Introduction
In vivo, the skeleton is constantly being
remodeled via a process involving the breakdown (resorption) and
build-up (synthesis) of bone, determined by a delicate balance
between osteoblast and osteoclast activities (1). As osteoclasts have key roles in the
regulation of bone mass and quality, the majority of adult skeletal
diseases are due to excess osteoclast activity, resulting in
osteopenia (2,3). Such diseases include osteoporosis,
periodontal disease, rheumatoid arthritis and aseptic prosthesis
loosening (4,5). For individuals with osteoporosis, a
condition characterized by low bone mass and skeletal fragility,
low trauma bone fractures represent life-threatening events,
particularly when they affect the vertebrae, proximal femur (hip),
distal forearm or proximal humerus (6).
Osteoclasts are large, multinucleated cells that
arise from the hematopoietic stem cell monocyte/macrophage lineage
(7). Osteoclast activation was
initiated by activation of the receptor activator of the nuclear
factor-κB (RANK) and RANK ligand (RANKL) signaling pathways
(8,9). RANK and RANKL belong to the tumor
necrosis factor (TNF) receptor superfamily (10,11). The binding of RANKL to RANK
recruits the adapter protein, TNF receptor-associated factor 6
(TRAF6), to the plasma membrane. RANK, RANKL and TRAF6 are
essential for osteoclastogenesis, as mice lacking these molecules
show profound bone resorption defects (12). The RANK/TRAF6 complex activates
several pathways, including NF-κB signaling and the
mitogen-activated protein kinase (MAPK) pathways involving
extracellular signal-related kinase (ERK), which induce expression
of nuclear factor of activated T cells c1 (NFATc1), considered one
of the master transcription factors controlling osteoclastogenesis
(13–15). Therefore, ERK and NFATc1, which
are closely regulated by MAPK activity, are essential for the
differentiation, survival and activation of osteoclasts (16–18).
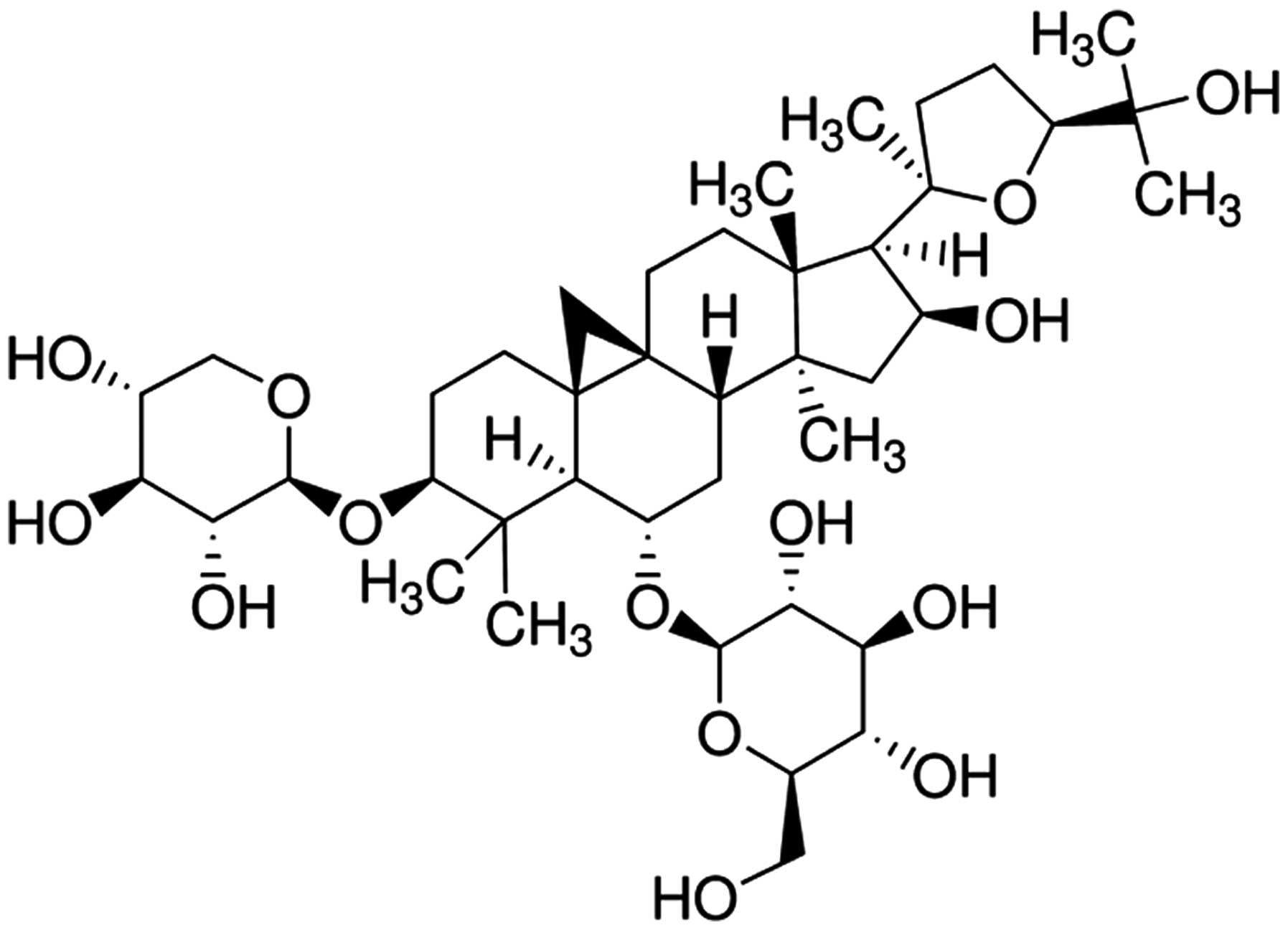
Astragaloside IV (AS-IV) (Fig. 1) is a saponin purified from
Astragalus membranaceus Bge., one of the most widely used
Chinese herbs (19). AS-IV has
been reported to have a wide range of treatment effects, with no
toxicity (20). Pharmacological
activities attributed to AS-IV include cardioprotective (21), anti-inflammatory (22), antioxidant (23), anti-asthmatic (24) and anticancer (25) effects. Some of these
pharmacological activities resulted from AS-IV-mediated inhibition
of ERK (26–28) and NF-κB (26,29–31) signaling pathways. Furthermore,
AS-IV has been reported to affect osteogenesis (32), and have anti-arthritis activity
(33). All these findings
indicated that AS-IV may have a negative effect on
osteoclastogenesis and may therefore have a significant potential
for the treatment of osteoclast-related diseases, including
osteoporosis. However, to the best of our knowledge, there is
little published information regarding this issue.
Therefore, the present study aimed to i) investigate
the potential therapeutic benefits of AS-IV on osteoclast-related
osteolytic bone diseases, ii) understand the underlying mechanisms
mediating the effects of AS-IV on osteoclast formation and
function, and iii) further elucidate the potential molecular
mechanisms of AS-IV in osteoclasts.
Materials and methods
Media, reagents and cells
Fetal bovine serum and α-modification of Eagle's
medium (α-MEM) were obtained from Gibco-BRL (Sydney, Australia).
The cell counting kit-8 (CCK-8) was purchased from Dojindo
(Kumamoto, Japan). Soluble human recombinant macrophage-colony
stimulating factor (M-CSF) and bacteria-derived recombinant mouse
RANKL were supplied by R&D Systems (Minneapolis, MN, USA).
AS-IV (purity >98%) was purchased from Sigma-Aldrich (St. Louis,
MO, USA) and dissolved in dimethyl sulfoxide prior to dilution to
the appropriate concentrations in culture medium (34). Western blot-specific antibodies
were obtained from Cell Signaling Technology (Cambridge, MA, USA).
RAW264.7 cells were purchased from the American Type Culture
Collection (Rockville, MD, USA).
Cytotoxicity assay
The cytotoxic effects of AS-IV were determined using
a CCK-8 assay. Bone marrow macrophages (BMMs) of C57/BL6 mice were
seeded in 96-well plates at a density of 8×103
cells/well, and cultured for 24 h. Cells were subsequently treated
with different concentrations of AS-IV for 24, 48, 72 and 96 h.
CCK-8 buffer (10 µl) was added to each well, and the cells
were incubated at 37°C for an additional 2 h. The absorbance was
subsequently measured at a wavelength of 450 nm (650 nm reference).
The half-maximal inhibitory concentration (IC50) value
was calculated.
BMM isolation and osteoclast culture
For primary cell culture, BMMs were obtained from
the femurs and tibias of 6-week-old C57/BL6 mice. Cells were
cultured in a T75 flask for 24 h. Floating cells were removed, and
the adherent cells were cultured for another 3 days. The BMMs were
subsequently seeded in a 96-well plate at a density of
8×103 cells/well in complete α-MEM supplemented with 30
ng/ml M-CSF, 50 ng/ml RANKL and different concentrations of AS-IV
(0, 25, 50 or 100 µM). Culture media were replaced every 2
days until mature osteoclasts had formed. Osteoclasts were
identified by positive staining for tartrate-resistant acid
phosphatase (TRAP). TRAP-positive cells with >3 nuclei were
counted under a microscope.
F-actin ring immunofluorescence
For F-actin ring immunofluorescent staining,
osteoclasts were fixed with 4% paraformaldehyde for 15 min at room
temperature and permeabilized for 5 min with 0.1% v/v Triton X-100.
Cells were incubated with rhodamine-conjugated phalloidin (1:100;
Invitrogen, Carlsbad, CA, USA) for 1 h at room temperature. Cells
were subsequently incubated with Hoechst 3342 dye (1:5,000) for
visualization of the nuclei, washed with phosphate-buffered saline
(PBS), and mounted with ProLong Gold anti-fade mounting medium
(both from Invitrogen). Fluorescence detection was performed on
Nikon A1Si spectral detector confocal system equipped with 20X
(dry) lenses (Nikon, Tokyo, Japan). Images were collected using
NIS-C elements software and analyzed using ImageJ Software
(National Institutes of Health, Bethesda, MD, USA).
Bone resorption pit assay
BMMs were seeded on bone slices in 96-well plates at
a density of 8×103 cells/well and stimulated (in
triplicate) with M-CSF (30 ng/ml), RANKL (50 ng/ml) and AS-IV (0,
25, 50 or 100 µM). Bone slices were imaged using
field-emission scanning electron microscopy (FESEM, Hitachi S-4800,
CamScan; Hitachi, Tokyo, Japan) with a magnification of 10 kV.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
assay
RT-qPCR was used to measure specific gene
expression. BMMs were plated in 6-well plates at a density of
1×105 cells/well and cultured in complete α-MEM
supplemented with 30 ng/ml M-CSF and 50 ng/ml RANKL. For the
concentration gradient assay, cells were incubated with AS-IV (0,
25 or 50 µM) for 5–7 days until mature osteoclasts formed.
For the time gradient assay, cells were incubated with 50 µM
AS-IV for 0, 1, 3 or 5 days. Total RNA was extracted using the
Qiagen RNeasy Mini kit (Qiagen, Valencia, CA, USA) and cDNA was
synthesized from 1 µg of total RNA using reverse
transcriptase (Takara Biotechnology, Otsu, Japan). qPCR was
performed using the SYBR Premix Ex Tag kit (Takara Biotechnology)
and an ABI 7500 Sequencing Detection system (Applied Biosystems,
Foster City, CA, USA) with 40 cycles of denaturation at 95°C for 5
sec and amplification at 60°C for 24 sec. GAPDH was used as the
reference gene, and all the reactions were run in triplicate. The
mouse primer sequences for cathepsin K (CtsK), TRAP, dendritic
cell-specific trans membrane protein (DC-STAMP), V-ATPase d2,
c-fos, NFATc1 and GAPDH were as follows: CtsK forward,
5′-CTTCCAATACGTGCA GCAGA-3′ and reverse,
5′-TCTTCAGGGCTTTCTCGTTC-3′; TRAP forward,
5′-CTGGAGTGCACGATGCCAGCGACA-3′ and reverse,
5′-TCCGTGCTCGGCGATGGACCAGA-3′; DC-STAMP forward,
5′-AAAACCCTTGGGCTGTTCTT-3′ and reverse, 5′-AATCATGGACGACTCCTTGG-3′;
V-ATPase d2 forward, 5′-AAGCCTTTGTTTGACGCTGT-3′ and reverse,
5′-TTCGATGCCTCTGTGAGATG-3′; c-fos forward, 5′-CCA
GTCAAGAGCATCAGCAA-3′ and reverse, 5′-AAGTAG TGCAGCCCGGAGTA-3′;
NFATc1 forward, 5′-CCGTTG CTTCCAGAAAATAACA-3′ and reverse,
5′-TGTGGGATG TGAACTCGGAA-3′; and GAPDH forward, 5′-ACCCAG
AAGACTGTGGATGG-3′ and reverse, 5′-CACATTGGG GGTAGGAACAC-3′.
NF-κB luciferase reporter assay
RAW264.7 cells were stably transfected with a
p-NF-κB-TA-Luc luciferase reporter construct. Briefly, cells were
plated in a 24-well plate at a density of 1×105
cells/well. The cells were treated 24 h later with different AS-IV
concentrations (0, 12.5, 25, 50, 100 or 200 µM) for 1 h,
prior to incubation with 50 ng/ml RANKL for a further 8 h. Cells
were subsequently lysed and luciferase activity was measured using
the Promega Luciferase Assay system (Promega, Madison, WI,
USA).
Western blotting
RAW264.7 cells were seeded in 6-well plates at a
density of 5×105 cells/well. After pretreatment with or
without AS-IV (200 µM) for 4 h, the cells were stimulated
with RANKL for 0, 5, 10, 20, 30 or 60 min. The cells were
subsequently washed twice in PBS and lysed in
radioimmuno-precipitation assay lysis buffer. The lysates were
centrifuged at 12,000 x g for 15 min and supernatants were
collected.
Protein concentrations were determined using the
bicinchoninic acid assay. Protein from each lysate (20 µg)
was resolved using 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis gels, and transferred to polyvinylidene difluoride
membranes (Millipore, Bedford, MA, USA). Membranes were
subsequently blocked with 5% skimmed milk in TBS-Tween-20 for 1 h,
and incubated with primary antibodies overnight at 4°C. The
membranes were incubated with horseradish peroxidase-conjugated
secondary antibodies (1:5,000) for 1 h. Antibody reactivity was
detected using an Odyssey infrared imaging system (LI-COR
Biosciences, Lincoln, NE, USA).
Titanium particle-induced calvarial
osteolysis model
An in vivo wear particle-induced osteolysis
model was generated as previously reported (35). All the animal care and
experimental procedures complied with Directive 2010/63/EU revising
Directive 86/609/EEC approved by Animal Care and Use Committee of
Zhengzhou University (approved on Feb 19th 2014, the approval
number is 001381). Twenty healthy 8-week-old C57BL/J6 mice were
randomly assigned to four groups: Sham PBS control (sham), Ti
particles with PBS (vehicle), and Ti particles with low (10
mg/kg/day) and high (25 mg/kg/day) concentrations of AS-IV (low and
high, respectively). AS-IV was used by intraperitoneal injection
every other day for 14 days. At the end of the experiment, the mice
were sacrificed, and the calvaria were excised and fixed in 4%
paraformaldehyde for micro-computed tomography (micro-CT)
analysis.
Micro-CT scanning
A high-resolution micro-CT scanner (Skyscan 1176;
Skyscan; Aartselaar, Belgium) was used with the following settings:
X-ray voltage, 50 kV; electric current, 500 mA; and rotation step,
0.7°. Following reconstruction, a square region of interest (ROI)
around the midline suture was chosen for further qualitative and
quantitative analyses. The bone volume against tissue volume
(BV/TV), number of porosities and percentage of total porosity were
determined for each sample as described previously (36).
Statistical analysis
The data Are expressed as mean ± standard deviation.
The results were analyzed using the analysis of variance and post
hoc tests with the SPSS 13.0 software (SPSS Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference between groups.
Results
AS-IV cytotoxicity
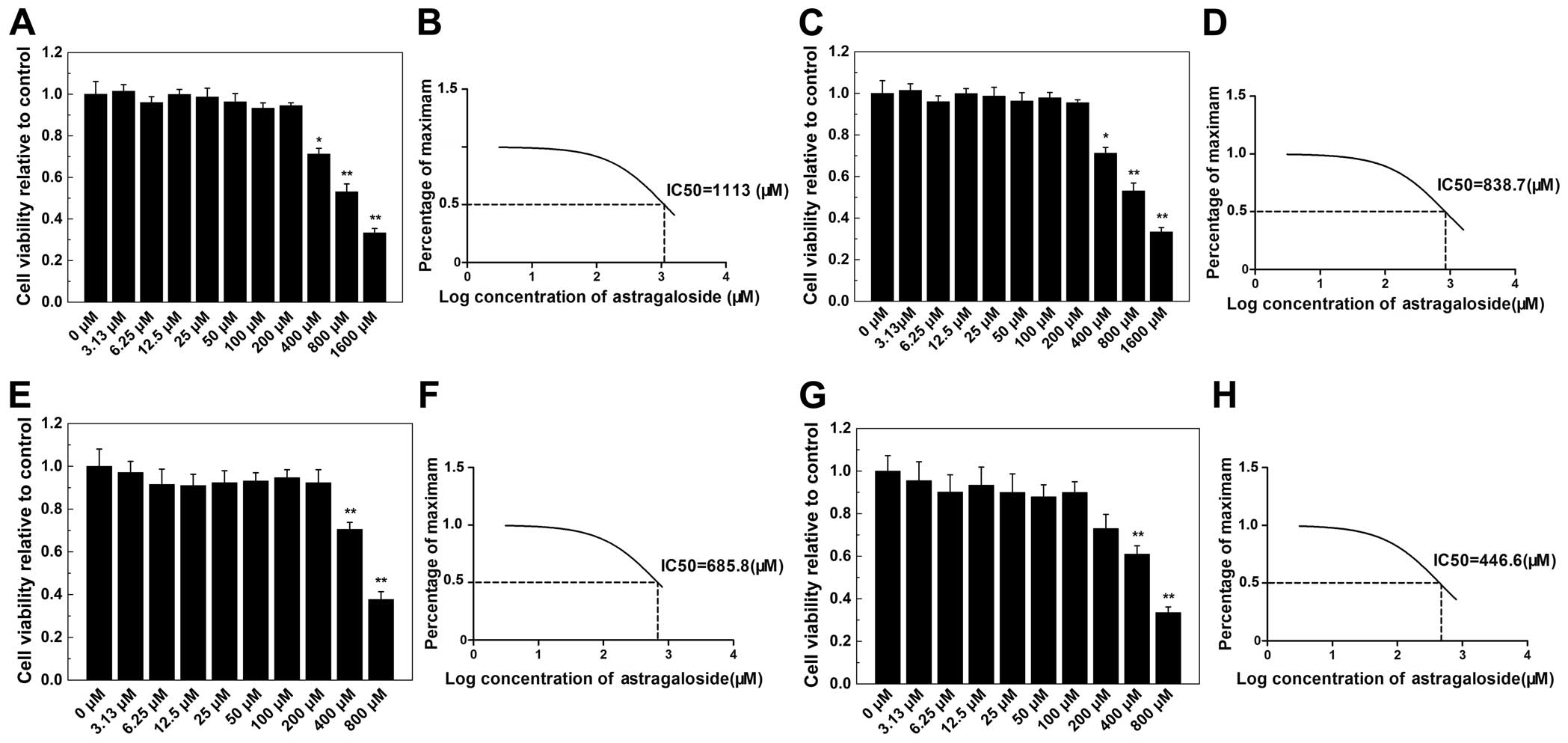
CCK-8 cell viability assays were performed to
examine the potential AS-IV cytotoxicity. The results showed that
AS-IV cytotoxicity was concentration- and time-dependent (Fig. 2). These assays indicated that the
maximum concentration used in the subsequent studies (200
µM) showed no cytotoxic effects in BMMs, even after 96-h
exposure. Based on these data, the AS-IV concentrations used in
subsequent experiments were considered non-cytotoxic.
Effect of AS-IV on
osteoclastogenesis
As osteoclasts have key roles in osteoclast-related
diseases (2), the effect of AS-IV
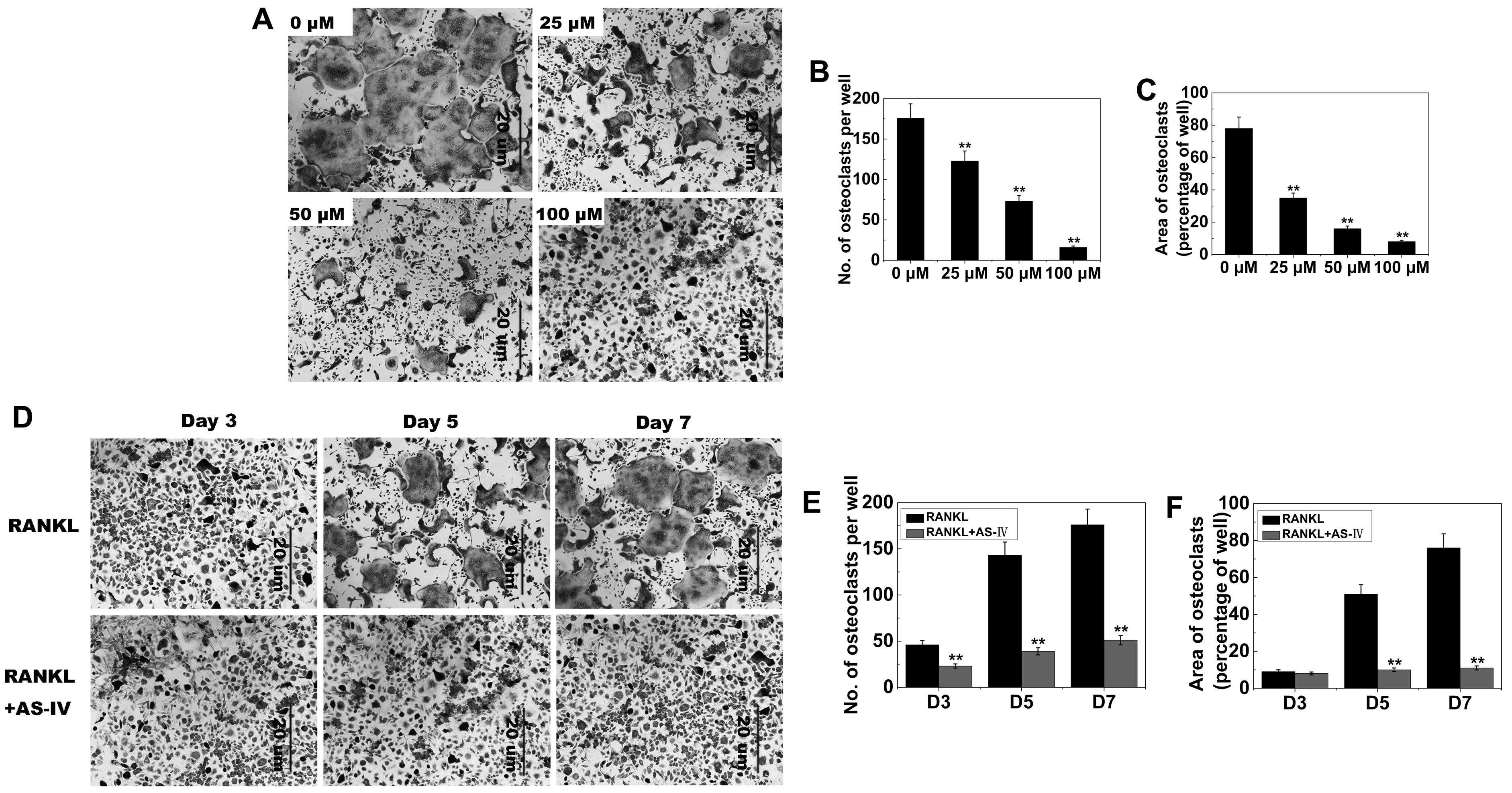
on osteoclastogenesis was investigated. BMMs were exposed to AS-IV
(0, 25, 50 and 100 µM) during osteoclast formation. The
control group formed numerous TRAP-positive multinucleated
osteoclasts (Fig. 3A). Osteoclast
formation was inhibited by AS-IV (Fig. 3A–C). Furthermore, comparison of
the control and 100 µM AS-IV cells after 3, 5 and 7 days
produced similar results (Fig.
3D–F).
F-actin ring formation
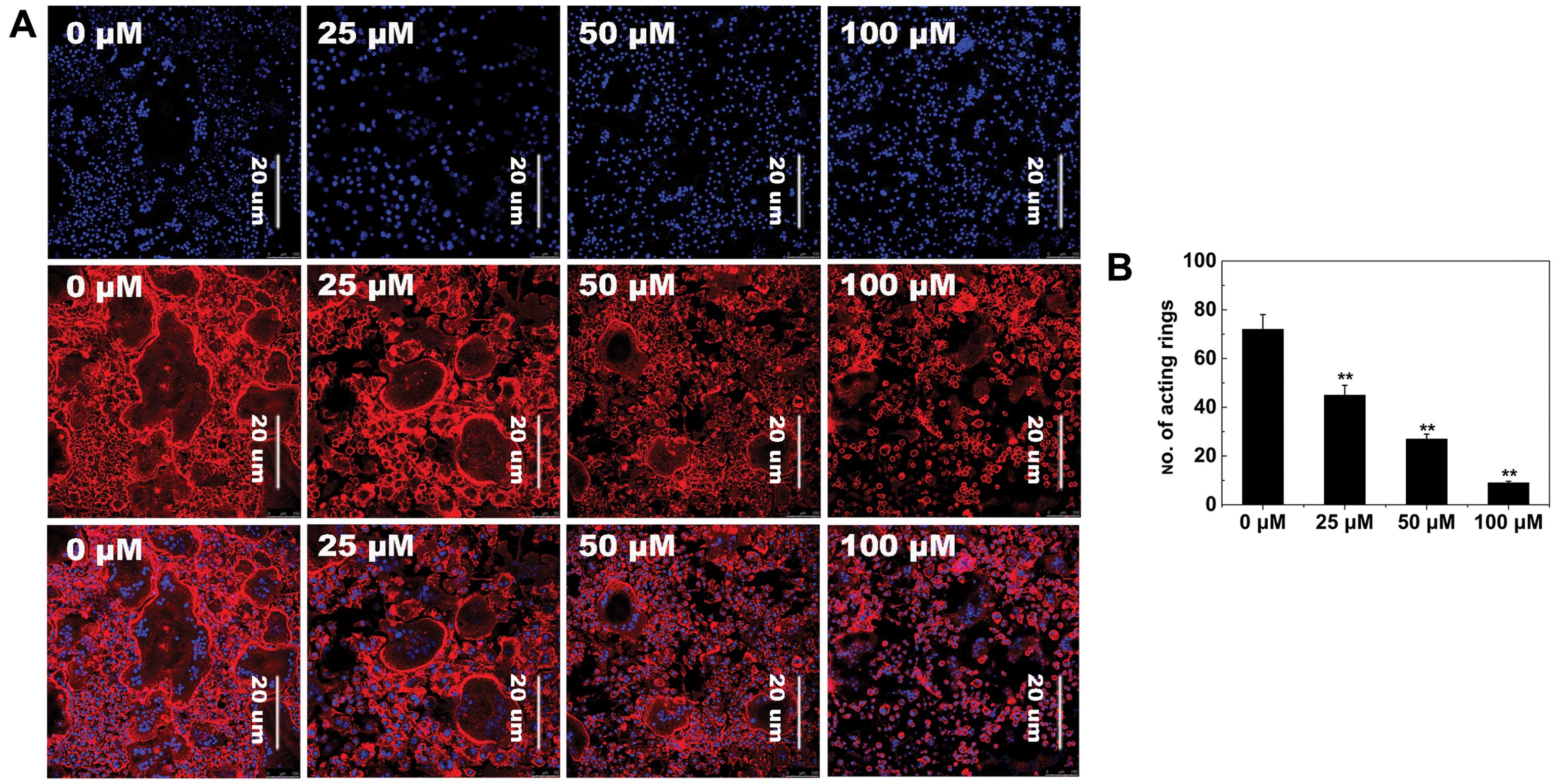
To further examine the effects of AS-IV on
osteoclastogenesis, RANKL-induced osteoclast F-actin ring formation
was studied, which is the most well-known characteristic of mature
osteoclasts and a prerequisite for osteoclast bone resorption
during osteoclastogenesis (37).
As expected, confocal microscopy revealed F-actin ring formation
and characteristic podosomal condensation in control osteoclasts.
However, the size and number of F-actin rings significantly
decreased in cells incubated with AS-IV (Fig. 4A), suggesting that AS-IV
suppressed F-actin ring formation.
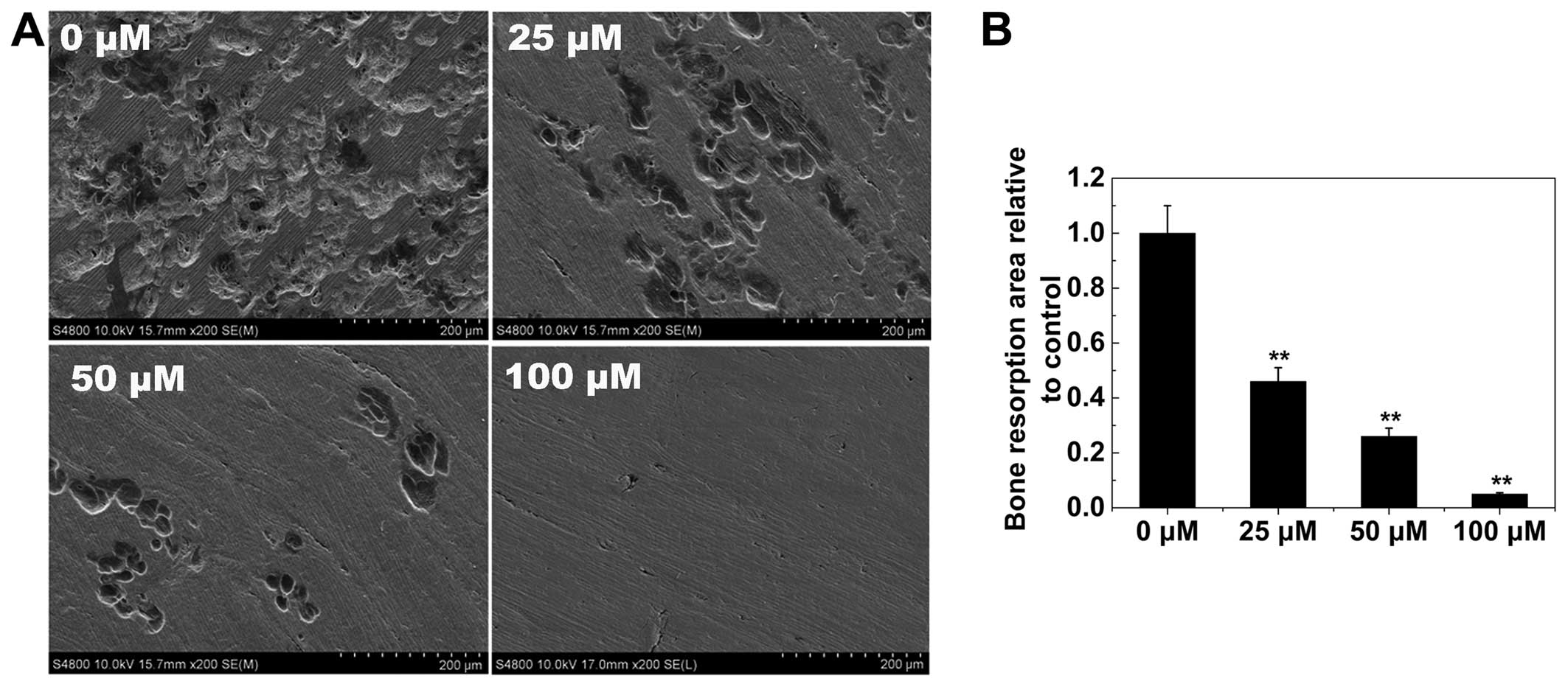
Osteoclast bone resorption
As the formation of a well-polarized F-actin ring is
an essential prerequisite for efficient bone resorption by
osteoclasts, we inferred that osteoclast bone resorption would also
be inhibited by AS-IV. Numerous bone resorption pits were observed
on the surface of control bone slices (Fig. 5). In bone slices exposed to AS-IV,
the resorption area was decreased. These findings demonstrated that
AS-IV impaired osteoclast bone resorption in vitro.
RANKL-induced gene expression
The expression levels of several specific genes are
upregulated during osteoclast differentiation. Thus, RT-qPCR was
used to examine and quantify the RANKL-induced mRNA expression of
osteoclast-related genes (including NFATc1, TRAP, V-ATPase d2,
CtsK, DC-STAMP and c-fos). The results showed that the expression
of these genes was inhibited by AS-IV in a dose- and time-dependent
manner (Fig. 6).
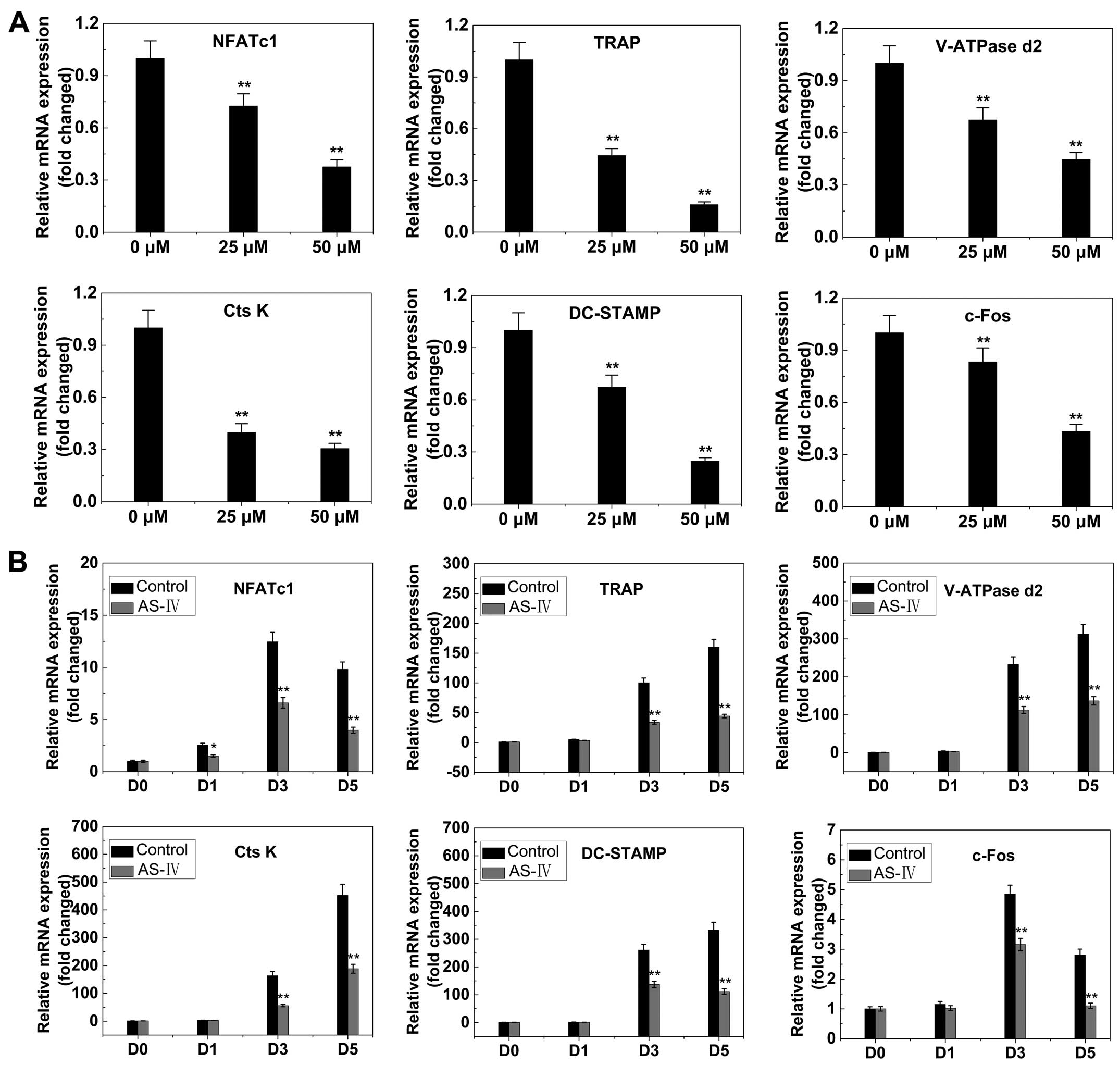 | Figure 6AS-IV suppresses RANKL-induced
expression of osteoclast-specific genes. Bone marrow macrophages
were cultured with macrophage colony-stimulating factor (30 ng/ml)
and RANKL (50 ng/ml), with or without AS-IV. NFATc1, TRAP, V-ATPase
d2, CtsK, DC-STAMP and c-fos expression levels were analyzed by
reverse transcription-quantitative polymerase chain reaction and
the results were normalized to the expression of
glyceraldehyde-3-phosphate dehydrogenase. (A) Levels of the
indicated mRNAs following exposure to AS-IV (0, 25 or 50
µM). (B) Levels of the indicated mRNAs following exposure to
50 µM AS-IV for 0, 1, 3 or 5 days. All the experiments were
performed at least three times. *P<0.05 and
**P<0.01, compared to 0 µM treatment
(control). AS-IV, astraga-loside IV; RANKL, receptor activator of
the nuclear factor-κB ligand; TRAP, tartrate-resistant acid
phosphatase; NFATc1, nuclear factor of activated T cells c1. |
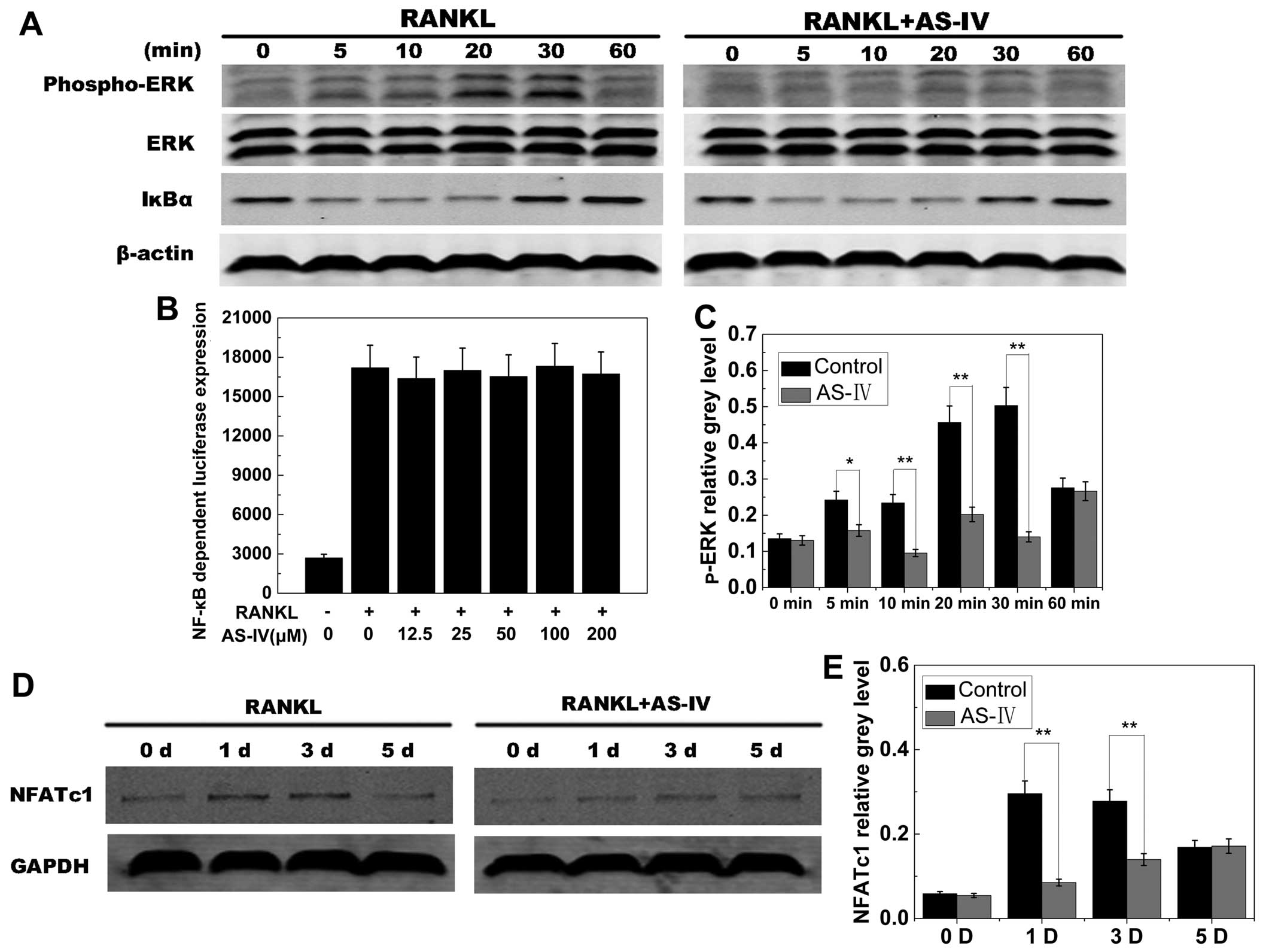
RANKL-induced ERK and NFATc1 expression
signaling
RANKL-induced activation of ERK is essential for
osteoclast differentiation and function (38,39). The effects of AS-IV on
RANKL-induced signaling pathways were therefore investigated. ERK
phosphorylation increased within 5–30 min of stimulation with RANKL
in the control group. However, ERK phosphorylation was
significantly reduced by exposure to AS-IV (Fig. 7A). Quantitative analysis confirmed
these results (Fig. 7C). These
results suggested that AS-IV inhibited phosphorylation of ERK
during osteoclast differentiation.
NFATc1 is an important master regulator of
osteoclastogenesis and osteoclast function (40). Therefore, the effects of AS-IV on
RANKL-induced NFATc1 expression were investigated. The data
presented in Fig. 6A and B
indicated that NFATc1 transcriptional activity increased when the
cells were stimulated by RANKL. AS-IV inhibited this activity in a
dose- and time-dependent manner. To confirm this effect of AS-IV on
NFATc1 expression, the NFATc1 protein level was examined using
western blot analysis. NFATc1 protein levels increased when cells
were exposed to RANKL, and AS-IV attenuated this increase (Fig. 7D and E), suggesting that AS-IV
suppressed RANKL-induced NFATc1 expression.
RANKL-induced NF-κB signaling
RANKL-induced NF-κB activation is also a dominant
mediator of osteoclast differentiation, resorption function and
survival (41–43). Western blot analysis and
luciferase assays were used to investigate the NF-κB signaling
pathway. Similar levels of IκBα phosphorylation and degradation
were observed in control and AS-IV groups (Fig. 7A). This observation was supported
by luciferase reporter gene assays (Fig. 7B). These data indicated that AS-IV
inhibited osteoclastogenesis without affecting the NF-κB signaling
pathway.
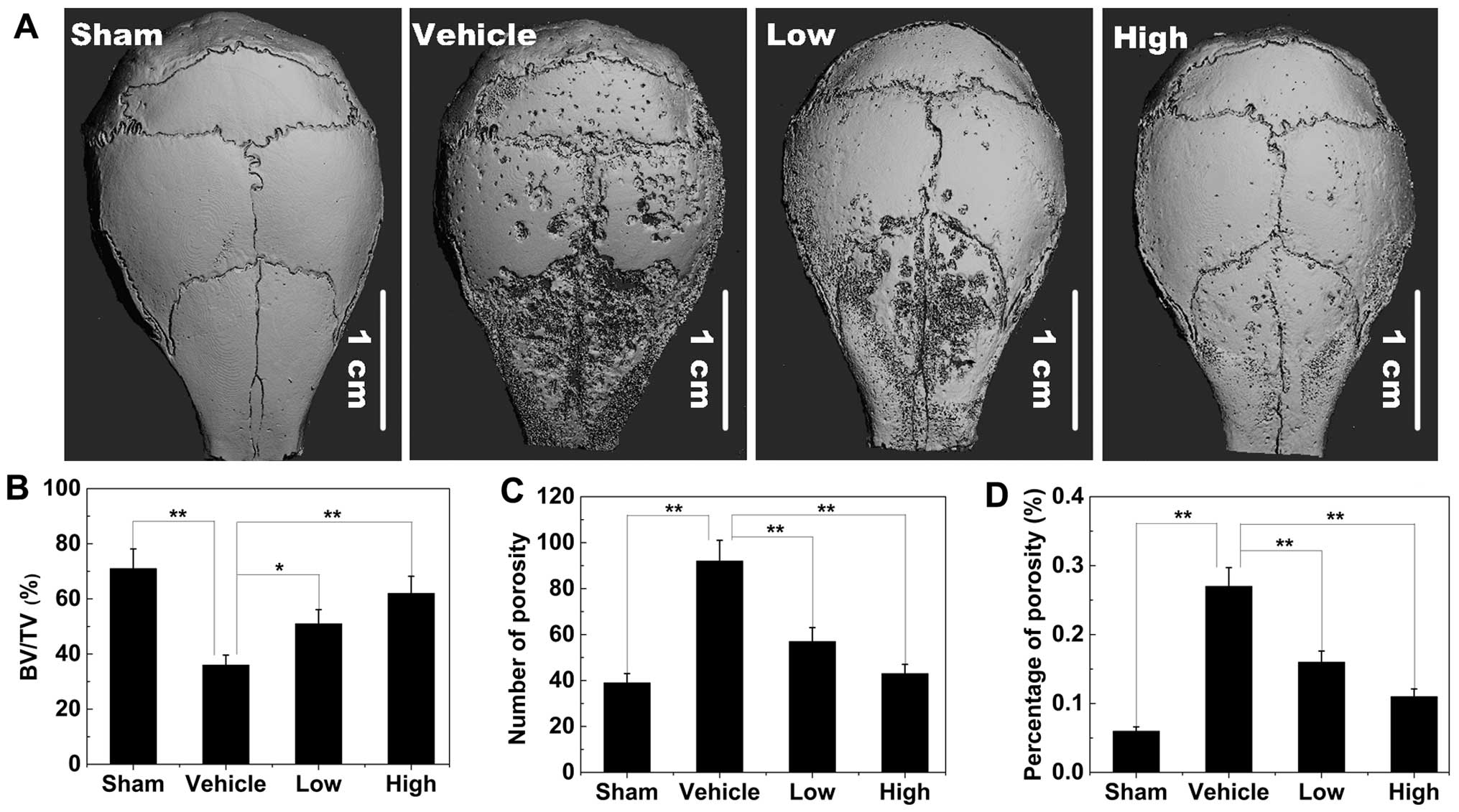
Titanium particle-induced osteolysis
To explore the effects of AS-IV on pathological
osteolysis, a Ti particle-induced mouse calvarial osteolysis model
was used. The degree of particle-induced osteolysis was assessed
using high-resolution micro-CT. Compared with the sham group (no Ti
particles), the vehicle group (administration of Ti particles in
PBS) showed significant calvarial osteolysis. When AS-IV (10 mg/kg,
low; 25 mg/kg, high) was administered with the Ti particles, this
osteolytic bone loss reduced (Fig.
8A). Quantitative analysis of bone parameters further
demonstrated that the high or low concentrations of AS-IV
significantly increased the BV/TV (Fig. 8B), and decreased the number of
porosities and the percentage of total porosity in the ROI in the
calvaria (Fig. 8C and D).
Discussion
Diseases associated with osteoclasts include
osteoporosis, rheumatoid arthritis, multiple myeloma,
periprosthetic osteolysis and metastatic cancers (2,44–46). During the last two decades,
numerous advances have been made in the treatment of
osteoclast-related diseases; however, treatment options are not
optimal thus far.
The bisphosphonates were the first drugs approved
specifically for the treatment and prevention of osteoclast-related
diseases. However, they often cause gastrointestinal toxicity,
including dyspepsia, abdominal pain, gastritis and esophagitis.
Other serious adverse effects include osteonecrosis of the jaw,
femur fractures and atrial fibrillation (47). Estrogens have also been used for
the treatment of osteoporosis, but can have serious adverse
effects, such as breast cancer, endometrial cancer and
thromboembolism (48). The first
effective bone anabolic agent teriparatide [parathyroid hormone
(PTH) 1–34] has also been used clinically. However, high cost and
the requirement for daily subcutaneous injections are major
limitations to its use (49).
Denosumab is a human monoclonal antibody that inhibits RANKL and,
consequently, osteoclastogenesis. However, cellulitis was more
frequent in patients taking denosumab compared with the placebo
(50) and patients also had a
high risk of fractures (51).
Strontium ranelate is also used clinically; however, it has the
common side effects of nausea, diarrhea, and mild and transient
elevation in creatine kinase, and is contraindicated in patients
with a high risk of thromboembolic events. In addition, a few cases
of hypersensitivity have been reported (52). Due to the limitations of present
therapies, attempts to develop improved treatment options are being
pursued.
Previous studies indicated that AS-IV had positive
effects on osteogenesis (32) and
arthritis (33), and to the best
of our knowledge, the present study demonstrated for the first time
that it significantly inhibited osteoclast differentiation and
formation, impaired F-actin ring formation, and significantly
decreased the number and area of bone resorption pits in
vitro. In the in vivo studies, three-dimensional
reconstruction of micro-CT images from mouse calvarias showed that
AS-IV treatment markedly suppressed Ti particle-induced osteolysis
in a dose-dependent manner.
Activation of RANK by its ligand leads to the
expression of osteoclast-specific genes during differentiation, and
the activation of resorption by mature osteoclasts (11). RANK signaling is mediated by
cytoplasmic factors that activate downstream pathways controlling
these various functions. At least five distinct protein
kinase-mediated signaling cascades are induced during
osteoclastogenesis and activation; inhibitor of NF-κB kinase (IKK),
c-Jun N-terminal kinase, p38, ERK and Src pathways (11). ERK is essential for osteoclast
differentiation, survival and activation (16,17,53,54). The inhibition of ERK has been
proven to possess a therapeutic potential for osteoclast-related
diseases (39). Activated ERK
stimulates transcription factors, such as NFATc1 (15), which is a master regulator of
osteoclast differentiation (40,55,56). Overexpression of NFATc1
accelerates RANKL-induced osteoclast differentiation and can also
increase osteoclast formation independently of RANKL. Additionally,
NFATc1-deficient embryonic stem cells failed to differentiate into
osteoclasts, even in the presence of RANKL (40). Using western blot analysis, AS-IV
was revealed to inhibit RANKL-induced ERK signaling. NF-κB was
unaffected and this result was confirmed using an NF-κB luciferase
reporter assay. AS-IV also inhibited NFATc1 mRNA and protein
expression. NFATc1 regulates the expression of a range of genes
associated with osteoclast differentiation and function. In the
present study, the expression of NFATc1-regulated genes (TRAP, CtsK
and V-ATPase d2) was downregulated by AS-IV, suggesting that AS-IV
not only affected the expression of NFATc1, but also affected the
expression of its downstream genes.
In conclusion, the present study demonstrated that
AS-IV had inhibitory effects on osteoclastogenesis and osteoclast
function in vitro and in vivo. Additionally, these
inhibitory effects appeared to operate via blockade of the ERK and
NFATc1 pathways. Taken together, the data strongly suggested that
AS-IV may be developed as a potential agent for the treatment of
osteoclast-related diseases, including osteoporosis.
Acknowledgments
The present study was supported by the Department of
Oncology, The First Affiliated Hospital of Zhengzhou
University.
Abbreviations:
|
AS-IV
|
astragaloside IV
|
|
BMMs
|
bone marrow macrophages
|
|
ERK
|
extracellular signal-regulated
kinase
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
MAPK
|
mitogen-activated protein kinase
|
|
M-CSF
|
macrophage colony-stimulating
factor
|
|
NFATc1
|
nuclear factor of activated T cells
c1
|
|
RANK
|
receptor activator of the nuclear
factor-κB
|
|
RANKL
|
receptor activator of the nuclear
factor-κB ligand
|
|
TNF
|
tumor necrosis factor
|
|
TRAF6
|
tumor necrosis factor
receptor-associated factor 6
|
References
|
1
|
Kim HS, Suh KS, Sul D, Kim BJ, Lee SK and
Jung WW: The inhibitory effect and the molecular mechanism of
glabridin on RANKL-induced osteoclastogenesis in RAW264.7 cells.
Int J Mol Med. 29:169–177. 2012.
|
|
2
|
Rodan GA and Martin TJ: Therapeutic
approaches to bone diseases. Science. 289:1508–1514. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim TH, Kim HJ, Lee SH and Kim SY: Potent
inhibitory effect of Foeniculum vulgare Miller extract on
osteoclast differentiation and ovariectomy-induced bone loss. Int J
Mol Med. 29:1053–1059. 2012.PubMed/NCBI
|
|
4
|
Guo H, Zhang J, Hao S and Jin Q:
Adenovirus-mediated small interfering RNA targeting tumor necrosis
factor-α inhibits titanium particle-induced osteoclastogenesis and
bone resorption. Int J Mol Med. 32:296–306. 2013.PubMed/NCBI
|
|
5
|
Hou GQ, Guo C, Song GH, Fang N, Fan WJ,
Chen XD, Yuan L and Wang ZQ: Lipopolysaccharide (LPS) promotes
osteoclast differentiation and activation by enhancing the MAPK
pathway and COX-2 expression in RAW264.7 cells. Int J Mol Med.
32:503–510. 2013.PubMed/NCBI
|
|
6
|
Kanis JA: Assessment of fracture risk and
its application to screening for postmenopausal osteoporosis:
Synopsis of a WHO report. Osteoporos Int. 4:368–381. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Udagawa N, Takahashi N, Akatsu T, Tanaka
H, Sasaki T, Nishihara T, Koga T, Martin TJ and Suda T: Origin of
osteoclasts: Mature monocytes and macrophages are capable of
differentiating into osteoclasts under a suitable microenvironment
prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci
USA. 87:7260–7264. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Purdue PE, Koulouvaris P, Potter HG,
Nestor BJ and Sculco TP: The cellular and molecular biology of
periprosthetic osteolysis. Clin Orthop Relat Res. 454:251–261.
2007. View Article : Google Scholar
|
|
9
|
Ren W, Wu B, Peng X, Hua J, Hao HN and
Wooley PH: Implant wear induces inflammation, but not osteoclastic
bone resorption, in RANK(−/−) mice. J Orthop Res. 24:1575–1586.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Raggatt LJ and Partridge NC: Cellular and
molecular mechanisms of bone remodeling. J Biol Chem.
285:25103–25108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Asagiri M and Takayanagi H: The molecular
understanding of osteoclast differentiation. Bone. 40:251–264.
2007. View Article : Google Scholar
|
|
13
|
Strait K, Li Y, Dillehay DL and Weitzmann
MN: Suppression of NF-kappaB activation blocks osteoclastic bone
resorption during estrogen deficiency. Int J Mol Med. 21:521–525.
2008.PubMed/NCBI
|
|
14
|
Zwerina J, Hayer S, Redlich K, Bobacz K,
Kollias G, Smolen JS and Schett G: Activation of p38 MAPK is a key
step in tumor necrosis factor-mediated inflammatory bone
destruction. Arthritis Rheum. 54:463–472. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meissner JD, Freund R, Krone D, Umeda PK,
Chang KC, Gros G and Scheibe RJ: Extracellular signal-regulated
kinase 1/2-mediated phosphorylation of p300 enhances myosin heavy
chain I/beta gene expression via acetylation of nuclear factor of
activated T cells c1. Nucleic Acids Res. 39:5907–5925. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grigoriadis AE, Wang ZQ, Cecchini MG,
Hofstetter W, Felix R, Fleisch HA and Wagner EF: c-Fos: A key
regulator of osteoclast-macrophage lineage determination and bone
remodeling. Science. 266:443–448. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mansky KC, Sankar U, Han J and Ostrowski
MC: Microphthalmia transcription factor is a target of the p38 MAPK
pathway in response to receptor activator of NF-kappa B ligand
signaling. J Biol Chem. 277:11077–11083. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim YW, Baek SH, Lee SH, Kim TH and Kim
SY: Fucoidan, a sulfated polysaccharide, inhibits osteoclast
differentiation and function by modulating RANKL signaling. Int J
Mol Sci. 15:18840–18855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xuying W, Jiangbo Z, Yuping Z, Xili M,
Yiwen Z, Tianbao Z and Weidong Z: Effect of astragaloside IV on the
general and peripartum reproductive toxicity in Sprague-Dawley
rats. Int J Toxicol. 29:505–516. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ren S, Zhang H, Mu Y, Sun M and Liu P:
Pharmacological effects of astragaloside IV: A literature review. J
Tradit Chin Med. 33:413–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jia Y, Zuo D, Li Z, Liu H, Dai Z, Cai J,
Pang L and Wu Y: Astragaloside IV inhibits doxorubicin-induced
cardiomyocyte apoptosis mediated by mitochondrial apoptotic pathway
via activating the PI3K/Akt pathway. Chem Pharm Bull (Tokyo).
62:45–53. 2014. View Article : Google Scholar
|
|
22
|
Tan S, Wang G, Guo Y, Gui D and Wang N:
Preventive effects of a natural anti-inflammatory agent,
astragaloside IV, on ischemic acute kidney injury in rats. Evid
Based Complement Alternat Med. 2013:2840252013.PubMed/NCBI
|
|
23
|
Hu JY, Han J, Chu ZG, Song HP, Zhang DX,
Zhang Q and Huang YS: Astragaloside IV attenuates hypoxia-induced
cardiomyocyte damage in rats by upregulating superoxide dismutase-1
levels. Clin Exp Pharmacol Physiol. 36:351–357. 2009. View Article : Google Scholar
|
|
24
|
Huang X, Tang L, Wang F and Song G:
Astragaloside IV attenuates allergic inflammation by regulation
Th1/Th2 cytokine and enhancement CD4(+)CD25(+)Foxp3 T cells in
ovalbumin-induced asthma. Immunobiology. 219:565–571. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qi H, Wei L, Han Y, Zhang Q, Lau AS and
Rong J: Proteomic characterization of the cellular response to
chemopreventive triterpenoid astragaloside IV in human
hepatocellular carcinoma cell line HepG2. Int J Oncol. 36:725–735.
2010.PubMed/NCBI
|
|
26
|
He CL, Yi PF, Fan QJ, Shen HQ, Jiang XL,
Qin QQ, Song Z, Zhang C, Wu SC, Wei XB, et al: Xiang-Qi-Tang and
its active components exhibit anti-inflammatory and anticoagulant
properties by inhibiting MAPK and NF-κB signaling pathways in
LPS-treated rat cardiac microvascular endothelial cells.
Immunopharmacol Immunotoxicol. 35:215–224. 2013. View Article : Google Scholar
|
|
27
|
Wang Q, Shao X, Xu W, Qi C, Gu L, Ni Z and
Mou S: Astragalosides IV inhibits high glucose-induced cell
apoptosis through HGF activation in cultured human tubular
epithelial cells. Ren Fail. 36:400–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zheng R, Deng Y, Chen Y, Fan J, Zhang M,
Zhong Y, Zhu R and Wang L: Astragaloside IV attenuates complement
membranous attack complex induced podocyte injury through the MAPK
pathway. Phytother Res. 26:892–898. 2012. View Article : Google Scholar
|
|
29
|
Gui D, Huang J, Guo Y, Chen J, Chen Y,
Xiao W, Liu X and Wang N: Astragaloside IV ameliorates renal injury
in streptozotocin-induced diabetic rats through inhibiting
NF-κB-mediated inflammatory genes expression. Cytokine. 61:970–977.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li M, Yu L, She T, Gan Y, Liu F, Hu Z,
Chen Y, Li S and Xia H: Astragaloside IV attenuates Toll-like
receptor 4 expression via NF-κB pathway under high glucose
condition in mesenchymal stem cells. Eur J Pharmacol. 696:203–209.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun L, Li W, Li W, Xiong L, Li G and Ma R:
Astragaloside IV prevents damage to human mesangial cells through
the inhibition of the NADPH oxidase/ROS/Akt/NF-κB pathway under
high glucose conditions. Int J Mol Med. 34:167–176. 2014.PubMed/NCBI
|
|
32
|
Bian Q, Huang JH, Liang QQ, Shu B, Hou W,
Xu H, Zhao YJ, Lu S, Shi Q and Wang YJ: The osteogenetic effect of
astragaloside IV with centrifugating pressure on the OCT-1 cells.
Pharmazie. 66:63–68. 2011.PubMed/NCBI
|
|
33
|
Wang B and Chen MZ: Astragaloside IV
possesses antiarthritic effect by preventing interleukin 1β-induced
joint inflammation and cartilage damage. Arch Pharm Res.
37:793–802. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xie J, Wang H, Song T, Wang Z, Li F, Ma J,
Chen J, Nan Y, Yi H and Wang W: Tanshinone IIA and astragaloside IV
promote the migration of mesenchymal stem cells by up-regulation of
CXCR4. Protoplasma. 250:521–530. 2013. View Article : Google Scholar
|
|
35
|
Qin A, Cheng TS, Lin Z, Cao L, Chim SM,
Pavlos NJ, Xu J, Zheng MH and Dai KR: Prevention of wear
particle-induced osteolysis by a novel V-ATPase inhibitor
saliphenylhalamide through inhibition of osteoclast bone
resorption. PLoS One. 7:e341322012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wedemeyer C, Xu J, Neuerburg C,
Landgraeber S, Malyar NM, von Knoch F, Gosheger G, von Knoch M,
Löer F and Saxler G: Particle-induced osteolysis in
three-dimensional micro-computed tomography. Calcif Tissue Int.
81:394–402. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qin A, Cheng TS, Pavlos NJ, Lin Z, Dai KR
and Zheng MH: V-ATPases in osteoclasts: Structure, function and
potential inhibitors of bone resorption. Int J Biochem Cell Biol.
44:1422–1435. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Monje P, Hernández-Losa J, Lyons RJ,
Castellone MD and Gutkind JS: Regulation of the transcriptional
activity of c-Fos by ERK. A novel role for the prolyl isomerase
PIN1. J Biol Chem. 280:35081–35084. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Seo SW, Lee D, Minematsu H, Kim AD, Shin
M, Cho SK, Kim DW, Yang J and Lee FY: Targeting extracellular
signal-regulated kinase (ERK) signaling has therapeutic
implications for inflammatory osteolysis. Bone. 46:695–702. 2010.
View Article : Google Scholar :
|
|
40
|
Takayanagi H, Kim S, Koga T, Nishina H,
Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T and Inoue J:
Induction and activation of the transcription factor NFATc1 (NFAT2)
integrate RANKL signaling in terminal differentiation of
osteoclasts. Dev Cell. 3:889–901. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Franzoso G, Carlson L, Xing L, Poljak L,
Shores EW, Brown KD, Leonardi A, Tran T, Boyce BF and Siebenlist U:
Requirement for NF-kappaB in osteoclast and B-cell development.
Genes Dev. 11:3482–3496. 1997. View Article : Google Scholar
|
|
42
|
Iotsova V, Caamaño J, Loy J, Yang Y, Lewin
A and Bravo R: Osteopetrosis in mice lacking NF-kappaB1 and
NF-kappaB2. Nat Med. 3:1285–1289. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee SH, Kim JK and Jang HD: Genistein
inhibits osteoclastic differentiation of RAW 264.7 cells via
regulation of ROS production and scavenging. Int J Mol Sci.
15:10605–10621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Inacio MC, Ake CF, Paxton EW, Khatod M,
Wang C, Gross TP, Kaczmarek RG, Marinac-Dabic D and Sedrakyan A:
Sex and risk of hip implant failure: Assessing total hip
arthroplasty outcomes in the United States. JAMA Intern Med.
173:435–441. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rizzoli R: A new treatment for
post-menopausal osteoporosis: Strontium ranelate. J Endocrinol
Invest. 28(Suppl): 50–57. 2005.PubMed/NCBI
|
|
46
|
Weitzmann MN and Pacifici R: Estrogen
regulation of immune cell bone interactions. Ann NY Acad Sci.
1068:256–274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Marx RE: The deception and fallacies of
sponsored randomized prospective double-blinded clinical trials:
The bisphosphonate research example. Int J Oral Maxillofac
Implants. 29:e37–e44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Maeda SS and Lazaretti-Castro M: An
overview on the treatment of postmenopausal osteoporosis. Arq Bras
Endocrinol Metabol. 58:162–171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nakamura T, Sugimoto T, Nakano T,
Kishimoto H, Ito M, Fukunaga M, Hagino H, Sone T, Yoshikawa H,
Nishizawa Y, et al: Randomized Teriparatide [human parathyroid
hormone (PTH) 1-34] Once-Weekly Efficacy Research (TOWER) trial for
examining the reduction in new vertebral fractures in subjects with
primary osteoporosis and high fracture risk. J Clin Endocrinol
Metab. 97:3097–3106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cummings SR, San Martin J, McClung MR,
Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A,
et al: FREEDOM Trial: Denosumab for prevention of fractures in
postmenopausal women with osteoporosis. N Engl J Med. 361:756–765.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hiligsmann M, Boonen A, Dirksen CD, Ben
Sedrine W and Reginster JY: Cost-effectiveness of denosumab in the
treatment of postmenopausal osteoporotic women. Expert Rev
Pharmacoecon Outcomes Res. 13:19–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Meunier PJ, Roux C, Ortolani S,
Diaz-Curiel M, Compston J, Marquis P, Cormier C, Isaia G, Badurski
J, Wark JD, et al: Effects of long-term strontium ranelate
treatment on vertebral fracture risk in postmenopausal women with
osteoporosis. Osteoporos Int. 20:1663–1673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gingery A, Bradley E, Shaw A and Oursler
MJ: Phosphatidylinositol 3-kinase coordinately activates the
MEK/ERK and AKT/NFkappaB pathways to maintain osteoclast survival.
J Cell Biochem. 89:165–179. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu X, Qu X, Wu C, Zhai Z, Tian B, Li H,
Ouyang Z, Xu X, Wang W, Fan Q, et al: The effect of enoxacin on
osteoclastogenesis and reduction of titanium particle-induced
osteolysis via suppression of JNK signaling pathway. Biomaterials.
35:5721–5730. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhai Z, Qu X, Li H, Yang K, Wan P, Tan L,
Ouyang Z, Liu X, Tian B, Xiao F, et al: The effect of metallic
magnesium degradation products on osteoclast-induced osteolysis and
attenuation of NF-κB and NFATc1 signaling. Biomaterials.
35:6299–6310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu F, Zhu Z, Mao Y, Liu M, Tang T and Qiu
S: Inhibition of titanium particle-induced osteoclastogenesis
through inactivation of NFATc1 by VIVIT peptide. Biomaterials.
30:1756–1762. 2009. View Article : Google Scholar : PubMed/NCBI
|