Introduction
The immune system-released activating agent (ISRAA)
is a 15 kDa novel immune mediator encoded by a gene located on
mouse chromosome 14 (GenBank accession no. EU552928) (1) and is believed to mediate signaling
between the nervous and the immune system. ISRAA was initially
considered to have a cytokine-like activity due to several factors,
including its relatively low molecular mass, its biological effects
on a broad spectrum of cell types, such as T and B cells and
monocytes/macrophages, the ability of the same cell populations to
respond to ISRAA by producing cytokines, such as interferon
(IFN)-γ, as well as the fact that ISRAA is lined up on chromosome
14 on the mouse system where several low molecular mass cytokines
are mapped (1). Although ISRAA
was identified in the mouse, the alignment has shown that the
molecule has conserved regions between many species (2). Furthermore, the function of ISRAA
has been found to be dose-dependent, as it has the ability to
stimulate the proliferation of human peripheral blood mononuclear
cells (hPBMCs) at a low concentration of 50 pg, while a higher
concentration of 5 µg has been shown to result in apoptosis
(2).
In general, these two pathways for proliferation and
apoptosis are controlled by a variety of (growth-deleted) factors
that bind to receptors on the cell surface and initiate a cascade
of events (signal transduction pathways), that convey the message
from the receptors to the nucleus where transcription factors bind
to DNA, turning either 'ON' or 'OFF' the production of proteins
that induce cell proliferation or death (3–5).
Such activities may be induced directly by the effective molecule,
e.g., ISRAA, or indirectly by the production of immune mediators,
such as cytokines (2). Cytokines
are low molecular mass proteins (~5–20 kDa) that are important in
cell signaling. They are released by cells and act on the same cell
(autocrine) or on other cells in a paracrine or endocrine manner.
Cytokines are produced by a broad range of cells, acting via
receptors and are particularly important for the immune system as
they modulate the balance between humoral and cell-mediated immune
responses. In addition, they regulate the growth, maturation and
responsiveness of particular cell populations. Thus, they are
important in health and disease, and more specifically, in host
responses to infection, immune responses, inflammation, trauma,
sepsis, cancer and reproduction (6).
The identification of ISRAA by our laboratory
prompted us to investigate the mechanisms through which the
activation of ISRAA initiates signaling cascades to induce
particular effects, either directly or indirectly via cytokine
production. We also wished to further characterize the signaling
pathways involved in order to gain insight into the mechanisms
through which ISRAA integrates the regulatory mechanisms and how
information flows through a system.
Understanding signaling pathways is challenging,
since there are hundreds of signaling pathways, as well as hundreds
or even thousands of different proteins used by the cell at the
same time. The fact that many signals are transient events further
complicates our understanding of signaling pathways. Furthermore,
the response of some pathways depends on the strength and the time
of the stimulus (7,8). In the present study, we stimulated
hPBMCs with ISRAA in order to examine the broad spectrum of
cytokines and explore the activated signaling pathways by detecting
phosphorylated proteins from second messengers down to the nuclear
translocation of transcription factors.
Materials and methods
Isolation of hPBMCs
This study was approved by the Ethics Committee of
Arabian Gulf University, Manama, Bahrain. Blood samples in EDTA
tubes (Becton, Dickinson and Co., San Diego, CA, USA) were obtained
from apparently healthy donors; none of the participants reported
any history of acute or chronic medical problems, and informed
consent was obtained from all donors. hPBMCs were purified using
the Ficoll-Hypaque technique by diluting the blood 1:2 with
phosphate-buffered saline. Subsequently, 10 ml of this diluted
blood were carefully layered onto the 3 ml Ficoll-Hypaque Plus
cushion (Pharmacia Biotech, Uppsala, Sweden) and centrifuged at 400
× g for 30–40 min at 18–20°C. The upper layer was drawn off using a
clean Pasteur pipette, leaving the lymphocyte layer undisturbed at
the interface, which was then washed twice using phosphate-buffered
saline and once with RPMI-1640 containing 1% FBS. The pellet was
reconstituted in 5 ml of complete RPMI-1640 (Life Technologies,
Grand Island, NY, USA) culture medium containing 10%
heat-inactivated fetal bovine serum, 2 mM L-glutamine, 10 mM HEPES
buffer and antibiotic (50 U/ml penicillin, 50 µg/ml
streptomycin). The viability of the mono-nuclear cells was
confirmed by the trypan blue exclusion test. The viable cell
numbers were counted using a hemocytometer. The viability of the
mononuclear cells was >95% on average, as previously
demonstrated (9).
The obtained mononuclear cells were rapidly plated
onto a tissue culture plate (Falcon 3001) at a concentration of
1×106 cells/ml and incubated in a 37°C, 95% air, 5%
CO2, 100% humidity incubator. Subsequently, the cells
were stimulated with 5 µg and 50 pg concentrations of ISRAA,
and 5 µg of phytohaemagglutinin (PHA; Cat. no. L8902;
Sigma-Aldrich, St. Louis, MO, USA) as a positive control (PC).
Cells without stimulation were used as a negative control (NC).
Each concentration was used in triplicate. The cells were collected
at 0, 5, 10, 15 and 30 min and at 1, 3, 6, 12, 24 and 48 h;
however, stimulation for 24 h was found to be the optimal time
point (data not shown).
Identification and production of
ISRAA
ISRAA used in this study was obtained as previously
described (1). The
QIAexpressionist™ protocol was used for the cloning of the full
length ISRAA gene and for high-level expression and purification of
the 6xHis-tagged protein according to the manufacturer's
instructions (Qiagen, Inc., Valencia, CA, USA). In brief, protein
expression and purification were performed by the transformation of
two constructs of bacterial expression vector (pQE-32 vector) in 5
ml Lysogeny broth (LB) culture medium; protein expression was
induced using isopropyl β-D-1-thiogalactopyranoside (IPTG) for one
construct, while the other construct was kept uninduced to serve as
a negative control. The expression was tested on a small scale
first, and was then tested on a large scale using 1 l LB culture
medium. Protein purification was performed by immobilized-metal
affinity chromatography (IMAC) as the initial purification step. In
addition, column chromatography under denaturing conditions with
Ni-NTA magnetic beads (Qiagen, Hilden, Germany) was carried out to
achieve further purification. Subsequently, the protein was
subjected to IMAC again to remove the protease and any cellular
proteins that bound to the metal affinity resin, followed by sodium
dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and
mass spectrometry to determine the expression and purification of
the ISRAA protein. Following elution, the purified protein showed
2.212 optical density (OD) using the 280 nm absorbance wavelength,
and western blot analysis was carried out using anti-His antibody
(Fig. 10) indicating a high
level of protein purification, which was 80–95%; polymyxin B was
used to neutralize the effect of the Gram-negative capsule
endotoxins. Purified recombinant protein when titrated showed that
there are two concentrations considered to be optimal in inducing
the proliferation and cytotoxicity of the mononuclear cells; these
two concentrations were 50 pg and 5 µg. To ensure that those
two effects are due to the ISRAA recombinant protein, polyclonal
antibodies to ISRAA were used, and the effect of ISRAA was
neutralized by the antibodies.
MTT cell proliferation and cytotoxicity
assay
The MTT Cell Proliferation Assay (ATCC, Manassas,
VA, USA) was used to measure the cell proliferation rate. The
protocol was performed to examine the proliferative and cytotoxic
effects of ISRAA on the cells according to manufacturer's
instructions. Cell suspension was harvested by centrifugation, and
was then resuspended at 1×106 cells/ml. A total of 100
µl of cell suspension was plated in each well in triplicate.
Three control wells of medium were left alone to provide the blanks
for the absorbance readings. Another three wells with cell
suspension alone as a negative control and three wells stimulated
with 5 µg of PHA as a positive control were used. A total of
10 µl of 50 pg and 5 µg ISRAA protein was added to
each well in triplicate. The plates were incubated at 37°C in 5%
CO2 for 24 h. Subsequently, 10 µl of 5 mg/ml MTT
reagent were added to each well, including controls and incubated
for 4 h at 37°C. A total of 100 µl of MTT solvent (acidic
isopropanol 0.04 M HCl in absolute isopropanol) was then added to
all wells. The plates were covered with tinfoil and agitated on an
orbital shaker for 15 min. Finally, the absorbance at 690 nm was
recorded, and the cytocide rate was calculated using the following
formula: cytocide rate (%) = (OD control - OD experiment)/OD
control x100. The assay was also used to determine the effects of
the mitogen-activated protein kinase (MAPK) pathway inhibitor,
U0126 (MEK1/2 inhibitor, from Cell Signaling Technology, Inc,
Danvers, MA, USA; Cat. no. 9903), on ISRAA-induced apoptosis. The
inhibitors were added at a concentration of 10 µM 1 h prior
to stimulation.
Measurement of CD45/CD3/CD8/CD4-positive
cell count by flow cytometry
To examine the effects of various concentrations of
ISRAA on the absolute counts of mature T lymphocytes
(CD3+) and the percentage of helper/inducer
(CD3+/CD4+) and suppressor/cytotoxic
(CD3+/CD8+) T cell subsets, MultiTEST CD3
FITC/CD8, PE/CD45, PerCP/CD4 APC with Trucount tubes (Becton,
Dickinson and Co.) containing a freeze-dried pellet of fluorescent
beads in a single-use tube were utilized. Samples were stained
directly in a Trucount tube. The lyophilized pellet in the tube was
dissolved releasing a known number of fluorescent beads. During
analysis, the absolute number (cells/µl) of positive cells
in the sample was determined by comparing cellular events to bead
events. A BD Biosciences flow cytometer was used for flow
cytometry, as previously descrbied (10). The BD FACSCalibur™ flow cytometer
was used with CaliBRITE™ beads and FACSComp version 4 and Multiset™
Software as recommended by the manufacturer. This method was also
used to determine the effects of the p42 MAPK pathway inhibitor
(U0126) on ISRAA-induced proliferation. The inhibitors were added
at a concentration of 10 µM 1 h prior to stimulation.
Detection of cytokines by flow
cytometry
To examine the effects of various concentrations of
ISRAA at separate intervals on cytokine production BD Cytometric
Bead Array (CBA), Human Soluble Protein and CBA Flex Set for the
cytokines, interleukin (IL)-4, IL-6, IL-10, IL-17A, IFN-γ, tumor
necrosis factor (TNF)-α, IL-8 and transforming growth factor
(TGF)-β (Becton, Dickinson and Co.) were used according to the
manufacturer's instructions and as previously described (11). To determine the pathway adopted by
ISRAA in the production of IL-6, pharmacological inhibitors of
MAPKs [U0126, MEK1/2 inhibitor and SB20358, p38 inhibitor (Cell
Signaling Technology, Inc; Cat. no. 5633)] were used and the levels
of IL-6 were monitored using CBA with flow cytometry.
Quantitative sandwich enzyme-linked
immunosorbent assay (ELISA) to detect IL-6
The hPBMCs were cultured and treated as described
above. All the cells were incubated at 37°C in 5% CO2
for 24 h. The supernatants were collected and analyzed for IL-6
expression using an ELISA kit (R&D Systems, Minneapolis, MN,
USA).
Bradford protein assay
To perform the western blot analysis and
immunofluorescence assay, the protein concentrations were measured
to standardize the volume of protein extraction used on each
reaction. The Bradford protein assay and nanogram measures were
used (Sigma-Aldrich). This assay was performed in a 96-well plate.
A total of 5 µl of a 0.1–1.4 mg/ml protein sample was used
as a standard. The protein standards (5 µl), the cell
lysates and buffer as a blank were added to the 96-well plate. The
reagent was gently mixed; subsequently, 250 µl of the
Bradford reagent were added and mixed in a shaker for ~30 sec. The
samples were incubated at room temperature for 5–45 min. The
absorbance was then measured at 595 nm. The protein concentration
of the unknown samples was determined by comparing the Net A595
values against the standard curve, as previously described
(12).
MAPK signaling pathway detection
MAPK activity was achieved by measuring the
phosphorylation of extracellular signal-regulated protein kinase
(ERK)1/2 pathways, as the main components of the MAPK downstream
signaling protein which plays a vital role in the IL-6 signaling
pathway. The hPBMCs were grown, counted and adjusted to
106 cells/ml in complete RPMI-1640 growth medium. The
cells were treated with 5 µg and 50 pg concentrations of
ISRAA in triplicate wells, and the cells were then collected at
intervals of 5, 10, 30 and 60 min. Total cell extracts from Jurkat
cells were treated with 12-O-tetradecanoylphorbol-13-acetate (TPA;
Cell Signaling Technology, Inc) as a positive control, and the cell
lysates were then tested for the level and the optimal time of
phosphorylation by western blot analysis of the cell lysates for
phosphorylated (p-)ERK1/2 [by incubating the membrane in p44/p42
MAPK (ERK/1/2 (Thr202/Tyr204; Cell Signaling Technology, Inc; Cat.
no. 4370)]. The pharmacological inhibitor, U0126 (MEK1/2 inhibitor)
(Cell Signaling Technology, Inc.), was used to examine the role of
MAPK in the production of IL-6 as a direct or indirect effect of
ISRAA. The cells were cultured (as described above), and then
treated with 10 µM of U0126 inhibitor for 60 min prior to
stimulation with ISRAA at intervals of 5, 10 and 30 min and then
examined by western blot analysis (see below) for p-ERK1/2
(13).
Signal transducer and activator of
transcription (STAT)3 pathway detection
The hPBMCs were grown, counted and adjusted to
106 cells/ml in complete RPMI-1640 growth medium. The
cells were treated with 50 pg of ISRAA in triplicate wells. The
cells were then collected after 0, 5, 10, 30 and 60 min. IFN-α
treated Jurkat cells were used as a positive control and tested for
the level and the optimal time of phosphorylation by western blot
analysis of the cell lysates with p-Stat3 (Tyr705) antibody (Cell
Signaling Technology, Inc.), as well as for the presence of
unphosphorylated STAT3.
Western blot analysis
The cell lysates were subjected to electrophoresis
on a 12% SDS-PAGE gel and transferred onto a nitrocellulose
membrane. The membranes were blocked in Tris-buffered saline with
5% milk and 0.1% Tween-20. The blots were probed with anti-STAT3
(Cat. no. 9130), anti-p-Stat3 (Tyr705; Cat. no. 9130) and
anti-p-p44/42 MAPK (ERK1/2) (Thr202/Tyr204; Cat. no. 4370)
antibodies overnight and incubated with horseradish
peroxidase-conjugated secondary antibody (Cat. no. 9130) (all from
Cell Signaling Technology, Inc.). Antibody/antigen complex was
visualized using an enhanced chemiluminescent ECL Western Blotting
Substrate (Cell Signaling Technology, Inc.) scanned by an image
analyzer (LAS-1000; Fujifilm, Tokyo, Japan), as previously
described (13).
Immunostaining and fluorescence
microscopy for ERK1/2
The hPBMCs were grown, counted and adjusted to
106 cells/ml in complete RPMI-1640 growth medium. The
cells were treated with 50 pg of ISRAA in triplicate wells. The
cells were collected after 0, 5, 10, 15, 30 and 60 min, and were
then fixed and stained directly in a multi-chamber slide. Using 4%
formaldehyde in PBS, the cells were fixed for 15 min at room
temperature, rinsed three times in PBS for 5 min each time and then
permeabilized with 0.1% Triton X-100 and 5% FCS in PBS at room
temperature for 60 min. The permeabilized cells were incubated with
diluted primary antibody [p-p44/42 MAPK (ERK1/2) (Thr202/Tyr204)
rabbit antibody; Cell Signaling Technology, Inc.] overnight at 4°C.
They were subsequently washed three times in PBS for 5 min each
time before the addition of the fluorochrome-conjugated secondary
antibody [anti-rabbit, IgG (H+L), F(ab')2 fragment (Alexa
Fluor® 488 Conjugate); Cat. no. 4412; Cell Signaling
Technology, Inc.], and were then kept for 1 h at room temperature
in the dark and washed with PBS. Prior to visualization by
fluorescence microscopy, the cells were covered with coverslip
slides with ProLong® Gold Antifade Reagent (Cell
Signaling Technology, Inc.; Cat. no. 9071). Images were obtained
using a fluorescence microscope (Leica DM-3000; Leica Microsystems,
Milton Keynes, UK), as previously described (13).
Statistical analysis
ANOVA was used to compare the levels of significance
between the different groups. The Student's t-test was used to
calculate the levels of significance. A value of p<0.05 was
considered to indicate a statistically significant difference.
Results
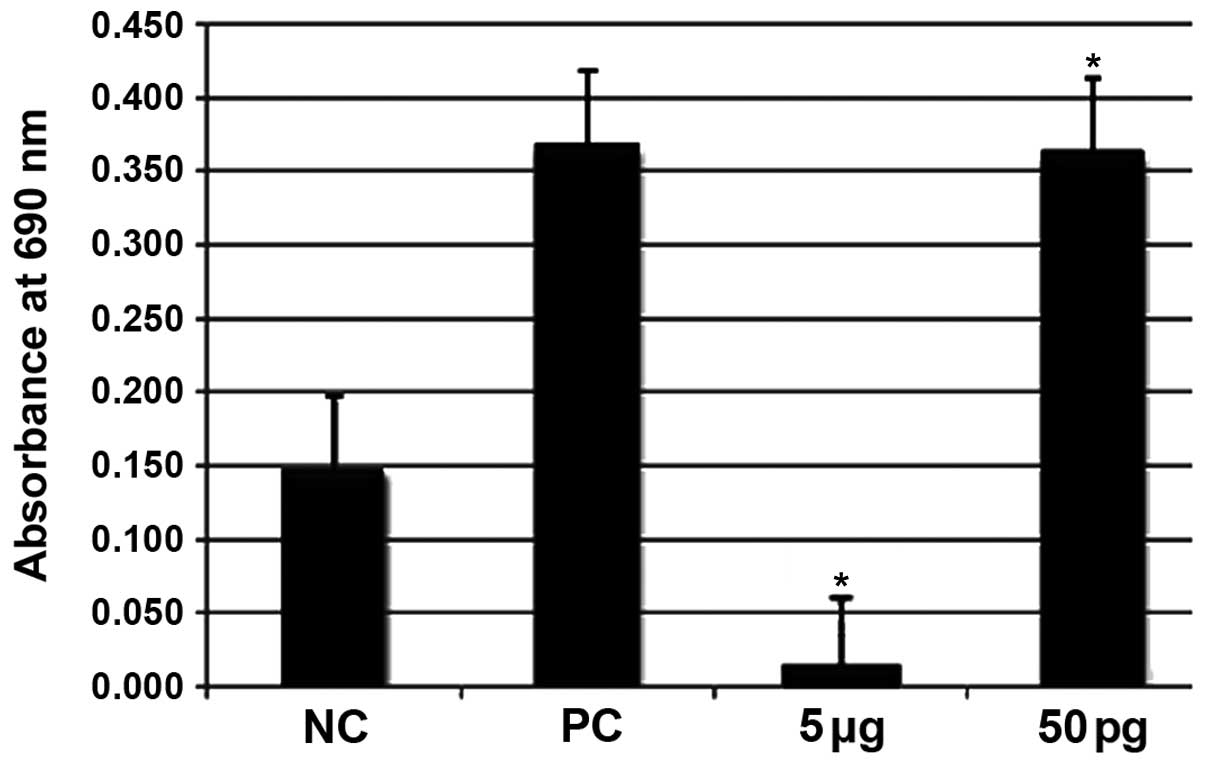
MTT cell proliferation and cytotoxicity
assays
The effects of ISRAA on the cell metabolic rate were
monitored by the MTT assay (Fig.
1). A significant decrease (p<0.05) in the number of cells
was observed following treatment with the highest concentration of
ISRAA (5 µg). However, a significant increase in cell
proliferation was observed following treatment with ISRAA at the
concentration of 50 pg (p<0.05) compared to the unstimulated
cells (negative control, NC). PHA was used as a positive control
(PC) and was found to exert significant proliferative effects
(p<0.05).
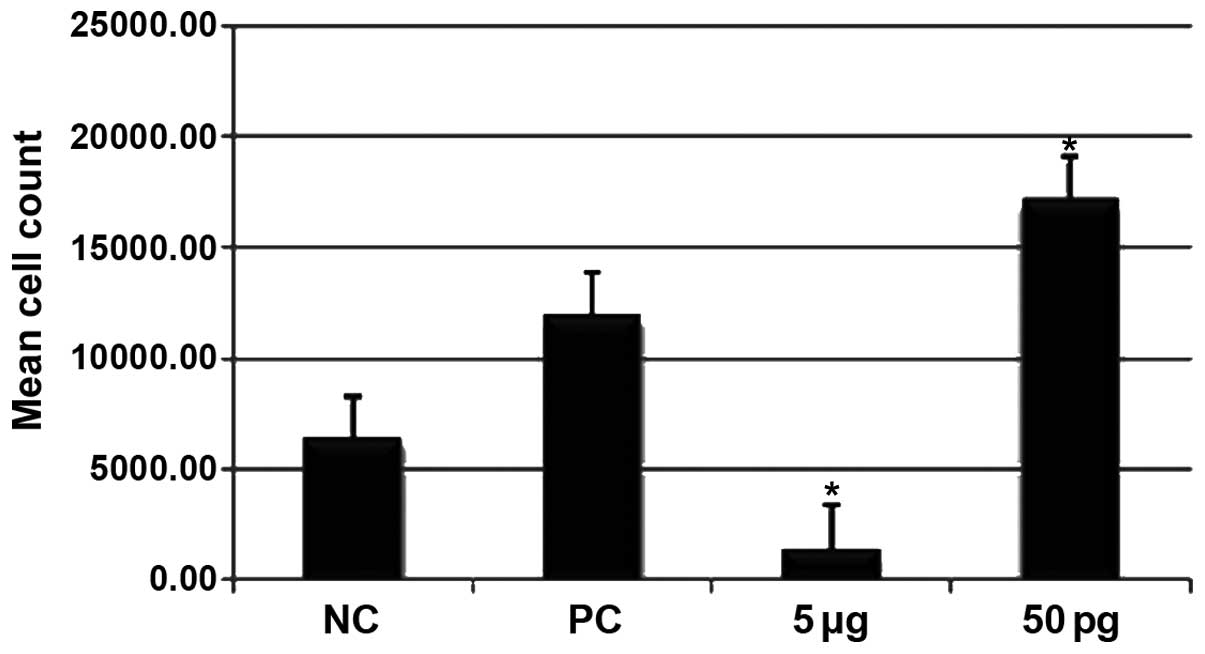
Effects of ISRAA on
CD45/CD3/CD4/CD8-positive hPBMC numbers determined by flow
cytometry
The cells treated with 50 pg ISRAA exhibited a
significant increase in the number of CD45/CD3/CD4/CD8-positive
cells compared to the unstimulated cells and the cells treated with
5 µg ISRAA (p<0.05). Stimulation with 5 µg ISRAA
resulted in a significant decrease in the CD45/CD3/CD4/CD8-positive
cell number (p<0.05) (Fig.
2).
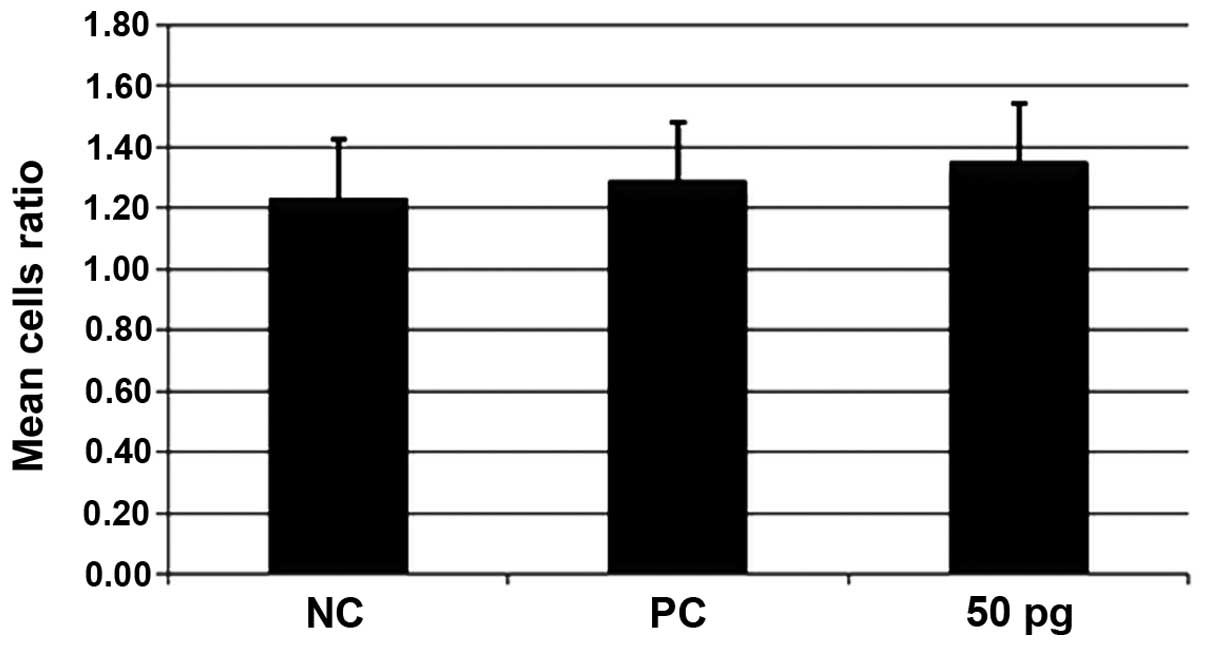
Effect of ISRAA on the ratio of CD4:CD8
proliferated hPBMCs
The hPBMCs were stimulated for 24 h with 50 pg and 5
µg of ISRAA. PHA was used as a positive control (PC) and
unstimulated cells served as a negative control (NC). The cells
were counted using the CD45/CD3/CD4/CD8 MultiTEST count by flow
cytometry. The cells stimulated with 50 pg of ISRAA exhibited the
ability to proliferate. In order to determine the subset of T
lymphocytes which proliferated more as a result of stimulation with
50 pg ISRAA, the ratio of the two subsets, i.e., the helper/inducer
(CD3+/CD4+) and suppressor/cytotoxic
(CD3+/CD8+) was calculated and the results
are shown in Fig. 3. The T/helper
and T/cytotoxic cells both increased in number at the same rate,
with no significant difference observed between the groups (postive
control and group treated with 50 pg ISRAA; p>0.05).
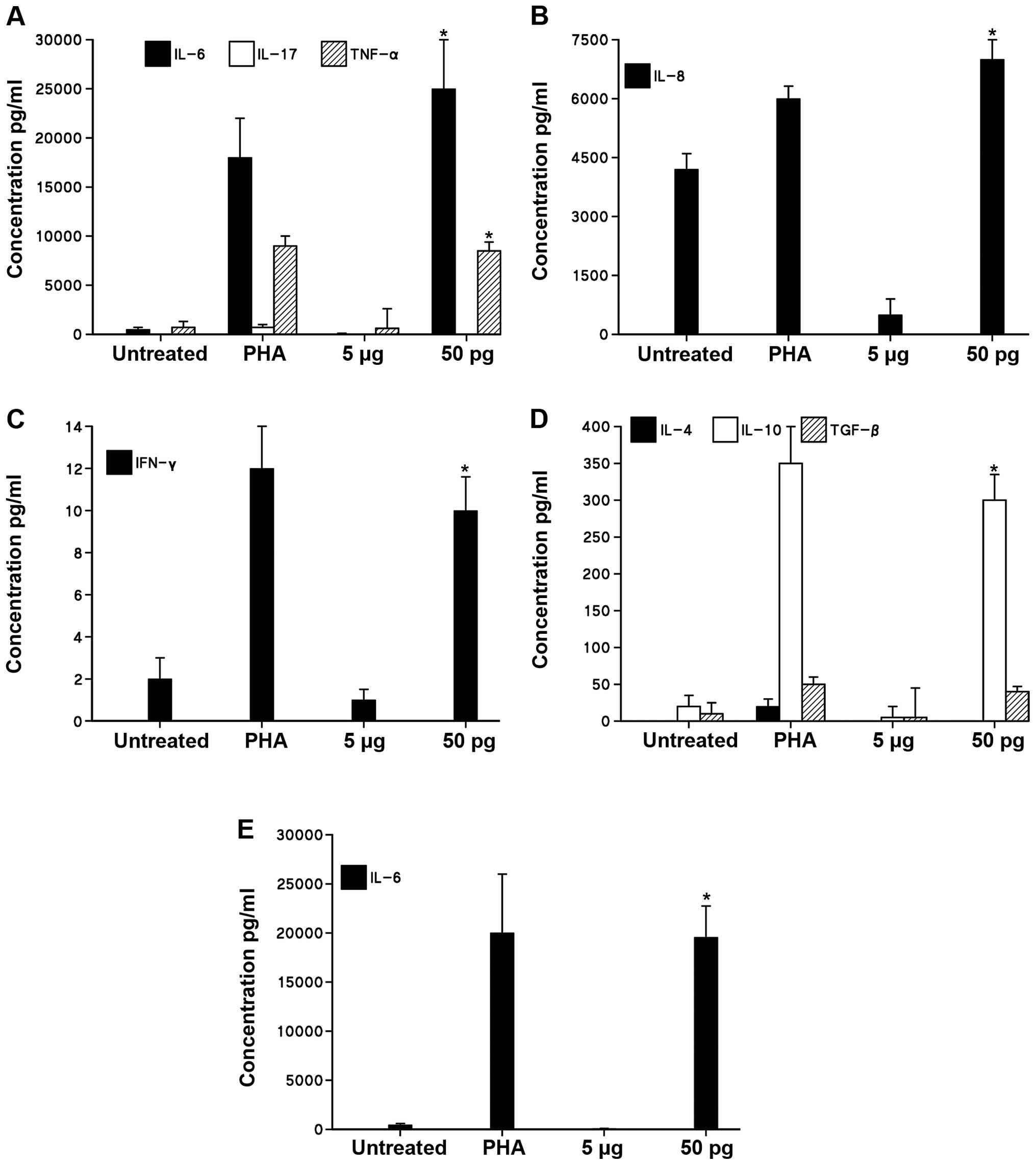
Cytokine production induced by ISRAA
To quantify and analyze the effects of ISRAA on the
expression of a group of pro-inflammatory (IL-6, IL-17, IFN-γ,
TNF-α) and anti-inflammatory cytokines (IL-8, IL-4, IL-10, TGF-β)
produced by the hPBMCs stimulated with 50 pg and 5 µg of
ISRAA, various techniques were used, such as CBA with flow
cytometry and quantitative sandwich ELISA. Kinetic analysis on
cytokine production demonstrated that a 24-h incubation period is
the optimal time point for the maximum induction of the measured
cytokines (data not shown).
As shown in Fig.
4, with the use of CBA with flow cytometry, our results
revealed that stimulation with 50 pg ISRAA markedly increased the
levels of the pro-inflammatory cytokines, IL-6 and TNF-α
(p<0.05), but not those of IL-17 compared to the unstimulated
cells (Fig. 4A). Stimulation with
ISRAA (50 pg) also induced a significant increase in the levels of
IL-8 (Fig. 4B) and IFN-γ
(Fig. 4C) (p<0.05). The
analysis of anti-inflammatory cytokines revealed a significant
induction of IL-10 (p<0.05), but not IL-4 or TGF-β (Fig. 4D). The toxic concentration of 5
µg ISRAA did not significantly alter the levels of all the
examined cytokines.
The cytokine IL-6 exhibited the highest detectable
cytokine levels, as shown by CBA with flow cytometry. These results
were confirmed by ELISA, which revealed a 4-fold increase in IL-6
production in the cells stimulated with 50 pg ISRAA compared to the
unstimulated cells (p<0.05; Fig.
4E).
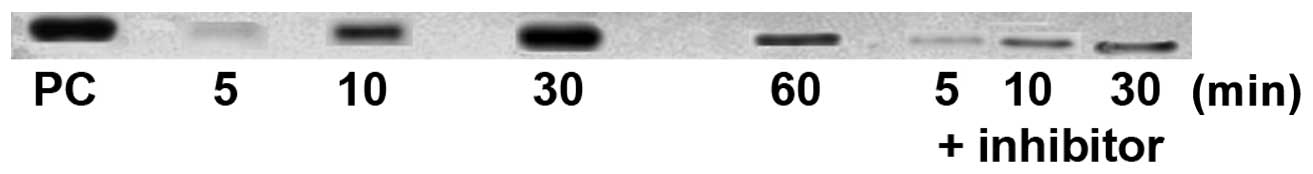
Western blot analysis of p-ERK1/2
The phosphorylation of ERK1/2 as a MAPK downstream
signaling protein was examined by western blot analysis of extracts
from cells treated with 50 pg ISRAA with or without ERK1/2
inhibitor (U0126) at intervals of 5, 10, 30 and 60 min. Total cell
extracts from Jurkat cells treated with TPA were used as a positive
control. The results revealed that detectable ERK1/2
phosphorylation began at 10 min and steadily continued to increase
until the 60-min time point following stimulation, while low levels
of phosphorylation or no phosphorylation were observed in cells
stimulated with ISRAA when the inhibitors were used (Fig. 5).
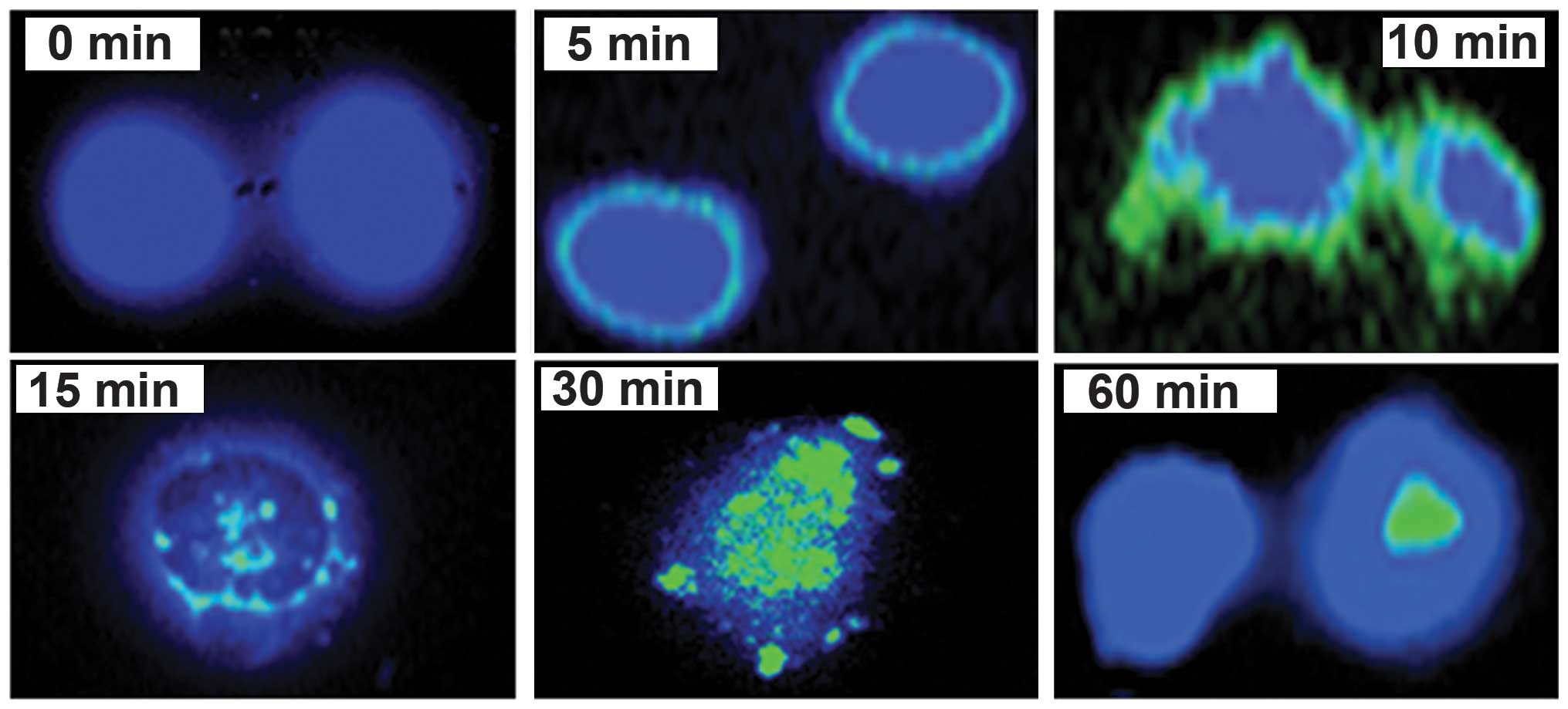
ERK1/2 translocation
Upon activation, ERK1/2 translocates to the nucleus
in order to be transcribed. Using immunofluorescence assay to
reveal the exact time required for p-ERK1/2 to translocate to the
nucleus, the results demonstrated that the phosphorylation and
dimerization of ERK1/2 began at around 5 min, and intensified at
around 10 min. It then began to translocate from the cytoplasm
towards the nucleus at 15 min where some of the phosphorylated
molecules entered the nucleus. This intensified at 30 min, and by
60 min, a complete translocation to the nucleus had occurred
(Fig. 6).
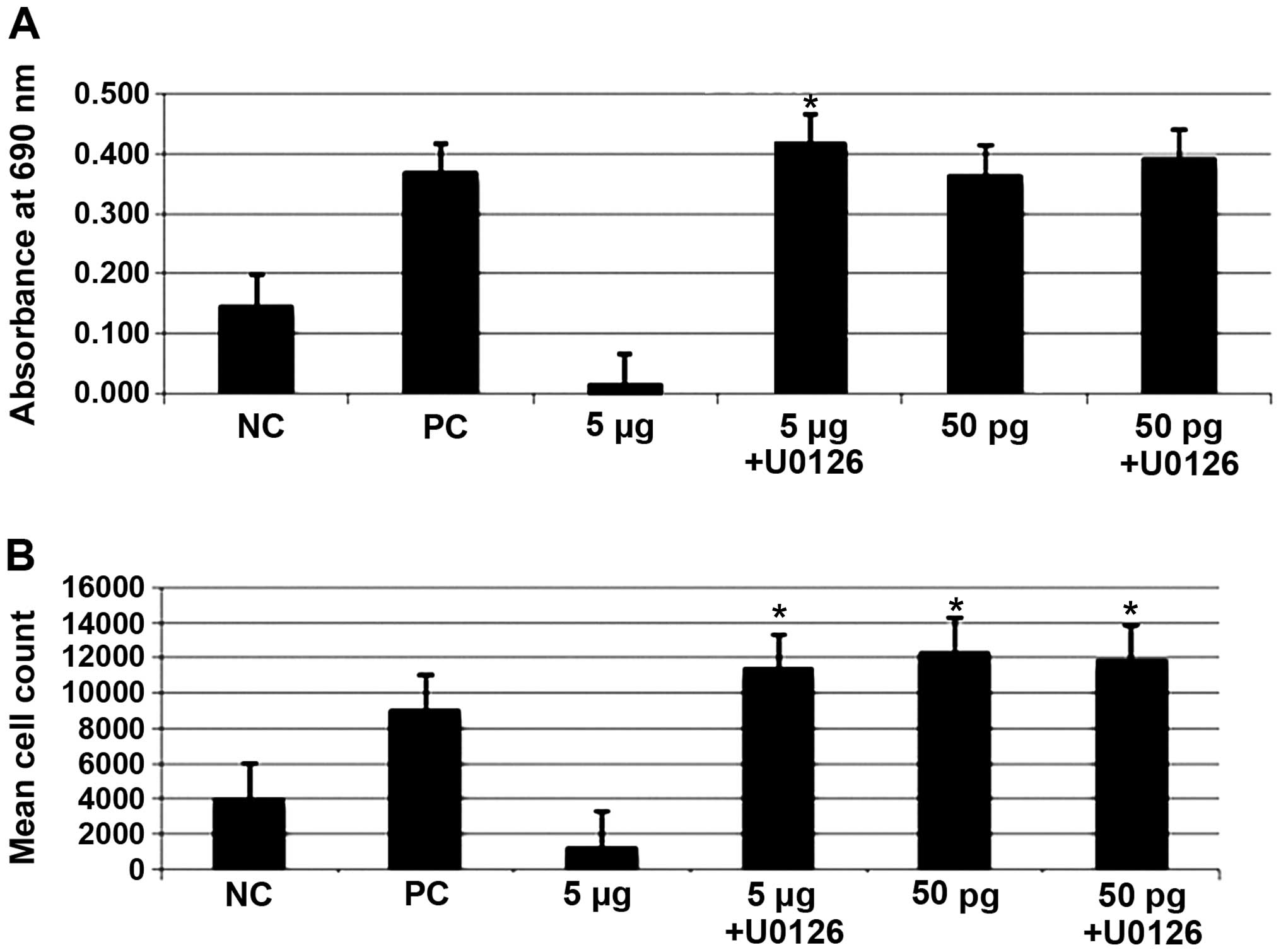
Effect of ERK1/2 inhibitor on cell
proliferation and apoptosis
The hPBMCs were cultured and treated with 5
µg or 50 pg of ISRAA with or without the p-ERK1/2 inhibitor.
Data were obtained using the MTT assay for cell death (Fig. 7A), and cell proliferation was
analyzed by taking the absolute counts of mature helper/inducer
(CD3+/CD4+) and suppressor/cytotoxic
(CD3+/CD8+) T lymphocyte subsets by flow
cytometry (Fig. 7B). As shown in
Fig. 7, the results revealed
that, compared to the unstimulated cells (negative control, NC),
there was a significant increase in hPBMC proliferation (p<0.05)
following stimulation with 50 pg of ISRAA with or without the
addition of the inhibitor. However, the toxic effect of the 5
µg concentration of ISRAA was significantly abrogated by the
addition of the inhibitor and, consequently, a significant increase
in proliferation was observed following the addition of the
inhibitor (p<0.05). PHA was used as a positive control (PC) and
did not significantly affect proliferation (p<0.05).
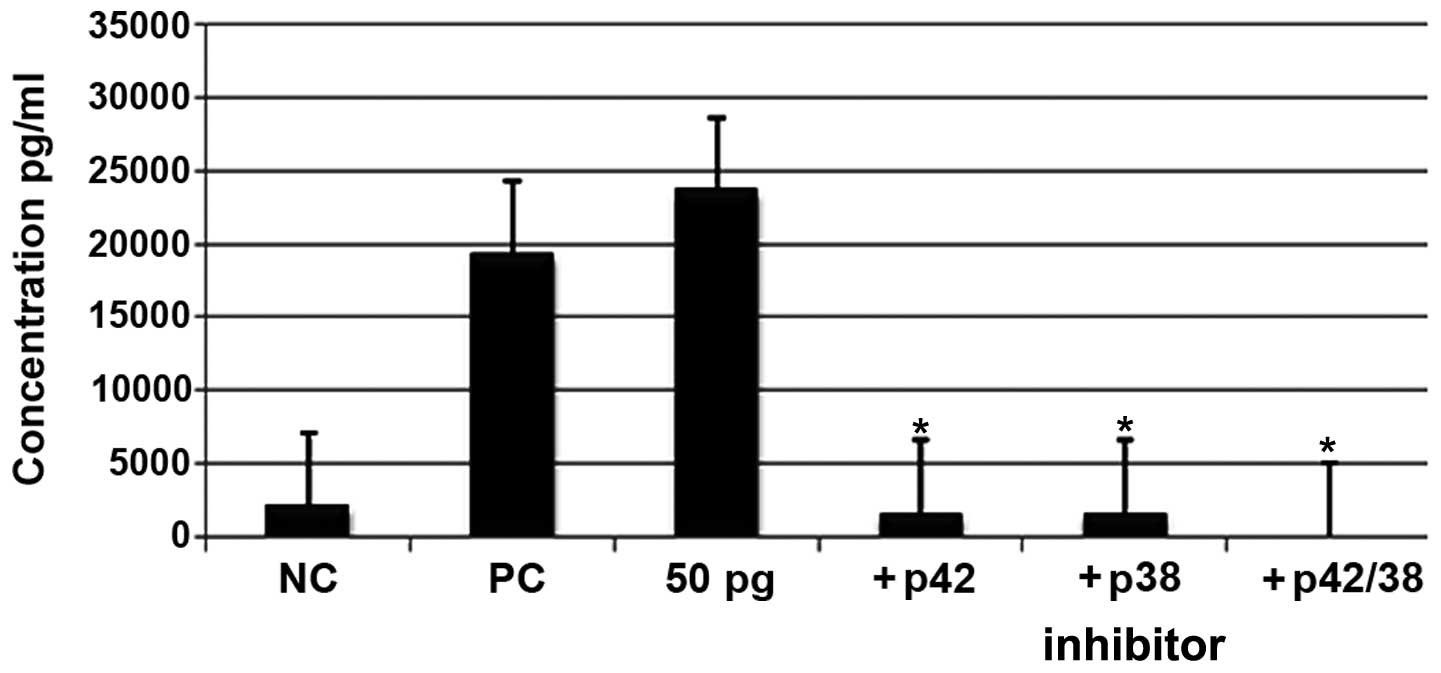
Effects of ERK1/2 inhibitors on the
production of IL-6
To determine the pathway adopted by ISRAA in the
production of IL-6, the pharmacological inhibitors of MAPKs, U0126
and SB20358, were used and the levels of IL-6 were monitored using
CBA with flow cytometry. Our results demonstrated the abrogation of
IL-6 production as a result of pre-incubation with the two blockers
prior to stimulation with ISRAA (p<0.05; Fig 8).
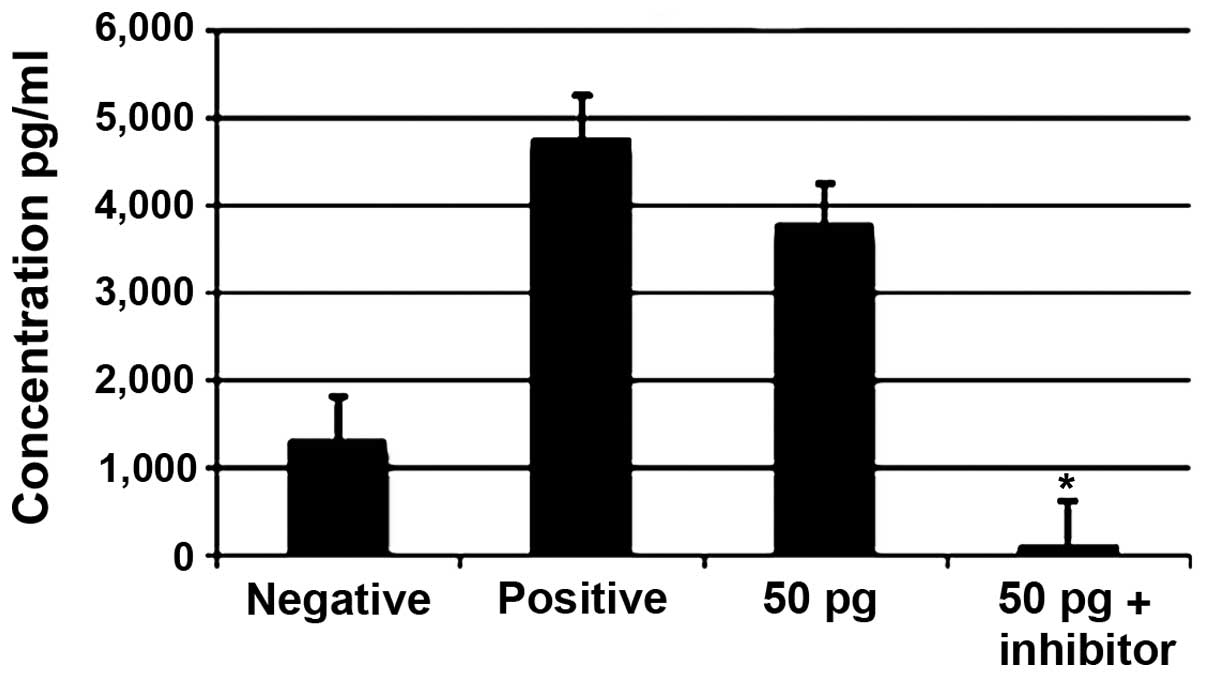
Effect of ERK1/2 inhibitors on the
production of TNF-α
Treatment with 50 pg of ISRAA with or without the
addition of the ERK1/2 inhibitor using the flow cytometric
technique revealed a significant decrease in the expression of
TNF-α when using the inhibitor compared with the increased level
observed in the cells treated with 50 pg of ISRAA (p<0.05;
Fig 9).
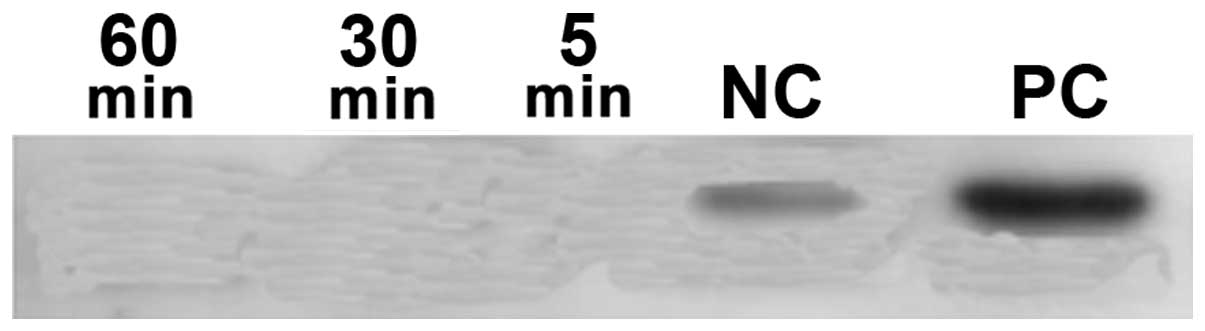
Phosphorylation of STAT3 proteins
IL-6 was originally cloned as an acute-phase
response factor resulting in the activation of the Janus kinase
(JAK)/STAT3 pathway. To examine the phosphorylation of the STAT3,
western blot analysis was performed using monoclonal antibodies to
phosphorylated and unphosphorylated STAT3. The results revealed
that STAT3 factors were ubiquitously expressed in an unstimulated
inactive unphosphorylated form. The examination of p-STAT3 revealed
no phosphorylation of STAT3 (Fig.
10).
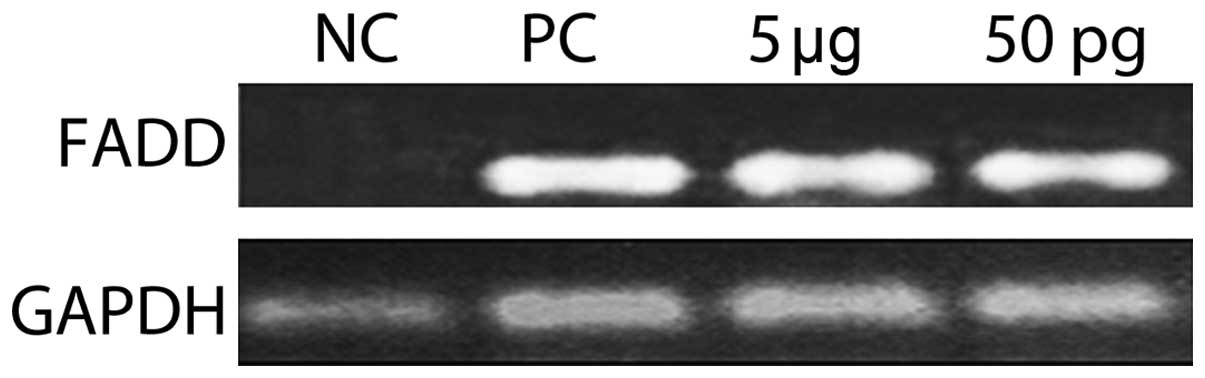
Fas-associated protein with death domain
(FADD) expression
The expression of the death domain revealed that
ISRAA activated FADD at a concentration of 5 µg and 50 pg
(Fig. 11).
Discussion
ISRAA is an immune mediator activated upon a nerve
stimulus triggered by an immune challenge (1). The human counterpart of mouse ISRAA
is not known; however, we recently demonstrated that ISRAA contains
an interspecies-conserved functional motif sharing 72% homology
with a motif present in the TNF receptor 1 (TNFR1), a receptor
connected to intracellular domains inducing dose-dependent signals
for survival or death, and the potential proliferative effects of
mouse ISRAA on human cells were recorded (2). In this study, we reconfirmed this
activity using different techniques and examined the induction of a
broad spectrum of pro-inflammatory and anti-inflammatory cytokines
in response to the stimulation of hPBMCs with ISRAA. Furthermore,
the signaling pathways used by ISRAA to induce cytokine production
were explored.
The results revealed increased levels of IL-6, IL-8,
IL-10, IFN-γ and TNF-α produced by the ISRAA-stimulated hPBMCs,
whereas no changes were observed in the production of the IL-4,
IL-17A and TGF-β cytokines in response to ISRAA stimulation. Since
ISRAA is involved in different cellular activities, including
death, growth and survival, complex signaling pathways may be
involved. Moreover, dissociated cytokine profiles were recorded to
induce such activities considering that cytokines are signaling
proteins with pleiotropic effects in diverse aspects of bodily
functions in health and disease and are used extensively in
intercellular communications to produce different activities. Among
the produced cytokines, IL-6 showed the highest measurable levels
in the current study.
IL-6 possesses a number of functional properties and
this is reflected by the terminology used to describe the
activities of this cytokine (IFN-β2, hepatocyte-stimulating factor,
cytotoxic T cell differentiation factor, B cell differentiation
factor, and B cell stimulatory factor 2) (14). It is well known for promoting
inflammatory events through the expansion and activation of T
cells, the differentiation of B cells, and the induction of
acute-phase reactants cells. By contrast, it also plays a
protective role during disease and counteracts the manifestation of
certain inflammatory responses (15–17). Thus, IL-6 functions not only as a
pro- but also as an anti-inflammatory cytokine, and, as a result,
plays a pivotal role during disease.
To understand the mechanisms through which ISRAA
transduces its potent biological responses, we briefly examined the
signaling pathway used by ISRAA to induce the production of IL-6.
The signaling pathways known to be involved in the production of
IL-6 have been mainly found to be the MAPK and/or JAK/STAT pathways
(18). Therefore, these two
pathways were analyzed after stimulating the hPBMCs cells with
ISRAA.
The ERK-MAPK pathway was the first signal
transduction cascade to be unraveled and delineated from the cell
membrane to the nucleus (4). The
pathway has been extensively investigated and is also known as the
classical mitogen kinase cascade (19–23,28). The cascade is initiated by the
small G protein Ras, which recruits the MAPKKKs A-Raf, B-Raf and
Raf-1, the MAPKKs MEK1 and 2, and the MAPKs ERKs, in addition to a
large number of a range of substrates, including membrane and
cytoskeletal proteins, cytosolic enzymes, and transcription factors
(24). Ras/MAPK regulates many
cellular processes and plays an important role in physiological
processes, such as proliferation, differentiation and development
(25,26). Moreover, Ras/MAPK has also been
found to control the signaling pathway leading to the
phosphorylation and inactivation of a pro-apoptotic Bcl-2 family
member in mammalian cells, Bad, which is targeted on serine 112 by
a MEK-dependent pathway, whereas the phosphorylation of two other
residues occurs in a MEK-independent manner (one of them is serine
136 by the PI3K/AKT pathway). Serine 112 phosphorylation has been
found to be required for the dissociation of Bad from Bcl-2
providing a key link between growth and survival pathways and
protection from apoptosis (27).
ERK1/2 signaling has been implicated as a key
regulator of cell proliferation, and for this reason, inhibitors of
the ERK pathway are used in clinical trials as potential anticancer
agents (28). In this study, we
examined the involvement of ERK1/2 and p38 kinase in mediating IL-6
production from hPBMCs using U0126 and SB203580 as inhibitors of
ERK1/2 and p38 kinase, respectively. We demonstrated that by
treating the cells with U0126 and SB203580 for 1 h prior to
stimulation with ISRAA, the differential effects of these compounds
were observed in the level of IL-6, which was markedly decreased,
suggesting the essential role and function of MAPKs in the level of
IL-6 produced by cells stimulated with ISRAA.
In the present study, western blot analysis and
immunofluorescence assay were used to investigate the levels and
intensity of p-ERK (Thr202/Tyr204) as a downstream target protein,
and the results revealed that P-ERK1/2 p-ERK1/2 as downstream
signals in the MAPK pathway were phosphorylated due to ISRAA
stimulation and a significant amount of p-ERK1/2 accumulated in the
nucleus. The localization of MAPK depends on its phosphorylation
state at the sites of action rather than on its activity.
Phosphorylation induces its cytodimerization, which is required for
its nuclear translocation. Upon activation, both kinases partly
translocate to the nucleus (29,30).
IL-6 signals via gp130 homodimerization which then
activates JAKs and recruits STAT proteins (31). STAT factors are ubiquitously
expressed in an unstimulated, inactive (unphosphorylated) form
(32), predominantly regulated by
post-transitional modifications, i.e., tyrosine and serine
phosphorylation. This is in accordance with our findings since
unphosphorylated STAT3 in the ISRAA-stimulated hPBMCs was noted,
and STAT3 was ubiquitously expressed in unstimulated cells
suggesting that ISRAA has a protein inhibitor of activated STAT
(PIAS)-like activity by acting as a negative regulator of the
JAK/STAT pathway, where PIAS1 and PIAS3 have been shown to inhibit
the activity of STAT1 and STAT3, respectively (33).
The effects of ISRAA on cells have been shown to be
dualistic, as high concentrations induce apoptosis and low
concentrations induce proliferation (2). This study demonstrated that ERK1/2
had no effect on the ISRAA-induced cell proliferation, while it was
shown that ERK1/2 is required for ISRAA-induced apoptosis since the
inhibition of ERK activities by a small molecule inhibitor almost
completely inhibited the apoptotic effects of the high dose of
ISRAA and reversed the suppressive effect of the cytotoxic dose.
This is in agreement with the results of other studies (34–36), suggesting that the ERK pathway
mediates the apoptosis induced by different stimuli in different
tissues even though ERK has generally been considered a survival
signaling pathway. However, the mechanisms through which ERK
mediates apoptosis remain unclear. We hypothesized that the ERK1/2
pathway is involved in apoptosis by increasing an upstream signal
for TNF-α production via the extrinsic pathway by activating the
death domain of the FADD pathway. This is supported by Jo et
al (37), who examined the
effect of ERK inhibition on TNF-α expression and subsequent
caspase-3 activation in cisplatin-induced acute renal failure in
mice. The authors demonstrated that the inhibition of ERK1/2
reduced TNF-α expression, caspase-3 activation and apoptosis in
kidney tissue, suggesting that the ERK1/2 pathway also participates
in apoptosis by increasing an upstream signal for TNF-α production.
In this study, we also demonstrated that the TNF-α level induced by
ISRAA was significantly decreased when ERK1/2 was inhibited,
indicating that ISRAA/ERK1/2 may use the extrinsic apoptotic
pathway by activating the death receptors via increasing the level
of TNF-α to induce apoptosis. These data have been recently
supported by an exhaustive structural analysis of ISRAA that
demonstrated features of novel signaling molecules (unpublished
data).
In conclusion, the present study confirmed the
dose-dependent apoptotic and proliferative effects of ISRAA on
hPBMCs. Furthermore, high measurable levels of dissociated cytokine
responses were detected, and the downstream signals of the MAPK
pathway (ERK1/2) were found to be critically involved. The results
also revealed that ISRAA functions as a PIAS-like protein,
functioning as a negative regulator of STAT3 action on the JAK/STAT
pathway. Since FADD was activated with both concentrations of ISRAA
(proliferative and toxic) and ERK1/2 activation was involved in the
IL-6 and TNF-α production and in apoptosis, but not in
proliferation, further research into the intrinsic pathways is
required to examine the effects of ISRAA on ERK and consequently on
different caspases, and p53 and Bcl-2 proteins, as well as on
survival signaling, such as the Ras/PI3K/Akt pathway and Fyn
expression. Experiments to define the proliferative and apoptotic
concentrations for each hPBMC population during proliferation and
apoptosis have been recently initiated in our laboratory.
Acknowledgments
This study received financial support from the
College of Medicine and Medical Sciences, Arabian Gulf University
(Bahrain).
References
|
1
|
Bakhiet M and Taha S: A novel nervous
system-induced factor inducing immune responses in the spleen.
Immunol Cell Biol. 86:688–699. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taha S, Fathallah MD and Bakhiet M: An
interspecies conserved motif of the mouse immune system-released
activating agent (ISRAA) induces proliferative effects on human
cells. Mol Med Rep. 10:75–81. 2014.PubMed/NCBI
|
|
3
|
Arch RH, Gedrich RW and Thompson CB: Tumor
necrosis factor receptor-associated factors (TRAFs) - a family of
adapter proteins that regulates life and death. Genes Dev.
12:2821–2830. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang F, Steelman LS, Shelton JG, Lee JT,
Navolanic PM, Blalock WL, Franklin R and McCubrey JA: Regulation of
cell cycle progression and apoptosis by the Ras/Raf/MEK/ERK pathway
(Review). Int J Oncol. 22:469–480. 2003.PubMed/NCBI
|
|
5
|
Elmore S: Apoptosis: a review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ibelgaufts H: Cytokines. Cytokines &
Cells Online Pathfinder Encyclopedia. Version 31.4. Spring/Summer.
2013 edition.
|
|
7
|
Pollard TD, Earnshaw WC,
Lippincott-Schwartz J and Johnson GT: Cell biology. 2nd edition.
Saunders/Elsevier; Philadelphia: 2008
|
|
8
|
Ubersax JA and Ferrell JE Jr: Mechanisms
of specificity in protein phosphorylation. Nat Rev Mol Cell Biol.
8:530–541. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sallusto F and Lanzavecchia A: Efficient
presentation of soluble antigen by cultured human dendritic cells
is maintained by granulocyte/macrophage colony-stimulating factor
plus interleukin 4 and downregulated by tumor necrosis factor
alpha. J Exp Med. 179:1109–1118. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cairo MS, Wagner EL, Fraser J, Cohen G,
van de Ven C, Carter SL, Kernan NA and Kurtzberg J:
Characterization of banked umbilical cord blood hematopoietic
progenitor cells and lymphocyte subsets and correlation with
ethnicity, birth weight, sex, and type of delivery: A Cord Blood
Transplantation (COBLT) Study report. Transfusion. 45:856–866.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morgan E, Varro R, Sepulveda H, et al:
Cytometric bead array: A multiplexed assay platform with
applications in various areas of biology. Clin Immunol.
110:252–266. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Leonard M, Ryan MP, Watson AJ, Schramek H
and Healy E: Role of MAP kinase pathways in mediating IL-6
production in human primary mesangial and proximal tubular cells.
Kidney Int. 56:1366–1377. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jones SA, Horiuchi S, Topley N, Yamamoto N
and Fuller GM: The soluble interleukin 6 receptor: Mechanisms of
production and implications in disease. FASEB J. 15:43–58. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rao MS, Sun Y, Escary JL, Perreau J,
Tresser S, Patterson PH, Zigmond RE, Brulet P and Landis SC:
Leukemia inhibitory factor mediates an injury response but not a
target-directed developmental transmitter switch in sympathetic
neurons. Neuron. 11:1175–1185. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamamori T, Fukada K, Aebersold R,
Korsching S, Fann MJ and Patterson PH: The cholinergic neuronal
differentiation factor from heart cells is identical to leukemia
inhibitory factor. Science. 246:1412–1416. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lo DC: A central role for ciliary
neurotrophic factor? Proc Natl Acad Sci USA. 90:2557–2558. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heinrich PC, Behrmann I, Haan S, Hermanns
HM, Müller-Newen G and Schaper F: Principles of interleukin
(IL)-6-type cytokine signalling and its regulation. Biochem J.
374:1–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Galabova-Kovacs G, Kolbus A, Matzen D,
Meissl K, Piazzolla D, Rubiolo C, Steinitz K and Baccarini M: ERK
and beyond: insights from B-Raf and Raf-1 conditional knockouts.
Cell Cycle. 5:1514–1518. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kolch W: Meaningful relationships: the
regulation of the Ras/Raf/MEK/ERK pathway by protein interactions.
Biochem J. 351(Pt 2): 289–305. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pagès G, Guérin S, Grall D, Bonino F,
Smith A, Anjuere F, Auberger P and Pouysségur J: Defective
thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice.
Science. 286:1374–1377. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pawson T: Regulation and targets of
receptor tyrosine kinases. Eur J Cancer. 38(Suppl 5): S3–S10. 2002.
View Article : Google Scholar
|
|
23
|
Rouse J, Cohen P, Trigon S, Morange M,
Alonso-Llamazares A, Zamanillo D, Hunt T and Nebreda AR: A novel
kinase cascade triggered by stress and heat shock that stimulates
MAPKAP kinase-2 and phosphorylation of the small heat shock
proteins. Cell. 78:1027–1037. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Galabova-Kovacs G, Catalanotti F, Matzen
D, Reyes GX, Zezula J, Herbst R, Silva A, Walter I and Baccarini M:
Essential role of B-Raf in oligodendrocyte maturation and
myelination during postnatal central nervous system development. J
Cell Biol. 180:947–955. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shaul YD and Seger R: The MEK/ERK cascade:
From signaling specificity to diverse functions. Biochim Biophys
Acta. 1773:1213–1226. 2007. View Article : Google Scholar
|
|
26
|
Yoon S and Seger R: The extracellular
signal-regulated kinase: Multiple substrates regulate diverse
cellular functions. Growth Factors. 24:21–44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kyriakis JM and Avruch J: Mammalian
mitogen-activated protein kinase signal transduction pathways
activated by stress and inflammation. Physiol Rev. 81:807–869.
2001.PubMed/NCBI
|
|
28
|
Roux PP and Blenis J: ERK and p38
MAPK-activated protein kinases: A family of protein kinases with
diverse biological functions. Microbiol Mol Biol Rev. 68:320–344.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brunet A, Roux D, Lenormand P, Dowd S,
Keyse S and Pouysségur J: Nuclear translocation of p42/p44
mitogen-activated protein kinase is required for growth
factor-induced gene expression and cell cycle entry. EMBO J.
18:664–674. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lenormand P, Sardet C, Pagès G, L'Allemain
G, Brunet A and Pouysségur J: Growth factors induce nuclear
translocation of MAP kinases (p42mapk and p44mapk) but not of their
activator MAP kinase kinase (p45mapkk) in fibroblasts. J Cell Biol.
122:1079–1088. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Heinrich PC, Behrmann I, Müller-Newen G,
Schaper F and Graeve L: Interleukin-6-type cytokine signalling
through the gp130/Jak/STAT pathway. Biochem J. 334:297–314. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mertens C, Zhong M, Krishnaraj R, Zou W,
Chen X and Darnell JE Jr: Dephosphorylation of phosphotyrosine on
STAT1 dimers requires extensive spatial reorientation of the
monomers facilitated by the N-terminal domain. Genes Dev.
20:3372–3381. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu X, Sun YL and Hoey T: Cooperative DNA
binding and sequence-selective recognition conferred by the STAT
amino-terminal domain. Science. 273:794–797. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bhat NR and Zhang P: Hydrogen peroxide
activation of multiple mitogen-activated protein kinases in an
oligodendrocyte cell line: Role of extracellular signal-regulated
kinase in hydrogen peroxide-induced cell death. J Neurochem.
72:112–119. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ishikawa Y and Kitamura M: Anti-apoptotic
effect of quercetin: Intervention in the JNK- and ERK-mediated
apoptotic pathways. Kidney Int. 58:1078–1087. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim YK, Kim HJ, Kwon CH, Kim JH, Woo JS,
Jung JS and Kim JM: Role of ERK activation in cisplatin-induced
apoptosis in OK renal epithelial cells. J Appl Toxicol. 25:374–382.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jo SK, Cho WY, Sung SA, Kim HK and Won NH:
MEK inhibitor, U0126, attenuates cisplatin-induced renal injury by
decreasing inflammation and apoptosis. Kidney Int. 67:458–466.
2005. View Article : Google Scholar : PubMed/NCBI
|