Introduction
Ischemia-reperfusion injury involves cellular
responses to anoxia/reoxygenation (A/R), which initiate a cascade
of cellular processes and molecular events that cause endothelial
cell apoptosis (1–7). Volatile anesthetics have been
demonstrated to induce caspase-dependent, mitochondrial-mediated
apoptosis in vitro (8).
However, preconditioning with volatile anesthetics, including
desflurane, halothane, isoflurane and sevoflurane has been reported
to protect against A/R injury both in vivo and in
vitro (9–11). Preconditioning with volatile
anesthetics has been reported to affect inflammation (12–16), and we have previously reported
that desflurane preconditioning protects human umbilical vein
endothelial cells (HUVECs) against A/R injury through a process
involving nuclear factor-κB (NF-κB) (17,18).
The Rel-NF-κB family of transcription factors has
been implicated in a variety of biological functions, including
cellular proliferation and apoptosis, and in the initiation and
propagation of innate and adaptive immune responses (19–22). Rel-NF-κB activates two distinct
NF-κB activation pathways: the canonical and non-canonical NF-κB
pathways (23,24). The canonical NF-κB pathway is
activated by the most stressful stimuli, and results in the IκB
kinase (IKK) complex-mediated degradation of IκB and the rapid
nuclear accumulation of p50-RelA and p50-cRel NF-κB complexes
(25). By contrast, the
non-canonical NF-κB pathway is activated by a group of tumor
necrosis factor (TNF) receptors, such as CD40 (26), lymphotoxin β receptor (LTβR)
(27) and BAFF receptor (BAFF-R)
(28). The activation of the
non-canonical NF-κB pathway results in the degradation of the
C-terminus of p100 into p52 and the trans-location of p52 into the
nucleus. In the nucleus, p52 combines with RelB, producing the
NF-κB complex (29).
The Nod-like receptor (NLR) family contributes
either directly or indirectly to a variety of hallmarks associated
with cancer, including inflammation, cell death, tumor growth,
angiogenesis, invasion and metastasis (30–34). NLRs have been traditionally
considered as pattern-recognition receptors (PRRs), as they are
activated in response to conserved structural motifs found in
microbes or pathogen-associated molecular patterns (PAMPs). There
is a subgroup of NLRs that negatively regulate inflammation
(35–37), currently including three NLR
family members, NLRP12, NLRX1 and NLRC3. NLRP12 (previously known
as Monarch-1, PYPAF7, or CLR19.3) is one of the first to be well
described and is the most well characterized member of this
subgroup. It has been demonstrated in vitro that the
overexpression of NLRP12 induces the transcription of an NF-κB
reporter construct (38),
suggesting that it is an inflammasome-forming NLR and a positive
regulator of NF-κB signaling. However, under physiological
conditions and in the context of human disease, the ability of
NLRP12 to form a functional inflammasome appears to occur only
under highly specific conditions (39,40). In fact, several studies have
evaluated NLRP12 inflammasome formation and have directly shown
that NLRP12 does not regulate IL-1β/IL-18 maturation (41–46). Studies on NLRP12 have indicated
that it functions as a negative regulator of inflammation by
modulating canonical and non-canonical NF-κB signaling (37,42,44–46). NLRP12 negatively regulates
non-canonical NF-κB signaling through its association with TRAF3
and NF-κB-inducing kinase (NIK) (37,42).
In the present study, we investigated whether the
protective effects of desflurane preconditiong against A/R injury
are mediated by the downregulation of the non-canonical NF-κB
signaling pathway.
Materials and methods
Primary culture of HUVECs
HUVECs were isolated from the human umbilical vein
vascular wall using collagenase (Roche Diagnostics, Indianapolis,
IN, USA) digestion, as previously described (47), and cultured in a humidified
atmosphere containing 95% O2 and 5% CO2 at
37°C in endothelial cell culture medium (ECM; Sciencell Research
Laboratories, Carlsbad, CA, USA) supplemented with 5% fetal bovine
serum (FBS; Gibco-Life Technologies, Grand Island, NY, USA), 1%
endothelial cell growth supplement (ECGS), 100 U/ml penicillin and
100 µg/ml streptomycin sulfate (all from Sciencell Research
Laboratories). Cells were passaged 3–6 times before being used in
the experiments. Ethics approval for the isolation of the HUVECs
was obtained from the Ethics Committee of Fudan University Shanghai
Cancer Center, Shanghai, China.
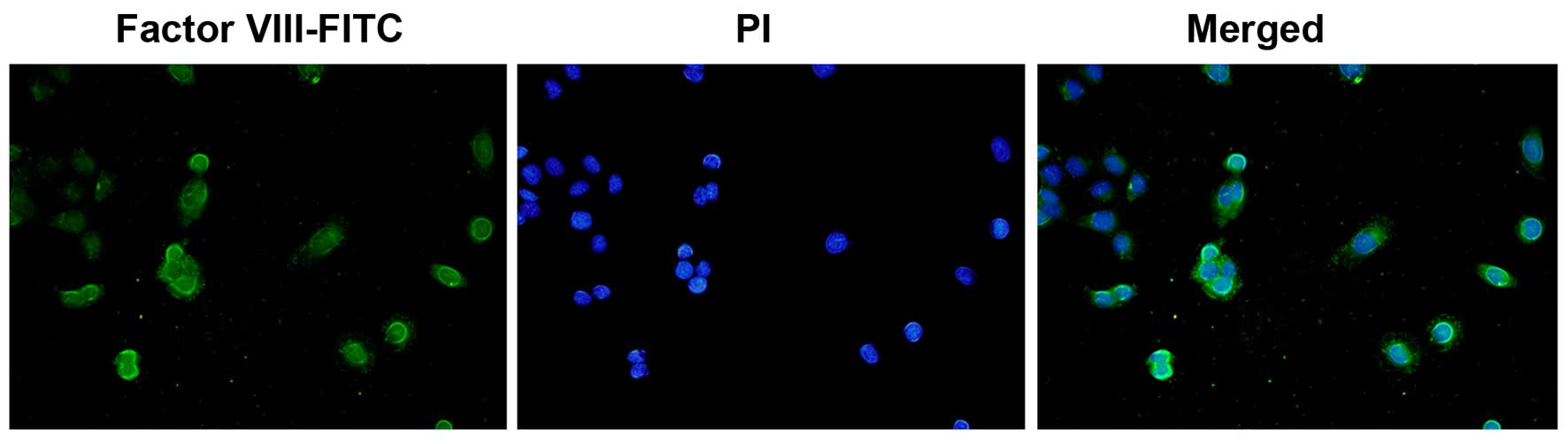
Immunofluorescence staining
Confluent endothelial cells in monolayer were fixed
with 95% cold ethanol for 5 min (the cells were grown on sterile
glass cover slides overnight at 37°C. The slides were briefly
washed 3 times for 5 min in PBS, and then fixed with 95% cold
ethanol and air dried). A drop of diluted anti-rabbit human factor
VIII antibody (Abcam, Cambridge, UK) was added (1:10 dilution),
allowed to react for 30 min in a moisture chamber, and then washed
3 times for 5 min in PBS. The slide was then incubated for 45 min
at 37°C with FITC-conjugated goat anti-rabbit globulin (Cwbiotech,
Shanghai, China) at a 1:50 dilution, and the washing procedure was
then repeated. A drop of mounting fluid consisting of 10% glycerol,
90% PBS and 0.25 mg/ml propidium iodide (PI; Cwbiotech) for
counterstaining the nuclei was added. The slides were examined on a
coverslip under an epifluorescence microscope (Olympus, Tokyo,
Japan) (Fig. 1), as previously
described (48).
Desflurane preconditioning and the A/R
regimen
An in vitro model of A/R, which has been
previously described (49), was
used in the present study. The HUVECs were subjected to a period of
anoxia, during which the cells were incubated in 95% N2
and 5% CO2 for 60 min, followed by 60 min reoxygenation,
during which time the cells were incubated in 95% O2 and
5% CO2. Prior to exposure to A/R, the cells were
incubated in the presence or absence of desflurane (1.0 MAC) for 30
min, and then allowed to rest for 15 min. Immediately after the A/R
protocol, the cells were incubated in the presence or absence of 10
ng/ml recombitant human TNF-α (rhTNF-α) (ProSpec, Ness Ziona,
Israel) for 60 min (Fig. 2).
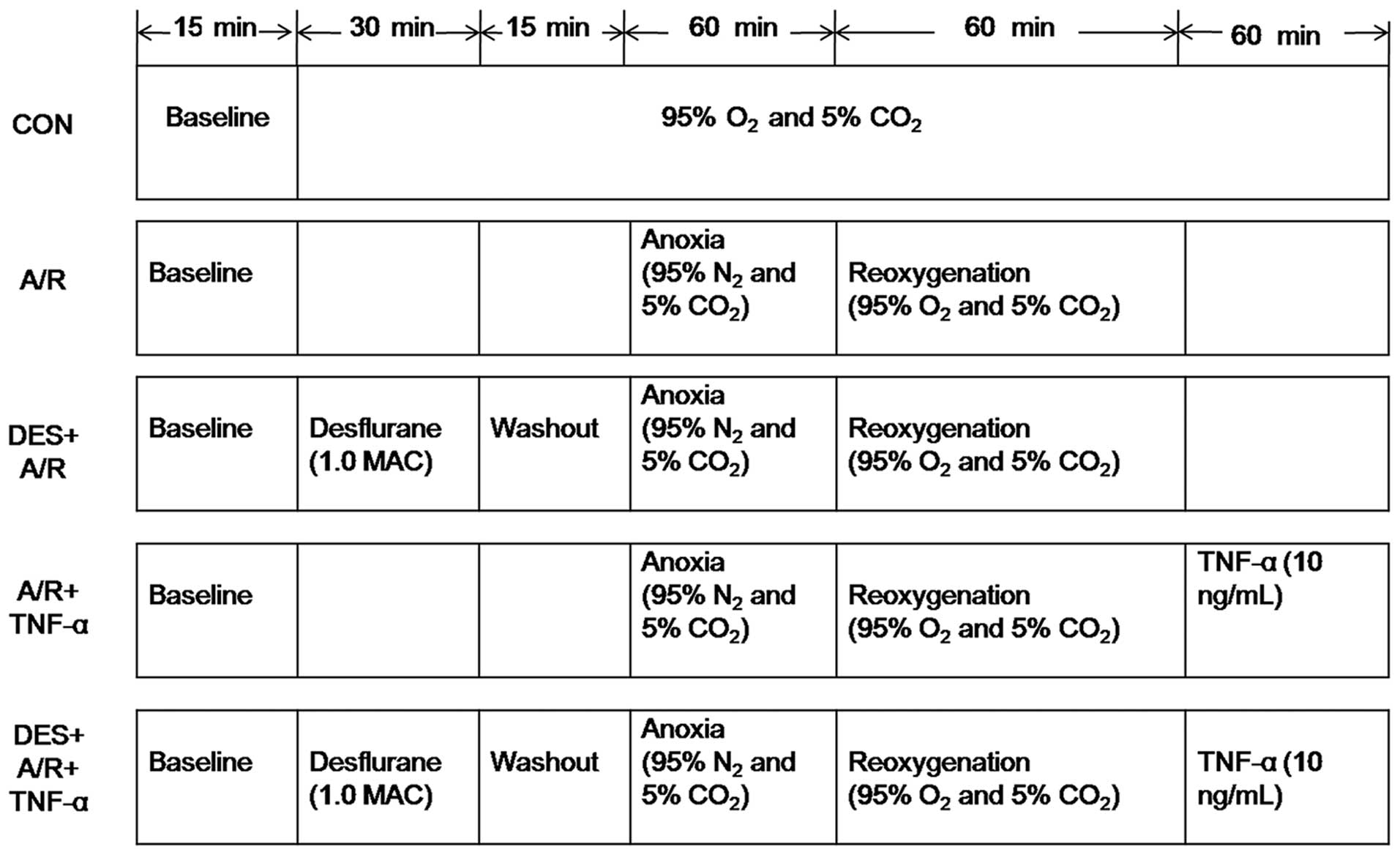 | Figure 2Desflurane preconditioning and
anoxia/reoxygenation exposure protocol. As described in the
Materials and methods, after 3 to 6 passages ex vivo, the
HUVECs were incubated in the presence or absence of 1.0 MAC
desflurane for 30 min, followed by a 15-min washout period before
A/R, and in the presence or absence of 10 ng/ml tumor necrosis
factor (TNF)-α for 60 min after A/R. CON, control; A/R,
anoxia/reoxygenation; DES+A/R, desflurane preconditioning and
anoxia/reoxygenation; A/R + TNF-α, anoxia/reoxygenation and tumor
necrosis factor-α (10 ng/ml); DES + A/R + TNF-α, desflurane
preconditioning, anoxia/rexoygenation and tumor necrosis factor-α
(10 ng/ml). |
Assessment of cell viability
An MTT assay was used, as tetrazolium salts are
cleaved to form a formazan dye only by metabolically active cells
and are particularly useful for quantifying the number of viable
cells. The HUVECs were seeded in 96-well plates (3×104
cells/well) and incubated overnight for complete cell adhesion. On
the second day, desflurane preconditioning and A/R exposure were
carried out as described above. At the end point of the experiment,
MTT (50 µl/well; Beyotime Institute of Biotechnology,
Haimen, China) was added to the medium followed by incubation at
37°C for a further 4 h. The medium was removed from all wells, and
the insoluble formazan product was dissolved in 150 µl of
DMSO for 10 min at room temperature. The optical density (OD) of
each culture/well was measured using a spectrophotometer
(UV-2450/2550, Shimadzu Corp., Tokyo, Japan) at 550 nm. The OD of
the cells in the control group represented 100% viability.
Flow cytometric analysis
Cell apoptosis was detected by flow cytometry. Cells
were double-stained with Annexin V-FITC and PI (Beyotime Institute
of Biotechnology) according to the manufacturer's instructions, and
cell fluorescence was analyzed on a FACSan flow cytometer. Annexin
V-FITC-positive cells reflected the relative proportion of
apoptotic cells.
PCR array
Total RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA). The expression of inflammatory
genes was examined by real-time PCR, utilizing the NF-κB signaling
pathway RT2 RNA QC PCR array (PAHS-025; Qiagen, Inc.,
Valencia, CA, USA), which profiled the expression of 84 key genes
related to NF-κB-mediated signal transduction. The expression of
the genes of interest (84 key genes related to NF-κB-mediated
signal transduction) was compared between the treated and untreated
cells. The fold change in expression for each gene between the
treated and untreated cells was calculated using the
2−∆∆Ct method (Shanghai Kangcheng Biological Engineering
Co., Ltd. Shanghai, China).
Immunoblot analysis
Cells were harvested and homogenized using RIPA
lysis buffer (Beyotime Institute of Biotechnology). Proteins were
separated on 8% SDS-PAGE gels and transferred onto polyvinylidene
fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The
membranes were blocked with 5% non-fat dry milk in TBST (containing
0.05% Tween-20), and incubated overnight at 4°C with the following
primary anti bodies: NLR family, pyrin domain containing 12
(NLRP12; sc-99175), (Santa Cruz Biotechnology, Santa Cruz, CA,
USA), Smac (2952), cellular inhibitor of apoptosis 1 (c-IAP1;
4592), NIK, IKKα, p100/p52, RelB (included in the Non-Canonical
Pathway Antibody Sampler kit) (all from Cell Signaling Technology,
Danvers, MA, USA), GAPDH (AG019; Beyotime Institute of
Biotechnology) and β-actin (A2668; Sigma, St. Louis, MO, USA). The
blots were then washed and incubated with horseradish
peroxidase-conjugated goat anti-mouse IgG (sc-2005; Santa Cruz
Biotechnology) for 1 h at room temperature. Immunoreactivity was
enhanced with a chemiluminescence kit (Millipore) and exposed to
film. GAPDH (Beyotime Institute of Biotechnology) or β-actin
(Sigma) were used as internal controls. The density of the bands on
the blots was quantified using a Bio-Rad imaging system (Bio-Rad
Laboratories, Hercules, CA, USA).
Statistical analysis
Data are expressed as the means ± SD, and were
analyzed using one-way analysis of variance (ANOVA), followed by
the Student-Newman-Keuls test. A P-value <0.05 was considered to
represent a statistically significant difference. All analyses were
conducted using SPSS 13.0 software (SPSS, Inc., Chicago, IL,
USA).
Results
Effect of desflurane preconditioning on
A/R-induced damage to HUVECs
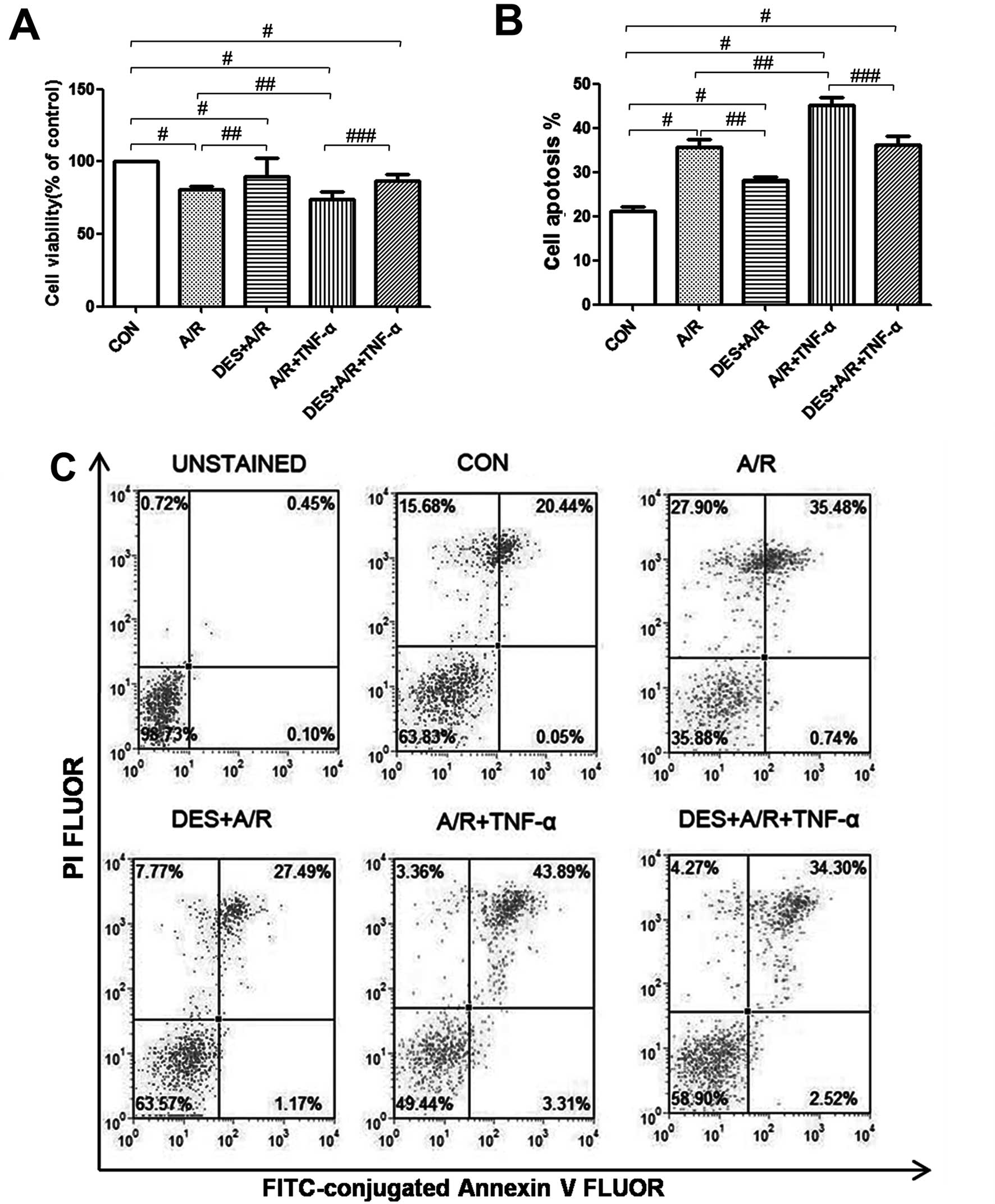
Desflurane preconditioning promotes
HUVEC survival during A/R
As previously established, exposure to A/R and/or
TNF-α reduces HUVEC viability (50). In this study, we detected HUVEC
viability by MTT assay, and found that our A/R protocol
significantly reduced HUVEC viability (P<0.05), and when A/R was
followed by incubation with TNF-α, HUVEC viability was further
reduced (P<0.05; Fig. 3A).
However, desflurane (1.0 MAC) preconditioning significantly
attenuated the effects of A/R or A/R and TNF-α on HUVEC viability
(P<0.05; Fig. 3A), suggesting
that desflurane preconditioning promotes HUVEC survival under
certain conditions of cellular stress.
Desflurane preconditioning decreases
the apoptosis of HUVECs exposed to A/R
The rate of apoptosis was determined by Annexin V
and PI staining, and analyzed by flow cytometry. Spontaneous
apoptosis was low in the HUVECs in the control group, whereas
exposure to A/R increased apop-tosis (P<0.05), and when A/R was
followed by incubation with TNF-α, HUVEC apoptosis increased even
further (P<0.05). Pre-treatment with desflurane (1.0 MAC)
attenuated the effects of A/R or A/R and THF-α on HUVEC apoptosis
(P<0.05) (Fig. 3B and C),
suggesting that desflurane preconditioning protects HUVECs against
A/R induced apoptosis.
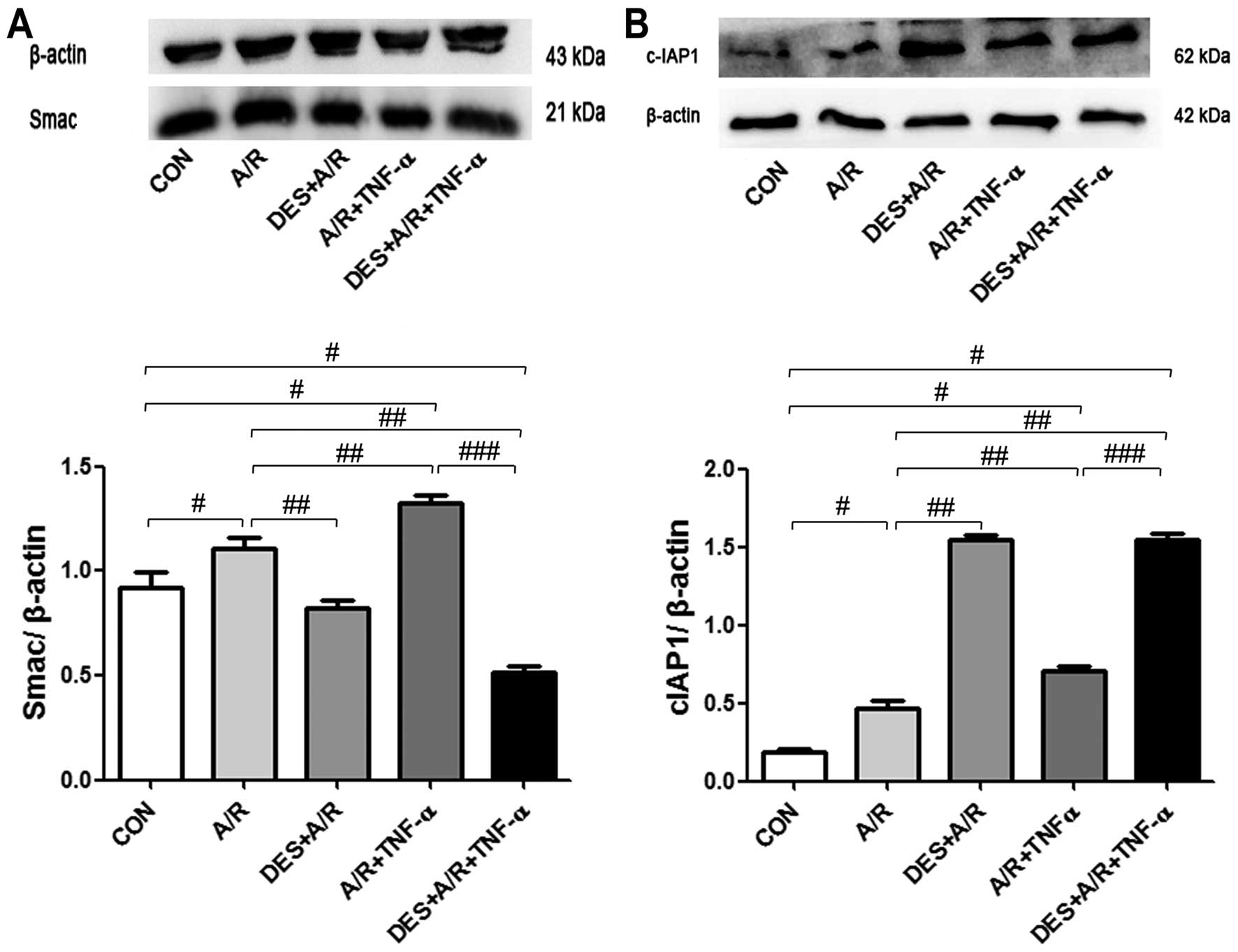
Desflurane preconditioning increases
the expression of cIAP1 and decreases the expression of Smac
In order to further elucidate the effects of
desflurane preconditioning on A/R-induced HUVEC apoptosis, we
examined Smac activation and cIAP1 inhibition, as these are
processes unique to apoptosis, which do not occur in other forms of
cell death. We detected increased levels of Smac and cIAP1 in the
HUVECs exposed to A/R and A/R plus TNF-α (P<0.05). Desflurance
preconditioning increased c-IAP1 levels (P<0.05) and decreased
the levels of Smac (P<0.05; Fig.
4), suggesting that desflurane preconditioning protects HUVECs
against A/R-induced apoptosis through a mechanism involving the
inhibition of Smac and the activation of cIAP1.
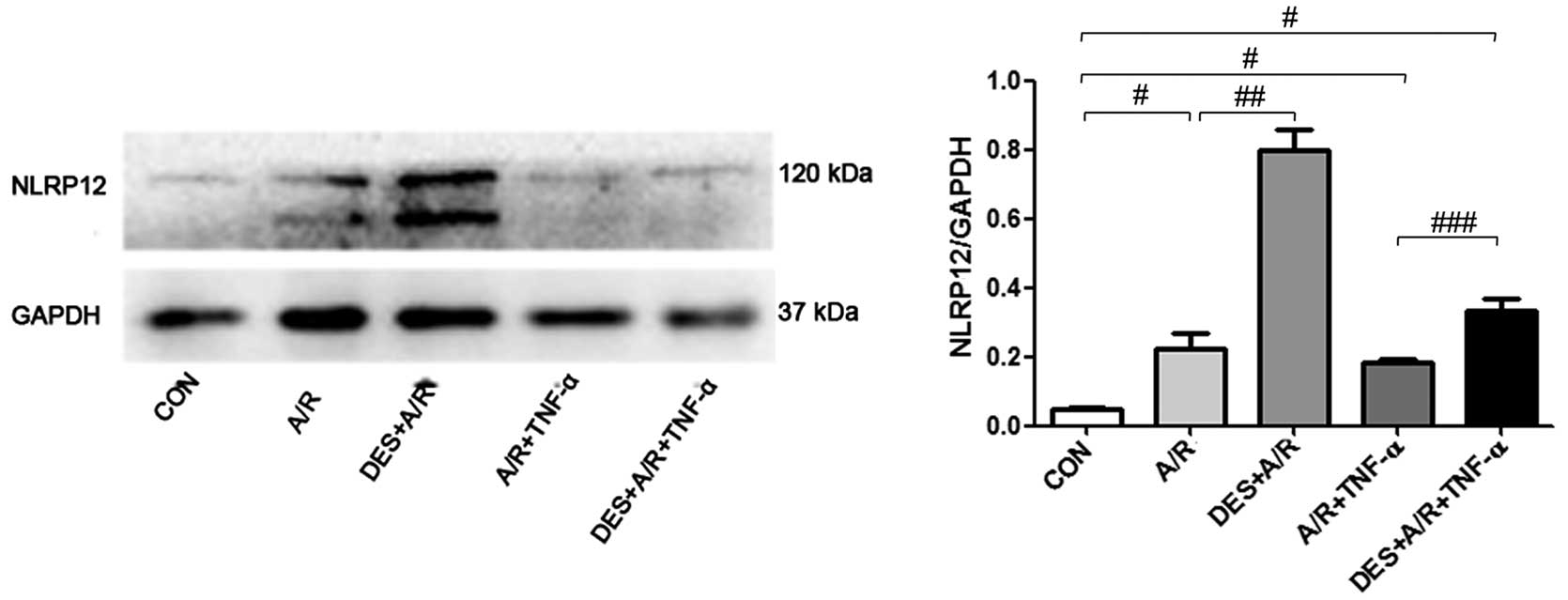
Desflurane preconditioning affects the
expression of inflammatory genes
In the HUVECs exposed to A/R or A/R plus TNF-α,
elevated protein levels of NLRP12 (a regulator of inflammation),
were detected by immunoblot analysis (Fig. 5). In addition, in the HUVECs
exposed to A/R or A/R plus TNF-α elevated mRNA levels of
interleukin (IL)-10 and NLRP12 were detected by PCR array.
Preconditioning with desflurane increased the mRNA level of IL-10
and NLRP12 in the cells exposed to A/R by 2.40- and 2.16-fold,
respectively (Table I), and
enhanced the protein level of NLRP12 (P=0.0013). In the cells
exposed to A/R or A/R plus TNF-α higher levels of NLRP12 were
noted, and desflurane preconditioning further increased the NLRP12
protein levels (P=0.0118; Fig.
5). These results suggest that the NLRP12 and IL-10 genes
associated with inflammation are invovled in the effects of
desflurane preconditioning.
 | Table IIL-10 and NLRP12 mRNA expression in
HUVECs in response to A/R and desflurane preconditioning. |
Table I
IL-10 and NLRP12 mRNA expression in
HUVECs in response to A/R and desflurane preconditioning.
| Gene symbol | Gene name | GenBank accession
no. | Description | Upregulation (fold
change) | Downregulation
(fold change) |
|---|
| IL-10 |
CSIF/IL-10/IL-10A/MGC126450/MGC126451/TGIF | NM_000572 | Interleukin 10 | 2.40 | |
| NLRP12 |
CLR19.3/FCAS2/NALP12/PAN6/PYPAF7/RNO/RNO2 | NM_033297 | NLR family, pyrin
domain containing 12 | 2.16 | |
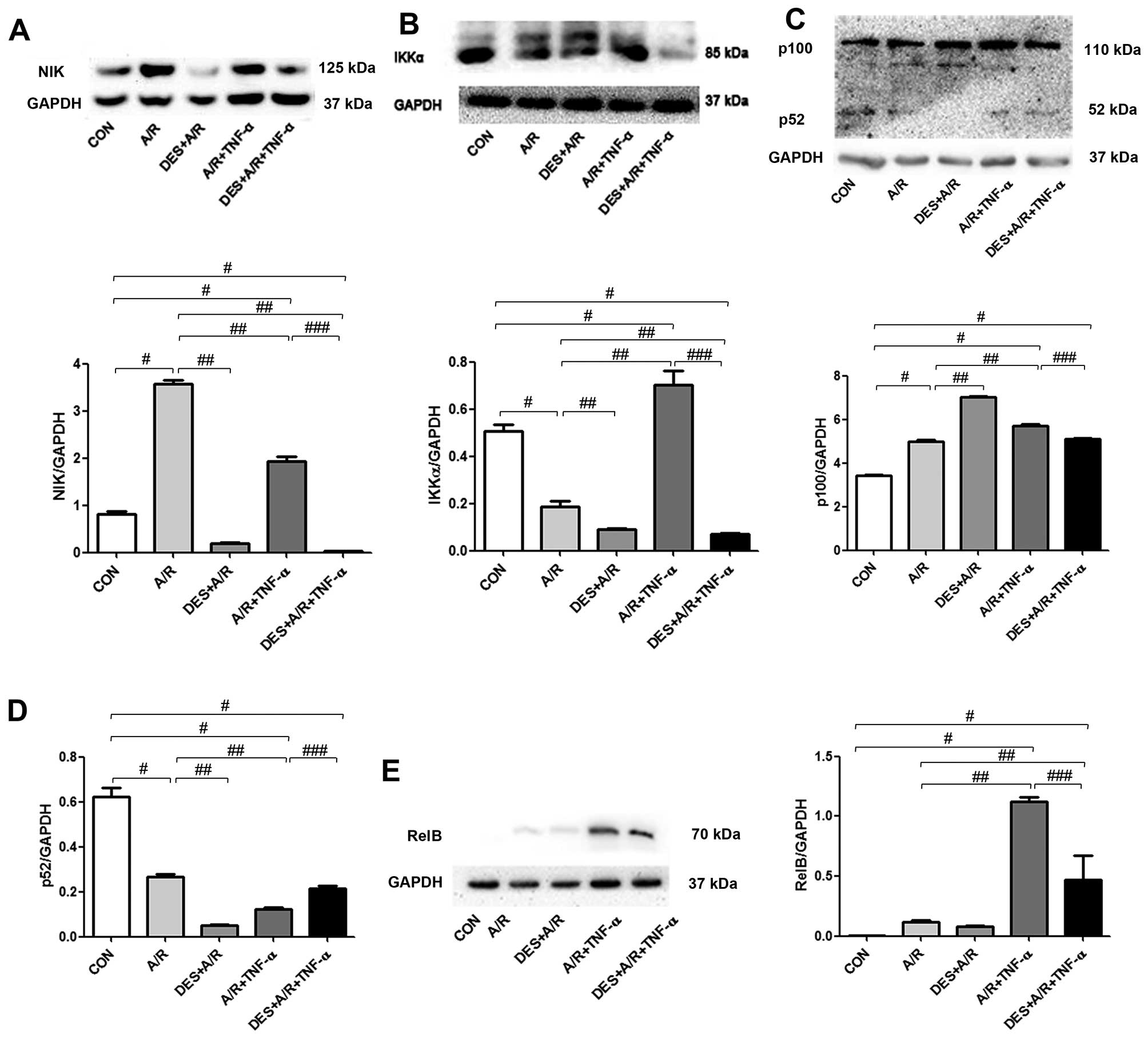
Desflurane preconditioning inhibits
the non-canonical NF-κB signaling pathway
To determine whether desflurane preconditioning has
an effect on the non-canonical NF-κB signaling pathway, we measured
the levels of NIK, IKKα, p52 and RelB in HUVECs exposed to A/R by
immunoblot analysis, as illustrated in Fig. 6. The HUVECs exposed to A/R had
greater levels of NIK and p100, and and reduced levels of p52 and
IKKα. Desflurance preconditioning reduced the level of NIK below
baseline levels, further increased p100 levels, and further reduced
p52 and IKKα levels. A/R in combination with TNF-α increased the
levels of NIK, IKKα, p100 and RelB. These changes were
significantly attenuated by desflurance preconditioning (all
P<0.05; Fig. 6).
Discussion
A/R has previously been reported to activate NF-κB
in HUVECs, with parallel increases in oxidase stress, inflammatory
responses and apoptosis (23,51–53). Apoptosis is mediated by an
increase in the permeability of the outer mitochondrial membrane
(54), leading to the release of
apoptogenic factors from the mitochondrial inter-membrane space
into the cytosol. Apoptogenic factors include Smac, which in turn
binds to and neutralizes caspase inhibitors of apoptosis proteins,
such as IAPs (57), thereby
activating caspases. A previous study indicated that desflurane
induced Aβ production and caspase activation under hypoxic
conditions (58). Our results,
however, suggest that desflurane preconditioning protects
endothelial cells by activating anti-apoptotic cIAP1 and decreasing
the expression of Smac, thus resulting in decreased levels of
apoptosis.
cIAP1 is an NF-κB responsive gene (59,60). Desflurane preconditioning has
previously been reported to activate the canonical NF-κB pathway
(17,18). However, whether volatile
anesthetics protect HUVECs against A/R injury through the
non-canonical NF-κB signaling pathway or crosstalk with other
pathways remains to be established. In the present study, with the
use of a human NF-κB signaling pathway array, we revealed that
NLRP12 expression was upregulated by desflurane
preconditioning.
It has previously been reported that NLRP12
suppresses the production of pro-inflammatory cytokines and
chemokines (55). Lich and Ting
(54) reported that NLRP12
suppressed 'non-canonical' NF-κB activation. Furthermore, this
alternative pathway is activated downstream of TLRs in addition to
the TNF family receptors (61,62). Unlike the canonical NF-κB
signaling pathway, which can be activated by multiple upstream
kinases, the non-canonical pathway is strictly dependent upon the
kinase NIK (63). Upon
activation, NIK recruits IKKα and NF-κB2/p100, which in turn leads
to the processing of p100 into its active form, p52. In the present
study, we found that desflurane preconditioning downregulated the
expression of NIK, IKKα, p52 and upregulated the expression of p100
during A/R-induced injury. These results validated those of
previous studies indicating that desflurane preconditioning
inhibits the non-canonical NF-κB signaling pathway and thus
protects cells against A/R induced cell injury (1,18).
In conclusion, in this study, we demonstrated that
desflurane preconditioning attenuated HUVEC inflammatory responses
to A/R. Desflurane preconditioning upregulated cIAP1 and NLRP12
expression, and downregulated Smac, NIK, IKKα and p52 expression,
ameliorating cellular stress processes and apoptosis during A/R. As
desflurane is increasingly applied in clinical settings, the role
of desflurane in A/R injury needs to be further characterized, in
an aim to obtain a deeper understanding of the cellular responses
to desflurane, which may support the extension of this therapeutic
strategy in the treatment of A/R-induced inflammatory
responses.
Acknowledgments
The present study was supported, in part, by a grant
from the National Natural Science Foundation of China (no.
30972838).
References
|
1
|
Yi J, Zheng Y, Miao C, Tang J and Zhu B:
Desflurane preconditioning induces oscillation of NF-κB in human
umbilical vein endothelial cells. PLoS One. 8:e665762013.
View Article : Google Scholar
|
|
2
|
Ding WG, Zhou HC, Cui XG, Li WZ, Guo YP,
Zhang B and Liu W: Anti-apoptotic effect of morphine-induced
delayed preconditioning on pulmonary artery endothelial cells with
anoxia/reoxygenation injury. Chin Med J (Engl). 121:1313–1318.
2008.
|
|
3
|
Yu EZ, Li YY, Liu XH, Kagan E and McCarron
RM: Antiapoptotic action of hypoxia-inducible factor-1 alpha in
human endothelial cells. Lab Invest. 84:553–561. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu Y, Li L, Yin W, Shen L, You B and Gao
H: Protective effect of proanthocyanidins on anoxia-reoxygenation
injury of myocardial cells mediated by the PI3K/Akt/GSK-3β pathway
and mitochondrial ATP-sensitive potassium channel. Mol Med Rep.
10:2051–2058. 2014.PubMed/NCBI
|
|
5
|
Rui T and Tang Q: IL-33 attenuates
anoxia/reoxygenation-induced cardiomyocyte apoptosis by inhibition
of PKCβ/JNK pathway. PLoS One. 8:e560892013. View Article : Google Scholar
|
|
6
|
Zhang C, Lin G, Wan W, Li X, Zeng B, Yang
B and Huang C: Resveratrol, a polyphenol phytoalexin, protects
cardiomyocytes against anoxia/reoxygenation injury via the
TLR4/NF-κB signaling pathway. Int J Mol Med. 29:557–563.
2012.PubMed/NCBI
|
|
7
|
Li WJ, Nie SP, Chen Y, Xie MY, He M, Yu Q
and Yan Y: Ganoderma atrum polysaccharide protects cardiomyocytes
against anoxia/reoxygenation-induced oxidative stress by
mitochondrial pathway. J Cell Biochem. 110:191–200. 2010.PubMed/NCBI
|
|
8
|
Zaugg M, Lucchinetti E, Garcia C, Pasch T,
Spahn DR and Schaub MC: Anaesthetics and cardiac preconditioning.
Part II. Clinical implications. Br J Anaesth. 91:566–576. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zaugg M, Lucchinetti E, Uecker M, Pasch T
and Schaub MC: Anaesthetics and cardiac preconditioning. Part I.
Signalling and cytoprotective mechanisms. Br J Anaesth. 91:551–565.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Piriou V, Chiari P, Lhuillier F, Bastien
O, Loufoua J, Raisky O, David JS, Ovize M and Lehot JJ:
Pharmacological preconditioning: comparison of desflurane,
sevoflurane, isoflurane and halothane in rabbit myocardium. Br J
Anaesth. 89:486–491. 2002.PubMed/NCBI
|
|
11
|
Haelewyn B, Zhu L, Hanouz JL, Persehaye E,
Roussel S, Ducouret P and Gérard JL: Cardioprotective effects of
desflurane: effect of timing and duration of administration in rat
myocardium. Br J Anaesth. 92:552–557. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Suleiman MS, Zacharowski K and Angelini
GD: Inflammatory response and cardioprotection during open-heart
surgery: the importance of anaesthetics. Br J Pharmacol. 153:21–33.
2008. View Article : Google Scholar
|
|
13
|
Wang H, Lu S, Yu Q, Liang W, Gao H, Li P,
Gan Y, Chen J and Gao Y: Sevoflurane preconditioning confers
neuroprotection via anti-inflammatory effects. Front Biosci (Elite
Ed). 3:604–615. 2011. View
Article : Google Scholar
|
|
14
|
Boost KA, Flondor M, Hofstetter C,
Platacis I, Stegewerth K, Hoegl S, Nguyen T, Muhl H and Zwissler B:
The beta-adrenoceptor antagonist propranolol counteracts
anti-inflammatory effects of isoflurane in rat endotoxemia. Acta
Anaesthesiol Scand. 51:900–908. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang Y, Li Z, Mo N, Li M, Zhuang Z, Wang
J, Wang Y and Guo X: Isoflurane preconditioning ameliorates renal
ischemia-reperfusion injury through antiinflammatory and
antiapoptotic actions in rats. Biol Pharm Bull. 37:1599–1605. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bedirli N, Demirtas CY, Akkaya T, Salman
B, Alper M, Bedirli A and Pasaoglu H: Volatile anesthetic
preconditioning attenuated sepsis induced lung inflammation. J Surg
Res. 178:e17–e23. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Biao Z, Zhanggang X, Hao J, Changhong M
and Jing C: The in vitro effect of desflurane preconditioning on
endothelial adhesion molecules and mRNA expression. Anesth Analg.
100:1007–1013. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Y, Zhang X, Zhu B and Xue Z: Desflurane
preconditioning inhibits endothelial nuclear factor-kappa-B
activation by targeting the proximal end of tumor necrosis
factor-alpha signaling. Anesth Analg. 106:1473–1479. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kopp EB and Ghosh S: NF-kappaB and rel
proteins in innate immunity. Adv Immunol. 58:1–27. 1995. View Article : Google Scholar
|
|
20
|
Tsung A, Hoffman RA, Izuishi K, Critchlow
ND, Nakao A, Chan MH, Lotze MT, Geller DA and Billiar TR: Hepatic
ischemia/reperfusion injury involves functional TLR4 signaling in
nonparenchymal cells. J Immunol. 175:7661–7668. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Donnahoo KK, Meldrum DR, Shenkar R, Chung
CS, Abraham E and Harken AH: Early renal ischemia, with or without
reperfusion, activates NFkappaB and increases TNF-alpha bioactivity
in the kidney. J Urol. 163:1328–1332. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baeuerle PA and Baltimore D: NF-kappaB:
ten years after. Cell. 87:13–20. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kokura S, Wolf RE, Yoshikawa T, Granger DN
and Aw TY: T-lymphocyte-derived tumor necrosis factor exacerbates
anoxiareoxygenation-induced neutrophil-endothelial cell adhesion.
Circ Res. 86:205–213. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Karakurum M, Shreeniwas R, Chen J, Pinsky
D, Yan SD, Anderson M, Sunouchi K, Major J, Hamilton T and Kuwabara
K: Hypoxic induction of interleukin-8 gene expression in human
endothelial cells. J Clin Invest. 93:1564–1570. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Loop T, Dovi-Akue D, Frick M, Roesslein M,
Egger L, Humar M, Hoetzel A, Schmidt R, Borner C, Pahl HL, et al:
Volatile anesthetics induce caspase-dependent,
mitochondria-mediated apoptosis in human T lymphocytes in vitro.
Anesthesiology. 102:1147–1157. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Coope HJ, Atkinson PG, Huhse B, Belich M,
Janzen J, Holman MJ, Klaus GG, Johnston LH and Ley SC: CD40
regulates the processing of NF-kappaB2 p100 to p52. EMBO J.
21:5375–5385. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ganeff C, Remouchamps C, Boutaffala L,
Benezech C, Galopin G, Vandepaer S, Bouillenne F, Ormenese S,
Chariot A, Schneider P, et al: Induction of the alternative NF-κB
pathway by lymphotoxin αβ (LTαβ) relies on internalization of LTβ
receptor. Mol Cell Biol. 31:4319–4334. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Claudio E, Brown K, Park S, Wang H and
Siebenlist U: BAFF-induced NEMO-independent processing of NF-kappa
B2 in maturing B cells. Nat Immunol. 3:958–965. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dejardin E: The alternative NF-kappaB
pathway from biochemistry to biology: pitfalls and promises
forfuture drug development. Biochem Pharmacol. 72:1161–1179. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zaki MH, Boyd KL, Vogel P, Kastan MB,
Lamkanfi M and Kanneganti TD: The NLRP3 inflammasome protects
against loss of epithelial integrity and mortality during
experimental colitis. Immunity. 32:379–391. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Allen IC, TeKippe EM, Woodford RM, Uronis
JM, Holl EK, Rogers AB, Herfarth HH, Jobin C and Ting JP: The NLRP3
inflammasome functions as a negative regulator of tumorigenesis
during colitis-associated cancer. J Exp Med. 207:1045–1056. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hu B, Elinav E, Huber S, Strowig T, Hao L,
Hafemann A, Jin C, Wunderlich C, Wunderlich T, Eisenbarth SC and
Flavell RA: Microbiota-induced activation of epithelial IL-6
signaling links inflammasome-driven inflammation with transmissible
cancer. Proc Natl Acad Sci USA. 110:9862–9867. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hu B, Elinav E, Huber S, Booth CJ, Strowig
T, Jin C, Eisenbarth SC and Flavell RA: Inflammation-induced
tumorigenesis in the colon is regulated by caspase-1 and NLRC4.
Proc Natl Acad Sci USA. 107:21635–21640. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen GY: Role of Nlrp6 and Nlrp12 in the
maintenance of intestinal homeostasis. Eur J Immunol. 44:321–327.
2014. View Article : Google Scholar :
|
|
35
|
Zhang L, Mo J, Swanson KV, Wen H,
Petrucelli A, Gregory SM, Zhang Z, Schneider M, Jiang Y, Fitzgerald
KA, et al: NLRC3, a member of the NLR family of proteins, is a
negative regulator of innate immune signaling induced by the DNA
sensor STING. Immunity. 40:329–341. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xia X, Cui J, Wang HY, Zhu L, Matsueda S,
Wang Q, Yang X, Hong J, Songyang Z, Chen ZJ and Wang RF: NLRX1
negatively regulates TLR-induced NF-kappaB signaling by targeting
TRAF6 and IKK. Immunity. 34:843–853. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lich JD, Williams KL, Moore CB, Arthur JC,
Davis BK, Taxman DJ and Ting JP: Monarch-1 suppresses non-canonical
NF-kappaB activation and p52-dependent chemokine expression in
monocytes. Journal of immunology. 178:1256–1260. 2007. View Article : Google Scholar
|
|
38
|
Wang L, Manji GA, Grenier JM, Al-Garawi A,
Merriam S, Lora JM, Geddes BJ, Briskin M, DiStefano PS and Bertin
J: PYPAF7, a novel PYRIN-containing Apaf1-like protein that
regulates activation of NF-kappa B and caspase-1-dependent cytokine
processing. J Biol Chem. 277:29874–29880. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vladimer GI, Weng D, Paquette SW, Vanaja
SK, Rathinam VA, Aune MH, Conlon JE, Burbage JJ, Proulx MK and Liu
Q: The NLRP12 inflammasome recognizes Yersinia pestis. Immunity.
37:96–107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ataide MA, Andrade WA, Zamboni DS, Wang D,
Souza Mdo C, Franklin BS, Elian S, Martins FS, Pereira D, Reed G,
et al: Malaria-induced NLRP12/NLRP3-dependent caspase-1 activation
mediates inflammation and hypersensitivity to bacterial
superinfection. PLoS Pathog. 10:e10038852014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Allen IC, McElvania-TeKippe E, Wilson JE,
Lich JD, Arthur JC, Sullivan JT, Braunstein M and Ting JP:
Characterization of NLRP12 during the in vivo host immune response
to Klebsiella pneumoniae and Mycobacterium tuberculosis. PloS One.
8:e608422013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Allen IC, Wilson JE, Schneider M, Lich JD,
Roberts RA, Arthur JC, Woodford RM, Davis BK, Uronis JM, Herfarth
HH, et al: NLRP12 suppresses colon inflammation and tumorigenesis
through the negative regulation of noncanonical NF-kappaB
signaling. Immunity. 36:742–754. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Allen IC, Lich JD, Arthur JC, et al:
Characterization of NLRP12 during the development of allergic
airway disease in mice. PloS one. 7:e306122012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zaki MH, Vogel P, Malireddi RK,
Body-Malapel M, Anand PK, Bertin J, Green DR, Lamkanfi M and
Kanneganti TD: The NOD-like receptor NLRP12 attenuates colon
inflammation and tumorigenesis. Cancer Cell. 20:649–660. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pinheiro AS, Eibl C, Ekman-Vural Z,
Schwarzenbacher R and Peti W: The NLRP12 pyrin domain: structure,
dynamics, and functional insights. J Mol Biol. 413:790–803. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Arthur JC, Lich JD, Ye Z, Allen IC, Gris
D, Wilson JE, Schneider M, Roney KE, O'Connor BP and Moore CB:
Cutting edge: NLRP12 controls dendritic and myeloid cell migration
to affect contact hypersensitivity. J Immunol. 185:4515–4519. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Baudin B, Bruneel A, Bosselut N and
Vaubourdolle M: A protocol for isolation and culture of human
umbilical vein endothelial cells. Nat Protoc. 2:481–485. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Takahashi K, Sawasaki Y, Hata J, Mukai K
and Goto T: Spontaneous transformation and immortalization of human
endothelial cells. In Vitro Cell Dev Biol. 26:265–274. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Koyama T, Temma K and Akera T:
Reperfusion-induced contracture develops with a decreasing [Ca2+]i
in single heart cells. Am J Physiol. 261:H1115–H1122.
1991.PubMed/NCBI
|
|
50
|
Mouithys-Mickalad A, Mathy-Hartert M, Du
G, Sluse F, Deby C, Lamy M and Deby-Dupont G: Oxygen consumption
and electron spin resonance studies of free radical production by
alveolar cells exposed to anoxia: inhibiting effects of the
antibiotic ceftazidime. Redox Rep. 7:85–94. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ichikawa H, Flores S, Kvietys PR, Wolf RE,
Yoshikawa T, Granger DN and Aw TY: Molecular mechanisms of
anoxia/reoxygenation-induced neutrophil adherence to cultured
endothelial cells. Circ Res. 81:922–931. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cepinskas G1, Lush CW and Kvietys PR:
Anoxia/reoxygenation-induced tolerance with respect to
polymorphonuclear leukocyte adhesion to cultured endothelial cells.
A nuclear factor-kappaB-mediated phenomenon. Circ Res. 84:103–12.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rupin A, Paysant J, Sansilvestri-Morel P,
Lembrez N, Lacoste JM, Cordi A and Verbeuren TJ: Role of NADPH
oxidase-mediated superoxide production in the regulation of
E-selectin expression by endothelial cells subjected to
anoxia/reoxygenation. Cardiovasc Res. 63:323–30. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lich JD and Ting JP: Monarch-1/PYPAF7 and
other CATERPILLER (CLR, NOD, NLR) proteins with negative regulatory
functions. Microbes Infect. 9:672–676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Williams KL, Lich JD, Duncan JA, Reed W,
Rallabhandi P, Moore C, Kurtz S, Coffield VM, Accavitti-Loper MA,
Su L, et al: The CATERPILLER protein monarch-1 is an antagonist of
toll-like receptor-, tumor necrosis factor alpha-, and
Mycobacterium tuberculosis-induced pro-inflammatory signals. J Biol
Chem. 280:39914–39924. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Green DR and Kroemer G: The
pathophysiology of mitochondrial cell death. Science. 305:626–629.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hennessy EJ, Saeh JC, Sha L, MacIntyre T,
Wang H, Larsen NA, Aquila BM, Ferguson AD, Laing NM and Omer CA:
Discovery of aminopiperidine-based Smac mimetics as IAP
antagonists. Bioorg Med Chem Lett. 22:1690–1694. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang B, Dong Y, Zhang G, Moir RD, Xia W,
Yue Y, Tian M, Culley DJ, Crosby G, Tanzi RE and Xie Z: The
inhalation anesthetic desflurane induces caspase activation and
increases amyloid beta-protein levels under hypoxic conditions. J
Biol Chem. 283:11866–11875. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mahoney DJ, Cheung HH, Mrad RL, Plenchette
S, Simard C, Enwere E, Arora V, Mak TW, Lacasse EC, Waring J and
Korneluk RG: Both cIAP1 and cIAP2 regulate TNFalpha-mediated
NF-kappaB activation. Proc Natl Acad Sci USA. 105:11778–11783.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Varfolomeev E and Vucic D: (Un)expected
roles of c-IAPs in apoptotic and NF-kappaB signaling pathways. Cell
Cycle. 7:1511–1521. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Vatsyayan J, Qing G, Xiao G and Hu J:
SUMO1 modification of NF-kappaB2/p100 is essential for
stimuli-induced p100 phosphorylation and processing. EMBO Rep.
9:885–890. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Heusch M, Lin L, Geleziunas R and Greene
WC: The generation of nfkb2 p52: mechanism and efficiency.
Oncogene. 18:64–6208. 1999. View Article : Google Scholar
|
|
63
|
Xiao G, Fong A and Sun SC: Induction of
p100 processing by NF-kappaB-inducing kinase involves docking
IkappaB kinase alpha (IKKalpha) to p100 and IKKalpha-mediated
phosphorylation. J Biol Chem. 279:30099–30105. 2004. View Article : Google Scholar : PubMed/NCBI
|