Introduction
The management of chronic wounds is a significant
drain on healthcare resources; the cost of wounds to the United
Kingdom healthcare system alone is estimated to be ~£1 billion per
year (1), notwithstanding the
economic loss and impaired quality of life experienced by people
with chronic ulcers. Identification of the molecular factors
underlying chronic and acute wounds is critical for developing
improved tailored treatments for wound healing.
In acute wounds caused by trauma to intact skin,
normal wound healing involves three overlapping dynamic phases of
inflammation (lasting 1-3 days), proliferation (lasting 3-14 days)
and remodelling (can last up to several months) (2,3).
However, in chronic wounds, 70% of which is represented by venous
leg ulcers, often underlying disease states reduce healing and this
dynamic process does not proceed in an orderly or timely manner to
produce anatomic and functional skin integrity within 3 months, as
observed in acute wounds (4).
The biology of skin healing in acute and chronic
wounds involves complex interactions between epidermal and dermal
cells, the extracellular matrix and plasma-derived proteins
(5). Currently, research is
focusing on understanding the role of angiogenesis in physiological
and pathological processes, such as inflammation, wound healing and
tumour angiogenesis.
Tumour endothelial marker-8 (TEM-8) is a highly
conserved type 1 transmembrane protein that was originally
identified based on its overexpression in the endothelial cells
lining the tumour vasculature of human colorectal cancer (6). The present understanding of the
physiological function of TEM-8 is limited; the high level of
conservation of TEM-8 among different species suggests that TEM-8
has a fundamental role in normal physiology, as well as
pathological processes. TEM-8 has been found to bind to collagens
and promote migration of endothelial cells in vitro
(7,8), and thus have a potential role in
angiogenesis (9–11) and wound healing.
TEM-8 is upregulated in tumour vasculature in mice
and humans (7,12,13), and is also expressed by tumour
cells themselves in certain cancer types (12,14,15). However, TEM-8 could not be
detected in the angiogenic corpus luteum of human ovaries (6,7)
and thus presents itself as a unique target for selectively
blocking pathological angiogenesis. Previous studies have shown
that the genetic disruption and antibody-mediated disruption of
TEM-8 in mice inhibited tumour angiogenesis and growth, but did not
perturb acute wound healing observed for ≤7 days (16,17). However, the role of TEM-8 in
chronic non-healing wounds has not been investigated previously.
Inhibition of angiogenesis is known to impair wound healing
(18–20) and previous studies have revealed
potential microenvironmental factors, specifically a reduction in
tissue growth factors, which is known to impair healing (21), have now been shown to also induce
TEM-8 expression (16,22).
The present study investigated the role of TEM-8 in
wound healing processes, specifically its expression in clinical
chronic wound samples and its influence on HaCaT growth and
migrational rates.
Materials and methods
Materials
A universal immunohistochemical kit, Elite ABC kit,
was purchased from Vector Laboratories (Peterborough, UK). The
total RNA isolation reagent was purchased from Sigma-Aldrich
(Dorset, UK) and reverse transcription kits (iScript) were obtained
from Bio-Rad Laboratories (Hemel Hempstead, UK).
Skin biopsies
Skin biopsies were obtained from patients attending
the University Hospital of Wales (UHW, Cardiff, UK) wound healing
clinic, as described previously (23,24). Ethical approval was granted by the
Local Research Ethics Committee. Written informed consent was
obtained from each patient.
Chronic wound tissue
Biopsies from 14 patients with chronic leg ulcers
were used during the study. Venous disease was diagnosed by duplex
ultrasonography and all the wounds were present for ≥6 months, with
no evidence of healing occurring 6 weeks before biopsy. The wounds
had a minimum area of 4 cm2 prior to biopsy and had no
clinical indications of infection. Using an aseptic technique, 6-mm
punch biopsies were removed, following the application of local
anesthetic (1% lidocaine), from the wound margin, incorporating
epidermis and dermis at the wound edge with adjacent granulation
tissue.
Acute wound tissue
Single wedge biopsies were obtained from 10 patients
with acute surgical wounds subsequent to undergoing excision of
pilonoidal disease. These wounds were judged to be clinically
noninfected. The biopsies were obtained from the edge of the
healing wound within 6 weeks from the surgical excision.
Normal skin tissue
To provide a comparison to wound tissue, normal skin
tissue was also examined. Under local anesthetic, 3-mm punch
biopsies were removed from the inner aspect of the upper arm of 10
healthy volunteers working within the Wound Healing Research Unit
(School of Medicine, Cardiff University, Cardiff, Wales).
Immunohistochemical staining
Frozen sections from wound tissues were first fixed
in an acetone/methanol solution and rehydrated in wash buffer
(MenaPath Autowash buffer; A. Menarini Diagnostics, Berkshire, UK)
prior to placing the samples into a wash buffer solution containing
10% horse serum to aid in the blocking of non-specific antigen
binding. An avidin/biotin complex (ABC) immunohistochemistry (IHC)
kit (Vector Laboratories, Nottingham, UK) was used in accordance
with the manufacturer's protocol. A polyclonal antibody to TEM-8,
previously generated (9), was
used and diluted in a buffer that contained 1% horse serum and 0.1%
Tween-20 at 1:40 dilution. After a 1-h incubation period with the
primary antibody, the slides were washed 4 times in a washing
buffer and a universal biotinylated secondary antibody was added
for 30 min. Following washing, avidin and biotin were added through
the addition of the ABC complex. A DAB colour developing system was
used to indirectly detect protein staining. Sections were
dehydrated through a series of graded alcohols, cleared in xylene,
mounted and evaluated on an Olympus microscope equipped with a
digital camera (Olympus, Southend-on-Sea, UK).
HaCaT cell line and culture
conditions
The HaCaT human keratinocyte cell line was purchased
from the German Cancer Research Institute (Heidelberg, Germany).
Cells were maintained in Dulbecco's modified Eagle's medium
supplemented with penicillin, streptomycin and 10% fetal calf serum
(PAA Laboratories Ltd., Somerset, UK). The cells were incubated at
37°C, 5% CO2 and 95% humidity.
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR)
Cells were grown to confluence in a
25-cm2 flask prior to RNA extraction using the total RNA
isolation reagent in accordance with the manufacturer's protocol.
Sample RNA was quantified using a spectrophotometer (WPA UV 1101;
Biotech Photometer, Cambridge, UK) and standardized to a
concentration of 500 ng prior to being used as a template to
reverse transcribe cDNA using an iScript cDNA synthesis kit
(Bio-Rad Laboratories). Following cDNA synthesis, samples were
probed using glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
primers to check the cDNA quality and confirm uniform sample cDNA
levels, together with those specific for TEM-8 transcript (Table I) as previously reported (9,11).
 | Table IPrimer and ribozyme sequences. |
Table I
Primer and ribozyme sequences.
| Primer/sequence | Forward | Reverse |
|---|
| TEM-8 ribozyme 1 |
ctgcagggggccatagagacggctgatgagccgtga |
actagtccacagctattatgtgtttcgtcctcacggac |
| TEM-8 ribozyme 3 |
gtgcagacttcttcaaaattgagtggatctgatgactccctga |
actagttttcaggctctgcaaggcatttcgtcctcacgga |
| TEM-8
conventional |
catttcaagttgtcgtgaga |
gacgcatattgttgttgaga |
| TEM-8
quantitative |
acagggtcctctgcagctt |
actgaacctgaccgtacactttcatgccaacttgttt |
| GAPDH
conventional |
agcttgtcatcaatggaaat |
cttcaccaccttcttgatgt |
| GAPDH
quantitative |
ctgagtacgtcgtggagtc |
actgaacctgaccgtacacagagatgatgacccttttg |
Conventional RT-PCR primers were designed using
Beacon designer Software (Beacon Designer, Palo Alto, CA, USA), to
allow amplification of regions that have no overlap with known
genes and span at least one intron. Primers were synthesized by
Invitrogen (Paisley, UK). Conditions for conventional RT-PCR to
amplify transcripts of TEM-8 were: 94°C for 40 sec, 54°C for 30
sec, 72°C for 50 sec and a final extension phase of 10 min for
34–36 cycles, with GAPDH used as the reference gene. The PCR
products were separated on a 0.8% agarose gel and stained with
ethidium bromide prior to examination under UV light.
RT-quantitative PCR (RT-qPCR)
RT-qPCR was used to determine the transcript
expression levels of TEM-8 in wound tissues. This methodology has
been reported previously (25).
Briefly, the iCycler IQ system (Bio-Rad, Camberley, UK) was used to
quantify the transcript level of TEM-8 within each of the clinical
samples. Results are provided as number of transcripts per
microlitre and are based on an internal standard run and amplified
in conjunction with the samples. Normalisation of samples was
achieved through comparison of sample GAPDH levels. The Amplifluor
system (Intergen Inc., New York, NY, USA) was used in conjunction
with a universal probe (UniPrimer), which recognised a specific
sequence (z sequence), incorporated into the primers. RT-qPCR
conditions were as follows: 95°C for 15 sec, 54°C for 20 sec and
60°C for 60 sec.
TEM-8 knockdown in the human HaCaT
keratinocyte cell line
Hammerhead ribozyme transgenes, specifically
targeted to TEM-8 transcripts were constructed based on the
secondary structure of TEM-8 mRNA, as described previously
(10) (Table I). Following the design and
synthesis by Invitrogen, two separate ribozymes, targeting
different regions of the TEM-8 transcript, were cloned into a
mammalian pEF6/His TOPO vector (Invitrogen) and transfected into
HaCaT cells, as previously reported (10). Following a period of blasticidin
selection (5 µg/ml), the cells were maintained in media
containing 0.5 µg/ml blasticidin. This process allowed the
generation of stably transfected HaCaT cells containing the
ribozyme transgene and expressing reduced TEM-8 levels
(HaCaTΔTEM-8 rib1 and HaCaTΔTEM-8 rib3) and
control HaCaT cells transfected with a closed pEF6 plasmid
(HaCaTpEF6). Suppression of TEM-8 expression in
HaCaTΔTEM-8 rib1 and HaCaTΔTEM-8 rib3 cells
was verified, in comparison to HaCaTpEF6 controls, using
RT-PCR.
In vitro growth assay
The effect of TEM-8 suppression on HaCaT cell growth
rates was assessed using an in vitro growth assay. Cells
were seeded at a density of 3,000 cells/well in 96-well plates.
Triplicate plates were set up and incubated for 3- and 5-day
periods before analyses. Following incubation, the plates were
fixed in 4% formaldehyde (v/v) and stained with 0.5% (w/v) crystal
violet and were subsequently treated with 10% acetic acid (v/v),
prior to colorimetric detection of cell density by
spectrophotometric analysis at 540 nm using a Bio-Tek ELx800
mutliplate reader (Bio-Tek Instruments Inc., Winnoski, VT,
USA).
Electric cell-substrate impedance sensing
(ECIS) analysis of HaCaT migration
The ECIS 9600 system (Applied Biophysics Inc., Troy,
NJ, USA) was used to detect and track HaCaT cell migration, as
described previously (26,27).
Briefly, cells were simultaneously plated in ECIS 8W10 arrays and
incubated until a confluent monolayer had formed over the array
electrodes. This monolayer was subsequently wounded electrically by
applying 6V for a 30-sec time-period to create a simultaneous
physical break in the cell monolayer of equal dimensions. The rate
of change in resistance as cells migrated back onto the electrode,
was subsequently monitored and measured using the ECIS software
provided. Prior to use, ECIS arrays were treated with L-cysteine
solution for 40 min followed by several washes with complete medium
to remove any particles present on the electrode.
Statistical analysis
The SigmaPlot 11 statistical package (Systat
Software, San Jose, CA, USA) were used to identify statistical
differences between the test groups using one-way analysis of
variance (ANOVA) or ANOVA on RANKS tests. P<0.05 was considered
to indicate a statistically significant difference. All the in
vitro functional assays were repeated a minimum of three
times.
Results
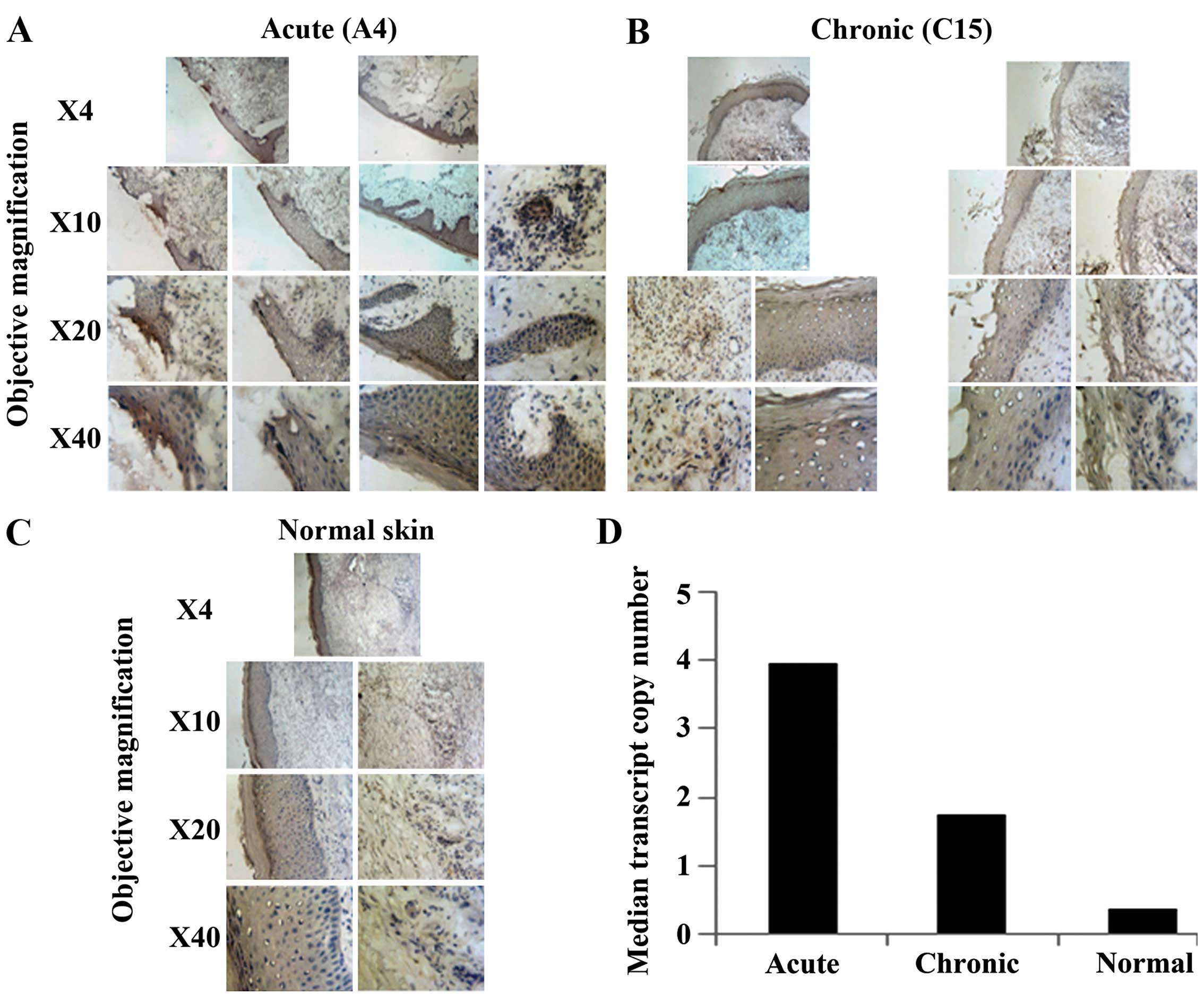
TEM-8 expression is decreased in
non-healing chronic venous leg ulcers compared to acute wounds
TEM-8 is minimally expressed in all layers of the
epidermis of normal skin. However, in acute wounds, expression of
TEM-8 increases within the cytoplasm of keratinocytes and
endothelial cells, consistent with its putative role in
angiogenesis. Expression was also increased in chronic wounds
compared to normal skin, but is lower compared to the acute wounds
(Fig. 1A–C).
Consistent with the immunohistochemical analyses,
RT-qPCR detection and normalisation of TEM-8 levels also revealed
higher TEM-8 expression in acute (median, 3.964; IQR,
12.049-0.0445) and chronic (median, 1.755; IQR, 7.426-0.144) wounds
and again, extremely low levels were observed in normal skin
(median, 0.381; IQR, 1.996-0.0629), although no significant
differences were observed within the group (P>0.05) (Fig. 1D).
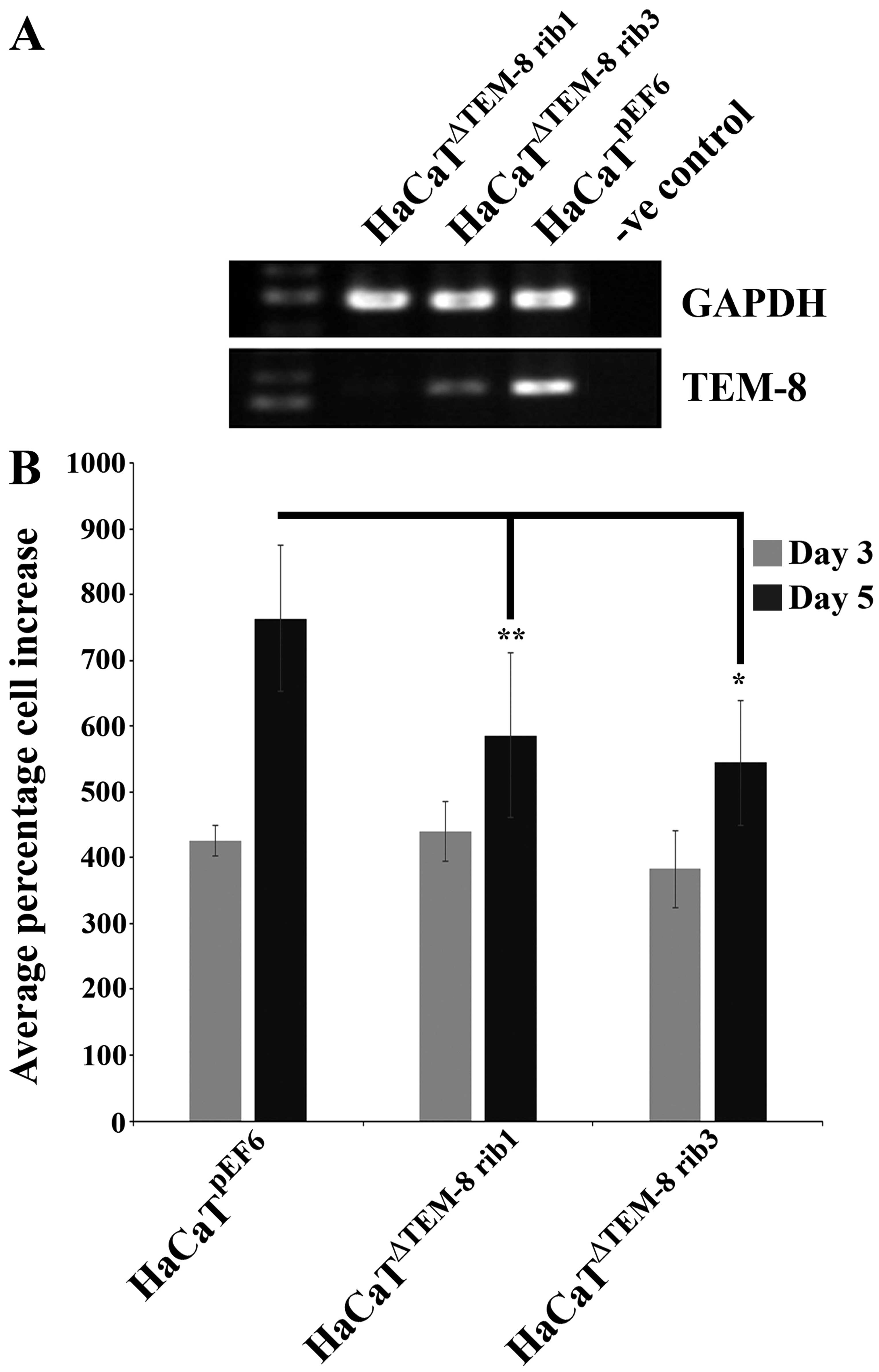
Suppression of TEM-8 expression reduces
the growth rate of HaCaT keratinocytes
To determine the effect of reduced TEM-8 expression
on keratinocyte cell growth, HaCaT keratinocytes were transfected
with ribozyme transgenes specifically targeted to the TEM-8
transcript to reduce TEM-8 transcript levels within HaCaT cells, as
assessed by RT-PCR (Fig. 2A).
In HaCaT keratinocytes in which TEM-8 expression was
reduced, a decrease was also observed in the in vitro growth
rate of the HaCaT cells (Fig.
2B). The growth rate of HaCaTΔTEM-8 rib1 and
HaCaTΔTEM-8 rib3 cells over a 5-day incubation period
was significantly lower compared to the HaCaTpEF6
control cells (P=0.004 and P=0.014, respectively). No significant
differences between the groups were observed over a 3-day
incubation period (P>0.05).
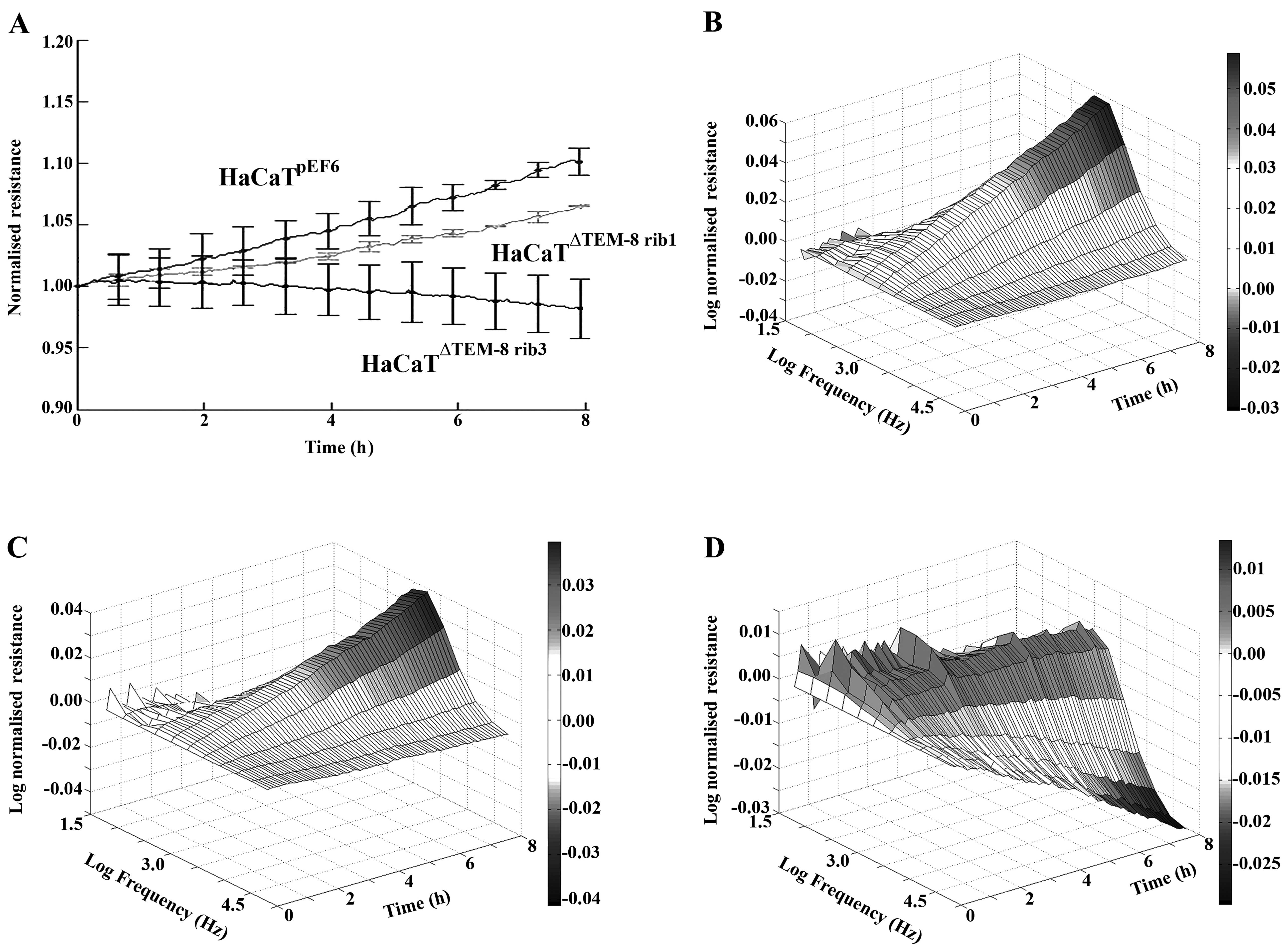
Suppression of TEM-8 expression reduces
the migrational rates of HaCaT cells
As TEM-8 expression was reduced in non-healing
wounds compared to acute wounds and suppression of TEM-8 expression
also reduced cell growth, the effects of TEM-8 knockdown on
keratinocyte migration directly were investigated. An ECIS assay
was carried out to examine the effects of TEM-8 suppression on
HaCaT migration following electrical wounding (Fig. 3). Substantial differences between
the migratory rates of HaCaTpEF6, HaCaTΔTEM-8
rib1 and HaCaTΔTEM-8 rib3 were observed as the
cell lines responded to re-colonise the wounded area of the array.
HaCaTpEF6 cells migrated at a steady rate following
wounding as indicated by the increase in resistance recorded across
the array. In contrast to this, HaCaTΔTEM-8 rib1 and
HaCaTΔTEM-8 rib3 cells showed a reduced capacity to
migrate into the wound and recover the monolayer over the
experimental time (Fig. 3A).
Three-dimensional analysis further demonstrated this trend, showing
changes in resistance across a range of tested frequencies and time
for HaCaTpEF6 (Fig.
3B), HaCaTΔTEM-8 rib1 (Fig. 3C) and HaCaTΔTEM-8 rib3
(Fig. 3D).
Discussion
TEM-8 is a cell surface receptor that has been shown
to promote tumour angiogenesis. Given the importance of
angio-genesis in tissue healing and repair, the role of TEM-8 in
acute and chronic wounds was investigated in the present study.
TEM-8 expression was significantly higher in acute
wounds, measured at 6 weeks, and chronic wounds, measured at 6
months, compared to normal skin tissue. Targeting TEM-8 expression,
using a ribozyme transgene system in the HaCaT keratinocyte cell
line, resulted in decreased growth after 5 days and decreased
migration within hours in an ECIS-based assay. A similar role for
TEM-8 in promoting cell migration was observed in previous studies
demonstrating that TEM-8 mediates spreading of endothelial cells
in vitro (8).
In other studies, TEM-8-KO and anti-TEM-8 antibodies
did not appear to affect acute wound closure rates or the amount of
vasculature present within the acute wound granulation tissue in
mice measured at 5–7 days (16,17). Therefore, it is important to note
that wound healing was assessed at different times (days versus
weeks/months) and the experiments reported in the present study
were performed in vitro on single HaCaT cell lines, and
therefore it is difficult to compare the direct effects on a single
cell line with observed physiological responses in animal models,
whereby multiple mechanisms and cells participate in wound
healing.
Notably, TEM-8 expression was 2-fold higher in the
acute versus chronic wounds, but nearly undetectable in normal skin
tissue, consistent with studies by Chaudhary and St Croix, which
demonstrated that local environmental stressors, including growth
factor deprivation, reduced levels of which are commonly observed
in non-healing wounds (21), led
to TEM-8 overexpression (16,22).
It has been proposed that TEM-8 may be involved in a
transient stress-mediated response (16,22), and numerous stressors, such as
hypoxia and low levels of growth factors, exist in the chronic
wound microenvironment (18–21,28). Persistent elevated levels of TEM-8
in chronic wounds suggest that TEM-8 could have a role in
pathological angiogenesis in non-healing wounds, as well as in
tumours.
The notion that tumours represent 'unhealed wounds'
is one of the oldest ideas in cancer biology (29) and it is perhaps not surprising
that TEM-8 could have a role in angiogenesis in both of these
pathological processes.
An improved understanding of the mechanism and role
TEM-8 has in acute and chronic wounds requires further study and
may allow for the development of more effective therapies for
tumour angiogenesis and also chronic wound healing.
Acknowledgments
The authors would like to acknowledge the A4B Scheme
of the Welsh Government, Ser Cymru, NRN life sciences research
network and Cancer Research Wales for kindly supporting the present
study.
References
|
1
|
Harding KG: The future of wound healing.
Wounds: Biology and Management. 191–199. 1998.
|
|
2
|
Clark RAF: Biology of dermal wound repair.
Dermatol Clin. 11:647–666. 1993.PubMed/NCBI
|
|
3
|
Singer AJ and Clark RAF: Cutaneous wound
healing. N Engl J Med. 341:738–746. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Margolis DJ, Allen-Taylor L, Hoffstad O
and Berlin JA: Diabetic neuropathic foot ulcers: Predicting which
ones will not heal. Am J Med. 115:627–631. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamada KM and Clark RAF: Provisional
matrix. The Molecular and Cellular Biology of Wound Repair. 51–93.
1996.
|
|
6
|
St Croix B, Rago C, Velculescu V, Traverso
G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C,
Vogelstein B, et al: Genes expressed in human tumor endothelium.
Science. 289:1197–1202. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nanda A, Carson-Walter EB, Seaman S,
Barber TD, Stampfl J, Singh S, Vogelstein B, Kinzler KW and St
Croix B: TEM8 interacts with the cleaved C5 domain of collagen α
3(VI). Cancer Res. 64:817–820. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Werner E, Kowalczyk AP and Faundez V:
Anthrax toxin receptor 1/tumor endothelium marker 8 mediates cell
spreading by coupling extracellular ligands to the actin
cytoskeleton. J Biol Chem. 281:23227–23236. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rmali KA, Al-Rawi MA, Parr C, Puntis MC
and Jiang WG: Upregulation of tumour endothelial marker-8 by
interleukin-1beta and its impact in IL-1beta induced angiogenesis.
Int J Mol Med. 14:75–80. 2004.PubMed/NCBI
|
|
10
|
Rmali KA, Puntis MCA and Jiang WG: TEM-8
and tubule formation in endothelial cells, its potential role of
its vW/TM domains. Biochem Biophys Res Commun. 334:231–238. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rmali KA, Watkins G, Harrison G, Parr C,
Puntis MCA and Jiang WG: Tumour endothelial marker 8 (TEM-8) in
human colon cancer and its association with tumour progression. Eur
J Surg Oncol. 30:948–953. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carson-Walter EB, Watkins DN, Nanda A,
Vogelstein B, Kinzler KW and St Croix B: Cell surface tumor
endothelial markers are conserved in mice and humans. Cancer Res.
61:6649–6655. 2001.PubMed/NCBI
|
|
13
|
Fernando S and Fletcher BS: Targeting
tumor endothelial marker 8 in the tumor vasculature of colorectal
carcinomas in mice. Cancer Res. 69:5126–5132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jinnin M, Medici D, Park L, Limaye N, Liu
Y, Boscolo E, Bischoff J, Vikkula M, Boye E and Olsen BR:
Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2
signaling in infantile hemangioma. Nat Med. 14:1236–1246. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang MY, Chaudhary A, Seaman S, Dunty J,
Stevens J, Elzarrad MK, Frankel AE and St Croix B: The cell surface
structure of tumor endothelial marker 8 (TEM8) is regulated by the
actin cytoskeleton. Biochim Biophys Acta. 1813:39–49. 2011.
View Article : Google Scholar :
|
|
16
|
Chaudhary A, Hilton MB, Seaman S, Haines
DC, Stevenson S, Lemotte PK, Tschantz WR, Zhang XM, Saha S, Fleming
T, et al: TEM8/ANTXR1 blockade inhibits pathological angiogenesis
and potentiates tumoricidal responses against multiple cancer
types. Cancer Cell. 21:212–226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cullen M, Seaman S, Chaudhary A, Yang MY,
Hilton MB, Logsdon D, Haines DC, Tessarollo L and St Croix B:
Host-derived tumor endothelial marker 8 promotes the growth of
melanoma. Cancer Res. 69:6021–6026. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brem H, Ehrlich P, Tsakayannis D and
Folkman J: Delay of wound healing by the angiogenesis inhibitor
TNP-470. Surg Forum. 48:714–716. 1997.
|
|
19
|
Brem H, Tsakayannis D and Folkman J: Time
dependent suppression of wound healing with the angiogenesis
inhibitor, AGM-1470. J Cell Biol. 115:403a1991.
|
|
20
|
McGrath MH and Emery JM III: The effect of
inhibition of angiogenesis in granulation tissue on wound healing
and the fibroblast. Ann Plast Surg. 15:105–122. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Higley HR, Ksander GA, Gerhardt CO and
Falanga V: Extravasation of macromolecules and possible trapping of
transforming growth factor-β in venous ulceration. Br J Dermatol.
132:79–85. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chaudhary A and St Croix B: Selective
blockade of tumor angio-genesis. Cell Cycle. 11:2253–2259. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Conway K, Ruge F, Price P, Harding KG and
Jiang WG: Hepatocyte growth factor regulation: An integral part of
why wounds become chronic. Wound Repair Regen. 15:683–692. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Conway KP, Price P, Harding KG and Jiang
WG: The role of vascular endothelial growth inhibitor in wound
healing. Int Wound J. 4:55–64. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang WG, Douglas-Jones A and Mansel RE:
Expression of peroxisome-proliferator activated receptor-gamma
(PPARgamma) and the PPARgamma co-activator, PGC-1, in human breast
cancer correlates with clinical outcomes. Int J Cancer.
106:752–757. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang WG, Martin TA, Lewis-Russell JM,
Douglas-Jones A, Ye L and Mansel RE: Eplin-alpha expression in
human breast cancer, the impact on cellular migration and clinical
outcome. Mol Cancer. 7:712008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Keese CR, Wegener J, Walker SR and Giaever
I: Electrical wound-healing assay for cells in vitro. Proc Natl
Acad Sci USA. 101:1554–1559. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hasan A, Murata H, Falabella A, Ochoa S,
Zhou L, Badiavas E and Falanga V: Dermal fibroblasts from venous
ulcers are unresponsive to the action of transforming growth
factor-β1. J Dermatol Sci. 16:59–66. 1997. View Article : Google Scholar
|
|
29
|
Dvorak HF: Tumors: Wounds that do not
heal. Similarities between tumor stroma generation and wound
healing. N Engl J Med. 315:1650–1659. 1986. View Article : Google Scholar : PubMed/NCBI
|