Introduction
Prostate cancer (PCa) is the most common malignancy
among men worldwide, and the natural course of the disease is
characterized by high heterogeneity, varying from indolent to
highly aggressive cancer that spreads at an early stage of the
disease and causes pain and ultimately mortality (1). Currently, the disease is stratified
as low-, intermediate- and high-risk based on the prostate-specific
antigen (PSA) level, Gleason score or clinical stage, whereas such
stratification is deemed insufficient to predict the clinical
outcome of PCa, which is largely determined by the presence of
metastasis, as even when grouped as high-risk, only 30% of the
patients develop metastases and succumb due to their disease
(2–4). The key problem in determining the
clinical outcome of PCa is its characteristic to metastasize to
bone, which essentially makes the disease incurable and leads to
significant morbidity prior to patient fatality (5,6).
Therefore, it is of great clinical significance to determine the
molecular mechanism underlying metastasis for the purpose of
disease prevention, identification of new therapeutic targets and
development of anti-metastatic therapies. Multiple steps, including
cellular disengagement and degradation of the surrounding
extracellular matrix, are believed to be involved in the formation
of distant secondary bone tumors, and the total processes are under
the control of multiple factors and molecular signaling pathways
(7).
MicroRNAs (miRNAs or miRs) are short non-coding RNA
strands with an average length of 22 nucleotides (8). Initially transcribed as RNA
hairpins, miRNAs are processed into mature miRs that regulate the
expression of genes at the post-transcriptional and the
translational level by binding to complementary messenger RNA
(9). To date, ~1,000 human miRs
have been identified and each of the miRNAs may target hundreds of
genes (9), making it a
complicated regulatory network of multiple molecular signaling
pathways that are important for the control of human cell behavior
(10). Increasing evidence
indicates that miRNAs are significantly involved in the regulation
of the development, progression and metastasis of a variety of
types of cancer, as tumor suppressors or oncogenes (oncomiRs)
(11). In PCa, several miRNAs
have been reported as mediators of metastasis. The dysregulation of
miR-221 and miR-222 is responsible for PCa
progression, poor prognosis and the formation of metastasis
(12). miR-21 was also
found to be overexpressed in PCa, functioning as a major oncogenic
regulator and contributing to tumor growth, invasiveness and
metastasis (13,14). Another study demonstrated that
downregulation of miR-146a, as well as upregulation of its
target, ROCK1, promote cell proliferation, invasion and metastasis
in the PCa cells (15).
To investigate the role of miRNAs in bone metastasis
of PCa, the miRNA expression profiles in primary and bone
metastatic PCa were first examined and compared, and identified
that miR-335 and -543 is associated with bone
metastasis. Furthermore, miR-335 and -543 repressed
migration and invasion in vitro, via targeting endothelial
nitric oxide synthase (eNOS).
Materials and methods
Tissue samples
Tissue samples were collected from histologically
confirmed PCa patients that were divided into two groups, primary
PCa with (n=20) and without (n=15) bone metastasis. Primary PCa
tissue samples were collected from all 35 enrolled patients (from
prostatectomy or transurethral resection in the treatment of local
prostate carcinoma) and skeletal metastatic tissue samples were
collected from the 20 patients with PCa (from the surgical
treatment of bone metastasis). The histological diagnosis was made
by at least two experienced pathologists, and bone metastasis was
diagnosed based on clinical symptoms and signs, as well as the
imaging tests, including bone scan, computed tomography and
magnetic resonance imaging. Those patients who had received
neoadjuvant hormone, radiation or chemotherapy prior to the
occurrence of tumor tissues were excluded from the study. The
clinical characteristics and demographic data of the subjects, such
as age, bone metastasis, total PSA level, free PSA level and the
Gleason score, are described in Table
I. The study was approved by the Institutional Ethical Board in
the Zhongshan Hospital of Dalian University (Dalian, Liaoning,
Chian), and written consent was obtained from each participant
prior to the start of the study.
 | Table IClinicopathological features of the
prostate cancer patients with or without bone metastasis recruited
in the study. |
Table I
Clinicopathological features of the
prostate cancer patients with or without bone metastasis recruited
in the study.
| Features | PCa with bone
metastatsis
(n=20) | PCa without bone
metastatsis
(n=15) | P-value |
|---|
| Age, years | 65.2±7.23 | 64.6±6.63 | 0.638 |
| Pre-operative PSA,
ng/ml | 48.2±6.25 | 50.5±7.51 | 0.359 |
| Gleason score | | | 0.991 |
| ≤6 | 2 | 1 | |
| 7 | 4 | 3 | |
| 8 | 6 | 4 | |
| 9 | 5 | 4 | |
| 10 | 3 | 3 | |
RNA extraction
All the tissue samples were embedded into paraffin
blocks, cut into slices and placed in 1.5 ml nuclease-free
microcentrifuge tubes. Subsequently, the sliced tissue samples were
deparaffinized three times in 1 ml limonene (Sigma-Aldrich, St.
Louis, MO, USA), and washed with 1 ml 100% ethanol twice prior to
air drying at room temperature. Samples were subsequently incubated
with digestion buffer (20 mM Tris-HCl, 10 mM EDTA and 1% SDS) and
proteinase K (Merck, Whitehouse Station, NJ, USA) at 55°C overnight
for the purpose of complete digestion. Subsequently, TRIzol reagent
(Invitrogen, Carlsbad, CA, USA) was used to isolate the RNA
following the manufacturer's instructions, and isolated RNA samples
were resuspended in RNase-free water. Finally, RNA quality and
quantity were evaluated using a NanoDrop (NanoDrop Technologies,
Wilmington, DE, USA) and agarose electrophoresis.
Microarray analysis
The miRNA microarray chips were purchased from
CapitalBio (CapitalBio Corp. Beijing, China), and each chip
contained 924 probes in triplicate, corresponding to 677 human, 461
mouse and 292 rat miRNAs described in the miRNA registry
(http://microrna.sanger.ac.uk; miRBase
release 10.0, 2007). The microarray analysis was carried out as
described previously (16). The
low-molecular-weight RNA was isolated using the polyethylene glycol
solution precipitation method, and dephosphorylated by alkaline
phosphatase (New England Biolabs, Ipswich, MA, USA) prior to
fluorescently labeling and precipitation. Subsequently, the RNA was
resuspended in 20 ml of hybridization buffer (36 SSC, 0.2% SDS and
15% formamide), and the hybridization was performed at 42°C
overnight. The resultant chips were scanned with a double-channel
laser scanner (LuxScan 10K/A), and the data was obtained from the
TIFF images using LuxScan™ 3.0 software (both from CapitalBio
Corp.). Raw data were normalized and analyzed using the
significance analysis of microarrays (SAM, version 2.1; Stanford
University, CA, USA) software.
Cell culture
PC-3, a metastatic PCa cell line, was purchased from
American Type Culture Collection (Manassas, VA, USA) and cultured
in F-12 medium supplemented with 10% fetal bovine serum (FBS) (both
from Gibco, Eggenstein, Germany). Cells were grown at a humidified
atmosphere of 5% CO2 at 37°C.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was used to confirm the expression level of
mRNAs. Reverse transcription was performed using a superscript III
first-strand synthesis system for an RT-PCR kit (Invitrogen), and
RT-qPCR was performed on a Rotor-Gene 2000 Real-time Cycler
detection system (Corbett Research, Sydney, Australia) supplied
with analytical software, using an Express SYBR-GreenER qPCR
Supermix Universal kit (Invitrogen), according to the
manufacturer's instructions. U6 mRNA levels were used for
normalization as an endogenous reference. The relative expression
levels of miR-335, miR-543 and eNOS in the tissue
samples were calculated using the 2−ΔΔCT method. Primer
sets for the RT-qPCR are described as followed: miR-335
forward, 5′-TCAAGAGCAATAACGAAAAATGT-3′ and reverse,
5′-GCTGTCAACGATACGCTACGT-3′; miR-543 forward,
5′-GTGCTCGGTTTGTAGGCAGT-3′ and reverse, 5′-GTGCCTTGTTTTGATGGCAG-3′;
eNOS forward, 5′-CCCTTCAGTGGCTGGTACAT-3′ and reverse
5′-CACGATGGTGACTTTGGCTA-3′; and U6 forward,
5′-CGCTTCGGCAGCACATATAC-3′ and reverse,
5′-TTCACGAATTTGCGTGTCAT-3′.
Western blotting
To evaluate the expression level of eNOS in the cell
line with or without overexpressed miR-335 and -543,
cells were seeded in 100-mm tissue culture dishes. After 48 h,
cells were washed with pre-chilled phosphate-buffered saline when
the confluence reached 60–70%, prior to being harvested in the
lysis buffer [62.5 mmol/l Tris-HCl (pH 6.8), 2% SDS, 10% glycerol,
and 5% 2-β-mercaptoethanol] (Sigma-Aldrich). Equal amounts of
lysates were loaded for SDS-polyacrylamide electrophoresis, and the
separated protein was transferred onto a PVDF membrane (Millipore,
Billerica, MA, USA), followed by blocking in 5% non-fat milk for 1
h at room temperature, and incubating with the eNOS (Cat. no.
sc-376751, dilution: 1:1,000) and β-actin (Cat. no. sc-47778,
dilution: 1:10,000; both from Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) primary antibodies for 1.5 h. Membranes were
washed three times (5 min each) in Tris-buffered saline Tween 20
(TBS-T) buffer and incubated for 1 h at room temperature with
horseradish peroxidase-conjugated secondary antibodies (Cat. no.
sc-51625, dilution: 1:10,000; Santa Cruz Biotechnology, Inc.).
Blots were washed three times (5 min each) in TBS-T and the signal
was detected using an ECL system (Applygen, Beijing, China).
β-actin was used to normalize the amount of each loading
sample.
Scratch assay
The cell migration was determined by the scratch
assay. The cells were cultivated to 90% confluence on 12-well
plates and were transfected with the miR-335 and
miR-543 mimics, and miR-335 inhibitors or control.
Subsequently, cell scrapers (Corning Inc., Corning, NY, USA) were
utilized to scratch the confluent cells 24 h post-transfection. The
procedures of cellular growth were subsequently observed. All the
experiments were repeated in triplicate.
Transwell migration
The Transwell migration chambers were used to
evaluate the PC-3 cell invasion. The cells were first seeded at a
density of 1×105 cells in serum-free media on the upper
chamber with the non-coated membrane (8 μm pore size;
Millipore, Zug, Switzerland). The lower chamber contained F-12
medium with 20% FBS as a chemo-attractant. The cells in the upper
chamber were discarded using cotton wool after 24 h and the
migration cells in the lower chamber were counted using a
microscope (Olympus, Tokyo, Japan). All the experiments were
repeated in triplicate.
Luciferase assay
The full-length 3′ untranslated region (3′UTR) of
eNOS was amplified by PCR from genomic DNA and cloned into the
pGL3-BS vector (Promega, Madison, WI, USA). The mutant construct of
eNOS 3′UTR was generated using a QuickChange mutagenesis kit
(Stratagene, Heidelberg, Germany). Co-transfection of the reporter
vectors and miRs, mimics or controls was performed using
Lipofectamine 2000 (Invitrogen). After 48 h, dual-luciferase
activity was measured using the Dual-Luciferase®
reporter assay system (Promega) according to the manufacturer's
instructions.
Statistical analysis
Statistics were assessed using SPSS 20.0 (SPSS,
Inc., Armonk, NY, USA). In the RT-qPCR and animal experiments, data
were compared by Student's t-test. The association between
downregulated miRNA expression and clinicopathological features in
primary PCa with or without metastasis, and bone metastatic PCa was
analyzed using the Spearman's rank correlation test. In the
metastasis assay-based experiments, the data were analyzed with
one-way analysis of variance. For understanding the association
between miRNAs, the significant correlations were determined using
the Kendall's rank correlation test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Identification of differentially
expressed miRNAs between primary PCa and bone metastasis using
microarray analysis
To identify the differentially expressed miRNAs that
could be responsible for the bone metastasis in PCa, 8 pairs of
primary and metastatic tissues (from the same patient) were
collected and their expression profiles were compared using a
microRNA microarray. A significantly upregulated expression of 14
miRNAs was revealed, as well as 4 downregulated miRNAs
(miR-543, -335, -196 and -19a) in bone
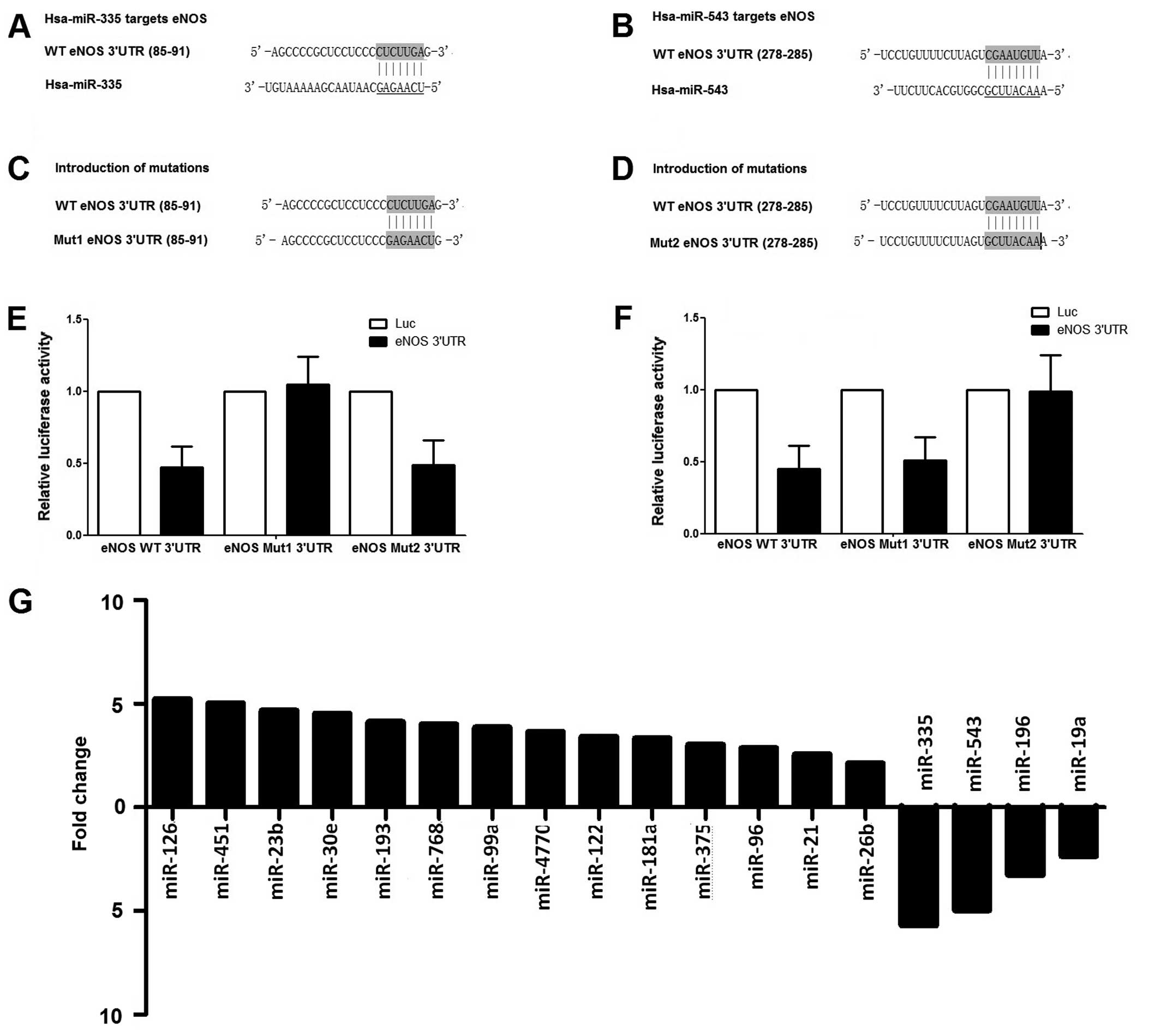
metastasis compared with primary PCa (Fig. 1G). By cluster analysis, no miRNA
expression pattern that was specific to the bone metastasis was
identified. The online miRNA database was searched for the
potential target genes that have been reported to be associated
with metastasis in the literature. Notably, eNOS was a potentially
shared target of miR-543 and miR-335, which were
substantially downregulated in the present miRNA microarray
analysis, by searching the online database www.targetscan.org (Fig.
1A and B). In addition, the 'seeds sequences' were replaced
with the mutants in the 3′UTR of eNOS (Fig. 1C and D), and their interactions
were tested with the microRNAs, finding that overexpression of
miR-543 and miR-335 could significantly suppress the
luciferase activity of those carrying wild-type 3′UTR of eNOS;
however, no inhibitory effect was shown on the luciferase activity
of those carrying the corresponding mutant (Fig. 1E and F). The data of the
luciferase assay demonstrated that miR-543 and
miR-335 could suppress the expression of eNOS by binding the
specific 'seed sequence' in the 3′UTR of the gene.
Verification of the downregulation of
miR-543 and miR-335 by RT-qPCR analysis in bone metastasis compared
with primary PCa
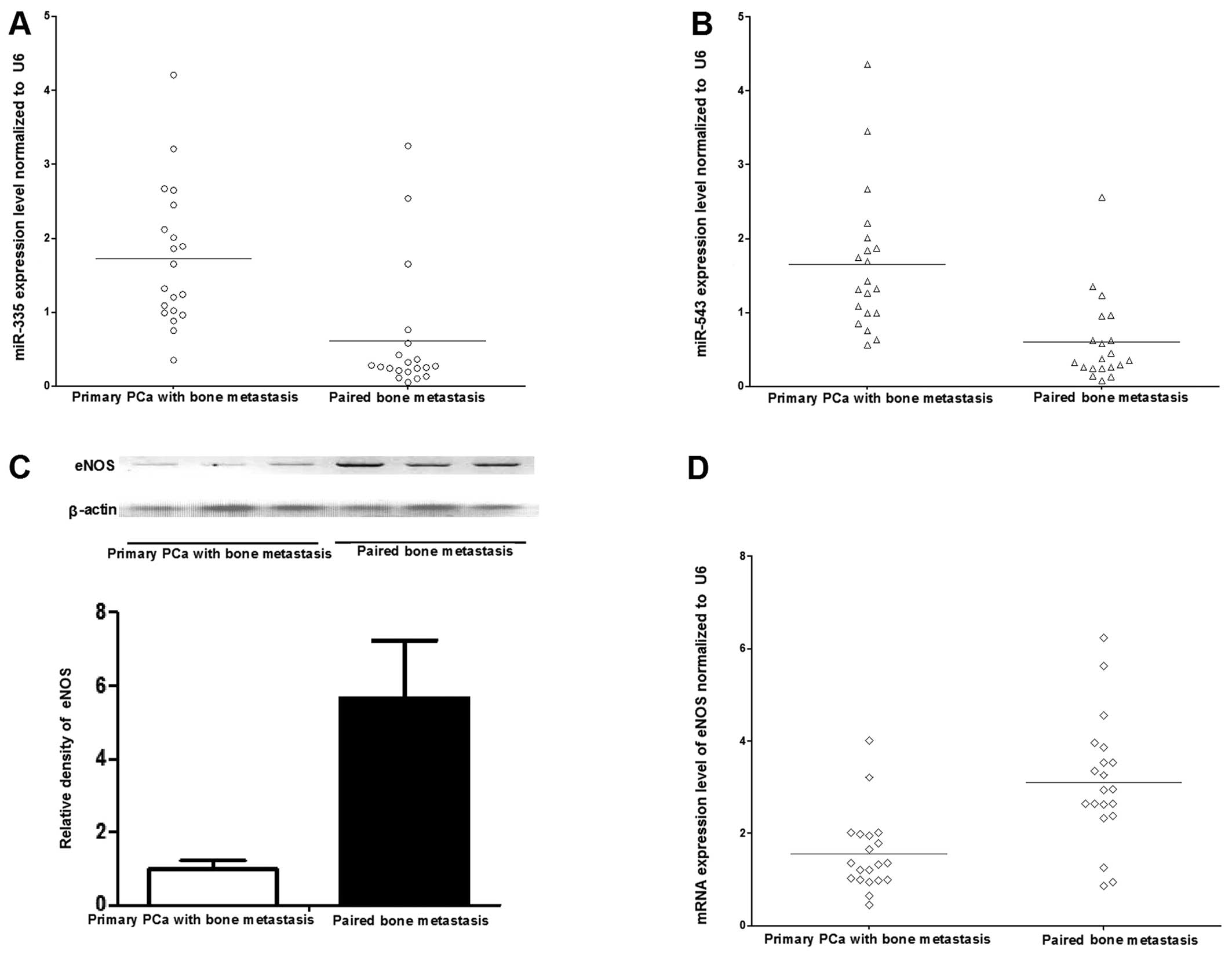
To confirm the microarray data, RT-qPCR was
conducted to evaluate the expression of the significantly
downregulated miRNAs (miR-543, -335, -196 and
-19a) in the independent samples of 20 primary PCa and their
paired bone metastasis samples. Among them, 20 samples were paired
samples of primary PCa and bone metastasis, including the 8 paired
samples that have been involved in the miRNA microarray analysis.
Subsequent to the quantification of the individual miRNA level in
each sample and normalization to U6 expression, RT-qPCR data
confirmed the downregulated expression of miR-543, and
miR-335 in the bone metastatic tissues, respectively
(Fig. 2A and B). The expression
levels of miR-543 and -335 were downregulated
significantly in metastasis samples versus primary PCa. Although
the expression of miR-196 and -19a was significantly
downregulated in bone metastasis compared with in primary PCa
samples in the microarray analysis, there were no statistically
significant differences regarding the two miRs by RT-qPCR analysis
(data not shown). Thus, the data indicated that there was a
significant downregulation of miR-543 and -335 when
PCa tumors metastasized to bone. As miR-543 and -335
levels were downregulated in bone metastasis, we postulated that
downregulation of miR-543 and -335 may also be
associated with an increased expression level of eNOS. Furthermore,
the mRNA and protein expression patterns of eNOS were determined in
the primary PCa and bone metastasis samples using RT-qPCR and
western blot analysis. The mRNA level of eNOS was significantly
upregulated in the bone metastasis compared with primary PCa
(Fig. 2D), and consistently, the
protein expression of eNOS was increased in the bone metastasis as
well (Fig. 2C).
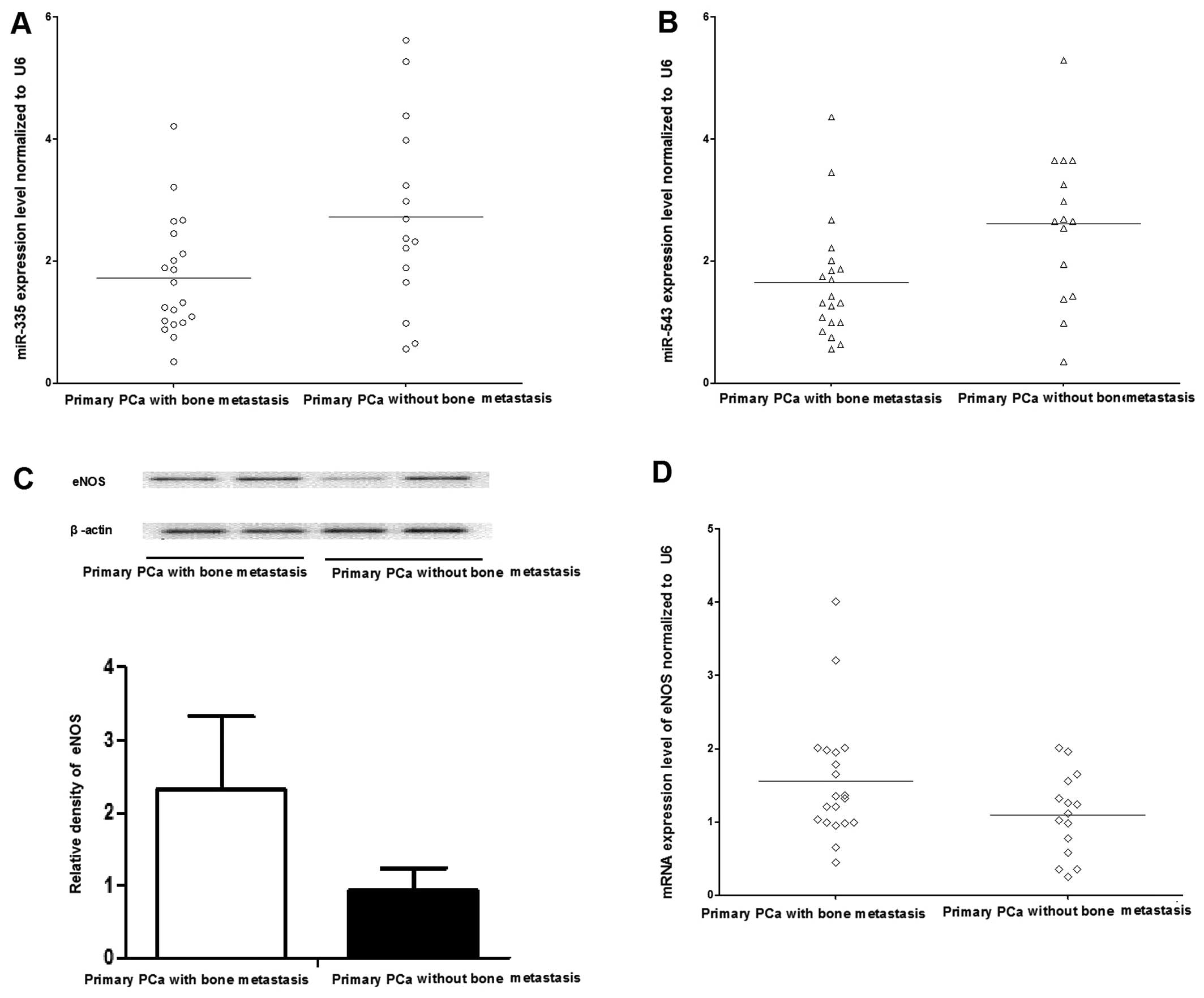
Firstly, a retrospective comparison was performed
between the aforementioned 20 PCa patients with bone metastasis and
15 PCa patients without bone metastatis. The distribution of age in
those patients with and without bone metastases was not
significantly different. The expression of miR-335 and
-543 in 20 patients with bone metastases was significantly
lower than that in the 15 patients without bone metastases (P=0.035
and P=0.028) (Fig. 3A and B).
Secondly, the mRNA and protein expression patterns of eNOS were
determined in the primary PCa with and without bone metastasis
samples using RT-qPCR and western blot analysis. The mRNA level of
eNOS was significantly upregulated in primary PCa without bone
metastasis compared with those with bone metastasis (Fig. 3D), and consistently, the protein
expression of eNOS was increased in the PCa with bone metastasis as
well (Fig. 3C). Thirdly, whether
the presence of bone metastasis was associated with the total serum
PSA levels or Gleason scores was evaluated, and no significant
difference was identified (Table
I). Finally, whether the expression levels of miR-335
and -543 were correlated with total serum level and Gleason
score in primary PCa was assessed, and there was no correlation
between the expression of miR-335/-543 and total PSA level
or Gleason score was noted (data not shown).
Upregulation of miR-335 and -543 reduces
the expression of eNOS and its effect on the skeletal
aggressiveness of PC-3 cells
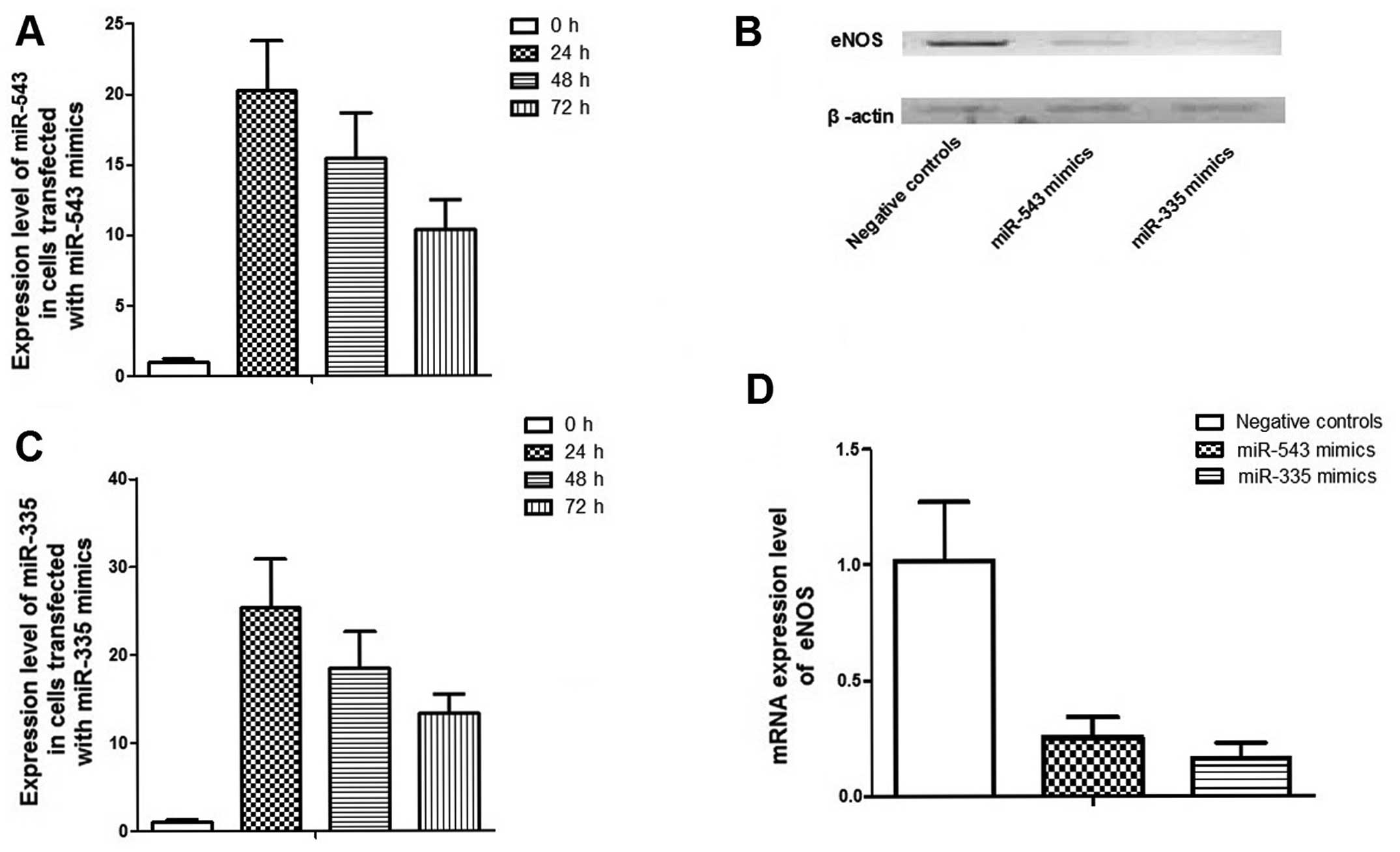
To investigate the role of miR-335 and
-543 in the control of PCa metastasis, scramble controls,
miR-335 and -543 mimics were transfected into PC-3
cells, which are characterized by bone metastasis. Fold changes in
the relative expression of miR-335 and -543
transfected PC-3 cell lines were much higher than that in the cells
transfected with the control (Fig. 4A
and B). Transfection of miR-335 and -543 mimics
could significantly suppress mRNA and protein expression levels of
eNOS in PC-3 cells (Fig. 4C and
D). Migration and invasion assays were performed in
vitro. Notably, cell migration was observed by the wound
healing assay and it was much slower when PC-3 cells were
transfected with miR-335 and -543 compared to the
PC-3 cells transfected with the control (Fig. 5). The invasive property of PC-3
cells was examined by the Transwell-Matrigel penetration assay,
which depicted that much fewer cells penetrated through the
gel-membrane section when PC-3 cells were transfected with
miR-335 and -543 compared to PC-3 cells transfected
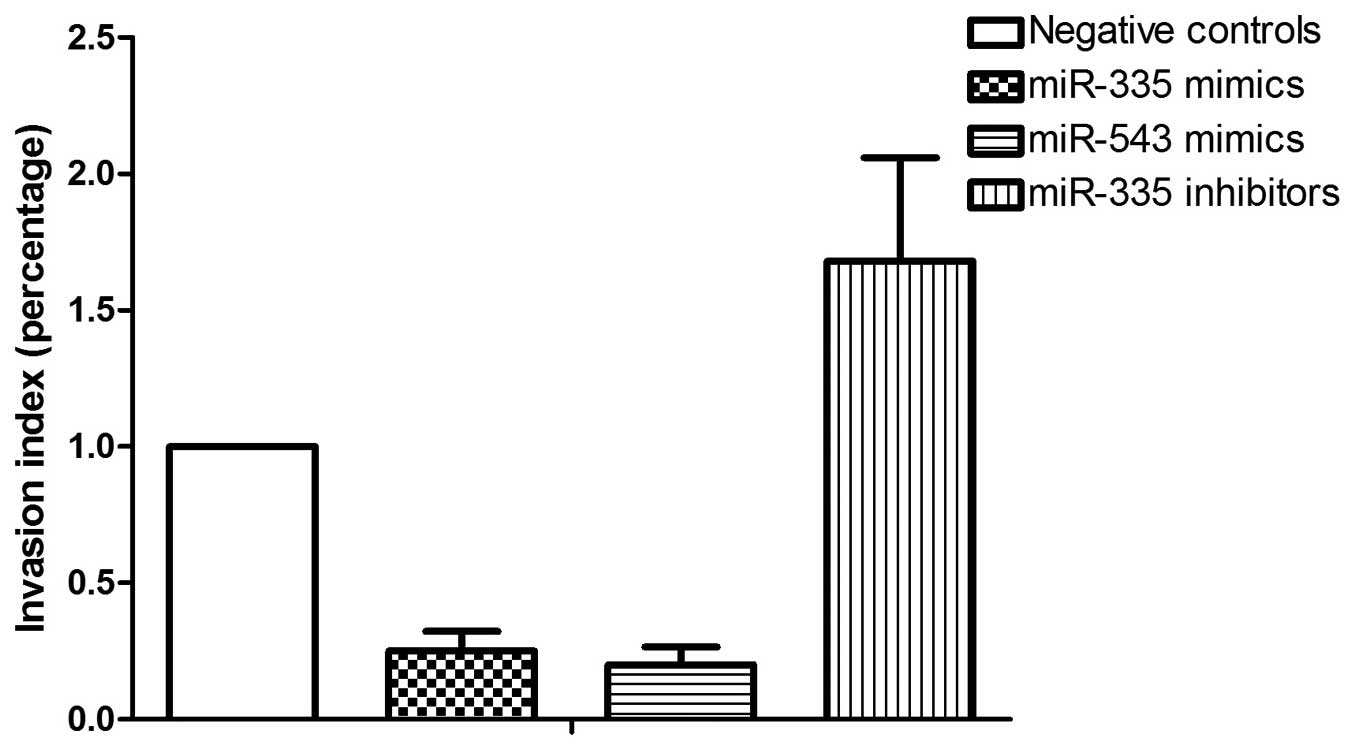
with the control (Fig. 6). The
invasive property of PC-3 cells was significantly inhibited by
miR-335 and -543, and was more evidently inhibited by
miR-335.
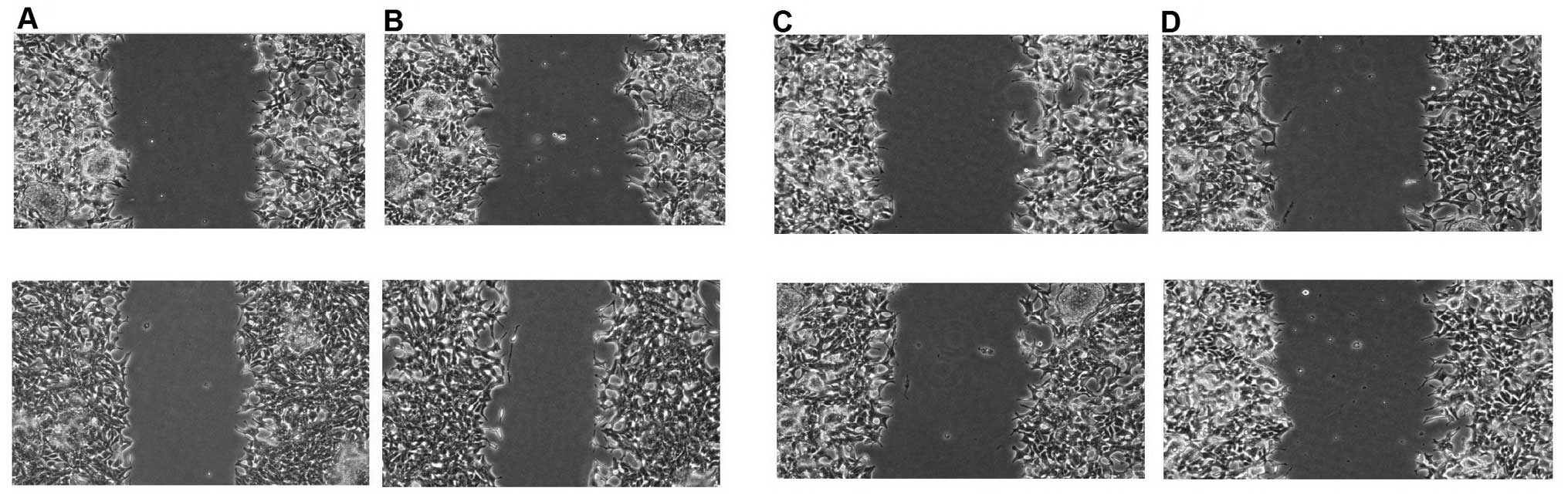 | Figure 5Migratory capability of PC-3
transfected with (A) the negative control (upper, 0 h; lower, 24
h), (B) miR-335 inhibitors (upper, 0 h; lower, 24 h), (C)
miR-543 mimics (upper, 0 h; lower, 24 h) and (D)
miR-335 mimics (upper, 0 h; lower, 24 h). |
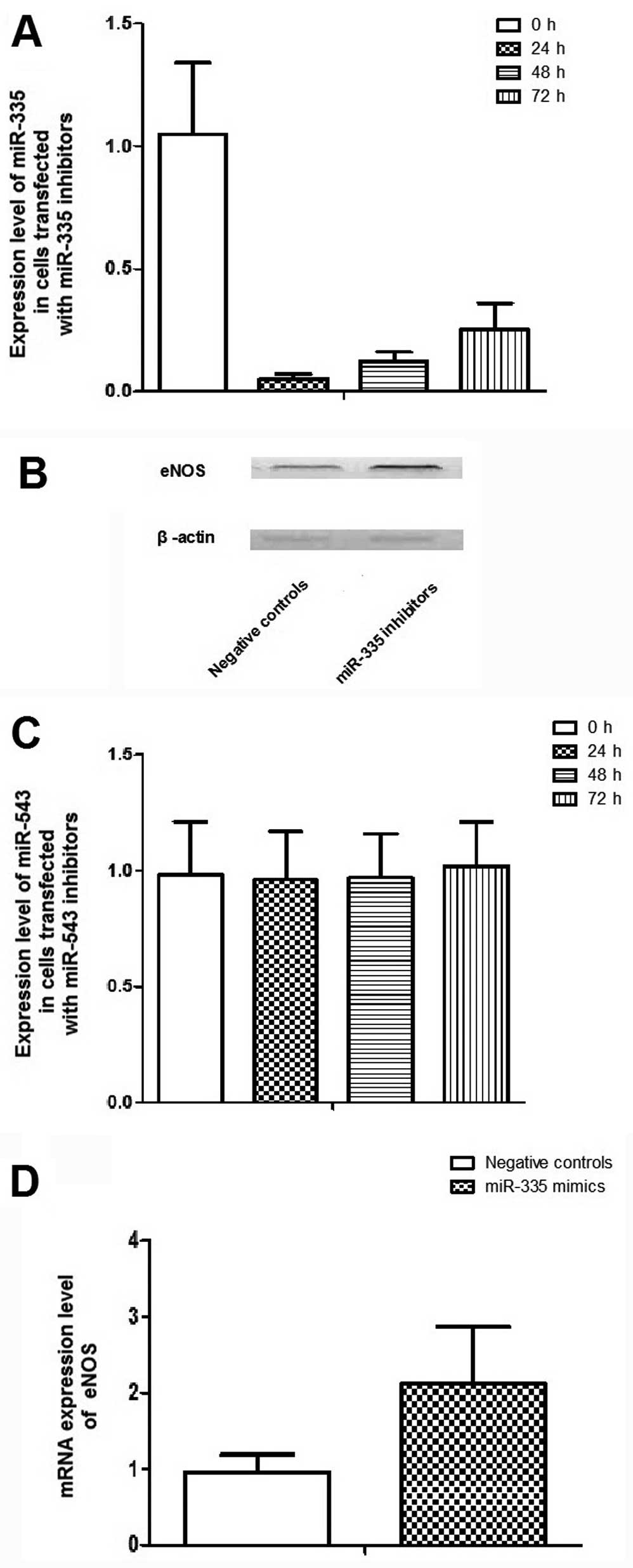
Downregulation of miR-335 by introduction
of its inhibitors promotes the expression of eNOS and its effect on
the skeletal aggressiveness of PC-3 cells
To further explore the role of miR-335 and
-543 in the control of PCa metastasis, scramble controls,
miR-335 and -543 inhibitors were transfected into
PC-3 cells. Fold changes in the relative expression of
miR-335 transfected PC-3 cell lines were much lower than
that of the cells transfected with the control, whereas the
endogenous expression level of miR-543 was too low to be
further lowered (Fig. 7).
Transfection of the miR-335 inhibitors could significantly
promote mRNA and protein expression levels of eNOS in PC-3 cells
(Fig. 7). Migration and invasion
assays were performed in vitro. Cell migration was observed
by the wound healing assay and it was much slower when the PC-3
cells were transfected with miR-335 and -543 compared
to the PC-3 cells transfected with the control, in a time-dependent
manner (Fig. 5).
Discussion
In the present study, the expression levels of
miR-335 and -543 were identified to be downregulated
in skeletal metastatic PCa tumors compared with the primary tumors.
Primary PCa patients without metastasis had significantly higher
expression levels of miR-335 and -543 compared to the
patients with bone metastasis. The upregulation of miR-335
and -543 reduced the aggressiveness of PC-3 cells, and
suppressed the expression of eNOS. These results suggest that
miR-335 and -543 may have a significant role in the
control of bone metastasis of PCa.
miRNAs have been repeatedly reported to be involved
in the regulation of cancer metastasis and invasiveness. Among
those, miR-335 has been identified as a potent suppressor of
tumor cell migration and invasion in multiple forms of cancer. For
example, in breast cancer cells, upregulation of miR-335
significantly decreased the invasive ability in a Boyden chamber
assay and ectopic overexpression of the same miR also reduced the
formation of metastasis in lung (17). Xu et al (18) reported that miR-335 may act
as a potent metastasis suppressor in gastric cancer cells via
directly targeting SP1 and indirectly modulating the Bcl-w-induced
activation of the phosphoinositide 3-kinase-Akt-Sp1 signaling
pathway, showing evidently altered expression of miR-335 at
different stages of gastric cancer and suggesting that the miR
could be a therapeutic target and prognostic factor for gastric
cancer. Using an RT-qPCR assay, miR-335 was reported to be
downregulated in breast cancer tissues and sera (19). Similarly, the low expression of
miR-335 was significantly associated with adrenocortical
carcinomas and acute myeloid leukemia (20,21). Sorrentino et al (22) demonstrated that downregulation of
miR-335 may be responsible for the development of
chemoresistance in ovarian cancer cells. miR-335 has also
been shown to indirectly regulate the myosin-driven motor activity
of the cytoskeleton through modulating the expression levels of
ROCK1, MAPK1 and LRG1 (23).
Subsequently, the same research group extended this by showing that
miR-335 can directly regulate actin filament assembly and
disassembly by targeting the Formin family of actin nucleators.
Even though the majority of research data in the literature favors
the tumor suppressive or anti-metastatic role of miR-335,
overexpression of miR-335 may also have an important role in
the pathogenesis of numerous types of cancer, including colonic
cancer, pediatric acute leukemia and multiple myeloma (24,25,19), and such discrepancy could be
attributed to the tissue specificity or ethnic diversity.
To the best of our knowledge, there is only one
study in the literature regarding the regulatory role of
miR-543 in malignant cells, where exogenous expression of
miR-543 in aggressive endometrial cancer cell lines was
found to impair tumor cell migration and invasion, endogenous
miR-543 expression is substantially decreased in malignant
versus normal endometrium tissue, and the levels of miR-543
inversely correlated with mRNA levels of Focal adhesion kinase
(FAK) and Twist homolog 1 (TWIST1), suggesting that FAK and TWIST1
may be effectors mediating the inhibitory effect of miR-543
on the migratory ability of tumor cells (26).
In the present study, 4 significantly downregulated
miRNAs were identified in bone metastatic PCa samples using
microarray analysis, and the downregulation of 2 among these was
confirmed in an expanded pool of samples using RT-qPCR, showing
that the expression of miR-335 and -543 were lower in
bone metastasis samples compared to primary PCa samples.
Furthermore, the expression of miR-335 and -543 were
further upregulated in the sample tissues collected from the
primary PCa without bone metastasis compared with the
aforementioned primary PCa with bone metastasis, exhibiting the
distinct expression patterns of miR-335 and miR-543
in the different types of tumor tissues: PCa without bone
metastasis > PCa with bone metastasis > bone metastatic
tissues.
Currently, the literature presents contradictory
findings regarding the role of nitric oxide (NO) production in the
control of cancer cell behavior. Certain studies have showed that
elevated NO could facilitate vascularization in tumor cells by
promoting angiogenesis, which is believed to be an important step
in the formation of metastasis. It also could enhance mutagenesis
through interacting with other free radicals to form cytotoxic
compounds, such as peroxynitrite, leading to DNA damage (27). Simultaneously, the upregulation of
NO that damages DNA may promote the growth of tumors via activating
oncogenes and/or inhibiting tumor suppressor genes (28). By contrast, it was also reported
that NOS activity was negatively correlated with tumor progression,
which could be attributed to the ability of NO to protect cells
from DNA damage by enhancing the activity of DNA-dependent
protein-kinase catalytic subunit (DNA-PKcs), destroying tumor cells
and stimulating apoptosis (29–31). Such discrepancy in the literature
could be largely explained by the differential sensitivity of tumor
cells to NO-mediated cytostasis or apoptosis, and clonal
accumulation of NO-resistant or NO-dependent cells in different
types of cancer (32). It was
also reported that eNOS, mapped to 7q35–7q36 in the human
chromosome, may have an important role in the progression of PCa
(33), and this is in line with
the present results that exogenous overexpression of miR-335
and -543 could synergistically downregulate eNOS in prostate
cancer cells, and suppress the migratory capability, as well as the
invasive ability, of the cancer cells by suppressing the gene.
In conclusion, the present findings suggest that
miR-335 and -543 may have significant roles in the
bone metastasis of PCa and may be involved in the regulation of the
migratory capability or invasive ability of the tumor cells. These
two miRs may also be clinically used as novel biomarkers in
discriminating different stages of human PCa and predicting the
possibility of metastasis or even as therapeutic targets in bone
metastasis of PCa.
Acknowledgments
The present study was fully sponsored by the Natural
Science Foundation of China with grant no. 81373762.
References
|
1
|
Ferlay J, Autier P, Boniol M, Heanue M,
Colombet M and Boyle P: Estimates of the cancer incidence and
mortality in Europe in 2006. Ann Oncol. 18:581–592. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Petrovich Z, Lieskovsky G, Stein JP,
Huberman M and Skinner DG: Comparison of surgery alone with surgery
and adjuvant radiotherapy for pT3N0 prostate cancer. BJU Int.
89:604–611. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Spahn M, Joniau S, Gontero P, Fieuws S,
Marchioro G, Tombal B, Kneitz B, Hsu CY, Van Der Eeckt K, Bader P,
et al: Outcome predictors of radical prostatectomy in patients with
prostate-specific antigen greater than 20 ng/ml: A European
multi-institutional study of 712 patients. Eur Urol. 58:1–7. 10–11.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wilt TJ, Brawer MK, Jones KM, Barry MJ,
Aronson WJ, Fox S, Gingrich JR, Wei JT, Gilhooly P, Grob BM, et al:
Prostate Cancer Intervention versus Observation Trial (PIVOT) Study
Group: Radical prostatectomy versus observation for localized
prostate cancer. N Engl J Med. 367:203–213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roodman GD: Mechanisms of bone metastasis.
N Engl J Med. 350:1655–1664. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mundy GR: Metastasis to bone: Causes,
consequences and therapeutic opportunities. Nat Rev Cancer.
2:584–593. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ye L, Kynaston HG and Jiang WG: Bone
metastasis in prostate cancer: Molecular and cellular mechanisms
(Review). Int J Mol Med. 20:103–111. 2007.PubMed/NCBI
|
|
8
|
Catto JW, Miah S, Owen HC, Bryant H, Myers
K, Dudziec E, Larré S, Milo M, Rehman I, Rosario DJ, et al:
Distinct microRNA alterations characterize high- and low-grade
bladder cancer. Cancer Res. 69:8472–8481. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pang Y, Young CY and Yuan H: MicroRNAs and
prostate cancer. Acta Biochim Biophys Sin (Shanghai). 42:363–369.
2010. View Article : Google Scholar
|
|
13
|
Wang G, Wang Y, Feng W, Wang X, Yang JY,
Zhao Y, Wang Y and Liu Y: Transcription factor and microRNA
regulation in androgen-dependent and -independent prostate cancer
cells. BMC Genomics. 9(Suppl 2): S222008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Si ML, Zhu S, Wu H, Lu Z, Wu F and Mo YY:
miR-21-mediated tumor growth. Oncogene. 26:2799–2803. 2007.
View Article : Google Scholar
|
|
15
|
Lin SL, Chiang A, Chang D and Ying SY:
Loss of mir-146a function in hormone-refractory prostate cancer.
RNA. 14:417–424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peng X, Guo W, Liu T, Wang X, Tu X, Xiong
D, Chen S, Lai Y, Du H, Chen G, et al: Identification of miRs-143
and -145 that is associated with bone metastasis of prostate cancer
and involved in the regulation of EMT. PLoS One. 6:e203412011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tavazoie SF, Alarcón C, Oskarsson T, Padua
D, Wang Q, Bos PD, Gerald WL and Massagué J: Endogenous human
microRNAs that suppress breast cancer metastasis. Nature.
451:147–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu Y, Zhao F, Wang Z, Song Y, Luo Y, Zhang
X, Jiang L, Sun Z, Miao Z and Xu H: MicroRNA-335 acts as a
metastasis suppressor in gastric cancer by targeting Bcl-w and
specificity protein 1. Oncogene. 31:1398–1407. 2012. View Article : Google Scholar :
|
|
19
|
Wang YX, Zhang XY, Zhang BF, Yang CQ, Chen
XM and Gao HJ: Initial study of microRNA expression profiles of
colonic cancer without lymph node metastasis. J Dig Dis. 11:50–54.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marcucci G, Maharry K, Radmacher MD,
Mrózek K, Vukosavljevic T, Paschka P, Whitman SP, Langer C, Baldus
CD, Liu CG, et al: Prognostic significance of, and gene and
microRNA expression signatures associated with, CEBPA mutations in
cytogenetically normal acute myeloid leukemia with high-risk
molecular features: A Cancer and Leukemia Group B Study. J Clin
Oncol. 26:5078–5087. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Soon PS, Tacon LJ, Gill AJ, Bambach CP,
Sywak MS, Campbell PR, Yeh MW, Wong SG, Clifton-Bligh RJ, Robinson
BG, et al: miR-195 and miR-483-5p identified as predictors of poor
prognosis in adrenocortical cancer. Clin Cancer Res. 15:7684–7692.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sorrentino A, Liu CG, Addario A, Peschle
C, Scambia G and Ferlini C: Role of microRNAs in drug-resistant
ovarian cancer cells. Gynecol Oncol. 111:478–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lynch J, Fay J, Meehan M, Bryan K, Watters
KM, Murphy DM and Stallings RL: MiRNA-335 suppresses neuroblastoma
cell invasiveness by direct targeting of multiple genes from the
non-canonical TGF-β signalling pathway. Carcinogenesis. 33:976–985.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ronchetti D, Lionetti M, Mosca L, Agnelli
L, Andronache A, Fabris S, Deliliers GL and Neri A: An integrative
genomic approach reveals coordinated expression of intronic
miR-335, miR-342, and miR-561 with deregulated host genes in
multiple myeloma. BMC Med Genomics. 1(37)2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang H, Luo XQ, Zhang P, Huang LB, Zheng
YS, Wu J, Zhou H, Qu LH, Xu L and Chen YQ: MicroRNA patterns
associated with clinical prognostic parameters and CNS relapse
prediction in pediatric acute leukemia. PLoS One. 4:e78262009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bing L, Hong C, Li-Xin S and Wei G:
MicroRNA-543 suppresses endometrial cancer oncogenicity via
targeting FAK and TWIST1 expression. Arch Gynecol Obstet.
290:533–541. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ying L and Hofseth LJ: An emerging role
for endothelial nitric oxide synthase in chronic inflammation and
cancer. Cancer Res. 67:1407–1410. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lala PK and Chakraborty C: Role of nitric
oxide in carcinogenesis and tumour progression. Lancet Oncol.
2:149–156. 2001. View Article : Google Scholar
|
|
29
|
Dhar A, Brindley JM, Stark C, Citro ML,
Keefer LK and Colburn NH: Nitric oxide does not mediate but
inhibits transformation and tumor phenotype. Mol Cancer Ther.
2:1285–1293. 2003.
|
|
30
|
Simeone AM, Colella S, Krahe R, Johnson
MM, Mora E and Tari AM: N-(4-Hydroxyphenyl)retinamide and nitric
oxide pro-drugs exhibit apoptotic and anti-invasive effects against
bone metastatic breast cancer cells. Carcinogenesis. 27:568–577.
2006. View Article : Google Scholar
|
|
31
|
Fabbri F, Brigliadori G, Ulivi P, Tesei A,
Vannini I, Rosetti M, Bravaccini S, Amadori D, Bolla M and Zoli W:
Pro-apoptotic effect of a nitric oxide-donating NSAID, NCX 4040, on
bladder carcinoma cells. Apoptosis. 10:1095–1103. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jadeski LC, Chakraborty C and Lala PK:
Role of nitric oxide in tumour progression with special reference
to a murine breast cancer model. Can J Physiol Pharmacol.
80:125–135. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qian J, Jenkins RB and Bostwick DG:
Genetic and chromosomal alterations in prostatic intraepithelial
neoplasia and carcinoma detected by fluorescence in situ
hybridization. Eur Urol. 35:479–483. 1999. View Article : Google Scholar : PubMed/NCBI
|