Introduction
Lung cancer is the most common type of cancer of the
respiratory system, with a 5-year overall survival rate of <15%
(1). Due to its malignant
tendency and the high rate of recurrence and metastasis, the
effects of surgical treatment are limited (2). Similar to other types of cancer
cells, lung cancer cells are also characterized by the features of
sustained proliferation and resistance to cell apoptosis. Non-small
cell lung cancer (NSCLC) is responsible for approximately 85% of
the total lung cancer cases. The prognosis is pessimistic as NSCLC
is not sensitive to the majority of conventional cytotoxic
treatments, such as chemotherapy and radiotherapy (3). Thus, the need for t he
identification of novel alternative therapeutic drugs for NSCLC is
urgent and of significance.
Apoptosis, also known as programmed cell death, is
believed to be triggered by multiple extra- and intracellular
factors. Osmotic pressure imbalance, reactive oxygen species (ROS),
mitochondrial damage and calcium overload have been shown to play
an important role in the initiation and regulation of apoptosis
(4). Calcium is one of the small
signaling molecules regulating various biological functions in
cells. Cell cycle regulation and cell death have been suggested to
closely correlate with the intracellular calcium ion
([Ca2+]i) concentration (5). Under pathological conditions, such
as ischemia-reperfusion injury and oxidative stress, the
[Ca2+]i level has been reported to markedly increase in
many types of cells. This condition is known as calcium overload,
which eventually leads to the activation of pro-apoptotic factors,
resulting in apoptosis (6,7).
The endoplasmic reticulum (ER) is considered the
critical organelle participating in multiple cellular biological
functions. Accumulating evidence indicates that in response to
pathological stimuli, the ER decides the cell fate by initiating
cell defense or activating apoptosis (8,9).
It has been reported that calcium is released from the ER to induce
calcium overload, leading to cell death (10). Located in the ER membrane, the
inositol 1,4,5-trisphosphate receptor (IP3R) has been suggested to
be involved in the regulation of cellular calcium homeostasis,
playing a role as the main calcium release channel in the majority
of cells (11). B-cell lymphoma-2
(Bcl-2) belongs to the family of anti-apoptotic proteins and is
located in the ER. Accumulating evidence has indicated that the
Bcl-2 protein plays a role in regulating the phosphorylation status
of IP3R. It appears that the phosphorylation of IP3R is reduced by
Bcl-2 and thus the IP3R-induced calcium release (IICR) is impaired
(12).
Curcumin has a long medical application history in
Traditional Chinese Medicine (TCM) since the ancient times.
Curcumin is extracted from turmeric (Curcuma longa L.) and
is known as
1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione in
modern pharmacology. In several recent studies, the suppression of
Bcl-2 expression has been suggested to be involved in the
apoptosis-inducing effects of curcumin on NSCLC (13). Furthermore, curcumin has been
reported to induce cell apoptosis by increasing the
[Ca2+]i level (14).
However, whether calcium overload-induced cell apoptosis is
involved in the anti-proliferative effects of curcumin has not yet
been elucidated. Thus, in the present study, we examined our
hypothesis that curcumin exacerbates IICR by suppressing Bcl-2
expression, thus inducing the apoptosis of NSCLC cells.
Materials and methods
Cell lines and treatment
The human lung cancer cell lines, A549 and H1299,
were provided by American Type Culture Collection (ATCC; Manassas,
VA, USA). The cells were cultured in RPMI-1640 medium (HyClone,
Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS;
Gibco, Carlsbad, CA, USA), 250 ng/ml amphotericin B, 100 g/ml
streptomycin, 100 U/ml penicillin and 2 mmol/l glutamine (all from
Sigma, St. Louis, MO, USA) in culture flasks (Corning Inc.,
Corning, NY, USA) which were maintained in a an incubator under a
humidified environment with 5% CO2 and 95% fresh air. In
order to determine the cytotoxicity of curcumin, the cells were
treated with serially diluted curcumin (Sigma) at concentrations of
0, 10, 20, 30, 40, 50, 60, 70 and 80 μmol/l for 24 h. In
order to examine the role of IP3R, a specific IP3R inhibitor,
xestospongin C (XSC; Sigma) was used to pre-treat the cancer cells
at a concentration of 10 μmol/l for 15 min to block
IP3R.
Cell viability assay
The viabilities of the A549 and H1299 cells were
assessed by a colorimetric
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Briefly, 1×105 cells were plated in a 96-well
plate and then incubated with curcumin at different concentrations
(0–80 μmol/l) for 24 h. After being washed with sterilized
phosphate-buffered saline (PBS; Bioss, Beijing, China), the cells
were incubated with MTT solution (Sigma) at a concentration of 5
mg/ml for 4 h. The cells were then dissolved by dimethyl sulfoxide
(DMSO; Sigma). The amount of formed formazan crystals was
investigated by measuring the absorbance value at 540 nm using a
plate reader (Bio-Rad, Hercules, CA, USA).
Measurement of cell apoptosis
Cell apoptosis was evaluated by Hoechst 33342
staining. The cells were collected, washed and fixed in 4%
paraformaldehyde for 20 min. The cells were stained with Hoechst
33342 (Sigma) at a final concentration of 10 μmol/l for 20
min in a dark chamber. The cells were then observed under a
fluorescence microscope (Carl Zeiss, Jena, Germany). Captured
images were analyzed using Image-Pro Plus software (Media
Cybernetics, Rockville, MD, USA). The amount of Hoechst positively
stained cells was used to indicate the percentage of apoptosis.
Determination of the [Ca2+]i
concentration
The [Ca2+]i level in the A549 and H1299
cells was determined by Fura-2/AM fluorescence staining. Briefly,
the harvested cells were incubated with Fura-2/AM (Beyotime
Institute of Biotechnology, Shanghai, China) at final concentration
of 5 μmol/l at 30°C for 30 min. A fluorescence microscope
(Carl Zeiss) was then used to observe the cells. The
[Ca2+]i level was represented by the mean fluorescence
intensity (MFI) after the captured images were analyzed using
Image-Pro Plus software (Media Cybernetics).
Evaluation of mitochondrial membrane
potential (ΔΨm)
In order to detect the ΔΨm, the collected cells were
incubated with rhodamine 123 (Sigma) at final concentration of 10
mg/l at 37°C for 30 min in a dark chamber. The fluorescence of
rhodamine 123 was detected using a FACSCalibur flow cytometer (BD
Biosciences, San Jose, CA, USA) at 530 nm. The fluorescence of
rhodamine 123 represented the levels of ΔΨm.
Western blot analysis
The harvested cells were washed with PBS and lysed
in RIPA buffer system (Santa Cruz Biotechnology, Inc. Santa Cruz,
CA, USA) on ice. Following centrifugation at 12,000 × g at 4°C for
10 min, the supernatant separated from the lysates was considered
as the extracted total protein. The protein concentration was
detected using the BCA kit (Thermo Scientific, Rockford, IL, USA)
according to the manufacturer’s instructions. A total of 20
μg of protein were loaded and separated by electrophoresis
on 10–15% sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE) and
then transferred onto PVDF membranes electronically. Non-specific
binding was eliminated by incubation with 0.05% Tween-20 and 5%
defatted milk. Specific antibodies against IP3R (Abcam, Cambridge,
MA, USA), phosphorylated (p-)IP3R (Ser1756; Cell Signaling
Technology, Inc., Beverly, MA, USA), Bcl-2 (Abcam), cleaved
caspase-3, cleaved caspase-9 (both from Santa Cruz Biotechnology,
Inc.) and GAPDH (Invitrogen, Carlsbad, CA, USA) were used to
incubate the membranes at 4°C for 8–12 h. The immunoblots were
detected by corresponding horseradish peroxidase-conjugated
secondary antibodies (Invitrogen) using the enhanced
chemiluminescence kit (Amersham Pharmacia Biotech, Piscataway, NJ,
USA). The intensities of the immunoblots were analyzed by Image-Pro
Plus software (Media Cybernetics).
Results
Curcumin inhibits the proliferation of
A549 and H1299 cells
The inhibitory effects of crucumin on the growth of
cancer cells were determined by MTT assay. As demonstrated in
Fig. 1, curcumin suppressed the
growth of the A549 and H1299 cells in a concentration-dependent
manner. Curcumin began to exert cytotoxic effects on the A549 cells
at a concentration of 50 μmol/l, while the H1299 cells
appeared to be more sensitive to curcumin, which began to exert
cytotoxic effects at a concentration of 40 μmol/l. Thus, the
concentrations of 50, 60 and 70 μmol/l curcumin were
selected to incubate the A549 cells, while the concentrations of
40, 50 and 60 μmol/l curcumin were selected to incubate the
H1299 cells.
 | Figure 1Curcumin inhibits the growth of A549
and H1299 lung cancer cells. Line chart demonstrates the results
from MTT assay. The blue line indicates the viable percentage of
A549 cells incubated with serially diluted curcumin at
concentrations of 0, 10, 20, 30, 40, 50, 60 70 and 80 μmol/l
for 24 h. The red line indicates the viable percentage of H1299
cells incubated with serially diluted curcumin at concentrations of
0, 10, 20, 30, 40, 50, 60 70 and 80 μmol/l for 24 h. Values
are presented as the means ± SD. Indicates that the difference is
significant when compared with the previous concentration
(*P<0.05). |
IP3R inhibitor impairs the
curcumin-induced apoptosis of A549 and H1299 cells
Fig. 2 shows the
captured images of Hoechst 33342 fluorescence staining of the A459
and H1299 cells. Following incubation with serially diluted
curcumin, the apoptosis of both the A549 and H1299 cells
significantly increased in a concentration-dependent manner.
However, in the XSC pre-treated A549 and H1299 cells, the apoptosis
induced by curcumin was significantly attenuated.
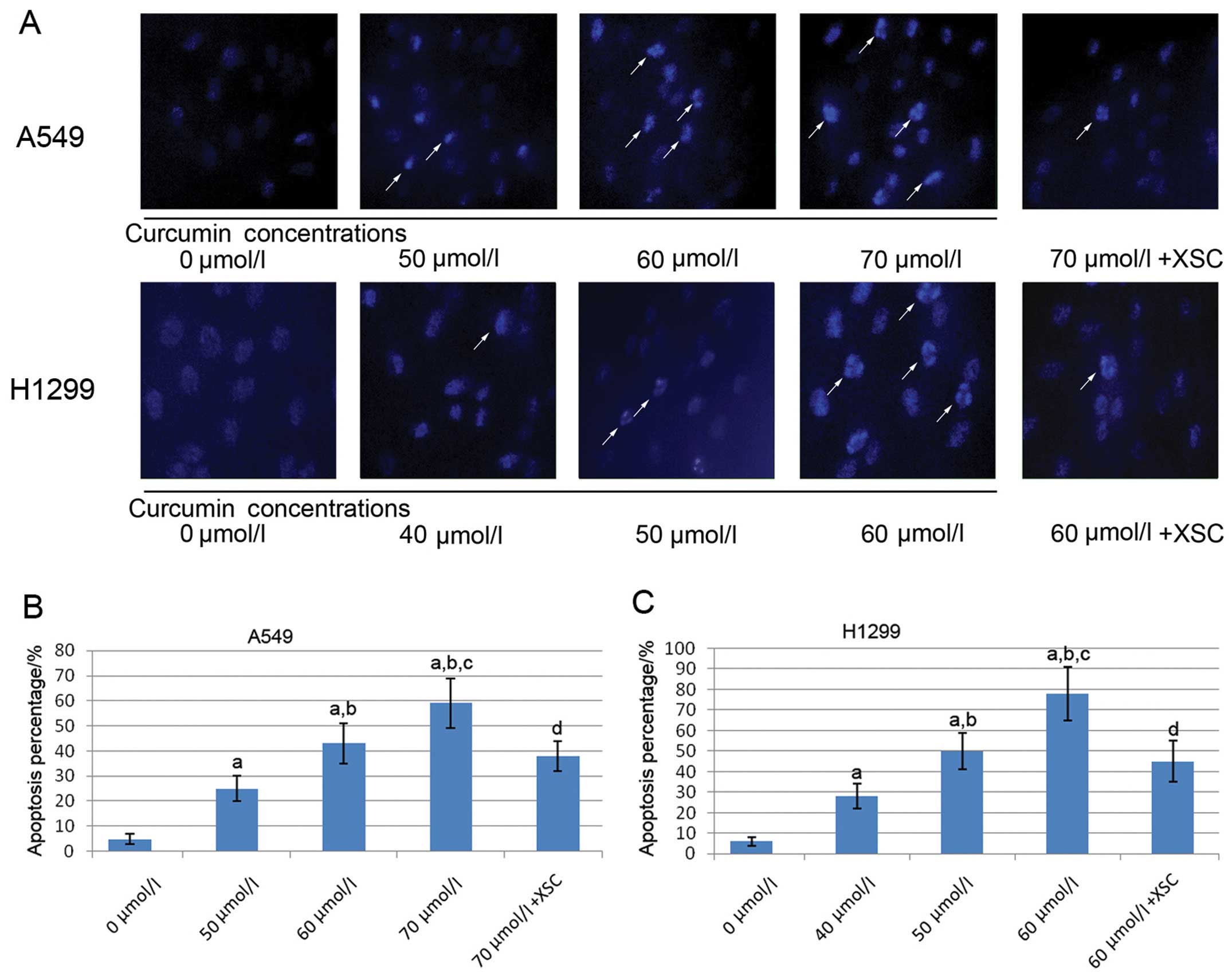 | Figure 2Effects of curcumin and xestospongin C
(XSC) on the apoptosis of A549 and H1299 lung cancer cells; 0, 50,
60 and 70 μmol/l indicate A549 cells incubated with curcumin
at concentrations of 0, 50, 60 and 70 μmol/l, respectively
for 24 h; 70 μmol/l + XSC indicates XSC-pre-treated A549
cells incubated with curcumin at 70 μmol/l for 24 h; 0, 40,
50 and 60 μmol/l indicate H1299 cells incubated with
curcumin at concentrations of 0, 40, 50 and 60 μmol/l,
respectively for 24 h; 60 μmol/l + XSC indicates
XSC-pre-treated H1299 cells incubated with curcumin at 60
μmol/l for 24 h. (A) Captured fluorescence images of A549
and H1299 cells stained with Hoechst 33342. White arrows indicate
apoptotic cells. Columns in (B and C) indicate apoptotic the
percentage of A549 cells and H1299 cells. a, Differences are
significantly different when compared with 0 μmol/l; b,
differences are significantly different when compared with 50
μmol/l (or 40 μmol/l for H1299 cells); c, differences
are significantly different when compared with 60 μmol/l (or
50 μmol/l for H1299); d, differences are significantly
different when compared with 70 μmol/l (or 60 μmol/l
for H1299 cells). |
The [Ca2+]i concentration is
elevated by treatment with curcumin, but this effect is reversed by
pre-treatment with XSC in the A549 and H1299 cells
The images of Fura-2/AM staining of the A549 and
H1299 cells are shown in Fig. 3.
As a calcium indicator, the intensities of Fura-2/AM staining were
used to determine the [Ca2+]i concentration. Trreatment
with curcumin significantly elevated the [Ca2+]i levels
in both the A549 and H1299 cells in a concentration-dependent
manner. However, following pre-treatment with XSC, the
curcumin-induced increase in the [Ca2+]i level was
impaired.
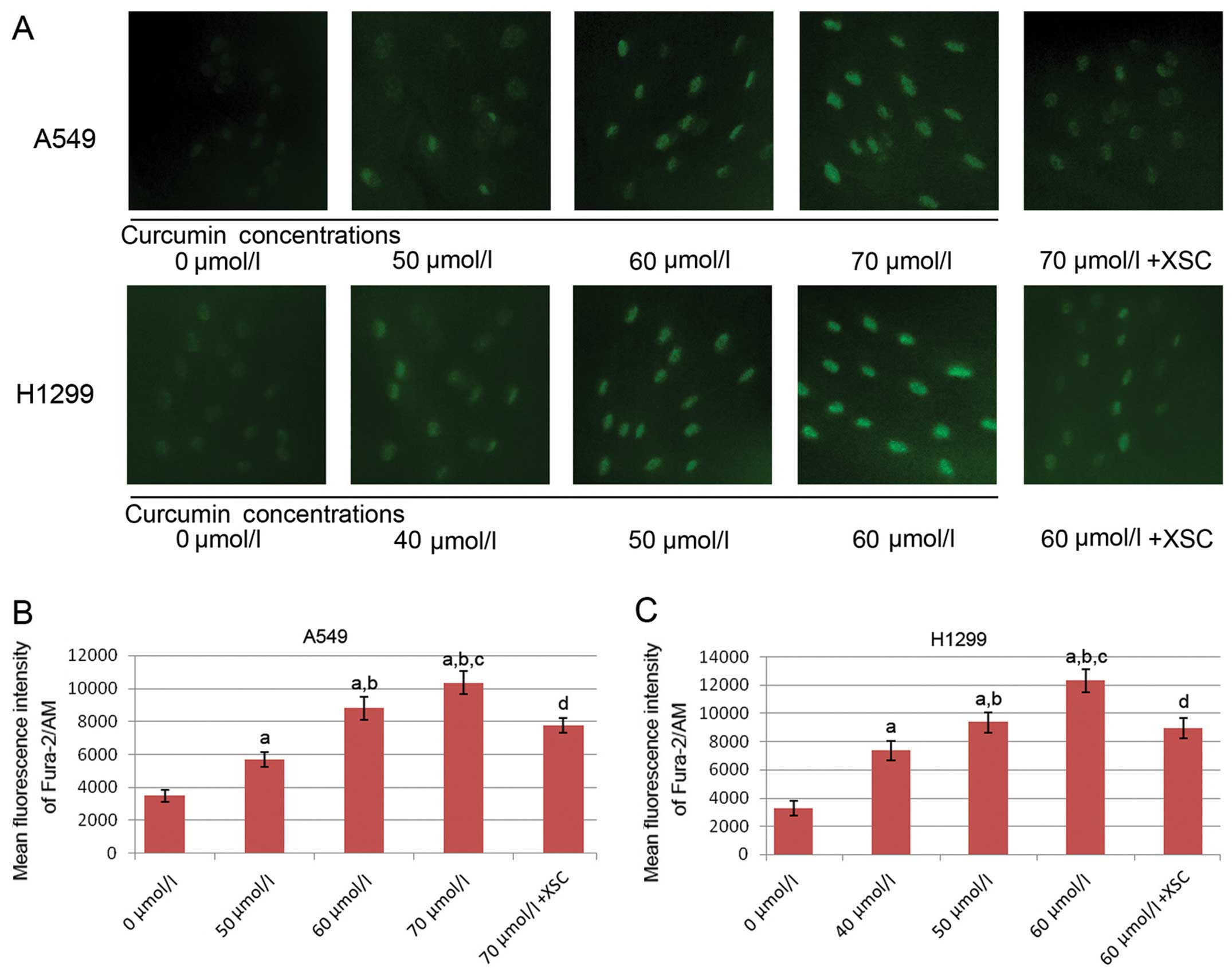 | Figure 3Effects of curcumin and xestospongin C
(XSC) on intracellular free calcium [Ca2+]i levels in
A549 and H1299 lung cancer cells; 0, 50, 60 and 70 μmol/l
indicate A549 cells incubated with curcumin at concentrations of 0,
50, 60 and 70 μmol/l, respectively for 24 h; 70
μmol/l + XSC indicates XSC-pre-treated A549 cells incubated
with curcumin at 70 μmol/l for 24 h; 0, 40, 50 and 60
μmol/l indicate H1299 cells incubated with curcumin at
concentrations of 0, 40, 50 and 60 μmol/l respectively for
24 h; 60 μmol/l + XSC indicates XSC-pre-treated H1299 cells
incubated with curcumin at 60 μmol/l for 24 h. (A) The
[Ca2+]i level was detected by Fura-2/AM fluorescence
staining. Positively stained calcium is shown as green zones in the
captured images under a microscope. Columns in (B and C) indicate
the mean fluorescence intensity of Fura-2/AM in A549 cells and
H1299 cells. a, Differences are significantly different when
compared with 0 μmol/l; b, differences are significantly
different when compared with 50 μmol/l (or 40 μmol/l
for H1299 cells); c, differences are significantly different when
compared with 60 μmol/l (or 50 μmol/l for H1299
cells); d, differences are significantly different when compared
with 70 μmol/l (or 60 μmol/l for H1299 cells). |
Protective effect of XSC on the
curcumin-induced decrease in ΔΨm in the A549 and H1299 cells
The flow cytometric assessment of rhodamine 123
staining was used to examine the ΔΨm level. The decrease in ΔΨm
indicated the increased permeability and impaired integrity of the
mitochondrial membrane which was considered as a pro-apoptotic
event. Treatment with curcumin decreased the ΔΨm in both the A549
and H1299 cells in a concentration-dependent manner (Fig. 4). However, the XSC pre-treated
A549 and H1299 cells showed a distinct resistance to the
curcumin-induced decrease in ΔΨm.
 | Figure 4Effects of curcumin and xestospongin
C (XSC) on mitochondrial membrane potential (ΔΨm) in A549 and H1299
lung cancer cells; 0, 50, 60 and 70 μmol/l indicate A549
cells incubated with curcumin at concentrations of 0, 50, 60 and 70
μmol/l, respectively for 24 h; 70 μmol/l + XSC
indicates XSC-pretreated A549 cells incubated with curcumin at 70
μmol/l for 24 h; 0, 40, 50 and 60 μmol/l indicate
H1299 cells incubated with curcumin at concentrations of 0, 40, 50
and 60 μmol/l, respectively for 24 h; 60 μmol/l + XSC
indicates XSC-pre-treated H1299 cells incubated with curcumin at 60
μmol/l for 24 h. Columns on the upper and lower panel show
the mean fluorescence intensity of rhodamine 123 staining of A549
and H1299 respectively. Rhodamine 123 is usually used as an
indicator stain of ΔΨm. Values are presented as the means ± SD. a,
Differences are significantly different when compared with 0
μmol/l; b, differences are significantly different when
compared with 50 μmol/l (or 40 μmol/l for H1299
cells); c, differences are significantly different when compared
with 60 μmol/l (or 50 μmol/l for H1299 cells); d,
differences are significantly different when compared with 70
μmol/l (or 60 μmol/l for H1299 cells). |
Effect of curcumin on Bcl-2 expression
and IP3R phosphorylation
Fig. 5 illustrates
the immunoblots of Bcl-2, IP3R and p-IP3R in the curcumin-treated
A549 and H1299 cells. We found that the expression of Bcl-2 was
reduced by treatment curcumin in a concentration-dependent manner.
We also found that the phosphorylation of IP3R was enhanced by
treatment with curcumin in a concentration-dependent manner.
Pre-treatment with XSC failed to affect the changes in Bcl-2
expression and IP3R phosphorylation in the curcumin-treated
cells.
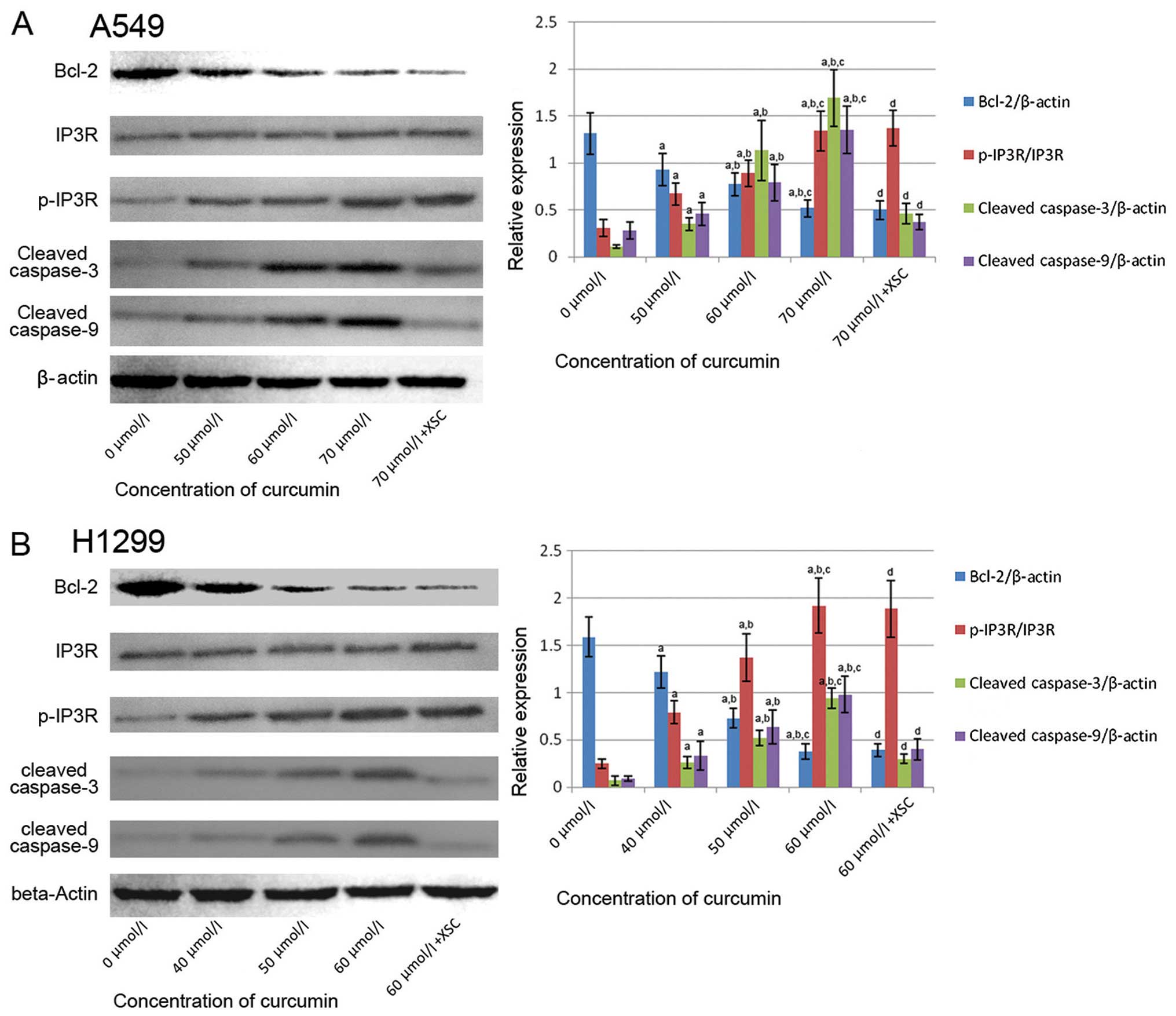 | Figure 5Effects of curcumin and xestospongin
C (XSC) on relative protein expression in A549 and H1299 cells; 0,
50, 60 and 70 μmol/l indicate A549 cells incubated with
curcumin at concentrations of 0, 50, 60 and 70 μmol/l,
respectively for 24 h; 70 μmol/l + XSC indicates
XSC-pre-treated A549 cells incubated with curcumin at 70
μmol/l for 24 h; 0, 40, 50 and 60 μmol/l indicate
H1299 cells incubated with curcumin at concentrations of 0, 40, 50
and 60 μmol/l, respectively for 24 h; 60 μmol/l + XSC
indicates XSC-pre-treated H1299 cells incubated with curcumin at 60
μmol/l for 24 h. The left panels in both (A and B)
demonstrated the immunoblots of Bcl-2. Inositol 1,4,5-triphosphate
receptor (IP3R), p-IP3R, cleaved caspase-3, cleaved caspase-9 and
β-actin in A549 and H1299 cells. Columns on the right panels in
both (A and B) indicate the ratio of Bcl-2/β-actin, p-IP3R/IP3R,
cleaved caspase-3/β-actin and cleaved caspase-9/β-actin
respectively. a, Differences are significantly different when
compared with 0 μmol/l; b, differences are significantly
different when compared with 50 μmol/ (or 40 μmol/
for H1299 cells); c, differences are significantly different when
compared with 60 μmol/ (or 50 μmol for H1299 cells);
d, differences are significantly different when compared with 70
μmol/ (or 60 μmol for H1299 cells). |
Curcumin initiates caspase cascade
activation which is reversed by pre-treatment with XSC
Fig. 5 also
demonstrates the immunoblots of cleaved caspase-3 and caspase-9 in
the A549 and H1299 cells. It was found that treatment with curcumin
stimulated the expression levels of cleaved caspase-3 and caspase-9
in a concentration-dependent manner. The cleavage of caspase-3 and
caspase-9 suggests the activation of caspase cascade, which is
generally accepted as the indicator of mitochondrial-dependent
apoptosis. However, in the XSC pre-treated A549 and H1299 cells,
the activation of the caspase cascade was markedly attenuated.
Discussion
In the present study, we investigated the
anti-proliferative effects of curcumin on NSCLC cells. Two NSCLC
cell lines, A549 and H1299, were used in this study. We found that
curcumin significantly inhibited the growth of the A549 and H1299
cells in a concentration-dependent manner. The cytotoxic effects of
curcumin on lung cancer cells were mediated by the induction of
apoptosis. Thus, we further investigated the possible mechanisms
involved. For the first time, to the best of our knowledge, our
results indicated that the phosphorylation status of IP3R played a
crucial role in this mitochondrial-dependent apoptosis
machinery.
Due to the inefficiency of conventional anticancer
therapies and drugs, the prognosis of NSCLC remains poor. It is
tremendously costly and time-consuming and requires much effort to
continuously seek for and develop new effective anticancer drugs,
as has been over the past decades (15,16). Mother nature may provide promising
solutions to this problem. To date, a number of natural products,
such as curcumin, resveratrol (17), ginsenosides (18), matrine (19), baicalin (20) have been found to exert potent
anticancer effects. Curcumin has been studied since the 1990s and
has been shown to exhibit various biological functions, including
anti-inflammatory, anti-microbial, anti-fibrotic, antioxidant and
anticancer activities (21).
Previous studies have indicated that curcumin inhibits the
proliferation of many types of human cancer cells (22,23). In this study, we found that
significantly curcumin inhibited the proliferation of A549 and
H1299 lunc cancer cells in a concentration-dependent manner.
The induction of apoptosis is the most common
approach to inhibit the proliferation of cancer cells, which is
also recognized as the cytotoxic effect of anticancer reagents. At
cytotoxic concentrations, above 50 μmol/l for A549 cells and
40 μmol/l for H1299 cells in the current study, curcumin
induced cell apoptosis in a concentration-dependent manner which
was evidenced by Hoechst staining. Mitochondrial-dependent
apoptosis is known as one of the most common apoptotic pathways
(24). At the early stage of
apoptosis, there are several featured changes in the dysfunctioned
mitochondria. The integrity of the mitochondrial membrane cannot be
maintained and then several soluble proteins, such as cytochrome
c are released into the cytosol due to increased
permeability of the membrane (25,26). This change is characterized by
loss of ΔΨm (27). In the present
study, following incubation with curcumin, the ΔΨm in both the A549
and H1299 cells decreased significantly as the concentration of
curcumin increased. After cytochrome c binds with
pro-caspase-9 and apoptosis protease activating factor-1 (APAF-1),
the apoptosome is formed to cleave caspase-9, triggering the
activation of the caspase cascade, resulting in cell apoptosis
(28). In the present study, the
expression levels of cleaved caspase-3 and cleaved caspase-9
increased significantly following treatment with curcumin,
indicating that the curcumin-induced apoptosis was
mitochondrial-dependent.
The intracellular calcium overload may be one of the
initiators of mitochondrial-dependent apoptosis (29). In cytosol, the excess accumulation
of calcium leads to mitochondrial damage directly and indirectly.
Formed calcium phosphate in the mitochondrial matrix interrupts the
functions of the respiratory chain, leading to the loss of ΔΨm
(30). Additionally, the
activities of some enzymes, such as Mn-superoxide dismutase (MnSOD)
and peroxidase are impaired by calcium accumulation, resulting in
the excessive production of ROS, which compromises the integrity of
the mitochondrial membrane (31).
In the present study, we found that the [Ca2+]i
concentration in both the A549 and H1299 cells was significantly
elevated following treatment with curcumin. This result suggested
that the calcium overload induced mitochondrial-dependent apoptosis
in the curcumin-treated NSCLC cells.
In order to investigate the role of IP3R in
curcumin-induced mitochondrial-dependent apoptosis, XSC, a specific
inhibitor of IP3R, was used to pre-treat the cells in this study.
As shown by our results, the XSC pre-treated A549 and H1299 cells
were resistant to curcumin-induced apoptosis. Mechanically, XSC
inhibited the increase in the [Ca2+]i level and
apoptotic proteins, indicating that IP3R was involved in the
calcium overload-induced mitochondrial-dependent apoptosis in the
curcumin-treated cells. IP3R is a universal intracellular calcium
release channel. The activation of IP3R is considered to be
dependent on its phosphorylation status (32). In most cases, IP3R is activated by
phosphorylation. IP3R is also believed to be located in the Golgi
apparatus, the nuclear envelop and the mitochondrial-associated ER
membranes (MAMs), which enables activated IP3R to mediate the
direct calcium transfer from the ER to the mitochondria (33).
It is generally accepted that the Bcl-2 protein is
anti-apoptotic, which inhibits the accumulation and oligomerization
of activated Bax and Bak, thus suppressing mitochondrial outer
membrane permeabilization (34).
Furthermore, Bcl-2 has been shown to play a role in the regulation
of calcium release from the ER (35). In a previous study, it was
suggested that Bcl-2 acts as a docking protein to facilitate the
interaction of IP3R and calcineurin, which then dephosphorylates
IP3R, decreasing the channel activity (36,37). In the present study, we found that
in the curcumin-treated A549 and H1299 cells, the IP3R
phosphorylation was enhanced and Bcl-2 expression was
downregulated. The changes in IP3R phosphorylation and Bcl-2
expression were not affected by XSC. These results suggested that
the downregulation of Bcl-2 upregulated the phosphorylation of
IP3R, thus increasing the IICR following treatment with
curcumin.
In the present study, we found that the
curcumin-induced NSCLC cell apoptosis was associated with calcium
overload, which further induced mitochondrial-dependent apoptosis.
Mechanically, the calcium overload in the curcumin-treated NSCLC
cells was mediated by IP3R. Bcl-2 may be involved in regulating the
phosphorylation status of IP3R. In conclusion, our findings provide
new insight, namely that curcumininduces the apoptosis of cancer
cells through a calcium signaling-mediated pathway.
References
|
1
|
Ike S, Tanaka F, Ueda M, et al: Evaluation
of improvement in prognosis in surgical cases of lung cancer based
on the 5-year survival rate. Nippon Kyobu Geka Gakkai Zasshi.
44:312–313. 1996.In Japanese.
|
|
2
|
Hung JJ, Jeng WJ, Hsu WH, Chou TY, Huang
BS and Wu YC: Predictors of death, local recurrence, and distant
metastasis in completely resected pathological stage-I
non-small-cell lung cancer. J Thorac Oncol. 7:1115–1123. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saisho S, Yasuda K, Maeda A, et al:
Post-recurrence survival of patients with non-small-cell lung
cancer after curative resection with or without induction/adjuvant
chemotherapy. Interact Cardiovasc Thorac Surg. 16:166–172. 2013.
View Article : Google Scholar :
|
|
4
|
Li JH, Yue W, Huang Z, et al: Calcium
overload induces C6 rat glioma cell apoptosis in sonodynamic
therapy. Int J Radiat Biol. 87:1061–1066. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakayama H, Fujio Y and Yamaguchi O:
Calcium dependent signaling in cardiac hypertrophy and cell death.
Clin Calcium. 23:505–515. 2013.In Japanese. PubMed/NCBI
|
|
6
|
Seo SR and Seo JT: Calcium overload is
essential for the acceleration of staurosporine-induced cell death
following neuronal differentiation in PC12 cells. Exp Mol Med.
41:269–276. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma TS: Sarcoplasmic reticulum calcium
ATPase overexpression induces cellular calcium overload and cell
death. Ann NY Acad Sci. 853:325–328. 1998. View Article : Google Scholar
|
|
8
|
Johnson GG, White MC and Grimaldi M:
Stressed to death: targeting endoplasmic reticulum stress response
induced apoptosis in gliomas. Curr Pharm Des. 17:284–292. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He Q, Lee DI, Rong R, et al: Endoplasmic
reticulum calcium pool depletion-induced apoptosis is coupled with
activation of the death receptor 5 pathway. Oncogene. 21:2623–2633.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deniaud A, Sharaf el dein O, Maillier E,
et al: Endoplasmic reticulum stress induces calcium-dependent
permeability transition, mitochondrial outer membrane
permeabilization and apoptosis. Oncogene. 27:285–299. 2008.
View Article : Google Scholar
|
|
11
|
Kasumu AW, Liang X, Egorova P, Vorontsova
D and Bezprozvanny I: Chronic suppression of inositol
1,4,5-triphosphate receptor-mediated calcium signaling in
cerebellar purkinje cells alleviates pathological phenotype in
spinocerebellar ataxia 2 mice. J Neurosci. 32:12786–12796. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rong YP, Aromolaran AS, Bultynck G, et al:
Targeting Bcl-2-IP3 receptor interaction to reverse Bcl-2’s
inhibition of apoptotic calcium signals. Mol Cell. 31:255–265.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Puliyappadamba VT, Cheriyan VT,
Thulasidasan AK, et al: Nicotine-induced survival signaling in lung
cancer cells is dependent on their p53 status while its
down-regulation by curcumin is independent. Mol Cancer. 9:2202010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang WH, Chiang IT, Ding K, et al:
Curcumin-induced apoptosis in human hepatocellular carcinoma j5
cells: Critical role of ca(+2)-dependent pathway. Evid Based
Complement Alternat Med. 2012:5129072012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zi X and Zhang R: Anti-cancer molecular
targets of natural products. Curr Cancer Drug Targets. 13:4852013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cragg GM, Grothaus PG and Newman DJ:
Impact of natural products on developing new anti-cancer agents.
Chem Rev. 109:3012–3043. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun W, Wang W, Kim J, et al: Anti-cancer
effect of resveratrol is associated with induction of apoptosis via
a mitochondrial pathway alignment. Adv Exp Med Biol. 614:179–186.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang W, Rayburn ER, Hao M, et al:
Experimental therapy of prostate cancer with novel natural product
anti-cancer ginsenosides. Prostate. 68:809–819. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu XY, Ruan LM, Mao WW, Wang JQ, Shen YQ
and Sui MH: Preparation of RGD-modified long circulating liposome
loading matrine, and its in vitro anti-cancer effects. Int J Med
Sci. 7:197–208. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Franek KJ, Zhou Z, Zhang WD and Chen WY:
In vitro studies of baicalin alone or in combination with Salvia
miltiorrhiza extract as a potential anticancer agent. Int J Oncol.
26:217–224. 2005.
|
|
21
|
Noorafshan A and Ashkani-Esfahani S: A
review of therapeutic effects of curcumin. Curr Pharm Des.
19:2032–2046. 2013.
|
|
22
|
Mehta HJ, Patel V and Sadikot RT: Curcumin
and lung cancer-a review. Target Oncol. May 21–2014.Epub ahead of
print. View Article : Google Scholar
|
|
23
|
Darvesh AS, Aggarwal BB and Bishayee A:
Curcumin and liver cancer: a review. Curr Pharm Biotechnol.
13:218–228. 2012. View Article : Google Scholar
|
|
24
|
Jiang L, Liu Y, Ma MM, Tang YB, Zhou JG
and Guan YY: Mitochondria dependent pathway is involved in the
protective effect of bestrophin-3 on hydrogen peroxide-induced
apoptosis in basilar artery smooth muscle cells. Apoptosis.
18:556–565. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sawai H and Domae N: Release of cytochrome
c from mitochondria precedes Bax translocation/activation in Triton
X-100-induced apoptosis. Leuk Res. 32:445–453. 2008. View Article : Google Scholar
|
|
26
|
Wang S and El-Deiry WS: Cytochrome c: a
crosslink between the mitochondria and the endoplasmic reticulum in
calcium-dependent apoptosis. Cancer Biol Ther. 3:44–46. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zamzami N and Kroemer G: Methods to
measure membrane potential and permeability transition in the
mitochondria during apoptosis. Methods Mol Biol. 282:103–115.
2004.PubMed/NCBI
|
|
28
|
Soengas MS, Alarcon RM, Yoshida H, et al:
Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor
inhibition. Science. 284:156–159. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pinton P, Giorgi C, Siviero R, Zecchini E
and Rizzuto R: Calcium and apoptosis: ER-mitochondria
Ca2+ transfer in the control of apoptosis. Oncogene.
27:6407–6418. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kurskii MD, Tugai VA and Fedoriv AN:
Effect of serotonin and calcium on separate components of
respiratory chain of mitochondria in some rabbit tissues. Ukr
Biokhim Zh. 42:584–588. 1970.In Ukrainian.
|
|
31
|
Komary Z, Tretter L and Adam-Vizi V:
Membrane potential-related effect of calcium on reactive oxygen
species generation in isolated brain mitochondria. Biochim Biophys
Acta. 1797:922–928. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vanderheyden V, Devogelaere B, Missiaen L,
De Smedt H, Bultynck G and Parys JB: Regulation of inositol
1,4,5-trispho-sphate-induced Ca2+ release by reversible
phosphorylation and dephosphorylation. Biochim Biophys Acta.
1793:959–970. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Giorgi C, Wieckowski MR, Pandolfi PP and
Pinton P: Mitochondria associated membranes (MAMs) as critical hubs
for apoptosis. Commun Integr Biol. 4:334–335. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hahn P, Lindsten T, Ying GS, et al:
Proapoptotic bcl-2 family members, Bax and Bak, are essential for
developmental photo-receptor apoptosis. Invest Ophthalmol Vis Sci.
44:3598–3605. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rong YP, Bultynck G, Aromolaran AS, et al:
The BH4 domain of Bcl-2 inhibits ER calcium release and apoptosis
by binding the regulatory and coupling domain of the IP3 receptor.
Proc Natl Acad Sci USA. 106:14397–14402. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hanson CJ, Bootman MD, Distelhorst CW,
Wojcikiewicz RJ and Roderick HL: Bcl-2 suppresses Ca2+
release through inositol 1,4,5-trisphosphate receptors and inhibits
Ca2+ uptake by mitochondria without affecting ER calcium
store content. Cell Calcium. 44:324–338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen R, Valencia I, Zhong F, et al: Bcl-2
functionally interacts with inositol 1,4,5-trisphosphate receptors
to regulate calcium release from the ER in response to inositol
1,4,5-trisphosphate. J Cell Biol. 166:193–203. 2004. View Article : Google Scholar : PubMed/NCBI
|



















