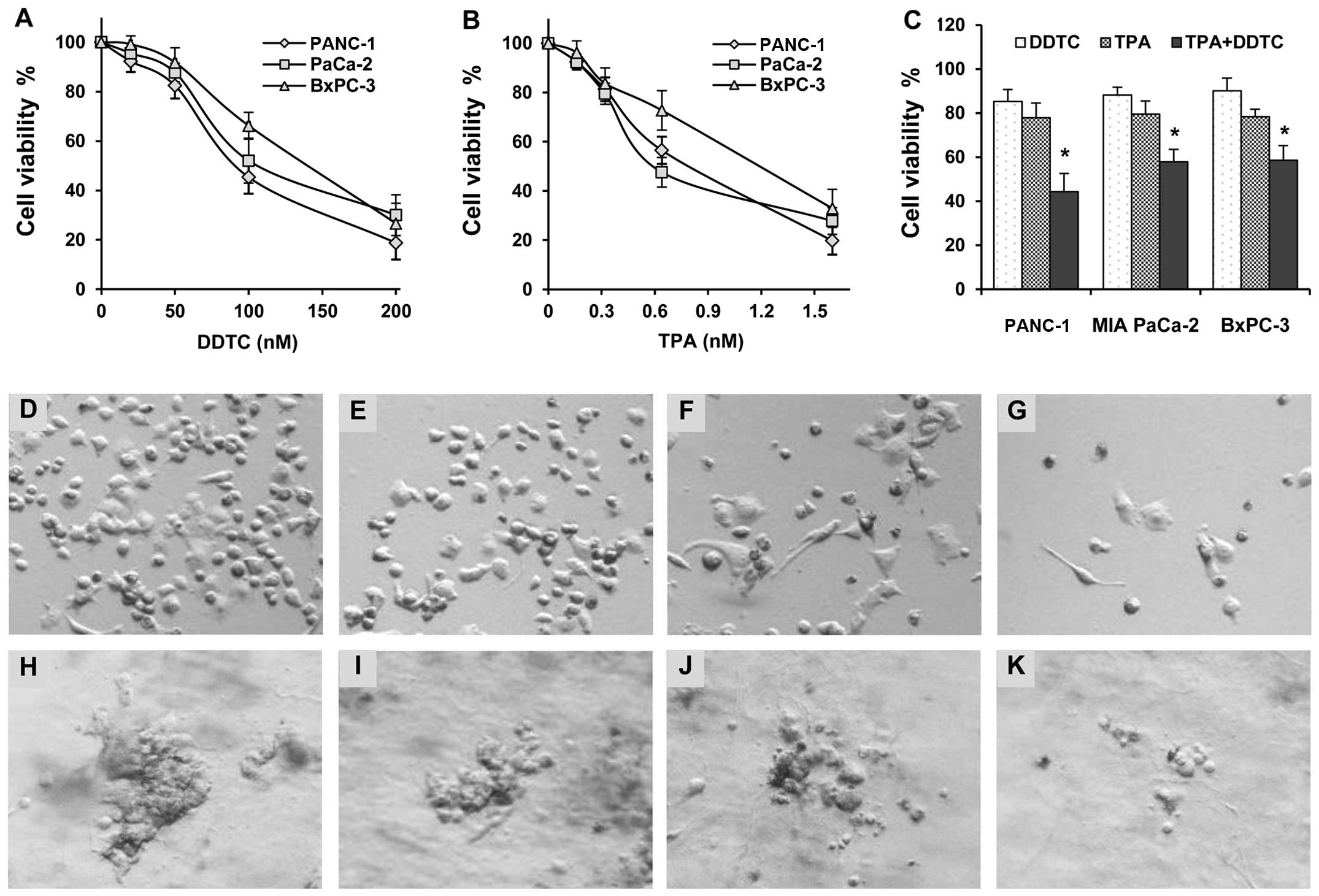
The effects of TPA and DDTC alone or in combination
on the growth of human pancreatic cancer cells were determined
using the trypan blue exclusion assay. Treatment of the human
prostate cancer cells, PANC-1, MIA PaCa-2 and BxPC-3, with TPA or
DDTC alone resulted in cancer cell growth inhibition in a
concentration-dependent manner (Fig.
2A and B). The PANC-1 cells were more sensitive than the MIA
PaCa-2 and BxPC-3 cells to the growth inhibitory effects induced by
TPA and DDTC (Fig. 2A and B). The
combination of DDTC and TPA had more potent inhibitory effects on
the growth of the cells than either agent alone (Fig. 2C). The number of viable PANC-1,
MIA PaCa-2 and BxPC-3 cells was significantly lower in the group
treated with the combination of both agents than in the groups
treated with either TPA or DDTC alone (P<0.001). We also
observed the morphology of the PANC-1 cells treated with TPA and/or
DDTC under a phase-contrast microscope (Fig. 2D–G). The effects of TPA and/or
DDTC on the apoptosis of the PANC-1 cells were determined by
morphological assessment and caspase-3 assay. Treatment with TPA or
DDTC alone resulted in a moderate increase in the number of
apoptotic cells (Table I). The
combination treatments with TPA and DDTC at various concentrations
had a more potent promoting effect on apoptosis than treatment with
either agent alone (Table I).
 | Table IEffects of TPA and DDTC alone or in
combination on the apoptosis of PANC-1 cells. |
Table I
Effects of TPA and DDTC alone or in
combination on the apoptosis of PANC-1 cells.
| Treatment | Apoptotic cells
(%) | Relative caspase-3
activity |
|---|
| Control | 2.1±0.2 | 1.0 |
| TPA (0.16 nM) | 4.0±0.3 | 1.7±0.2 |
| TPA (0.32 nM) | 7.2±0.3 | 4.1±0.5 |
| DDTC (20 nM) | 5.6±0.4 | 2.7±0.3 |
| DDTC (50 nM) | 10.7±1.1 | 5.2±0.5 |
| TPA (0.16 nM) +
DDTC (20 nM) | 19.7±2.6a | 9.8±0.8a |
| TPA (0.32 nM) +
DDTC (50 nM) | 32.5±2.4a | 13.0±1.0a |
Effects of TPA and DDTC on PANC-1 cells
in 3D culture
A 3D cell culture model was used to determine the
effects of TPA and DDTC alone or in combination on the formation
and growth of 3D tissue-like structures. The PANC-1 cells formed a
tissue-like morphology in 3D culture in the extracellular matrix
gel (Fig. 2H). Treatment with
DDTC or TPA alone had an inhibitory effect on the formation and
growth of tissue-like structures (Fig. 2I and J). DDTC and TPA in
combination had a more potent inhibitory effect on the formation of
tissue-like structures (Fig.
2K).
Effects of TPA and/or DDTC on NF-κB
activation and the expression of Bcl-2
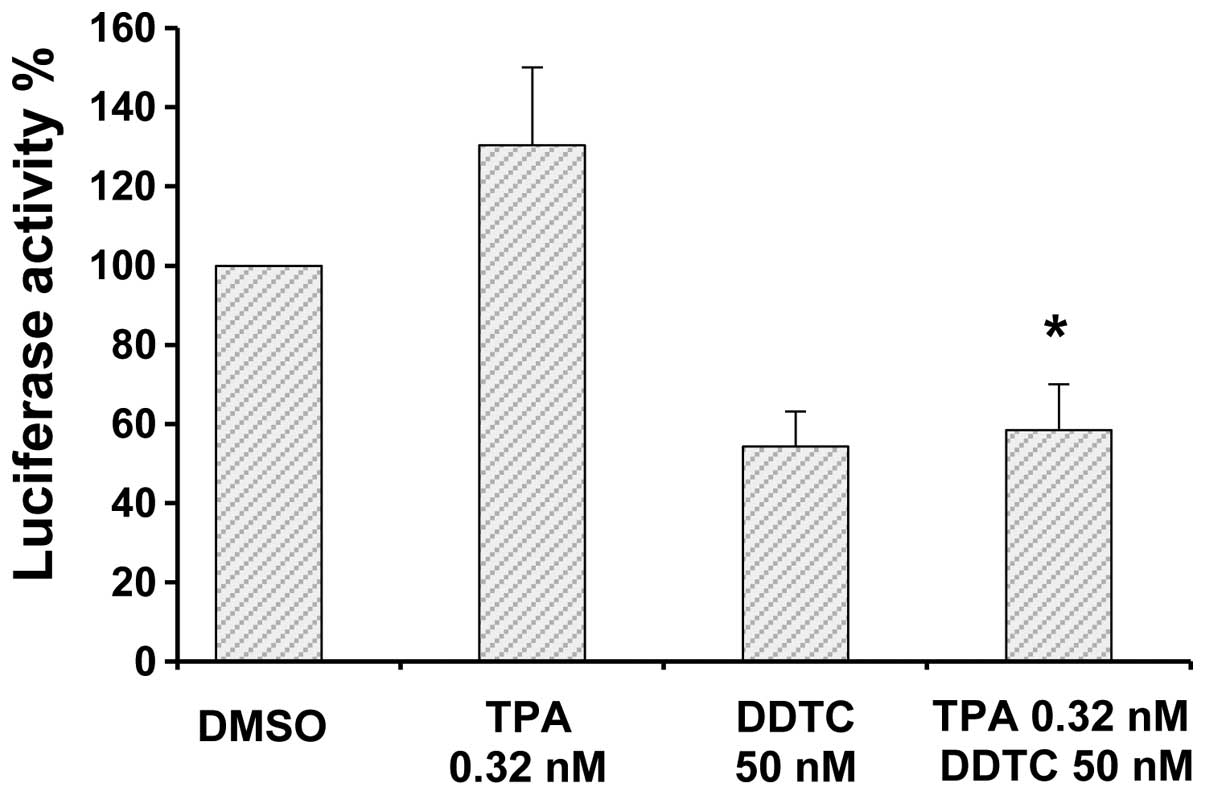
The effects of TPA and DDTC alone or in combination
on the activation of NF-κB were determined by luciferase reporter
gene expression assay. Treatment of the PANC-1 cells with DDTC
resulted in a marked decrease in NF-κB activity, while treatment
with TPA alone caused an increase in the activity of NF-κB
(Fig. 3). The stimulatory effects
of TPA on NF-κB were markedly suppressed by treatment with DDTC
(combination treatment; Fig. 3).
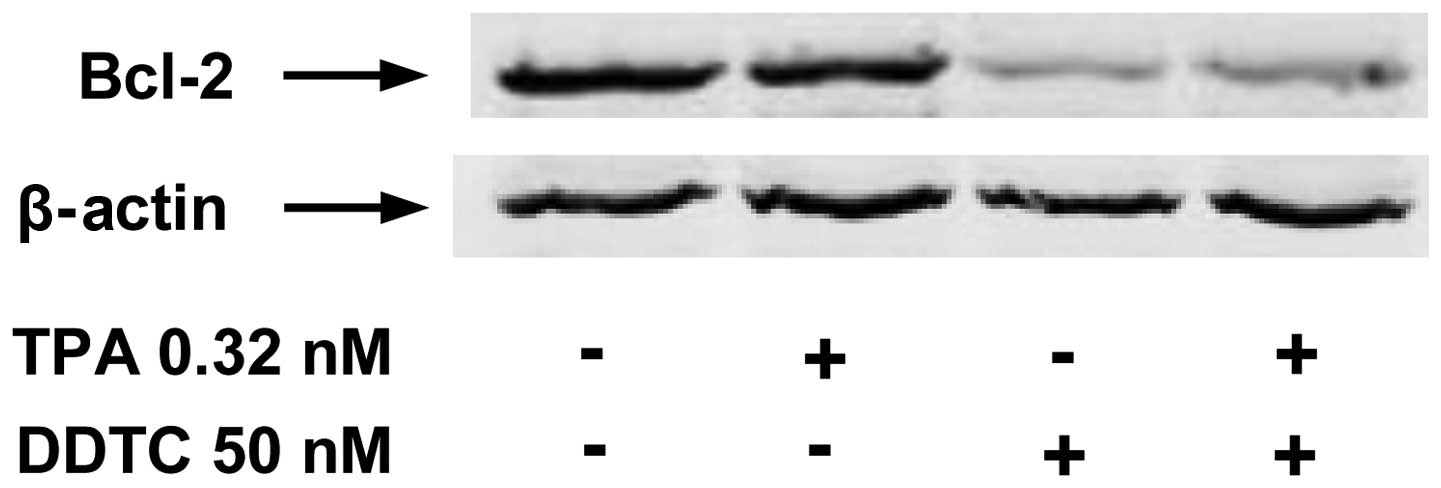
The expression of Bcl-2, a downstream target of the NF-κB pathway
was measured by western blot analysis. Treatment with TPA alone had
little or no effect on the level of Bcl-2 (Fig. 4). However, treatment of the PANC-1
cells with DDTC alone or in combination with TPA resulted in a
marked decrease in the level of Bcl-2 (Fig. 4).
Inhibitory effect of TPA or DDTC alone or
in combination on the growth of PANC-1 xenograft tumors in NCr nude
mice
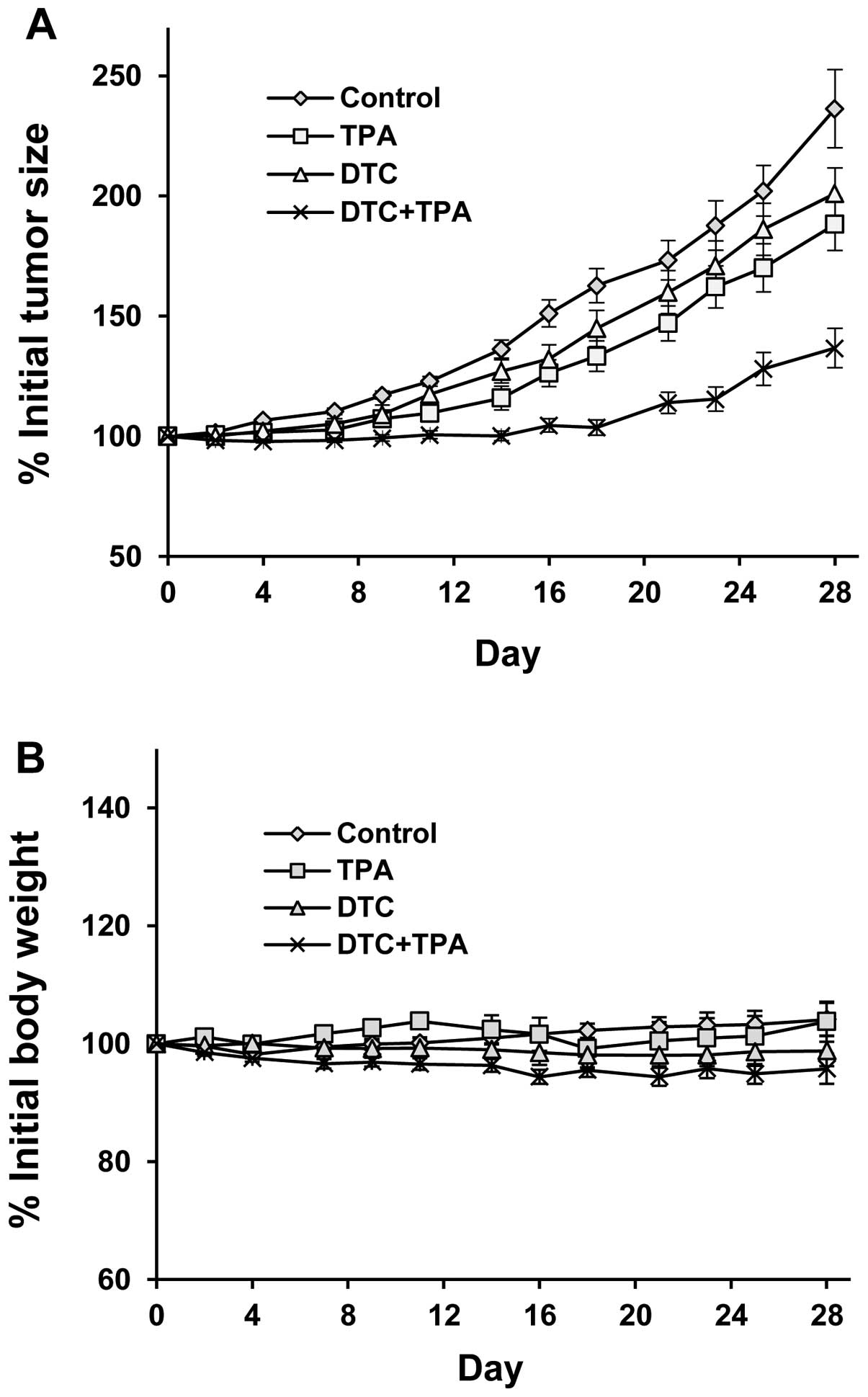
NCr nude mice bearing PANC-1 xenograft tumors were
treated with daily an i.p. injection of TPA or DDTC alone or a
combination of both for 28 days. Tumor growth was also observed in
the control group (Fig. 5A).
Treatment with i.p. injections of TPA in combination with DDTC had
a more prominent inhibitory effect on the growth of PANC-1 tumors
than either agent used individually (Fig. 5A). Statistical analysis using
ANOVA with the Tukey-Kramer multiple comparison test revealed that
the differences in the percentage of the initial tumor size at the
end of the experiment were statistically significant between the
control group and the group treated with the combination of both
agents (P<0.001), as well as between the control group and the
TPA-treated group (P<0.05). The percentage of the initial tumor
size in the group treated with the combination of both agents was
significantly smaller than that in the groups treated with TPA
alone (P<0.05) or DDTC alone (P<0.01). Treatment with TPA or
DDTC alone or in combination did not significantly affect the body
weight of the animals (Fig. 5B).
Statistical analysis using ANOVA with the Tukey-Kramer multiple
comparison test revealed that the difference in the percentage of
the initial body weight between any 2 groups was not statistically
significant (P>0.05).
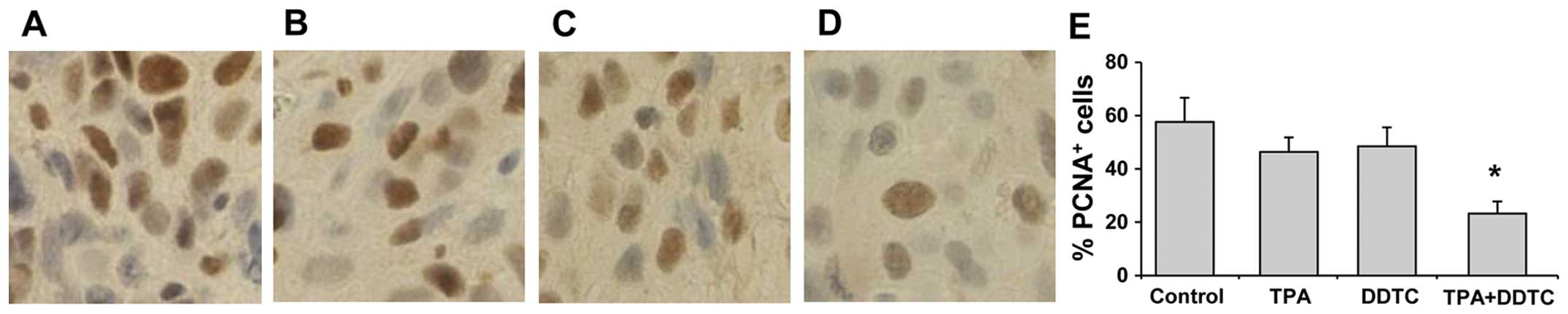
Inhibitory effects of TPA and/or DDTC on
cell proliferation in PANC-1 tumors
The effects of TPA and DDTC on PANC-1 tumor growth
were investigated by determining the expression of PCNA in the
tumor cells. Immunohistochemistry of PCNA in the paraffin-embedded
sections of PANC-1 tumors revealed that treatment of the mice with
TPA or DDTC alone reduced the number of PCNA-positive cells in the
tumors (Fig. 6B and C). Combined
treatment with TPA and DDTC had a more potent inhibitory effect on
the number of PCNA-positive cells than treatment with either agent
alone (Fig. 6D). The differences
in the number of PCNA-positive cells were statistically significant
between the group treated with the combination of both agents and
the group treated with TPA alone (P<0.01), as well as between
the group treated with the combination of both agents and the group
treated with DDTC alone (P<0.01; Fig. 6E).
Discussion
Although previous studies have shown that treatment
with TPA or DDTC alone inhibits pancreatic cancer cell growth
(13–17,30,31), to the best of our knowledge, the
effects and mechanisms of action of these two agents in combination
on the growth and the apoptosis of pancreatic cancer cells in
vitro and in vivo have not yet been reported. In the
present study, we demonstrated that TPA in combination with DDTC
exerted potent growth inhibitory and apoptosis-promoting effects on
pancreatic cancer cells. We also demonstrated that the combination
of both agents markedly inhibited the growth of PANC-1 xenograft
tumors in NCr nude mice. To the best of our knowledge, this is the
first study indicating a strong inhibitory effect of the
combination of TPA and DDTC on pancreatic cancer cell growth.
In the present study, we determined the effects of
TPA and DDTC alone or in combination on PANC-1 cells in 3D
cultures. Compared to conventional 2D monolayer cell cultures, the
3D culture system mimics the structural architecture and functional
differentiation of tumor tissues (33,34). It is well known that cell-cell and
cell-matrix interactions within the 3D microenvironment are
important to the physiological function and response of cancer
cells to anticancer agents (33,34). In the present study, we found that
PANC-1 cells formed a 3D tissue-like morphology in the
extracellular matrix Matrigel. Treatment of the PANC-1 cells with
TPA and DDTC in combination had a more prominent inhibitory effect
on the formation of a tissue-like morphology in the 3D cultures
than treatment with either agent alone.
The inhibition of NF-κB activation has been found to
enhance the anticancer activities of TPA in leukemia (19) and prostate cancer cells (20). NF-κB is an important cellular
regulator of growth and apoptosis. This transcription factor has
been connected with multiple aspects of oncogenesis, including the
control of apoptosis, the cell cycle, cell differentiation and cell
migration (18,35,36). A number of studies have indicated
that the activation of NF-κB suppresses cell death pathways and
that the activation of NF-κB is required to protect cells from the
apoptotic cascade. Chemotherapeutic agents, such as 5-fluorouracil
(5-FU), etoposide, docetaxel and gemcitabine have been reported to
activate NF-κB in cancer cells (37–40). The activation of NF-κB may be a
protective response to treatment with chemotherapeutic agents,
while the inhibition of NF-κB has been shown to enhance the
anticancer activity of chemotherapeutic agents (37–40). In the present study, luciferase
reporter assay revealed that TPA increased NF-κB activity.
Treatment with DDTC markedly inhibited NF-κB activity, decreased
the expression of Bcl-2 and enhanced the effects of TPA on the
PANC-1 cells. These findings indicate that TPA in combination with
pharmacological inhibitors of NF-κB may thus be an effective
strategy for improving the therapeutic efficacy of TPA in
pancreatic cancer.
Previous research by our team demonstrated that the
peak blood levels of TPA ± SD value in several patients who
received an intravenous (i.v.) infusion of TPA (0.125
mg/m2) was 1.75±0.55 ng/ml and ranged between 0.3 and
5.2 ng/ml. The concentrations of TPA used to obtain an inhibitory
effect on pancreatic cancer cells in the present study (0.1–1
ng/ml; 0.16–1.6 nM) are clinically achievable (9,41).
Concentrations of DDTC used in some previous in vitro
studies have ranged from nanomolar (nM) to micromolar (μM)
and the treatment time has varied between 30 min to 24 h (21,26,44,45). Instead of using a high
concentration and a short treatment time, we found that treatment
with lower concentrations (50–200 nM) of DDTC for 72 h markedly
inhibited the growth and induced the apoptosis of pancreatic cancer
cells. The concentrations of DDTC used in the present study were
much lower than the blood levels of DDTC in humans (42,43).
A strong inhibitory effect of TPA and DDTC on the
growth of PANC-1 xenograft tumors in nude mice was observed in the
present study. Treatment of NCr nude mice with i.p. injections of
TPA and DDTC in combination more potently inhibited the growth of
PANC-1 tumors than treatment with either agent alone.
Immunohistochemical analysis revealed that cancer cell growth
(proliferation), as reflected by the expression of PCNA, was
significantly lower in the tumors from the mice treated with TPA +
DDTC than in the tumors from the mice treated with either TPA or
DDTC alone. At the doses used in the present study, TPA and DDTC
alone or in combination appeared to be non-toxic as no differences
in body weight were observed in the animals following treatment.
Furthermore, no abnormalities were observed in the major organs at
the end of the experiment (data not shown). Further studies are
required to establish the plasma levels of TPA and DDTC in relation
to their combined inhibitory effect on pancreatic tumors in
suitable animal models.
In conclusion, in the present study, we demonstrated
that TPA in combination with DDTC markedly inhibited pancreatic
cancer cell growth and induced the apoptosis of human pancreatic
cancer cells. In addition, we found that treatment of NCr nude mice
with a combination of TPA and DDTC inhibited the growth of
xenograft PANC-1 tumors. TPA in combination with pharmacological
inhibitors of NF-κB, such as DDTC, may thus be an effective
approach with which to inhibit the growth of pancreatic cancer.
Acknowledgments
The present study was supported by grants from the
Guangdong Province Leadership Project, the Rutgers Cancer Institute
of New Jersey (CCSG P30-CA072720 RSD), the China National Science
Foundation (81272452 and 21102020), the Guangdong Province Project
(2012B091100342), and the Guangzhou City Project (2013J4500014).
The authors dedicate this study to Dr Allan H. Conney, an
outstanding and widely recognized cancer researcher who passed away
on September 10, 2013.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang DX, Dai YD, Yuan SX and Tao L:
Prognostic factors in patients with pancreatic cancer. Exp Ther
Med. 3:423–432. 2012.PubMed/NCBI
|
|
3
|
Cao H, Le D and Yang LX: Current status in
chemotherapy for advanced pancreatic adenocarcinoma. Anticancer
Res. 33:1785–1791. 2013.PubMed/NCBI
|
|
4
|
Arslan C and Yalcin S: Current and future
systemic treatment options in metastatic pancreatic cancer. J
Gastrointest Oncol. 5:280–295. 2014.PubMed/NCBI
|
|
5
|
Di Marco M, Di Cicilia R, Macchini M,
Nobili E, Vecchiarelli S, Brandi G and Biasco G: Metastatic
pancreatic cancer: Is gemcitabine still the best standard
treatment? (Review). Oncol Rep. 23:1183–1192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Felice F, Musio D, Raffetto N and
Tombolini V: Neoadjuvant strategy as initial treatment in
resectable pancreatic cancer: concrete evidence of benefit.
Anticancer Res. 34:4673–4676. 2014.PubMed/NCBI
|
|
7
|
Yutani S, Komatsu N, Yoshitomi M, Matsueda
S, Yonemoto K, Mine T, Noguchi M, Ishihara Y, Yamada A, Itoh K and
Sasada T: A phase II study of a personalized peptide vaccination
for chemotherapy-resistant advanced pancreatic cancer patients.
Oncol Rep. 30:1094–1100. 2013.PubMed/NCBI
|
|
8
|
Han ZT, Zhu XX, Yang RY, Sun JZ, Tian GF,
Liu XJ, Cao GS, Newmark HL, Conney AH and Chang RL: Effect of
intravenous infusions of 12-O-tetradecanoylphorbol-13-acetate (TPA)
in patients with myelocytic leukemia: preliminary studies on
therapeutic efficacy and toxicity. Proc Natl Acad Sci USA.
95:5357–5361. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Strair RK, Schaar D, Goodell L, Aisner J,
Chin KV, Eid J, Senzon R, Cui XX, Han ZT, Knox B, Rabson AB, Chang
R and Conney A: Administration of a phorbol ester to patients with
hematological malignancies: preliminary results from a phase I
clinical trial of 12-O-tetradecanoylphorbol-13-acetate. Clin Cancer
Res. 8:2512–2518. 2002.PubMed/NCBI
|
|
10
|
Schaar D, Goodell L, Aisner J, Cui XX, Han
ZT, Chang R, Martin J, Grospe S, Dudek L, Riley J, Manago J, Lin Y,
Rubin EH, Conney A and Strair RK: A phase I clinical trial of
12-O-tetradecanoylphorbol-13-acetate for patients with
relapsed/refractory malignancies. Cancer Chemother Pharmacol.
57:789–795. 2006. View Article : Google Scholar
|
|
11
|
Zheng X, Ryan A, Patel N, Klemons S,
Hansson A, Shih WJ, Lin Y, Huberman E, Chang RL and Conney AH:
Synergistic stimulatory effect of
12-O-tetradecanoylphorbol-13-acetate and capsaicin on macrophage
differentiation in HL-60 and HL-525 human myeloid leukemia cells.
Int J Oncol. 26:441–448. 2005.PubMed/NCBI
|
|
12
|
Zheng X, Chang RL, Cui XX, Avila GE,
Hebbar V, Garzotto M, Shih WJ, Lin Y, Lu SE, Rabson AB, Kong AN and
Conney AH: Effects of 12-O-tetradecanoylphorbol-13-acetate (TPA) in
combination with paclitaxel (Taxol) on prostate Cancer LNCaP cells
cultured in vitro or grown as xenograft tumors in immunodeficient
mice. Clin Cancer Res. 12:3444–3451. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Avila GE, Zheng X, Cui XX, Ryan AD,
Hansson A, Suh J, Rabson AB, Chang RL, Shih WJ, Lin Y, Crowell P,
Lu YP, Lou YR and Conney AH: Inhibitory effects of
12-O-tetradecanoylphorbol-13-acetate alone or in combination with
all-trans retinoic acid on the growth of cultured human pancreas
cancer cells and pancreas tumor xenografts in immunodeficient mice.
J Pharmacol Exp Ther. 315:170–187. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bond JA, Gescher AJ, Verschoyle RD,
Lemoine NR, Errington R, Wiltshire M, Smith PJ and Wynford Thomas
D: Cytotoxic action of phorbol esters on human pancreatic cancer
cells. Int J Cancer. 121:1445–1454. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Salabat MR, Ding XZ, Flesche JB, Ujiki MB,
Robin TP, Talamonti MS, Bell RH Jr and Adrian TE: On the mechanisms
of 12-O-tetradecanoylphorbol-13-acetate-induced growth arrest in
pancreatic cancer cells. Pancreas. 33:148–155. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Detjen KM, Brembeck FH, Welzel M, Kaiser
A, Haller H, Wiedenmann B and Rosewicz S: Activation of protein
kinase C alpha inhibits growth of pancreatic cancer cells via
p21(cip)-mediated G(1) arrest. J Cell Sci. 113:3025–3035. 2000.
|
|
17
|
Zheng X, Cui XX, Gao Z, Verano M, Huang
MT, Liu Y, Rabson AB and Conney AH: Effects of
12-O-tetradecanoylphorbol-13-acetate in combination with
gemcitabine on Panc-1 pancreatic cancer cells cultured in vitro or
Panc-1 tumors grown in immunodeficient mice. Int J Oncol.
41:2269–2275. 2012.PubMed/NCBI
|
|
18
|
Carbone C and Melisi D: NF-κB as a target
for pancreatic cancer therapy. Expert Opin Ther Targets. 16(Suppl
2): S1–S10. 2012. View Article : Google Scholar
|
|
19
|
Hansson A, Marín YE, Suh J, Rabson AB,
Chen S, Huberman E, Chang RL, Conney AH and Zheng X: Enhancement of
TPA-induced growth inhibition and apoptosis in myeloid leukemia
cells by BAY 11-7082, an NF-kappaB inhibitor. Int J Oncol.
27:941–948. 2005.PubMed/NCBI
|
|
20
|
Zheng X, Chang RL, Cui XX, Avila G, Huang
MT, Liu Y, Kong AN, Rabson AB and Conney AH: Inhibition of
NF-kappaB by (E)3-[(4-methylphenyl)-sulfonyl]-2-propenenitrile
(BAY11-7082; BAY) is associated with enhanced
12-O-tetradecanoylphorbol-13-acetate-induced growth suppression and
apoptosis in human prostate cancer PC-3 cells. Int J Oncol.
32:257–264. 2008.
|
|
21
|
Matsuno T, Kariya R, Yano S, Morino-Koga
S, Taura M, Suico MA, Shimauchi Y, Matsuyama S, Okamoto Y, Shuto T,
Kai H and Okada S: Diethyldithiocarbamate induces apoptosis in
HHV-8-infected primary effusion lymphoma cells via inhibition of
the NF-κB pathway. Int J Oncol. 40:1071–1078. 2012.
|
|
22
|
Cvek B and Dvorak Z: Targeting of nuclear
factor-kappaB and proteasome by dithiocarbamate complexes with
metals. Curr Pharm Des. 13:3155–3167. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Suh JJ, Pettinati HM, Kampman KM and
O’Brien CP: The status of disulfiram: a half of a century later. J
Clin Psychopharmacol. 26:290–302. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eneanya DI, Bianchine JR, Duran DO and
Andresen BD: The actions of metabolic fate of disulfiram. Annu Rev
Pharmacol Toxicol. 21:575–596. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Langeland BT and McKinley-McKee JS: The
effects of disulfiram on equine hepatic alcohol dehydrogenase and
its efficiency against alcoholism: vinegar effect. Alcohol Alcohol.
31:75–80. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pang H, Chen D, Cui QC and Dou QP: Sodium
diethyldithiocarbamate, an AIDS progression inhibitor and a
copper-binding compound, has proteasome-inhibitory and
apoptosis-inducing activities in cancer cells. Int J Mol Med.
19:809–816. 2007.PubMed/NCBI
|
|
27
|
Bradberry SM and Vale JA: Therapeutic
review: do diethyldi-thiocarbamate and disulfiram have a role in
acute nickel carbonyl poisoning? J Toxicol Clin Toxicol.
37:259–264. 1999. View Article : Google Scholar
|
|
28
|
Lövborg H, Oberg F, Rickardson L, Gullbo
J, Nygren P and Larsson R: Inhibition of proteasome activity,
nuclear factor-KappaB translocation and cell survival by the
antialcoholism drug disulfiram. Int J Cancer. 118:1577–1580. 2006.
View Article : Google Scholar
|
|
29
|
Zhang JJ, Xu ZM, Zhang CM, Dai HY, Ji XQ,
Wang XF and Li C: Pyrrolidine dithiocarbamate inhibits nuclear
factor-κB pathway activation, and regulates adhesion, migration,
invasion and apoptosis of endometriotic stromal cells. Mol Hum
Reprod. 17:175–181. 2011. View Article : Google Scholar
|
|
30
|
Han J, Liu L, Yue X, Chang J, Shi W and
Hua Y: A binuclear complex constituted by diethyldithiocarbamate
and copper(I) functions as a proteasome activity inhibitor in
pancreatic cancer cultures and xenografts. Toxicol Appl Pharmacol.
273:477–483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dalla PE, Donadelli M, Costanzo C,
Zaniboni T, Dando I, Franchini M, Arpicco S, Scarpa A and Palmieri
M: Gemcitabine response in pancreatic adenocarcinoma cells is
synergistically enhanced by dithiocarbamate derivatives. Free Radic
Biol Med. 50:926–933. 2011. View Article : Google Scholar
|
|
32
|
Wei X, Du ZY, Cui XX, Verano M, Mo RQ,
Tang ZK, Conney AH, Zheng X and Zhang K: Effects of cyclohexanone
analogues of curcumin on growth, apoptosis and NF-κB activity in
PC-3 human prostate cancer cells. Oncol Lett. 4:279–284.
2012.PubMed/NCBI
|
|
33
|
Tsunoda T, Ishikura S, Doi K, Matsuzaki H,
Iwaihara Y and Shirasawa S: Resveratrol induces luminal apoptosis
of human colorectal cancer HCT116 cells in three-dimensional
culture. Anticancer Res. 34:4551–4555. 2014.PubMed/NCBI
|
|
34
|
Akeda K, Nishimura A, Satonaka H, Shintani
K, Kusuzaki K, Matsumine A, Kasai Y, Masuda K and Uchida A:
Three-dimensional alginate spheroid culture system of murine
osteosarcoma. Oncol Rep. 22:997–1003. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Z and Rigas B: NF-κB, inflammation
and pancreatic carcinogenesis: NF-κB as a chemoprevention target
(Review). Int J Oncol. 29:185–192. 2006.PubMed/NCBI
|
|
36
|
Hu YQ, Si LJ, Ye ZS, Lin ZH and Zhou JP:
Inhibitory effect of ARHI on pancreatic cancer cells and NF-κB
activity. Mol Med Rep. 7:1180–1184. 2013.PubMed/NCBI
|
|
37
|
Kwon OH, Kim JH, Kim SY and Kim YS:
TWEAK/Fn14 signaling mediates gastric cancer cell resistance to
5-fluorouracil via NF-κB activation. Int J Oncol. 44:583–590.
2014.
|
|
38
|
Kaewpiboon C, Srisuttee R, Malilas W, Moon
J, Kaowinn S, Cho IR, Johnston RN, Assavalapsakul W and Chung YH:
Extract of Bryophyllum laetivirens reverses etoposide resistance in
human lung A549 cancer cells by downregulation of NF-κB. Oncol Rep.
31:161–168. 2014.
|
|
39
|
Fujioka S, Son K, Onda S, Schmidt C,
Scrabas GM, Okamoto T, Fujita T, Chiao PJ and Yanaga K:
Desensitization of NFκB for overcoming chemoresistance of
pancreatic cancer cells to TNF-α or paclitaxel. Anticancer Res.
32:4813–4821. 2012.PubMed/NCBI
|
|
40
|
Zhang W, Chen H, Liu DL, Li H, Luo J,
Zhang JH, Li Y, Chen KJ, Tong HF and Lin SZ: Emodin sensitizes the
gemcitabine-resistant cell line Bxpc-3/Gem to gemcitabine via
downregulation of NF-κB and its regulated targets. Int J Oncol.
42:1189–1196. 2013.PubMed/NCBI
|
|
41
|
Cui XX, Chang RL, Zheng X, Woodward D,
Strair R and Conney AH: A sensitive bioassay for measuring blood
levels of 12-O-tetradecanoylphorbol-13-acetate (TPA) in patients:
preliminary pharmacokinetic studies. Oncol Res. 13:169–174.
2002.
|
|
42
|
Gandara DR, Nahhas WA, Adelson MD,
Lichtman SM, Podczaski ES, Yanovich S, Homesley HD, Braly P, Ritch
PS, Weisberg SR, et al: Randomized placebo-controlled multicenter
evaluation of diethyldithiocarbamate for chemoprotection against
cisplatin-induced toxicities. J Clin Oncol. 13:490–496.
1995.PubMed/NCBI
|
|
43
|
Qazi R, Chang AY, Borch RF, Montine T,
Dedon P, Loughner J and Bennett JM: Phase I clinical and
pharmacokinetic study of diethyldithiocarbamate as a chemoprotector
from toxic effects of cisplatin. J Natl Cancer Inst. 80:1486–1488.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mashiba H and Matsunaga K: Augmented
inhibition of MethA tumor cell proliferation in combined use of
diethyldithiocarbamate with catalase or by a nondialysable fraction
from co-incubation. Toxicol Lett. 66:97–104. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Han YH, Moon HJ, You BR, Kim SZ, Kim SH
and Park WH: The effects of buthionine sulfoximine,
diethyldithiocarbamate or 3-amino-1,2,4-triazole on propyl
gallate-treated HeLa cells in relation to cell growth, reactive
oxygen species and glutathion. Int J Mol Med. 24:261–268.
2009.PubMed/NCBI
|