Introduction
Lung cancer is one of the most common malignancies
and major causes of cancer-related fatalities worldwide, and the
majority of the patients with lung cancer present with advanced
disease (1,2). Over the past few years, despite the
significant advances that have been made in the treatment of
advanced lung cancer, such as chemotherapy and targeted therapy,
the majority of these patients succumb to cancer metastasis
(3). Therefore, it is of
importance to explore the underlying mechanisms of lung cancer
metastasis.
Cancer metastasis is a complex process, during which
the acquisition of migratory potential by cancer cells is a
fundamental prerequisite (4).
Epithelial-to-mesenchymal transition (EMT), a conversion in cell
phenotype, has been recognized as one of the universal mechanisms
by which cancer cells acquire the migratory and invasive capacities
(5). During the process of EMT,
epithelial cells acquire the fibroblastoid appearance due to
downregulation of epithelial markers and upregulation of
mesenchymal markers, thus, generating a migratory phenotype. Given
the role of EMT in the onset of the metastatic cascade, controlling
EMT is currently considered as a promising strategy to inhibit
cancer metastasis and improve patient survival. However, the drug
that can effectively block the occurrence of EMT has not been
reported.
Bufalin is one of the main effective components of
the traditional Chinese medicine Chan Su, which is obtained from
the skin and parotid venom glands of the Chinese toad (6). Our previous study and others have
shown that bufalin exerts anticancer effects by inducing cell cycle
arrest, cell differentiation and cell apoptosis in various types of
human cancer cells, such as leukemia, prostate, gastric, lung and
hepatocellular carcinoma cells (7–14).
Recently, several studies have suggested that bufalin inhibits cell
migration, invasion and metastasis in several types of cancer,
including hepatocellular carcinoma and osteosarcoma cells,
partially through suppression of protein kinase B (AKT) and
extracellular signal-regulated kinase (ERK), c-Jun N-terminal
kinase (JNK) and p38 mitogen-activated protein (MAP) kinases
signaling pathways (15–17). These signaling pathways are also
involved in transforming growth factor-β (TGF-β)-induced EMT and
migration (18). However, the
effect of bufalin on EMT and migration of lung cancer cells
mediated by TGF-β remains unclear.
In the present study, bufalin inhibits
TGF-β-triggered EMT and the consequent cell migration of lung
cancer A549 cells by downregulation of the TGF-β receptors, thus,
providing novel evidence for its anticancer effect.
Materials and methods
Cell culture
The human lung cancer A549 cell line was purchased
from the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). The cells were cultured in RPMI-1640 medium
(Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum
(FBS), penicillin (100 U/ml) and streptomycin (100 mg/ml) at 37°C,
in a humidified incubator with 5% CO2.
Reagents and antibodies
Recombinant human TGF-β was purchased from R&D
Systems (Minneapolis, MN, USA). Bufalin and SB431542 were purchased
from Sigma-Aldrich (St. Louis, MO, USA). Anti-E-cadherin (3195),
anti-vimentin (5741), anti-phospho-Smad2 (Ser465/467; 3108),
anti-Smad2 (5339), anti-phospho-AKT (Ser473; 9271), anti-AKT
(9272), anti-phospho-p38 (Thr180/Tyr182; 9216), anti-p38 (9218),
anti-phospho-JNK (Thr183/Tyr185; 9251), anti-JNK (9252), anti-TβRI
(3712) and anti-TβRII (11888) antibodies were purchased from Cell
Signalling Technology (Danvers, MA, USA). Anti-N-cadherin
(ab12221), anti-fibronectin (ab6328), anti-Twist (ab50887),
anti-Twist2 (ab57997), anti-Snail (ab135708), anti-Slug (ab27568),
anti-phospho-Smad3 (Ser423/425; ab52903) and anti-Smad3 (ab28379)
antibodies were purchased from Abcam (Cambridge, MA, USA).
Anti-actin (sc-1616-R), anti-phospho-ERK1/2 (Thr202/Tyr204;
sc-16982-R), anti-ERK1/2 (sc-154) and anti-zinc finger E-box
binding homeobox 2 (ZEB2; sc-271984) antibodies were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Cell viability assay
Cell viability was determined by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Cells were seeded at 2×104 cells/well in 96-well
plates and incubated overnight, and were treated with various
concentrations of bufalin for 24 h. Subsequently, 20 µl MTT
(5 mg/ml) was added to each well and the cells were incubated for
another 4 h at 37°C. Finally, the cells were lysed in 200 µl
dimethyl sulfoxide for 20 min at room temperature to solubilize the
crystals, and the optical density (OD) was measured at 570 nm with
a microplate reader (Bio-Rad Laboratories, Hercules, CA, USA). The
experiment was performed three times and in triplicate.
Flow cytometric analysis
Cells were seeded in 6-well plates and exposed to 5
ng/ml TGF-β alone or in combination with 50 nM bufalin for 24 h.
The cells were collected and fixed with ice-cold 70% ethanol for 12
h, and subsequently incubated with 20 µg/ml RNase A at 37°C
for 30 min and 10 µg/ml propidium iodide for 30 min in the
dark. Finally, the samples were evaluated by flow cytometry and the
data were analyzed using CellQuest software (Becton-Dickinson, San
Jose, CA, USA).
Western blot analysis
Cells were rinsed twice with phosphate-buffered
saline (PBS) and lysed in 1% Triton lysis buffer [1% Triton X-100,
50 mM Tris-Cl (pH 7.4), 150 mM NaCl, 10 mM ethylene diaminete
traacetic acid, 100 mM NaF, 1 mM Na3VO4, 1 mM
phenylmethyl sulfonyl fluoride and 2 µg/ml protinin] on ice.
Subsequently, the protein concentrations were determined using the
Lowry method. Total cell proteins (30–50 µg) were separated
by sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and electrophoretically transferred to nitrocellulose
membranes (Millipore, Bedford, MA, USA). The membranes were blocked
with 5% skimmed milk in Tris-buffered saline Tween-20 (TBST) buffer
[10 mM Tris (pH 7.4), 150 mM NaCl and 0.1% Tween-20] for 2 h at
room temperature and incubated with the primary antibodies at 4°C
overnight. Subsequent to rinsing thoroughly with TBST buffer, the
membrane was incubated with the corresponding horseradish
peroxidase-conjugated secondary antibodies for 30 min at room
temperature. Finally, following extensive rinsing with TBST buffer,
proteins on the membranes were visualized by an enhanced
chemiluminescence reagent (SuperSignal Western Pico
Chemiluminescent substrate; Pierce, Rockford, IL, USA) in the
Electrophoresis Gel Imaging analysis system (DNR Bio-Imaging
Systems, Jerusalem, Israel).
Immunofluorescence
The cells were seeded on coverslips, which were
placed in the 6-well plate in advance. Following treatment with or
without TGF-β (5 ng/ml) for 48 h, the cells were fixed with 4%
paraformaldehyde for 15 min, permeabilized with 0.5% Triton X-100
for 10 min, blocked with 1% bovine serum albumin for 1 h at room
temperature and incubated with anti-E-cadherin and anti-vimentin
antibody at 4°C overnight. Subsequently, the cells were rinsed
thoroughly with PBS, and were incubated with Alexa Fluor
546-conjugated goat anti-rabbit IgG (A-11010) or Alexa Fluor
488-conjugated goat anti-rabbit IgG (A-11034) (Molecular Probes,
Eugene, OR, USA) for 1 h at room temperature in the dark.
4′,6-Diamidino-2-phenylindole (Sigma-Aldrich) was used to stain the
nuclei for 5 min at room temperature. Following mounting with the
antifade mounting medium (Beyotime Institute of Biotechnology,
Haimen, China), the cells were visualized by fluorescence
microscopy (BX60; Olympus, Tokyo, Japan).
Wound healing assay
Cells were seeded in a 6-well plate and allowed to
grow to nearly 100% confluence in culture medium. Subsequently, a
cell-free line was manually created by scratching the confluent
cell monolayers with a 200-µl pipette tip. The wounded cell
monolayers were washed three times with PBS and incubated in
RPMI-1640 with 10% FBS containing 5 ng/ml TGF-β alone or in
combination with 50 nM bufalin for 24 h. Five scratched fields were
randomly chosen and the images were captured by bright-field
microscope (IX51; Olympus). The percentage of wound closure was
measured using Adobe Photoshop CS2 (Adobe Systems Inc., San Jose,
CA, USA). The experiment was performed three times and in
triplicate.
Transwell migration assay
A 24-well chemotaxis chamber (8 nM pore size;
Corning Inc., Corning, NY, USA) was used in the experiment.
Briefly, 1×104 cells in 200 µl serum-free medium
containing 5 ng/ml TGF-β alone or in combination with 50 nM bufalin
were seeded in the upper chamber, and 500 µl culture medium
supplemented with 2.5% FBS was added to the bottom well. After
incubation for 24 h, non-migrated cells were removed from the upper
surface of the chamber with a wet cotton swab and cells on the
lower surface of the chamber were stained using the Wright-Giemsa
method. The migrated cells were counted in five random fields under
bright-field microscope (DMI3000 B; Leica Microsystems, Wetzlar,
Germany). The experiment was performed three times and in
triplicate.
Statistical analysis
All the statistical analyses were performed using
the SPSS software (SPSS for Windows, version 16.0; SPSS, Inc.,
Chicago, IL, USA). Differences between two groups were evaluated by
Student's t-test. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
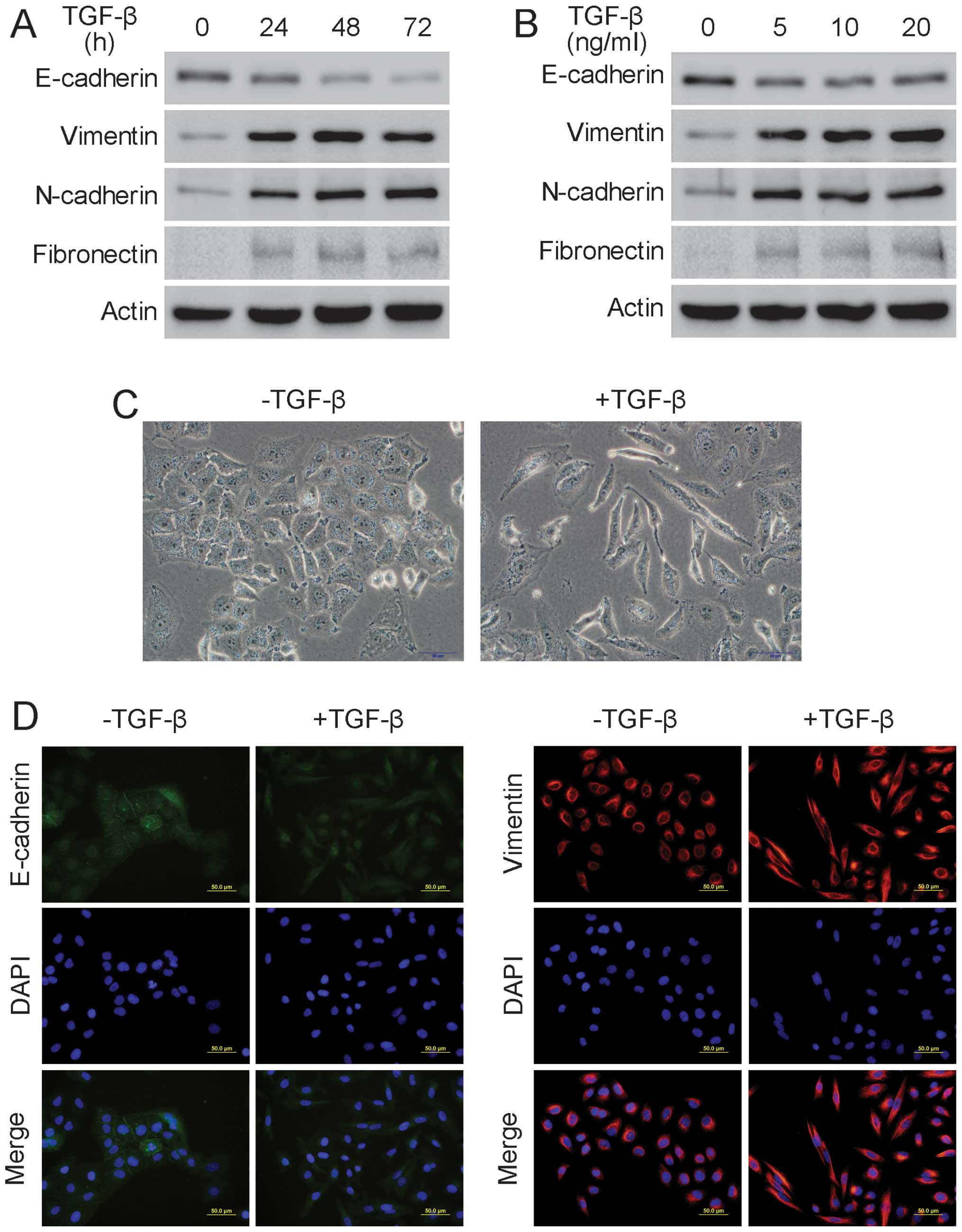
TGF-β induces EMT and promotes migration
in A549 cells
To determine the appropriate concentration and
duration for TGF-β to induce EMT, A549 cells were treated with 5
ng/ml TGF-β for the indicated durations or were incubated with
various TGF-β concentrations for 24 h. Western blot analysis showed
that the epithelial marker E-cadherin was downregulated and that
the mesenchymal markers vimentin, N-cadherin and fibronectin were
upregulated when the A549 cells were treated with 5 ng/ml TGF-β for
24 h, suggesting that EMT had occurred (Fig. 1A and B). Additionally, following
treatment with 5 ng/ml TGF-β for 24 h, A549 cells underwent clear
morphological changes, including disappearance of intercellular
junction, cell elongation and spindle-like appearance, indicating
that EMT had occurred (Fig. 1C).
Additionally, the immunofluorescence assay showed that there was an
evident decrease in E-cadherin and a significant increase in
vimentin after the A549 cells were treated with 5 ng/ml TGF-β for
24 h, further confirming the occurrence of EMT in A549 cells
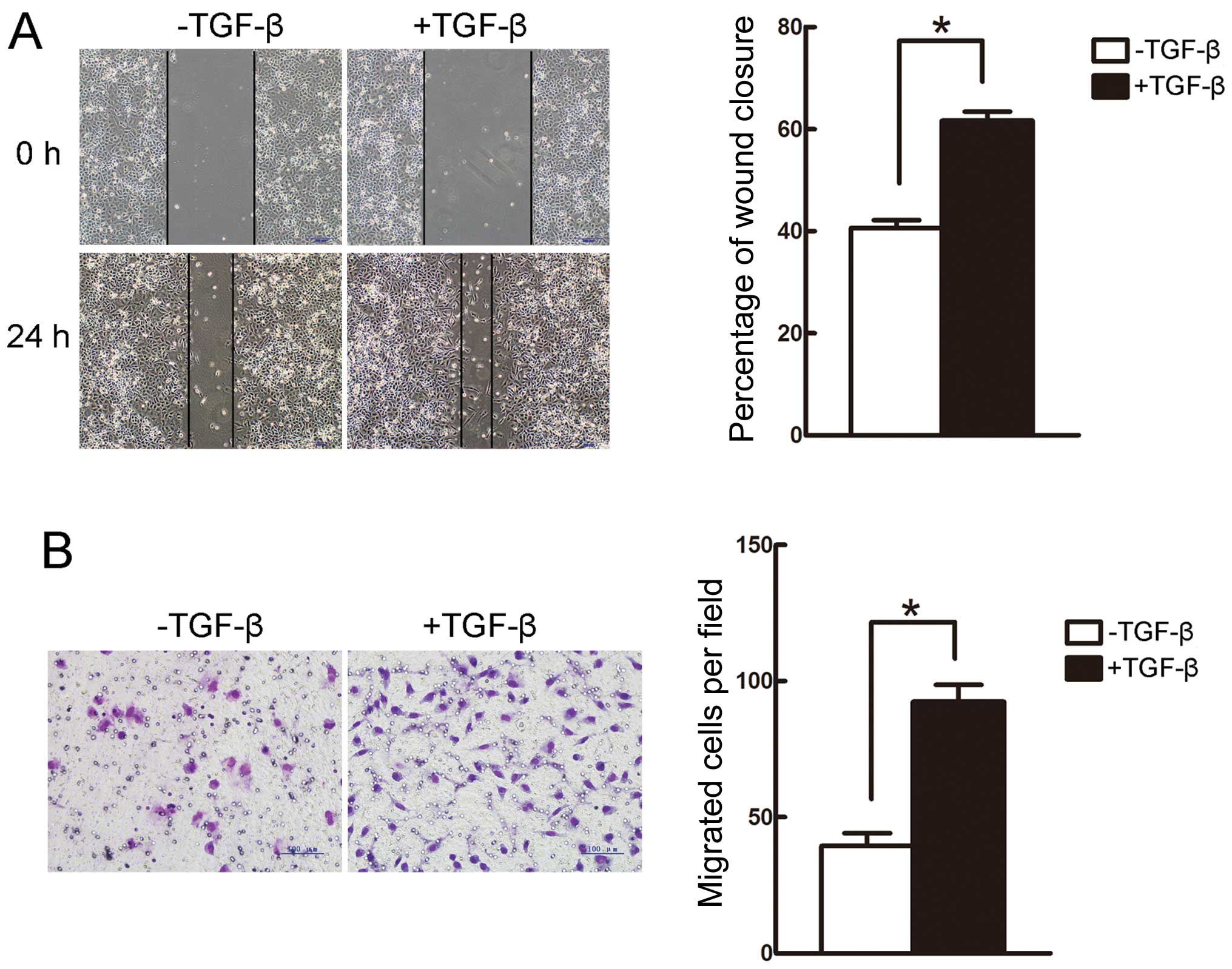
(Fig. 1D). Furthermore, the wound
healing and Transwell assays revealed that the migratory capacity
of A549 cells was enhanced following incubation with TGF-β for 24 h
(Fig. 2A and B). Therefore,
treatment with 5 ng/ml TGF-β for 24 h was used in the following
experiments.
Bufalin suppresses TGF-β-induced EMT in
A549 cells
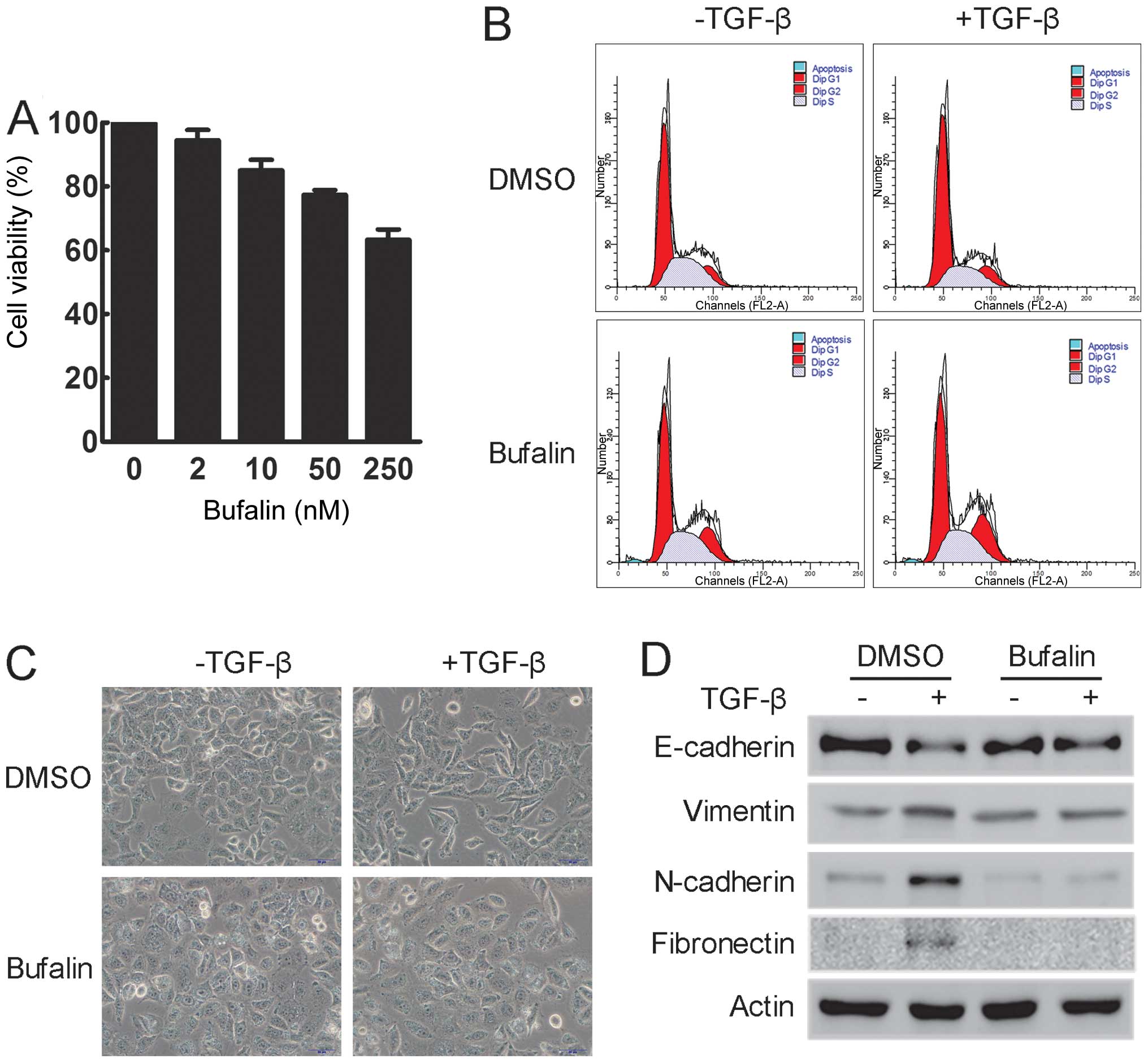
Since bufalin is mainly known as a cytotoxic agent,
the effect of bufalin on cell viability was examined. A549 cells
were treated with various concentrations of bufalin for 24 h. The
MTT assay showed that incubation with 50 nM bufalin for 24 h did
not significantly suppress the cell viability of A549 cells
(Fig. 3A). Additionally, as shown
by flow cytometry, treatment with 50 nM bufalin for 24 h had
minimal effect on the cell cycle distribution of A549 cells and did
not induce apoptosis in A549 cells (Fig. 3B). Thus, 50 nM bufalin was used in
the following experiments.
To determine the effect of bufalin during
TGF-β-induced EMT, the morphological changes in A549 cells treated
with TGF-β alone or in combination with bufalin were examined.
Treatment with TGF-β induced prominent morphological changes in
A549 cells, including cell elongation and spindle-like appearance,
indicating that A549 cells had undergone EMT. These changes were
clearly inhibited by concomitant treatment with bufalin, as
evidenced by a decrease in elongated and spindle-like cells
(Fig. 3C). In addition, western
blot analysis showed that the expression of epithelial markers,
such as E-cadherin, was significantly reduced, while that of
mesenchymal markers, such as vimentin, N-cadherin and fibronectin,
was increased following incubation with TGF-β. However,
simultaneous treatment with bufalin suppressed all these changes
(Fig. 3D). These data suggest
that bufalin can effectively inhibit TGF-β-induced EMT in A549
cells.
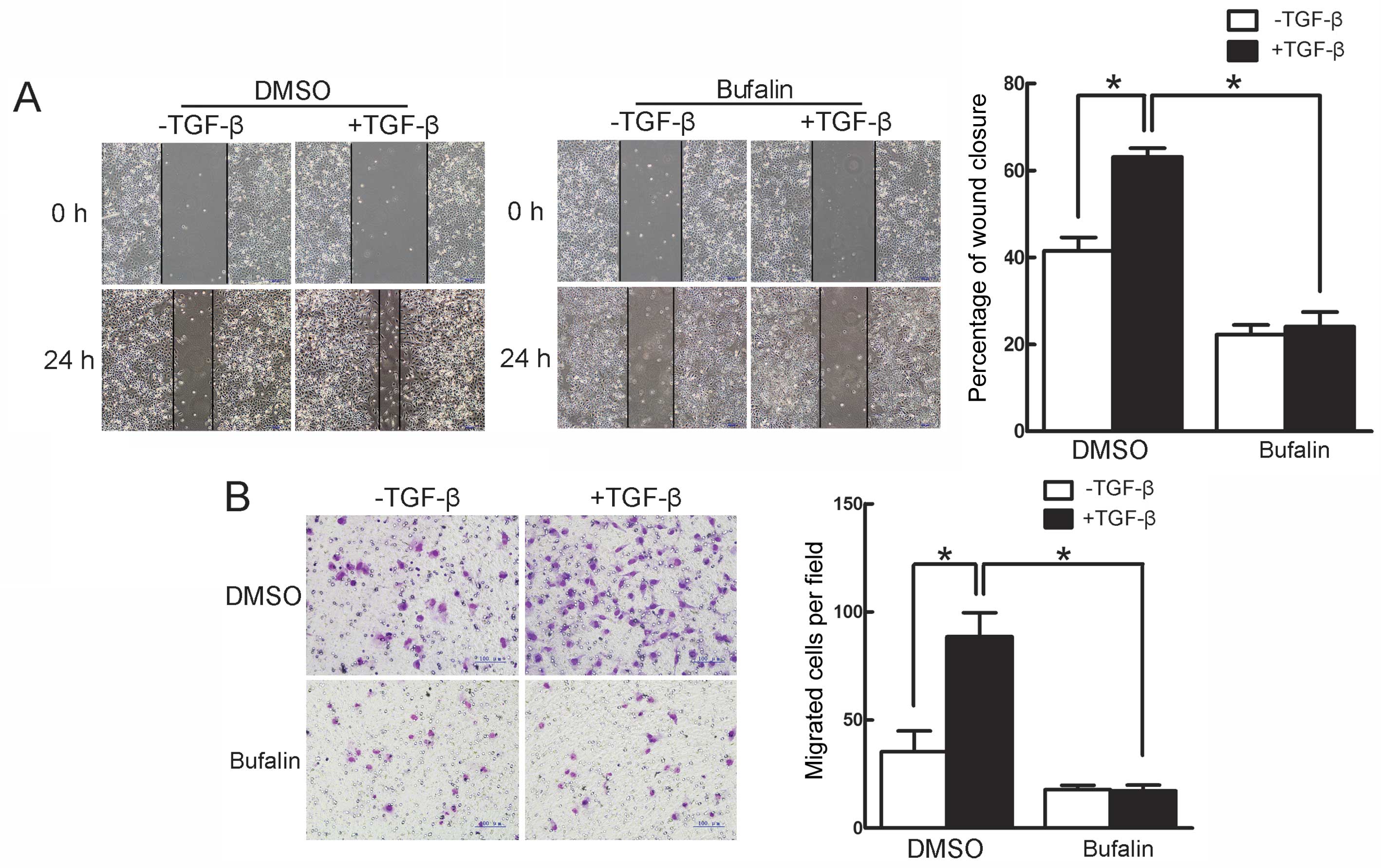
Bufalin inhibits TGF-β-induced migration
in A549 cells
The effect of bufalin on TGF-β-induced migration in
A549 cells was further investigated by the wound healing and
Transwell assays. In the wound healing assay, TGF-β facilitated the
closure of the scratched area on the cell monolayers, which was
inhibited by bufalin (Fig. 4A).
The Transwell assay showed that TGF-β significantly increased the
cells that migrated to the lower side of the filter, whereas
concomitant incubation with bufalin evidently suppressed the
TGF-β-induced increase in migrated cells (Fig. 4B). Thus, these findings
demonstrate that TGF-β-induced migration in A549 cells is
efficiently suppressed by bufalin.
Bufalin inhibits TGF-β-induced
upregulation of transcripton factors via downregulating TGF-β
receptors
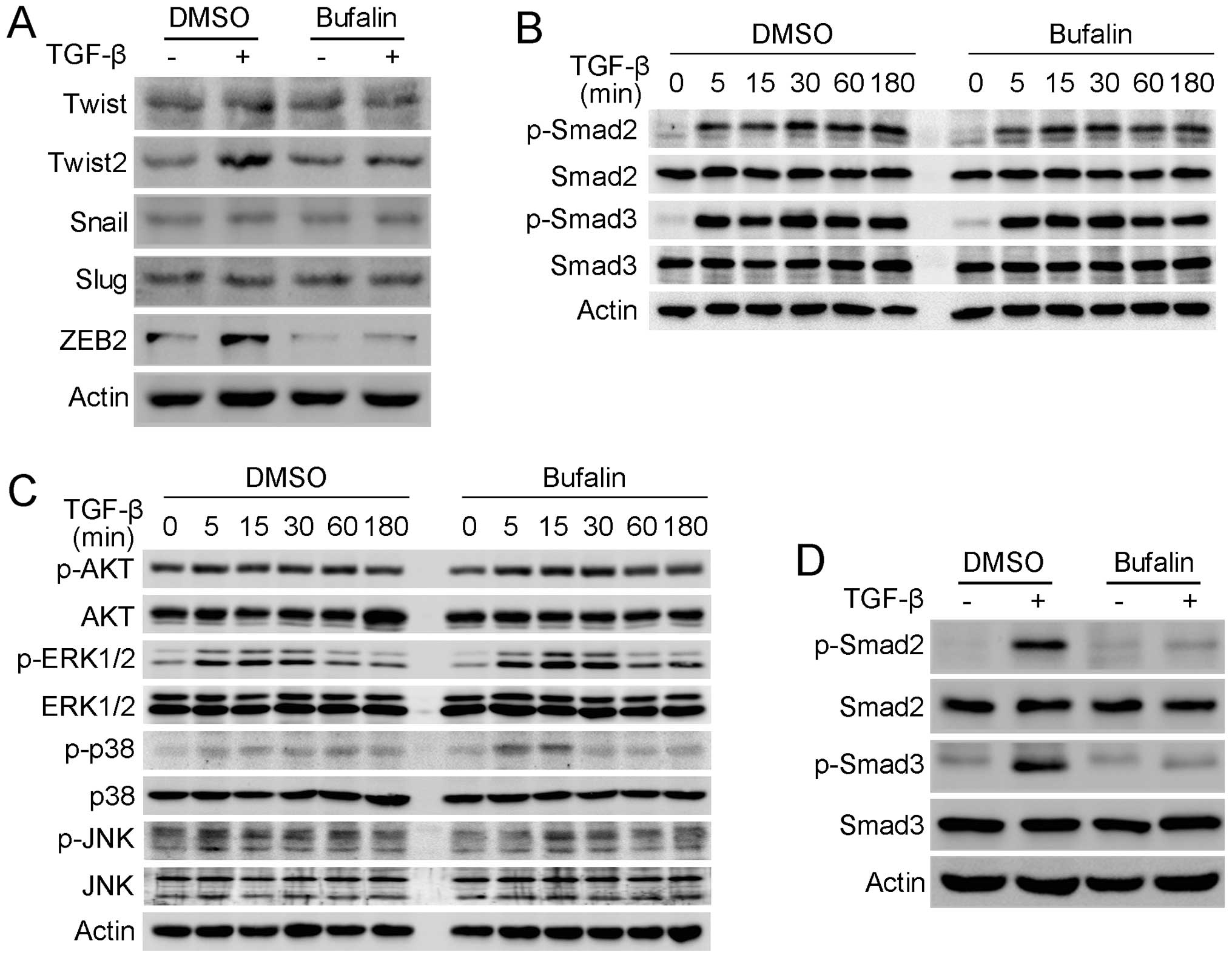
In order to reveal the mechanism by which bufalin
inhibits TGF-β-mediated EMT and migration in A549 cells, the
changes in EMT-related transcription factors induced by bufalin
were investigated. Western blot analysis showed that TGF-β induced
the upregulation of Twist2 and ZEB2, but not Twist, Snail or Slug
in the A549 cells, and the TGF-β-induced-upregulation of Twist2 and
ZEB2 was prominantly suppressed when A549 cells were concomitantly
treated with TGF-β and bufalin (Fig.
5A). As TGF-β functions mainly through Smad and
Smad-independent signaling pathways, the change of these signaling
pathways was investigated. Smad and Smad-independent signaling
pathways were not inhibited following treatment with bufalin for 3
h. The phosphorylation of AKT, ERK, p38 and JNK MAP kinases
terminated within 1 h, while the phosphorylation of Smad2 and Smad3
continued until 3 h (Fig. 5B and
C). Subsequently, the effect of bufalin on the phosphorylation
of Smad2 and Smad3 was further examined after A549 cells were
treated for 24 h. Western blot analysis demonstrated that bufalin
inhibited the phosphorylation of Smad2 and Smad3 activated by TGF-β
after incubation with bufalin for 24 h, while their total protein
levels remained unchanged (Fig.
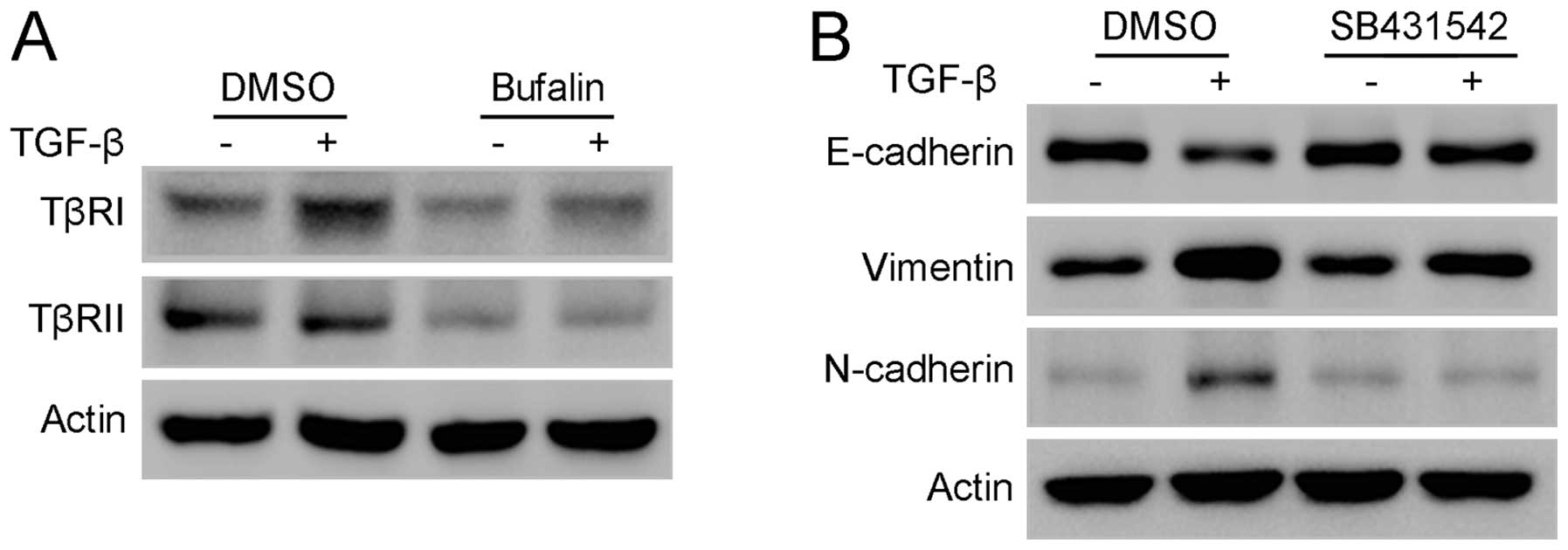
5D). As Smad2 and Smad3 are directly activated by TGF-β
receptors, the changes of TGF-β receptor I (TβRI) and TGF-β
receptor II (TβRII) were studied further. Western blot analysis
showed that TβRI and TβRII were significantly downregulated
following treatment with bufalin for 24 h (Fig. 6A). In addition, SB431542, a potent
inhibitor of the phosphorylation of TβRI, significantly attenuated
TGF-β-stimulated EMT in A549 cells, mimicking the effect of bufalin
on A549 cells (Fig. 6B). Taken
together, these results indicate that bufalin suppresses
TGF-β-induced EMT and migration in A549 cells by downregulating
TGF-β receptors.
Discussion
As EMT promotes cancer cell metastasis, its
mechanism has been studied extensively. EMT can be induced by
numerous cytokines and growth factors, particularly TGF-β (19). TGF-β is a pleiotropic cytokine
that regulates various biological processes, such as embryogenesis,
adult tissue homeostasis, fibrosis and cancer progression (20). TGF-β binds to TβRII on the cell
surface, resulting in the assembly of a hetero-tetrameric receptor
complex, in which TβRII phosphorylates and activates TβRI.
Activated TβRI phosphorylates the receptor-activated Smad proteins
(R-Smads), mainly Smad2 and Smad3, which translocate into the
nucleus and bind to DNA with transcriptional coactivators or
corepressors to control the expression of target genes (20). Additionally, TGF-β also activates
Smad-independent signaling pathways, such as AKT, ERK, JNK and p38
MAP kinases to exert its diverse function (21). Several studies have shown that
TGF-β induces EMT and enhances the migratory capacity in different
cell types (22–24). In the present study, TGF-β
stimulates morphological changes, characteristic of EMT, in a
time-dependent manner accompanied by downregulation of E-cadherin
and upregulation of vimentin, N-cadherin and fibronectin in A549
cells. In addition, the present results demonstrate that TGF-β
significantly enhances the migratory potential of A549 cells as a
result of EMT.
Bufalin has been shown to inhibit cell migration in
certain types of cancer cells (17,25). To the best of our knowledge, the
present study shows for the first time that bufalin effectively
suppresses TGF-β-induced-EMT and migration in A549 cells.
Transcription factors, such as Twist, Twist2, Snail, Slug and ZEB2,
repress the expression of E-cadherin and have a key role in EMT and
migration (26–30). Additionally, Smad and
Smad-independent signaling pathways are involved in TGF-β-induced
EMT and upreguation of EMT-related transcription factors (18). Furthermore, studies have
established the indispensable role of Smad signaling, mainly Smad2
and Smad3, in TGF-β-stimulated EMT (31,32). Smad signaling initiates Twist2 and
ZEB2 transcription following activation by TGF-β (33,34). The present results show that
bufalin significantly inhibits the upregulation of Twist2 and ZEB2,
but does not affect Smad-independent signaling pathways, including
AKT, ERK, p38 and JNK MAP kinases, during TGF-β-mediated EMT in
A549 cells. By contrast, the TGF-β-induced phosphorylation of Smad2
and Smad3 is significantly suppressed following treatment with
bufalin for 24 h. This may help the understanding of why the
upregulation of Twist2 and ZEB2 during TGF-β-induced EMT is
inhibited by bufalin. Smad2 and Smad3 are directly phosphorylated
by TβRI, which is phosphorylated and activated by TGF-β-bound TβRII
(20). Numerous studies have
suggested that TGF-β signaling is precisely controlled through the
modulation of TGF-β receptors (35–37). However, the detailed mechanisms of
the regulation of TGF-β receptors by bufalin remain unclear. The
present results show that TβRI and TβRII are significantly
downregulated following treatment of A549 cells with bufalin, and
that SB43152, the specific inhibitor of the phosphorylation of
TβRI, has a similar effect to bufalin on A549 cells. Thus, TGF-β
receptors may be the target for bufalin to inhibit TGF-β
signaling.
In conclusion, the present results indicate that
bufalin suppresses TGF-β-induced EMT and migratory capacity in
human lung cancer A549 cells through downregulating TGF-β
receptors. These findings warrant further assessment of bufalin in
clinically relevant models to explore its potential role in the
treatment of metastatic lung cancer.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81172369, 81172198
and 81472193).
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reck M, Heigener DF, Mok T, Soria JC and
Rabe KF: Management of non-small-cell lung cancer: Recent
developments. Lancet. 382:709–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yin PH, Liu X, Qiu YY, Cai JF, Qin JM, Zhu
HR and Li Q: Anti-tumor activity and apoptosis-regulation
mechanisms of bufalin in various cancers: New hope for cancer
patients. Asian Pac J Cancer Prev. 13:5339–5343. 2012. View Article : Google Scholar
|
|
7
|
Zhang L, Nakaya K, Yoshida T and Kuroiwa
Y: Induction by bufalin of differentiation of human leukemia cells
HL60, U937, and ML1 toward macrophage/monocyte-like cells and its
potent synergistic effect on the differentiation of human leukemia
cells in combination with other inducers. Cancer Res. 52:4634–4641.
1992.PubMed/NCBI
|
|
8
|
Jing Y, Watabe M, Hashimoto S, Nakajo S
and Nakaya K: Cell cycle arrest and protein kinase modulating
effect of bufalin on human leukemia ML1 cells. Anticancer Res.
14(3A): 1193–1198. 1994.PubMed/NCBI
|
|
9
|
Yu CH, Kan SF, Pu HF, Jea Chien E and Wang
PS: Apoptotic signaling in bufalin- and cinobufagin-treated
androgen-dependent and -independent human prostate cancer cells.
Cancer Sci. 99:2467–2476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li D, Qu X, Hou K, Zhang Y, Dong Q, Teng
Y, Zhang J and Liu Y: PI3K/Akt is involved in bufalin-induced
apoptosis in gastric cancer cells. Anticancer Drugs. 20:59–64.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qi F, Inagaki Y, Gao B, Cui X, Xu H,
Kokudo N, Li A and Tang W: Bufalin and cinobufagin induce apoptosis
of human hepatocellular carcinoma cells via Fas- and
mitochondria-mediated pathways. Cancer Sci. 102:951–958. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Y, Qu X, Wang P, Tian X, Luo Y, Liu S
and Lu X: WT1 down-regulation during K562 cell differentiation and
apoptosis induced by bufalin. Zhonghua Xue Ye Xue Za Zhi.
23:356–359. 2002.PubMed/NCBI
|
|
13
|
Qu X, Liu Y, Ma Y, Zhang Y, Li Y and Hou
K: Up-regulation of the Cbl family of ubiquitin ligases is involved
in ATRA and bufalin-induced cell adhesion but not cell
differentiation. Biochem Biophys Res Commun. 367:183–189. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu Z, Sun H, Ma G, Wang Z, Li E and Liu Y
and Liu Y: Bufalin induces lung cancer cell apoptosis via the
inhibition of PI3K/Akt pathway. Int J Mol Sci. 13:2025–2035. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang ZJ, Yang YK and Wu WZ: Bufalin
attenuates the stage and metastatic potential of hepatocellular
carcinoma in nude mice. J Transl Med. 12:572014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qiu DZ, Zhang ZJ, Wu WZ and Yang YK:
Bufalin, a component in Chansu, inhibits proliferation and invasion
of hepatocellular carcinoma cells. BMC Complement Altern Med.
13:1852013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chueh FS, Chen YY, Huang AC, Ho HC, Liao
CL, Yang JS, Kuo CL and Chung JG: Bufalin-inhibited migration and
invasion in human osteosarcoma U-2 OS cells is carried out by
suppression of the matrix metalloproteinase-2, ERK, and JNK
signaling pathways. Environ Toxicol. 29:21–29. 2014. View Article : Google Scholar
|
|
18
|
Moustakas A and Heldin CH: Induction of
epithelial-mesen-chymal transition by transforming growth factor β.
Semin Cancer Biol. 22:446–454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu J, Lamouille S and Derynck R:
TGF-β-induced epithelial to mesenchymal transition. Cell Res.
19:156–172. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Massagué J: TGFbeta in Cancer. Cell.
134:215–230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang YE: Non-Smad pathways in TGF-β
signaling. Cell Res. 19:128–139. 2009. View Article : Google Scholar :
|
|
22
|
Kim JH, Jang YS, Eom KS, Hwang YI, Kang
HR, Jang SH, Kim CH, Park YB, Lee MG, Hyun IG, et al: Transforming
growth factor β1 induces epithelial-to-mesenchymal transition of
A549 cells. J Korean Med Sci. 22:898–904. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ellenrieder V, Hendler SF, Boeck W,
Seufferlein T, Menke A, Ruhland C, Adler G and Gress TM:
Transforming growth factor β1 treatment leads to an
epithelial-mesenchymal transdifferentiation of pancreatic cancer
cells requiring extracellular signal-regulated kinase 2 activation.
Cancer Res. 61:4222–4228. 2001.PubMed/NCBI
|
|
24
|
Takai E, Tsukimoto M, Harada H, Sawada K,
Moriyama Y and Kojima S: Autocrine regulation of TGF-β1-induced
cell migration by exocytosis of ATP and activation of P2 receptors
in human lung cancer cells. J Cell Sci. 125:5051–5060. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hong SH, Kim GY, Chang YC, Moon SK, Kim WJ
and Choi YH: Bufalin prevents the migration and invasion of T24
bladder carcinoma cells through the inactivation of matrix
metalloproteinases and modulation of tight junctions. Int J Oncol.
42:277–286. 2013.
|
|
26
|
Fang X, Cai Y, Liu J, Wang Z, Wu Q, Zhang
Z, Yang CJ, Yuan L and Ouyang G: Twist2 contributes to breast
cancer progression by promoting an epithelial-mesenchymal
transition and cancer stem-like cell self-renewal. Oncogene.
30:4707–4720. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cano A, Pérez-Moreno MA, Rodrigo I,
Locascio A, Blanco MJ, del Barrio MG, Portillo F and Nieto MA: The
transcription factor snail controls epithelial-mesenchymal
transitions by repressing E-cadherin expression. Nat Cell Biol.
2:76–83. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bolós V, Peinado H, Pérez-Moreno MA, Fraga
MF, Esteller M and Cano A: The transcription factor Slug represses
E-cadherin expression and induces epithelial to mesenchymal
transitions: A comparison with Snail and E47 repressors. J Cell
Sci. 116:499–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Comijn J, Berx G, Vermassen P, Verschueren
K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D and van Roy
F: The two-handed E box binding zinc finger protein SIP1
downregulates E-cadherin and induces invasion. Mol Cell.
7:1267–1278. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Valcourt U, Kowanetz M, Niimi H, Heldin CH
and Moustakas A: TGF-β and the Smad signaling pathway support
transcriptomic reprogramming during epithelial-mesenchymal cell
transition. Mol Biol Cell. 16:1987–2002. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brandl M, Seidler B, Haller F, Adamski J,
Schmid RM, Saur D and Schneider G: IKK(α) controls canonical
TGF(β)-SMAD signaling to regulate genes expressing SNAIL and SLUG
during EMT in panc1 cells. J Cell Sci. 123:4231–4239. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsuji T, Ibaragi S, Shima K, Hu MG,
Katsurano M, Sasaki A and Hu GF: Epithelial-mesenchymal transition
induced by growth suppressor p12CDK2-AP1 promotes tumor cell local
invasion but suppresses distant colony growth. Cancer Res.
68:10377–10386. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Katoh M and Katoh M: Integrative genomic
analyses of ZEB2: Transcriptional regulation of ZEB2 based on
SMADs, ETS1, HIF1alpha, POU/OCT, and NF-kappaB. Int J Oncol.
34:1737–1742. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang S, Hölzel M, Knijnenburg T,
Schlicker A, Roepman P, McDermott U, Garnett M, Grernrum W, Sun C,
Prahallad A, et al: MED12 controls the response to multiple cancer
drugs through regulation of TGF-β receptor signaling. Cell.
151:937–950. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang L, Zhou F, García de Vinuesa A, de
Kruijf EM, Mesker WE, Hui L, Drabsch Y, Li Y, Bauer A, Rousseau A,
et al: TRAF4 promotes TGF-β receptor signaling and drives breast
cancer metastasis. Mol Cell. 51:559–572. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Eichhorn PJ, Rodón L, Gonzàlez-Juncà A,
Dirac A, Gili M, Martínez-Sáez E, Aura C, Barba I, Peg V, Prat A,
et al: USP15 stabilizes TGF-β receptor I and promotes oncogenesis
through the activation of TGF-β signaling in glioblastoma. Nat Med.
18:429–435. 2012. View
Article : Google Scholar : PubMed/NCBI
|