Introduction
Seaweed comprises abundant bioactive antioxidants,
soluble dietary fibers, proteins, minerals, vitamins,
phytochemicals and polyunsaturated fatty acids (1). It has been reported that these
bioactive components (e.g., fucoxanthin, fucoidan and other
extracts) exert a variety of effects in humans, such as
anti-obesity (2), anticoagulant
(3) and antitumor effects
(4). The value of these
properties as a function of bioactive substances in seaweed has
been studied with regards to food, pharmaceuticals and medicinal
purposes (1). In particular,
Hizikia fusiformis extract was found to protect against
injury to the liver and stomach in rats; this was proven by
studying its molecular mechanisms of action (5,6).
Moreover, it has been reported that a glycoprotein from Pyropia
yezoensis (P. yezoensis) exerts anti-inflammatory effects in
RAW 264.7 mouse macrophages (7).
In addition, a protein isolated from P. yezoensis was shown
to exert chemoprotective effects on acetaminophen
(n-acetyl-p-aminophenol, APAP)-induced liver injury in rats
(8). APAP is an over-the-counter
drug that is widely used for its analgesic and antipyretic effects.
APAP is safe when used at therapeutic levels; however, an acute or
cumulative overdose can cause severe liver injury and possibly
liver failure (9). Studies on
APAP toxicity have been investigated both in vitro (10–13) and in vivo (8,14–17). The ability of seaweed to exert
chemoprotective effects against APAP means they are advantageous to
various organisms.
Recently, the association between the molecular
structure and function of seaweed was reported (18). Choi et al (18) reported that the synthetic peptide
(SP) ALEGGKSSGGGEATRDPEPT, which is present at the N-terminus of
mature P. yezoensis protein 1 (PYP1), demonstrated
chemoprotective effects against APAP-induced Chang liver cell
death.
In the present study, 3 proteins (PYP1, PYP1-AC and
PYP1-B) were derived from the cDNA that encodes PYP1 and were then
investigated for their chemoprotective effects against APAP-induced
Chang liver cell injury. Moreover, the N-terminal 11 residue
sequence of SP, ALEGGKSSGGG, which represents a common sequence
among all 3 peptides, was synthesized and compared with the
PYP1s.
Materials and methods
Molecular cloning of cDNA and the gene
encoding PYP1
To determine the N-terminal amino acid sequence of
PYP1 (18), the P.
yezoensis expressed sequence tag (EST) database of the Kazusa
DNA institute (Chiba, Japan) was surveyed. Since the resultant
information indicated that both mature and immature mRNAs were
present, cDNA encoding these variants was cloned. Briefly, the
cultivation of P. yezoensis gametophytes and the
amplification of cDNA from total RNA were performed as previously
described by Uji et al (19). DNA fragments corresponding to the
open reading frames (ORFs) of mature and immature variants were
then amplified by polymerase chain reaction (PCR) using the
following primer sets: PYP1-F and PYP1-R, PYP1-F and PYP1-AC-R, and
PYP1-F and PYP1-B-R (Table I).
The PCR conditions were as follows: 30 cycles at 98°C for 10 sec
and 68°C for 2 min using PrimeSTAR HS DNA polymerase with GC buffer
(Takara Bio., Otsu, Japan). Separation, purification, cloning and
sequence analysis were performed as previously described by Uji
et al (19), with the
exception of the pENTR/SD/D-TOPO vector (Invitrogen/Life
Technologies, Carlsbad, CA, USA), which was used for the cloning
and construction of plasmids that were expressed in bacteria as
entry plasmids. To isolate the genomic fragment containing PYP ORF
information, genomic DNA was prepared from gametophytes using a
DNeasy Plant Mini kit (Qiagen, Hilden, Germany), and genomic PCR
was performed as described above, using the PYP1-F and PYP1-R
primers. The amplified fragment was inserted into a pCR-Blunt
II-TOPO cloning kit (Clontech Laboratories, Inc., Mountain View,
CA, USA) and sequenced.
 | Table IPrimers used for PCR. |
Table I
Primers used for PCR.
| Primer | Sequence |
|---|
| PYP1-F |
5′-CACCATGGCGTTCGTGTCTGGGTTCAC-3′ |
| PYP1-R |
5′-CTTGCCCTCAGCCTTCTTCTTG-3′ |
| PYP1-AC-R |
5′-GTACGAGCGCGAGGTTGCGG-3′ |
| PYP1-B-R |
5′-ACGTACCGGCTCAGGGTCAC-3′ |
Expression analysis
To analyze the expression profile of the PYP1
gene in both gametophytes and sporophytes of P. yezoensis,
several generations were cultured and used for total RNA extraction
in order to amplify the cDNA, as previously described by Uji et
al (19). Following the
synthesis of the first-strand cDNA using a PrimeScript II First
Strand cDNA Synthesis kit (Takara Bio), reverse-transcription PCR
(RT-PCR) was performed using Phusion High-Fidelity DNA polymerase
(New England BioLabs, Inc., Beverley, MA, USA) with the primer sets
described above, under the following conditions: 98°C for 1 min and
30 cycles at 98°C for 10 sec, 55°C for 30 sec, and 72°C for 1
min.
Construction of PYP1 expression
plasmids
Gateway Technology (Invitrogen/Life Technologies)
was employed to construct the expression plasmids for the PYP1 and
PYP1 variants in Escherichia coli (E. coli). To
produce the destination vector, pQE-82L (Qiagen) was digested with
SmaI in the multi-cloning site and ligated using Restriction
fragment analysis (RFA; Invitrogen/Life Technologies). The
resultant plasmid was designated as pQE80L-DES. LR recombination
reactions were then performed with entry plasmids and pQE82L-DES
according to the manufacturer’s instructions, thereby, producing
pQE82L-PYP1, pQE82L-PYP1-AC and pQE82L-PYP1-B.
Overexpression and purification of
recombinant PYP1, PYP1-AC and PYP1-B
Plasmids (pQE82L-PYP1, pQE82L- PYP1-AC and
pQE82L-PYP1-B) were transformed into the E. coli strain DH5α
and incubated on ice for 30 min. S.O.C medium (200 µl;
Invitrogen Life Technologies) was added followed by incubation for
1 h at 37°C. The mixture was then spread on a plate with LB medium
containing 100 µg/ml ampicillin and incubated for 16 h at
37°C. After confirming the transformation in E. coli DH5α
cells, the plasmids were transformed into the E. coli strain
BL21 (DE3) in a similar manner.
For the induction of the expression of PYP1, PYP1-AC
and PYP1-B, LB medium (20 ml) containing 100 µg/ml
ampicillin was inoculated and grown at 37°C overnight. Cultures
were inoculated into 1 ml of LB medium containing ampicillin (100
µg/ml; 1:50) with fresh LB culture. The cultures were grown
at 37°C until an optical density (OD)600 of 0.5 was
reached. One milliliter of sample was taken immediately prior to
induction, and expression was induced by the addition of isopropyl
β-D-1-thiogalactopyranoside (IPTG) to a final concentration of 1
mM. The cells were harvested by centrifugation at 4,000 × g for 20
min, resuspended in binding buffer (20 mM Tris-HCl, pH 8.0) and
lysed by ultrasonic disruption (Sonics & Materials Inc.,
Newtown, CT, USA). The sonicated extracts were then separated into
soluble and insoluble fractions by centrifugation at 4,000 × g for
20 min at 4°C.
To purify the recombinant proteins, soluble
fractions containing the PYP1s were loaded onto an Ni-NTA column
(Qiagen) with Ni-NTA His•Bind Resin (Merck Millipore, Darmstadt,
Germany) using lysis buffer (50 mM
NaH2PO4·H2O, 300 mM NaCl and 10 mM
imidazole). After complete loading, weakly bound proteins were
removed with wash buffer (50 mM NaH2PO4, 300
mM NaCl and 20 mM imidazole). Proteins were subsequently eluted
from a Ni-NTA spin kit (Qiagen) with elution buffer (50 mM
NaH2PO4, 300 mM NaCl and 250 mM imidazole),
according to the manufacturer’s instructions. The concentrations of
the protein samples collected during purification and purified
samples were measured using the BCA method using a BCA protein
assay kit (Pierce Biotechnology, Rockford, IL, USA).
All protein samples were resolved by 17% sodium
dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). For
N-terminal sequencing analysis, proteins were transferred onto
polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA,
USA) using an electrophoresis power supply (GE Healthcare
Bio-Sciences AB, Uppsala, Sweden).
Peptide synthesis
The N-terminal 11-residue sequence, which was a
common sequence in all the PYP1s (ALEGGKSSGGG), was synthesized by
Peptron (Daejeon, Korea). Purification of the SP was performed on a
Shimadzu Prominence HPLC system and controlled using the software
package Class-VP, 6.14 with a C18 column (Shiseido Capcell Pak;
Shiseido, Tokyo, Japan) in 0.1% trifluoroacetic acid (TFA)/water
and a gradient of 10–70% acetonitrile in 0.1% TFA, at a flow rate
of 1 ml/min and ultraviolet (UV) detection at 220 nm. The molecular
mass was confirmed at 918 kDa (it matched the sequence mass) using
mass analysis (HP 1100 Series LC/MSD; Agilent Technologies, Inc.,
Santa Clara, CA, USA).
Cell culture
The Chang liver cell line (HPV-18) was obtained from
the American Type Culture Collection (ATCC, Rockville, MD, USA).
The cells were cultured in minimum essential medium (MEM)
supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT,
USA), 100 U/ml penicillin and 100 mg/ml streptomycin. The cultures
were maintained in a humidified incubator at 37°C in 5%
CO2. The medium was replaced every 2 days.
Cell proliferation assay
The effects of PYP1, PYP1-AC, PYP1-B and SP
treatment on cell proliferation in the cells treated with 15 mM
APAP were colorimetrically determined by MTS assays using CellTiter
96 AQueous One Solution reagent (Promega, Madison, WI, USA). The
cells were seeded in 96-well plates at a density of
1.5×105 cells/well. Following incubation for 24 h, the
attached cells were maintained in serum-free medium (SFM) for 6 h,
and this was followed by treatment with PYP1, PYP1-AC, PYP1-B or SP
(0–1,000 pg/ml) for an additional 24 h. The cells were then
incubated with MTS solution at 37°C for 30 min, and the absorbance
was measured at 490 nm using a microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA). The OD490 values
of the control cells were designated as 100%.
4,6-Diamidio-2-phenylindole (DAPI)
staining assay
The cells were washed twice with phosphate-buffered
saline (PBS) and fixed with 4% paraformaldehyde. The fixed cells
were incubated at 37°C for 20 min and washed twice with PBS. The
cells were then stained with 1 µg/ml DAPI and incubated at
room temperature for 20 min in the dark. The stained cells were
observed under a fluorescence microscope (ECLIPSE TS100-F; Nikon
Corp., Tokyo, Japan).
Statistical analysis
Data were evaluated by one-way analysis of variance
(ANOVA) using the Statistical Package for the Social Sciences
version 10.0 (SPSS, Inc., Chicago, IL, USA). Significant
differences between means were identified using Duncan’s multiple
range test (P<0.05). A P-value <0.05 was considered to
indicate a statistically significant difference.
Results
Identification and characterization of
multiple PYP1 mRNA transcripts
The SP, ALG EGKSSGGGEATRDPEPT, corresponding to the
N-terminus of mature PYP1, has been shown to exhibit
chemoprotective activity against APAP-induced Chang liver cell
death (18). In order to obtain a
full-length cDNA encoding PYP1 for use in inducing expression in
bacteria, the P. yezoensis EST database of the Kazusa DNA
Research Institute (http://est.kazusa.or.jp/en/plant/porphyra/EST/) was
surveyed. A comparison of nucleotide sequences from the genomic
PYP1 gene revealed the presence of non-spliced introns,
which were determined to cause the various lengths of PYP1
mRNAs (Fig. 1A), indicating that
alterative splicing produces mature and immature PYP1
mRNAs.
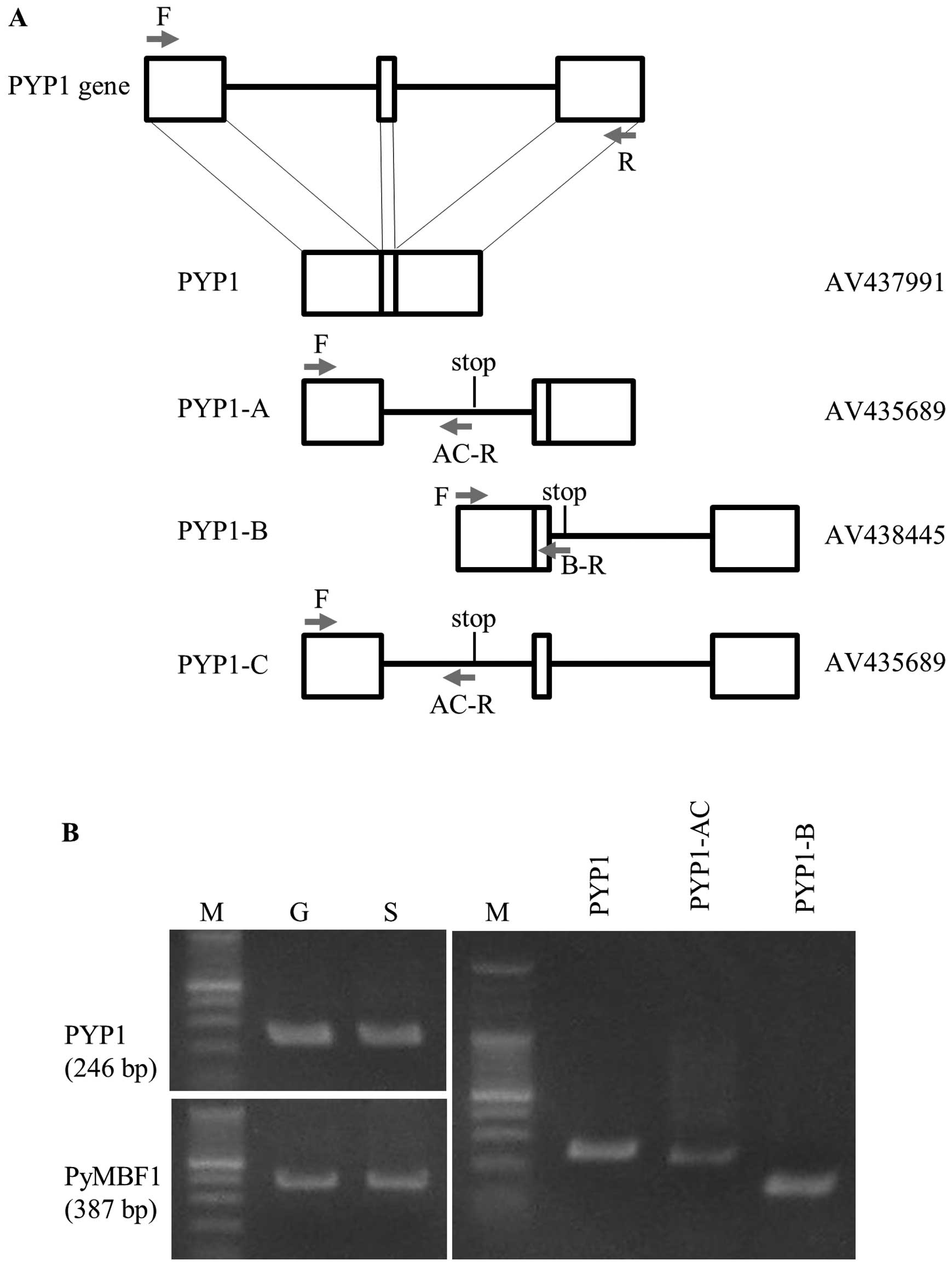 | Figure 1Identification and expression of
mature and immature Pyropia yezoensis protein 1
(PYP1) gene transcripts. (A) Schematic representation of
PYP1 and its variants produced by complete and alternative
splicing, respectively, compared with the genomic organization of
the PYP1 gene. Empty boxes represent exons, while bars
represent introns. Positions of stop codons are indicated in the
schemes of each variant. AV numbers determined by the Kazusa DNA
Research Institute are names of clones whose sequences were
determined previously. Arrows indicate the positions of primers
used for RT-PCR; (Table I): F,
PYP1-F; R, PYP1-R; AC-R, PYP1-AC-R; B-R, PYP1-B-R. (B) Gel images
showing the results from RT-PCR. Comparison of PYP1 gene
expression between gametophytic (G) and sporophytic (S) generations
(left panel). The expression pattern of PyMBF1 (19) is presented as a reference. The
presence of transcripts from the mature form and splice variants in
the gametophytic generation can be noted (right panel). The sizes
of the RT-PCR fragments for PYP1, PYP1-AC and PYP1-B are 246, 232
and 141 bp, respectively. M, protein marker. |
As shown in Fig.
1A, the PYP1 gene contains 2 introns; thus, the coding
region is divided into 3 exons. Three types of immature cDNA
contain either the first or second intron, or both introns.
Proteins derived from the cDNA containing the first, second, or
both introns were designated as PYP1-A, PYP1-B and PYP1-C,
respectively (Fig. 1A). PYP1-A
and PYP1-C encode the same protein, as they both contain the first
intron, resulting in 3 PYP1 proteins: one is mature and the other
two are variants, but all share an amino acid sequence
corresponding to the first exon (Fig.
2). We refer to this mixture of PYP1-A and PYP1-C as
PYP1-AC.
RT-PCR was employed to examine the expression of the
PYP1 gene in gametophytic and sporophytic generations of
P. yezoensis. Since a homology search of P. yezoensis
in the EST database resulted in ESTs derived from mRNAs of the
gametophytic generation being found (data not shown), the
generation-specific expression of the PYP1 gene was
speculated. However, Fig. 1B
(left panels) clearly illustrates the expression of the PYP1
gene in the gametophytic and sporophytic generations. Moreover, the
presence of transcripts corresponding to PYP1-AC and PYP1-B was
confirmed by RT-PCR (Fig. 1B,
right panel), thus indicating that P. yezoensis-treated
cells may contain all 3 PYP1 proteins.
Preparation of bacterially expressed PYP1
proteins
To evaluate the functional activities of the 3 PYP1
proteins, PYP1, PYP1-AC and PYP1-B plasmids for the induction of
expression in bacteria were constructed, which produced proteins
with a 6xHis tag.
Expression and purification of PYP1,
PYP1-AC and PYP1-B
The results of SDS-PAGE revealed purified soluble
proteins (Fig. 3). PYP1, PYP1-AC
and PYP1-B were first purified with Ni-NTA His•Bind Resin (Merck
Millipore) (Fig. 3A-a, B-a and
C-a). PYP1, PYP1-AC and PYP1-B were then further purified using
a Ni-NTA Spin kit (Qiagen), which produced proteins with the
following molecular weights: 15, 12 and 5 kDa, respectively
(Fig. 3A-b, B-b and C-b). This
two-step process used to extract PYP1s was effective.
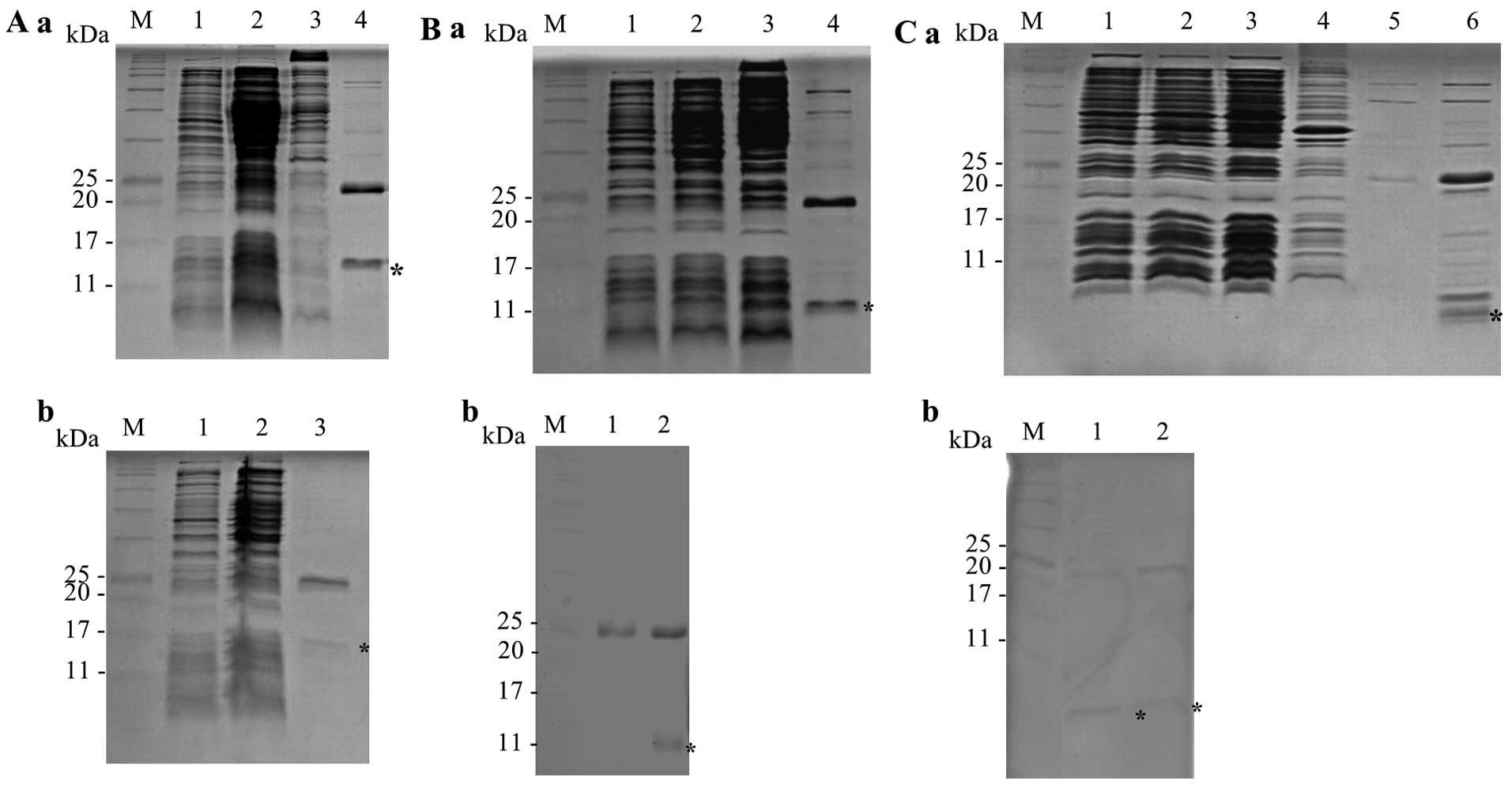 | Figure 3SDS-PAGE analysis of recombinant
PYP1s expressed in the E. coli strain BL21. Samples were
resolved by 17% SDS-PAGE. Recombinant proteins are indicated by an
asterisk. (A) Recombinant PYP1. (a) M, protein marker; lane 1,
before IPTG induction; lane 2, after IPTG induction; lane 3,
insoluble protein; lane 4, first purified, soluble protein; (b) M,
protein marker; lane 1, before IPTG induction; lane 2, after IPTG
induction; lane 3, second purified, soluble protein. (B)
Recombinant PYP1-AC. (a) M, protein marker; lane 1, before IPTG
induction; lane 2, after IPTG induction; lane 3; soluble protein;
lane 4, first purified, soluble protein; (b) M, protein marker;
lane 1, control vector; lane 2, second purified, soluble protein.
(C) Recombinant PYP1-B. (a) M, protein marker; lane 1, before IPTG
induction; lane 2, after IPTG induction; lane 3, soluble protein;
lane 4, insoluble protein; lane 5, first purified, soluble protein
fraction 1; lane 6, first purified, soluble protein fraction 2; (b)
M, protein marker; lanes 1 and 2, second purified, soluble protein.
PYP1, Pyropia yezoensis protein 1. |
Physiological activity of PYP1, PYP1-AC
and PYP1-B
The effects and toxicity of PYP1, PYP1-AC and PYP1-B
on Chang liver cells were determined by MTS assays. As shown in
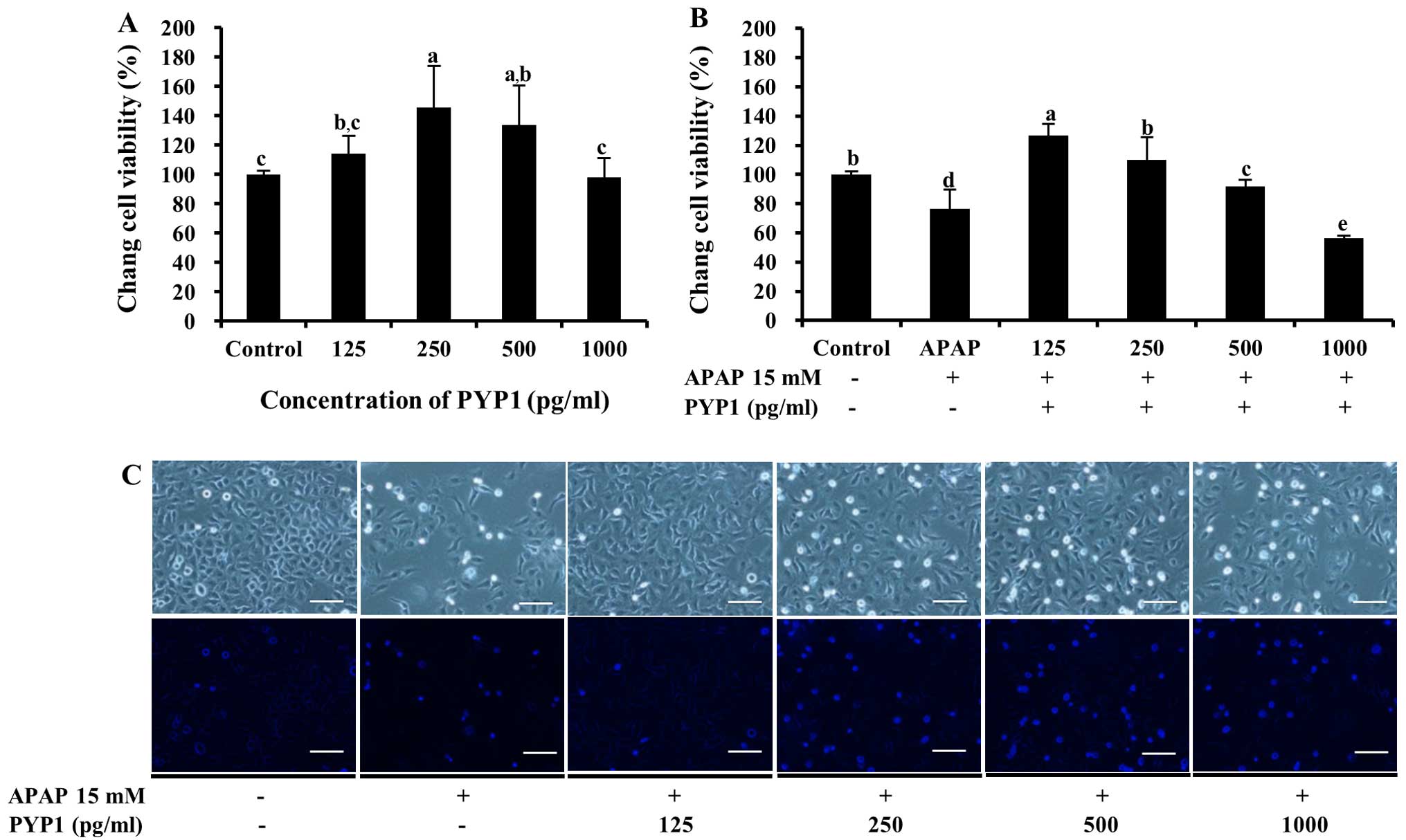
Fig. 4A, PYP1 was non-toxic.
Moreover, treatment with 125–500 pg/ml PYP1 increased cell
viability from 114.2±12.5 to 145.0±28.2% (P<0.05) compared with
the control. To determine whether PYP1 protects the Chang liver
cells against APAP-induced death, cell viability was examined
following treatment with APAP. The cells treated with 15 mM APAP
demonstrated a viability of 76.6±13.3%. By contrast, when the cells
were treated with 125–500 pg/ml PYP1, cell viability increased
significantly to 127.0±7.8, 110.2±15.6 and 91.0±4.6% that of the
control, respectively (Fig. 4B,
P<0.05). The same result was noted in the cells stained with
DAPI, according to fluorescence microscopy (Fig. 4C). Similar results were obtained
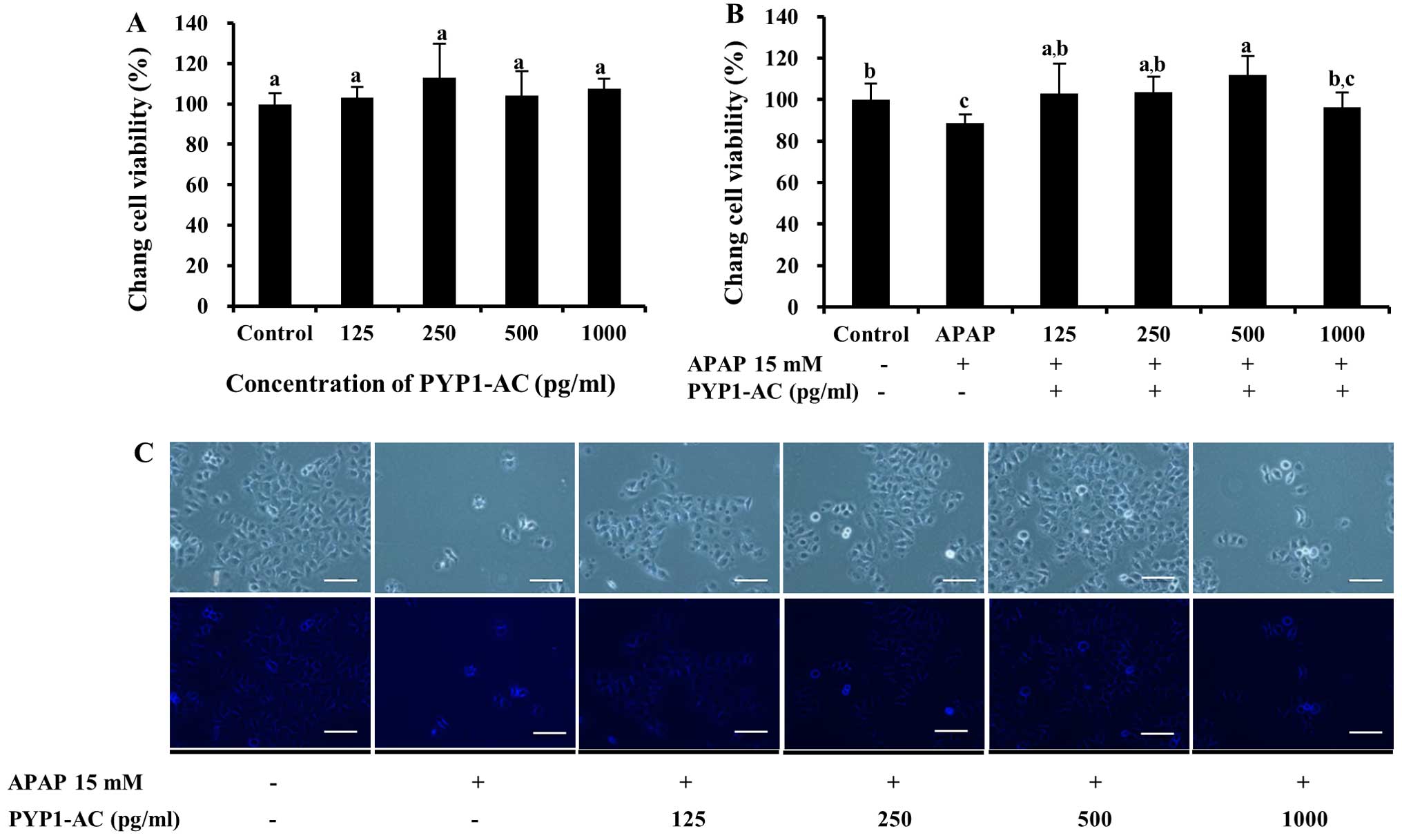
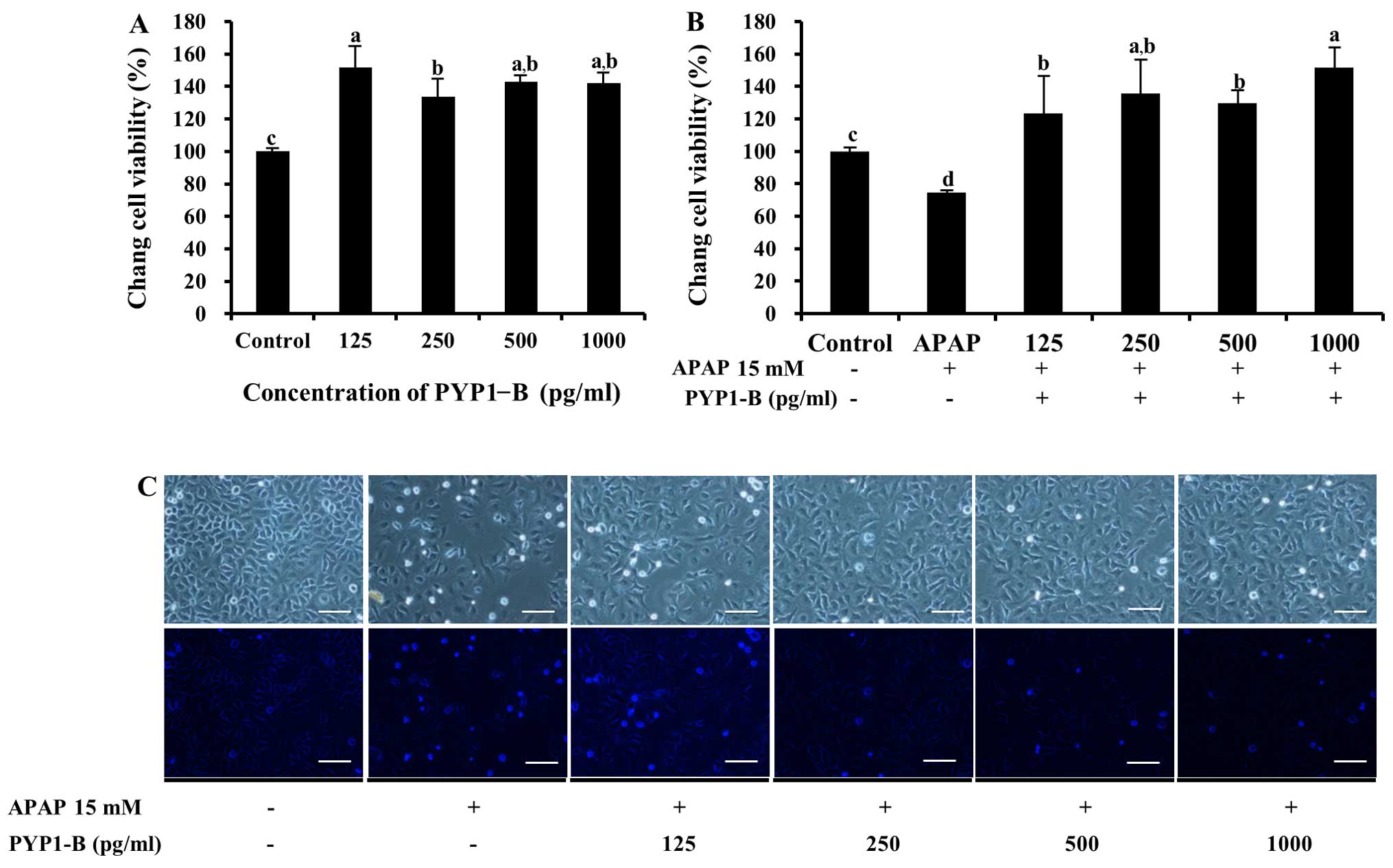
for both PYP1-AC and PYP1-B (Figs.
5 and 6). No significant
differences were observed with regard to cytotoxicity by PYP1-AC
(P<0.05, Fig. 5A); however,
cell viability increased significantly following treatment with
125–500 pg/ml PYP1-AC to 102.7±14.5, 103.5±7.6 and 111.7±9.3%
compared to that of the control, respectively (P<0.05, Fig. 5B). These results are presented as
photomicrographic images in Fig.
5C. Cell viability increased significantly to 151.4±13.6%
following treatment with 125–1000 pg/ml PYP1-B (P<0.05, Fig. 6A). In addition, treatment with
PYP1-B resulted in the highest value (151.4±12.6%) of all the APAP
treatment groups (P<0.05, Fig.
6B). The effect of PYP1-B was also demonstrated by examining
cellular morphology (Fig. 6C).
These results suggest that recombinant PYP1s may be used to protect
liver cells against APAP-induced cytotoxicity.
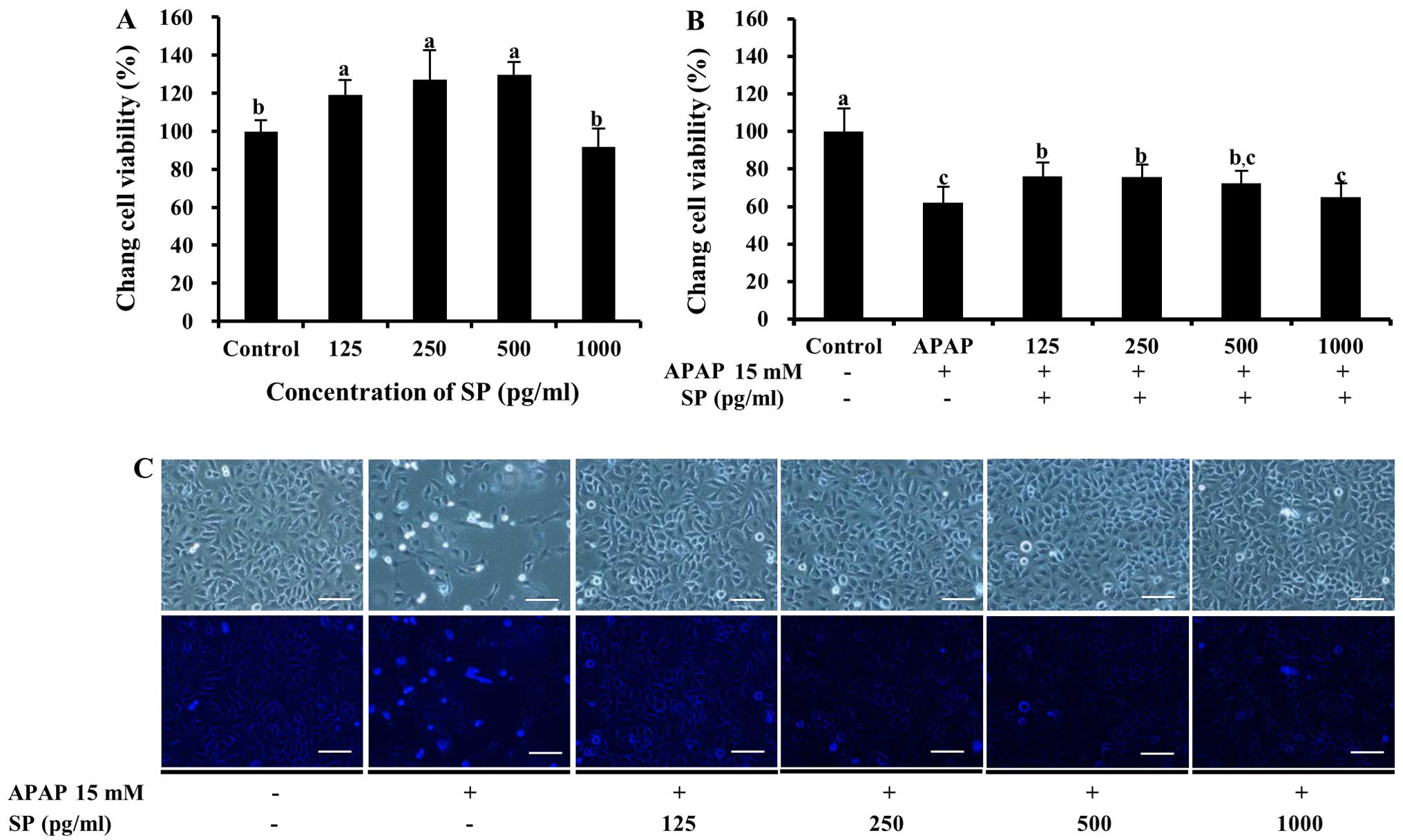
Physiological activity of SP
To determine whether SP, which contains the
N-terminal 11-residue sequence, protects Chang liver cells against
APAP-induced death, cell viability was examined following treatment
with APAP. As shown in Fig. 7A,
SP was not toxic to the cells and resulted in cellular
proliferation following treatment with 125–500 pg/ml SP. Notably,
following treatment with both APAP and SP, cell viability decreased
significantly compared with that of the control (P<0.05,
Fig. 7B). Lower values upon SP
treatment (64.9±7.5 to 75.9±7.5%, 125–1,000 pg/ml SP) compared to
those observed with PYP1, PYP1-AC and PYP1 were observed in the
APAP-treated cells. However, treatment with SP led to a significant
increase in cell viability compared to treatment with APAP alone
(61.8±8.6%; P<0.05, Fig. 7B).
The cells treated with SP appeared to increase in number compared
to the cells treated with APAP only (Fig. 7C).
Discussion
In a previous study of ours (18), we suggested that PYP1, a novel
protein from red alga P. yezoensis, had a sequence homology
with that of hypothetical, unknown proteins from Chondrus
crispus (Rhodophyta) and Emiliania huxleyi
(Haptophyceae). The physicochemical characteristics of PYP1 are
similar to those of late embryogenesis abundant (LEA) proteins. LEA
proteins protect against protein denaturation caused by various
environmental factors, such as desiccation, freezing, heat, salt
and osmotic stress (20). Since
P. yezoensis is one of the marine red algae that regulate
environmental stress responses, it is frequently used to study
molecular mechanisms (21).
Although APAP is an over-the-counter drug that is
widely used for analgesic and antipyretic purposes, an overdose can
cause liver injury, liver failure and even death (22). For these reasons, a variety of
studies have investigated APAP metabolism in the liver, and various
protective factors against APAP-induced liver toxicity, such as
genistein (23) and manganese
superoxide dismutase (SOD) (24),
have been noted. Few researchers have studied the mechanisms of
action of PYP since Hwang et al (8) reported the effects of PYP against
APAP-induced liver cell injury. Currently, only one study using a
particular PYP peptide has reported its regulation of multiple cell
growth-related signals in MCF-7 cells (25). PYP has also been shown to
stimulate the proliferation of IEC-6 normal intestinal epithelial
cells and is associated with the insulin-like growth factor I
(IGF-IR) and epidermal growth factor receptor (EGFR) signaling
pathways (26,27).
In the present study, three PYP1 proteins and one SP
containing the N-terminal 11-residue of the PYP1s were used to
investigate the chemoprotective activities of recombinant PYP1s
against APAP-induced cytotoxicity. We noted that SP, as well as
PYP1 proteins, exhibited highly positive and supportive
chemoprotective activities against APAP-induced toxicity. In
addition, SP had a similar effect against methotrexate-induced cell
death in Chang liver cells (data not shown). Therefore, these
results suggest that the PYP peptide exerts protective effects
against liver cell injury.
In general, macroalgae represent ideal starting
materials for the generation of marine protein-derived bioactive
peptides due to their high protein content (28). As these bioactive peptides act on
numerous physiological functions (29), we should focus on macroalgae from
natural materials. These peptides are involved in the modulation of
cell proliferation-associated molecules (30). In addition, peptides from
macroalgae have been reported to possess several biological traits,
including ACE inhibitory and anti-hypertensive effects (31,32). Of note, Suetsuna and Saito
(33) reported that PYP inhibits
antimutagenesis and Ca2+ precipitation, lowers plasma
and hepatic cholesterol, improves hepatic function, reduces blood
sugar and exhibits antioxidant and SOD-like qualities; PYP also has
angiotensin-converting-enzyme (ACE) inhibitory and
anti-hypertensive effects. Our findings demonstrated that PYP1s, as
bioactive peptides, have a positive effect against hepatic
toxicity. However, the potential of this bioactive peptide in
vivo remains to be elucidated; a specific biological activity
observed in vitro is only an indicator of the potential
active component in vivo (28). Therefore, further studies are
required to address the biological activity of PYP1s in vivo
and in signaling pathways.
Acknowledgments
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF), funded by the Ministry of Education (2012R1A6A1028677). We
are grateful to Ms. Megumi Akita for her technical assistance.
References
|
1
|
Mohamed S, Hashim SN and Rahman HA:
Seaweeds: A sustainable functional food for complementary and
alternative therapy. Trends Food Sci Technol. 23:83–96. 2012.
View Article : Google Scholar
|
|
2
|
Maeda H, Hosokawa M, Sashima T, Funayama K
and Miyashita K: Fucoxanthin from edible seaweed, Undaria
pinnatifida, shows antiobesity effect through UCP1 expression in
white adipose tissues. Biochem Biophys Res Commun. 332:392–397.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Colliec S, Fischer AM, Tapon-Bretaudiere
J, Boisson C, Durand P and Jozefonvicz J: Anticoagulant properties
of a fucoidan fraction. Thromb Res. 64:143–154. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Funahashi H, Imai T, Mase T, Sekiya M,
Yokoi K, Hayashi H, Shibata A, Hayashi T, Nishikawa M, Suda N, et
al: Seaweed prevents breast cancer? Jpn J Cancer Res. 92:483–487.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hwang HJ, Kim IH and Nam TJ: Effect of a
glycoprotein from Hizikia fusiformis on acetaminophen-induced liver
injury. Food Chem Toxicol. 46:3475–3481. 2008a. View Article : Google Scholar
|
|
6
|
Choi EY, Hwang HJ, Kim IH and Nam TJ:
Protective effects of a polysaccharide from Hizikia fusiformis
against ethanol toxicity in rats. Food Chem Toxicol. 47:134–139.
2009. View Article : Google Scholar
|
|
7
|
Shin ES, Hwang HJ, Kim IH and Nam TJ: A
glycoprotein from Porphyra yezoensis produces anti-inflammatory
effects in liposaccharide-stimulated macrophages via the TLR4
signaling pathway. Int J Mol Med. 28:809–815. 2011.PubMed/NCBI
|
|
8
|
Hwang HJ, Kwon MJ, Kim IH and Nam TJ:
Chemoprotective effects of a protein from the red algae Porphyra
yezoensis on acetaminophen-induced liver injury in rats. Phytother
Res. 22:1149–1153. 2008b. View
Article : Google Scholar
|
|
9
|
Lee WM: Acetaminophen and the U.S. Acute
Liver Failure Study Group: Lowering the risks of hepatic failure.
Hepatology. 40:6–9. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shon YH and Nam KS: Protective effect of
moutan cortex extract on acetaminophen-induced cytotoxicity in
human Chang liver cells. Biol Pharm Bull. 25:1427–1431. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rajaraman G, Chen J and Chang TKH:
Ginkgolide A contributes to the potentiation of acetaminophen
toxicity by Ginkgo biloba extract in primary cultures of rat
hepatocytes. Toxicol Appl Pharmacol. 217:225–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hwang HJ, Kwon MJ and Nam TJ:
Chemoprotective effect of insulin-like growth factor I against
acetaminophen-induced cell death in Chang liver cells via ERK1/2
activation. Toxicology. 230:76–82. 2007. View Article : Google Scholar
|
|
13
|
Gong Y, Wang G, Gong Y, Yan J, Chen Y and
Burczynski FJ: Hepatoprotective role of liver fatty acid binding
protein in acetaminophen induced toxicity. BMC Gastroenterol.
14:442014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ray SD, Kumar MA and Bagchi D: A novel
proanthocyanidin IH636 grape seed extract increases in vivo Bcl-XL
expression and prevents acetaminophen-induced programmed and
unprogrammed cell death in mouse liver. Arch Biochem Biophys.
369:42–58. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cover C, Fickert P, Knight TR,
Fuchsbichler A, Farhood A, Trauner M and Jaeschke H:
Pathophysiological role of poly(ADP-ribose) polymerase (PARP)
activation during acetaminophen-induced liver cell necrosis in
mice. Toxicol Sci. 84:201–208. 2005. View Article : Google Scholar
|
|
16
|
Oliveira FA, Chaves MH, Almeida FRC, Lima
RCP Jr, Silva RM, Maia JL, Brito GAAC, Santos FA and Rao VS:
Protective effect of α- and β-amyrin, a triterpene mixture from
Protium heptaphyllum (Aubl.) March. trunk wood resin, against
acetaminophen-induced liver injury in mice. J Ethnopharmacol.
98:103–108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Raghavendran HRB, Sathivel A and Devaki T:
Protective effect of Sargassum polycystum (brown alga) against
acetaminophen-induced lipid peroxidation in rats. Phytother Res.
19:113–115. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi YH, Yamaguchi K, Oda T and Nam TJ:
Chemical and mass spectrometry characterization of the red alga
Pyropia yezoensis chemoprotective protein (PYP): Protective
activity of the N-terminal fragment of PYP1 against
acetaminophen-induced cell death in Chang liver cells. Int J Mol
Med. 35:271–276. 2015.
|
|
19
|
Uji T, Hirata R, Mikami K, Mizuta H and
Saga N: Molecular characterization and expression analysis of
sodium pump genes in the marine red alga Porphyra yezoensis. Mol
Biol Rep. 39:7973–7980. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Battaglia M, Olvera-Carrillo Y,
Garciarrubio A, Campos F and Covarrubias AA: The enigmatic LEA
proteins and other hydrophilins. Plant Physiol. 148:6–24. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blouin NA, Brodie JA, Grossman AC, Xu P
and Brawley SH: Porphyra: a marine crop shaped by stress. Trends
Plant Sci. 16:29–37. 2011. View Article : Google Scholar
|
|
22
|
Xie Y, McGill MR, Dorko K, Kumer SC,
Schmitt TM, Forster J and Jaeschke H: Mechanisms of
acetaminophen-induced cell death in primary human hepatocytes.
Toxicol Appl Pharmacol. 279:266–274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan YJ, Rong Y, Li PF, Dong WL, Zhang DY,
Zhang L and Cui MJ: Genistein protection against
acetaminophen-induced liver injury via its potential impact on the
activation of UDP-glucuronosyltransferase and antioxidant enzymes.
Food Chem Toxicol. 55:172–181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ramachandran A, Lebofsky M, Weinman SA and
Jaeschke H: The impact of partial manganese superoxide dismutase
(SOD2)-deficiency on mitochondrial oxidant stress, DNA
fragmentation and liver injury during acetaminophen hepatotoxicity.
Toxicol Appl Pharmacol. 251:226–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park SJ, Ryu J, Kim IH, Choi YH and Nam
TJ: Induction of apoptosis by a peptide from Poryphyra yezoensis:
Regulation of the insulin-like growth factor I receptor signaling
pathway in MCF-7 cells. Int J Oncol. 45:1011–1016. 2014.PubMed/NCBI
|
|
26
|
Lee MK, Kim IH, Choi YH and Nam TJ: A
peptide from Porphyra yezoensis stimulates the proliferation of
IEC-6 cells by activating the insulin-like growth factor I receptor
signaling pathway. Int J Mol Med. 35:533–538. 2015.
|
|
27
|
Lee MK, Kim IH, Choi YH, Choi JW, Kim YM
and Nam TJ: The proliferative effects of Pyropia yezoensis peptide
on IEC-6 cells are mediated through the epidermal growth factor
receptor signaling pathway. Int J Mol Med. 35:909–914.
2015.PubMed/NCBI
|
|
28
|
Harnedy PA and FitzGerald RJ: Bioactive
proteins, peptides, and amino acids from macroalgae. J Phycol.
47:218–232. 2011. View Article : Google Scholar
|
|
29
|
Murray BA and FitzGerald RJ: Angiotensin
converting enzyme inhibitory peptides derived from food proteins:
Biochemistry, bioactivity and production. Curr Pharm Des.
13:773–791. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Morse ANC: GABA-mimetic peptides form
marine algae and cyanobacteria as potential diagnostic and
therapeutic agents. Bioactive Compounds From Marine Organisms -
With emphasis on the Indian Ocean. Thompson MF, Sarojini R and
Nagabhushanam R: Oxford & IBH Publishing Co.; New Delhi, India:
pp. 167–172. 1991
|
|
31
|
Suetsuna K: Purification and
identification of angiotensin I-converting enzyme inhibitors from
the red alga Porphyra yezoensis. J Mar Biotechnol. 6:163–167.
1998.PubMed/NCBI
|
|
32
|
Saito M and Hagino H: Antihypertensive
effect of oligopeptides derived from nori (Porphyra yezoensis) and
Ala-Lys-Tyr-Ser-Tyr in rats. J Jpn Soc Nutr Food Sci. 58:177–184.
2005. View Article : Google Scholar
|
|
33
|
Suetsuna K and Saito M: Enzyme-decomposed
materials of laver and uses thereof. US Patent 6217879B1. April
17–2001
|