Introduction
Lung cancer is a leading cause of cancer mortality
worldwide. Lung cancer is generally divided into non-small cell
lung cancer cells (NSCLC) and SCLC, in which the NSCLC constitutes
80–85% of lung cancers. The three major NSCLC subtypes are
adenocarcinoma, squamous cell carcinoma and large cell carcinoma.
NSCLC, which is characterized by slow tumor cell growth and
dissemination, is refractory to chemotherapy and chest
radiotherapy. Several driven genes have been identified in NSCLC,
such as EGFR, c-MET and the ALK-EML4 fusion
gene (1). Treatment for lung
cancer is mainly based on tumor stage, tumor pathology and
molecular pathology. Understanding thoroughly the molecular
mechanism underlying the aberrant cellular events driving lung
cancer progressions is crucial for developing novel treatments.
Mammary serine protease inhibitor (maspin), also
known as Serpin B5, was first identified in 1994 (2). Maspin belongs to the serine protease
inhibitor superfamily, and is predominantly localized in the
cytoplasm, but is also localized in the nucleus and membrane, and
is secreted. The exact roles of maspin in cancer are far more
complicated than initially thought due to the conflicting
experimental and clinical data that have been reported. In prostate
cancer, the expression of maspin is frequently absent (3). Overexpression of maspin is
considered an independent factor in predicting a favorable
prognosis in breast cancer and lung squamous cell cancer (4–6).
However, it has been also reported that the increased nuclear
maspin expression predicts a favorable prognosis, whereas the
enhanced cytoplasmic expression is associated with early-relapsing
and unfavorable prognosis in breast cancer (7). Enhanced expression of maspin is
correlated with unfavorable prognosis in pancreatic, ovarian and
colorectal cancer (8–10). High maspin expression correlates
with an unfavorable outcome in colorectal cancer in stage III and
IV, suggesting that maspin may have a stage-specific function
possibly associated with cancer cell dissemination and/or
metastatic outgrowth (11). The
maspin expression in the cytoplasm has recently been reported as an
independent unfavorable prognostic indicator of patients with lung
adenocarcinoma with tumor size <3 cm (12). The complexity in the clinical
significance of maspin expression indicates that maspin expression
is possibly influenced by a variety of factors including cell type,
genetic background and endogenous expression. The downregulated
maspin expression may be due to aberrant cytosine methylation and
chromatin condensation of the maspin promoter in cancer cells
(13). A recent bioinformatic
study suggests that maspin is not commonly underexpressed in
cancer, and the perturbation of genes near maspin, such as
PHLPP1, may explain the poor survival in patients with low
maspin expression (14).
However, it is generally believed that maspin
functions as a tumor suppressor. Maspin suppresses multiple
malignant behaviors of cancer cells, including cell proliferation,
apoptosis, migration, invasion and angiogenesis. Maspin inhibits
breast cancer progression by increased apoptosis. Increased maspin
expression sensitizes the apoptotic response of tumor cells to
various chemical reagents (15,16). The effect of maspin on apoptosis
originates from the cytoplasmic fraction and is mediated by Bax
(17). Overexpression of Bax also
enhances apoptosis through the mitochondrial permeability
transition. A mitochondrial death signaling pathway is induced that
involves the localization of maspin to the mitochondria in breast
cancer cells (18).
Overexpression of maspin sensitizes prostate cancer cells to
apoptosis by inhibiting hypoxia-induced AKT activation (19). Maspin inhibits tumor metastasis by
suppressing the invasion and motility abilities in breast and
prostate cancer cells (20).
Maspin could inhibit cancer-induced bone matrix remodeling and
induce prostate cancer cell re-differentiation in vivo
(21). Maspin regulates the cell
surface-bound uPA/uPAR-dependent cell detachment in prostate cancer
cells (20,22). Maspin can directly bind to
integrin β1, leading to the inactivation of integrin β1 and
inhibition of the migration ability in cancer cells (23).
Maspin expression is significantly higher in NSCLC,
such as squamous cell carcinoma and adenocarcinoma, compared to in
SCLC (24). In NSCLC patients,
the expression of maspin is also an independent prognostic factor.
A statistically significant longer overall survival has been found
in patients with a higher expression of nuclear maspin, and
unfavorable prognosis is present in patients with a higher
intensity of cytoplasmic maspin expression (25). It has also been reported that
nuclear maspin, but not the maspin expression in both the nucleus
and cytoplasm, correlates with improved survival of lung
adenocarcinoma, and maspin nuclear localization inversely
correlates with vascular endothelial growth factor-A (26). Maspin is a molecular target of
p63, which is a critical factor for cell invasion and progression
in the absence of wild-type p53 or in the presence of mutant p53 in
NSCLC cells (27). High frequency
of co-expression of maspin and p63, as well as maspin and p53, has
been detected in squamous cell carcinoma (28). Restoration of maspin expression
suppresses cell invasion in NCSLC cells (29).
The aim of the present study was to understand the
function of maspin in different NSCLC cell lines. The etopic
expression of maspin was established successfully in the A549 and
SPC-A1 cell lines. Multiple cellular functions were investigated,
including cell growth, migration and invasion. The expression of
maspin in NSCLC, in particular, the adenocarcinoma cell lines, was
heterogeneous. While the expression of maspin was almost absent in
the A549 and SPC-A1 cells, the expression of maspin in the PC-9 and
H460 cell lines was intact. Ectopic expression of maspin in A549
cells significantly inhibited cell migration and invasion
abilities; however, ectopic expression of maspin in SPC-A1 cells
affected cell growth via targeting the AKT signaling molecules.
Materials and methods
Tissue culture
Cell lines PC-9, A549, SPC-A1 and H460 were cultured
in RPMI-1640 (HyClone, Logan, UT, USA) supplemented with 10% fetal
bovine serum (FBS), 100 U/ml penicillin, 100 µg/ml
streptomycin and 2 mM glutamine. All the cells were cultured at
37°C in a humidified atmosphere of 5% CO2.
Cell transfection
The maspin overexpression vector was constructed on
the backbone of the MSCV vector. Full-length coding sequences (CDS)
of maspin was amplified by polymerase chain reaction (PCR) from
human normal breast tissue and cloned into the MSCV vector
(MSCV-hMaspin). Phoenix A packaging cells were transfected with
MSCV-hMaspin or MSCV by FuGENE HD (Roche, Beijing, China). Virus
supernatants were collected and target cells were infected with the
virus supernatants. For obtaining stable maspin-expressing cells,
the cells were selected for two weeks in the presence of puromycin
(5 µg/ml).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated using TRIzol reagent (Tiangen
Biotech Co., Ltd., Beijing, China) according to the manufacturer's
instructions. RNA yield and purity were determined by NanoDrop 1000
(Thermo Fisher Scientific, Suzhou, China). Equivalent amounts of
RNA (2 µg) were reverse-transcribed with SuperScript M-MLV
(Promega, Shanghai, China). Triplicates were performed for all
RT-qPCR reactions with a LightCycler 480 System (Roche). Primers
for RT-qPCR were designed using Primer-BLAST (PubMed). Primers were
synthesized from Invitrogen (Beijing, China). The reference
(β-actin) and target genes were run together. The reaction with was
set up with 2X LC480 SYBR-Green I Master mix (Roche), according to
the manufacturer's instructions. For data analysis, a target gene
transcript was quantified in comparison to the reference gene
(β-actin).
Western blot analysis
Whole-cell extracts were prepared with
radioimmunoprecipitation assay buffer according to the standard
instructions. Extracts (5 µg) were separated on an SDS-PAGE
gel and transferred to nitrocellulose membranes. Following
blocking, membranes were probed with individual antibodies (Abs).
Membranes were washed and further probed with an appropriate
secondary Ab. Proteins were detected and scanned with an Odyssey
system (LI-COR Biosciences, Lincoln, NE, USA). Band density was
normalized to the tubulin or β-actin reference. Abs against uPA
(sc-14019) were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Human maspin Abs (554292) was purchased from BD
Pharmingen (San Diego, CA, USA). Abs against ERK (#4695), AKT
(#4691), p-AKT (ser473, #4060), p-PDK1 (#3438) and intergrin-β1
(#9699) were obtained from Cell Signaling Technology (Beverly, MA,
USA). β-tubulin (SAM1002) were purchased from Sunshine Bio Science
and Technology Co., Ltd. (Guangzhou, China). β-actin (AT0001) was
purchased from CMCTAG (Milwaukee, WI, USA).
Cell growth assay
The cell growth assay was performed with the
xCELLigence RTCA instrument (Roche). In the assay, impedance for
indicated times was continuously monitored by the system, and the
value was indicated as 'cell index', which was determined by the
number of cells seeded, the overall size and morphology of the
cells, and the degree to which the cells interact with the sensor
surface. The assay was set up according to the manufacturer's
instructions. Following running the background blank with 100
µl RPMI-1640 supplemented with 10% FBS in each well of the
E-Plate, cells were seeded in wells (8,000 cells/well for A549
cells and 25,000 cells/well for SPC-A1 cells) and the program was
run. The cell index was continuously monitored by the system, and
data was collected and analyzed by RTCA software 1.2.
Cell migration and invasion assay
The cell migration assay was also performed with the
xCELLigence RTCA instrument. In this assay, a CIM-plate assembled
with an upper and lower chamber was used. RPMI-1640 (180 µl)
supplemented with 10% FBS was added to each well on the lower
chamber. Cells were suspended in the serum-free media and added
into the upper chamber. Following attachment, cell migration
towards the lower chamber containing RPMI-1640 supplemented with
10% FBS was continuously monitored, and data was collected and
analyzed by RTCA software 1.2. For the cell invasion assay, wells
of the upper chamber were pre-coated with Matrigel (cat. no.
356234; BD Biosciences, Shanghai, China) for ≥4 h.
Gel electrophoresis and zymography
Cells were plated in 100-mm dishes until a 70–80%
confluence was reached. Cells were rinsed twice with
phosphate-buffered saline and fed with serum-free medium.
Conditioned medium was collected after 24 h and centrifuged to
remove the cell debris. The conditioned medium was subsequently
mixed with non-reducing sample buffer (without β-mercaptoethanol),
and loaded on an 8% SDS-PAGE gel containing 0.1% gelatin. Following
electrophoresis, the gels were washed twice in 2.5% Triton X-100
for 30 min at room temperature to remove SDS. The gels were
incubated at 37°C overnight in substrate buffer containing 50 mM
Tris-HCl and 10 mM CaCl2 (pH 8.0), and stained with 0.5%
Coomassie blue R250 in 50% methanol and 10% glacial acetic acid for
30 min and destained. Upon renaturation of the enzyme, the
gelatinases digested the gelatin in the gel to produce clear bands
against an intensely stained background.
Cell cycle analysis
In order to estimate cell cycle, cells were detected
using flow cytometry. After 48-h continuous culture, cells were
harvested and fixed with 70% ethanol for 24 h at 4°C. Subsequently,
the single cell suspensions were prepared to stain DNA using
propidium iodide staining, based on the manufacturer's
instructions. Cell cycle was measured by FACSCalibur (BD
Biosciences) with at least three independent experiments
performed.
Statistical analysis
All the experiments were repeated at least three
times. The data are expressed as the mean ± standard deviation from
experiments in replicate. All the statistical analysis was
performed by GraphPad software (GraphPad Software, La Jolla, CA,
USA). The differences between groups were evaluated using the
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Analysis of maspin expression in
individual lung cancer cell lines
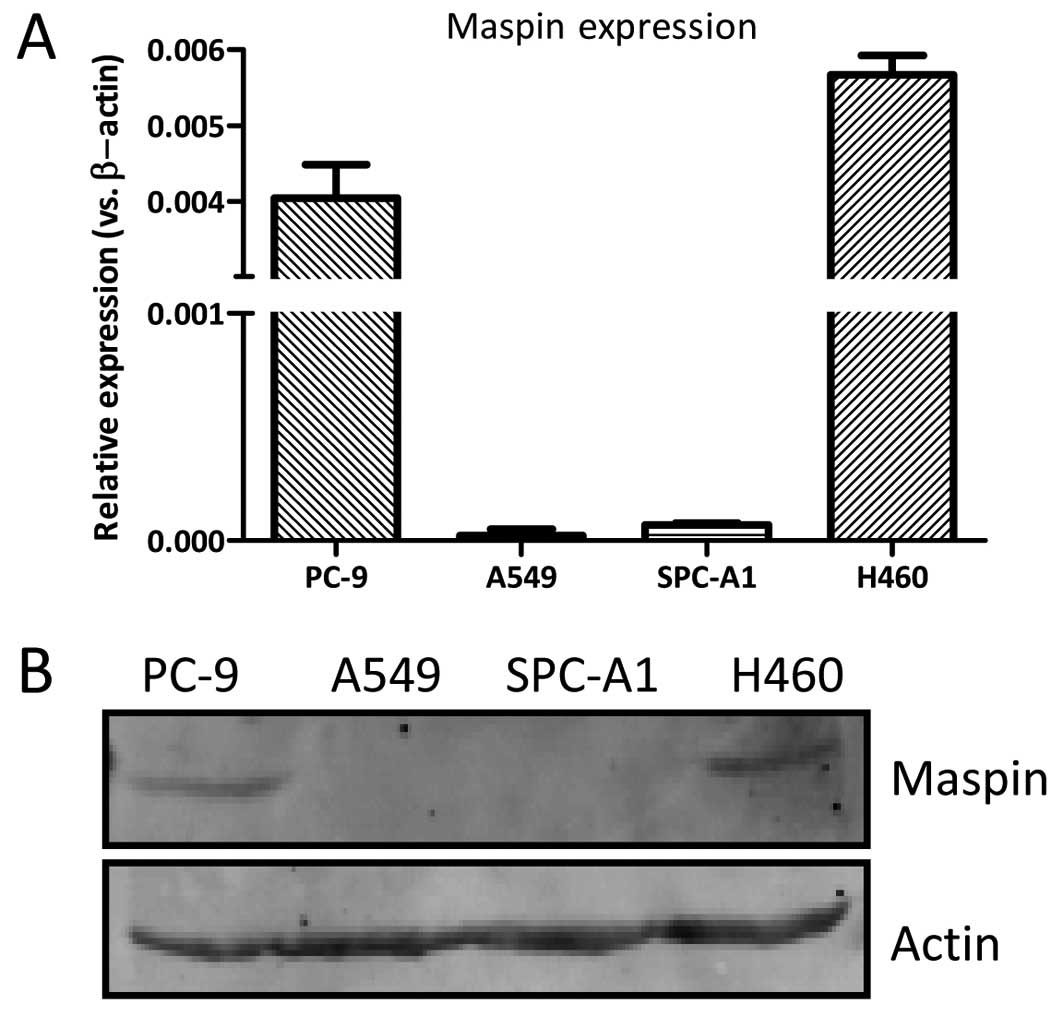
The maspin mRNA expression level was measured in the
NSCLC PC-9, A549, SPC-A1 and H460 cells by RT-qPCR. As shown in
Fig. 1, the maspin expression was
detected in the H460 and PC-9 cells, while it was barely detected
in the A549 and SPC-A1 cells. The protein expression of maspin in
the H460 and PC-9 cells was detectable, while the maspin expression
in the A549 and SPC-A1 cells could not be detected. The expression
of maspin was comparable in the H460 and PC-9 cells.
Establishing maspin-overexpressing cell
lines
In order to generate a stable maspin-overexpressing
cell line, a maspin expression vector was constructed on the
backbone of the MSCV vector. Full-length CDS of the maspin gene was
amplified by PCR and cloned into the MSCV vector (MSCV-hMaspin).
Phoenix A packaging cells were transfected with MSCV-hMaspin or the
control vector (MSCV), and followed by infecting target cells A549
(A549-hMaspin and A549-ctrl) and SPC-A1 (SPC-A1-hMaspin and
SPC-A1-ctrl) with the virus supernatants, respectively. The stably
transfected cell lines were established by puromycin selection for
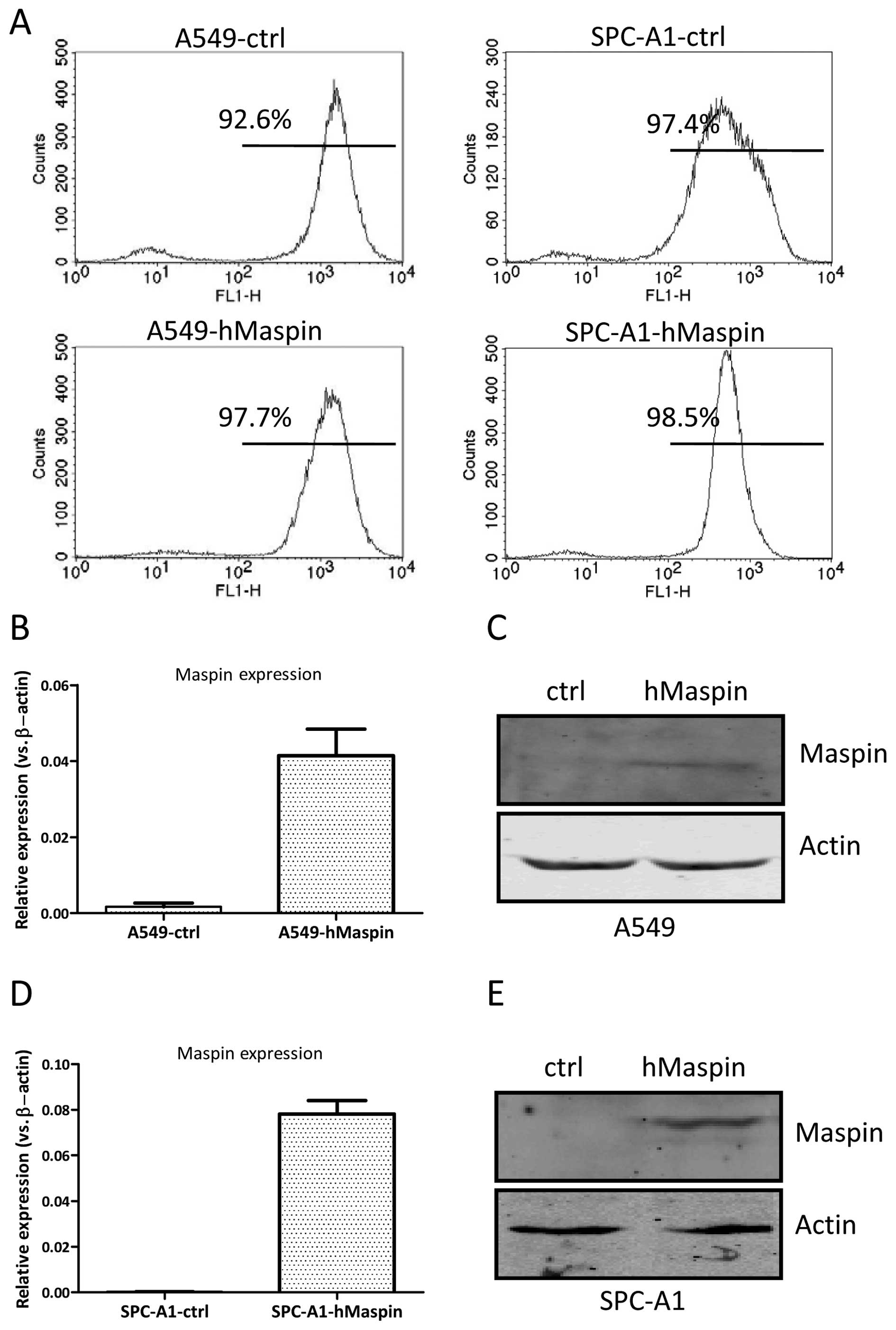
two weeks. As shown in Fig. 2A,
the purities of the established cells were measured by the
frequencies of green fluorescent protein expression by flow
cytometry analysis, and were 97.7 and 96.2% for A549-hMaspin and
A549-ctrl cells, and 98.5 and 97.4% for SPC-A1-hMaspin and
SPC-A1-ctrl cells, respectively.
The selected monoclones were further expanded and
examined for maspin expression by RT-qPCR. The maspin mRNA
expression level was increased ~20-fold in A549-hMaspin cells,
compared to that of the A549-ctrl cells (Fig. 2B). The maspin expression in the
whole-cell extracts of the A549-hMaspin cells became detectable
(Fig. 2C). Similarly, the maspin
mRNA expression level was increased ~100-fold in SPC-A1-hMaspin
cells, compared to that of the SPC-A1-ctrl cells (Fig. 2D). The maspin expression in the
whole-cell extracts of the SPC-A1-hMaspin cells could also be
detected (Fig. 2E). Taken
together, these results indicate that the stable maspin
overexpressing cell lines, A549-hMaspin and SPC-A1-hMaspin, were
established successfully.
Maspin overexpression does not affect
A549 cell growth
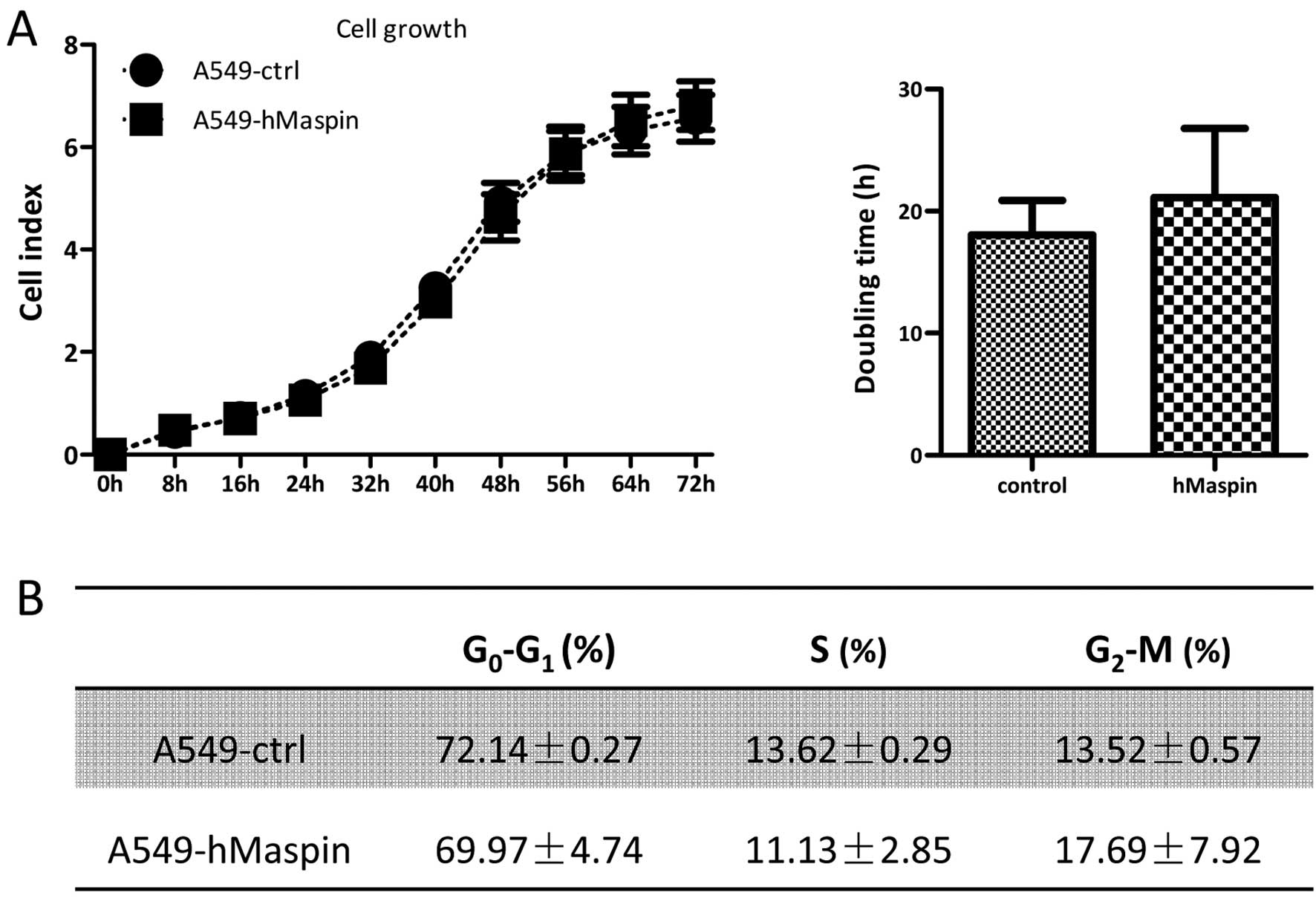
In order to test whether the overexpression of
maspin may affect cell growth, a real-time xCELLigence system using
E-plates was carried out on the A549-ctrl and A549-hMaspin cells.
As shown in Fig. 3A, there was no
significant difference between the cell index of A549-hMaspin and
A549-ctrl cells during the 72-h continuous monitoring. The doubling
times of A549-hMaspin and A549-ctrl were 21.12±5.66 and 18.5±2.82
h, respectively. The cell cycle analysis and cellular DNA content
measurement were examined by flow cytometry, and the distributions
of G0-G1, S and G2-M phases in the
A549-ctrl and A549-hMaspin cells were comparable (Fig. 3B).
Maspin overexpression attenuates the
migration and invasion abilities of the A549 cells
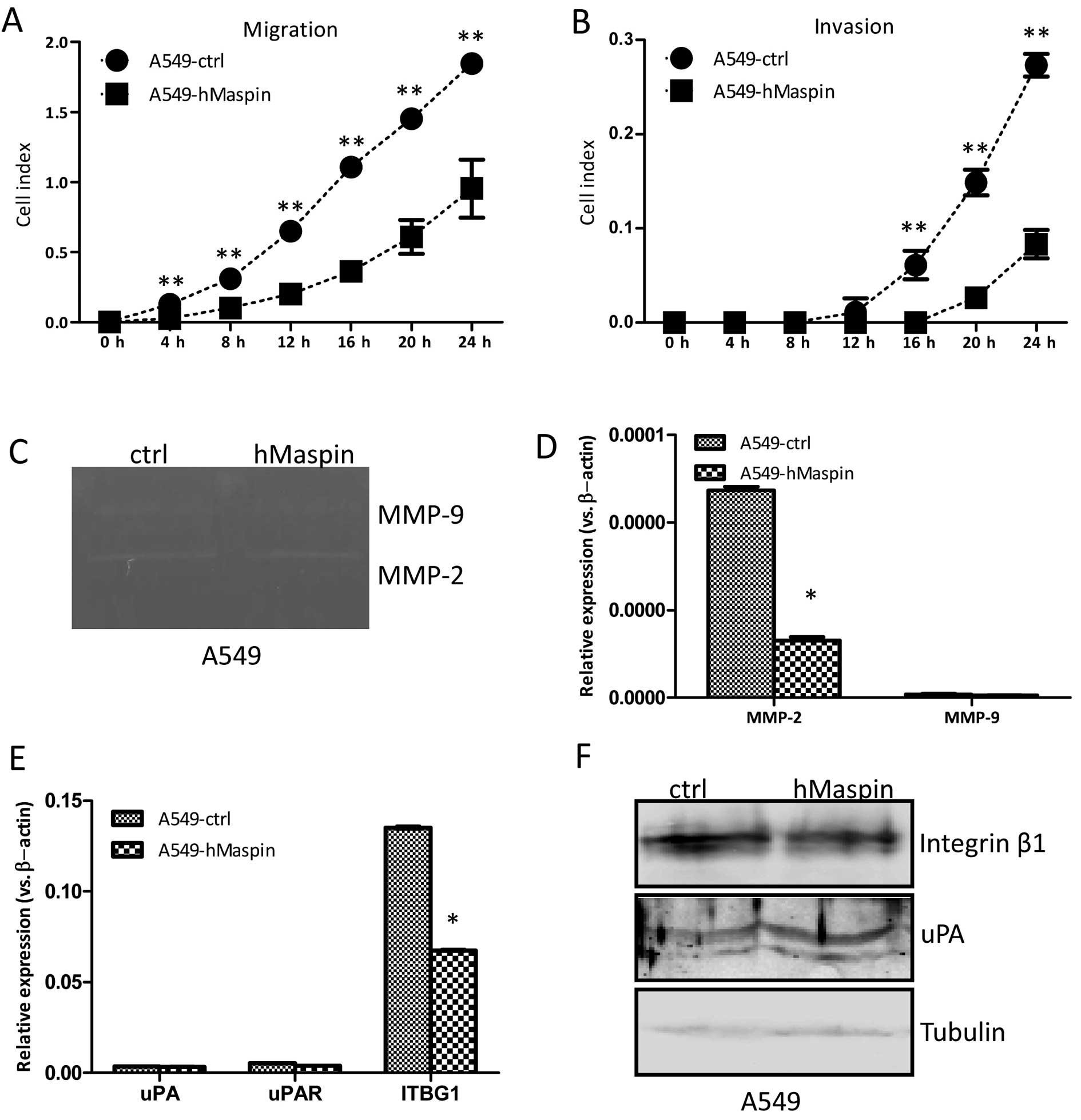
The migration ability affected by the overexpression
of maspin was examined by the real-time xCELLigence system using
CIM-plates. After 8-h culturing, the A549 cells overexpressing
maspin migrated significantly slower compared to the A549-ctrl
cells, and there was a significantly statistical difference in the
migration assay between the two established cell lines (Fig. 4A).
The invasion ability affected by the overexpression
of maspin was also measured by the xCELLigence system using
matrigel (dilution at 1:30)-coated CIM-plates. After 16 h
culturing, the A549 cells overexpressing maspin invaded through the
Matrigel slower compared to the A549-ctrl cells, and there were
statistically significant differences in the invasion ability
between the two established cell lines (Fig. 4B). The gelatin zymography assay
was further performed to explore the relative amounts of active
matrix metalloproteinases (MMPs). As shown in Fig. 4C, the MMP-2 activities were
decreased in the A549-hMaspin cells compared to that of the
A549-ctrl cells. Furthermore, the expression of MMP-2 in the
A549-hMaspin cells at the mRNA level was markedly decreased, while
the expression of MMP-9 remained unchanged (Fig. 4D). The expression of uPA and uPAR,
which are involved in the migration and invasion of cancer cells,
was not changed in the A549-hMaspin cells (Fig. 4E). The integrin β1 expression at
the protein level was significantly decreased in the A549-hMaspin
cells compared to that of the A549-ctrl cells (Fig. 4F). Thus, the results here
indicated that overexpression of maspin suppressed the migration
and invasion abilities of the A549 cells, which was associated with
the reduced activities of MMP-2, and the downregulated integrin β1
expression.
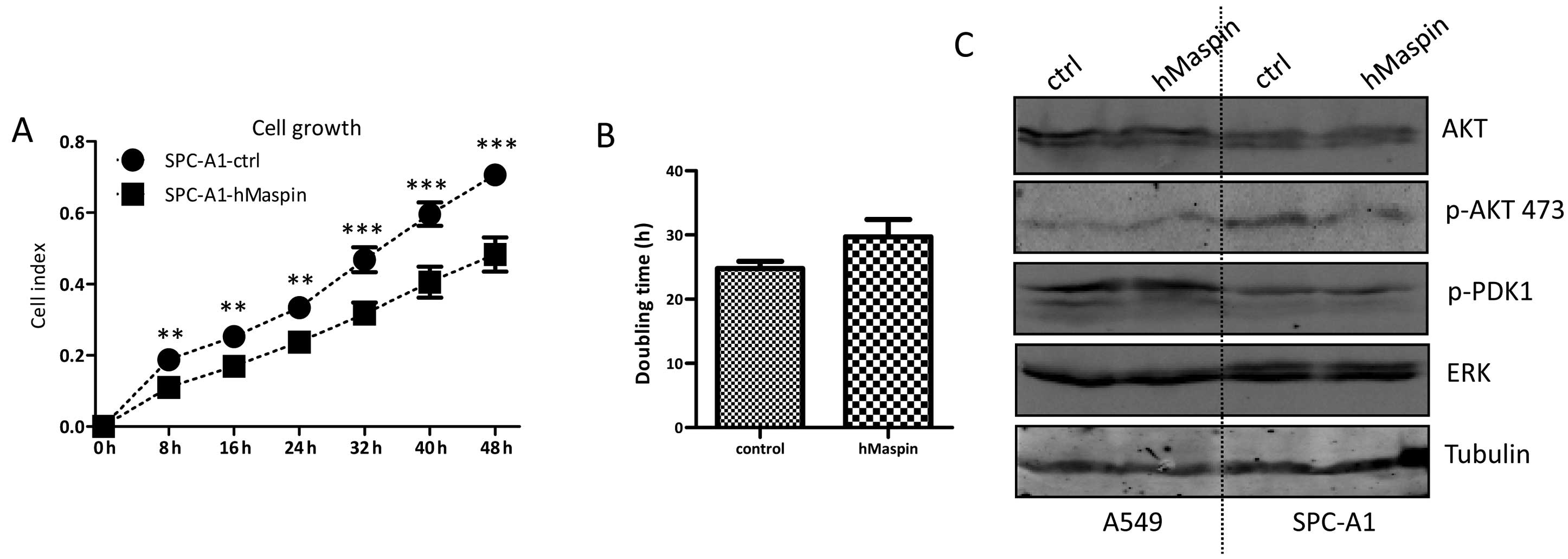
Maspin overexpression inhibits SPC-A1
cell growth
Similarly, the cell growth of SPC-A1 cells affected
by the maspin over-expression was detected. The SPC-A1 cells
overexpressing maspin grew much slower than that of the SPC-A1-ctrl
cells, and there was a statistically significant difference between
the two established cell lines during the 72-h continuous
monitoring (Fig. 5A). The
doubling times of SPC-A1-hMaspin and SPC-A1-ctrl were 24.77±1.11
and 29.73±2.08 h, respectively (Fig.
5B).
The PI3K/AKT signaling pathway has been implicated
in the tumor cell proliferation and apoptosis resistance. In order
to understand whether the ATK signaling is involved in the
decreased cell growth of the SPC-A1 cells overexpressing maspin,
the key molecules in the AKT signaling were examined by western
blotting. The expression of AKT was comparable in the SPC-A1-ctrl
and SPC-A1-hMaspin cells. However, the expression of phosphorylated
AKT was clearly decreased in SPC-A1-hMaspin cells. The expression
of phosphorylated PDK1 and ERK was not affected by the
overexpression of maspin in the SPC-A1 cells (Fig. 5C). These key molecules in the AKT
signaling were also examined in the A549-ctrl and A549-hMaspin
cells, and there was no significant difference between the two cell
lines.
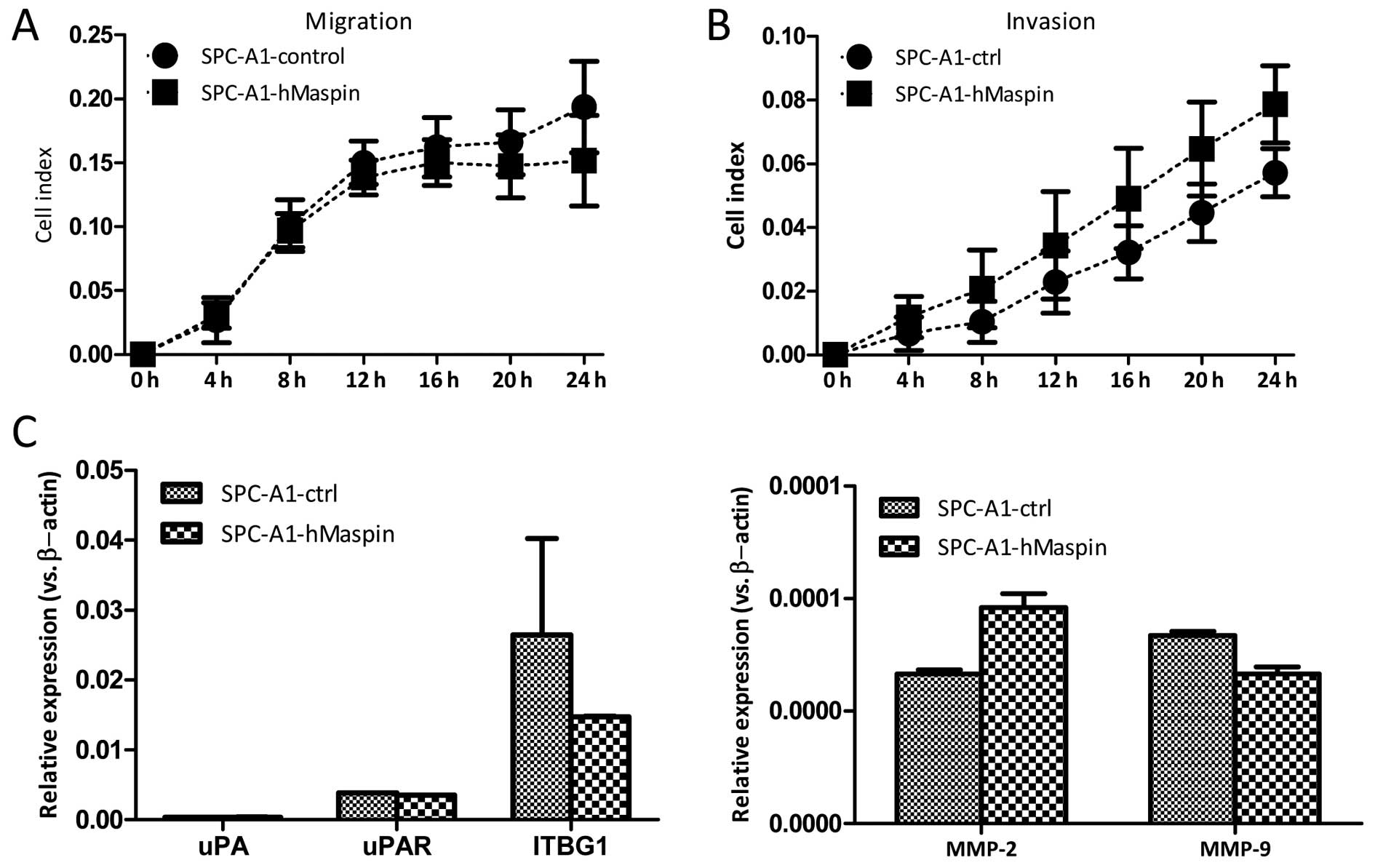
Maspin overexpression does not affect the
migration and invasion abilities of SPC-A1 cells
The migration and invasion abilities were also
investigated in the SPC-A1-ctrl and SPC-A1-hMaspin cells using the
real-time xCELLigence system. As opposed to the A549 cells, the
overexpression of maspin did not affect either the migration or the
invasion ability of the SPC-A1 cells. There was no significant
difference between the two established cell lines during the 24-h
continuous monitoring for the migration and the invasion assay
(Fig. 6A and B). Furthermore, the
mRNA expression of the MMP-2, MMP-9, uPA, uPAR and
ITGB1 genes, which are involved in the migration and
invasion of cancer cells, was comparable between the SPC-A1-ctrl
and SPC-A1-hMaspin cells (Fig.
6C).
Discussion
Maspin acts as a comprehensive molecule in diverse
types of cancer, including prostate, breast and pancreatic cancer.
Although the significance of maspin expression varies in different
types of cancer, it is usually believed that the decreased maspin
expression is associated with an unfavorable prognosis in lung
cancer, particularly in NSCLC (5,30,31).
The maspin expression was examined in PC-9, A549,
SPC-A1 and H460 cell lines. All four cell lines were derived from
NSCLC patients, in which the first three cell lines were from
adenocarcinoma and the H460 cell line was from large cell cancer of
lung. A clear heterogeneity was observed among the four cell lines.
The maspin expression was high in PC-9 and H460 cells; however, the
expression was barely detected in the A549 and SPC-A1
adenocarcinoma cell lines, suggesting that the behaviors of maspin
were also extremely complicated in lung cancer.
Maspin was also ectopically expressed in the A549
and SPC-A1 cells, which were almost void of maspin expression, and
identified that maspin functioned in a different way in the two
cell lines. Overexpression of maspin suppressed the cell migration
and invasion abilities of A549 cells, which was associated with the
downregulation of integrin β1 and inactivation of MMP-2. However,
maspin had no effect on the cell growth of A549 cells. When
overexpression of maspin occurred in the SPC-A1 cells, reduced cell
growth was observed, accompanied by the reduced phosphoyration of
AKT. In contrast to that of the A549 cells, maspin overexpression
had no effect on cell migration and invasion of the SPC-A1
cells.
The human MMP family consists of ≥26 proteases,
which are subdivided into collagenases, gelatinases, stromelysins
and matrilysins. MMP-2 and MMP-9 are well-known gelatinases and are
involved in cancer invasion and metastasis due to the strong
proteolytic activity of the extracellular matrix. While MMP-2
promotes cleavage of the extracellular matrix proteins, MMP-9
modulates permeability of the vascular endothelium. The critical
roles of MMPs and their inhibitors in the growth and progression of
lung cancer have been reported (32). In the present study,
overexpression of maspin in the A549 cells led to a clear reduction
of the MMP-2 mRNA level, whereas that of MMP-9 was
unchanged. The decreased MMP-2 activity was also observed in the
maspin-overexpressing A549 cells, indicated by the gelatinase
zymography assay. The migration and invasion abilities of the A549
cells were largely inhibited by the maspin overexpression. These
data are in line with the previous data that maspin overexpression
suppresses the invasion ability and inhibits the expression of
MMP-2 in malignant melanoma cells (33). Although several reports indicate
that maspin could target the extracellular uPA/uPAR complex
(34,35), which further affects the migration
and invasion abilities of certain cancer cells, the expression of
uPA/uPAR was not affected by the overexpression of maspin in A549
cells. In the present study, the expression of integrin β1 was
downregulated in the maspin-overexpressing A549 cells. Integrin β1,
encoded by the ITGB1 gene, belongs to the family of
heterodimeric transmembrane cell surface receptors that contain 18
α and 8 β subunits. There is a direct interaction between maspin
and integrin β1 by the reactive centre loop of maspin (23). Maspin could integrate with the
plasminogen activation system and integrin β1, thus, regulating
cell adhesion and migration (23,36). Integrin β1-silencing suppresses
lung cancer cell invasion and metastasis in vitro and in
vivo (37). Integrin
β1-silencing in A549 cells causes a defective activation of the
EGFR signaling cascade, leading to impaired cell proliferation,
migration and invasive behavior in vitro. Integrin β1
overexpression in lung cancer cells also has a key role in
chemoresistance (38,39). In the present study, in the A549
cells, maspin negatively regulated the integrin β1 expression.
Taken together, the decreased activities of MMP-2 together with the
downregulated integrin β1 contributed to the diminished migration
and invasion abilities of A549 lung adenocarcinoma cells with high
expression of maspin.
The overexpression of maspin affected the migration
and invasion abilities of A549 cells; however, these observations
were not found in the SPC-A1 cells. Rather, the high maspin
expression suppressed the cell growth of SPC-A1 cells. Several
studies suggest that maspin inhibits the survival pathway by
influencing the response to cell death in lung cancer cells. The
PI3K/Akt signaling pathway has essential roles in lung cancer cell
proliferation and survival (40).
Maspin modulates the prostate cancer cell apoptotic and angiogenic
response to hypoxia through targeting Akt signaling (19). In lung cancer cells, maspin also
modulates Akt phosphorylation and chemoresistance (41). In the present study, a slow cell
growth was detected in the SPC-A1 cells overexpressing maspin by a
real-time xCELLigence system. In addition, reduced phosphorylation
of Akt was identified in the SPC-A1 cells overexpressing maspin.
However, a clear change in the cell proliferation and apoptosis was
not detected by the Ki-67 and TUNEL assays (data not shown),
compared to the control cells.
Overexpression of maspin in A549 cells did not
affect the phosphorylation of Akt. A549 cells harbor the
KRAS gene mutation (p.G12S), while SPC-A1 cells are
wild-type for the K-ras gene. Following EGF binding to its
receptor and activation of tyrosine kinases, the K-ras protein
becomes activated and transduces the activation signals to the
nucleus by mitogen-activated protein kinases and PI3K/AKT-mediated
cascades. The K-ras gene mutation in the NSCLC cells leads
to the aberrant activation of the Akt signaling pathway (42). It appears that the constitutively
activated Akt due to the K-ras gene mutation was not
affected by the overexpression of maspin in A549 cells.
Taken together, maspin overexpression in lung
adenocarcinoma cells affected several aspects of malignant
phenotypes, including cell growth, migration and invasion. In the
A549 cells carrying the K-ras gene mutation, maspin
negatively regulated the expression of MMP-2 and integrin β1, and
influenced the migration and invasion abilities. In SPC-A1 cells
carrying the wild-type K-ras gene, maspin inhibited
phosphorylation of Akt, and mainly influenced the cell growth.
Maspin functioned differently in lung adenocarcinoma cells due to
the diverse genetic background.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 81172433).
References
|
1
|
Schiller JH, Gandara DR, Goss GD and Vokes
EE: Non-small-cell lung cancer: Then and now. J Clin Oncol.
31:981–983. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zou Z, Anisowicz A, Hendrix MJ, Thor A,
Neveu M, Sheng S, Rafidi K, Seftor E and Sager R: Maspin, a serpin
with tumor-suppressing activity in human mammary epithelial cells.
Science. 263:526–529. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zou Z, Zhang W, Young D, Gleave MG, Rennie
P, Connell T, Connelly R, Moul J, Srivastava S and Sesterhenn I:
Maspin expression profile in human prostate cancer (CaP) and in
vitro induction of Maspin expression by androgen ablation. Clin
Cancer Res. 8:1172–1177. 2002.PubMed/NCBI
|
|
4
|
Katakura H, Takenaka K, Nakagawa M, Sonobe
M, Adachi M, Ito S, Wada H and Tanaka F: Maspin gene expression is
a significant prognostic factor in resected non-small cell lung
cancer (NSCLC). Maspin in NSCLC. Lung Cancer. 51:323–328. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lonardo F, Li X, Kaplun A, Soubani A,
Sethi S, Gadgeel S and Sheng S: The natural tumor suppressor
protein maspin and potential application in non small cell lung
cancer. Curr Pharm Des. 16:1877–1881. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mohsin SK, Zhang M, Clark GM and Craig
Allred D: Maspin expression in invasive breast cancer: Association
with other prognostic factors. J Pathol. 199:432–435. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Joensuu KM, Leidenius MH, Andersson LC and
Heikkilä PS: High expression of maspin is associated with early
tumor relapse in breast cancer. Hum Pathol. 40:1143–1151. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maass N, Hojo T, Ueding M, Lüttges J,
Klöppel G, Jonat W and Nagasaki K: Expression of the tumor
suppressor gene Maspin in human pancreatic cancers. Clin Cancer
Res. 7:812–817. 2001.PubMed/NCBI
|
|
9
|
Sood AK, Fletcher MS, Gruman LM, Coffin
JE, Jabbari S, Khalkhali-Ellis Z, Arbour N, Seftor EA and Hendrix
MJ: The paradoxical expression of maspin in ovarian carcinoma. Clin
Cancer Res. 8:2924–2932. 2002.PubMed/NCBI
|
|
10
|
Baek JY, Yeo HY, Chang HJ, Kim KH, Kim SY,
Park JW, Park SC, Choi HS, Kim DY and Oh JH: Serpin B5 is a
CEA-interacting biomarker for colorectal cancer. Int J Cancer.
134:1595–1604. 2014. View Article : Google Scholar
|
|
11
|
Snoeren N, Emmink BL, Koerkamp MJ, van
Hooff SR, Goos JA, van Houdt WJ, de Wit M, Prins AM, Piersma SR,
Pham TV, et al: Maspin is a marker for early recurrence in primary
stage III and IV colorectal cancer. Br J Cancer. 109:1636–1647.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takagi Y, Matsuoka Y, Shiomi T, Nosaka K,
Takeda C, Haruki T, Araki K, Taniguchi Y, Nakamura H and Umekita Y:
Cytoplasmic maspin expression is a predictor of poor prognosis in
patients with lung adenocarcinoma measuring <3 cm.
Histopathology. 66:732–739. 2014. View Article : Google Scholar
|
|
13
|
Narayan M and Twining S: Focus on
molecules: Maspin. Exp Eye Res. 90:2–3. 2010. View Article : Google Scholar
|
|
14
|
Teoh SS, Vieusseux J, Prakash M, Berkowicz
S, Luu J, Bird CH, Law RH, Rosado C, Price JT, Whisstock JC, et al:
Maspin is not required for embryonic development or tumour
suppression. Nat Commun. 5(3164)2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li X, Chen D, Yin S, Meng Y, Yang H,
Landis-Piwowar KR, Li Y, Sarkar FH, Reddy GP, Dou QP, et al: Maspin
augments proteasome inhibitor-induced apoptosis in prostate cancer
cells. J Cell Physiol. 212:298–306. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tahmatzopoulos A, Sheng S and Kyprianou N:
Maspin sensitizes prostate cancer cells to doxazosin-induced
apoptosis. Oncogene. 24:5375–5383. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu J, Yin S, Reddy N, Spencer C and Sheng
S: Bax mediates the apoptosis-sensitizing effect of maspin. Cancer
Res. 64:1703–1711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Latha K, Zhang W, Cella N, Shi HY and
Zhang M: Maspin mediates increased tumor cell apoptosis upon
induction of the mitochondrial permeability transition. Mol Cell
Biol. 25:1737–1748. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McKenzie S, Sakamoto S and Kyprianou N:
Maspin modulates prostate cancer cell apoptotic and angiogenic
response to hypoxia via targeting AKT. Oncogene. 27:7171–7179.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sheng S, Carey J, Seftor EA, Dias L,
Hendrix MJ and Sager R: Maspin acts at the cell membrane to inhibit
invasion and motility of mammary and prostatic cancer cells. Proc
Natl Acad Sci USA. 93:11669–11674. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cher ML, Biliran HR Jr, Bhagat S, Meng Y,
Che M, Lockett J, Abrams J, Fridman R, Zachareas M and Sheng S:
Maspin expression inhibits osteolysis, tumor growth, and
angiogenesis in a model of prostate cancer bone metastasis. Proc
Natl Acad Sci USA. 100:7847–7852. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen EI and Yates JR III: Maspin and tumor
metastasis. IUBMB Life. 58:25–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ravenhill L, Wagstaff L, Edwards DR, Ellis
V and Bass R: G-helix of maspin mediates effects on cell migration
and adhesion. J Biol Chem. 285:36285–36292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bircan A, Bircan S, Kapucuoglu N, Songur
N, Ozturk O and Akkaya A: Maspin, VEGF and p53 expression in small
biopsies of primary advanced lung cancer and relationship with
clinico-pathologic parameters. Pathol Oncol Res. 16:553–561. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Berardi R, Santinelli A, Onofri A,
Brunelli A, Pierantoni C, Pisa E, Pagliacci A, Stramazzotti D,
Zuccatosta L, Mazzanti P, et al: Maspin expression is a favorable
prognostic factor in non-small cell lung cancer. Anal Quant Cytol
Histol. 34:72–78. 2012.PubMed/NCBI
|
|
26
|
Frey A, Soubani AO, Adam AK, Sheng S, Pass
HI and Lonardo F: Nuclear, compared with combined nuclear and
cytoplasmic expression of maspin, is linked in lung adenocarcinoma
to reduced VEGF-A levels and in Stage I, improved survival.
Histopathology. 54:590–597. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim S, Han J, Kim J and Park C: Maspin
expression is transactivated by p63 and is critical for the
modulation of lung cancer progression. Cancer Res. 64:6900–6905.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Choy B, Findeis-Hosey JJ, Li F, McMahon
LA, Yang Q and Xu H: High frequency of coexpression of maspin with
p63 and p53 in squamous cell carcinoma but not in adenocarcinoma of
the lung. Int J Clin Exp Pathol. 6:2542–2547. 2013.PubMed/NCBI
|
|
29
|
Beltran AS and Blancafort P: Reactivation
of MASPIN in non-small cell lung carcinoma (NSCLC) cells by
artificial transcription factors (ATFs). Epigenetics. 6:224–235.
2011. View Article : Google Scholar
|
|
30
|
Berardi R, Morgese F, Onofri A, Mazzanti
P, Pistelli M, Ballatore Z, Savini A, De Lisa M, Caramanti M,
Rinaldi S, et al: Role of maspin in cancer. Clin Transl Med.
2(8)2013.PubMed/NCBI
|
|
31
|
Takanami I, Abiko T and Koizumi S:
Expression of maspin in non-small-cell lung cancer: Correlation
with clinical features. Clin Lung Cancer. 9:361–366. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ali-Labib R, Louka ML, Galal IH and Tarek
M: Evaluation of matrix metalloproteinase-2 in lung cancer.
Proteomics Clin Appl. 8:251–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Denk AE, Bettstetter M, Wild PJ, Hoek K,
Bataille F, Dietmaier W and Bosserhoff AK: Loss of maspin
expression contributes to a more invasive potential in malignant
melanoma. Pigment Cell Res. 20:112–119. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bernardo MM, Meng Y, Lockett J, Dyson G,
Dombkowski A, Kaplun A, Li X, Yin S, Dzinic S, Olive M, et al:
Maspin reprograms the gene expression profile of prostate carcinoma
cells for differentiation. Genes Cancer. 2:1009–1022. 2011.
View Article : Google Scholar
|
|
35
|
Yin S, Lockett J, Meng Y, Biliran H Jr,
Blouse GE, Li X, Reddy N, Zhao Z, Lin X, Anagli J, et al: Maspin
retards cell detachment via a novel interaction with the
urokinase-type plasminogen activator/urokinase-type plasminogen
activator receptor system. Cancer Res. 66:4173–4181. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Endsley MP, Hu Y, Deng Y, He X, Warejcka
DJ, Twining SS, Gonias SL and Zhang M: Maspin, the molecular bridge
between the plasminogen activator system and beta1 integrin that
facilitates cell adhesion. J Biol Chem. 286:24599–24607. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang XM, Li J, Yan MX, Liu L, Jia DS, Geng
Q, Lin HC, He XH, Li JJ and Yao M: Integrative analyses identify
osteopontin, LAMB3 and ITGB1 as critical pro-metastatic genes for
lung cancer. PLoS One. 8:e557142013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jahangiri A, Aghi MK and Carbonell WS: β1
integrin: Critical path to antiangiogenic therapy resistance and
beyond. Cancer Res. 74:3–7. 2014. View Article : Google Scholar :
|
|
39
|
Kanda R, Kawahara A, Watari K, Murakami Y,
Sonoda K, Maeda M, Fujita H, Kage M, Uramoto H, Costa C, et al:
Erlotinib resistance in lung cancer cells mediated by integrin
β1/Src/Akt-driven bypass signaling. Cancer Res. 73:6243–6253. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wojtalla A and Arcaro A: Targeting
phosphoinositide 3-kinase signalling in lung cancer. Crit Rev Oncol
Hematol. 80:278–290. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nam E and Park C: Maspin suppresses
survival of lung cancer cells through modulation of Akt pathway.
Cancer Res Treat. 42:42–47. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bentley C, Jurinka SS, Kljavin NM,
Vartanian S, Ramani SR, Gonzalez LC, Yu K, Modrusan Z, Du P,
Bourgon R, et al: A requirement for wild-type Ras isoforms in
mutant KRas-driven signalling and transformation. Biochem J.
452:313–320. 2013. View Article : Google Scholar : PubMed/NCBI
|