Introduction
Inflammation, acts physiologically to protect normal
host function. It is a complex defense response to eliminate
foreign pathogens or damaged cells, and to initiate tissue healing
involving the activation of various immune cells. Inflammation is
mediated by a number of signaling molecules, including
pro-inflammatory mediators and cytokines primarily generated
through overactivated macrophages (1,2).
The induction of pro-inflammatory gene expression in activated
macrophages in response to a variety of stimuli, such as
lipopolysaccharide (LPS), is mediated by the activation of cellular
signaling pathways, such as nuclear factor-κB (NF-κB),
phosphatidylinositiol 3-kinase (PI3K)/Akt and mitogen-activated
protein kinases (MAPKs) (3,4).
NF-κB is a transcription factor that plays a central role in the
regulation of a number of inflammatory-related cytotoxic factors,
including inducible nitric oxide (NO) synthase (iNOS) and
cyclooxygenase-2 (COX-2), and pro-inflammatory cytokines, including
tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β). The
numerous pathway components, including PI3K/Akt and MAPKs, such as
extracellular signal-regulated kinase (ERK), c-Jun NH2-terminal
kinase (JNK) and p38 MAPK play a crucial role in the expression of
pro-inflammatory genes during the inflammatory process through the
regulation of the nuclear translocation of NF-κB (5,6).
Therefore, treatments aimed at inhibiting the NF-κB, PI3K/Akt and
MAPK signaling pathways may be a feasible approach in the treatment
of certain inflammatory diseases.
Several lines of evidence have revealed the
participation of reactive oxygen species (ROS) in inflammation.
Under physiological conditions, ROS are formed as a natural
byproduct of the normal metabolism of oxygen and play important
roles in cell signaling and homeostasis. However, the excessive
production of ROS, which may trigger a cascade of radical
reactions, causes oxidative damage to cellular macromolecules,
thereby resulting in significant damage to cell structures
(4,7). During oxidative stress, various
antioxidants and cytoprotective proteins, termed phase-2
detoxification enzymes, provide an anti-oxidant defense mechanism
against oxidative stress-induced cellular damage. Heme oxygenase-1
(HO-1) is a representative phase-2 detoxification enzyme with
anti-inflammatory, antioxidant and cytoprotective functions that
are regulated by the activation of nuclear factor
(erythroid-derived 2)-like 2 (Nrf2), which interacts with the
antioxidant response element (ARE) (8–10).
A wide variety of natural compounds have been shown to activate
Nrf2 and its downstream target genes, such as HO-1 for
cytoprotection against ROS generation and cellular damage (11,12). Thus, ROS, as well as Nrf2/HO-1
signaling may be an interesting target for the prevention or
therapy of inflammation-related diseases.
Ginseng, the root of Panax ginseng C.A.
Meyer, is a well-known medicinal herb in used in traditional Asian
medicine for over 2,000 years. It is held in high regard for its
tonic properties. Ginseng has been reported to have a variety of
biological properties, including antioxidant, anti-inflammatory,
anti-allergic, anti-diabetic, immunomodulatory and antitumor
properties, as well as cardiovascular and neuroprotective
properties (13–21). Accumulating evidence has indicated
that most of the pharmacological functions of ginseng are
attributed to a variety of ginsenosides, which are triterpenoid
saponins (22). Wild ginseng is
not field-cultivated domestically, but grows naturally and is
harvested from wherever is found. Commonly, cultivated ginseng is
systematically farmed on open land and harvested after a 4–6-year
cultivation period. On the other hand, wild ginseng grows in the
wild and deep in the mountains with fluctuating daily temperatures
and minor exposure to direct sunlight and is very difficult to
excavate. It is widely accepted that differences in environmental
exposure may result in a variation of the bioactive compounds
present in wild ginseng, known to be pharmacologically more
effective than field-cultivated ginseng. For example, it has been
shown that the concentrations of saponins found in wild ginseng are
generally 2-6-fold higher than those of field-cultivated ginseng
(23). Moreover, previous studies
have indicated that wild ginseng extract enhances the host defense,
and has more prominent anti-inflammatory and anticancer activities
than cultivated ginseng (24,25). Additionally, wild ginseng may be
more effective as an anxiolytic and anti-depressant than cultivated
ginseng (26). However, wild
ginseng is dilatory in growth and is more sensitive to
environmental changes than cultivated ginseng. The resultant low
yields and high costs hamper the efforts to meet the increasing
market demand, and thus the use of wild ginseng in traditional
medicine is very limited. On the other hand, cultured wild ginseng
roots, the substitute for natural wild ginseng, are easily obtained
by a plant cell culture technique. Cultured wild ginseng has been
shown to contain similar or higher levels of ginsenosides than
those found in cultivated ginseng, but in different ginsenoside
ratios (27,28).
In this study, the anti-inflammatory properties of
total saponins extracted from cultured wild ginseng roots (TSWG)
were investigated. We examined the effects of TSWG on the
production of NO and pro-inflammatory cytokines, such as TNF-α and
IL-1β, in LPS-stimulated mouse RAW 264.7 macrophages. We also
determined whether there is a link between the activation of the
NF-κB and MAPK signaling pathways and the anti-inflammatory
properties of TSWG. Additionally, we investigated whether TSWG has
any effect on the LPS-induced generation of ROS and the Nrf2/HO-1
signaling pathway.
Materials and methods
Preparation of TSWG
The tissue cultured wild ginseng adventitious roots
were supplied by the Research Center for the Development of
Advanced Horticultural Technology, Chunbuk National University
(Cheongju, Korea), where large-scale cultures of wild ginseng
adventitious roots have been developed. Air-dried roots of cultured
wild ginseng were ground to pass through an 80-mesh sieve. The
samples were extracted twice with methanol by refluxing at 80°C for
2 h, and then the methanol extract was suspended in water and
partitioned sequentially with n-hexane, chloroform, ethyl
acetate and n-butanol. Subsequently, the water-saturated
n-butanol fraction was evaporated to dryness in a vacuum.
The recovered crude saponins were loaded onto a Diaion®
HP-20 MCI gel (Sigma-Aldrich Chemical Co., St. Louis, MO, USA), and
the sugar residues were then removed with 40% CH3OH. The
fractions were eluted with 60–80% CH3OH, collected and
then dried to obtain TSWG. TSWG was diluted with the medium, to the
desired concentration prior to use.
Cell culture and MTT assay
RAW 264.7 murine macrophage-like cells were
purchased from American Tissue Culture Collection (ATCC, Manassas,
VA, USA). The cells were grown in Dulbecco’s modified Eagle’s
medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1%
(v/v) penicillin (100 U/ml)/streptomycin (100 μg/ml) under a
humidified condition of 5% CO2 at 37°C. An inflammatory
response was induced in the RAW 264.7 cells by LPS (purchased from
Sigma-Aldrich Chemical Co.). DMEM, FBS and penicillin/streptomycin
were purchased from Gibco-BRL (Grand Island, NY, USA). Cell
viability was measured based on the formation of blue formazan that
is metabolized from colorless
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Sigma-Aldrich Chemical Co.) by mitochondrial dehydrogenases, which
are active only in live cells. Briefly, the RAW 264.7 cells
(5×105 cells/ml) were seeded in a 96-well plate. The
cells were pre-treated with various concentrations of TSWG for 1 h
and then stimulated with 100 ng/ml LPS for 24 h. Following
incubation with TSWG and LPS, the cultured medium was changed to
fresh medium and the cells were incubated with 0.5 mg/ml MTT
solution for 3 h. The supernatant was discarded and the formazan
blue, which was formed in the cells, was dissolved with dimethyl
sulfoxide (DMSO). The optical density was measured at 540 nm using
an enzyme-linked immunosorbent assay (ELISA) plate reader (Dynatech
Laboratories, Chantilly, VA, USA).
Measurement of NO production
The concentrations of NO in the culture supernatants
were determined by measuring the levels of nitrite, which is a
major stable product of NO, using Griess reagent (Sigma-Aldrich
Chemical Co.). For this purpose, the RAW 264.7 cells were seeded in
each well of a 96-well plate. The cells were pre-treated with the
indicated concentrations of TSWG for 1 h and then stimulated with
100 ng/ml LPS. Following incubation for 24 h, the supernatant of
each well was mixed with the same volume of Griess reagent, for 10
min at room temperature in the dark. Nitrite levels were determined
using an ELISA plate reader at 540 nm, and nitrite concentrations
were calculated by referencing a standard curve generated with
known concentrations of sodium nitrite.
Measurement of TNF-α and IL-1β
production
The inhibitory effects of TSWG on the production of
TNF-α and IL-1β were examined using ELISA kits (R&D Systems
Inc., Minneapolis, MN, USA). The cell culture conditions were the
same as those used for the nitrite measurement assay. Following
incubation with TSWG and LPS for 24 h, the concentrations of TNF-α
and IL-1β in the culture medium were determined using a selective
ELISA kit, according to the manufacturer’s instructions.
Reverse transcription-polymerase chain
reaction (RT-PCR)
The cells were either incubated with TSWG (400
μg/ml) alone for 24 h, or pre-treated with the indicated
concentrations of TSWG for 1 h prior to stimulation with LPS for 24
h. Total RNA from the cultured cells was isolated using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA). cDNA was synthesized from
1 μg of total RNA, using AccuPower® RT PreMix
(Bioneer, Daejeon, Korea) containing moloney murine leukemia virus
reverse transcriptase. iNOS, IL-1β and TNF-α genes were amplified
from the cDNA by PCR. A sample of each amplified product was
subjected to 1.0% agarose gel electrophoresis and stained with
ethidium bromide (EtBr, Sigma-Aldrich Chemical Co.) and visualized
using ultraviolet (UV) illumination. The glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) housekeeping gene transcript was used as a
control. The primers used for PCR were as follows: iNOS forward,
5′-ATG TCC GAA GCA AAC ATC AC-3′ and reverse, 5′-TAA TGT CCA GGA
AGT AGG TG-3′; IL-1β forward, 5′-GGG CTG CTT CCA AAC CTT TG-3′ and
reverse, 5′-GCT TGG GAT CCA CAC TCT CC-3′; TNF-α forward, 5′-TCT
CAT CAG TTC TAT GGC CC-3′ and reverse, 5′-GGG AGT AGA CAA GGT ACA
AC-3′; and GAPDH forward, 5′-AGG CCGGTG CTG AGT ATG TC-3′ and
reverse, 5′-TGC CTG CTT CAC CAC CTT CT-3′.
Protein extraction and western blot
analysis
For total protein extraction, the cells were lysed
in lysis buffer [25 mM Tris-Cl (pH 7.5), 250 mM NaCl, 5 mM
ethylenediaminetetraacetic acid (EDTA), 1% NP-40, 1 mM
phenylmethylsulfonyl fluoride (PMSF) and 5 mM dithiothreitol (DTT)]
for 1 h. The insoluble materials were discarded by centrifugation
at 13,000 x g for 20 min at 4°C. In a parallel experiment, nuclear
and cytosolic proteins were prepared using nuclear extraction
reagents (Pierce, Rockford, IL, USA) according to the
manufacturer’s instructions. The protein concentration of the cell
lysate was determined using detergent-compatible protein assay
obtained from Bio-Rad (Hercules, CA, USA). Equal amounts of protein
were separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE). The separated protein was transferred
onto nitrocellulose membranes (Schleicher & Schuell BioScience,
Inc., Keene, NH, USA) and subsequently blocked with Tris-buffered
saline (10 mM Tris-Cl, pH 7.4) containing 0.5% Tween-20 and 5%
non-fat dry milk for 1 h at room temperature. The proteins were
probed with primary antibodies at 4°C overnight. After probing with
the primary antibodies (Table I),
the membranes were incubated with horseradish peroxidase-conjugated
anti-rabbit immunoglobulin G (IgG; sc-2004, 1:1,000), anti-mouse
IgG (sc-2005, 1:1,500) and anti-goat IgG (sc-2350, 1:1,500) as
secondary antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA). Immunoreactive bands were detected using the enhanced
chemiluminescence (ECL) detection system (Amersham Corp.) and
exposed to X-ray film. Primary antibodies were purchased from Santa
Cruz Biotechnology, Inc., Cell Signaling Technology, Inc. (Danvers,
MA, USA) and Abcam (Cambridge, UK) (Table I). Lamin B and actin were used as
internal controls for the nuclear and cytosolic fractions,
respectively.
 | Table IAntibodies used in the present
study. |
Table I
Antibodies used in the present
study.
| Antibody | Dilution | Product no. | Species of origin
and supplier |
|---|
| iNOS | 1:500 | SC-7271 | Mouse monoclonal,
Santa Cruz Biotechnology, Inc. |
| TNF-α | 1:500 | #3707S | rabbit polyclonal,
Cell Signaling Technology, Inc. |
| IL-1β | 1:500 | SC-7884 | Rabbit polyclonal,
Santa Cruz Biotechnology, Inc. |
| NF-κB p65 | 1:500 | SC-109 | Rabbit polyclonal,
Santa Cruz Biotechnology, Inc. |
| IκBα | 1:500 | SC-371 | Rabbit polyclonal,
Santa Cruz Biotechnology, Inc. |
| Akt | 1:500 | SC-8312 | Rabbit polyclonal,
Santa Cruz Biotechnology, Inc. |
| p-Akt | 1:500 | SC-101629 | Rabbit polyclonal,
Santa Cruz Biotechnology, Inc. |
| ERK | 1:1,000 | SC-154 | Rabbit polyclonal,
Santa Cruz Biotechnology, Inc. |
| p-ERK | 1:500 | #9106S | Mouse monoclonal,
Cell Signaling Technology, Inc. |
| p38 | 1:1,000 | SC-535 | Rabbit polyclonal,
Santa Cruz Biotechnology, Inc. |
| p-p38 | 1:500 | #9211S | Rabbit polyclonal,
Cell Signaling Technology, Inc. |
| JNK | 1:1,000 | #9252S | Rabbit polyclonal,
Cell Signaling Technology, Inc. |
| p-JNK | 1:500 | #9255S | Mouse monoclonal,
Cell Signaling Technology, Inc. |
| Nrf2 | 1:500 | SC-13032 | Rabbit polyclonal,
Santa Cruz Biotechnology, Inc. |
| p-Nrf2 | 1:500 | ab76026 | Rabbit monoclonal,
Abcam, Inc. |
| HO-1 | 1:500 | SC-136960 | Mouse monoclonal,
Santa Cruz Biotechnology, Inc. |
| Lamin B | 1:500 | SC-6216 | Goat polyclonal,
Santa Cruz Biotechnology, Inc. |
| β-actin | 1:1,000 | sc-1616 | Goat polyclonal,
Santa Cruz Biotechnology, Inc. |
Immunofluorescence staining
For the detection of the trans-location of NF-κB
p65, the RAW 264.7 cells were grown on glass coverslips for 24 h.
The cells were pre-treated with 400 μg/ml TSWG for 30 min
prior to stimulation with 100 ng/ml LPS. Following incubation for
30 min, the cells were fixed with 3.7% paraformaldehyde, treated
with 0.2% Triton X-100 and blocked with 2% bovine serum albumin
(BSA). The cells were then sequentially incubated with anti-NF-κB
p65 antibody (Table I),
fluorescein isothiocyanate-conjugated donkey anti-rabbit IgG
(1:500; #111-095-003, Jackson ImmunoResearch Laboratories, Inc.,
West Grove, PA, USA) and 4,6-diamidino-2-phenylindole (DAPI;
Sigma-Aldrich Chemical Co.) solution. They were then examined under
a fluorescence microscope (Carl Zeiss, Jena, Germany).
Measurement of intracellular ROS
generation
The intracellular accumulation of ROS was determined
using the fluorescent probe, 2′,7′-dichlorodihydrofluorescein
diacetate (H2DCFDA; Sigma-Aldrich Chemical Co.).
Briefly, the RAW 264.7 cells were pre-treated with 400 μg/ml
TSWG for 30 min prior to stimulation with 100 ng/ml LPS for 30 min.
The cells were then incubated for 4 h at 37°C in phosphate-buffered
saline (PBS) containing 20 mM H2DCFDA to label the intracellular
ROS. ROS production in the cells was monitored using a flow
cytometer (FACSCalibur; Becton-Dickinson, San Jose, CA, USA) using
CellQuest Pro software, as previously described (29).
Data analysis
The results are expressed as the means ± standard
deviation (SD). Differences in the mean values between groups were
analyzed by one-way analysis of variance followed by Dunnett’s
test. A P-value of <0.05 was considered to indicate a
statistically significant difference.
Results
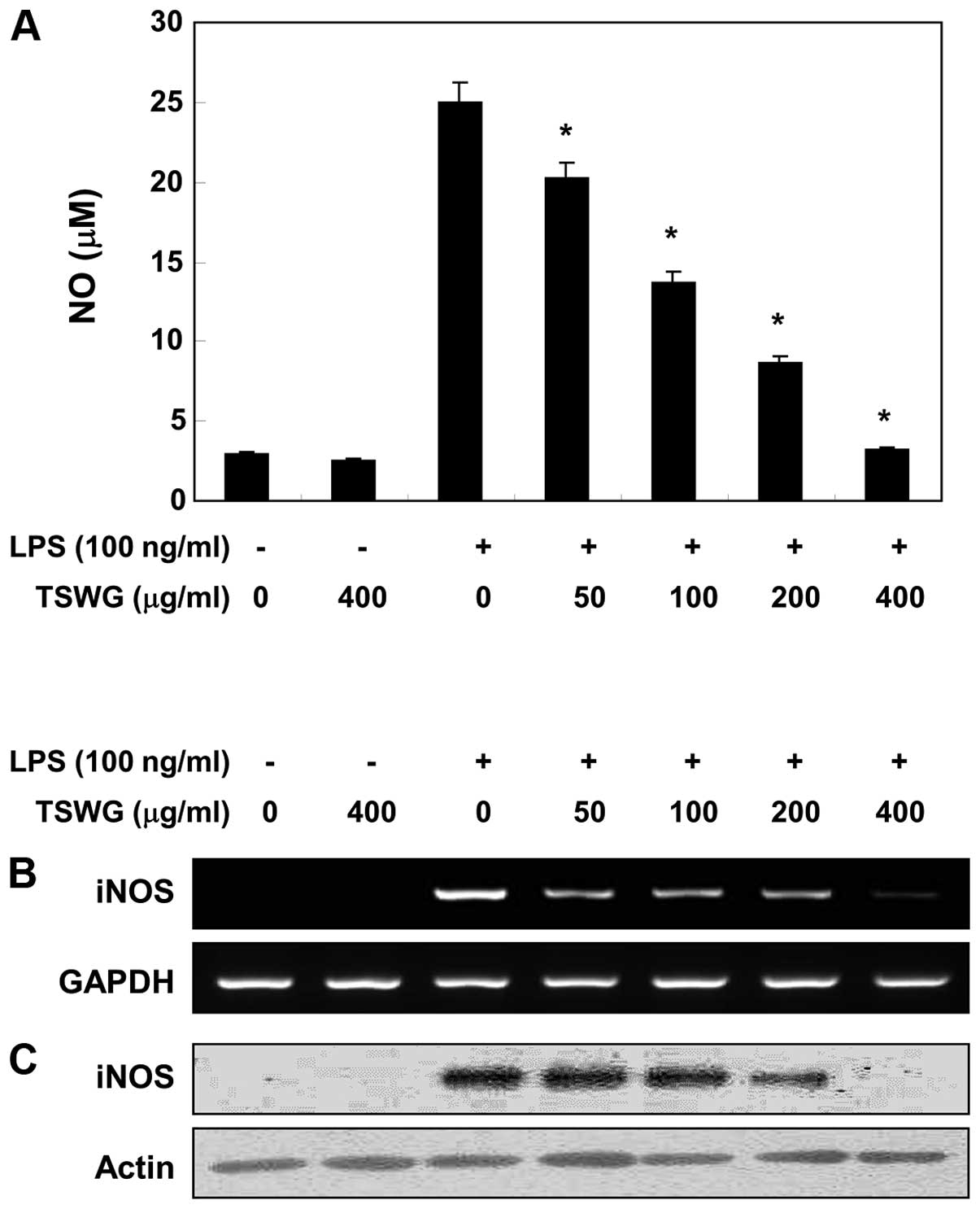
Effect of TSWG on NO production in
LPS-stimulated RAW 264.7 cells
Treatment of the cells with LPS alone markedly
induced NO production compared with the untreated controls,
according to the NO detection assay using Griess reagent. However,
pre-treatment with TSWG significantly suppressed the levels of NO
production in the LPS-stimulated RAW 264.7 microglial cells, in a
concentration-dependent manner (>400 μg/ml) (Fig. 1A). We then performed RT-PCR and
western blot analysis to determine whether the inhibition of NO
production by TSWG in the LPS-stimulated RAW 264.7 cells was
associated with decreased levels of iNOS, which produces NO as a
key mediator of inflammation. TSWG did not induce iNOS expression
at the mRNA and protein level in the unstimulated RAW 264.7 cells,
while stimulation with LPS increased iNOS expression (Fig. 1B and C). However, TSWG induced a
significant decrease in iNOS expression in the LPS-stimulated RAW
264.7 cells in a concentration-dependent manner. These results
indicated that TSWG downregulated NO production in the
LPS-stimulated RAW 264.7 cells by inhibiting iNOS expression.
Additionally, TSWG regulated the expression of iNOS at the
transcriptional level.
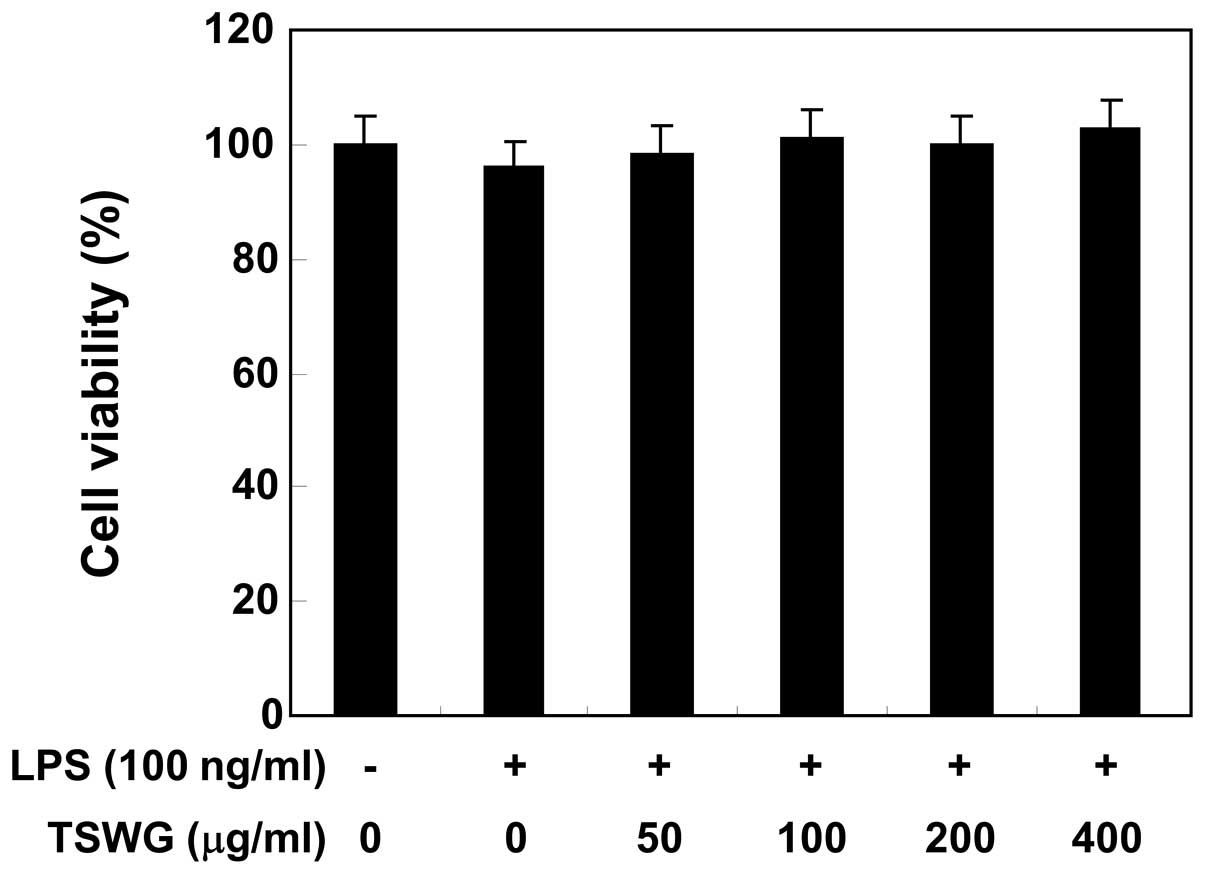
Cytotoxicity of TSWG
MTT assays were performed using the RAW 264.7 cells
treated with TSWG for 24 h in the presence or absence of LPS (100
ng/ml), to exclude the possibility that the inhibition of NO
production was due to cytotoxicity caused by TSWG. TSWG alone did
not affect cell viability at the concentrations used to inhibit NO
production (Fig. 2). Furthermore,
co-treatment with TSWG and LPS had no cytotoxic effects. These
results clearly indicated that the inhibition of NO production in
the LPS-stimulated RAW 264.7 cells was not due to the cytotoxic
effects of TSWG.
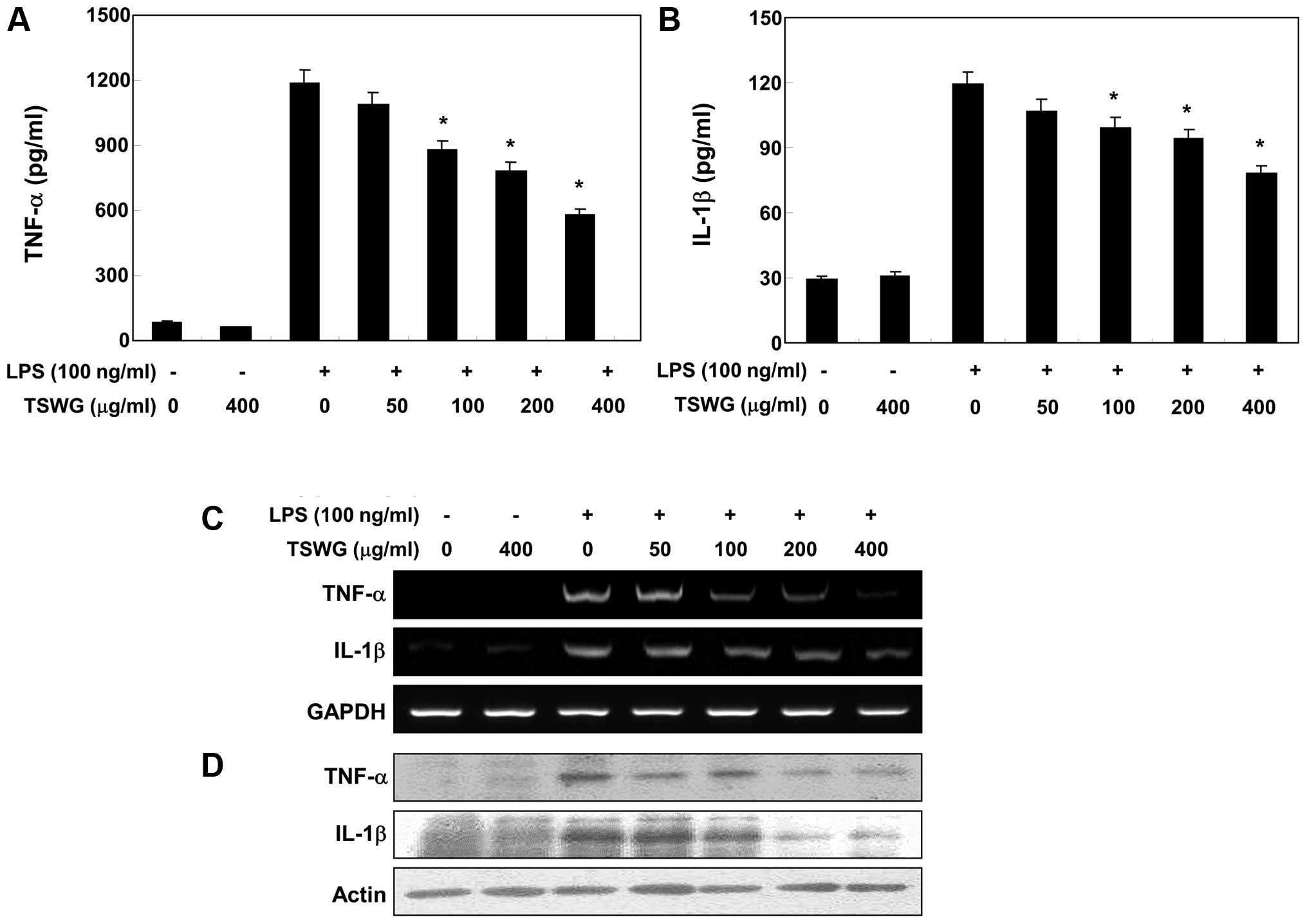
Effect of TSWG on the production of
pro-inflammatory cytokines in LPS-stimulated RAW 264.7 cells
We further quantified the production of inflammatory
cytokines, such as IL-1β and TNF-α in the culture medium using
ELISA to identify the anti-inflammatory properties of TSWG. When
the RAW 264.7 cells were treated with TSWG alone, there were no
changes observed in the production of IL-1β and TNF-α. The
production of IL-1β and TNF-α was markedly increased by LPS
stimulation; however, pre-treatment with TSWG significantly
decreased the production of cytokines in a concentration-dependent
manner (Fig. 3A and B). Since
cytokine secretion was reduced by treatment with TSWG, we evaluated
whether the inhibitory effects of TSWG on TNF-α and IL-1β
expression are associated with the inhibitory effects on the
release of TNF-α and IL-1β. Our results indicated that the
LPS-induced increase in the TNF-α and IL-1β mRNA levels was
reversed by TSWG in a concentration-dependent manner (Fig. 3C). In a parallel experiment, the
elevated protein levels of TNF-α and IL-1β resulting from
stimultion with LPS were also decreased following treatment with
TSWG (Fig. 3D). Our results thus
indicate that TSWG negatively regulates the production of
pro-inflammatory cytokines, such as IL-1β and TNF-α at the
transcriptional level.
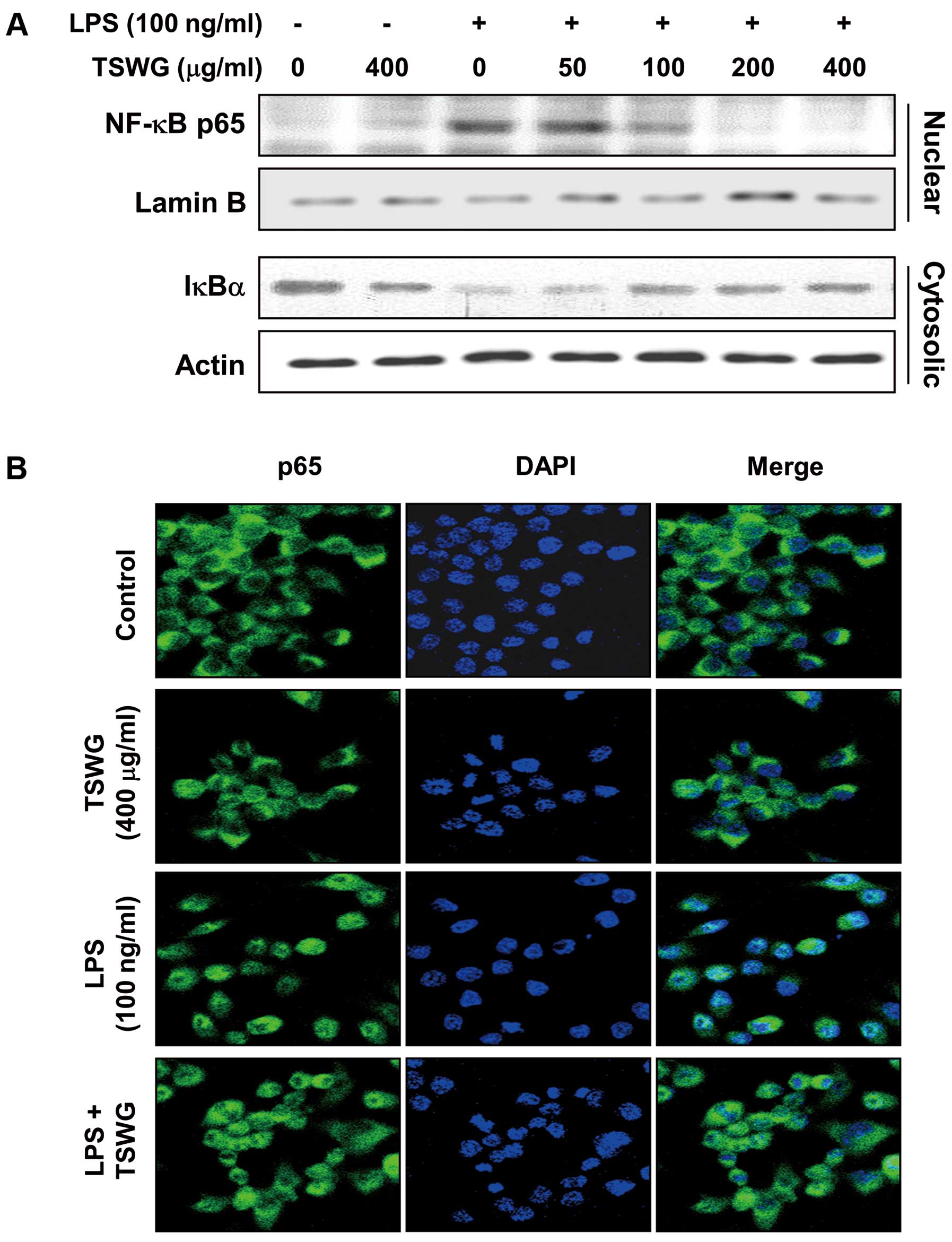
Effect of TSWG on LPS-induced NF-κB
translocation in the RAW 264.7 cells
Our results revealed that TSWG regulated the
expression of iNOS and that of the pro-inflammatory cytokines,
TNF-α and IL-1β, at the transcriptional level. We then evaluated
the expression of nuclear NF-κB p65, a subunit of NF-κB, and
cytosolic IκBα to further characterize the mechanisms of
TSWG-mediated anti-inflammatory responses. We first investigated
whof NF-κB p65. According to the results of western blot analysis,
nuclear NF-κB p65 expression was not specifically increased by
TSWG. However, the amount of NF-κB p65 in the nucleus was markedly
increased following exposure to LPS for 30 min (Fig. 4A). However, pre-treatment with
TSWG decreased the expression of nuclear NF-κB p65, causing its
epxression to return to basal levels. In a parallel experiment,
stimulation with LPS decreased IκBα expression in the cytosol;
however, pre-treatment with TSWG somewhat amplified cytosolic IκBα
expression in a concentration-dependent manner. We visualized the
location of NF-κB p65 in the cells by immunofluorescence staining,
as experimental evidence of the TSWG-induced NF-κB inactivation.
The cells in the control group and TSWG-treated group showed
cytosolic NF-κB p65, while stimulation with LPS facilitated the
NF-κB p65 translocation to the nucleus; however, the cells in the
TSWG pre-treated group showed that NF-κB p65 was arrested in the
cytosol (Fig. 4B). These results
indicated that treatment with TSWG suppressed NF-κB activity in the
LPS-stimulated RAW 264.7 cells by inhibiting the nuclear
translocation of NF-κB and the degradation of IκBα.
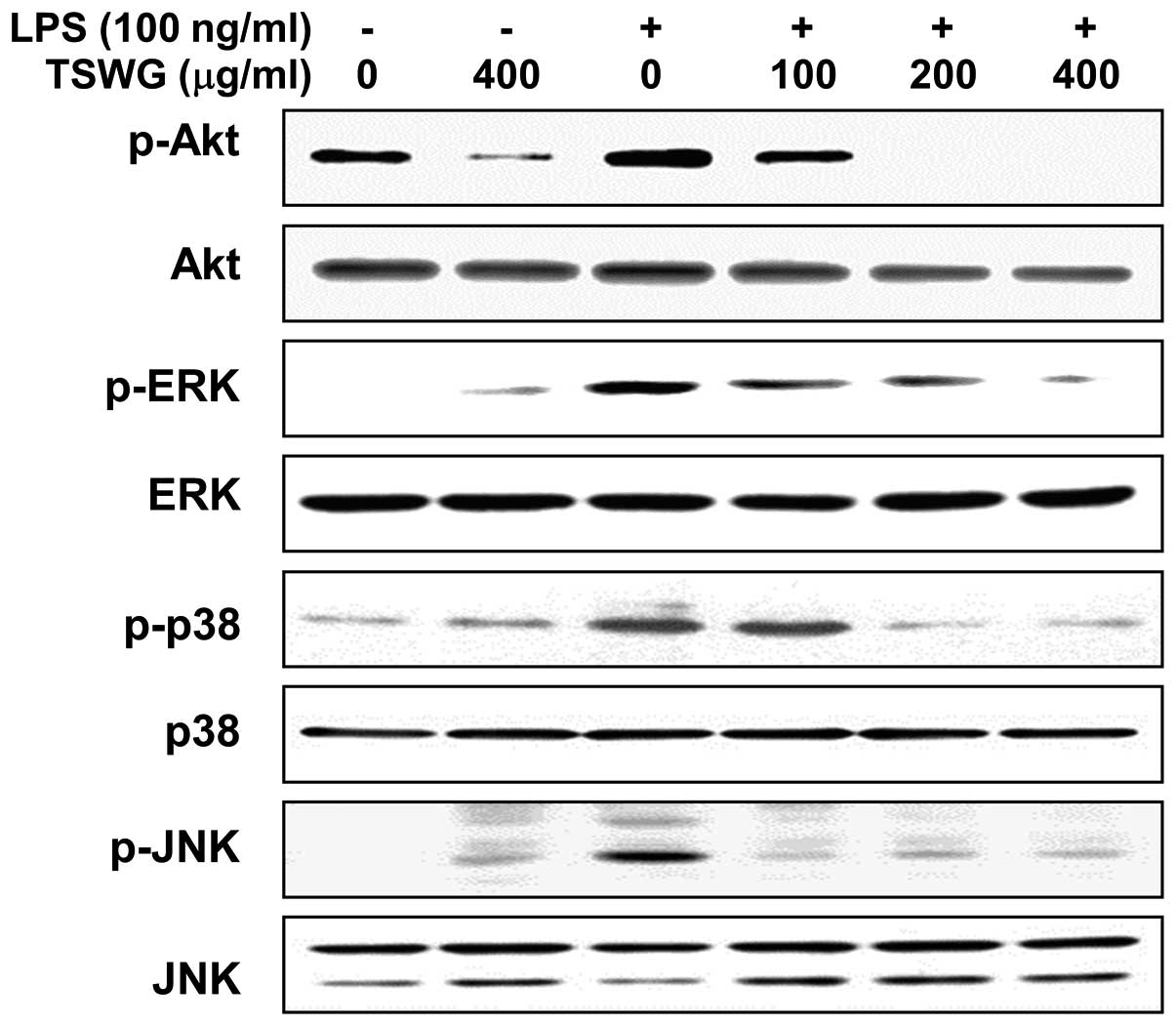
Inhibition of LPS-induced Akt and MAPK
activation by TSWG in LPS-stimulated RAW 264.7 cells
Previous studies have demonstrated that the PI3K/Akt
and MAPK signaling pathways are involved in the expression of
LPS-induced pro-inflammatory genes (3,4).
We therefore investigated the effects of TSWG on the LPS-induced
activation of Akt and MAPKs, such as ERK, JNK and p38 MAPK by
western blot analysis. Stimulation with LPS for 1 h resulted in a
significant increase in the phosphorylated forms of Akt, ERK, JNK
and p38 MAPK compared with the control group without altering their
unphosphorylated forms (Fig. 5).
However, pre-treatment with TSWG for 1 h attenuated the
phosphorylation of Akt and MAPKs in a concentration-dependent
manner (Fig. 5). These results
suggested that the TSWG-induced inactivation of Akt and MAPKs may
cause the suppression of the inflammatory response in
LPS-stimulated RAW 264.7 cells.
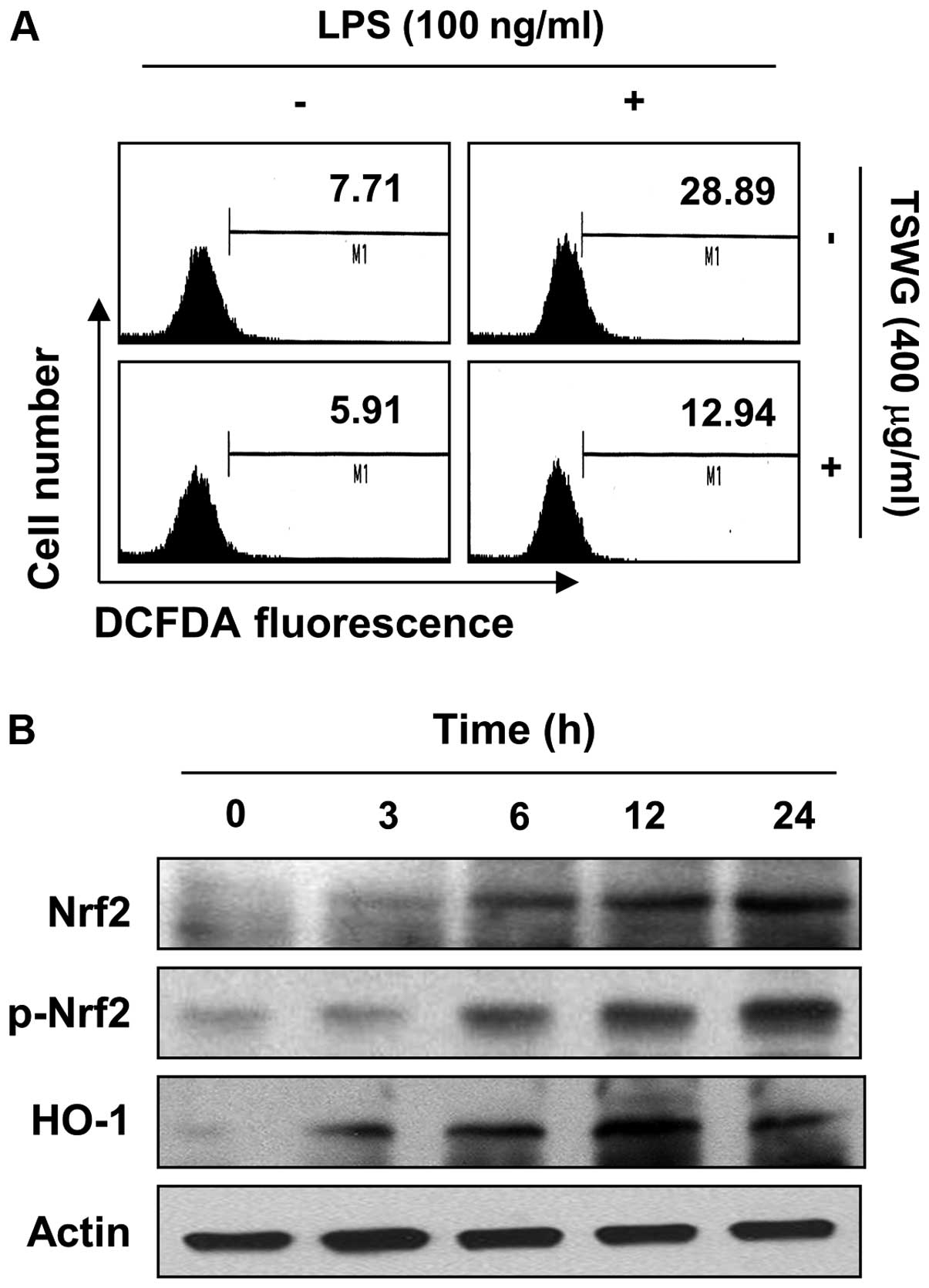
Suppression of LPS-induced ROS formation
by TSWG in RAW 264.7 cells
Several studies have reported that the LPS-mediated
activation of NF-κB leads to the enhanced production of ROS as a
common second messenger, thereby contributing to the sustained
oxidant production during chronic inflammation (5,6).
The level of the generation of intracellular ROS was accordingly
assessed to determine whether TSWG reduces the level of LPS-induced
oxidative stress in RAW 264.7 cells. LPS significantly enhanced ROS
production, while pretreatment with TSWG considerably reversed the
LPS-induced cellular production of ROS (Fig. 6A). Treatment of the RAW 264.7
cells alone also decreased the ROS levels when compared with the
untreated control cells. These results indicated that TSWG was
capable of abrogating the LPS-induced increase in ROS production in
RAW 264.7 cells.
Induction of Nrf-2 and HO-1 expression by
TSWG in RAW 264.7 cells
Since TSWG prevented the generation of ROS, we
hypothesized that pre-treatment with TSWG may facilitate the
expression of antioxidant enzymes in the RAW 264.7 cells. We then
examined whether the expression of Nrf2 and HO-1 is regulated by
TSWG. Following treatment with TSWG, the RAW 264.7 cells showed a
gradual increase in the total, as well as in the phosphorylated
Nrf2 levels in a time-dependent manner, and this strongly
correlated with the increase in HO-1 expression (Fig. 6B). Thee results indicated that
TSWG induced HO-1 expression throug the activation of Nrf2.
Discussion
Macrophage defensive systems play essential roles in
teh host defense against bacterial infection by recognition of the
threats through specific receptors, particularly the Toll-like
receptor 4 (TLR4) complex. However, the enhanced production of
inflammatory molecules, including NO, ROS and cytokines by
macrophages can cause damage to the host (30,31). In response to macrophage
activation by LPS, the principal component of the outer membrane of
Gram-negative bacteria (32),
TLR4 recruits downstream-associated adaptor molecules, including
myeloid differentiation factor (MyD88) (33,34). The activation of the
MyD88-dependent pathway results in the rapid activation of the
NF-κB, PI3K/Akt and MAPK pathways, which coordinately regulate
inflammation-associated gene activities responsible for host
defense. Furthermore, these pro-inflammatory products play key
roles in the pathogeneses of various acute and chronic inflammatory
diseases (35,36). Therefore, the suppression of the
aberrant activation of macrophages may have valuable therapeutic
potential for the treatment of various inflammation-associated
diseases caused by macrophage activation.
Macrophages have been found to be activated in acute
and chronic bacterial inflammatory diseases (1,2).
The overproduction of NO in activated macrophages, a potent and
critical mediator of inflammatory diseases, is mediated primarily
by iNOS, causing tissue injury (2,37).
Therefore, the modulation of the iNOS-mediated release of NO from
macrophages is considered one of the strategies to develop
therapeutic compounds against various inflammatory diseases. In
this study, we demonstrated that TSWG exerted its anti-inflammatory
effects by suppressing the LPS-induced iNOS mRNA and protein
expression, and the subsequent production of NO in RAW 264.7 cells
(Fig. 1). These data indicated
that the inhibition of NO production in response to TSWG was a
result of an inhibition of iNOS gene expression at the
transcriptional level. Activated macrophages also exert cytotoxic
effects by releasing various pro-inflammatory cytokines, including
TNF-α and IL-1β and ROS, that may participate in the inflammatory
process (1,2). Moreover, ROS are important
contributors to the production of TNF-α and IL-1β in LPS-stimulated
macrophages (38,39). In response to LPS, the production
of TNF-α and IL-1β was markedly upregulated, but treatment with
TSWG significantly inhibited the induction of these cytokines by
LPS (Fig. 3A and B). In addition,
the results from the RT-PCR and western blot analysis revealed that
TSWG decreased the TNF-α and IL-1β mRNA levels with a corresponding
decrease in the protein levels (Fig.
3C and D). A prerequisite for the proper evaluation of the
biological properties of candidate agents is the exact
determination of cytotoxicity associated with the prolonged
incubation of the cells. In the current study, the concentrations
of TSWG used to inhibit the production of NO, TNF-α and IL-1β did
not affect cell viability, as measured by MTT assay (Fig. 2), thereby indicating that the
anti-inflammatory effects of TSWG on LPS-stimulated RAW 264.7 cells
were not simply due to a cytotoxic action of TSWG.
There are several well established highly complex
inflammatory signaling pathways. Among these, NF-κB is the major
transcriptional factor in the production of pro-inflammatory
molecules and TLRs are the major pattern recognition receptors to
detect conserved microbial products (40,41). In unstimulated macrophages, NF-κB
is retained in the cytoplasm by IκB; however, when LPS binds TLR4,
IκB is rapidly phosphorylated by the IκB kinase complex, which
results in IκB degradation and the translocation of NF-κB to the
nucleus to initiate inflammatory-associated gene expression,
including that of iNOS and pro-inflammatory cytokines (33,39,41). Moreover, upon the activation of
Akt by PI3K, phosphorylated Akt promotes the activation of NF-κB
and MAPKs to trigger the transcription of pro-inflammatory
regulators in LPS-stimulated macrophages (5,6,42).
Thus, suppressing the activation of NF-κB by inhibiting IκBα
degradation may be a target for anti-inflammatory therapeutic
candidates. To investigate whether the inhibition of NF-κB
activation by TSWG was associated with the inactivation of the
PI3K/Akt and MAPK pathways, the effects of TSWG on the LPS-induced
nuclear translocation of NF-κB and the degradation of IκB were
assessed. The results revealed that TSWG significantly attenuated
the LPS-induced IκB degradation and the nuclear translocation of
NF-κB p65 in the LPS-stimulated RAW 264.7 macrophages (Fig. 4). Our results study also
demonstrated that pre-treatment with TSWG significantly suppressed
the phosphorylation of MAPKs, including ERK, JNK and p38 MAPK, as
well as that of Akt in a concentration-dependent manner (Fig. 5). Taken together, these results
indicated that the inhibition of NF-κB activity by TSWG was
mediated through the inactivation of PI3K/Akt and MAPKs by the
deficiencies in the phosphorylation of Akt or MAPKs. This may lead
to the inhibition of pro-inflammatory mediators and cytokine
expression in LPS-stimulated macrophages.
HO-1 is one of the phase II enzymes, which are
protective proteins expressed to preserve cells against oxidative
stress and inflammation. The de novo induction of this
protein is mainly due to transcriptional activation mediated by
Nrf2 through its interaction with ARE, a regulatory sequence for
the transcriptional activation of genes encoding various
antioxidant enzymes and phase II detoxifying enzymes. Moreover, it
has been shown that HO-1 suppresses the production of
pro-inflammatory mediators, including iNOS, as well as that of
cytokines in activated macrophages (8,10–12). Therefore, the activation of the
Nrf2/HO-1 pathway is a promising target for the treatment of
inflammatory, as well as oxidative stress-mediated diseases. Our
results indicated that TSWG induced HO-1 expression in a
concentration-dependent manner, which was associated with the
activation of Nrf2 (Fig. 6B).
Consistent with this, we found that the LPS-induced generation of
intracellular ROS was markedly abolished by pre-treatment with TSWG
in the RAW 264.7 cells (Fig. 6A).
Our findings revealed that TSWG increased Nrf2 activation and HO-1
expression, which may lead to the prevention of oxidative stress,
as well as the supportive negative regulation of pro-inflammatory
molecules in LPS-stimulated RAW 264.7 cells.
In conclusion, the present data demonstrate that
TSWG possesses anti-inflammatory properties, such as the
suppression of NO, TNF-α and IL-1β production through the
downregulation of their corresponding gene expression in
LPS-stimulated RAW 264.7 macrophages. TSWG also significantly
inhibited the LPS-induced inflammation-associated signaling
pathways, including the nuclear translocation of NF-κB and the
phosphorylation of Akt and MAPKs. In addition, TSWG suppressed the
increased generation of intracellular ROS in LPS-stimulated RAW
264.7 cells and promoted HO-1 protein expression by promoting Nrf2
activation. Although further studies are required to clearly
elucidate the exact mechanisms through which TSWG inhibits
oxidative stress and the possible crosstalk between the NF-κB and
Nrf2/HO-1 signaling pathways, this study may provide a molecular
basis for the therapeutic use of TSWG against various inflammatory
diseases.
Acknowledgments
This study was supported by the National Research
Foundation of Korea (NRF) grant funded by the Korea government
(MSIP) (no. NRF-2014R1A2A1A09006983) and the Blue-Bio Industry
Regional Innovation Center (RIC08-06-07) at Dongeui University as a
RIC program under the Ministry of Trade, Industry and Energy and
Busan city.
References
|
1
|
Zhang X and Mosser DM: Macrophage
activation by endogenous danger signals. J Pathol. 214:161–178.
2008. View Article : Google Scholar
|
|
2
|
Cinel I and Opal SM: Molecular biology of
inflammation and sepsis: a primer. Crit Care Med. 37:291–304. 2009.
View Article : Google Scholar
|
|
3
|
Guha M and Mackman N: LPS induction of
gene expression in human monocytes. Cell Signal. 13:85–94. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Korbecki J, Baranowska-Bosiacka I,
Gutowska I and Chlubek D: The effect of reactive oxygen species on
the synthesis of pros-tanoids from arachidonic acid. J Physiol
Pharmacol. 64:409–421. 2013.PubMed/NCBI
|
|
5
|
Ito K: Impact of post-translational
modifications of proteins on the inflammatory process. Biochem Soc
Trans. 35:281–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ivanenkov YA, Balakin KV and Tkachenko SE:
New approaches to the treatment of inflammatory disease: focus on
small-molecule inhibitors of signal transduction pathways. Drugs R
D. 9:397–434. 2008. View Article : Google Scholar
|
|
7
|
Geronikaki AA and Gavalas AM: Antioxidants
and inflammatory disease: synthetic and natural antioxidants with
anti-inflammatory activity. Comb Chem High Throughput Screen.
9:425–442. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaspar JW, Niture SK and Jaiswal AK:
Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol
Med. 47:1304–1309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee HS, Lee GS, Kim SH, Kim HK, Suk DH and
Lee DS: Anti-oxidizing effect of the dichloromethane and hexane
fractions from Orostachys japonicus in LPS-stimulated RAW 264.7
cells via upregulation of Nrf2 expression and activation of MAPK
signaling pathway. BMB Rep. 47:98–103. 2014. View Article : Google Scholar :
|
|
10
|
Zhao CR, Gao ZH and Qu XJ: Nrf2-ARE
signaling pathway and natural products for cancer chemoprevention.
Cancer Epidemiol. 34:523–533. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Paine A, Eiz-Vesper B, Blasczyk R and
Immenschuh S: Signaling to heme oxygenase-1 and its
anti-inflammatory therapeutic potential. Biochem Pharmacol.
80:1895–1903. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Su ZY, Shu L, Khor TO, Lee JH, Fuentes F
and Kong AN: A perspective on dietary phytochemicals and cancer
chemoprevention: oxidative stress, nrf2, and epigenomics. Top Curr
Chem. 329:133–162. 2013. View Article : Google Scholar
|
|
13
|
Zhang D, Yasuda T, Yu Y, Zheng P, Kawabata
T, Ma Y and Okada S: Ginseng extract scavenges hydroxyl radical and
protects unsaturated fatty acids from decomposition caused by
iron-mediated lipid peroxidation. Free Radic Biol Med. 20:145–150.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yun TK: Experimental and epidemiological
evidence on non-organ specific cancer preventive effect of Korean
ginseng and identification of active compounds. Mutat Res.
523–524:63–74. 2003. View Article : Google Scholar
|
|
15
|
Yun TK: Experimental and epidemiological
evidence of the cancer-preventive effects of Panax ginseng C.A.
Meyer. Nutr Rev. 54:S71–S81. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dey L, Xie JT, Wang A, Wu J, Maleckar SA
and Yuan CS: Anti-hyperglycemic effects of ginseng: comparison
between root and berry. Phytomedicine. 10:600–605. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Attele AS, Zhou YP, Xie JT, Wu JA, Zhang
L, Dey L, Pugh W, Rue PA, Polonsky KS and Yuan CS: Antidiabetic
effects of Panax ginseng berry extract and the identification of an
effective component. Diabetes. 51:1851–1858. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu ZQ, Luo XY, Liu GZ, Chen YP, Wang ZC
and Sun YX: In vitro study of the relationship between the
structure of ginsenoside and its antioxidative or prooxidative
activity in free radical induced hemolysis of human erythrocytes. J
Agric Food Chem. 51:2555–2558. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie JT, McHendale S and Yuan CS: Ginseng
and diabetes. Am J Chin Med. 33:397–404. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nag SA, Qin JJ, Wang W, Wang MH, Wang H
and Zhang R: Ginsenosides as anticancer agents: In vitro and in
vivo activities, structure-activity relationships, and molecular
mechanisms of action. Front Pharmacol. 3:252012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim JH: Cardiovascular diseases and Panax
ginseng: A review on molecular mechanisms and medical applications.
J Ginseng Res. 36:16–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shibata S: Chemistry and cancer preventing
activities of ginseng saponins and some related triterpenoid
compounds. J Korean Med Sci. 16(Suppl): S28–S37. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee B, Park J, Kwon S, Park MW, Oh SM,
Yeom MJ, Shim I, Lee HJ and Hahm DH: Effect of wild ginseng on
scopolamine-induced acetylcholine depletion in the rat hippocampus.
J Pharm Pharmacol. 62:263–271. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mizuno M, Yamada J, Terai H, Kozukue N,
Lee YS and Tsuchida H: Differences in immunomodulating effects
between wild and cultured Panax ginseng. Biochem Biophys Res
Commun. 200:1672–1678. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park BG, Jung HJ, Cho YW, Lim HW and Lim
CJ: Potentiation of antioxidative and anti-inflammatory properties
of cultured wild ginseng root extract through probiotic
fermentation. J Pharm Pharmacol. 65:457–464. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee B, Kim H, Shim I, Lee H and Hahm DH:
Wild ginseng attenuates anxiety- and depression-like behaviors
during morphine withdrawal. J Microbiol Biotechnol. 21:1088–1096.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park JS, Hwang SY, Lee WS, Yu KW, Paek KY,
Hwang BY and Han K: The therapeutic effect of tissue cultured root
of wild Panax ginseng C.A. Mayer on spermatogenetic disorder. Arch
Pharm Res. 29:800–807. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li H, Lee JH and Ha JM: Effective
purification of ginsenosides from cultured wild ginseng roots,red
ginseng, and white ginseng with macroporous resins. J Microbiol
Biotechnol. 18:1789–1791. 2008.PubMed/NCBI
|
|
29
|
Song JJ, Lim HW, Kim K, Kim KM, Cho S and
Chae SW: Effect of caffeic acid phenethyl ester (CAPE) on
H2O2 induced oxidative and inflammatory
responses in human middle ear epithelial cells. Int J Pediatr
Otorhinolaryngol. 76:675–679. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu YC, Yeh WC and Ohashi PS: LPS/TLR4
signal transduction pathway. Cytokine. 42:145–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin YC, Huang DY, Chu CL and Lin WW:
Anti-inflammatory actions of Syk inhibitors in macrophages involve
non-specific inhibition of toll-like receptors-mediated JNK
signaling pathway. Mol Immunol. 47:1569–1578. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rietschel ET, Kirikae T, Schade FU, Ulmer
AJ, Holst O, Brade H, Schmidt G, Mamat U, Grimmecke HD, Kusumoto S
and Zähringer U: The chemical structure of bacterial endotoxin in
relation to bioactivity. Immunobiology. 187:169–190. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takeda K, Kaisho T and Akira S: Toll-like
receptors. Annu Rev Immunol. 21:335–376. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
West TE, Ernst RK, Jansson-Hutson MJ and
Skerrett SJ: Activation of Toll-like receptors by Burkholderia
pseudomallei. BMC Immunol. 9:462008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Janeway CA Jr and Medzhitov R:
Lipoproteins take their toll on the host. Curr Biol. 9:R879–R882.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Glushkova OV, Parfenyuk SB, Khrenov MO,
Novoselova TV, Lunin SM, Fesenko EE and Novoselova EG: Inhibitors
of TLR-4, NF-κB, and SAPK/JNK signaling reduce the toxic effect of
lipopolysaccharide on RAW 264.7 cells. J Immunotoxicol. 10:133–140.
2013. View Article : Google Scholar
|
|
37
|
Kobayashi Y: The regulatory role of nitric
oxide in proinflam-matory cytokine expression during the induction
and resolution of inflammation. J Leukoc Biol. 88:1157–1162. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Haddad JJ and Land SC: A non-hypoxic,
ROS-sensitive pathway mediates TNF-alpha-dependent regulation of
HIF-1alpha. FEBS Lett. 505:269–274. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ryan KA, Smith MF Jr, Sanders MK and Ernst
PB: Reactive oxygen and nitrogen species differentially regulate
Toll-like receptor 4-mediated activation of NF-kappa B and
interleukin-8 expression. Infect Immun. 72:2123–2130. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Doyle SL and O’Neill LA: Toll-like
receptors: from the discovery of NFkappaB to new insights into
transcriptional regulations in innate immunity. Biochem Pharmacol.
72:1102–1113. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kawai T and Akira S: Signaling to
NF-kappaB by Toll-like receptors. Trends Mol Med. 13:460–469. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim BH, Oh I, Kim JH, Jeon JE, Jeon B,
Shin J and Kim TY: Anti-inflammatory activity of compounds isolated
from Astragalus sinicus L. in cytokine-induced keratinocytes and
skin. Exp Mol Med. 46:e872014. View Article : Google Scholar : PubMed/NCBI
|