Introduction
The opioid growth factor (OGF)-OGF receptor (OGFr)
axis has been characterized in animal and human cancer cell lines
(1,2), as well as in animal and human tumors
(3). The OGFr-OGF axis is a
tonically active pathway, and the knockdown of the receptor with
small interfering RNA (siRNA) technology increases cell
proliferation (1,4–6).
Treatment with OGF or low-dose naltrexone (LDN) in cell culture or
in mouse models has been shown to significantly decrease cell
proliferation (7). Additionally,
it has been demonstrated that OGF and LDN function in combination
with standard chemotherapeutic agents to suppress tumor growth
(7). Using several human breast
cancer cell lines, OGF has been shown to inhibit cell replication
alone or in combination with paclitaxel (4). Moreover, lower concentrations of
paclitaxel, which are known to be cytotoxic, may be used in
combination with OGF with similar therapeutic results (4). In order to better understand the
function of the OGF-OGFr regulatory axis, mutations identified
through the Catalogue of Somatic Mutations in Cancer (COSMIC)
(8,9) database were selected for their
location and characterized. At the present time, to the best of our
knowledge, there is no information correlating function and
receptor mutation. It is important to identify potential mutations
that alter the function of the axis, specifically the role that OGF
plays in the inhibition of cell replication. This information would
facilitate personalized medical treatment by enabling the
identification of cancer responsive to treatment with OGF and LDN.
Mutation data were obtained from the Sanger Institute’s COSMIC
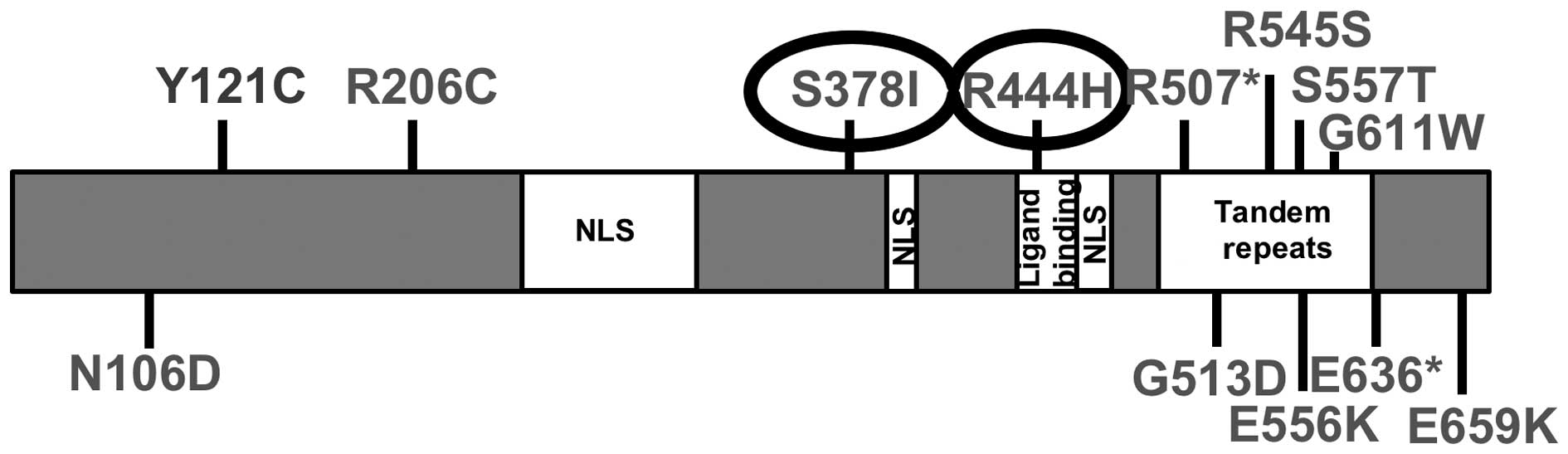
database (http://cancer.sanger.ac.uk/cosmic) (9). Thirteen mutations in OGFr are listed
in the COSMIC (8,9) database. Fig. 1 demonstrates the identified
mutations and their location in relation to the known functional
regions of OGFr. The functional domains of OGFr have not yet been
completely defined; however, of the identified mutations, S378I is
a putative phosphorylation site, and residue R444H falls within the
potential ligand-binding domain. For these reasons, we chose to
explore the functional changes associated with the mutation S378I,
identified in a kidney cancer sample, and R444H, identified in a
lung cancer sample.
Materials and methods
Cell culture
Transformed African green monkey kidney cells, COS-7
cells, were purchased from the American Type Culture Collection
(ATCC, Manassas, VA, USA) and cultured in Dulbecco’s modified
Eagle’s medium supplemented with 10% fetal calf serum and
antibiotics (5,000 U/ml penicillin and 5 mg/ml streptomycin). The
cells were grown in a humidified atmosphere of 5%
CO2/95% air at 37°C.
Plasmids
OGFr-enhanced green fluorescent protein (EGFP) was
previously generated (10) by
cloning OGFr into the pEGFP-N1 plasmid using EcoRI and
SalI. A QuikChange Site-Directed Mutagenesis kit (200518;
Agilent Technologies, Santa Clara, CA, USA) was utilized to
generate the mutations in OGFr-EGFP using the following primers:
S378I forward, 5′-ggaagataggccagagcccttaatccccaaagaga-3′ and
reverse, 5′-ctctc tttggggattaagggctctggcctatcttcc-3′; and R444H
forward, 5′-gcag ccctgccaccaacccctgg-3′ and reverse,
5′-ccaggggttggtggcaggg ctgc-3′. Mutations were confirmed by
sequencing. All transfections were performed in 6-well plates with
5 µg of DNA and 5 µl of Lipofectamine 2000/well for 4
h. After 4 h, the medium was removed and replaced with fresh
complete, medium.
Localization studies
The cells (2×105) were seeded on glass
coverslips and allowed to attach for 24 h prior to transfection.
The cells were transfected with the empty vector (EGFP) control,
wild-type OGFr (OGFr-EGFP) or the mutated OGFr plasmids (S378I-EGFP
or R444H-EGFP). At 18–24 h post-transfection, the cells were washed
in phosphate-buffered saline (PBS), counterstained with Hoechst
33342 (H3570; Molecular Probes/Life Technologies, Carlsbad, CA,
USA) and fixed in 4% paraformaldehyde (pH 7.4) for 30 min at room
temperature. The coverslips were imaged using an Olympus IX-81
epifluorescence microscope at x40 magnification. At least 30 images
were collected per group. The images were exported as .tiff files
and analyzed using CellProfiler to quantify the nuclear/cytoplasmic
ratio. Images were converted to gray scale and split by channel.
Nuclei were identified as primary objects by intensity of Hoechst
33342 staining. Based on the identified primary objects, the
intensity of EGFP in the nucleus was analyzed and used to set a
threshold for EGFP-positive cells. For the cells that were
identified as positive, a fixed cytoplasm of 25 pixels around the
nucleus was measured for EGFP intensity. The nuclear to cytoplasmic
ratio was found by dividing the average EGFP intensity of the
nucleus by the average EGFP intensity of the defined cytoplasm.
Growth curves
The COS-7 cells were seeded at 2×105
cells per well in 6-well plates and allowed to attach for 24 h
prior to transfection with wild-type, or mutant OGFr, or the empty
control vector for comparison. The cells were treated with sterile
water, OGF (M6638; Sigma-Aldrich, St. Louis, MO, USA) or naltrexone
(N3136; Sigma-Aldrich). The drugs were dissolved in sterile water
and the concentrations represent the final dilution. Twenty-four
hours after transfection (as described above), the cells were
harvested by trypsinization, counted and seeded at
2.5×104 cells/well in 24-well plates. The cells were
placed under selection for neomycin resistance using G418
(Geneticin; Invitrogen Life Technologies). The cells were counted
every 24 h for 120 h. At least 3 wells per group were counted for
each time point; the experiments were conducted at least twice.
5-Bromo-2′-deoxyuridine (BrdU) assay
To examine the effects of OGFr mutation on DNA
synthesis, BrdU assays were conducted. The cells (2×105)
were seeded on coverslips and allowed to attach for 24 h, after
which time they were transfected. At 15–24 h post-transfection, the
cells were pulsed with 30 µM BrdU for 3 h. The cells were
then washed in PBS, and fixed in acetone:methanol (1:1 vol:vol) for
20 min at −20°C. The coverslips were stained with Alexa Fluor 488
anti-GFP antibody at 1:200 (A21311) and Alexa Fluor 596 anti-BrdU
antibody at 1:200 (B35132), and counterstained with DAPI (D1306)
(all from Life Technologies). The coverslips were imaged using an
Olympus IX-81 epifluorescence microscope, and at least 30 images
were collected per group. The number of dual-labeled cells
(positive for BrdU and GFP) was divided by the total number of
GFP-positive cells to calculate the BrdU index.
Data analyses
All data (nuclear/cytoplasmic ratios, cell numbers
and BrdU indexes) were compared using one-way analysis of variance
(ANOVA) with Newman-Keuls post hoc tests. A P-value <0.05 was
considered to indicate a statistically signficant difference.
Results
The COSMIC database was examined for mutations
identified in OGFr. There were 13 mutations identified in various
types of cancer (Fig. 1). Of
these 13 mutations, S378I and R444H were selected for further
characterization due to their potential roles in cell cycle
regulation. S3781 is a putative phosphorylation site that has been
identified in a number of large phosphoproteomic studies (11–17), while R444H is located within the
purported ligand-binding domain (unpublished data). Our hypothesis
was that these mutations would significantly alter the function of
the receptor if either region or residue played a critical role in
the function of OGFr. In order to examine this hypothesis,
site-directed mutagenesis was utilized to modify wild-type OGFr
with each mutation. The mutated plasmids were then transfected into
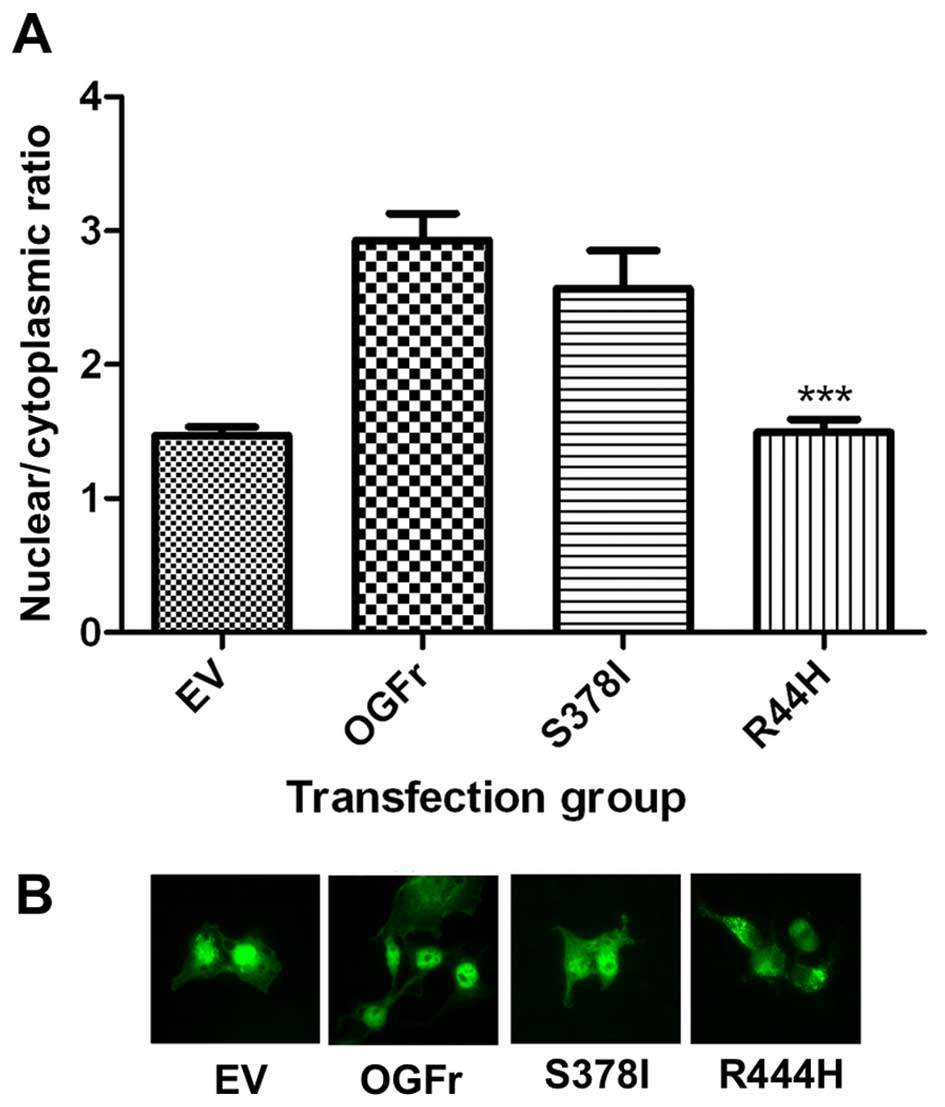
COS-7 cells. The localization of each mutant was then compared to
the localization of wild-type OGFr (Fig. 2). The R444H mutation resulted in a
significant decrease in nuclear localization, while S378I showed no
significant change in localization, as demonstrated in Fig. 2B in the representative images of
each group. If R444H is located within the ligand-binding region,
it would be expected to alter ligand binding, and this could result
in decreased nuclear localization.
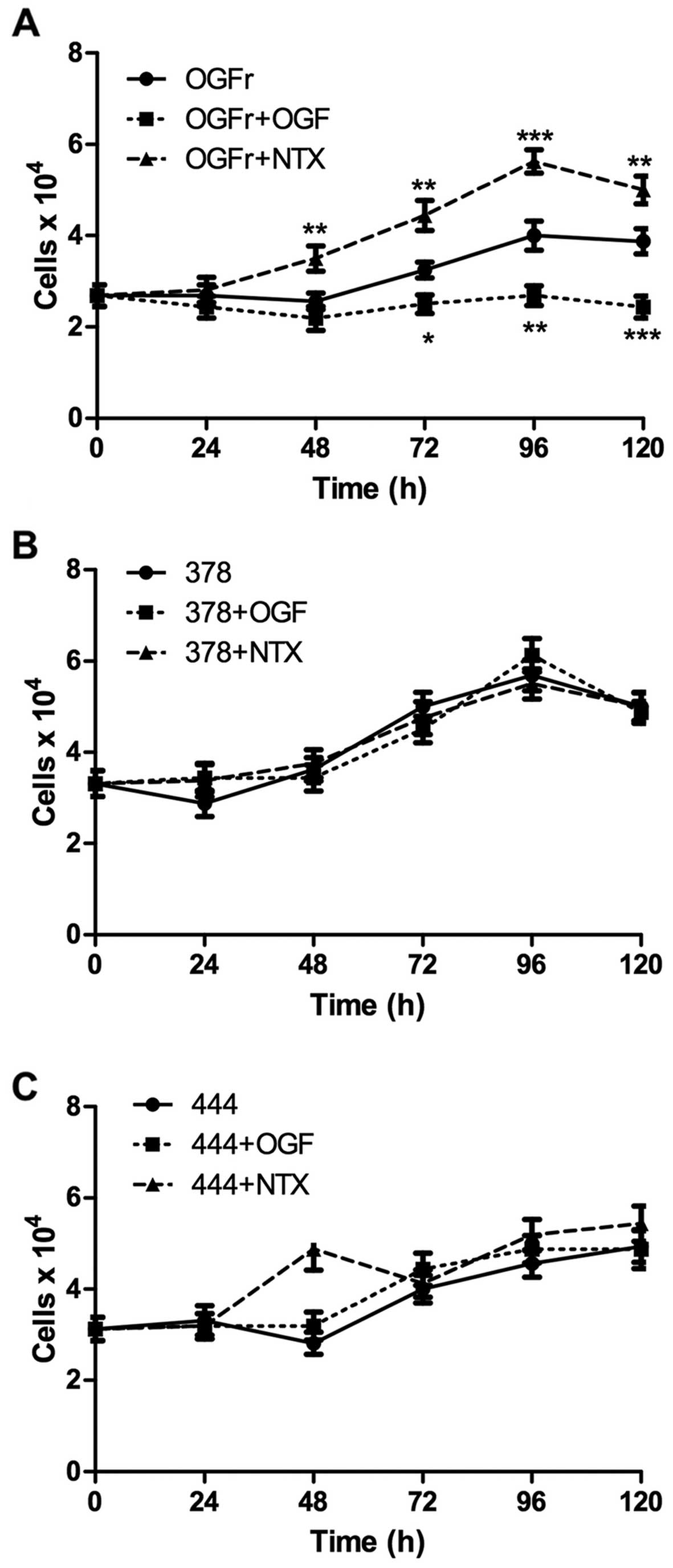
In order to examine the effects of mutated OGFr on
cell growth, the COS-7 cells were transiently transfected with
mutant plasmids. To inhibit the growth of untransfected cells,
cells were placed under G418 selection. The transfected cells were
treated with sterile water, OGF (10−6 M) or naltexrone
(NTX; 10−6 M). The overexpression of wild-type OGFr
responded to OGF and NTX by decreasing and increasing cell
replication, respectively; whereas the cells transfected with
mutations for S378I and R444H demonstrated a loss of response to
OGF or NTX, indicating that the mutated OGFr lost the ability to be
modulated by the agonist (OGF), as well as by the opioid receptor
antagonist (NTX) (Fig. 3). In
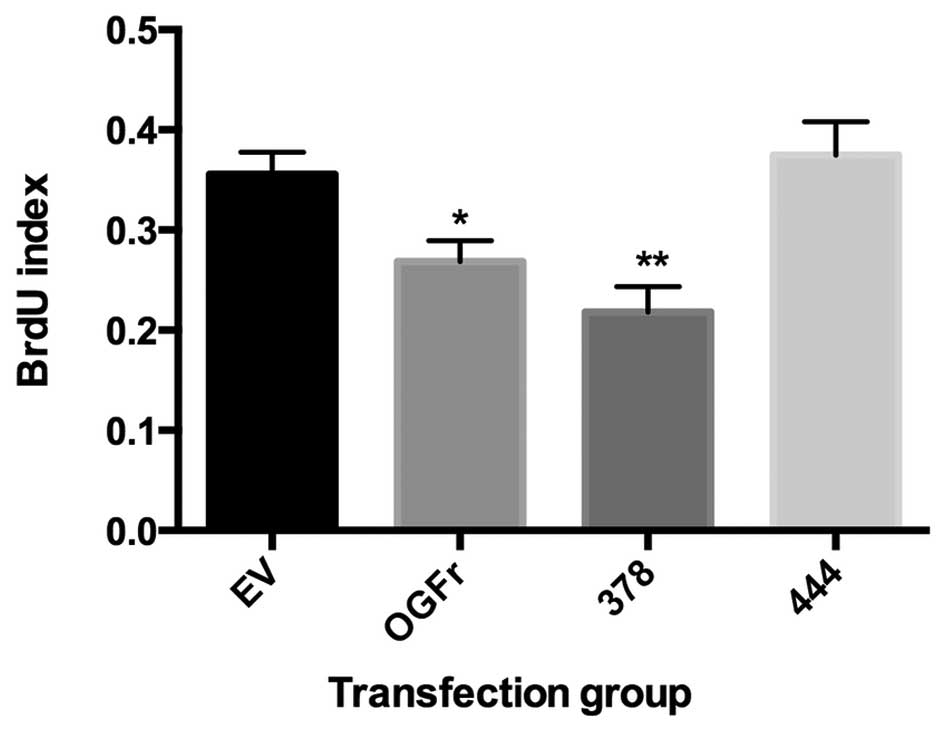
order to further examine the effects of mutated OGFr on cell
proliferation, DNA synthesis was measured using a BrdU assay. The
overexpression of OGFr, as well as the S378I mutation,
significantly decreased BrdU incorporation; whereas the
overexpression of R444H had no significant effect on the
incorporation of BrdU (Fig. 4).
These results indicate that the growth activity attributed to the
OGF interaction with OGFr is altered by both mutations, S378I and
R444H; moreover, the blockade by NTX resulting in enhanced cell
replication is diminished by both mutations. Furthermore, the OGFr
mutation R444H alters the localization of the receptor to the
nucleus.
Discussion
The OGF-OGFr axis has been characterized and
identified as a determinant in a variety of human cancers arising
from all germ layer derivations (1). To further characterize the axis with
regard to cancer, the COSMIC (8,9)
database was surveyed to identify mutations reported in human
cancer samples. Of the 13 identified missense mutations which had
been identified at the time, two were located in regions of
interest. S378I is a putative phosphorylation site that has been
identified in numerous large phosphoproteomic studies (11–15,17,18). S378I has been reported to be
exclusively phosphorylated in the cytoplasm of HeLa cells (15) and differentially phosphorylated
throughout the cell cycle (12,16). It has also been identified as a
phosphorylated residue in additional cell lines, such as Jurkat T
cell leukemia cells (11) and
MV4-11 leukemia cells (14), as
well as in normal liver tissue (13). S378I has also been found as a
phosphorylated residue in two mouse studies which examined melanoma
(17) and normal brain tissue
(18). The number of sites this
residue has been identified as being phosphorylated, as well as the
differential phosphorylation between the nucleus and the cytoplasm
and throughout the cell cycle, reinforces its potential importance.
Furthermore, in the present study, mutations were characterized in
an asynchronous population of cells. At least for the S378I
mutation, the results may be exacerbated in a synchronized
population of cells. Further investigations are warranted.
R444H was characterized as it is localized within a
region identified as a potential ligand-binding domain (unpublished
data). If this region is verified as a ligand binding domain, then
a residue substitution may alter ligand binding, as well as the
downstream function of the receptor.
Both mutations were characterized for changes in
localization. R444H showed a significant decrease in nuclear
localization, while S378I showed no significant change in
localization. It has previously been demonstrated that the function
of the receptor is dependent on its nuclear localization (10), indicating that R444H has the
potential to significantly alter the function of the receptor. The
function of both mutations was characterized using growth curves.
The cells were selected such that only cells which had plasmids
were capable of replicating. Cells with either mutation had an
inhibited response to excess ligand, OGF or the receptor
antagonist, NTX, suggesting a loss of regulation. The mutations
were also characterized with regard to the overall function of the
receptor. It has been previously demonstrated that the
overexpression of OGFr significantly decreases BrdU incorporation
(10). S378I showed no
significant change in BrdU incorporation. Again this may be due to
the asynchronous population of cells. R444H demonstrated a complete
loss of growth inhibition, indicating that the receptor had lost
its function. These experiments indicate that R444H renders the
receptor inactive and that S378I may alter the response to OGF and
NTX. These functions are critical for modulating the OGF-OGFr axis
in cancer therapy. These data demonstrate that cancer mutations in
OGFr can inhibit receptor function, and thus extend our knowledge
of the role played by the OGF-OGFr pathway in mediating cancer
growth.
Although only these two mutations were characterized
for the reasons explained above, important information may be
obtained by further characterization of these mutations, as well as
others that have been identified. At the time this study commenced,
13 missense mutations had been identified in the database; however,
the database has now been updated to include 111 mutations, 49 of
which are missense (9). The
increase in the number of mutations suggests that OGFr does in fact
play a critical role as a biological regulatory pathway. In the
updated catalog of mutations, many appear to occur in the tandem
repeat region of OGFr, suggesting that this region may have
functional importance. However, at this time, the function of the
tandem repeats is unknown. Further mutation analyses are warranted
for the tandem repeat region, as well as other regions of the
protein.
Acknowledgments
This study was supported in part by funding from the
Paul K. and Anna M. Shockey Foundation.
Abbreviations:
|
OGF
|
opioid growth factor
|
|
OGFr
|
opioid growth factor receptor
|
|
BrdU
|
5-bromo-2′-deoxyuridine
|
|
NTX
|
naltrexone
|
|
PBS
|
phosphate-buffered saline
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
References
|
1
|
Zagon IS, Donahue RN and McLaughlin PJ:
Opioid growth factor-opioid growth factor receptor axis is a
physiological determinant of cell proliferation in diverse human
cancers. Am J Physiol Regul Integr Comp Physiol. 297:R1154–R1161.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kren NP, Zagon IS and McLaughlin PJ:
Modulation of the opioid growth factor receptor alters the
proliferation and progression of cancer. Trends Cancer Res.
9:53–64. 2013.
|
|
3
|
Fanning J, Hossler CA, Kesterson JP,
Donahue RN, McLaughlin PJ and Zagon IS: Expression of the opioid
growth factor-opioid growth factor receptor axis in human ovarian
cancer. Gynecol Oncol. 124:319–324. 2012. View Article : Google Scholar
|
|
4
|
Zagon IS, Porterfield NK and McLaughlin
PJ: Opioid growth factor-opioid growth factor receptor axis
inhibits proliferation of triple negative breast cancer. Exp Biol
Med (Maywood). 238:589–599. 2013. View Article : Google Scholar
|
|
5
|
Donahue RN, McLaughlin PJ and Zagon IS:
Cell proliferation of human ovarian cancer is regulated by the
opioid growth factor-opioid growth factor receptor axis. Am J
Physiol Regul Integr Comp Physiol. 296:R1716–R1725. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Campbell AM, Zagon IS and McLaughlin PJ:
Astrocyte proliferation is regulated by the OGF-OGFr axis in vitro
and in experimental autoimmune encephalomyelitis. Brain Res Bull.
90:43–51. 2013. View Article : Google Scholar
|
|
7
|
Donahue RN, McLaughlin PJ and Zagon IS:
Low-dose naltrexone suppresses ovarian cancer and exhibits enhanced
inhibition in combination with cisplatin. Exp Biol Med (Maywood).
236:883–895. 2011. View Article : Google Scholar
|
|
8
|
Forbes SA, Bindal N, Bamford S, Cole C,
Kok CY, Beare D, Jia M, Shepherd R, Leung K, Menzies A, et al:
COSMIC: mining complete cancer genomes in the Catalogue of Somatic
Mutations in Cancer. Nucleic Acids Res. 39:D945–D950. 2011.
View Article : Google Scholar :
|
|
9
|
Bamford S, Dawson E, Forbes S, Clements J,
Pettett R, Dogan A, Flanagan A, Teague J, Futreal PA, Stratton MR
and Wooster R: The COSMIC (Catalogue of Somatic Mutations in
Cancer) database and website. Br J Cancer. 91:355–358.
2004.PubMed/NCBI
|
|
10
|
Cheng F, McLaughlin PJ, Verderame MF and
Zagon IS: Dependence on nuclear localization signals of the opioid
growth factor receptor in the regulation of cell proliferation. Exp
Biol Med (Maywood). 234:532–541. 2009. View Article : Google Scholar
|
|
11
|
Mayya V, Lundgren DH, Hwang SI, Rezaul K,
Wu L, Eng JK, Rodionov V and Han DK: Quantitative phosphoproteomic
analysis of T cell receptor signaling reveals system-wide
modulation of protein-protein interactions. Sci Signal. 2:ra462009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dephoure N, Zhou C, Villén J, Beausoleil
SA, Bakalarski CE, Elledge SJ and Gygi SP: A quantitative atlas of
mitotic phosphorylation. Proc Natl Acad Sci USA. 105:10762–10767.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han G, Ye M, Zhou H, Jiang X, Feng S,
Jiang X, Tian R, Wan D, Zou H and Gu J: Large-scale phosphoproteome
analysis of human liver tissue by enrichment and fractionation of
phosphopeptides with strong anion exchange chromatography.
Proteomics. 8:1346–1361. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oppermann FS, Gnad F, Olsen JV, Hornberger
R, Greff Z, Kéri G, Mann M and Daub H: Large-scale proteomics
analysis of the human kinome. Mol Cell Proteomics. 8:1751–1764.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Olsen JV, Blagoev B, Gnad F, Macek B,
Kumar C, Mortensen P and Mann M: Global, in vivo, and site-specific
phosphorylation dynamics in signaling networks. Cell. 127:635–648.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Olsen JV, Vermeulen M, Santamaria A, Kumar
C, Miller ML, Jensen LJ, Gnad F, Cox J, Jensen TS, Nigg EA, et al:
Quantitative phosphoproteomics reveals widespread full
phosphorylation site occupancy during mitosis. Sci Signal.
3:ra32010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zanivan S, Gnad F, Wickström SA, Geiger T,
Macek B, Cox J, Fässler R and Mann M: Solid tumor proteome and
phosphoproteome analysis by high resolution mass spectrometry. J
Proteome Res. 7:5314–5326. 2008. View Article : Google Scholar
|
|
18
|
Wiśniewski JR, Nagaraj N, Zougman A, Gnad
F and Mann M: Brain phosphoproteome obtained by a FASP-based method
reveals plasma membrane protein topology. J Proteome Res.
9:3280–3289. 2010. View Article : Google Scholar
|