Introduction
The deleterious effects of alcohol consumption have
been noted in recent years. Though alcohol is a well-described
immunomodulatory drug, there are inconsistencies concerning its use
due to its adverse effects and associated pathologies. Excessive or
chronic alcohol consumption has been associated with negative
clinical outcomes and linked to one-third of all mortalities caused
by traumatic injury each year (1,2).
Intoxicated trauma and/or burn patients are at higher risk of
suffering from infectious complications, as well as pneumonia,
sepsis, organ and multiple organ failure (MOF) (3–6).
Regarding chronic alcohol consumption, alcohol adversely affects
both recovery and outcome after trauma due to the increased risk of
infectious complications compared with patients who are not not
chronically intoxicated (7).
Other studies have reported no increase in the risk of
post-traumatic complications or worse outcome in multiple trauma
patients who are primarily alcohol intoxicated (8,9).
Moreover, experimental and epidemiological data have confirmed that
moderate alcohol consumption is associated with reduced risk of
cardiovascular disease events, diabetes and coagulopathy in
patients with severe, traumatic brain injury (10–12).
Alcohol is mainly metabolized in the liver, but
large amounts are circulated through the lungs through bronchial
circulation (13); alcohol
systemically affects the liver and the respiratory system.
Increased pro-inflammatory cytokine release, which has been closely
linked to detrimental effects on health, has been reported after
heavy or chronic alcohol consumption (14,15). By contrast, studies on acute
and/or low-dose to moderate alcohol intake have indicated
potentially beneficial anti-inflammatory effects (16–20). Johansson et al and others
have reported on the alcohol-reduced release of interleukin-8
(IL-8) in human endothelial and epithelial cells, their decreased
interaction with neutrophils and reduced translocation of nuclear
factor-κB (NF-κB) components into the nucleus (21,22). Previously, we have shown that
acute alcohol consumption diminished both liver and systemic IL-6
increase, and also hepatic neutrophil infiltration in a model of
acute inflammation in vivo (18).
On the one hand, alcohol may increase host
susceptibility to infections due to its hypo-inflammatory effects;
however, on the other hand, it hides its therapeutic potential
under acute inflammatory conditions. Nevertheless, its therapeutic
use is limited in clinical settings owing to its entry into the
central nervous system (CNS) and the questions which remain to be
answered on the dose- and time-dependent mode of action.
Ethyl pyruvate (EtP), the ester formed from ethanol
(EtOH) linked to pyruvate, is a well-tolerated non-toxic compound
which exerts similar anti-inflammatory effects as pyruvate
(23,24). Concerning the lungs, EtP has been
shown to protect against ventilation-induced neutrophil
infiltration and the accompanying deleterious oxidative stress
(25). Hepatic injury in animals
with severe acute pancreatitis, but also sepsis-or
hemorrhage-induced organ injuries in vivo, were prevented by
EtP (26–29). Collectively, these results suggest
that due to its stability, lack of adverse effects, wide
therapeutic window and apparently no signs of intoxication, EtP
will be useful in a clinical setting for the treatment of acute
inflammation. Thus, in the present study, human alveolar epithelial
and liver cells were exposed to proinflammatory stimuli and
subsequently treated with alcohol or EtP in order to i) clarify the
comparability of both agents concerning their anti-inflammatory
potential, ii) evaluate the adequate dose, and iii) assess the
cellular entity-specific efficacy of both agents.
Materials and methods
Cell culture
The human hepatocellular carcinoma cell line Huh7
and human lung adenocarcinoma cell line A549 were both purchased
from Cell Line Services (Heidelberg, Germany). Cells were cultured
at 37°C under 5% CO2 conditions in RPMI-1640 medium
(Seromed, Berlin, Germany) supplemented with 10% heat-inactivated
fetal calf serum (FCS), 100 IU/ml penicillin, 100 µg/ml
streptomycin (Gibco, Karlsruhe, Germany) and 20 mM HEPES buffer
(Sigma, Steinheim, Germany) (30,31). Culture media was changed every 2
or 3 days.
The isolation of polymorphonuclear neutrophils
(PMNs) from healthy volunteers was in accordance with the
Declaration of Helsinki and was approved by the Institutional
Ethics Committee of Goethe University (Frankfurt, Germany). PMNs
were isolated by density-gradient centrifugation (Polymorphprep;
Nycomed, Oslo, Norway) according to the manufacturer's instructions
(30,31). Thereafter, PMNs were cultured in
RPMI-1640 medium as described above, and subsequently the number of
neutrophils as well as their viability were determined by trypan
blue exclusion assay. Cell cultures with a purity of >95% were
used for experiments.
Cell stimulation
The concentrations of IL-1β and tumor necrosis
factor (TNF), as well as EtOH, EtP and sodium pyruvate (NaP), used
in this study are based on our previous work, and that of others,
to allow for better comparison of data (30,31). The cell lines were stimulated with
either recombinant IL-1β (1 ng/ml; R&D Systems, Wiesbaden,
Germany) for 24 h or TNF (10 ng/ml; Sigma) for 4 h. After
stimulation they were incubated with EtOH, EtP or NaP. EtOH was
used at 85 and 170 mM (corresponding to 0.5–1 vol/vol percent,
corresponding to 4–7.9 mg EtOH/ml) as described previously
(22,32,33). Likewise, the concentrations of EtP
(2.5 and 10 mM) and NaP (10 mM) were chosen from previous work
(32,34). The cells were stimulated with
either EtOH, EtP or NaP for 1 h to study the effects of acute
alcohol exposure.
Cell viability
A549 and Huh7 cell viability was assessed by
measuring cytoplasmic lactate dehydrogenase (LDH) activity
(30,31). When the plasma membrane is
damaged, the cells release LDH into the cell culture supernatant.
The activity of LDH in supernatants collected from cells treated
with EtOH, EtP, NaP, IL-1β and TNF was determined enzymatically
according to the manufacturer's instructions [cytotoxicity
detection kit (LDH); Roche]. A549 and Huh7 viability was >95% at
the time and doses chosen for the treatment of the cells in each
case. Moreover, no significant detachment of the cells was observed
by microscopic evaluation of the cell layers using a Zeiss inverted
fluorescence microscope (AXIO Observer Z1; Carl Zeiss AG,
Oberkochen, Germany).
A trypan blue exclusion assay was used to determine
the viability level of neutrophils (30,31). Briefly, isolated neutrophils were
stained with 0.4% trypan blue and approximately 100 cells were
counted after each isolation. The mean percentage of viability was
>99%.
Quantification of cytokine
production
To determine the effects of EtOH, EtP and NaP on
pro-inflammatory cytokine production, A549 or Huh7 cells were
pre-incubated with either IL-1β (24 h) or TNF (4 h). EtOH, EtP or
NaP were applied for 1 h after stimulation. Subsequently, IL-6 and
IL-8 were measured in culture supernatants from Huh7 and A549
cells, respectively, using the Quantikine assays (R&D Systems)
according to the manufacturer's instuctions. ELISA was performed
using an Infinite M200 microplate reader (Tecan, Männedorf,
Switzerland).
Ribonucleic acid (RNA) isolation, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
After stimulation with IL-1β or TNF and incubation
with EtOH, EtP or NaP, total RNA from A549 or Huh7 cells was
isolated using the RNeasy-system (Qiagen, Hilden, Germany)
according to the manufacturer's instructions. Residual amounts of
the remaining DNA were removed using the RNase-Free DNase kit
according to the manufacturer's instructions (Qiagen). The RNA was
stored at −80°C. The quality and amount of RNA were determined
photometrically using a NanoDrop ND-1000 device (NanoDrop
Technologies, Wilmington, DE, USA).
RNA was subsequently used for RT-qPCR as described
previously (17). Briefly, 100 ng
total RNA was reverse transcribed using the affinity script
qPCR-cDNA synthesis kit (Stratagene, La Jolla, CA, USA) following
the manufacturer's instructions. To determine the mRNA expression
of Bax and Bcl-2, RT-qPCR was carried out on a Stratagene MX3005p
qPCR system (Stratagene) using gene-specific primers for human Bax
and Bcl-2 (Bax: NM_004324, Hs.624291, ord. no. PPH00078B; and
Bcl-2: NM_00633, Hs.150749, ord. no. PPH00079B, respectively)
purchased from SABiosciences (SuperArray; Frederick, MD, USA). As a
reference gene, the expression of GAPDH with human GAPDH
(NM_002046, UniGene no. Hs.592355, cat. no. PPH00150E; SuperArray;
SABiosciences) was determined. PCR was set up with 1X
RT2 SYBR-Green/Rox qPCR master mix (SABiosciences) in a
25 µl volume according to the manufacturer's instructions. A
two-step amplification protocol consisting of initial denaturation
at 95°C for 10 min followed by 40 cycles with 15 sec denaturation
at 95°C and 60 sec annealing/extension at 60°C was chosen. A
melting-curve analysis was applied to control the specificity of
amplification products. This method has been described previously
(30).
Relative expression levels of the target mRNA in
each sample were calculated using the comparative threshold-cycle
(CT) method (ΔCT method). Briefly, the amount of target mRNA in
each sample was normalized to the amount of GAPDH mRNA, to
provide ΔCT, and then to a calibrator consisting of samples
obtained from unstimulated but pretreated cells. Relative Bax/Bcl-2
was then calculated.
CD54 surface expression
After stimulation with IL-1β or TNF and incubation
with EtOH, EtP or NaP, A549 or Huh7 cells were washed in PBS [0.5%
bovine serum albumin (BSA)]. Thereafter, cells were incubated with
a fluorescein-conjugated monoclonal antibody directed against
CD54/intercellular adhesion molecule (ICAM)-1 (BBA20; R&D
Systems) for 60 min at 4°C as described previously (30,31). CD54 expression was determined by
flow cytometry using a FACSCalibur (1×104 cells/scan
were counted; BD Biosciences, Heidelberg, Germany) and expressed as
mean fluorescence units (MFU). A mouse IgG1 fluorescein antibody
(IC002F; R&D Systems) was used as the isotype control (30,31).
Monolayer adhesion assay
In order to analyze PMN adhesion to pretreated A549
or Huh7 cells, A549 as well as Huh7 cells were transferred to
24-well multiplates (Falcon Primaria; Becton-Dickinson, San Jose,
CA, USA) in complete RPMI-1640 medium. After reaching a confluence
of ~80%, A549 or Huh7 cells were stimulated with either IL-1β or
TNF, and thereafter incubated with either EtOH, EtP or NaP for 1 h.
Subsequently, freshly isolated PMNs (5×104 cells/well)
were carefully added to the A549 or Huh7 monolayers or to an empty
plastic surface as controls. After 60 min, the non-adherent PMNs
were washed off 3 times using pre-warmed (37°C) complete RPMI-1640
medium. The remaining PMNs were fixed with 1% glutaraldehyde. The
adherent PMNs were then counted in 5 different fields of a defined
size (5×0.25 mm2) using a phase contrast microscope (×20
objective). The mean cellular adhesion rate was calculated. This
assay was performed as described previously (30,31).
Western blot analysis for intracellular
signaling
After stimulation with IL-1β or TNF and subsequent
incubation with EtOH, EtP or NaP, A549 or Huh7 cells were
homogenized in lysis buffer at 4°C, followed by centrifugation for
30 min at 4°C at 20,000 × g. Supernatants were stored at −80°C for
later analysis. Lysates (50 µg protein) were separated by
electrophoresis on 12% polyacrylamide SDS gels and transferred to
nitrocellulose membranes (Amersham-Buchler, Braunschweig, Germany).
Phosphorylated Akt was detected using mouse monoclonal p-Akt
antibody (cat. no. 4051S), and Akt using mouse monoclonal Akt
antibody (cat. no. 4051S), respectively. Phosphorylated NF-ĸB was
detected using mouse monoclonal p-NF-ĸB p65 (Ser536) antibody (cat.
no. 3036S) and NF-ĸB using mouse monoclonal NF-ĸB p65 antibody
(cat. no. 6956S), respectively (all antibodies were from Cell
Signaling Technology, Frankfurt, Germany). Determination of β-actin
with the anti-β-actin antibody (Sigma, Taufkirchen, Germany) served
as a loading control. Blots were blocked (10% non-fat dry milk in 1
mM Tris, 150 mM NaCl, pH 7.4) for 1 h, incubated 1 h at room
temperature with primary antibody (diluted according to the
manufacturer's instructions in blocking buffer with 0.5% Tween-20
and 0.5% BSA), and subsequently incubated for 1 h with horseradish
peroxidase-conjugated secondary antibody (sc-2005; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) diluted at a ratio of
1:1,000 in blocking buffer with 0.5% Tween-20 and 0.5% bovine serum
albumin at room temperature. Proteins were detected with ECL™
western blot detection reagents (GE Healthcare, Munich, Germany).
Blots were digitized, and the integrated density of individual
bands was determined. By densitometric measurement, the amount of
protein expression was normalized to β-actin, as previously
described (35). The measured
levels of a phosphorylated protein may change with treatment or
through gel-loading errors, and thus the total level of the
non-phosphorylated protein were also determined. Thus, the
phosphorylated protein fraction relative to the total protein
fraction serves also as an internal loading control. The activation
state of Akt or p65 was calculated as the ratio of phosphorylated
and total (phosphorylated plus non-phosphorylated) protein values
of densitometric results in percentage form (phospho/total ×
100).
Statistical analysis
Differences between groups were determined by
Wilcoxon-Mann-Whitney U-test. A p-value <0.05 was considered to
indicate a statistically significant difference. Data are
represented as the means ± standard error of the means (SEM).
Statistical analyses were performed using GraphPad Prism 6
(GraphPad Software, Inc., San Diego, CA, USA). All experiments were
performed 3 times.
Results
Determination of cytotoxicity
In order to evaluate the possible toxic effects of
the drugs that were used, after treatment as described in Materials
and methods, the LDH activity in supernatants was determined.
Treating A549 and Huh7 with IL-1β or TNF did not have a toxic
effect. Moreover, treating both cell lines with either EtOH, EtP or
NaP for 1 h did not exert any observable toxic effect either (data
not shown).
Measurement of the secretory potential of
A549 and Huh7 cells
In order to examine the secretory potential of the
pro-inflammatory cytokines, IL-8 release by A549 as well as the
IL-6 release by Huh7 cells was determined. Previously, the cells
had been stimulated with IL-1β or TNF and were subsequently
incubated with EtOH, EtP or NaP.
Lungs
IL-1β and TNF induced a significant release of IL-8
in A549 cells, from 1.55±0.38 to 36.10±2.76 ng/ml IL-8 (p<0.05;
Fig. 1A) and 0.89±0.03 to
8.07±0.58 ng/ml IL-8 (p<0.05; Fig.
1B), respectively. Treatment with EtOH for 1 h significantly
reduced IL-8 release to 27.33±0.21 ng/ml at a low dose (85 mM) and
to 24.32±1.52 ng/ml at a high dose (170 mM) of EtOH compared to
IL-1β-stimulated control samples (p<0.05; Fig. 1A). Treatment with a low dose of
EtOH for 1 h significantly reduced IL-8 release to 6.59±0.67 ng/ml,
and with high dose to 6.25±0.52 ng/ml, compared to TNF-stimulated
control samples (p<0.05; Fig.
1B). IL-8 release from IL-1β-stimulated A549 cells was
significantly decreased by both doses of EtP compared to
IL-1β-stimulated control samples which were not pretreated [low
dose EtP (2.5 mM): 28.34±2.78 and high dose (10 mM): 22.26±1.69
ng/ml, p<0.05; Fig. 1A].
Treatment with low-dose EtP significantly reduced IL-8 release to
5.36±0.95 ng/ml, and with high-dose EtP to 6.06±0.80 ng/ml,
compared to TNF-stimulated control samples (p<0.05; Fig. 1B). NaP significantly reduced IL-8
release after both IL-1β and TNF stimulation compared to control
samples, results that were comparable to EtOH and EtP treatment
(Fig. 1A and B).
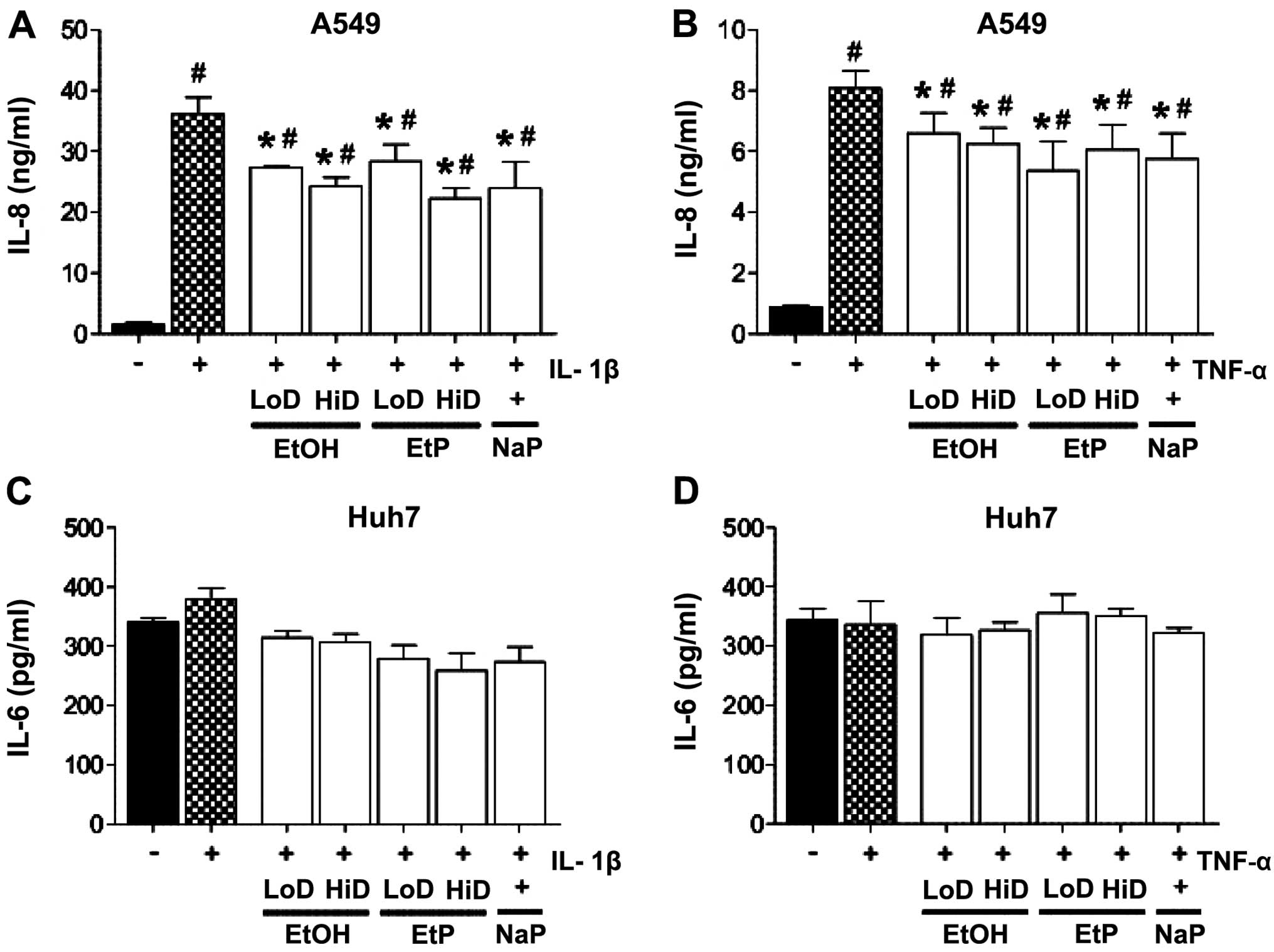 | Figure 1Effects of ethanol (EtOH), ethyl
pyruvate (EtP) or sodium pyruvate (NaP) on interleukin-8 (IL-8) and
IL-6 release from alveolar epithelial cells (A549) and
hepatocellular carcinoma cells (Huh7) after IL-1β (A and C) or
tumor necrosis factor (TNF) (B and D) stimulation. After
stimulation with either IL-1β (1 ng/ml) for 24 h or TNF (10 ng/ml)
for 4 h, cells were treated with EtOH (low dose, LoD, 85 mM; high
dose, HiD, 170 mM), EtP (LoD, 2.5 mM; HiD, 10 mM) or NaP (10 mM)
for 1 h. After the incubation periods, supernatants were analyzed
for IL-8 and IL-6 concentrations. The data are presented as the
means ± SEM. #p<0.05 vs. not pretreated and
unstimulated cells; *p<0.05 vs. not pretreated but
stimulated control. |
Liver
In Huh7 cells, neither stimulation with IL-1β nor
TNF induced an increase in IL-6 secretion. Similarly, the effects
of EtOH, EtP and NaP treatment did not markedly affect IL-6 release
from Huh7 cells (Fig. 1C and
D).
PMN adherence
Lungs
The adhesion rates of PMNs to either the IL-1β- or
TNF-stimulated A549 monolayers were significantly enhanced compared
with the adhesion rates of PMNs to unstimulated A549 cells
(increased to 156 and 143%, respectively, p<0.05; Fig. 2A and B). The treatment of
stimulated A549 cells with low-dose EtOH (85 mM) significantly
reduced PMN adhesion, and reached levels that were comparable to
PMN adherence of A549 cells which were not pretreated and not
stimulated (p<0.05; Fig. 2A and
B). The treatment of IL-1β-stimulated A549 cells with high-dose
EtOH (170 mM) did not markedly reduce PMN adhesion, whereas this
reduction was significant in the TNF-stimulated A549 cells
(p<0.05; Fig. 2A and B). The
treatment of both IL-1β- and TNF-stimulated A549 cells with both
high and low doses of EtP significantly diminished PMN adhesion in
most cases (p<0.05). Treatment with NaP exerted comparable
effects to treatment with high-dose EtP (Fig. 2A and B).
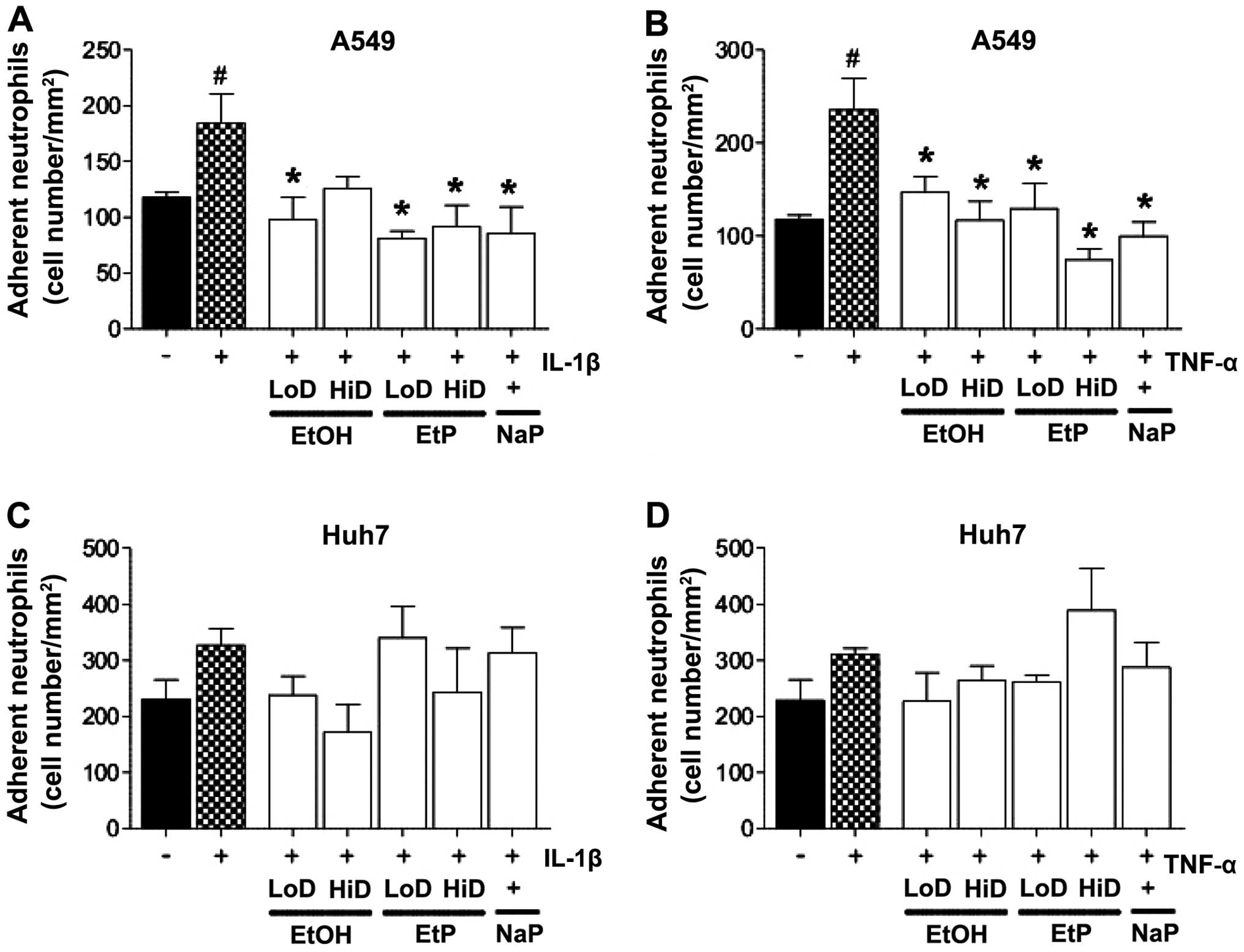 | Figure 2Effects of ethanol (EtOH), ethyl
pyruvate (EtP) or sodium pyruvate (NaP) on the adhesiveness of
neutrophils to alveolar epithelial cells (A549) and hepatocellular
carcinoma cells (Huh7) after interleukin-1β (IL-1β) (A and C) or
tumor necrosis factor (TNF) (B and D) stimulation. After
stimulation with either IL-1β (1 ng/ml) for 24 h or TNF (10 ng/ml)
for 4 h, cells were treated with EtOH (low dose, LoD, 85 mM; high
dose, HiD, 170 mM), EtP (LoD, 2.5 mM; HiD, 10 mM) or NaP (10 mM)
for 1 h. After the incubation periods, neutrophils were added and
the adhesion capacity was analyzed. The data are presented as the
means ± SEM. #p<0.05 vs. not pretreated and
unstimulated cells; *p<0.05 vs. not pretreated but
stimulated control. |
Liver
Experiments concerning the stimulation and
subsequent treatment of Huh7 cells did not deliver statistically
significant data (Fig. 2C and
D).
CD54 protein expression
Lungs
IL-1β or TNF stimulation of A549 cells induced a
significant increase in cell-surface CD54 protein expression
compared to unstimulated A549 cells (IL-1β: 22.84±2.99 vs.
2.61±0.53 MFU; and TNF: 20.30±3.05 vs. 2.61±0.53 MFU, p<0.05;
Fig. 3A and B). Treating
stimulated A549 cells with EtOH, EtP or NaP reduced the increased
adhesion rates (Fig. 3A and B),
although this reduction was not as significant in comparison to the
previously described reduction in PMN adhesion in stimulated A549
cells.
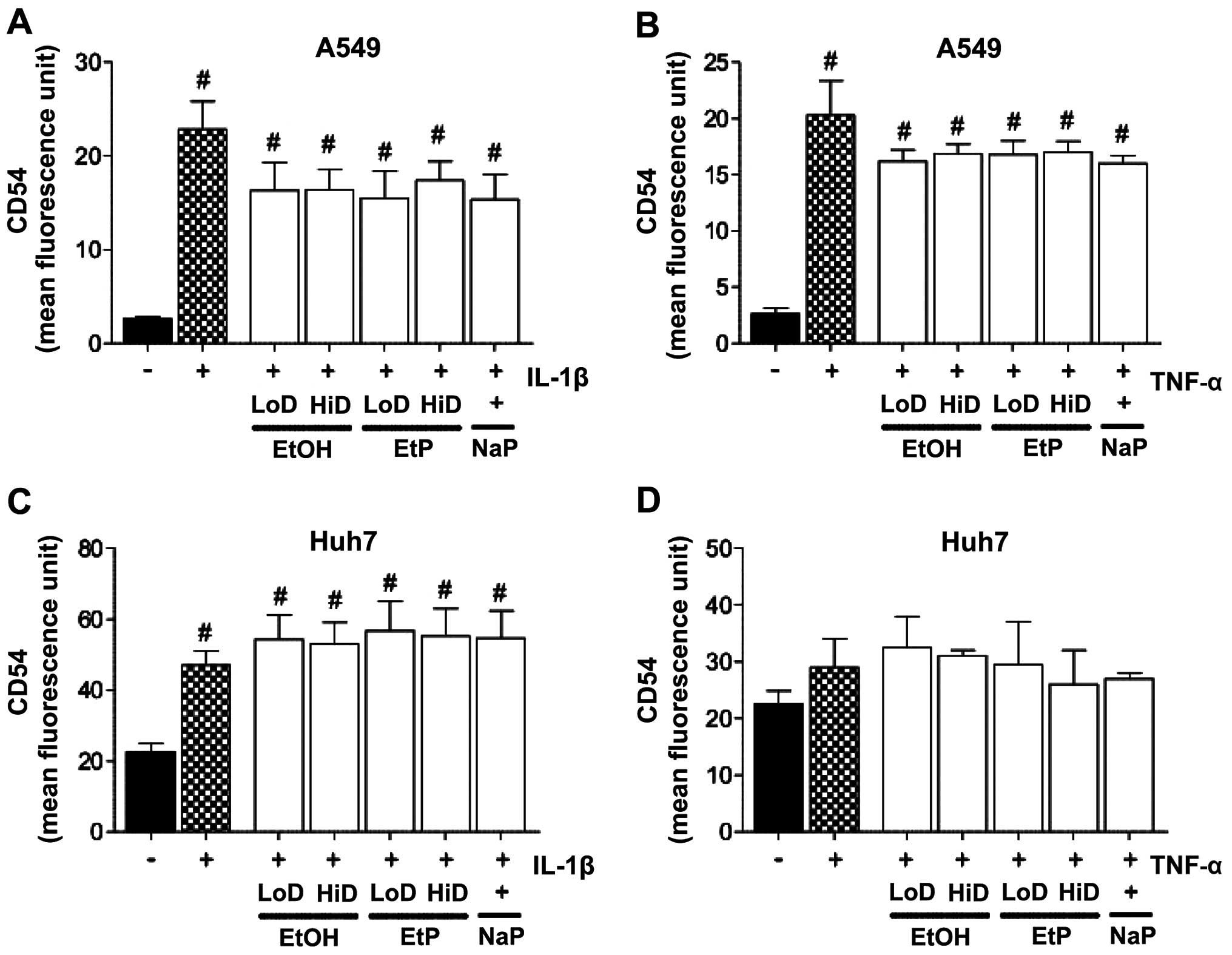 | Figure 3Effects of ethanol (EtOH), ethyl
pyruvate (EtP) or sodium pyruvate (NaP) on the surface expression
of CD54 in alveolar epithelial cells (A549) and hepatocellular
carcinoma cells (Huh7) after interleukin-1β (IL-1β) (A and C) or
tumor necrosis factor (TNF) (B and D) stimulation. After
stimulation with either IL-1β (1 ng/ml) for 24 h or TNF (10 ng/ml)
for 4 h, cells were treated with EtOH (low dose, LoD, 85 mM; high
dose, HiD, 170 mM), EtP (LoD, 2.5 mM; HiD, 10 mM) or NaP (10 mM)
for 1 h. After the incubation periods, CD54 expression was
evaluated (represented as mean fluorescence units, MFUs). The data
are presented as the means ± SEM. #p<0.05 vs. not
pretreated and unstimulated cells. |
Liver
We noted that the stimulation of Huh7 cells with
IL-1β induced a significant increase in CD54 expression compared to
unstimulated cells (47.00±4.00 vs. 22.50±2.39 MFU, p<0.05)
(Fig. 3C). However, as shown in
Fig. 3D, TNF stimulation did not
markedly affect CD54 expression. Treatment of the stimulated Huh7
cells with EtOH, EtP or NaP did not induce considerable changes in
the CD54 expression levels (Fig. 3C
and D).
Gene expression of apoptosis-related
Bax/Bcl-2
Lungs
RT-qPCR demonstrated that an increase in the
Bax/Bcl-2 ratio occurred in A549 cells after they were stimulated
with either IL-1β or TNF relative to the unstimulated control cells
(1.81 or 1.67) (Fig. 4A and B).
The Bax/Bcl-2 ratio was significantly decreased by 38.3% in
IL-1β-stimulated A549 cells after treatment with high-dose EtOH
compared to A549 cells which were stimulated but not pretreated
(p<0.05) (Fig. 4A). Low-dose
EtOH reduced the Bax/Bcl-2 ratio, but this reduction was not
designated significant. Treatment with high-dose EtP caused a
significant reduction in the Bax/Bcl-2 ratio after stimulation of
cells with IL-1β (reduced by 40.1%) compared to stimulated A549
control (p<0.05) (Fig. 4A).
The IL-1β-induced increase in Bax/Bcl-2 ratio was not significantly
altered by NaP.
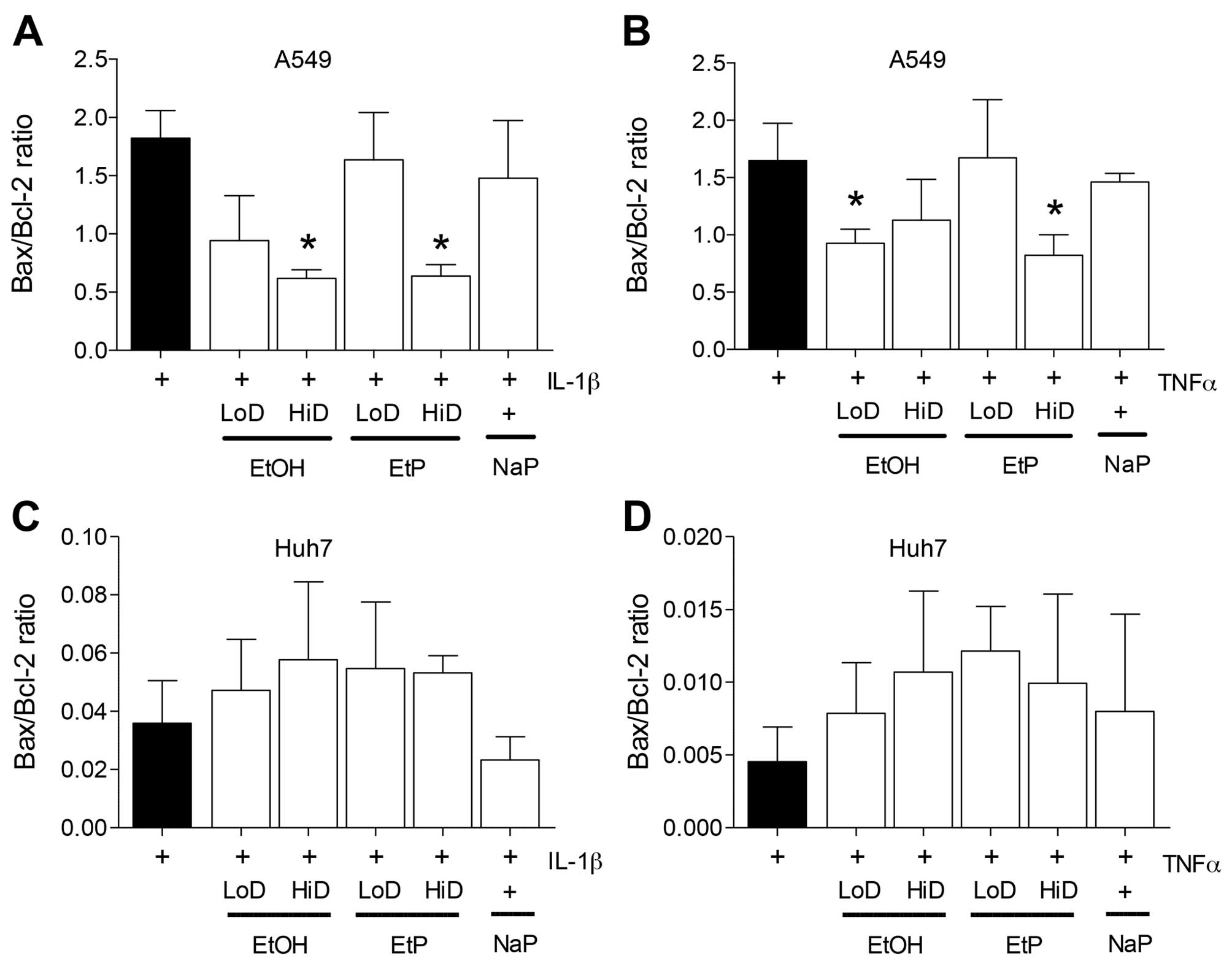 | Figure 4Effects of ethanol (EtOH), ethyl
pyruvate (EtP) or sodium pyruvate (NaP) on apoptosis of alveolar
epithelial cells (A549) or hepatocellular carcinoma cells (Huh7)
after interleukin-1β (IL-1β) (A and C) or tumor necrosis factor
(TNF) (B and D) stimulation. After stimulation with either IL-1β (1
ng/ml) for 24 h or TNF (10 ng/ml) for 4 h, cells were treated with
EtOH (low dose, LoD, 85 mM; high dose, HiD, 170 mM), EtP (LoD, 2.5
mM; HiD, 10 mM) or NaP (10 mM) for 1 h. After the incubation
periods, Bax/Bcl-2 gene expression ratio was analyzed. The data are
presented as the means ± SEM. *p<0.05 vs. not
pretreated but stimulated control. |
After stimulation of A549 cells with TNF, we noted
that the Bax/Bcl-2 ratio was significantly decreased by low-dose
EtOH as well a high dose of EtP (p<0.05) (Fig. 4B), whereas NaP did not induce
significant changes in Bax/Bcl-2 expression (Fig. 4B).
Liver
The Bax/Bcl-2 ratio was not significantly altered in
Huh7 after stimulation of cells with IL-1β or TNF (Fig. 4C and D).
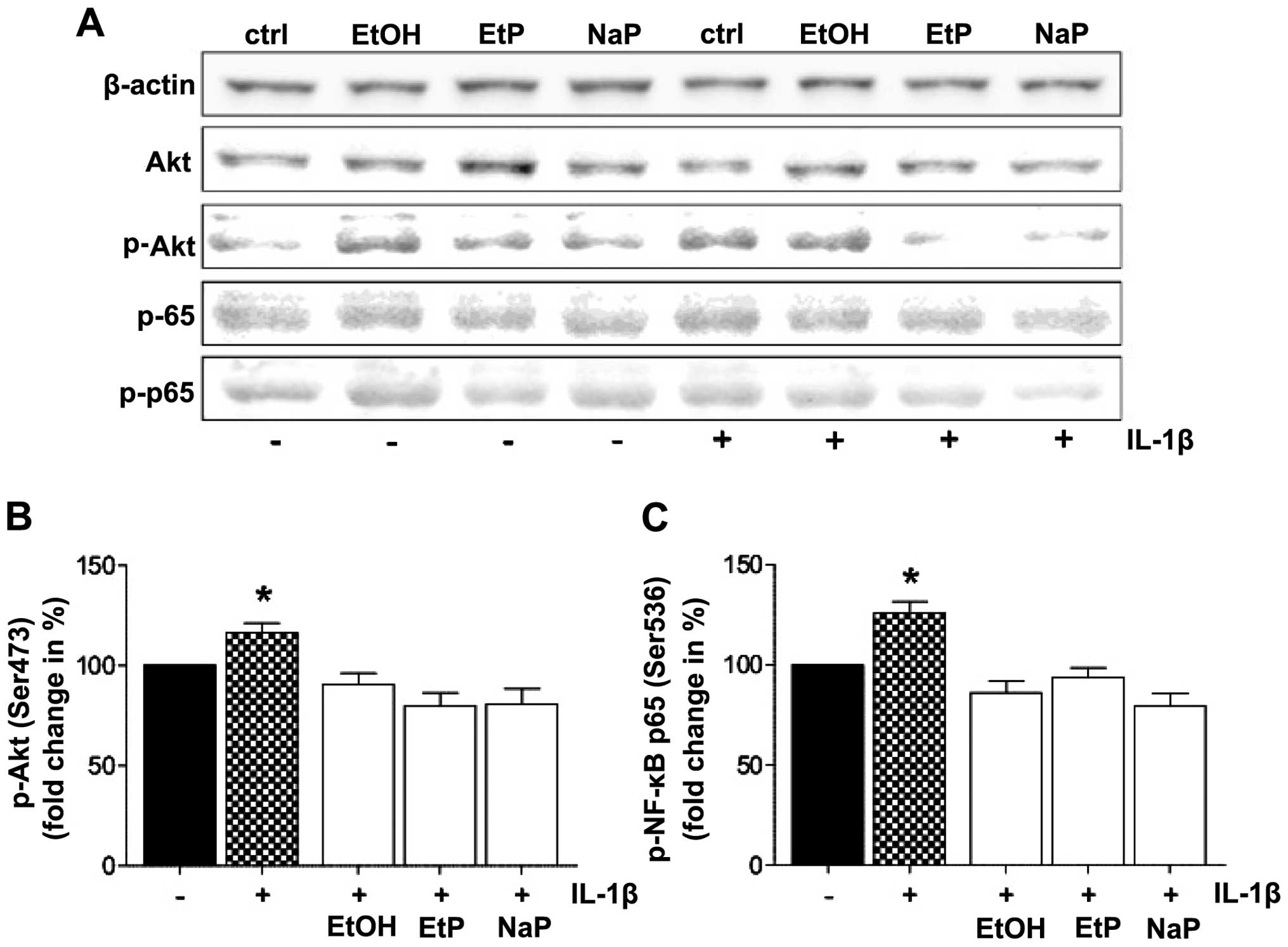
Protein expression of Akt and NF-κB
To analyze the signaling cascades involved in the
effects described above, the role of Akt and NF-κB in stimulated
and treated A549 cells was evaluated by detection of phosphorylated
and unphosphorylated Akt and NF-κB by western blot analysis in cell
homogenates (Fig. 5). The protein
levels of Akt were similar in all groups. The relative protein
levels of phosphorylated Akt were barely detectable in unstimulated
controls, whereas the expression of phosphorylated Akt was
increased by IL-1β stimulation (Fig.
5A). The relative protein levels of phosphorylated Akt were
reduced by treatment with EtOH, EtP and NaP (Fig. 5A). The expression levels of
unphosphorylated and phosphorylated NF-κB were similar to those
results obtained from analyses of Akt. The quantification of Akt
and NF-κB expression revealed that both Akt and NF-ĸB
phosphorylation, after stimulation of A549 cells with IL-1β, was
significantly enhanced compared to unstimulated A549 cells
(Fig. 5B and C). This increase in
both Akt and NF-κB phosphorylation after IL-1β stimulation was
decreased by EtOH, EtP and NaP treatment, and reached levels of
phosphorylation that were comparable to the expression levels of
unstimulated cells (Fig. 5B and
C).
Discussion
In the present study, we evaluated the effects of
acute alcohol or EtP on the pro-inflammatory responses induced by
IL-1β or TNF stimulation of human lung epithelial or liver cells,
respectively. Exposure of lung epithelial cells to either EtOH or
EtP significantly reduced IL-1β- or TNF-induced IL-8 release.
Similarly, the adhesion capacity of isolated neutrophils to
stimulated lung cells was significantly reduced by EtOH or EtP, and
this result was paralleled by notably reduced CD54 expression. The
Bax/Bcl-2 ratio indicated that both EtOH and the high dose of EtP
reduced the apoptosis of lung cells. The underlying
anti-inflammatory mechanisms appear to involve the reduced
phosphorylation of Akt and NF-κB p65. The exposure of liver cells
to either EtOH or EtP did not induce similar effects to those
observed in lung cells. In summary, EtP reduced to EtOH levels the
inflammatory response in lung epithelial cells under acute
inflammatory conditions. However, due to the low impact on the
hepatocellular cells which we noted, the data suggest that EtP and
EtOH have different effects depending on the cellular entity and
also on the use of the pro-inflammatory stimulus.
Although alcohol is a well-described
immunomodulatory drug, serious inconsistencies regarding its use
and subsequent effects on associated pathologies have been
previously noted. Despite numerous reports on the deleterious
effects of chronic or excessive alcohol abuse, others and our group
have reported that moderate or acute use may exert beneficial
effects (15–17,20,36). The negative effects of alcohol
consumption have been linked to an increased inflammatory response,
including increased cytokine release (14,15,37,38). Pro-inflammatory cytokines such as
IL-1β, TNF, IL-6 and IL-8 have been identified as important
contributors to the pathogenesis of organ damage, including lung as
well as liver injury in models of acute inflammation (17–19,39–42). Together with IL-6, IL-8 binds to
molecules that are involved in neutrophil activation, trafficking
and infiltration of tissues in models of acute lung and liver
injury (18,43). Interestingly, both IL-6 and IL-8
are modulated by alcohol: while chronic alcohol consumption has
been proven to increase their expression and release, acute alcohol
consumption reduced the lipopolysaccharide (LPS)-induced IL-6
release from macrophages (44).
Short-term alcohol exposure reduced IL-8 release from human
umbilical vein cells (HUVECs) or lung epithelial cells, but
considerable inhibitory effects on the cellular interactions with
neutrophils were also noted, suggesting that reduced nuclear
translocation of NF-κB components was also involved (21,22). In the present study, IL-8 release
from lung epithelial cells after stimulation with either IL-1β or
TNF was markedly reduced by EtOH and EtP treatment (Fig. 1). These data suggest that EtOH and
EtP both exerted potent anti-inflammatory effects on lung
epithelial cells in our in vitro model of acute
inflammation. In line with these findings, Bhatty et al
(2001) demonstrated that treating mice with ~87 mM alcohol and then
challenging them intraperitoneally with non-pathogenic E.
coli suppressed the production of the majority of known
pro-inflammatory cytokines (45).
Neutrophils, as essential components of the host
defense and the innate immune system, are responsible for combating
infections; it has been demonstrated that reducing neutrophil
presence at sites of inflammation improved organ integrity, as
activated neutrophils have the potential to harm the injured tissue
(46). Moreover, reduced tissue
infiltration by neutrophils was closely associated with decreased
CD54 expression (47). Jonsson
and Palmblad have demonstrated that increased CD54 expression in
HUVECs after stimulation with LPS resulted in enhanced adhesion
rates of neutrophils to endothelial monolayers (22). In line with these findings, we
have shown in the present study that stimulating lung epithelial
cells with pro-inflammatory cytokines markedly increased neutrophil
adhesion to these cells as well as the expression of CD54 (Figs. 2 and 3). Treating these cells with EtOH
decreased cytokine-induced adhesion capacity, and CD54 expression
rates were also lowered but the decrease was not so considerable.
It has previously been noted that treating lung epithelial cells
with alcohol after they were stimulated with IL-6 decreased
neutrophil adhesion to these cells, but CD54 expression was not
markedly altered (30,31). These findings suggest that other
mechanisms aside from CD54 downregulation are involved in reducing
neutrophil adhesion, possibly CD31 or CD62L.
As well as functional effects, it has been noted
that treating stimulated lung epithelial cells with alcohol reduced
apoptotic changes induced by the stimulation with pro-inflammatory
cytokines. Acute alcohol exposure markedly diminished tissue damage
in an in vivo model of acute inflammation (18). Interestingly, here, there are
anti-apoptotic effects of alcohol on lung epithelial cells. Our
analysis of underlying pathways demonstrated predominantly reduced
activation of Akt and NF-κB after acute alcohol exposure of
stimulated lung epithelial cells (Fig. 5). The mechanism via NF-κB for the
anti-inflammatory effects of alcohol on lung epithelial cells has
been suggested previously (22,32). However, to the best of our
knowledge, the involvement of Akt had not been described
previously.
While beneficial results were observed for lung
cells, no similar data were observed for liver cells, suggesting a
cell-type specific mode of action for the substances that were
applied (Fig. 1). In our previous
in vivo studies, we have shown that acute alcohol
consumption exerted liver-protective effects, through diminished
systemic and local anti-inflammatory effects in our model of acute
inflammation (18,19). Previously, we have also
demonstrated that acute alcohol ingestion diminished the neutrophil
infiltration of the liver in the same in vivo model
(18). In this study, we noted
that stimulating liver cells with IL-1β significantly enhanced CD54
expression compared with control. We noted increased adhesion rates
of PMNs to stimulated liver cells, but the data confirmed that
although there was a tendency to increased PMN adhesion to Huh7,
this effect was not significant. One rationale is that, compared
with lung cells, Huh7 had stronger baseline CD54 expression as well
as higher baseline adhesion rates of PMNs to untreated controls.
Possibly, due to this, the findings regarding Huh7 cells were not
as prominent as they were in A549 cells. This is one explanation as
to why the observed data in Huh7 was not as clear as data from A549
cells. With regard to the anti-apoptotic effects of alcohol, liver
cells were not as sensitive to alcohol either (Fig. 4). It is possible that stimulating
Huh7 cells with IL-1β or TNF does not induce apoptosis, as was
observed in lung epithelial cells. Additional cell lines should be
used in future studies in order to monitor liver tissue.
However, given the adverse effects of alcohol
regarding its entry into the CNS but also the risk of addiction,
its practical use in clinical settings as a therapy option is
rather limited. Comparing the therapeutic potential of EtP with
EtOH, EtP is a safe and well-tolerated drug (23). Moreover, the anti-inflammatory
potential of EtOH was mimicked by EtP in lung epithelial cells.
Johansson and Palmblad have demonstrated this anti-inflammatory
potential previously (48).
However, no underlying mechanism was described previous to the
present study, to the best of our knowledge. In the present study,
we noted that EtP, like EtOH, reduced both Akt and NF-κB
phosphorylation, which possibly resulted in diminished
pro-inflammatory cytokine release, reduced neutrophil adhesion
rates to stimulated lung epithelial cells or lowered CD54
expression. Therefore, EtP likely represents a useful therapeutic
tool that should be investigated in further studies. Regarding NaP,
its effects often mimicked those of EtP. However, NaP appears to be
anti-proliferative rather than anti-apoptotic. These findings
suggest that the pyruvate moiety of both molecules has potent
anti-inflammatory potential, whereas the ethyl moiety of EtP or
EtOH is responsible for the anti-apoptotic effects. However, this
hypothesis should be evaluated in further studies.
Notably, the previously described effects were
observed only in lung epithelial cells in our study, whereas liver
cells appeared relatively unaffected by various treatment options,
and the stimuli used. It is possible that the hepatocellular cell
line Huh7, which was used in the present study, was not sensitive
to the stimuli that were chosen. Furthermore, treatment with either
EtOH, EtP or NaP may simply be cell-type specific. This remains a
limitation of our study and will be the subject of further studies.
In future studies, to exclude the possible effects of an unknown
lack of sensitivity of Huh7 cells to certain substances, and in
order to further strengthen the data from this study, the
experiments should be performed on more than one 'tissue-specific'
cell line. While the same treatment may be beneficial for one
tissue entity, it may be disadvantageous when using another
treatment strategy (e.g., timing) or other tissues. Therefore,
applying different treatment strategies to other cell lines
deriving from various tissues will markedly improve the findings.
Furthermore, the effects of prolonged incubation periods with both
EtOH and pyruvate were not evaluated in this study and remain to be
seen. Modulations of CD54 expression were rather inconsistent
regarding the expected and observed adhesion rates from neutrophils
to lung or liver cells. Other adhesion-related proteins and cell
lines should thus be evaluated in future studies. Another limiting
factor of this study is that the protein activation state was
evaluated by western blot analysis and not by a protein kinase
activity assay. This remains to be elucidated in future
studies.
In conclusion, the pro-inflammatory cytokine release
from lung epithelial cells induced by inflammatory cytokines was
reduced by both EtOH and EtP treatment. Moreover, both EtOH and EtP
reduced the expression of the intercellular adhesion molecules,
resulting in associated decreased neutrophil adhesion rates to lung
epithelial monolayers. Additionally, both substances exerted
anti-apoptotic potential. We suggest that the underlying mechanisms
for these effects involve the down-regulated activation of Akt and
NF-κB. Due to similarly strong effects as well as pathways
involved, and also its stability, EtP is likely a potential
therapeutic tool which can be used in a pre-clinical setting for
the treatment of acute inflammation.
Acknowledgments
We would like to thank Kerstin Kontradowitz,
Alexander Schaible and Katrin Jurida for outstanding technical
assistance. This study was supported by DFG PE908/3-1 and
RE3304/5-1.
References
|
1
|
Li G, Keyl PM, Smith GS and Baker SP:
Alcohol and injury severity: reappraisal of the continuing
controversy. J Trauma. 42:562–569. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rehm J, Room R, Graham K, Monteiro M, Gmel
G and Sempos CT: The relationship of average volume of alcohol
consumption and patterns of drinking to burden of disease: an
overview. Addiction. 98:1209–1228. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brattström O, Granath F, Rossi P and
Oldner A: Early predictors of morbidity and mortality in trauma
patients treated in the intensive care unit. Acta Anaesthesiol
Scand. 54:1007–1017. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Griffin R, Poe AM, Cross JM, Rue LW III
and McGwin G Jr: The association between blood alcohol level and
infectious complications among burn patients. J Burn Care Res.
30:395–399. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Messingham KA, Faunce DE and Kovacs EJ:
Alcohol, injury, and cellular immunity. Alcohol. 28:137–149. 2002.
View Article : Google Scholar
|
|
6
|
Moore EC, Padiglione AA, Wasiak J, Paul E
and Cleland H: Candida in burns: risk factors and outcomes. J Burn
Care Res. 31:257–263. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jurkovich GJ, Rivara FP, Gurney JG,
Fligner C, Ries R, Mueller BA and Copass M: The effect of acute
alcohol intoxication and chronic alcohol abuse on outcome from
trauma. JAMA. 270:51–56. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nau C, Wutzler S, Dorr H, Lehnert M,
Lefering R, Laurer H, Wyen H and Marzi I: Liver cirrhosis but not
alcohol abuse is associated with impaired outcome in trauma
patients - a retrospective, multicentre study. Injury. 44:661–666.
2013. View Article : Google Scholar
|
|
9
|
Zeckey C, Dannecker S, Hildebrand F,
Mommsen P, Scherer R, Probst C, Krettek C and Frink M: Alcohol and
multiple trauma: is there an influence on the outcome? Alcohol.
45:245–251. 2011. View Article : Google Scholar
|
|
10
|
Berger K, Ajani UA, Kase CS, Gaziano JM,
Buring JE, Glynn RJ and Hennekens CH: Light-to-moderate alcohol
consumption and risk of stroke among U.S. male physicians. N Engl J
Med. 341:1557–1564. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lustenberger T, Inaba K, Barmparas G,
Talving P, Plurad D, Lam L, Konstantinidis A and Demetriades D:
Ethanol intoxication is associated with a lower incidence of
admission coagulopathy in severe traumatic brain injury patients. J
Neurotrauma. 28:1699–1706. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sesso HD: Alcohol and cardiovascular
health: recent findings. Am J Cardiovasc Drugs. 1:167–172. 2001.
View Article : Google Scholar
|
|
13
|
Kaphalia L and Calhoun WJ: Alcoholic lung
injury: metabolic, biochemical and immunological aspects. Toxicol
Lett. 222:171–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Burnham EL, Kovacs EJ and Davis CS:
Pulmonary cytokine composition differs in the setting of alcohol
use disorders and cigarette smoking. Am J Physiol Lung Cell Mol
Physiol. 304:L873–L882. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cook RT: Alcohol abuse, alcoholism, and
damage to the immune system - a review. Alcohol Clin Exp Res.
22:1927–1942. 1998.
|
|
16
|
Mandrekar P, Catalano D, White B and Szabo
G: Moderate alcohol intake in humans attenuates monocyte
inflammatory responses: inhibition of nuclear regulatory factor
kappa B and induction of interleukin 10. Alcohol Clin Exp Res.
30:135–139. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Relja B, Henrich D, Wetzel G, Sander AL,
Jakob H, Maraslioglu M, Marzi I and Lehnert M: Effects of acute
ethanol gavage on intestinal integrity after
hemorrhage/resuscitation. Scand J Gastroenterol. 48:448–458. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Relja B, Höhn C, Bormann F, Seyboth K,
Henrich D, Marzi I and Lehnert M: Acute alcohol intoxication
reduces mortality, inflammatory responses and hepatic injury after
haemorrhage and resuscitation in vivo. Br J Pharmacol.
165:1188–1199. 2012. View Article : Google Scholar :
|
|
19
|
Relja B, Wilhelm K, Wang M, Henrich D,
Marzi I and Lehnert M: Acute ethanol gavage attenuates
hemorrhage/resuscitation-induced hepatic oxidative stress in rats.
Oxid Med Cell Longev. 2012:9834272012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Szabo G, Mandrekar P, Girouard L and
Catalano D: Regulation of human monocyte functions by acute ethanol
treatment: decreased tumor necrosis factor-alpha, interleukin-1
beta and elevated interleukin-10, and transforming growth
factor-beta production. Alcohol Clin Exp Res. 20:900–907. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Johansson AS, Lidén J, Okret S and
Palmblad JE: Effects of ethanol on cytokine generation and NFkappaB
activity in human lung epithelial cell. Biochem Pharmacol.
70:545–551. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jonsson AS and Palmblad JE: Effects of
ethanol on NF-kappaB activation, production of myeloid growth
factors, and adhesive events in human endothelial cells. J Infect
Dis. 184:761–769. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fink MP: Ethyl pyruvate. Curr Opin
Anaesthesiol. 21:160–167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kao KK and Fink MP: The biochemical basis
for the anti-inflammatory and cytoprotective actions of ethyl
pyruvate and related compounds. Biochem Pharmacol. 80:151–159.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li LF, Kao KC, Yang CT, Huang CC and Liu
YY: Ethyl pyruvate reduces ventilation-induced neutrophil
infiltration and oxidative stress. Exp Biol Med (Maywood).
237:720–727. 2012. View Article : Google Scholar
|
|
26
|
Cai B, Brunner M, Wang H, Wang P, Deitch
EA and Ulloa L: Ethyl pyruvate improves survival in awake
hemorrhage. J Mol Med Berl. 87:423–433. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cai B, Deitch EA, Grande D and Ulloa L:
Anti-inflammatory resuscitation improves survival in hemorrhage
with trauma. J Trauma. 66:1632–1639; discussion 1639–1640. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luan ZG, Zhang H, Ma XC, Zhang C and Guo
RX: Therapeutic treatment with ethyl pyruvate attenuates the
severity of liver injury in rats with severe acute pancreatitis.
Pancreas. 41:729–737. 2012.PubMed/NCBI
|
|
29
|
Ulloa L, Ochani M, Yang H, Tanovic M,
Halperin D, Yang R, Czura CJ, Fink MP and Tracey KJ: Ethyl pyruvate
prevents lethality in mice with established lethal sepsis and
systemic inflammation. Proc Natl Acad Sci USA. 99:12351–12356.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Relja B, Omid N, Kontradowitz K, Jurida K,
Oppermann E, Störmann P, Werner I, Juengel E, Seebach C and Marzi
I: Decreased inflammatory responses of human lung epithelial cells
after ethanol exposure are mimicked by ethyl pyruvate. Mediators
Inflamm. 2014:7815192014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Relja B, Omid N, Schaible A, Perl M, Meier
S, Oppermann E, Lehnert M and Marzi I: Pre- or post-treatment with
ethanol and ethyl pyruvate results in distinct anti-inflammatory
responses of human lung epithelial cells triggered by
interleukin-6. Mol Med Rep. 12:2991–2998. 2015.PubMed/NCBI
|
|
32
|
Johansson AS, Johansson-Haque K, Okret S
and Palmblad J: Ethyl pyruvate modulates acute inflammatory
reactions in human endothelial cells in relation to the NF-kappaB
pathway. Br J Pharmacol. 154:1318–1326. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maiya R, Buck KJ, Harris RA and Mayfield
RD: Ethanol-sensitive sites on the human dopamine transporter. J
Biol Chem. 277:30724–30729. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Famili A, Ammar DA and Kahook MY: Ethyl
pyruvate treatment mitigates oxidative stress damage in cultured
trabecular meshwork cells. Mol Vis. 19:1304–1309. 2013.PubMed/NCBI
|
|
35
|
Relja B, Meder F, Wilhelm K, Henrich D,
Marzi I and Lehnert M: Simvastatin inhibits cell growth and induces
apoptosis and G0/G1 cell cycle arrest in hepatic cancer cells. Int
J Mol Med. 26:735–741. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ajisaka H, Okajima M, Goto Y, Taniguchi T
and Inaba H: Effects of acute low-dose ethanol on inflammatory
reactions to endotoxin-induced shock in rats. J Toxicol Sci.
37:649–654. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maraslioglu M, Oppermann E, Blattner C,
Weber R, Henrich D, Jobin C, Schleucher E, Marzi I and Lehnert M:
Chronic ethanol feeding modulates inflammatory mediators,
activation of nuclear factor-κB, and responsiveness to endotoxin in
murine Kupffer cells and circulating leukocytes. Mediators Inflamm.
2014:8086952014. View Article : Google Scholar
|
|
38
|
Maraslioglu M, Weber R, Korff S, Blattner
C, Nauck C, Henrich D, Jobin C, Marzi I and Lehnert M: Activation
of NF-κB after chronic ethanol intake and haemorrhagic
shock/resuscitation in mice. Br J Pharmacol. 170:506–518. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Do-Umehara HC, Chen C, Urich D, Zhou L,
Qiu J, Jang S, Zander A, Baker MA, Eilers M, Sporn PH, et al:
Suppression of inflammation and acute lung injury by Miz1 via
repression of C/EBP-δ. Nat Immunol. 14:461–469. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Allen TC and Kurdowska A: Interleukin 8
and acute lung injury. Arch Pathol Lab Med. 138:266–269. 2014.
View Article : Google Scholar
|
|
41
|
Monção-Ribeiro LC, Cagido VR, Lima-Murad
G, Santana PT, Riva DR, Borojevic R, Zin WA, Cavalcante MC, Riça I,
Brando-Lima AC, et al: Lipopolysaccharide-induced lung injury: Role
of P2X7 receptor. Respir Physiol Neurobiol. 179:314–325. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu T, Wang DX, Zhang W, Liao XQ, Guan X,
Bo H, Sun JY, Huang NW, He J, Zhang YK, et al: Andrographolide
protects against LPS-induced acute lung injury by inactivation of
NF-κB. PLoS One. 8:e564072013. View Article : Google Scholar
|
|
43
|
Reiss LK, Uhlig U and Uhlig S: Models and
mechanisms of acute lung injury caused by direct insults. Eur J
Cell Biol. 91:590–601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Karavitis J, Murdoch EL, Gomez CR, Ramirez
L and Kovacs EJ: Acute ethanol exposure attenuates pattern
recognition receptor activated macrophage functions. J Interferon
Cytokine Res. 28:413–422. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bhatty M, Jan BL, Tan W, Pruett SB and
Nanduri B: Role of acute ethanol exposure and TLR4 in early events
of sepsis in a mouse model. Alcohol. 45:795–803. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Segel GB, Halterman MW and Lichtman MA:
The paradox of the neutrophil's role in tissue injury. J Leukoc
Biol. 89:359–372. 2011. View Article : Google Scholar
|
|
47
|
Relja B, Töttel E, Breig L, Henrich D,
Schneider H, Marzi I and Lehnert M: Effects of green tea catechins
on the pro-inflammatory response after haemorrhage/resuscitation in
rats. Br J Nutr. 105:1791–1797. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Johansson AS and Palmblad J: Ethyl
pyruvate modulates adhesive and secretory reactions in human lung
epithelial cells. Life Sci. 84:805–809. 2009. View Article : Google Scholar : PubMed/NCBI
|