Introduction
Inflammation is a multifaceted process involving
changes at the cellular, tissue and systemic levels and it is
coordinated through a complex network of cytokine pathways. Many of
the key cytokines that are involved in inflammatory processes have
been identified and their functions determined. Research is,
however, continuing into the activation of the intracellular
signaling pathways that are activated and the interactions between
immunoregulatory factors and other cytokines and growth factors
(1).
Pathogen recognition by innate immune cells is
mediated by pattern recognition receptors that recognize conserved
pathogen-associated molecular patterns. Humans have various pattern
recognition receptors, which include Toll-like receptors (TLRs),
nucleotide-binding oligomerization domain (NOD)-like receptors
(NLRs) and retinoic acid-inducible gene (RIG)-1-like receptors
(2,3). These receptors transduce signals
leading to the activation of nuclear factor (NF)-κB, which
subsequently drives the induction of several pro-inflammatory
cytokines and chemokines (4–6).
TLRs play a major role in microbial detection. The regulation of
various genes, encoding inflammatory cytokines, such as interleukin
(IL)-1β, IL-6, IL-8, IL-12 and tumor necrosis factor (TNF)-α occurs
through TLRs on macrophages and other immunocompetent cells
(7). NF-κB is a key regulator of
pro-inflammatory gene expression. In unstimulated cells, NF-κB is
retained in the cytoplasm by binding to a family of inhibitory
proteins, the inhibitors of NF-κB (IκB). Upon cell stimulation, the
phosphorylation and degradation of IκBα leads to the translocation
of free NF-κB to the nucleus (8,9).
Nitric oxide (NO) is produced from L-arginine by the action of the
enzyme nitric oxide synthase (NOS). Several isoforms of the enzyme
exist, the most important of which are the constitutive form
(cNOS), which is present in endothelial cells and neurons, and
inducible NO synthase (iNOS), which is found in a variety of cells,
including macrophages and neutrophils. The latter is not normally
expressed, but it is induced by inflammatory cytokines and
bacterial lipopolysaccharide (LPS) (10,11).
Activins are dimeric growth and differentiation
factors that belong to the transforming growth factor (TGF)-β
superfamily of structurally related signaling proteins. Activins
are either heterodimers or homodimers of inhibin β subunits (βAβA,
βBβB or βAβB). Biological signaling by activins is mediated by
receptor complexes consisting of two different activin
serine/threonine kinase receptors (ActRs): type I (ActR-I) and type
II (ActR-II). Activin-responsive genes have been implicated in the
control of homeostasis, development, proliferation, apoptosis,
differentiation and inflammation in diverse cellular systems
(12,13). Activin produced by microglia acts
as an anti-inflammatory cytokine, presumably modulating
inflammation in an autocrine manner (14). Activin A decreases the production
of inflammatory factors and phagocytosis in activated macrophages
by suppressing the maturation of LPS-stimulated macrophages or
LPS-TLR4 signal transduction (15).
Human melanocytes are not merely pigment-producing
cells; they also act as phagocytes that contribute to inflammatory
responses (16), and secrete
agents of a wide range of signaling molecules, including cytokines,
pro-opiomelanocortin (POMC) peptides, catecholamines and NO in
response to ultraviolet (UV) irradiation and other stimuli.
Potential targets of these secretory products are keratinocytes,
lymphocytes, fibroblasts, mast cells and endothelial cells, all of
which express receptors for these signaling molecules (17). However, the regulatory effects of
activin A on normal human melanocytes as anti-inflammatory factors
remain unclear.
In this study, we examined the mechanisms through
which activin regulates the LPS-induced transcription of TLRs,
cytokines and NOS in normal human melanocytes, and its effects on
NF-κB and mitogen-activated protein kinase (MAPK) signaling.
Materials and methods
Cell culture
Normal human melanocytes were purchased from Cascade
Biologics (Gibco, Carlsbad, CA, USA) and cultured in Gibco Medium
254 containing human melanocyte growth supplement (Invitrogen,
Grand Island, NY, USA). The cells were incubated at 37°C in a
humidified atmosphere of 5% CO2 in 95% air.
Cell viability assay
Cell proliferation was measured using CellTiter 96
AQueous One Solution (Promega, Madison, WI, USA). The cells were
seeded (5×103 cells/well) in 96-well plates and
incubated with Escherichia coli LPS (E. coli 0111:
B4; Sigma-Aldrich Co., St. Louis, MO, USA) and activin A (ProSpec
(Protein-Specialists), East Brunswick, NJ, USA) for 24 h. Cell
viability was determined by colorimetric assay using PMS/MTS
solution. The absorbance was measured at 490 nm, with background
subtraction at 650 nm.
Treatment with LPS and activin A
Normal human melanocytes were treated for 20 h with
activin A (10 or 25 ng/ml) prior to exposure to LPS (1
µg/ml) for 4 h. At each time point, total RNA and protein
were isolated from the cultured melanocytes.
RNA extraction and reverse
transcription-quantitative-PCR (RT-qPCR)
Total RNA was purified from the cultured cells using
an RNeasy Mini kit according to the manufacturer's instructions
(Qiagen, Hilden, Germany). First-Strand cDNA synthesis was
performed with 1 µg of total RNA, which was transcribed into
cDNA using a reverse transcription system with random hexamers
(Promega) according to the manufacturer's instructions. The primer
sequences are listed in Table I.
Quantitative PCR (qPCR) was performed on a StepOnePlus Real-Time
PCR system with Power SYBR-Green PCR Master Mix (Applied
Biosystems, Foster City, CA, USA). PCR was performed with 1
µl of cDNA in a 20-µl reaction mixture containing 10
µl of Power SYBR-Green PCR Master Mix, 2 µl of
primers, and 7 µl of PCR grade water. The reaction
conditions were as follows: denaturation at 95°C for 10 min,
followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The
crossing points of the target genes with β-actin were calculated
using the formula 2−(target gene − β-actin) and the
relative amounts were quantified.
 | Table IPrimers used in RT-qPCR. |
Table I
Primers used in RT-qPCR.
| Gene | Primer
sequences | Product length
(bp) |
|---|
| TLR1 |
5′-GCCCAAGGAAAAGAGCAAAC-3′
5′-AAAGCAGCAATATCAACAGGAG-3′ | 135 |
| TLR2 |
5′-TCTCCCATTTCCGTCTTTTT-3′
5′-GGTCTTGGTGTTCATTATCTTC-3′ | 125 |
| TLR3 |
5′-TAAACTGAACCATGCACTCT-3′
5′-TATGACGAAAGGCACCTATC-3′ | 101 |
| TLR4 |
5′-GAAGCTGGTGGCTGTGGA-3′
5′-TGATGTAGAACCCGCAAG-3′ | 213 |
| TLR5 |
5′-TTGCTCAAACACCTGGACAC-3′
5′-CTGCTCACAAGACAAACGAT-3′ | 149 |
| TLR6 |
5′-GTGCCATTACGAACTCTA-3′
5′-CTTGTTGGGAATGCTGTT-3′ | 109 |
| TLR7 |
5′-CTGACCACTGTCCCTGAG-3′
5′-AACCCACCAGACAAACCA-3′ | 264 |
| TLR8 |
5′-AACATCAGCAAGACCCAT-3′
5′-GACTCCTTCATTCTCCCT-3′ | 65 |
| TLR9 |
5′-CGCCAACGCCCTCAAGACA-3′
5′-GGCGCTTACATCTAGTATTTGC-3′ | 79 |
| TLR10 |
5′-CTCCCAACTTTGTCCAGAAT-3′
5′-TGGTGGGAATGCAATAGAAT-3′ | 132 |
| IL-1β |
5′-TGATGGCTTATTACAGTGGCAATG-3′
5′-GTAGTGGTGGTCGGAGATTCG-3′ | 140 |
| IL-6 |
5′-GTGTTGCCTGCTGCCTTC-3′
5′-AGTGCCTCTTTGCTGCTTTC-3′ | 194 |
| IL-8 |
5′-GACATACTCCAAACCTTTCCAC-3′
5′-CTTCTCCACAACCCTCTGC-3′ | 160 |
| TNF-α |
5′-ATCTTCTCGAACCCCGAGTG-3′
5′-GGGTTTGCTACAACATGGGC-3′ | 51 |
| iNOS |
5′-TGGATGCAACCCCATTGTC-3′
5′-CCCGCTGCCCCAGTTT-3′ | 59 |
| β-actin |
5′-GCGAGAAGATGACCCAGATC-3′
5′-GGATAGCACAGCCTGGATAG-3′ | 77 |
Immunoblot analysis
The cells were collected and washed with cold PBS
then lysed using lysis buffer [20 mM Tris-HCl (pH 7.5), 150 mM
NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium
pyrophosphate, 1 mM β-glycerophosphate, 1 mM
Na3VO4 and 1 µg/ml leupeptin]
containing 1 mM PMSF (Cell Signaling Technology, Inc., Boston, MA,
USA). The protein concentration was determined using a BCA protein
assay kit (Thermo Fisher Scientific, Rockford, IL, USA) according
to the manufacturer's instructions. Protein (30 µg) were
fractionated by 12% SDS-PAGE and transferred by electrophoresis
onto nitrocellulose membranes. The membranes were blocked with 5%
non-fat dry milk for 1 h at room temperature and then incubated
overnight with antibodies against iNOS (AB5382), NF-κB p65 (#8242),
phosphorylated (p-)NF-κB p65 (#3031), IκBα (#9242), p-IκBα (#9246),
p38 MAPK (#9228), p-p38 MAPK (#9215), MEK (#4694), p-MEK (#9154),
ERK (#4696) and p-ERK1/2 (#4376; Cell Signaling Technology) and
β-actin (A5441; Sigma-Aldrich Co.), diluted 1:1,000 with
Tris-buffered saline containing 0.05% Tween-20 (TBS-T). After
washing with TBS-T for 1 h, the membranes were incubated for 1 h at
room temperature with horseradish peroxidase-conjugated secondary
antibodies diluted 1:2,500 in TBS-T. The membranes were
subsequently washed with TBS-T for 1 h, and proteins were detected
using an Enhanced Chemiluminescence kit (Santa Cruz Biotechnology
Inc., Santa Cruz, CA, USA). Protein expression was analyzed using a
Davinch-Chemi™ Chemiluminescence Imaging system (Davinch-K Co.,
Ltd., Seoul, Korea).
Statistical analyses
The experiments were repeated 3 times independently.
All values are expressed as the means ± SEM. Data were compared by
a non-parametric Kruskal-Wallis one-way analysis of variance
(ANOVA). Values of P<0.05 and P<0.01 were considered to
indicate statistically significant differences.
Results
Effects of activin on the proliferation
of normal human melanocytes
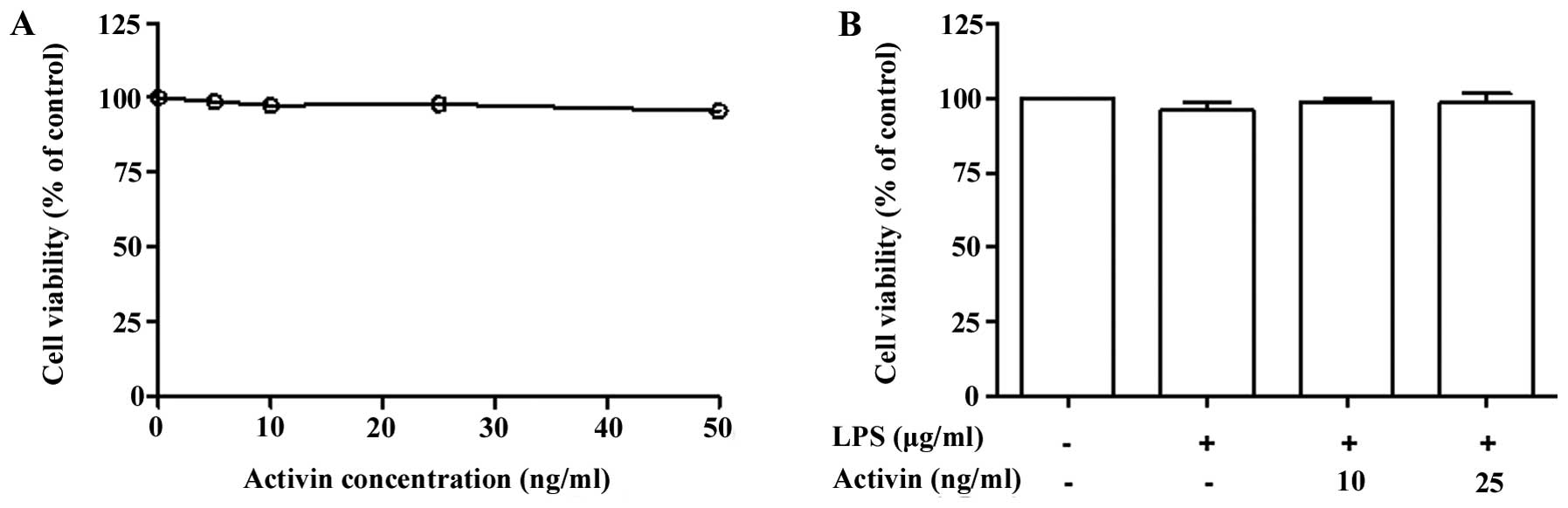
Normal human melanocytes were treated with various
concentrations of activin A (0–50 ng/ml) for 24 h and cell
proliferation was examined by PMS/MTS solution. Activin did not
influence the proliferation rate (cell viability) at concentrations
of 10 and 25 ng/ml (Fig. 1A).
Co-stimulation of the normal human melanocytes with LPS (1
µg/ml) and activin A (10 and 25 ng/ml) for 24 h did not
produce any cytotoxic effects (Fig.
1B).
Activin inhibits the LPS-induced increase
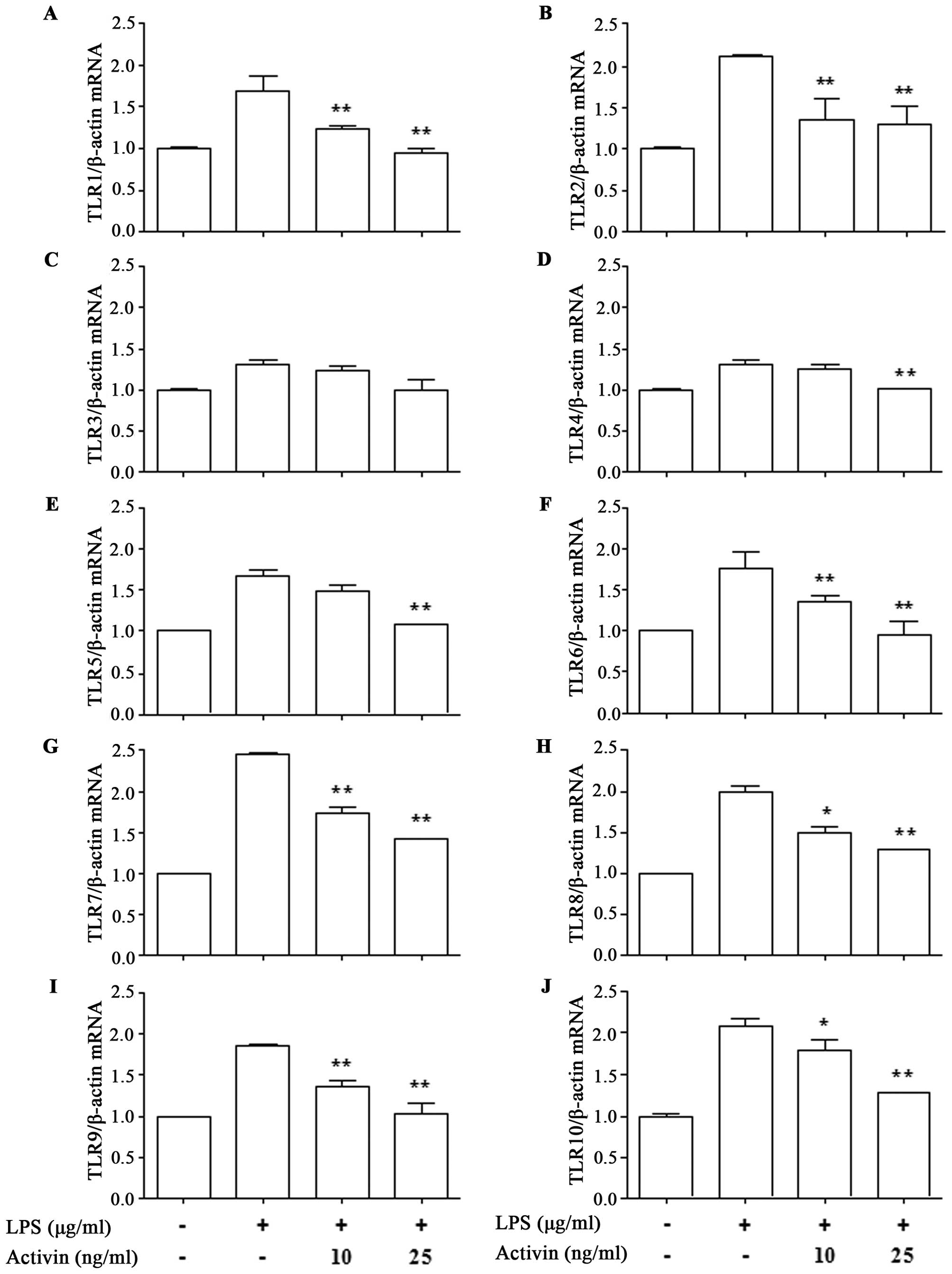
in the mRNA expression of TLRs in normal human melanocytes
To investigate the effects of activin on the
expression of TLR1-10 in normal human melanocytes in the presence
of LPS, the mRNA expression of TLR was measured by RT-qPCR. LPS
increased the mRNA expression of TLR1-10 compared with the control.
However, activin suppressed the LPS-induced increase in the mRNA
expression of all TLRs except TLR3 (Fig. 2).
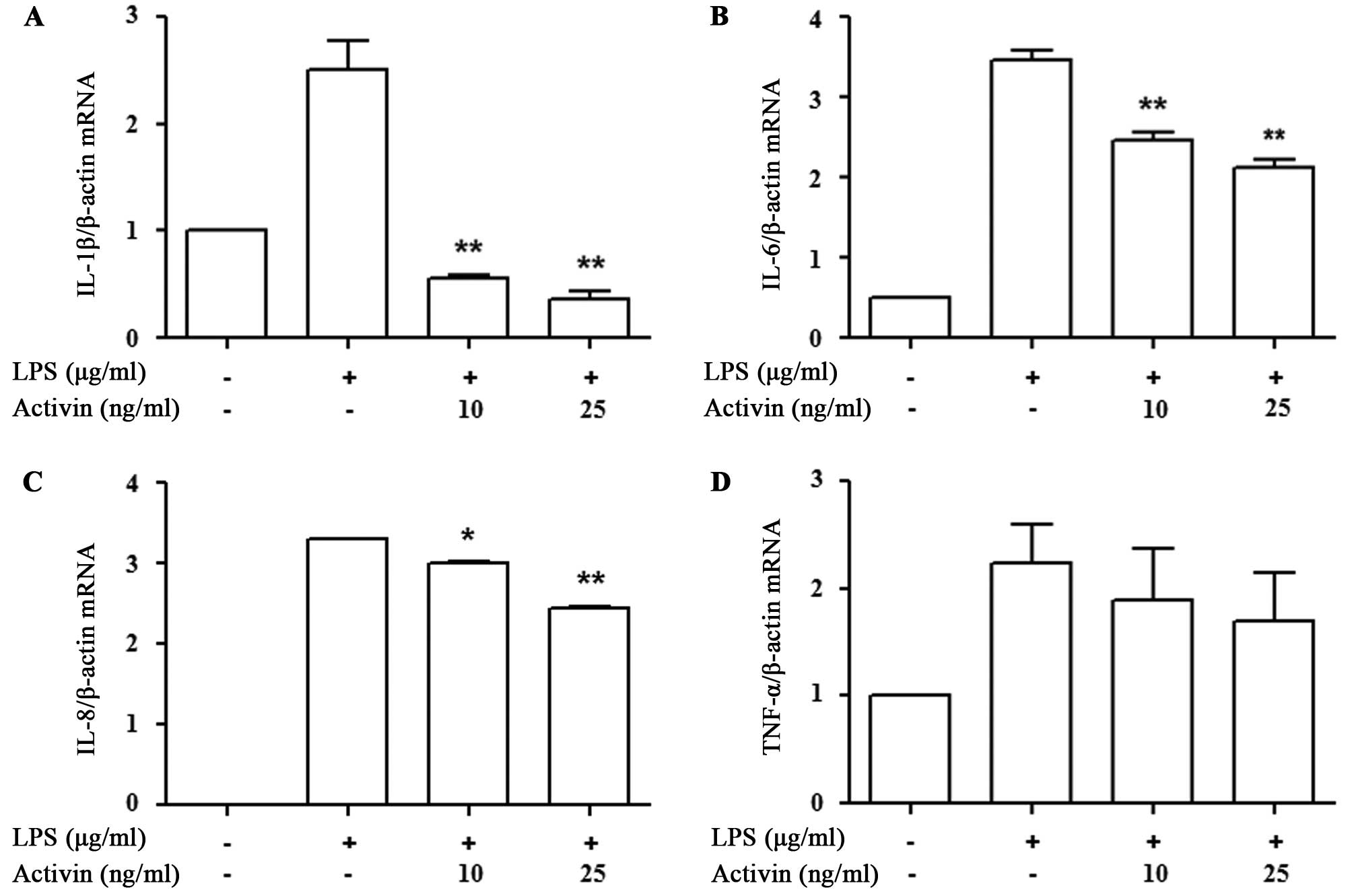
Activin inhibits the LPS-induced increase
in the mRNA expression of cytokines in normal human
melanocytes
We then determined whether activin A affects
inflammatory cytokine mRNA transcription. The expression of
cytokines was measured by RT-qPCR. LPS increased cytokine mRNA
expression compared with the controls, while activin A suppressed
the LPS-induced increase in the mRNA expression of IL-1β, IL-6, and
IL-8. The decrease in the mRNA expression of TNF-α following the
administration of activin A did not reach statistical significance
(Fig. 3).
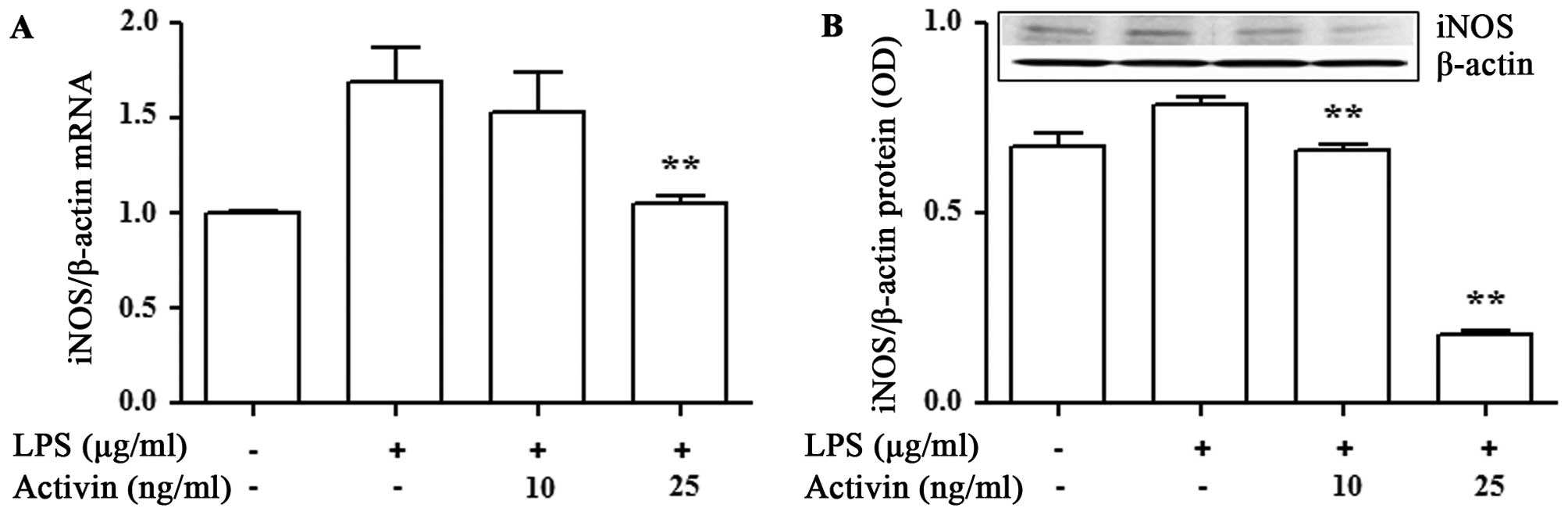
Activin inhibits the LPS-induced increase
in the mRNA and protein expression of iNOS in normal human
melanocytes
To examine the anti-inflammatory activity of
activin, we examined its effects on the mRNA and protein expression
of iNOS in the LPS-stimulated normal human melanocytes. The mRNA
and protein expression of iNOS was measured by RT-qPCR and
immunoblot analysis, respectively. LPS increased the mRNA and
protein expression of iNOS compared with the controls. However,
activin suppressed the LPS-induced increase in the mRNA and protein
expression of iNOS (Fig. 4).
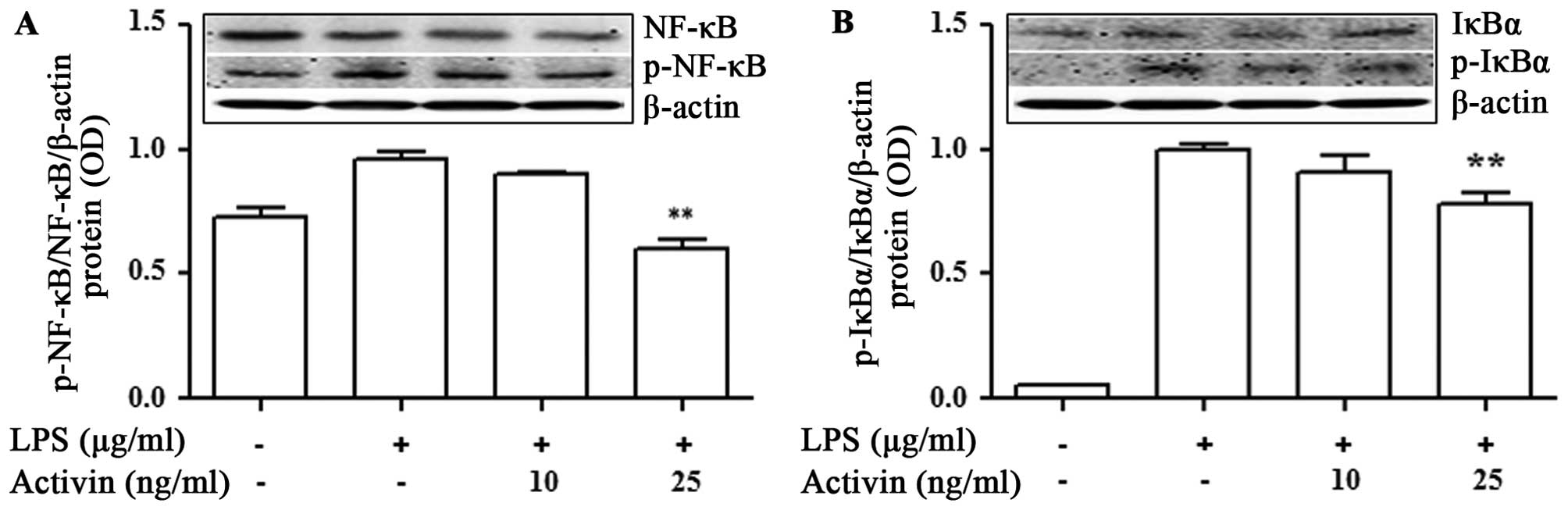
Activin inhibits the LPS-induced
activation of NF-κB signaling and IκBα degradation in normal human
melanocytes
To investigate the phenomena involved in the
inhibition of NF-κB activity, the effects of activin on IκBα
degradation were examined. The NF-κB p65 and IκBα protein levels
were measured by immunoblot analysis. LPS promoted the activation
of NF-κB p65 and IκBα compared with the controls. No phosphorylated
IκBα was detected in the unstimulated normal human melanocytes. The
LPS-induced phosphorylation of NF-κB and IκBα was inhibited by
activin A (Fig. 5).
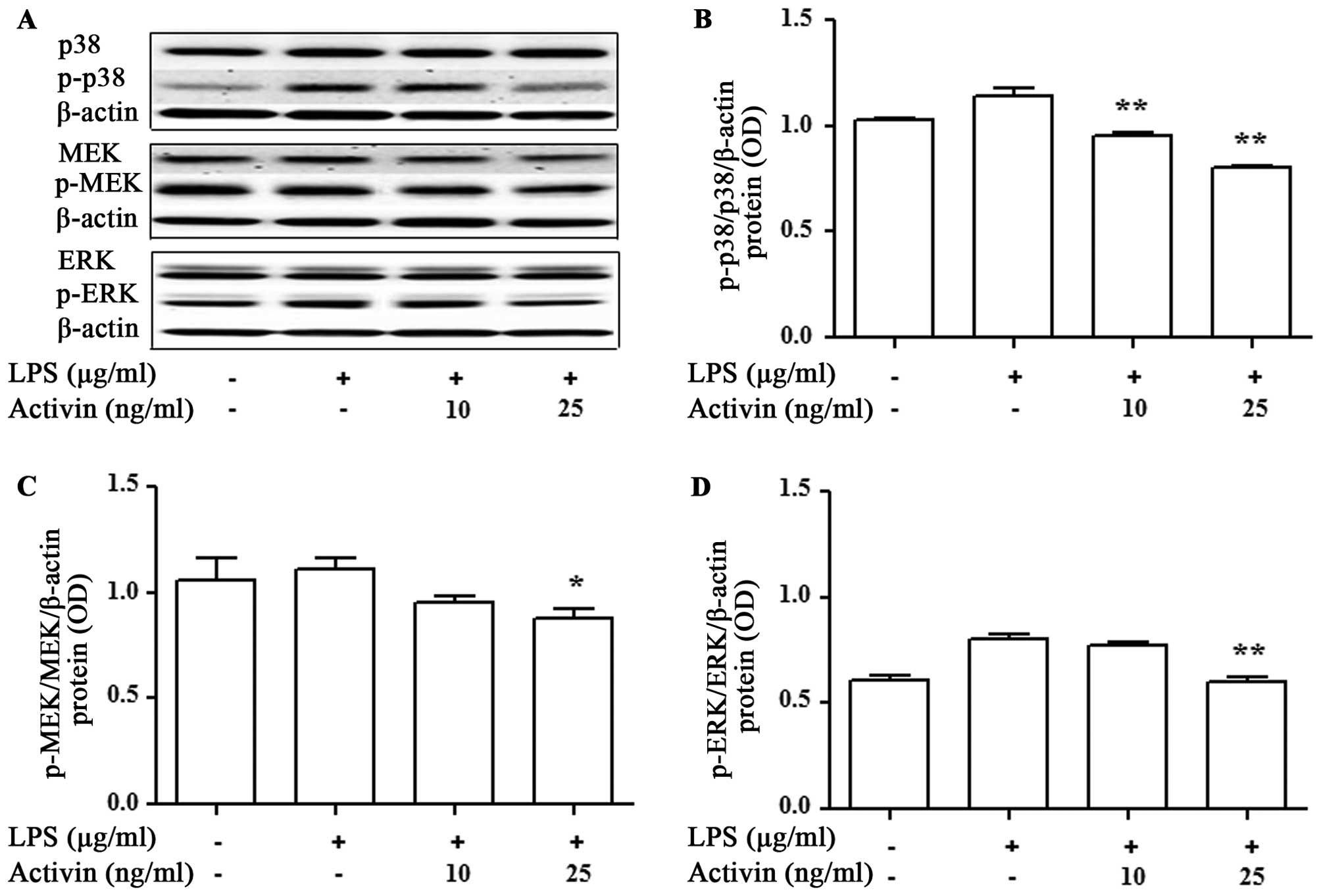
Activin inhibits the LPS-induced
activation of p38 MAPK and MEK/ERK in normal human melanocytes
We then evaluated the activation of signaling
molecules related to inflammation by activin. The expression levels
of p38 MAPK, MEK and ERK were measured by immunoblot blot analysis.
LPS promoted the activation of p38 MAPK, MEK and ERK compared with
the controls, while activin inhibited the LPS-induced activation of
p38 MAPK, MEK and ERK in normal human melanocytes (Fig. 6).
Discussion
Activin plays an important physiological role in
cell differentiation and inflammation (18). However, its effects on normal
human melanocytes remain unclear. In a previous study, LPS
inhibited melanocyte proliferation in a dose-dependent manner when
applied for 5 days (19). Activin
(25 ng/ml) has been shown to suppress the growth of normal human
melanocytes when applied for 4 days (20). In this study, we investigated the
effects of LPS and activin A on the proliferation of normal human
melanocytes. The cells stimulated with activin (10 and 25 ng/ml)
for 24 h did not show any cytotoxicity. Therefore, these
concentrations of activin were deemed to be appropriate for our
experiments.
Humans have various pattern recognition receptors,
including TLRs, NLRs and RIG-1-like receptors (21–23). Human melanocytes constitutively
express mRNA and protein for TLRs 2–5, 7, 9 and 10 (19–24,25); however, the mRNA expression of
NODs and RIG-1 in melanocytes is unclear. In the present study, we
demonstrate that activin regulates TLR gene transcription in normal
human melanocytes. Activin inhibited the LPS-induced mRNA
expression of TLRs (except TLR3). These results suggest that
activin plays a regulatory role in inflammation at the level of TLR
transcription. However, to the best of our knowledge, the
modulation of LPS-activated TLRs by activin has not been previously
reported in normal human melanocytes.
Activin A acts as an anti-inflammatory cytokine
produced by microglia and macrophages, and it is involved in
regulation of the acute-phase response in inflammatory diseases, in
an autocrine or paracrine manner (14,15). Activin was shown to significantly
inhibit the LPS-induced production of IL-6, IL-18, iNOS and
IL-1β-converting enzyme (ICE), in vivo and in vitro
(14). In the present study, we
found that activin suppressed the LPS-induced increase in the mRNA
expression of IL-1β, IL-6 and IL-8, whereas the mRNA levels of
TNF-α were not altered significantly. These results suggest that
activin modulates the transcription of inflammatory cyto-kines in
normal human melanocytes. In a previous study, no mRNA expression
of IL-2, IL-3, IL-4, IL-9, IL-12p40, IFN-α, or IFN-γ was detected
in human melanocytes (18).
Human melanocytes produce NO in response to UV
radiation and bacterial LPS. NO is produced through the enzymatic
action of NOS, of which both the constitutive (cNOS) and inducible
(iNOS) isoforms exist. In the control unstimulated melanocytes,
iNOS expression was detected, which suggests that, unlike in most
cell types, low levels of iNOS are constitutively expressed in
melanocytes (26). Normal dermal
fibroblasts express both endothelial NOS (eNOS) and iNOS mRNA
(27). Cultured normal human
melanocytes have been shown to express iNOS when stimulated with
LPS/cytokines (28). To determine
whether activin exerts an inhibitory effect on iNOS production in
melanocytes, we investigated its effects on iNOS mRNA expression in
the presence of LPS. Our results revealed that, under these
conditions, activin blocked the stimulatory effect of LPS on iNOS
mRNA and protein expression. These findings suggest that activin
inhibits iNOS at the transcriptional and translational levels in
normal human melanocytes exposed to inflammatory stimuli, such as
LPS.
NF-κB plays a crucial role in the expression of a
number of the genes involved in immune and inflammatory responses
(29). LPS has been shown to
induce the nuclear translocation of NF-κB in human melanocytes
(19). Activated NF-κB acts as a
transcription factor that increases the expression of several
inflammation-associated genes, including iNOS, COX-2, IL-1β, IL-6
and TNF-α (30). In this study,
we observed that activin blocked the degradation of IκBα,
suggesting that it attenuates the LPS-stimulated translocation of
NF-κB in normal human melanocytes by blocking IκBα degradation. The
phosphorylation and activation of 3 major MAPKs (p38 MAPK, JNK and
ERK1/2) has been shown to initiate the expression
inflammation-associated genes in LPS-stimulated macrophages.
Activated p38 induces TNF-α and iNOS production by modulating NF-κB
(31). It has been demonstrated
that activin stimulates the production of IL-1β, IL-6 and TNF-α
(32) by modulating IκB
degradation, the nuclear translocation of NF-κB, and the
phosphorylation of p38 MAPK and ERK1/2 (33,34). In this study, we found that
activin inhibited the LPS-induced phosphorylation of p38 MAPK and
MEK/ERK in normal human melanocytes. Taken together, our results
demonstrated that in LPS-stimulated normal human melanocytes,
activin exerted anti-inflammatory effects by modulating MAPK
phosphorylation and inactivating NF-κB by blocking IκBα
degradation.
In conclusion, the findings of the present study
demonstrated that activin inhibited LPS-induced TLR, cytokine and
iNOS expression in normal human melanocytes. Moreover, activin
exerted anti-inflammatory effects on LPS-activated normal human
melanocytes by modulating MAPK phosphorylation and NF-κB
inactivation.
Abbreviations:
|
IκB
|
inhibitors of NF-κB
|
|
IL
|
interleukin
|
|
iNOS
|
inducible nitric oxide synthase
|
|
LPS
|
lipopolysaccharide
|
|
MAPK
|
mitogen-activated protein kinase
|
|
NF-κB
|
nuclear factor-κB
|
|
TLR
|
Τoll-like receptor
|
References
|
1
|
Phillips DJ, Jones KL, Scheerlinck JY,
Hedger MP and de Kretser DM: Evidence for activin A and follistatin
involvement in the systemic inflammatory response. Mol Cell
Endocrinol. 180:155–162. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marshak-Rothstein A and Rifkin IR:
Immunologically active autoantigens: the role of toll-like
receptors in the development of chronic inflammatory disease. Annu
Rev Immunol. 25:419–441. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takeuchi O and Akira S: Pattern
recognition receptors and inflammation. Cell. 140:805–820. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Akira S, Takeda K and Kaisho T: Toll-like
receptors: critical proteins linking innate and acquired immunity.
Nat Immunol. 2:675–680. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Medzhitov R: Toll-like receptors and
innate immunity. Nat Rev Immunol. 1:135–145. 2001. View Article : Google Scholar
|
|
6
|
Barton GM and Medzhitov R: Toll-like
receptor signaling pathways. Science. 300:1524–1525. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kawai T and Akira S: TLR signaling. Semin
Immunol. 19:24–32. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee YM, Seon MR, Cho HJ, Kim JS and Park
JH: Benzyl isothiocyanate exhibits anti-inflammatory effects in
murine macrophages and in mouse skin. J Mol Med Berl. 87:1251–1261.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zaidi SF, Yamamoto T, Refaat A, Ahmed K,
Sakurai H, Saiki I, Kondo T, Usmanghani K, Kadowaki M and Sugiyama
T: Modulation of activation-induced cytidine deaminase by curcumin
in Helicobacter pylori-infected gastric epithelial cells.
Helicobacter. 14:588–595. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marletta MA: Approaches toward selective
inhibition of nitric oxide synthase. J Med Chem. 37:1899–1907.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nathan C and Xie QW: Nitric oxide
synthases: Roles, tolls, and controls. Cell. 78:915–918. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mathews LS: Activin receptors and cellular
signaling by the receptor serine kinase family. Endocr Rev.
15:310–325. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mather JP, Moore A and Li RH: Activins,
inhibins, and follistatins: further thoughts on a growing family of
regulators. Proc Soc Exp Biol Med. 215:209–222. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sugama S, Takenouchi T, Kitani H, Fujita M
and Hashimoto M: Activin as an anti-inflammatory cytokine produced
by microglia. J Neuroimmunol. 192:31–39. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang SY, Tai GX, Zhang PY, Mu DP, Zhang XJ
and Liu ZH: Inhibitory effect of activin A on activation of
lipopolysaccharide-stimulated mouse macrophage RAW264.7 cells.
Cytokine. 42:85–91. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Plonka PM, Passeron T, Brenner M, Tobin
DJ, Shibahara S, Thomas A, Slominski A, Kadekaro AL, Hershkovitz D,
Peters E, et al: What are melanocytes really doing all day long?
Exp Dermatol. 18:799–819. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsatmali M, Ancans J and Thody AJ:
Melanocyte function and its control by melanocortin peptides. J
Histochem Cytochem. 50:125–133. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mattei S, Colombo MP, Melani C, Silvani A,
Parmiani G and Herlyn M: Expression of cytokine/growth factors and
their receptors in human melanoma and melanocytes. Int J Cancer.
56:853–857. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ahn JH, Park TJ, Jin SH and Kang HY: Human
melanocytes express functional Toll-like receptor 4. Exp Dermatol.
17:412–417. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Stove C, Vanrobaeys F, Devreese B, Van
Beeumen J, Mareel M and Bracke M: Melanoma cells secrete
follistatin, an antagonist of activin-mediated growth inhibition.
Oncogene. 23:5330–5339. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sabroe I, Read RC, Whyte MK, Dockrell DH,
Vogel SN and Dower SK: Toll-like receptors in health and disease:
complex questions remain. J Immunol. 171:1630–1635. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cook DN, Pisetsky DS and Schwartz DA:
Toll-like receptors in the pathogenesis of human disease. Nat
Immunol. 5:975–979. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Strober W, Murray PJ, Kitani A and
Watanabe T: Signalling pathways and molecular interactions of NOD1
and NOD2. Nat Rev Immunol. 6:9–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu N, Zhang S, Zuo F, Kang K, Guan M and
Xiang L: Cultured human melanocytes express functional toll-like
receptors 2–4, 7 and 9. J Dermatol Sci. 56:113–120. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin SH and Kang HY: Activation of
toll-like receptors 1, 2, 4, 5, and 7 on human melanocytes modulate
pigmentation. Ann Dermatol. 22:486–489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsatmali M, Graham A, Szatkowski D, Ancans
J, Manning P, McNeil CJ, Graham AM and Thody AJ:
alpha-melanocyte-stimulating hormone modulates nitric oxide
production in melanocytes. J Invest Dermatol. 114:520–526. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang R, Ghahary A, Shen YJ, Scott PG and
Tredget EE: Human dermal fibroblasts produce nitric oxide and
express both constitutive and inducible nitric oxide synthase
isoforms. J Invest Dermatol. 106:419–427. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rocha IM and Guillo LA: Lipopolysaccharide
and cytokines induce nitric oxide synthase and produce nitric oxide
in cultured normal human melanocytes. Arch Dermatol Res.
293:245–248. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gupta SC, Sundaram C, Reuter S and
Aggarwal BB: Inhibiting NF-κB activation by small molecules as a
therapeutic strategy. Biochim Biophys Acta. 1799:775–787. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wan F and Lenardo MJ: The nuclear
signaling of NF-kappaB: current knowledge, new insights, and future
perspectives. Cell Res. 20:24–33. 2010. View Article : Google Scholar
|
|
31
|
Kaminska B: MAPK signalling pathways as
molecular targets for anti-inflammatory therapy - from molecular
mechanisms to therapeutic benefits. Biochim Biophys Acta.
1754:253–262. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yamashita N, Nakajima T, Takahashi H,
Kaneoka H, Mizushima Y and Sakane T: Effects of activin A on IgE
synthesis and cytokine production by human peripheral mononuclear
cells. Clin Exp Immunol. 94:214–219. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Murase Y, Okahashi N, Koseki T, Itoh K,
Udagawa N, Hashimoto O, Sugino H, Noguchi T and Nishihara T:
Possible involvement of protein kinases and Smad2 signaling
pathways on osteoclast differentiation enhanced by activin A. J
Cell Physiol. 188:236–242. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sugatani T, Alvarez UM and Hruska KA:
Activin A stimulates IkappaB-alpha/NFkappaB and RANK expression for
osteoclast differentiation, but not AKT survival pathway in
osteoclast precursors. J Cell Biochem. 90:59–67. 2003. View Article : Google Scholar : PubMed/NCBI
|