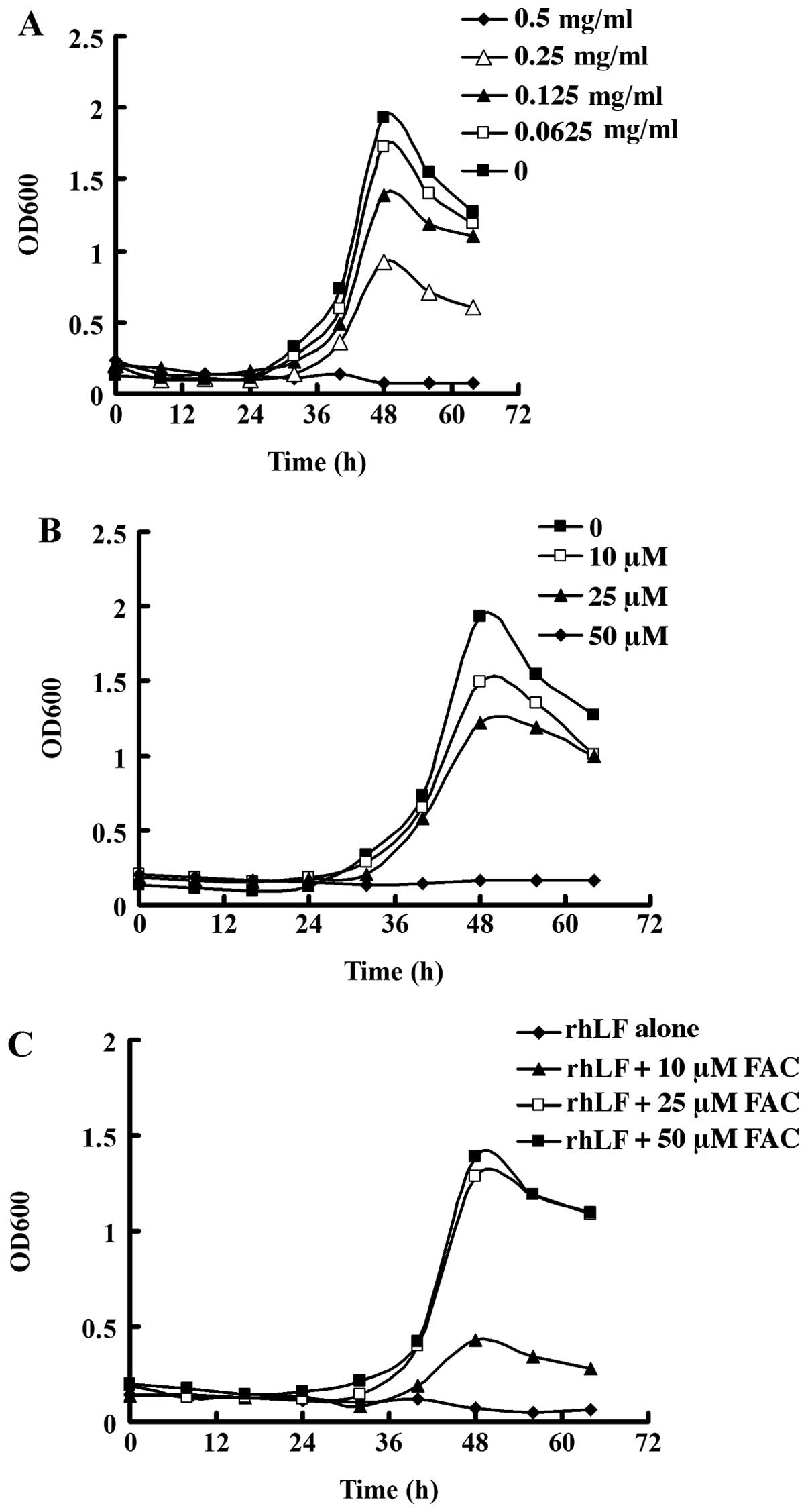
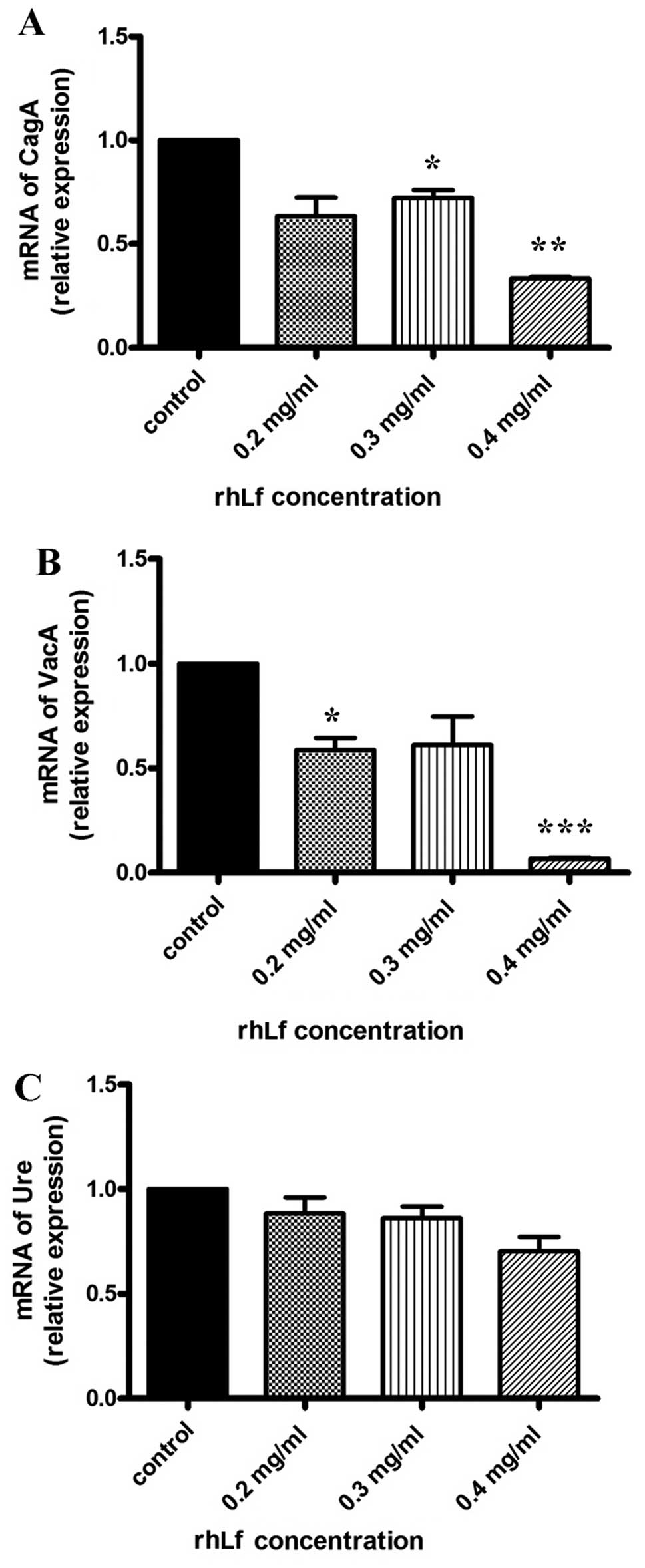
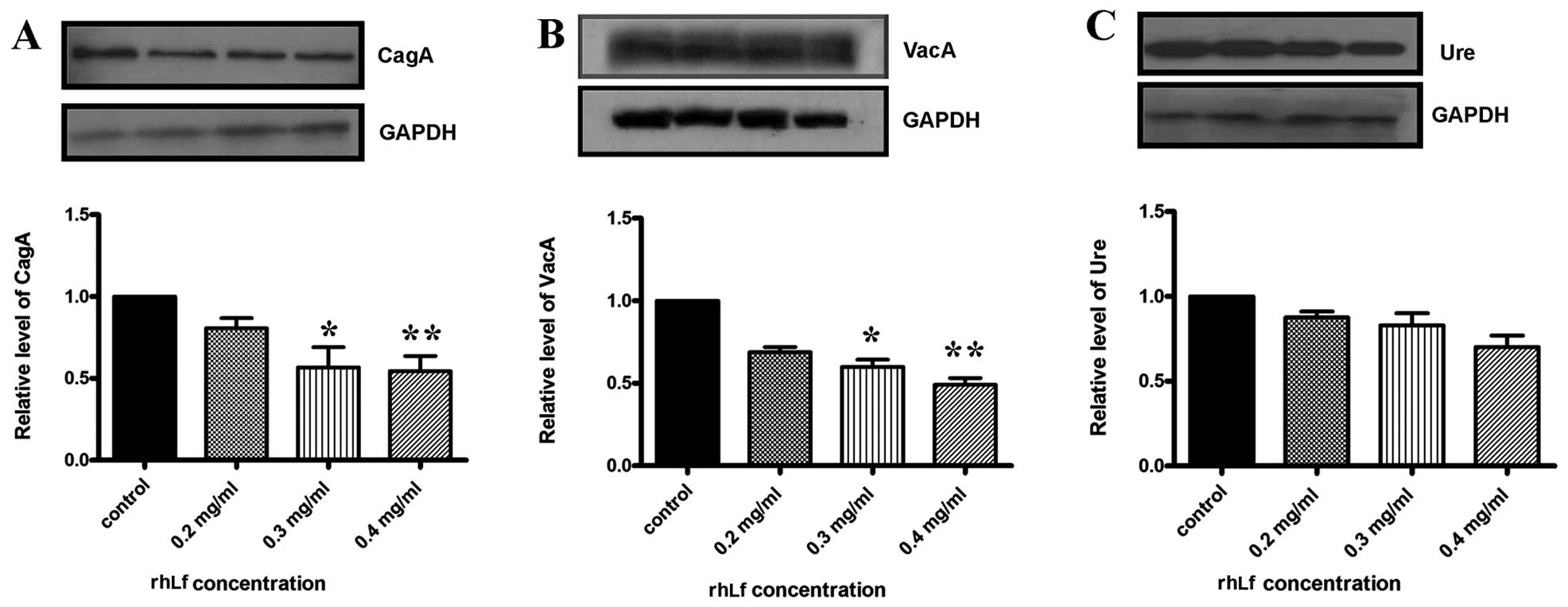
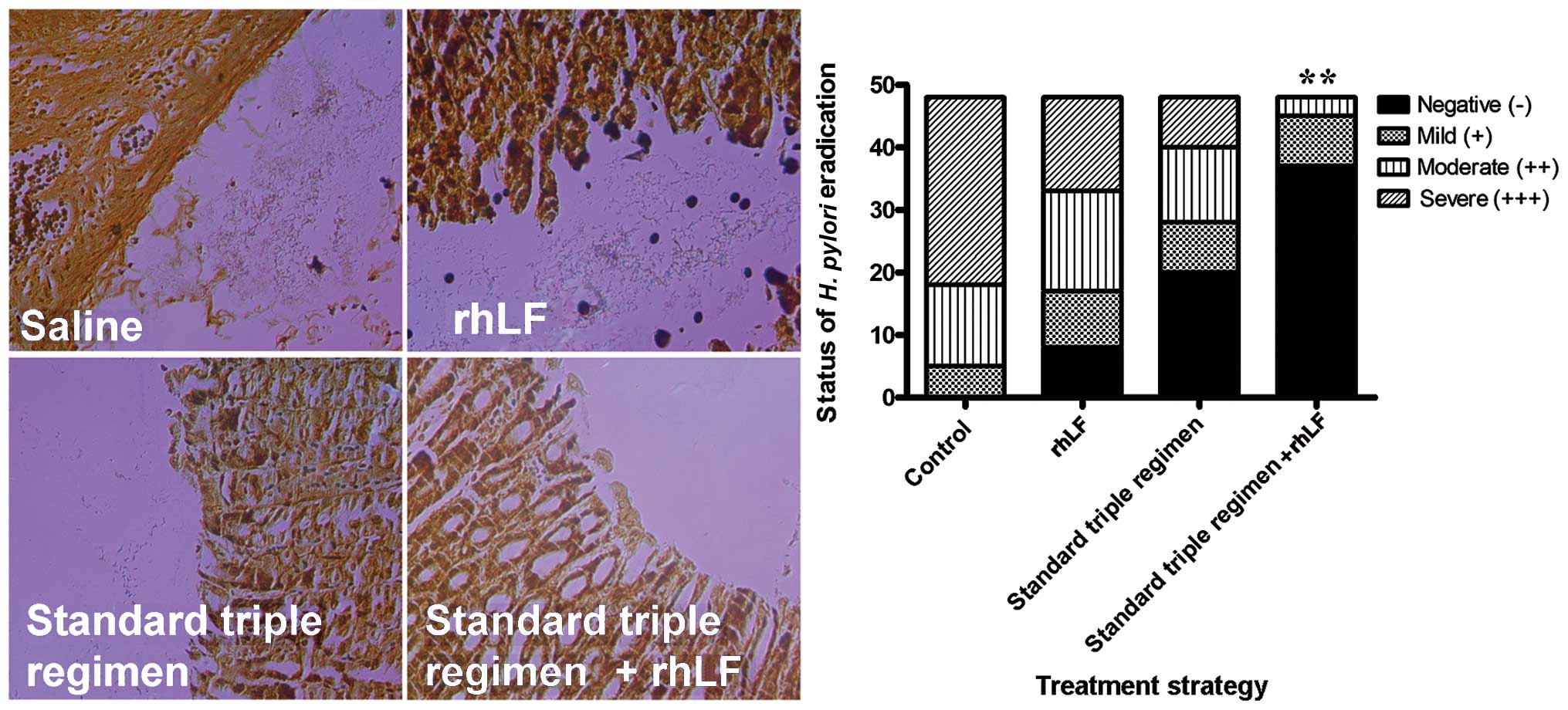
|
1
|
Bytzer P, Dahlerup JF, Eriksen JR, Jarbøl
DE, Rosenstock S and Wildt S; Danish Society for Gastroenterology:
Diagnosis and treatment of Helicobacter pylori infection. Dan Med
Bull. 58:C42712011.PubMed/NCBI
|
|
2
|
Olokoba AB, Obateru OA and Bojuwoye MO:
Helicobacter pylori eradication therapy: A review of current
trends. Niger Med J. 54:1–4. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murphy G, Michel A, Taylor PR, Albanes D,
Weinstein SJ, Virtamo J, Parisi D, Snyder K, Butt J, McGlynn KA, et
al: Association of seropositivity to Helicobacter species and
biliary tract cancer in the ATBC study. Hepatology. 60:1963–1971.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leung WK and Graham DY: Rescue therapy for
Helicobacter pylori. Curr Treat Options Gastroenterol. 5:133–138.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gisbert JP and Pajares JM: Review article:
Helicobacter pylori ‘rescue’ regimen when proton pump
inhibitor-based triple therapies fail. Aliment Pharmacol Ther.
16:1047–1057. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dresner D, Coyle W, Nemec R, Peterson R,
Duntemann T and Lawson JM: Efficacy of ciprofloxacin in the
eradication of Helicobacter pylori. South Med J. 89:775–778. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sanaka M, Kuyama Y, Yamanaka M and Iwasaki
M: Decrease in serum concentrations of Helicobacter pylori IgG
antibodies during antituberculosis therapy: the possible
eradication by rifampicin and streptomycin. Am J Gastroenterol.
94:1983–1984. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kusters JG, van Vliet AH and Kuipers EJ:
Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev.
19:449–490. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vorland LH: Lactoferrin: a multifunctional
glycoprotein. APMIS. 107:971–981. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Farnaud S and Evans RW: Lactoferrin: a
multifunctional protein with antimicrobial properties. Mol Immunol.
40:395–405. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ikeda M, Nozaki A, Sugiyama K, Tanaka T,
Naganuma A, Tanaka K, Sekihara H, Shimotohno K, Saito M and Kato N:
Characterization of antiviral activity of lactoferrin against
hepatitis C virus infection in human cultured cells. Virus Res.
66:51–63. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ellison RT III, Giehl TJ and LaForce FM:
Damage of the outer membrane of enteric Gram-negative bacteria by
lactoferrin and transferrin. Infect Immun. 56:2774–2781.
1988.PubMed/NCBI
|
|
13
|
Drago-Serrano ME, de la Garza-Amaya M,
Luna JS and Campos-Rodriguez R: Lactoferrin-lipopolysaccharide
(LPS) binding as key to antibacterial and antiendotoxic effects.
Int Immunopharmacol. 12:1–9. 2012. View Article : Google Scholar
|
|
14
|
Di Mario F, Aragona G, Bò ND, Ingegnoli A,
Cavestro GM, Moussa AM, Iori V, Leandro G, Pilotto A and Franzè A:
Use of lactoferrin for Helicobacter pylori eradication. Preliminary
results. J Clin Gastroenterol. 36:396–398. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sachdeva A and Nagpal J: Meta-analysis:
efficacy of bovine lacto-ferrin in Helicobacter pylori eradication.
Aliment Pharmacol Ther. 29:720–730. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Bortoli N, Leonardi G, Ciancia E, Merlo
A, Bellini M, Costa F, Mumolo MG, Ricchiuti A, Cristiani F, Santi
S, et al: Helicobacter pylori eradication: a randomized prospective
study of triple therapy versus triple therapy plus lactoferrin and
probiotics. Am J Gastroenterol. 102:951–956. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guttner Y, Windsor HM, Viiala CH and
Marshall BJ: Human recombinant lactoferrin is ineffective in the
treatment of human Helicobacter pylori infection. Aliment Pharmacol
Ther. 17:125–129. 2003. View Article : Google Scholar
|
|
18
|
Miehlke S, Reddy R, Osato MS, Ward PP,
Conneely OM and Graham DY: Direct activity of recombinant human
lactoferrin against Helicobacter pylori. J Clin Microbiol.
34:2593–2594. 1996.PubMed/NCBI
|
|
19
|
Peek RM Jr and Blaser MJ: Helicobacter
pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer.
2:28–37. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vergara M, Vallve M, Gisbert JP and Calvet
X: Meta-analysis: comparative efficacy of different proton-pump
inhibitors in triple therapy for Helicobacter pylori eradication.
Aliment Pharmacol Ther. 18:647–654. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Altintas E, Sezgin O, Ulu O, Aydin O and
Camdeviren H: Maastricht II treatment scheme and efficacy of
different proton pump inhibitors in eradicating Helicobacter
pylori. World J Gastroenterol. 10:1656–1658. 2004.PubMed/NCBI
|
|
22
|
Dial EJ, Hall LR, Serna H, Romero JJ, Fox
JG and Lichtenberger LM: Antibiotic properties of bovine
lactoferrin on Helicobacter pylori. Dig Dis Sci. 43:2750–2756.
1998. View Article : Google Scholar
|
|
23
|
Censini S, Lange C, Xiang Z, Crabtree JE,
Ghiara P, Borodovsky M, Rappuoli R and Covacci A: cag, a
pathogenicity island of Helicobacter pylori, encodes type
I-specific and disease-associated virulence factors. Proc Natl Acad
Sci USA. 93:14648–14653. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Christie PJ and Vogel JP: Bacterial type
IV secretion: conjugation systems adapted to deliver effector
molecules to host cells. Trends Microbiol. 8:354–360. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fischer W, Puls J, Buhrdorf R, Gebert B,
Odenbreit S and Haas R: Systematic mutagenesis of the Helicobacter
pylori cag pathogenicity island: essential genes for CagA
translocation in host cells and induction of interleukin-8. Mol
Microbiol. 42:1337–1348. 2001. View Article : Google Scholar
|
|
26
|
Papini E, Satin B, Norais N, de Bernard M,
Telford JL, Rappuoli R and Montecucco C: Selective increase of the
permeability of polarized epithelial cell monolayers by
Helicobacter pylori vacuolating toxin. J Clin Invest. 102:813–820.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cover TL and Blanke SR: Helicobacter
pylori VacA, a paradigm for toxin multifunctionality. Nat Rev
Microbiol. 3:320–332. 2005. View Article : Google Scholar : PubMed/NCBI
|