Introduction
1α,25-Dihydroxyvitamin D3
[1,25(OH)2D3], the active form of vitamin D,
has long been known for its effects on bone mineralization and
calcium homeostasis. However, its physiological importance outside
of bone health and calcium homeostasis effects has received
increasing attention and it has been recognized as a key regulator
of cell proliferation, differentiation, apoptosis and
immunomodulation (1–4). In this regard, several studies have
identified that vitamin D may have a role in multiple chronic lung
diseases, such as asthma, chronic obstructive pulmonary disease
(COPD), respiratory infections, lung cancer, interstitial lung
disease, cystic fibrosis and pulmonary arterial hypertension (PAH)
(5–12).
Vitamin D3 is a steroid prehormone that requires two
hydroxylation steps in the liver and kidney to generate the
biological active form, 1,25(OH)2D3. The
biological effects of 1,25(OH)2D3 are
mediated through the vitamin D receptors (VDRs), which belong to
the superfamily of steroid/thyroid nuclear hormone receptors and
are expressed widely in the body, including the lungs and cells of
the immune system (8). Following
ligand binding, the VDR forms a heterodimer with retinoid X
receptor (RXR) and binds to the vitamin D responsive element in the
regulatory region of the target genes to activate or repress their
transcription (13,14).
Pulmonary fibroblasts have key roles in the
formation and maintenance of lung structure and function, and are
involved in tissue repair and remodeling, which is a key feature of
a number of chronic lung diseases such as asthma, COPD, pulmonary
fibrosis and PAH (15–20). Transforming growth factor-β1
(TGF-β1) is secreted by numerous cell types and implicated in a
wide range of cell functions, critically regulating cell
proliferation, growth, differentiation, apoptosis, cell movement,
and extracellular matrix secretion and deposition (21–24). Additionally, TGF-β1 is a crucial
regulator of fibroblast phenotype and function. TGF-β1-stimulated
fibroblasts undergo phenotypic transition and differentiate into
myofibroblasts, the key effector cells in fibrotic states, which
are characterized by the expression of α-smooth muscle actin
(α-SMA) fibers, contributing to the progression of pulmonary
fibrogenesis (25,26). Therefore, inhibition of fibroblast
proliferation and differentiation may prove to be a common and
effective approach to attenuate pulmonary fibrosis.
Previous studies demonstrated that
1,25(OH)2D3 was capable of inhibiting the
TGF-β-mediated tissue remodeling responses in cultured lung
fibroblasts and blocking myofibroblastic transformation of
fibroblasts by TGF-β1 (18,27). In addition, several studies showed
cross-talk between the TGF-β and vitamin D signaling pathways. VDR
binds to the MH1 domain of Smad3, which belongs to a receptor of
the TGF-β superfamily, thereby enhancing Smad3 ligand-induced
transactivation (28–31). However, the molecular mechanisms
are far from understood. MicroRNAs (miRNAs) have attracted
increasing attention due to their significant roles in diverse
biological processes, including developmental processes, cell
proliferation, differentiation, apoptosis, stress responses, and
cancer initiation and progression (32–37). miRNAs are short non-coding RNAs
with wide gene regulatory activity at the post-transcriptional
level, which forms RNA silencing complexes with several proteins to
cause mRNA degradation or translation inhibition, or both processes
(32,33). The relatively few studies of
miRNAs in lung fibrogenesis include miR-155, Let-7
family, miR-29 family, miR-21, miR-30 family,
miR-145 and miR-27b (38–42). The present study aimed to evaluate
the ability of 1,25(OH)2D3 to inhibit the
differentiation of lung fibroblasts and the participation of
miR-27b in this process. The first aim of the study was to
determine the role of 1,25(OH)2D3 in
regulating the differentiation of human lung fibroblasts induced by
TGF-β1. Furthermore, whether miR-27b may be relevant for the
regulating effect of 1,25(OH)2D3 was also
determined.
Materials and methods
Materials and reagents
The α-SMA (cat. no. sc-53015), VDR (cat. no.
sc-13133) and β-actin antibodies (cat. no. sc-47778) were all
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Secondary antibodies for goat anti-mouse immunoglobulin G (IgG)
conjugated with horseradish peroxidase (HRP) and goat anti-mouse
IgG labeled with Cy3 were sourced from Boster (Hubei, China).
1,25(OH)2D3 (cat. no. D1530) and human
recombinant TGF-β1 (cat. no. 240-B-002/CF) were obtained from
Sigma-Aldrich (St. Louis, MI, USA) and R&D Systems
(Minneapolis, MN, USA), respectively. Scrambles, miRNA mimics and
inhibitors were from Ambion (Austin, TX, USA). α-SMA, VDR and
β-actin primers were produced by BGI-Beijing (Beijing, China).
Cell culture
MRC5 human lung fibroblasts and 293A cells were
obtained from the Cell Resource Center of the Institute of Basic
Medical Sciences, Chinese Academy of Medical Sciences (Beijing,
China). These two types of cells were incubated in Dulbecco's
modified Eagle's medium (DMEM) with 4.5 g/l of glucose supplemented
(Gibco, Carlsbad, CA, USA), supplemented with 100 U/ml penicillin,
100 μg/ml streptomycin and 10% fetal bovine serum (FBS;
Gibco) at 37°C in a humidified atmosphere with 5% CO2.
MRC5 cells were cultured to ~80% confluence, serum-starved for 24
h, and were treated for 48 h in 2% FBS medium with an ethanol
vehicle or 1,25(OH)2D3 (100 nM) in the
absence or presence of rhTGF-β1 (10 ng/ml).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA), according to the manufacturer's
instructions. cDNA was generated from total RNA using PrimeScript
RT Master mix (5X) (Takara, Dalian, China), according to the
manufacturing instructions. To quantify mRNA expression, SYBR
Premix Ex Taq (2X) (Takara) was used. The qPCR thermal cycling
protocol was programmed in the CFX96™ Real-Time PCR Detection
system (Bio-Rad, Hercules, CA, USA) and consisted of an initial
denaturation step at 95°C for 30 sec, followed by 40 cycles of
denaturation for 5 sec at 95°C, and annealing and extension for 30
sec at 60°C. A primer pair for the detection of human β-actin was
used as the internal control. RT-qPCR primers were based on GenBank
published sequences and were as follows: α-SMA sense, 5′-GGC GGT
GCT GTC TCT CTA TG-3′ and antisense, 5′-CCC ATC AGG CAA CTC GTA
AC-3′; VDR sense, 5′-GGC CGG ACC AGA AGC CTT T-3′ and antisense,
5′-CAG CCT TCA CAG GTC ATA GCA-3′; β-actin sense, 5′-ACT GGA ACG
GTG AAG GTG AC-3′ antisense, 5′-GGC ACG AAG GCT CAT CAT-3′.
miRNA extracts were prepared using an mirVana™ miRNA
Isolation kit (Ambion) and subsequently reverse transcribed using a
TaqMan miRNA Reverse Transcription kit (Applied Biosystems, Foster
City, CA, USA) in accordance with the manufacturer's instructions.
miRNA expression levels were quantified using a TaqMan Universal
Master mix II (2X) (Applied Biosystems), normalized to U6 snRNA.
Results are presented as fold changes in gene expression calculated
using the ΔΔCt method.
Western blotting
Cells were plated in 60-mm dishes in 10% FBS-DMEM,
cultured until nearly 80% confluence and serum-starved for 24 h,
and subsequently the cells were treated as indicated. After 48 h
culture, cells were washed twice with ice-cold phosphate-buffered
saline (PBS) and were subsequently harvested with cell lysis buffer
with protease inhibitors [20 mmol/l Tris-HCl (pH 7.5), 150 mmol/l
NaCl, 1% Triton X-100, 1 mmol/l EDTA, 1 mmol/l EGTA, 20 mol/l
Na4P2O7, 2 mol/l
Na3VO4, 0.1% SDS, 10% glycerol, 2
μg/ml leupeptin and 1 mmol/l PMSF]. Upon centrifugation at
13,000 × g for 5 min at 4°C, the supernatants were collected and
the total protein concentrations were determined by the
bicinchoninic acid protein assay kit (Beyotime, Shanghai, China)
following the manufacturer's instructions. SDS-PAGE (10%) gels were
prepared and 10–20 μg/lane of cellular proteins was loaded.
The resolved proteins were transferred to a polyvinylidene
difluoride membrane and incubated with primary antibodies (α-SMA,
1:100 dilution; VDR, 1:50 dilution) following the manufacturer's
instructions. Following incubation with HRP-conjugated secondary
antibodies (1:1,000 dilution), the blotting bands were visualized
with ECL chemiluminescent kit (Thermo Scientific, Rockford, IL,
USA) and quantified with a ChemiDoc™ XRS+ Imaging System
(Bio-Rad).
Immunofluorescence
Fibroblasts growing on cover slides were fixed in 4%
paraformaldehyde for 1 h. Following being permeabilized with 0.2%
Triton X-100 in Tris-buffered saline (TBS) for 30 min, the cells
were blocked in TBS containing 2% bovine serum albumin for 1 h.
Cells were subsequently incubated with anti α-SMA antibody (1:50
dilution) at 4°C overnight. Cells were washed 3 times and were
incubated at room temperature with Cy3-conjugated goat anti-mouse
secondary antibody (1:50 dilution) for 1 h. Following this, cells
were counterstained with 4′,6-diamidino-2-phenylindole (Boster) for
nuclear staining, 5 min in the dark and were washed 3 times.
Negative controls were carried out by omitting the primary
antibody. Fluorescent images were captured with a laser scanning
confocal microscope (Olympus FV1000; Olympus, Tokyo, Japan).
Cell transfection
Transfection was performed with scramble,
miR-27b mimic (50 nM) or miR-27b inhibitor (100 nM)
(Ambion). All the transfection experiments were conducted with
Lipofectamine 2000 (Invitrogen), following the manufacturer's
instructions. Cells in medium containing 10% FBS were seeded in
each well and incubated at 37°C. The transfection mixture was
incubated at room temperature for 10 min, and was added to a 6-well
plate. After 24 h, cells were serum-starved for an additional 24 h,
following which they were treated with vehicle control,
1,25(OH)2D3 or TGF-β1 for 48 h in 2% FBS
medium. Cells were subsequently harvested and total RNA, miRNA or
proteins were extracted and analyzed by RT-qPCR or western blot
analysis.
3′ Untranslated region (3′UTR) luciferase
assay
A synthetic oligonucleotide was inserted at the
3′UTR region of the luciferase reporter gene of the pMIR-target
vector. The wild-type and mutant 3′UTR of the VDR genes were
amplified by PCR and subcloned in the pMIR-target vectors. The
constructs were co-transfected into 293A cells along with scramble
(50 nM), miR-27b mimic (50 nM) or miR-27b inhibitor
(100 nM) using Lipofectamine 2000, according to the manufacturer's
instructions. Luciferase activities were measured with the Dual
Luciferase Reporter Assay system (Promega, Madison, WI, USA) 48 h
after transfection.
Statistical analysis
Values are expressed as means ± standard error of
the mean derived from at least three independent experiments.
Comparison of two groups was made with an unpaired, two-tailed
Student's t-test. Comparison of multiple groups was made with a
one-way analysis of variance followed by Dunnett or Tukey test.
P<0.05 was considered to indicate a statistically significant
difference. All the statistical analyses were performed and graphs
were plotted using the GraphPad Prism 5 software (GraphPad
Software, San Diego, CA, USA).
Results
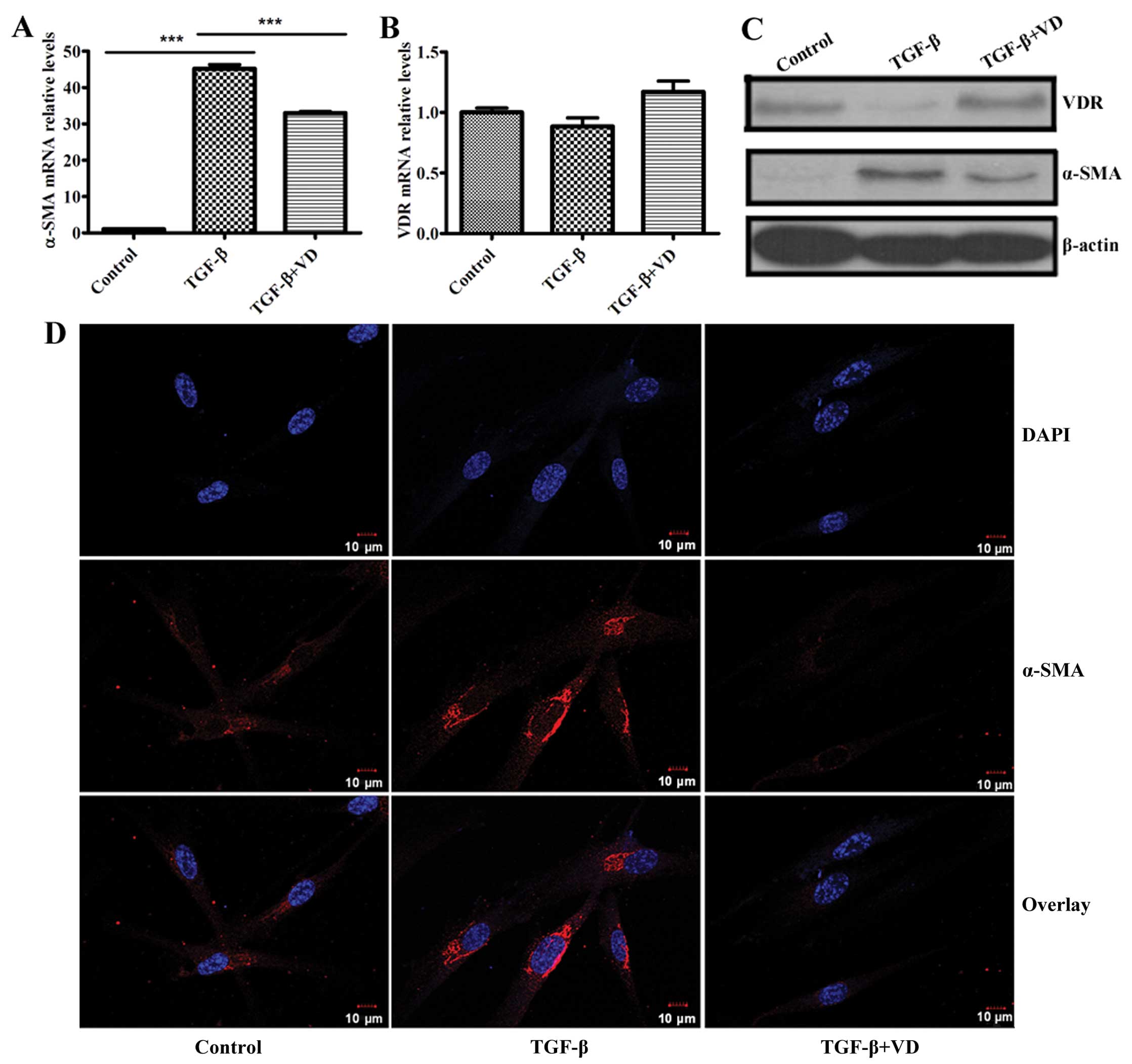
1,25(OH)2D3
inhibits differentiation and upregulates VDR protein expression of
human lung fibroblasts induced by TGF-β1
Previous studies have documented that
1,25(OH)2D3 opposed the effect of TGF-β1 on
mouse lung fibroblasts differentiation, as demonstrated by its
ability to inhibit TGF-β1-induced expression of α-SMA (27). Consequently, whether
1,25(OH)2D3 could inhibit α-SMA expression of
human lung fibroblasts induced by TGF-β1 was examined. Based on
previous experiments, the optimum concentration of
1,25(OH)2D3 (100 nM) was chosen as the dose
for the present experiments. Notably, RT-qPCR and western blot
analysis revealed that TGF-β1 significantly upregulated α-SMA
expression at the mRNA and protein levels in MRC5 cells; however,
1,25(OH)2D3 markedly inhibited this effect
(Fig. 1A and C). Confocal
immunofluorescence analysis of MRC5 fibroblasts also revealed that
TGF-β1 increased levels of α-SMA, and the process was inhibited by
1,25(OH)2D3 (Fig. 1D).
Several studies showed cross-talk between the TGF-β
and vitamin D signaling pathways (28–31) and TGF-β contributed to the
decreased expression of VDR in dermal fibroblasts of healthy
volunteers (43). Furthermore,
1,25(OH)2D3 had a significant effect in
vivo on the TGF-β signaling pathway by altering levels of VDR
and Smad3, and subsequently affecting the bioactive of TGF-β1
(44). Based on this evidence, we
speculated that VDR may be a negative regulator of fibroblast
differentiation induced by TGF-β. TGF-β significantly decreased the
protein expression of VDR, and treatment of TGF-β-stimulated
fibroblasts with 1,25(OH)2D3 effectively
upregulated the VDR protein level (Fig. 1C). However, the levels of the VDR
transcripts did not change (Fig.
1B).
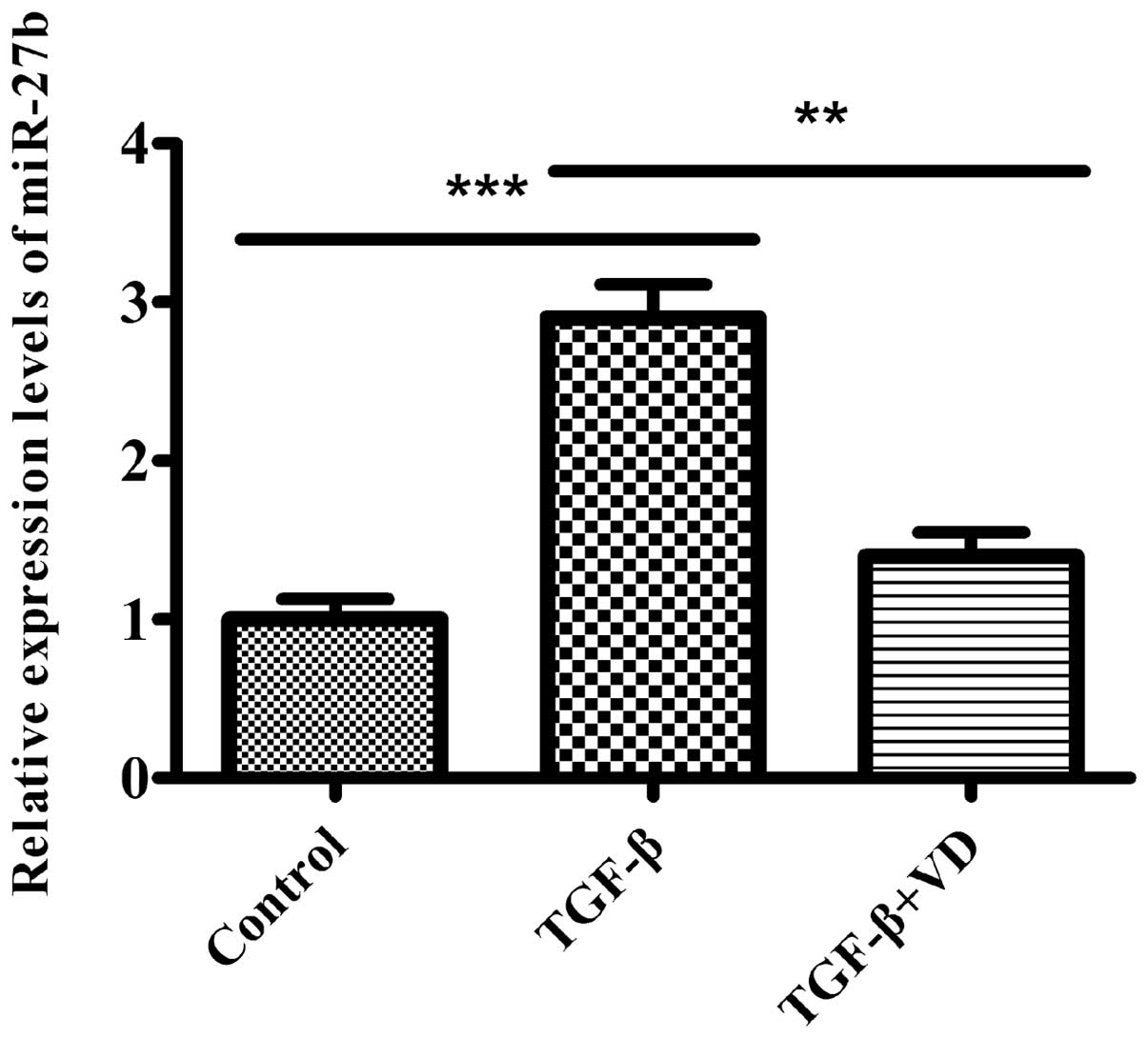
1,25(OH)2D3
downregulates TGF-β1-induced miR-27b expression in human lung
fibroblasts
As TGF-β1 is a critical cytokine in the pathogenesis
of human lung fibroblast differentiation, and a previous study
reported that miR-27b was downregulated in the lungs of mice
that were administered with bleomycin, a widely used animal model
of lung fibrosis (45), we
hypothesized that miR-27b participates in this process and
could be relevant for the inhibitory effect of
1,25(OH)2D3 on the differentiation of lung
fibroblasts induced by TGF-β1. Therefore, the levels of
miR-27b were examined in MRC5 cells treated with the ethanol
vehicle or 1,25(OH)2D3 in the absence or
presence of TGF-β1. In the present study, miR-27b expression
levels were significantly higher in MRC5 cells induced by TGF-β1.
However, treatment of TGF-β-stimulated fibroblasts with
1,25(OH)2D3 effectively decreased
miR-27b expression (Fig.
2). These data suggest that miR-27b may have a role in
regulating the differentiation phenotype of the pulmonary
fibroblasts.
miR-27b regulates differentiation and VDR
expression of human lung fibroblasts
To determine whether miR-27b regulates the
differentiation phenotype of the pulmonary fibroblasts, human lung
fibroblasts were transfected with scramble, miR-27b mimic or
miR-27b inhibitor and evaluated α-SMA levels in these cells.
Overexpression of miR-27b markedly increased the baseline
levels of the α-SMA transcripts and α-SMA proteins in lung
fibroblasts (Fig. 3A-a and c).
The miR-27b inhibitor decreased the α-SMA levels in lung
fibroblasts (Fig. 3B-a and c).
Given that TGF-β1 upregulated miR-27b in lung fibroblasts,
these data suggest that miR-27b may mediate TGF-β1-induced
α-SMA expression. TGF-β1-treated lung fibroblasts were transfected
with miR-27b inhibitor and subsequently evaluated α-SMA
levels in the cells. miR-27b inhibitor attenuated
TGF-β1-induced α-SMA expression at the mRNA and protein levels in
lung fibroblasts (Fig. 3C-a and
c). These data indicate that TGF-β1-mediated α-SMA expression
requires, at least in part, the induction of miR-27b. In
addition, these initial experiments have demonstrated that
1,25(OH)2D3 reduced α-SMA expression and
downregulated miR-27b expression in human lung fibroblasts
induced by TGF-β1. When the miR-27b mimic was transfected
into the human lung fibroblasts, the reduced expression of α-SMA
protein in 1,25(OH)2D3-treated cells was
attenuated by the miR-27b mimic (Fig. 3D-a and c).
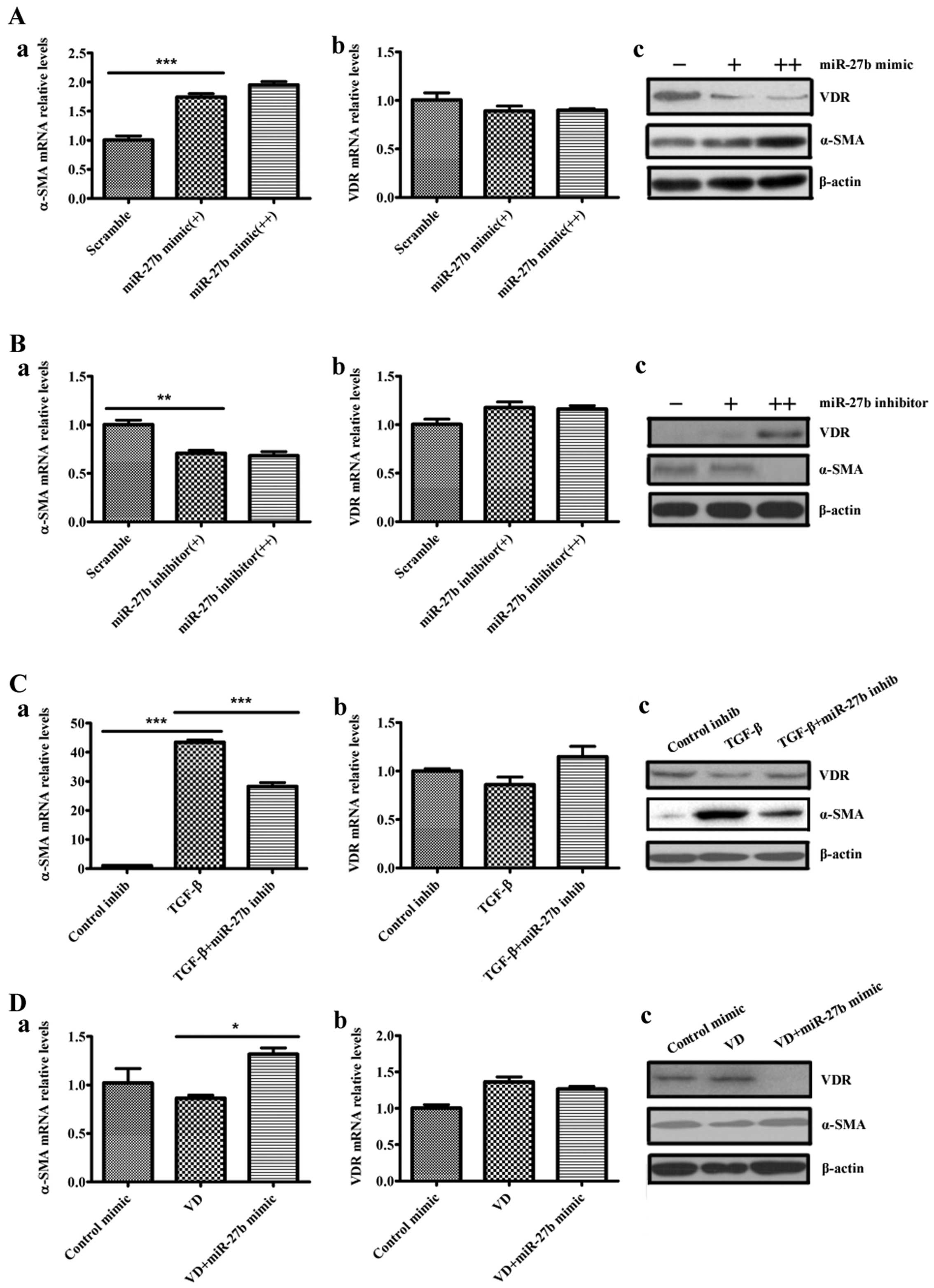 | Figure 3miR-27b regulates
differentiation and vitamin D receptor (VDR) expression of human
lung fibroblasts. (A) Human lung fibroblasts were transfected with
50 nM scramble, 50 nM miR-27b mimic (+) or 100 nM
miR-27b mimic (++). At 48 h after transfection, levels of
α-smooth muscle actin (α-SMA) and VDR were determined by (a and b)
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and (c) western blot analysis, normalized to β-actin
expression. ***P<0.001 miR-27b mimic (+) vs.
scramble. (B) Human lung fibroblasts were transfected with 100 nM
scramble, 100 nM miR-27b inhibitor (+) or 200 nM
miR-27b inhibitor (++), and 48 h post transfection, RNA or
whole cell lysates were harvested. The expression levels of α-SMA
and VDR were determined by (a and b) RT-qPCR and (c) western blot
analysis, normalized to β-actin expression. **P<0.01
miR-27b inhibitor (+) vs. scramble. (C) Human lung
fibroblasts were transfected with 100 nM control inhibitor (control
inhib) or 100 nM miR-27b inhibitor (miR-27b inhib).
At 24 h after transfection, cells were serum-starved for an
additional 24 h, after which they were treated with transforming
growth factor-β1 (TGF-β1) for 48 h. RNA or whole cell lysates were
subsequently harvested. The expression levels of α-SMA and VDR were
determined by (a and b) RT-qPCR and (c) western blot analysis,
normalized to β-actin expression. ***P<0.001 TGF-β
vs. control inhib; ***P<0.001 TGF-β + miR-27b
inhib vs. TGF-β. (D) Human lung fibroblasts were transfected with
50 nM control mimic or 50 nM miR-27b mimic. At 24 h after
transfection, cells were serum-starved for an additional 24 h,
after which they were treated with 100 nM 1α,25-dihydroxyvitamin
D3 [1,25(OH)2D3] (VD) for 48 h.
RNAs or whole cell lysates were subsequently harvested. The
expression levels of α-SMA and VDR were determined by (a and b)
RT-qPCR and (c) western blot analysis, normalized to β-actin
expression. *P<0.05 VD + miR-27b mimic vs.
VD. |
Given that VDR can be a negative regulator of
fibroblast differentiation induced by TGF-β, whether miR-27b
regulates VDR gene expression was further investigated. MRC5 cells
were transfected with scramble, miR-27b mimic or
miR-27b inhibitor, and VDR levels were evaluated in these
cells. The miR-27b mimic reduced VDR protein levels in lung
fibroblasts (Fig. 3A-c). The
miR-27b inhibitor increased the VDR protein levels in these
cells (Fig. 3B-c). However,
miR-27b had no effect on the levels of the VDR transcripts
(Fig. 3A-b and B-b), suggesting
that miR-27b may affect the VDR translation, but not the
transcripts in lung fibroblasts. These initial experiments have
demonstrated that TGF-β1 upregulates miR-27b and decreases
VDR protein expression, but 1,25(OH)2D3
opposed the above effects of TGF-β1. The reduced expression of VDR
protein in TGF-β1-treated cells was attenuated by the
miR-27b inhibitor (Fig.
3C-c). Furthermore, the miR-27b mimic resulted in a
decrease of VDR protein expression in lung fibroblasts treated with
1,25(OH)2D3 (Fig. 3D-c). Similarly, miR-27b had
no effect on the levels of the VDR transcripts (Fig. 3C-b and D-b). Taken together, these
data suggest that miR-27b promotes the differentiation of
human lung fibroblasts by suppressing VDR protein expression.
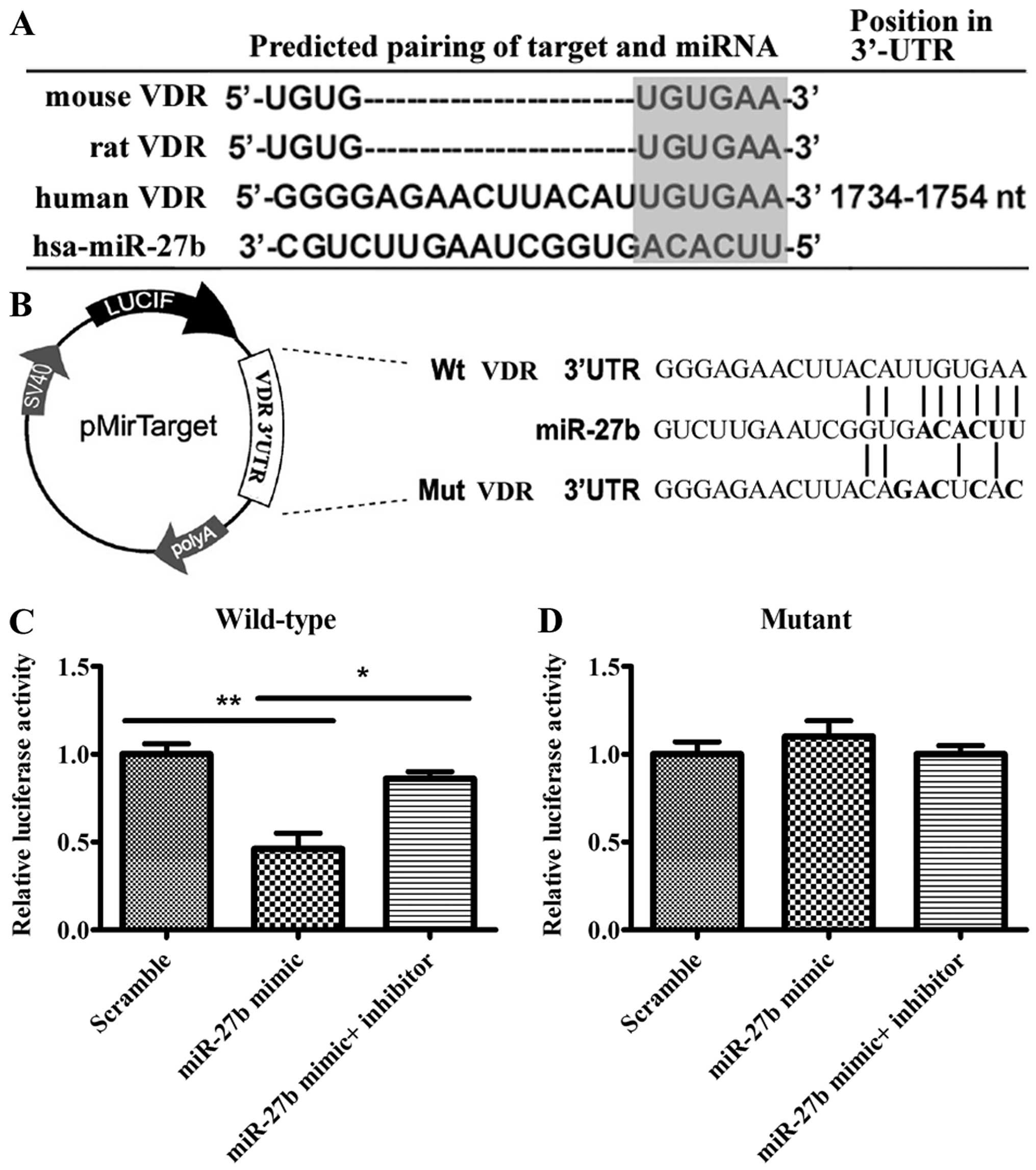
miR-27b directly targets VDR 3′UTR
The aforementioned results demonstrate that
miR-27b can regulate VDR expression; however, they do not
prove that there is a direct interaction between miR-27b and
the mRNA of VDR. To further substantiate that miR-27b
targets VDR directly, whether there was a perfect match between the
seed sequence of miR-27b and a region in the 3′UTR of VDR
was verified. In addition, this binding site is identical among
mammals (Fig. 4A). These results
suggested that VDR may be regulated by miR-27b, thus this
prediction was evaluated. Luciferase reporter constructs were used,
incorporating a wild-type or mutant 3′UTR of VDR, in which the
sequence corresponding to the seed region was altered (Fig. 4B). The reporter vectors were
subsequently co-transfected into 293A cells with scramble,
miR-27b mimic or miR-27b mimic/miR-27b
inhibitor. The miR-27b mimic significantly decreased
luciferase activity and introduction of the miR-27b
inhibitor increased luciferase activit (Fig. 4C). The miR-27b target site
was subsequently mutated to confirm that miR-27b was binding
to this sequence. Of note, the effects of the miR-27b mimic
or miR-27b inhibitor were abolished (Fig. 4D). Taken together, the results
confirm that miR-27b targets VDR 3′UTR specifically and
directly.
Discussion
Pulmonary fibroblasts have important roles in lung
tissue repair and remodeling. Fibroblasts often differentiate into
myofibroblasts that possess enhanced fibrotic, contractile and
migratory activities. TGF-β1 stimulated the proliferation of lung
fibroblasts and their differentiation, as highlighted by increased
expression and organization of α-SMA, a marker of myofibroblasts
and a primary contributor to the contractile force in
myofibroblasts (46,47). New emerging studies regarding the
roles of miRNAs in pulmonary fibrosis and in their regulation of
TGF-β signaling are increasing (38,48–50). miR-27b has been studied in
various cancer cells (51–55),
however, miR-27b has not been well studied in lung
fibroblasts. Previous studies suggested that miR-27b was
identified as a pro-angiogenic miRNA (56) and modulated fibrotic responses
(41). The aim of the present
study was to investigate the role and mechanism of miR-27b
regulating human lung fibroblasts differentiation induced by
TGF-β1.
Firstly, 1,25(OH)2D3 inhibited
the effect of TGF-β1 on human lung fibroblast differentiation, as
demonstrated by its ability to inhibit TGF-β1-induced expression of
α-SMA. These results are consistent with findings from a study of
vitamin D inhibition of pro-fibrotic effects of TGF-β1 in mouse
lung fibroblasts (27). The
present study identified that miR-27b expression levels were
significantly higher in MRC5 cells induced by TGF-β1, however,
treatment of TGF-β-stimulated fibroblasts with
1,25(OH)2D3 effectively decreased
miR-27b expression. This suggests that miR-27b may
have a role in regulating the differentiation phenotype of the
pulmonary fibroblasts. By contrast, two studies observed that TGF-β
treatment downregulated miR-27b expression (41,57), which may be caused by different
types of cells, and additional investigations are required to
demonstrate the function.
There is evidence showing that VDR is involved in
pathological fibrogenesis. VDR belongs to the superfamily of
steroid/thyroid nuclear hormone receptors. Following ligand
binding, the VDR forms a heterodimer with RXR, or Smad3, a receptor
of TGF-β/Smad signalling. Previous studies showed cross-talk
between the TGF-β and vitamin D signaling pathways and VDR binds to
the MH1 domain of Smad3, enhancing Smad3 ligand-induced
transactivation (28–31). 1,25(OH)2D3
had a significant effect in vivo on the TGF-β signaling
pathway by altering levels of VDR and Smad3, and subsequently
affecting the bioactive of TGF-β (44). Additionally, a recent study
identified VDR as a negative regulator of fibroblast activation
that interfered with the pro-fibrotic effects of TGF-β (43). The present study further showed
that reduction of the VDR protein was mediated by TGF-β1 in human
lung fibroblasts, whereas 1,25(OH)2D3
effectively upregulated VDR protein expression, which was in
accordance with the study of activation of VDR by paricalcitol
reducing the stimulatory effects of TGF-β on skin fibroblasts
(43).
In addition, the experiments of lung fibroblasts
transfected with miR-27b mimic or miR-27b inhibitor
and luciferase reporter assays reveal that miR-27b directly
targets VDR 3′UTR and inhibits VDR gene expression to promote
differentiation of human lung fibroblasts characterized by
expression of α-SMA. The present in vitro study showed that
the overexpression of miR-27b decreased VDR protein
expression and increased expression of fibroblast differentiation
marker, α-SMA, while reducing levels of miR-27b had opposing
effects. Studies of fibrosis have not reached a consensus on the
importance of miR-27b in disease pathology. An in
vitro study suggested that miR-27b may be profibrotic in
activated hepatic stellate cells (58). Additionally, cardiomyocyte
overexpression of miR-27b induces cardiac hypertrophy in
mice (57). These two studies
were similar to the present results. By contrast, a recent study
suggested that miR-27b overexpression markedly repressed
fibrotic responses in pulmonary epithelial cells (41).
In conclusion, 1,25(OH)2D3
inhibits differentiation and downregulates miR-27b
expression in human lung fibroblasts induced by TGF-β1.
Furthermore, miR-27b overexpression decreased the expression
of VDR protein and increased the expression of α-SMA, while
reducing levels of miR-27b had opposing effects. Notably,
miR-27b has abilities for targeting the 3′UTR of VDR and
negatively regulating VDR protein expression, which effects
differentiation of human lung fibroblasts. Thus,
1,25(OH)2D3 inhibits lung fibroblast
differentiation induced by TGF-β1 via miR-27b targeting VDR
3′UTR, and this may be used as a novel treatment strategy in
differentiation pathways.
Abbreviations:
|
1,25(OH)2D3
|
1α,25-dihydroxyvitamin
D3
|
|
VDR
|
vitamin D receptor
|
|
α-SMA
|
α-smooth muscle actin
|
|
RXR
|
retinoid X receptor
|
|
TGF-β
|
transforming growth factor-β
|
|
miRNA
|
microRNA
|
|
UTR
|
untranslated region
|
|
qPCR
|
quantitative polymerase chain
reaction
|
References
|
1
|
Holick MF: Vitamin D deficiency. N Engl J
Med. 357:266–281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Etten E, Stoffels K, Gysemans C,
Mathieu C and Overbergh L: Regulation of vitamin D homeostasis:
Implications for the immune system. Nutr Rev. 66(Suppl 2):
S125–S134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haroon M and Fitzgerald O: Vitamin D and
its emerging role in immunopathology. Clin Rheumatol. 31:199–202.
2012. View Article : Google Scholar
|
|
4
|
Messa P, Alfieri C and Rastaldi MP: Recent
insights into vitamin D and its receptor. J Nephrol. 24(Suppl 18):
S30–S37. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Førli L, Bjortuft O and Boe J: Vitamin D
status in relation to nutritional depletion and muscle function in
patients with advanced pulmonary disease. Exp Lung Res. 35:524–538.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Black PN and Scragg R: Relationship
between serum 25-hydroxyvitamin D and pulmonary function in the
third national health and nutrition examination survey. Chest.
128:3792–3798. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Foong RE and Zosky GR: Vitamin D
deficiency and the lung: Disease initiator or disease modifier?
Nutrients. 5:2880–2900. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hansdottir S and Monick MM: Vitamin D
effects on lung immunity and respiratory diseases. Vitam Horm.
86:217–237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herr C, Greulich T, Koczulla RA, Meyer S,
Zakharkina T, Branscheidt M, Eschmann R and Bals R: The role of
vitamin D in pulmonary disease: COPD, asthma, infection, and
cancer. Respir Res. 12:312011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Finklea JD, Grossmann RE and Tangpricha V:
Vitamin D and chronic lung disease: A review of molecular
mechanisms and clinical studies. Adv Nutr. 2:244–253. 2011.
View Article : Google Scholar :
|
|
11
|
Hughes DA and Norton R: Vitamin D and
respiratory health. Clin Exp Immunol. 158:20–25. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Demir M, Uyan U, Keçeoçlu S and Demir C:
The relationship between vitamin D deficiency and pulmonary
hypertension. Prague Med Rep. 114:154–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lips P: Vitamin D physiology. Prog Biophys
Mol Biol. 92:4–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haussler MR, Jurutka PW, Mizwicki M and
Norman AW: Vitamin D receptor (VDR)-mediated actions of
1α,25(OH)2 vitamin D3: Genomic and
non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab.
25:543–559. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Duffield JS, Lupher M, Thannickal VJ and
Wynn TA: Host responses in tissue repair and fibrosis. Annu Rev
Pathol. 8:241–276. 2013. View Article : Google Scholar :
|
|
16
|
Hetzel M, Bachem M, Anders D, Trischler G
and Faehling M: Different effects of growth factors on
proliferation and matrix production of normal and fibrotic human
lung fibroblasts. Lung. 183:225–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Coen M, Gabbiani G, Bochaton-Piallat ML
and Chen YE: Myofibroblast-mediated adventitial remodeling: An
underestimated player in arterial pathology. Arterioscler Thromb
Vasc Biol. 31:2391–2396. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sartore S, Chiavegato A, Faggin E, Franch
R, Puato M, Ausoni S and Pauletto P: Contribution of adventitial
fibroblasts to neointima formation and vascular remodeling: From
innocent bystander to active participant. Circ Res. 89:1111–1121.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu X, Nelson A, Wang X, Farid M, Gunji Y,
Ikari J, Iwasawa S, Basma H, Feghali-Bostwick C and Rennard SI:
Vitamin D modulates prostaglandin E2 synthesis and degradation in
human lung fibroblasts. Am J Respir Cell Mol Biol. 50:40–50.
2014.
|
|
20
|
Stenmark KR, Frid MG and Yeager ME:
Fibrocytes: Potential new therapeutic targets for pulmonary
hypertension? Eur Respir J. 36:1232–1235. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kajdaniuk D, Marek B, Borgiel-Marek H and
Kos-Kudła B: Transforming growth factor β1 (TGFβ1) in physiology
and pathology. Endokrynol Pol. 64:384–396. 2013. View Article : Google Scholar
|
|
22
|
Ma L and Chung WK: The genetic basis of
pulmonary arterial hypertension. Hum Genet. 133:471–479. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Biernacka A, Dobaczewski M and
Frangogiannis NG: TGF-β signaling in fibrosis. Growth Factors.
29:196–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suwanabol PA, Seedial SM, Zhang F, Shi X,
Si Y, Liu B and Kent KC: TGF-β and Smad3 modulate PI3K/Akt
signaling pathway in vascular smooth muscle cells. Am J Physiol
Heart Circ Physiol. 302:H2211–H2219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Scotton CJ and Chambers RC: Molecular
targets in pulmonary fibrosis: The myofibroblast in focus. Chest.
132:1311–1321. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo W, Shan B, Klingsberg RC, Qin X and
Lasky JA: Abrogation of TGF-beta1-induced fibroblast-myofibroblast
differentiation by histone deacetylase inhibition. Am J Physiol
Lung Cell Mol Physiol. 297:L864–L870. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ramirez AM, Wongtrakool C, Welch T,
Steinmeyer A, Zügel U and Roman J: Vitamin D inhibition of
pro-fibrotic effects of transforming growth factor beta1 in lung
fibroblasts and epithelial cells. J Steroid Biochem Mol Biol.
118:142–150. 2010. View Article : Google Scholar
|
|
28
|
Ding N, Yu RT, Subramaniam N, Sherman MH,
Wilson C, Rao R, Leblanc M, Coulter S, He M, Scott C, et al: A
vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic
response. Cell. 153:601–613. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yanagi Y, Suzawa M, Kawabata M, Miyazono
K, Yanagisawa J and Kato S: Positive and negative modulation of
vitamin D receptor function by transforming growth factor-beta
signaling through smad proteins. J Biol Chem. 274:12971–12974.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yanagisawa J, Yanagi Y, Masuhiro Y, Suzawa
M, Watanabe M, Kashiwagi K, Toriyabe T, Kawabata M, Miyazono K and
Kato S: Convergence of transforming growth factor-beta and vitamin
D signaling pathways on SMAD transcriptional coactivators. Science.
283:1317–1321. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Subramaniam N, Leong GM, Cock TA, Flanagan
JL, Fong C, Eisman JA and Kouzmenko AP: Cross-talk between
1,25-dihydroxyvitamin D3 and transforming growth factor-beta
signaling requires binding of VDR and Smad3 proteins to their
cognate DNA recognition elements. J Biol Chem. 276:15741–15746.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stefani G and Slack FJ: Small non-coding
RNAs in animal development. Nat Rev Mol Cell Biol. 9:219–230. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gangaraju VK and Lin H: MicroRNAs: Key
regulators of stem cells. Nat Rev Mol Cell Biol. 10:116–125. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bauersachs J and Thum T: Biogenesis and
regulation of cardiovascular microRNAs. Circ Res. 109:334–347.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lino Cardenas CL, Kaminski N and Kass DJ:
Micromanaging microRNAs: Using murine models to study microRNAs in
lung fibrosis. Drug Discov Today Dis Models. 10:e145–e151. 2013.
View Article : Google Scholar
|
|
39
|
Thum T, Gross C, Fiedler J, Fischer T,
Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, et
al: MicroRNA-21 contributes to myocardial disease by stimulating
MAP kinase signalling in fibroblasts. Nature. 456:980–984. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vettori S, Gay S and Distler O: Role of
microRNAs in fibrosis. Open Rheumatol J. 6:130–139. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Graham JR, Williams CM and Yang Z:
MicroRNA-27b targets gremlin 1 to modulate fibrotic responses in
pulmonary cells. J Cell Biochem. 115:1539–1548. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang S, Cui H, Xie N, Icyuz M, Banerjee S,
Antony VB, Abraham E, Thannickal VJ and Liu G: miR-145 regulates
myofibroblast differentiation and lung fibrosis. FASEB J.
27:2382–2391. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zerr P, Vollath S, Palumbo-Zerr K, Tomcik
M, Huang J, Distler A, Beyer C, Dees C, Gela K, Distler O, et al:
Vitamin D receptor regulates TGF-β signalling in systemic
sclerosis. Ann Rheum Dis. 74:e202015. View Article : Google Scholar
|
|
44
|
Aschenbrenner JK, Sollinger HW, Becker BN
and Hullett DA: 1,25-(OH(2))D(3) alters the transforming growth
factor beta signaling pathway in renal tissue. J Surg Res.
100:171–175. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xie T, Liang J, Guo R, Liu N, Noble PW and
Jiang D: Comprehensive microRNA analysis in bleomycin-induced
pulmonary fibrosis identifies multiple sites of molecular
regulation. Physiol Genomics. 43:479–487. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier
C and Brown RA: Myofibroblasts and mechano-regulation of connective
tissue remodelling. Nat Rev Mol Cell Biol. 3:349–363. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hinz B, Phan SH, Thannickal VJ, Prunotto
M, Desmoulière A, Varga J, De Wever O, Mareel M and Gabbiani G:
Recent developments in myofibroblast biology: Paradigms for
connective tissue remodeling. Am J Pathol. 180:1340–1355. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fernandez IE and Eickelberg O: The impact
of TGF-β on lung fibrosis: From targeting to biomarkers. Proc Am
Thorac Soc. 9:111–116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pandit KV, Milosevic J and Kaminski N:
MicroRNAs in idiopathic pulmonary fibrosis. Transl Res.
157:191–199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhou L, Wang L, Lu L, Jiang P, Sun H and
Wang H: Inhibition of miR-29 by TGF-beta-Smad3 signaling through
dual mechanisms promotes transdifferentiation of mouse myoblasts
into myofibroblasts. PLoS One. 7:e337662012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gu J, Wang Y and Wu X: MicroRNA in the
pathogenesis and prognosis of esophageal cancer. Curr Pharm Des.
19:1292–1300. 2013.
|
|
52
|
Jiang J, Lv X, Fan L, Huang G, Zhan Y,
Wang M and Lu H: MicroRNA-27b suppresses growth and invasion of
NSCLC cells by targeting Sp1. Tumour Biol. 35:10019–10023. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Jin L, Wessely O, Marcusson EG, Ivan C,
Calin GA and Alahari SK: Prooncogenic factors miR-23b and miR-27b
are regulated by Her2/Neu, EGF, and TNF-α in breast cancer. Cancer
Res. 73:2884–2896. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ye J, Wu X, Wu D, Wu P, Ni C, Zhang Z,
Chen Z, Qiu F, Xu J and Huang J: miRNA-27b targets vascular
endothelial growth factor C to inhibit tumor progression and
angiogenesis in colorectal cancer. PLoS One. 8:e606872013.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Goto Y, Kojima S, Nishikawa R, Enokida H,
Chiyomaru T, Kinoshita T, Nakagawa M, Naya Y, Ichikawa T and Seki
N: The microRNA-23b/27b/24-1 cluster is a disease progression
marker and tumor suppressor in prostate cancer. Oncotarget.
5:7748–7759. 2014.PubMed/NCBI
|
|
56
|
Urbich C, Kuehbacher A and Dimmeler S:
Role of microRNAs in vascular diseases, inflammation, and
angiogenesis. Cardiovasc Res. 79:581–588. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang J, Song Y, Zhang Y, Xiao H, Sun Q,
Hou N, Guo S, Wang Y, Fan K, Zhan D, et al: Cardiomyocyte
overexpression of miR-27b induces cardiac hypertrophy and
dysfunction in mice. Cell Res. 22:516–527. 2012. View Article : Google Scholar :
|
|
58
|
Ji J, Zhang J, Huang G, Qian J, Wang X and
Mei S: Overexpressed microRNA-27a and 27b influence fat
accumulation and cell proliferation during rat hepatic stellate
cell activation. FEBS Lett. 583:759–766. 2009. View Article : Google Scholar : PubMed/NCBI
|