Introduction
Atherosclerosis is a common, complex, chronic
disease with high morbidity and mortality, and it is characterized
by the formation of atheromatous plaque in the intimal layer. A
growing body of evidence indicates that the deregulation of cell
behavior, such as proliferation and the migration of vascular
smooth muscle cells (VSMCs) play essential roles in the
pathogenesis of atherosclerosis (1). The formation of atheromatous plaque
is believed to be initiated through the activation of endothelial
cells by various metabolic risk factors, such as hyperlipidemia,
hypertension or pro-inflammatory mediators. The aberrant activation
of endothelial cells permits circulating monocytes to infiltrate
the intima, leading to the formation of foam cells by the
phagocytosis of low-density lipoprotein (LDL) cholesterol and
oxidized phospholipids. Subsequently, atherosclerotic plaque is
formed by proliferated VSMCs, physiologically located in the media
of vessel walls, migrated to the intima (1–3).
Migrated VSMCs in the intima can produce extracellular matrix
(ECM), which is the major component of the fibrous cap of
atherosclerotic plaque (4).
MicroRNAs (miRNAs or miRs) are a class of endogenous
and small (approximately 22 nt) non-coding RNAs that suppress gene
expression BY binding to the 3′ untranslated region (3′UTR) of mRNA
transcripts to promote mRNA degradation or the translational
inhibition of target mRNAs (5).
To date, approximately 1,000 human miRNAs have been discovered and
are believed to regulate up to 30% of gene expression, and are
thereby involved in the regulation of a wide range of biological
activities, including cell growth, apoptosis and differentiation.
In recent years, accumulating evidence indicates that miRNAs play
crucial roles in the control of VSMC function and the response to
vascular injury by targeting transcriptional factors or key factors
along certain signaling pathways in VSMC proliferation and
migration (6,7). Indeed, a group of miRNAs, including
miR-143/145, miR-221/222, miR-24, miR-26a, miR-1, miR-146a and
miR-21, have been found to modulate VSMC differentiation, the
phenotypic switch and neointimal formation under variable
conditions (8–15).
Among these miRNAs, miR-155 is one of the most
commonly studied miRNAs in the development of atherosclerosis
(16,17), and the downregulation of miR-155
has been reported to be involved in the susceptibility to
atherosclerosis (18).
Nevertheless, a previous study on miR-155 focused on its role in
endothelial cells (19), and one
of its target genes is endothelial nitric oxide synthase (eNOS);
miR-155 downregulates eNOS expression by decreasing eNOS mRNA
stability and by binding to its 3′UTR in human umbilical vein
endothelial cells (HUVECs), and the knockdown of miR-155 prevents
the cytokine-induced downregulation of eNOS expression, the
reduction in nitric oxide (NO) production and the impairment of
endothelium-dependent vascular relaxation (20). A previous study demonstrated that
eNOS also plays a functional role in regulating the biological
behaviors of VSMCs (21), which
is consistent with other previous studies showing that the NO
signaling pathway is involved in the regulation of the cellular
activities of VSMCs (22,23).
In the present study, we proved that miR-155
suppresses the expression level of eNOS by binding to the 3′UTR
directly in VSMCs. Thus, it plays a significant role in the
development of atherosclerosis.
Materials and methods
Sample collection
Samples were collected from atherosclerotic lesions
from patients who received coronary artery bypass surgery or
carotid endarterectomy (n=12) and reference left internal mammary
arteries were harvested during coronary artery bypass surgery (n=9)
at the Fourth Affiliated Hospital of China Medical University,
Shenyang, China. Subjects with other cardiovascular diseases, such
as aortic dissection and aneurysms were excluded from the study.
The study protocol was approved by the Ethics Committee of China
Medical University, and written informed consent was obtained from
each participant prior to enrollment.
Cell culture
Human aortic SMCs (HASMCs) were cultured in SmGM-2
growth medium (both from Lonza, Walkersville, MD, USA) in 5% fetal
bovine serum (FBS) following the manufacturer’s instructions.
Isolation of RNA and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated using the miRNeasy mini kit
(Qiagen, Valencia, CA, USA) following the manufacturer’s
instructions. The quantity and quality of the RNA were then
determined using the NanoDrop spectrophotometer (NanoDrop
Technologies, Wilmington, DE, USA) and agarose electrophoresis.
Subsequently, the cDNA of the target gene was synthesized using
reverse transcriptase (Invitrogen, Carlsbad, CA, USA) and amplified
using primer set, 5′-CTGTTAATGCTAATCGTGATAG-3′ and
5′-GCAGGGTCCGAGGT-3′ (miR-155); primer set, 5′-CTC
GCTTCGGCAGCACA-3′ and 5′-AACGCTTCACGAATTT GCGT-3′ (U6); primer set,
5′-CCCTTCAGTGGCTGGT ACAT-3′ and 5′-CACGATGGTGACTTTGGCTA-3′ (eNOS).
The SYBR-Green real-time detection system (from Bio-Rad
Laboratories, Hercules, CA, USA) was used to quantify the signals.
U6, an internal control, was used to normalize the miR-155 levels.
The samples were all normalized to the endogenous control, U6. All
fold changes were calculated using the ΔΔCt method.
Targeted miRNA overexpression
miR-115 mimics (5′-ttaaugctaatcgtgataggggt-3′) and
anti-eNOS siRNA (5′-tgtggaaagacaaggcagca-3′) were purchased from
Ambion (Austin, TX, USA), and the VSMCs were transfected with high
efficiency using the transfection Lipofectamine 2000 (Invitrogen).
Successful transfection (>90% of all cells) was confirmed by
visual fluorescent microscopic analysis. The in vitro
experiments detailed below included a Cy3-labeled control scrambled
oligo (negative control) known to have no effect on any human miRNA
to minimize the non-specific effect, and cohorts transfected with
30 or 100 pmol of miR-155 mimics, 100 pmol of anti-eNOS siRNAs or
40 ng pcDNA-eNOS.
Prediction of the target of miR-155
We identified the potential target genes of miR-155
by searching the online microRNA database, http://targetscan.org/, and narrowed down the
candidate genes by analyzing the pathological or physiological
function of the candidate genes.
Boyden chamber chemotaxis assays
To evaluate the migratory capability of the VSMCs, a
modified Boyden chamber assay was conducted, as previously
described (24). Briefly, 6-well
Transwell migration chambers with 8 μm pores
(Becton-Dickinson, Franklin Lakes, NJ, USA) were used, and
6×104 HASMCs transfected with miR-155 mimics, anti-eNOS
siRNA, pcDNA-eNOS or the control were serum-starved for 24 h and
then placed in the upper chamber in 1 ml of medium. A total of 2 ml
of SmGM-2 medium were added to the lower chamber, and 24 h later,
the migrated cells were fixed, stained with trypan blue and
counted.
Proliferation and cell survival
assays
Amodified3-[4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay was performed to examine the HASMC viability,
as previously described (25).
Briefly, the HASMCs transfected with miR-155 mimics, anti-eNOS
siRNAs or pcDNA-eNOS were cultured in 96-well plates for 24 h and
then 10 μl of MTT AB solution (Millipore, Billerica, MA,
USA) were added, followed by incubation for 4 h. The absorbance was
determined at 570 nm (reference wavelength, 630 nm).
Apoptosis assays
Programmed cell death rates were assessed using a
commercially available apoptosis assay kit (Becton-Dickinson). The
differentially transfected HASMCs were treated with 10%
H2O2 in serum-free medium for 6 h.
Subsequently, 1×105 cells were harvested and stained
with 10 μl FITC Annexin V and 10 μl propidium iodide
and FACS sorted within 1 h [BD FACSCalibur, 530 nm (FL1) and
>575 nm (FL3); Becton-Dickinson].
Western blot analysis
The differentially treated HASMCs were harvested and
lysed, and the cell lysates were subjected to 10% polyacrylamide
gel electrophoresis (PAGE). The separated proteins were transferred
onto a polyvinylidene difluoride (PVDF) membrane which was blocked
with 5% non-fat milk solution under room temperature for 1 h.
Subsequently, the PVDF membrane was incubated with primary
antibodies directed against human eNOS (1:500; ab66127) and β-actin
(1:1,000; ab6276) (Abcam, Cambridge, MA, USA), and an
HRP-conjugated goat-anti-rabbit secondary antibody (1:2,000) (Cell
Signaling Technology, Danvers, MA, USA). Exposed films were scanned
and fluorescence signals were detected using the ECL kit (Applygen,
Beijing, China) and integrated band densities were
densitometrically analyzed with the background subtracted.
Luciferase assay
The full-length of human eNOS 3′UTR was
PCR-amplified from the genomic DNA sample. The PCR product was then
cloned using the TA cloning kit (Invitrogen), and the accuracy of
the insert was confirmed by Sanger sequencing. Subsequently, the
QuickChange XL site-directed mutagenesis kit (Stratagene, La Jolla,
CA, USA) was used to introduce the variant. Finally, the 3′UTR of
Renilla luciferase in the vector pRL-SV40 (Promega, Madison,
WI, USA) was substituted with the generated wild-type and mutant
3′UTR of eNOS. Luciferase assay was performed using the HASMCs, and
the cells were seeded at 1×105 cells/well in 24-well
plates. After 12 h, the cells were transfected with miR-155 mimics
together with pRL-SV40 containing the wild-type or mutant 3′UTR of
eNOS using Lipofectamine 2000 (Invitrogen) according to
manufacturer’s instructions. Twenty-four hours after transfection,
the cells were harvested by the addition of passive lysis buffer.
Luciferase activities in the cell lysate were determined using the
Dual-Luciferase assay system (Promega) on a TD-20/20 luminometer
(Turner BioSystems, Sunnyvale, CA, USA).
Determination of the concentration of NO
and cyclic guanosine monophosphate (cGMP) in HASMCs
The concentrations of NO and cGMP were determined in
the HASMCs transfected with miR-155 mimics or anti-eNOS siRNA using
the nitric oxide assay kit (Abcam) and the cGMP assay kit (Cell
Signaling Technology).
Overexpression of eNOS
The coding sequence of eNOS was amplified using the
following primers: 5′-ATAAGAATGCGGCCGCATGGGCAACTTGAAGAGCGTGGC-3′
and 5′-GGTCTAGATCAGGGGCTGTTGGTGTCTGAGCC-3′. The PCR product was
purified and inserted into the NotI and XbaI
restrictive sites in pcDNA3.1. The accuracy of the insert was
verified using direct Sanger sequencing. The plasmid containing
eNOS was transfected into the HASMCs using Lipofectamine 2000.
Statistical analysis
Data are presented as the means ± SD. All in
vitro experiments included at least triple replicates per
group. Data were subjected to the Kolmogorov-Smirnov test to
determine distribution. Groups were compared using the two-tailed
Student’s t-test for parametric data (two groups comparison) or
one-way ANOVA (multiple groups comparison). A value of P<0.05
was considered to indicate a statistically significant difference.
Data analysis was performed using SPSS 20.0 software (SPSS Inc.,
Chicago, IL, USA).
Results
Comparison of miR-155 between
atherosclerotic lesions and normal control samples
Twelve samples from atherosclerotic lesions and 9
normal control samples were collected and the demographic
parameters and clinical characteristics are presented in Table I. No difference was noted
regarding the age, gender, body mass index (BMI), and blood
pressure and blood glucose levels. The expression levels of
miR-145, miR-221, miR-26a, miR-1, miR-146a, miR-21 and miR-155 were
determined and compared between the 2 groups by RT-qPCR, and no
difference was noted regarding the expression levels of these miRs
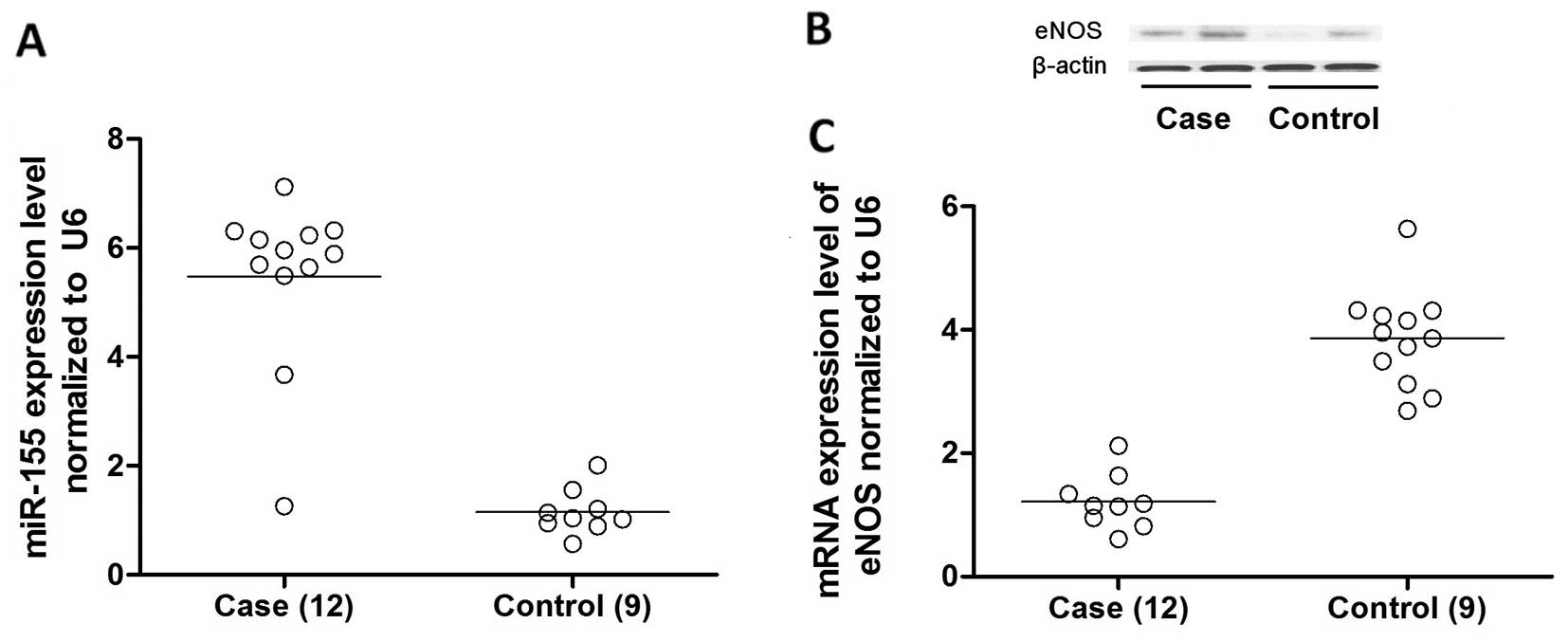
(data not shown) except for miR-155. The relative expression of
miR-155 in the atherosclerotic plaque samples was significantly
upregulated to approximately 500% compared with normal controls
(Fig. 1A).
 | Table IDemographic parameters and clinical
characteristics of the cases and controls enrolled in this
study. |
Table I
Demographic parameters and clinical
characteristics of the cases and controls enrolled in this
study.
| Variables | Controls (n=9) | Cases (n=12) | P-value |
|---|
| Age (years) | 61.4±6.3 | 62.1±6.7 | 0.811 |
| Gender (M/F) | 6/3 | 9/3 | 0.677 |
| Blood pressure
(mmHg) |
| Systolic BP | 142±11.6 | 151±17.2 | 0.192 |
| Diastolic BP | 92±10.2 | 96±11.4 | 0.416 |
| Glucose
(mg/dl) | 138±14.8 | 146±15.1 | 0.241 |
| Body mass index
(kg/m2) | 27.3±4.7 | 28.5±4.8 | 0.574 |
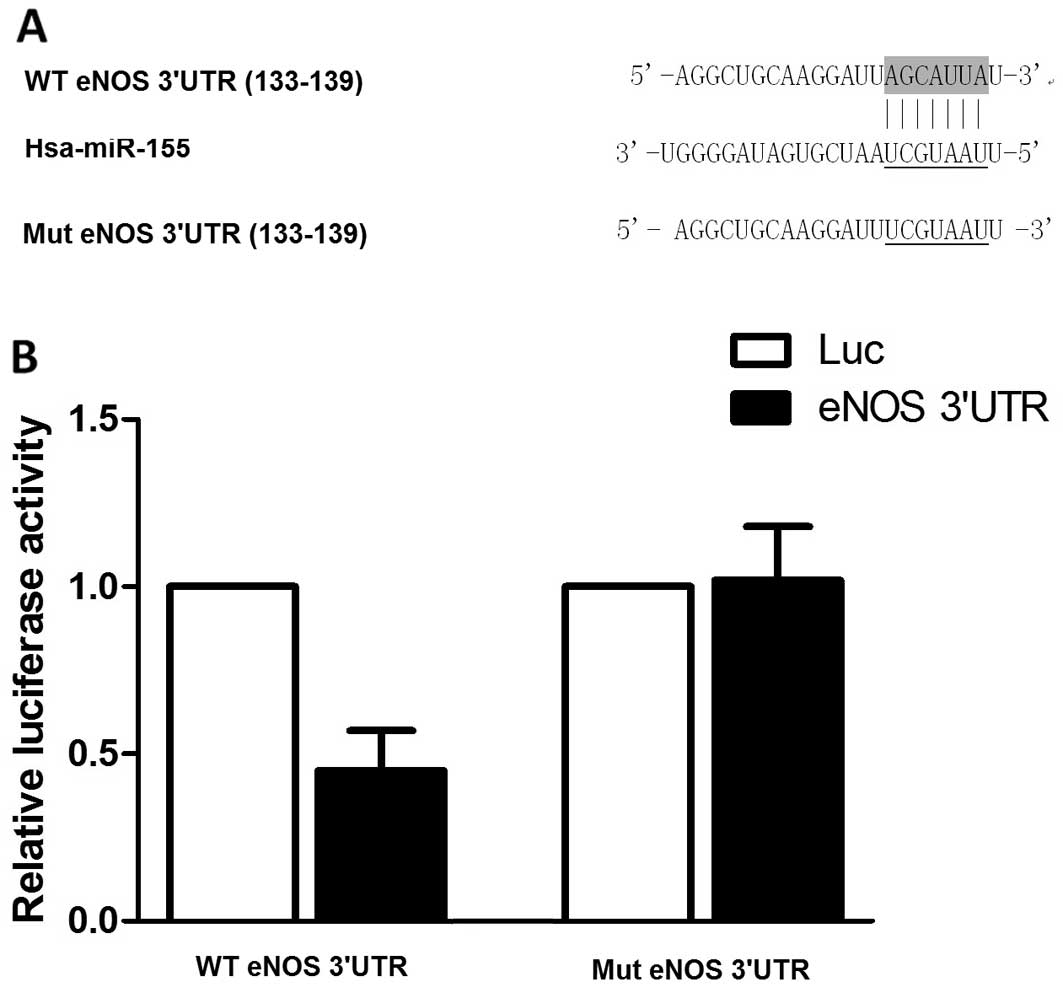
eNOS is a direct target of miR-155 in
VSMCs
Based on the computational analysis using an online
target predicting tool (www.targetscan.org), we identified that miR-155 was
able to bind to the 3′UTR of eNOS mRNA, indicating that this gene
may be a potential molecular target of miR-155 (Fig. 2A). To determine whether miR-155
regulates the expression of eNOS by directly interacting with the
3′UTR of the gene in VSMCs, we subcloned the eNOS 3′UTR into a
luciferase reporter plasmid and performed luciferase assay using
the HASMCs. The results revealed that co-transfection of the
miR-155 mimics with the eNOS 3′UTR reporter resulted in the
inhibition of luciferase activity (Fig. 2B). However, miR-155 failed to
suppress the activity of the mutant eNOS 3′UTR reporter (Fig. 2B). In agreement with the reporter
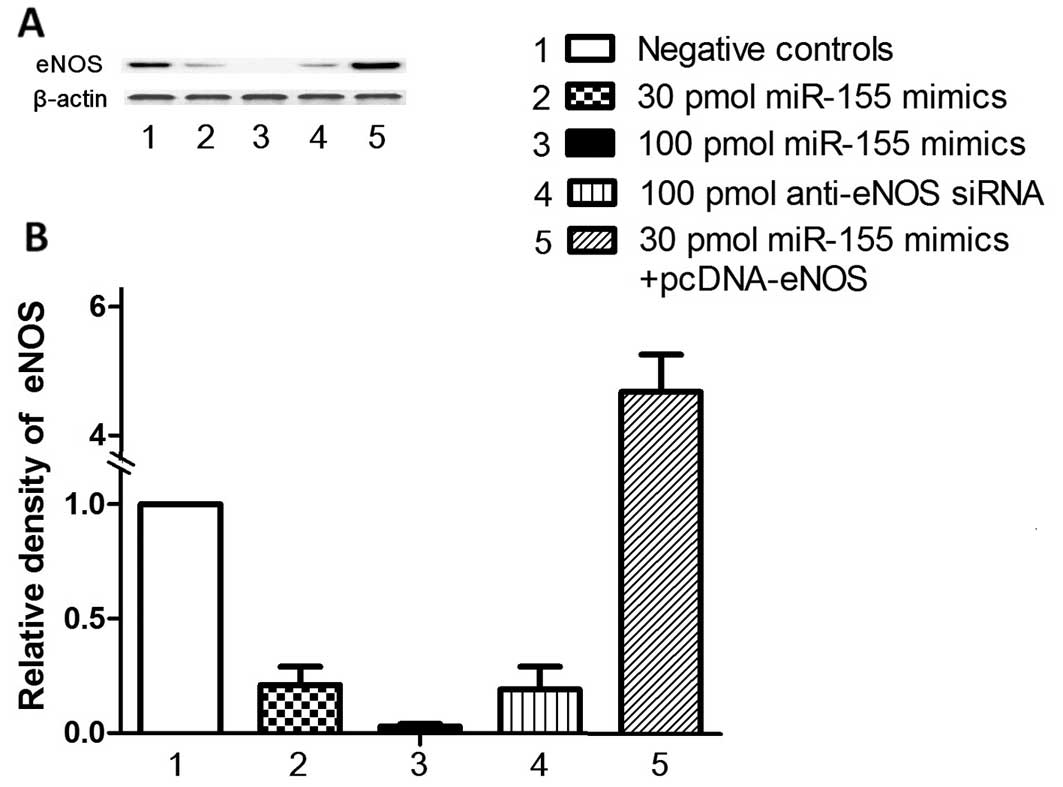
assays, the overexpression of miR-155 following transfection with
miR-155 mimics suppressed eNOS protein expression in a
dose-dependent manner (Fig. 3A).
These data indicate that eNOS is a direct target of miR-155.
miR-155 downregulates endogeneous eNOS
expression in VSMCs by destabilizing eNOS mRNA
To further determine whether miR-155 suppresses eNOS
expression in the VSMCs, we then examined the effects of miR-155
overexpression on the endogenous eNOS levels in the HASMCs
transfected with miR-155 mimics. The overexpression of miR-155 for
48 h reduced the mRNA and protein expression levels of eNOS in the
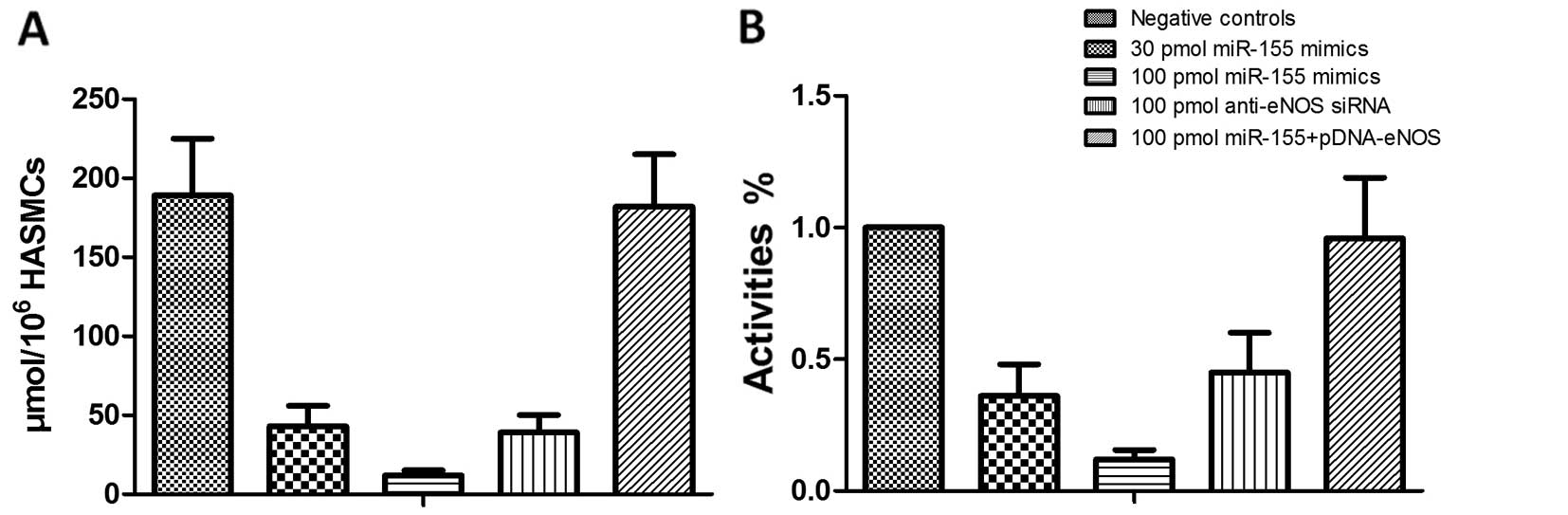
HASMCs in a dose dependent manner (Fig. 3). Importantly, the downregulation
of eNOS expression correlated with the decrease in cGMP activity
and NO production in the HASMCs (Fig.
4). The results revealed that the overexpression of miR-155 in
the HASMCs significantly downregulated both the mRNA and protein
expression levels of eNOS by promoting the degradation of eNOS
mRNA.
Effects of miR-155 overexpression on VSMC
proliferation
MTT assay was used to evaluate the proliferation of
HASMCs, and HASMCs were transfected with either miR-155 mimics or
the control prior to MTT assay. The results revealed that the
exogenous expression of miR-155 significantly suppressed the
expression level of eNOS (Fig.
3A), and substantially promoted the proliferation of the HASMCs
(Fig. 5A). Following incubation
for 48 h, a significantly enhanced effect on the proliferation of
the transfected cells was observed compared with the controls, and
the promoting rates were 250% (P<0.01) for transfection with 100
pmol miR-155 mimics and 220% (P<0.01) for transfection with 100
pmol anti-eNOS siRNAs, respectively, indicating that the
overexpression of miR-155 promoted the proliferation of the HASMCs
in vitro, and its effect was stronger than that of anti-eNOS
siRNA. The ectopic over-expression of eNOS significantly reverse
the increase in cell viability induced by transfection with miR-155
mimics and anti-eNOS siRNA (Fig.
5A).
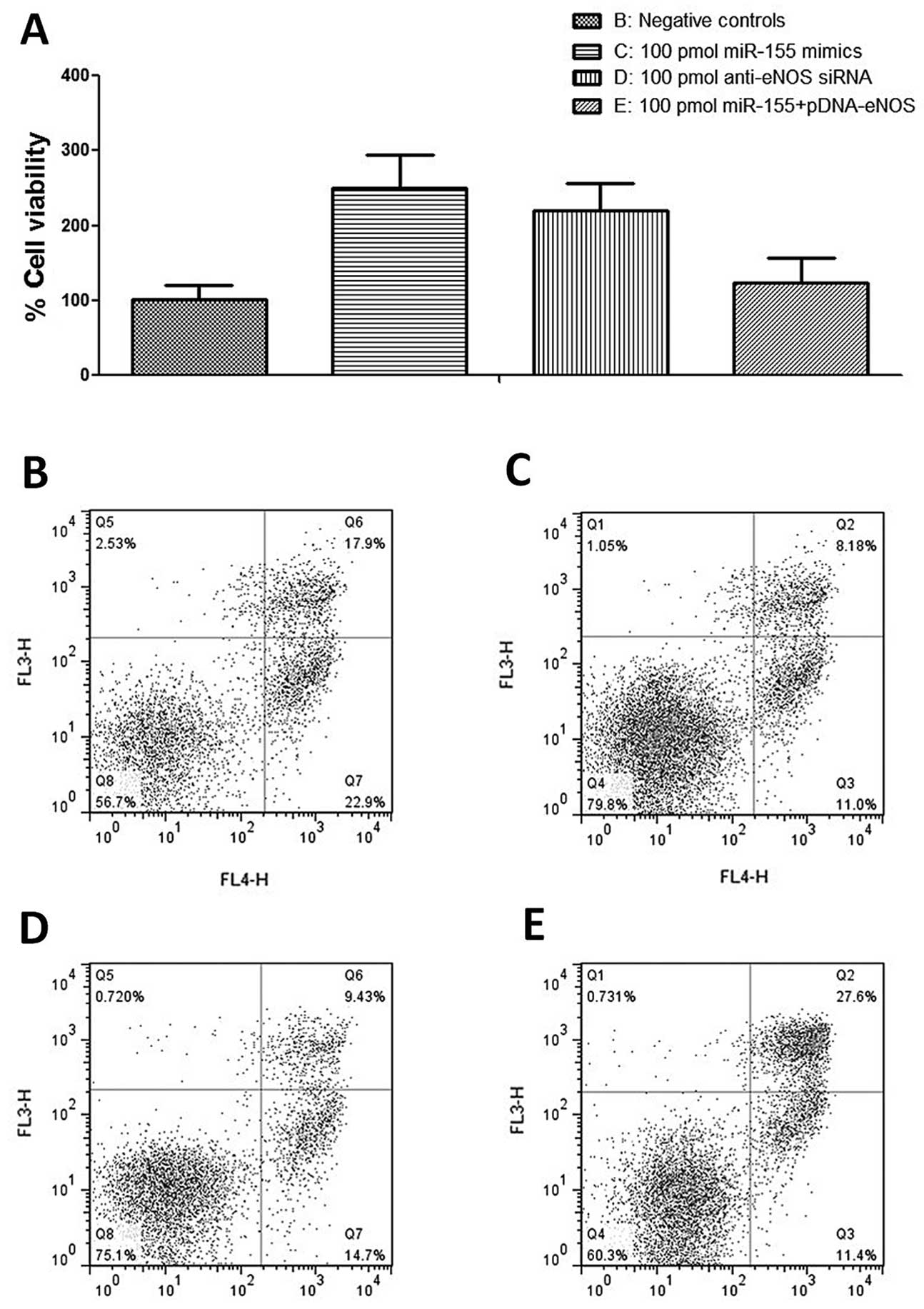
Increased expression of miR-155 protects
HASMCs against apoptosis
As eNOS functions as a pro-apoptotic protein and the
exogenous expression of miR-155 causes a reduction in the enzyme,
we then examined the effects of miR-155 on the apoptosis of HASMCs
by flow cytometry. In the HASMCs, we found that transfection with
miR-155 mimics and anti-eNOS siRNA similarly caused a reduction in
the proportion of apoptotic and live cells (Fig. 5B-E). The ectopic over-expression
of eNOS significantly reversed the decrease in apoptosis caused by
transfection with miR-155 mimics and anti-eNOS siRNA (Fig. 5B-E).
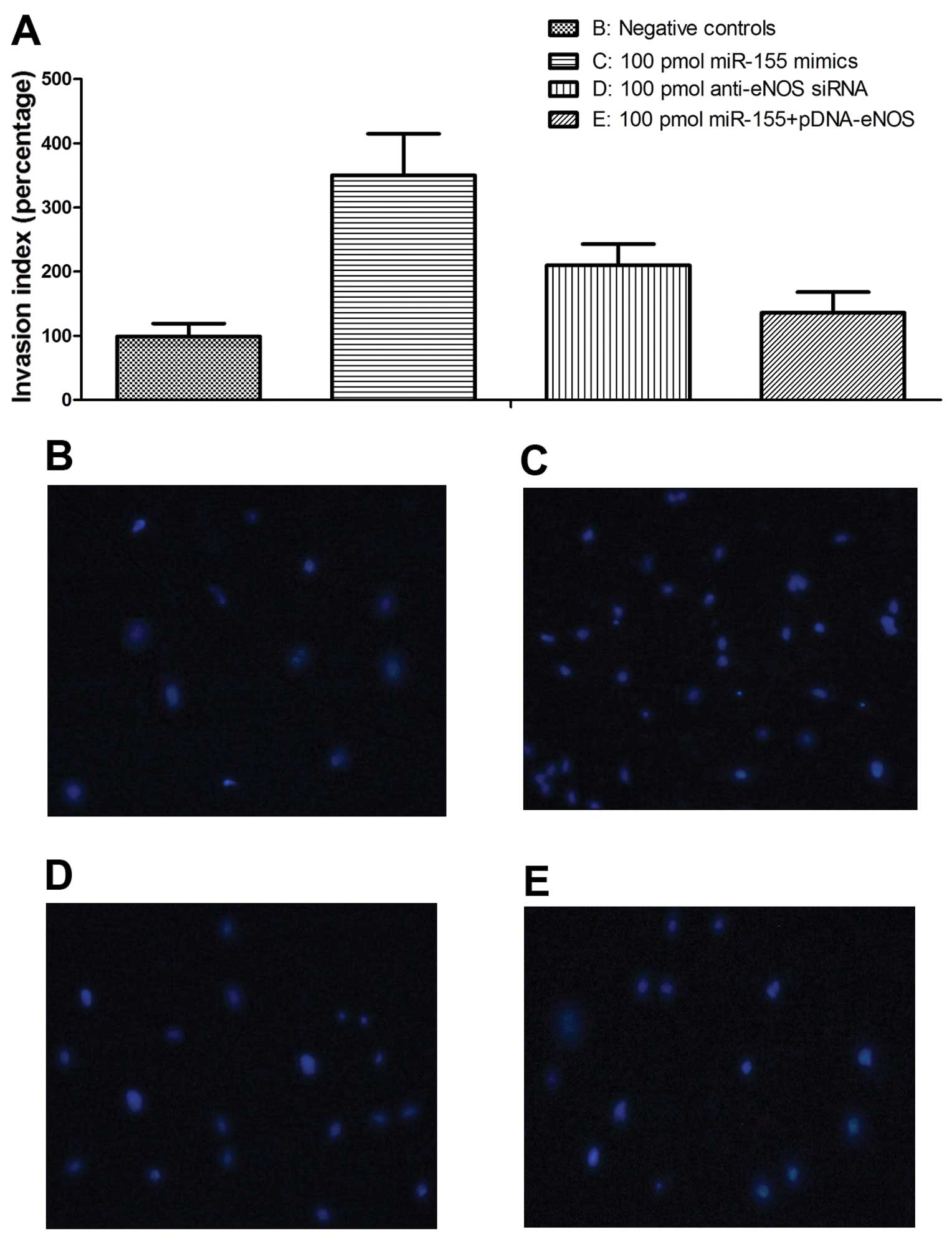
Effects of miR-155 overexpression on VSMC
proliferation and migration
To evaluate the role of miR-155 in the regulation of
VSMC migration, we performed a Transwell migration assay. The
results revealed that the exogenous expression of miR-155
significantly enhanced the migratory ability of the HCASMCs
(Fig. 6A). Following incubation
for 48 h, a significantly enhanced effect on the migratory ability
of the transfected cells was observed compared with the controls,
and the promoting rates were 350% (P<0.01) for transfection with
100 pmol miR-155 mimics and 210% (P<0.01) for transfection with
100 pmol anti-eNOS siRNAs, respectively, indicating that the
overexpression of miR-155 promotes the migratory ability of HASMCs
in vitro, and that its effect was stronger than that of
anti-eNOS siRNA. The ectopic overexpression of eNOS significantly
reversed the increase in the migratory ability caused by
transfection with miR-155 mimics and anti-eNOS siRNA (Fig. 6B-E).
Discussion
The proliferation and migration of VSMCs during the
development of intimal hyperplasia are critical for the development
of atherosclerotic lesions. In spite of rapid progress in our
understanding of the role of miRNAs in VSMC biology (7), the molecular mechanism(s) underlying
VSMC proliferation, migration, and proliferative vascular disease
remain largely unknown. The present study identified miR-155 as a
novel regulator of human VSMC proliferation and migration. We
demonstrated that miR-155 is substantially upregulated in human
atherosclerotic lesions and that the introduction of miR-155
significantly promoted the proliferation of VSMCs by inhibiting
apoptosis. Additionally, the introduction of miR-155 promoted the
migratory capability of the VSMCs. Furthermore, the ectopic
overexpression of eNOS significantly reversed the increase in
proliferation and in the migration of VSMCs caused by transfection
with miR-155 mimics or anti-eNOS siRNAs. Thus, this study provides
evidence implicating miR-155 as a novel key regulator in human VSMC
biology.
The expression of miR-155 has been reported to be
significantly upregulated in human and mouse atherosclerotic
lesions, but circulating levels of miR-155 have been shown to be
reduced in patients with coronary artery disease (17,26–28). The expression of miR-155 has been
identified in VSMCs, endothelial cells and in activated
macrophages, and thereby may affect most of the cell types involved
in atherosclerosis (29–31). The majority of previous studies on
miR-155 focused on its role in the activation of monocytes or
macrophages during the development of atherosclerosis. It has been
demonstrated that miR-155 is significantly upregulated in monocytes
and dendritic cells in response to lipopolysaccharide (LPS)
stimulation (32,33) and during the pro-inflammatory
activation of macrophages, a small group of miRNAs, including
miR-155, is specifically upregulated (29,34). In endothelial cells, miR-155 has
been shown to be upregulated in response to a variety of stimuli,
targeting the angiotensin II type 1 receptor and Ets-1, and
reducing the pro-inflammatory activity of angiotensin II in
endothelial cells (29). In
VSMCs, the suppression of angiotensin II type 1 receptor by miR-155
may also attenuate the effects of angiotensin II on vascular
remodeling (35). Data from
animal experiments studying the effects of miR-155 on
atherosclerosis are conflicting. Bone marrow cells are thought to
be able to contribute to the formation of atherosclerotic plaque by
relocating to the lesion and differentiating into VSMCs. It has
been demonstrated that LDL-R−/− mice carrying
miR-155−/− bone marrow cells on a high-cholesterol diet
developed slightly, but significantly more atherosclerosis. Whereas
ApoE−/− mice harboring miR-155−/− bone marrow
cells had markedly less hypercholesterolemia-induced lesions
compared with the controls (28,36). Therefore, miR-155 may have
different effects on lesion formation depending on the stage of
atherosclerosis. In the present study, we confirmed the
upregulation of miR-155 and the corresponding downregulation of its
potential target, eNOS, in atherosclerotic lesions. Furthermore,
eNOS was found to be a target gene of miR-155 in VSMCs by
luciferase assay. The exogenous introduction of miR-155
substantially suppressed the expression of eNOS in the HASMCs,
which could, at least partially, explain the proliferation- and
migration-promoting effects of miR-155 in HASMCs.
NO produced by enzymatic reaction catalyzed by eNOS
is an important homeostatic mechanism in the cardiovascular system.
It has been reported that VSMC progenitor cells are recruited to
injured arteries and significantly contribute to teh pathological
neointimal VSMC accumulation in eNOS-deficient mice, and the same
research group also demonstrated that eNOS deficiency increases the
recruitment of mononuclear cells, including monocytes and
lymphocytes into the arterial wall, and enhanced VSMC-rich
neointimal lesion formation in a carotidartery ligation (CAL) model
(21). These observations,
together with those of previous studies showing that the NO
signaling pathway is involved in the induction of the apoptosis of
VSMCs (22,23), led us to the hypothesis that the
proliferation of VSMCs is accelerated by the deficiency of eNOS. To
examine this hypothesis, the present study investigated the effects
of eNOS deficiency, suppressed by the upregulation of miR-155, on
the proliferation and migration of VSMCs, which are both critical
for the formation of atherosclerotic plaque. Although the effects
of eNOS/NO in cardiovascular systems have been investigated
intensively, its role in the regulation of VSMCs in the
cardiovascular system is only limitedly understood (37,38). The formation of neointima seems to
result from a combination of cellular infiltration and local
proliferation, and the accumulation of VSMCs, migrated from the
medial layer, at the site of atherosclerotic lesions may depend on
the balance between apoptosis and proliferation. Our data
demonstrated that eNOS deficiency, surppressed by miR-155, promoted
cell proliferation by inhibiting the apoptosis of VSMCs. In
addition to its ability to recruit circulating progenital cells
into the lesion that then differentiate into smooth muscle cells,
eNOS/NO deficiency also transports the VSMCs to the atheromatous
lesion, and the lack of eNOS is also capable of inhibiting
apoptosis and thereby accelerating the proliferation of VSMCs in
situ. This result presented a new aspect of the vascular
protective function of the eNOS/NO system and should be considered
in interventions directed at VSMC accumulation in the neointimal
lesion, which is a major component in the chain of events, causing
the formation of atherosclerosis (39).
Taken together, the present study identified miR-155
as a novel regulator of human VSMCs by targeting, at least in part,
the eNOS pathway. miR-155 expression was substantially upregulated
in proliferative human VSMCs and we found that the restoration of
eNOS expression markedly inhibited both VSMC proliferation and
migration in response. This study provides significant novel
insight into the molecular mechanisms associated with VSMC
proliferation and migration, and suggests a potential therapeutic
target for the prevention and treatment of human vascular diseases,
such as atherosclerosis and restenosis.
References
|
1
|
Pidkovka NA, Cherepanova OA, Yoshida T,
Alexander MR, Deaton RA, Thomas JA, Leitinger N and Owens GK:
Oxidized phospholipids induce phenotypic switching of vascular
smooth muscle cells in vivo and in vitro. Circ Res. 101:792–801.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berliner JA and Watson AD: A role for
oxidized phospholipids in atherosclerosis. N Engl J Med. 353:9–11.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Libby P, Ridker PM and Hansson GK:
Progress and challenges in translating the biology of
atherosclerosis. Nature. 473:317–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lacolley P, Regnault V, Nicoletti A, Li Z
and Michel JB: The vascular smooth muscle cell in arterial
pathology: A cell that can take on multiple roles. Cardiovasc Res.
95:194–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hata A: Functions of microRNAs in
cardiovascular biology and disease. Annu Rev Physiol. 75:69–93.
2013. View Article : Google Scholar
|
|
6
|
Kang H and Hata A: MicroRNA regulation of
smooth muscle gene expression and phenotype. Curr Opin Hematol.
19:224–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McDonald RA, Hata A, MacLean MR, Morrell
NW and Baker AH: MicroRNA and vascular remodelling in acute
vascular injury and pulmonary vascular remodelling. Cardiovasc Res.
93:594–604. 2012. View Article : Google Scholar :
|
|
8
|
Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu
Q, Deitch EA, Huo Y, Delphin ES and Zhang C: MicroRNA-145, a novel
smooth muscle cell phenotypic marker and modulator, controls
vascular neointimal lesion formation. Circ Res. 105:158–166. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cordes KR, Sheehy NT, White MP, Berry EC,
Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN and Srivastava D:
miR-145 and miR-143 regulate smooth muscle cell fate and
plasticity. Nature. 460:705–710. 2009.PubMed/NCBI
|
|
10
|
Davis BN, Hilyard AC, Nguyen PH, Lagna G
and Hata A: Induction of microRNA-221 by platelet-derived growth
factor signaling is critical for modulation of vascular smooth
muscle phenotype. J Biol Chem. 284:3728–3738. 2009. View Article : Google Scholar :
|
|
11
|
Liu X, Cheng Y, Zhang S, Lin Y, Yang J and
Zhang C: A necessary role of miR-221 and miR-222 in vascular smooth
muscle cell proliferation and neointimal hyperplasia. Circ Res.
104:476–487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang M, Li W, Chang GQ, Ye CS, Ou JS, Li
XX, Liu Y, Cheang TY, Huang XL and Wang SM: MicroRNA-21 regulates
vascular smooth muscle cell function via targeting tropomyosin 1 in
arteriosclerosis obliterans of lower extremities. Arterioscler
Thromb Vasc Biol. 31:2044–2053. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan MC, Hilyard AC, Wu C, Davis BN, Hill
NS, Lal A, Lieberman J, Lagna G and Hata A: Molecular basis for
antagonism between PDGF and the TGFbeta family of signalling
pathways by control of miR-24 expression. EMBO J. 29:559–573. 2010.
View Article : Google Scholar :
|
|
14
|
Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen
H, Dean DB and Zhang C: MicroRNA expression signature and
antisense-mediated depletion reveal an essential role of microRNA
in vascular neointimal lesion formation. Circ Res. 100:1579–1588.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun SG, Zheng B, Han M, Fang XM, Li HX,
Miao SB, Su M, Han Y, Shi HJ and Wen JK: miR-146a and Krüppel-like
factor 4 form a feedback loop to participate in vascular smooth
muscle cell proliferation. EMBO Rep. 12:56–62. 2011. View Article : Google Scholar
|
|
16
|
Zhang E and Wu Y: Dual effects of miR-155
on macrophages at different stages of atherosclerosis: LDL is the
key? Med Hypotheses. 83:74–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tian FJ, An LN, Wang GK, Zhu JQ, Li Q,
Zhang YY, Zeng A, Zou J, Zhu RF, Han XS, et al: Elevated
microRNA-155 promotes foam cell formation by targeting HBP1 in
atherogenesis. Cardiovasc Res. 103:100–110. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hao L, Wang XG, Cheng JD, You SZ, Ma SH,
Zhong X, Quan L and Luo B: The up-regulation of endothelin-1 and
down-regulation of miRNA-125a-5p, -155, and -199a/b-3p in human
atherosclerotic coronary artery. Cardiovasc Pathol. 23:217–223.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Weber M, Kim S, Patterson N, Rooney K and
Searles CD: MiRNA-155 targets myosin light chain kinase and
modulates actin cytoskeleton organization in endothelial cells. Am
J Physiol Heart Circ Physiol. 306:H1192–H1203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun HX, Zeng DY, Li RT, Pang RP, Yang H,
Hu YL, Zhang Q, Jiang Y, Huang LY, Tang YB, et al: Essential role
of microRNA-155 in regulating endothelium-dependent vasorelaxation
by targeting endothelial nitric oxide synthase. Hypertension.
60:1407–1414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang LN, Wilson DW, da Cunha V, Sullivan
ME, Vergona R, Rutledge JC and Wang YX: Endothelial NO synthase
deficiency promotes smooth muscle progenitor cells in association
with upregulation of stromal cell-derived factor-1alpha in a mouse
model of carotid artery ligation. Arterioscler Thromb Vasc Biol.
26:765–772. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boyle JJ, Weissberg PL and Bennett MR:
Tumor necrosis factor-alpha promotes macrophage-induced vascular
smooth muscle cell apoptosis by direct and autocrine mechanisms.
Arterioscler Thromb Vasc Biol. 23:1553–1558. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gough PJ, Gomez IG, Wille PT and Raines
EW: Macrophage expression of active MMP-9 induces acute plaque
disruption in apoE-deficient mice. J Clin Invest. 116:59–69. 2006.
View Article : Google Scholar
|
|
24
|
Ishida N, Hayashi K, Hattori A, Yogo K,
Kimura T and Takeya T: CCR1 acts downstream of NFAT2 in
osteoclastogenesis and enhances cell migration. J Bone Miner Res.
21:48–57. 2006. View Article : Google Scholar
|
|
25
|
Erl W, Hristov M, Neureuter M, Yan ZQ,
Hansson GK and Weber PC: HMG-CoA reductase inhibitors induce
apoptosis in neointima-derived vascular smooth muscle cells.
Atherosclerosis. 169:251–258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fichtlscherer S, De Rosa S, Fox H,
Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Röxe T,
Müller-Ardogan M, et al: Circulating microRNAs in patients with
coronary artery disease. Circ Res. 107:677–684. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Raitoharju E, Lyytikäinen LP, Levula M,
Oksala N, Mennander A, Tarkka M, Klopp N, Illig T, Kähönen M,
Karhunen PJ, et al: miR-21, miR-210, miR-34a, and miR-146a/b are
up-regulated in human atherosclerotic plaques in the Tampere
Vascular Study. Atherosclerosis. 219:211–217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nazari-Jahantigh M, Wei Y, Noels H, Akhtar
S, Zhou Z, Koenen RR, Heyll K, Gremse F, Kiessling F, Grommes J, et
al: MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in
macrophages. J Clin Invest. 122:4190–4202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martin MM, Buckenberger JA, Jiang J,
Malana GE, Nuovo GJ, Chotani M, Feldman DS, Schmittgen TD and Elton
TS: The human angiotensin II type 1 receptor +1166 A/C polymorphism
attenuates microRNA-155 binding. J Biol Chem. 282:24262–24269.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
O’Connell RM, Taganov KD, Boldin MP, Cheng
G and Baltimore D: MicroRNA-155 is induced during the macrophage
inflammatory response. Proc Natl Acad Sci USA. 104:1604–1609. 2007.
View Article : Google Scholar
|
|
31
|
Zhu N, Zhang D, Chen S, Liu X, Lin L,
Huang X, Guo Z, Liu J, Wang Y, Yuan W, et al: Endothelial enriched
microRNAs regulate angiotensin II-induced endothelial infammation
and migration. Atherosclerosis. 215:286–293. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ceppi M, Pereira PM, Dunand-Sauthier I,
Barras E, Reith W, Santos MA and Pierre P: MicroRNA-155 modulates
the inter-leukin-1 signaling pathway in activated human
monocyte-derived dendritic cells. Proc Natl Acad Sci USA.
106:2735–2740. 2009. View Article : Google Scholar
|
|
33
|
Taganov KD, Boldin MP, Chang KJ and
Baltimore D: NF-kappaB-dependent induction of microRNA miR-146, an
inhibitor targeted to signaling proteins of innate immune
responses. Proc Natl Acad Sci USA. 103:12481–12486. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Graff JW, Dickson AM, Clay G, McCaffrey AP
and Wilson ME: Identifying functional microRNAs in macrophages with
polarized phenotypes. J Biol Chem. 287:21816–21825. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Heeneman S, Sluimer JC and Daemen MJ:
Angiotensin-converting enzyme and vascular remodeling. Circ Res.
101:441–454. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Donners MM, Wolfs IM, Stöger LJ, van der
Vorst EP, Pöttgens CC, Heymans S, Schroen B, Gijbels MJ and de
Winther MP: Hematopoietic miR155 deficiency enhances
atherosclerosis and decreases plaque stability in hyperlipidemic
mice. PLoS One. 7:e358772012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Naseem KM: The role of nitric oxide in
cardiovascular diseases. Mol Aspects Med. 26:33–65. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Albrecht EW, Stegeman CA, Heeringa P,
Henning RH and van Goor H: Protective role of endothelial nitric
oxide synthase. J Pathol. 199:8–17. 2003. View Article : Google Scholar
|
|
39
|
Casscells W: Migration of smooth muscle
and endothelial cells. Critical events in restenosis. Circulation.
86:723–729. 1992. View Article : Google Scholar : PubMed/NCBI
|