Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic,
progressive form of lung disease which is characterized by the
abnormal and excessive deposition of collagen (fibrosis) in the
pulmonary interstitium, mainly on the walls of the alveoli
(1). The annual incidence of IPF
was estimated at 6.8–8.8 per 100,000 individuals and 16.3–17.4 per
100,000 individuals using narrow and broad case definitions,
respectively, in the USA, and 0.22–7.4 per 100,000 individuals in
Europe (2). The prognosis for
patients with IPF is quite poor, and the current median survival
rate following diagnosis is close to 3 years (3,4).
Although extensive investigations have been conducted, the cause
and pathogenesis of IPF are not yet completely understood (5). However, new concepts have been
recently proposed: IPF is no longer thought to be the result of
inflammatory mechanisms, as previously considered, but is rather
thought to be the result of a fibro-proliferative and aberrant
wound healing cascade (6).
Despite the increase in the number of clinical trials for IPF, the
majority of the results from these trials, including those using
corticosteroids, or a combination of N-acetylcysteine, warfarin and
bosentan, have been disappointing (7). Although pirfenidone and nintedanib
attenuate the decline in pulmonary function, there are currently no
effective pharmacological therapies to reduce the mortality rate
(7–10). Thus, the discovery of novel agents
with therapeutic potential for IPF is desirable.
Citrus fruits (e.g., oranges) are important fruit
tree crops worldwide and are among the most commonly consumed
fruits, and the global Citrus industry is worth approximately $9
billion/year (11). The benefits
of Citrus fruits are partly due to their phytochemical components
(12), which include a wide
variety of non-nutritive phytochemicals, such as flavonoids,
alkaloids, anthocyanins, phenolic acids, carotenoids and tannins,
together with nutritive components, such as sugars, proteins,
vitamins, fibers and minerals (13,14). Flavonoids, which are common
polyphenols and one of the main chemical constituents of the
Citrus genus, have been widely investigated for their
possible role in the prevention of cardiovascular disease and
cancer (15). Another important
active component of Citrus fruits are adrenergic amines, such as
octopamine, synephrine and tyramine, which are a type of simple
alkaloid, and they exert their effects on the cardiovascular system
through adrenergic stimulation (16,17). Synephrine, a main constituent of
Citrus fruits, stimulates lipolysis, raises the metabolic rate, and
promotes the oxidation of fat, and therefore helps to reduce fat
mass in obese individuals, and has been widely used in weight loss
and weight management as well as in sports performance products
(17). Furthermore, previous
studies have revealed that the use of Citrus extract and synephrine
appears to be safe, and no serious adverse effects have been
directly attributed to these ingredients (17–19).
Within our research project aimed at discovering the
active molecules which exert anti-fibrotic effects, we previously
screened a number of Chinese herbal drugs that have been used to
treat lung disease, and discovered that the pericarp of Citrus
reticulata (C. reticulata) exerted inhibitory effects on
pulmonary fibrosis in vitro and in vivo (20,21). In traditional Chinese medicine,
the pericarp of C. reticulata has medical functions: it
regulates Qi and expels phlegm, and has been used for the treatment
of lung-related diseases for a long time (22). In another previous study of ours,
we revealed that the alkaline extract of 75% ethanol soluble
fraction from C. reticulata was responsible for the
inhibitory effects on pulmonary fibrosis in vitro and in
vivo (23). Considering the
chemical properties of the alkaline extract, and the alkalinity of
alkaloids, we speculated that the active compounds in the alkaline
extract may be the amines contained in C. reticulata.
As a continuation of this project, and motivated by
our previous encouraging results, we conducted further research to
clarify whether these amines are responsible for the inhibitory
effects observed on pulmonary fibrosis. In the present study, the
main amines in the alkaline extract were isolated, and their
structures were determined based on nuclear magnetic resonance
(NMR) and mass spectra (MS). Furthermore, the hydrochlorides of the
isolated amines were prepared, and their anti-proliferative
activity was assayed using a human embryonic lung fibroblast (hELF)
culture system. The effects of the amine hydrochloride, which
possessed potent inhibitory activity in vitro, were further
evaluated using a rat model of bleomycin-induced pulmonary
fibrosis, and the preliminary mechanisms were also
investigated.
Materials and methods
Plant material
The pericarp of Citrus reticulata Blanco
(Rutaceae) was obtained from the Department of Medicinal Materials,
Affiliated Jiangsu Province Hospital of Traditional Chinese
Medicine, Nanjing University of Chinese Medicine (Nanjing, China),
and identified by Professor Z.N. Gong from the School of Life
Science in Nanjing Normal University (Nanjing, China). The voucher
specimen (no. TCM130917) was deposited in the hospital.
Chemicals
The chemicals and reagents were purchased from the
following sources: bleomycin A5 hydrochloride from Nippon Kayaku
Co., Ltd. (Tokyo, Japan); prednisone from Hubei HolleyPharm Co.,
Ltd. (Hubei, China); hydroxyproline test kits from the Nanjing
Jiancheng Bioengineering Institute (Nanjing, China); transforming
growth factor (TGF)-β1 from Strept Avidin-Biotin Complex
(SABC); immunohistochemical test kit from Roche Diagnostics
(Indianapolis, IN, USA); penicillin and streptomycin from Wuhan
Boster Biological Technology, Ltd. (Wuhan, China); DMEM from
HyClone (Logan, UT, USA); fetal bovine serum (FBS) from Hangzhou
Ever Green Organism Engineering Materials Co., Ltd. (Hangzhou,
China).
Extraction, isolation and
purification
The alkaline extract of C. reticulata was
prepared according to our previously reported procedure (23). A portion of the alkaline extract
(10 g) was added to a silica gel (125 g) column, and eluted with
CHCl3-triethylamine-MeOH (99.2:0.4:0.4, 98.6:0.4:1 and
97.6:0.4:2) successively to yield 5 fractions: fr-1 (0.4 g), fr-2
(0.6 g), fr-3 (3.9 g), fr-4 (2.5 g) and fr-5 (2.2 g). Fr-3 was
further chromatographed on a medium-pressure column with
CHCl3-triethylamine-MeOH (99.5:0.4:0.1), to obtain
amines 1 (22 mg) and 2 (40 mg). Fr-4 was applied to the same
medium-pressure column with CHCl3-triethylamine-MeOH
(99.5:0.4:0.2) to produce compounds 2 (43 mg) and 3 (45 mg).
Repeated medium-pressure column chromatography of fr-5 with
CHCl3-triethylamine-MeOH (99.5:0.4:0.2) produced amines
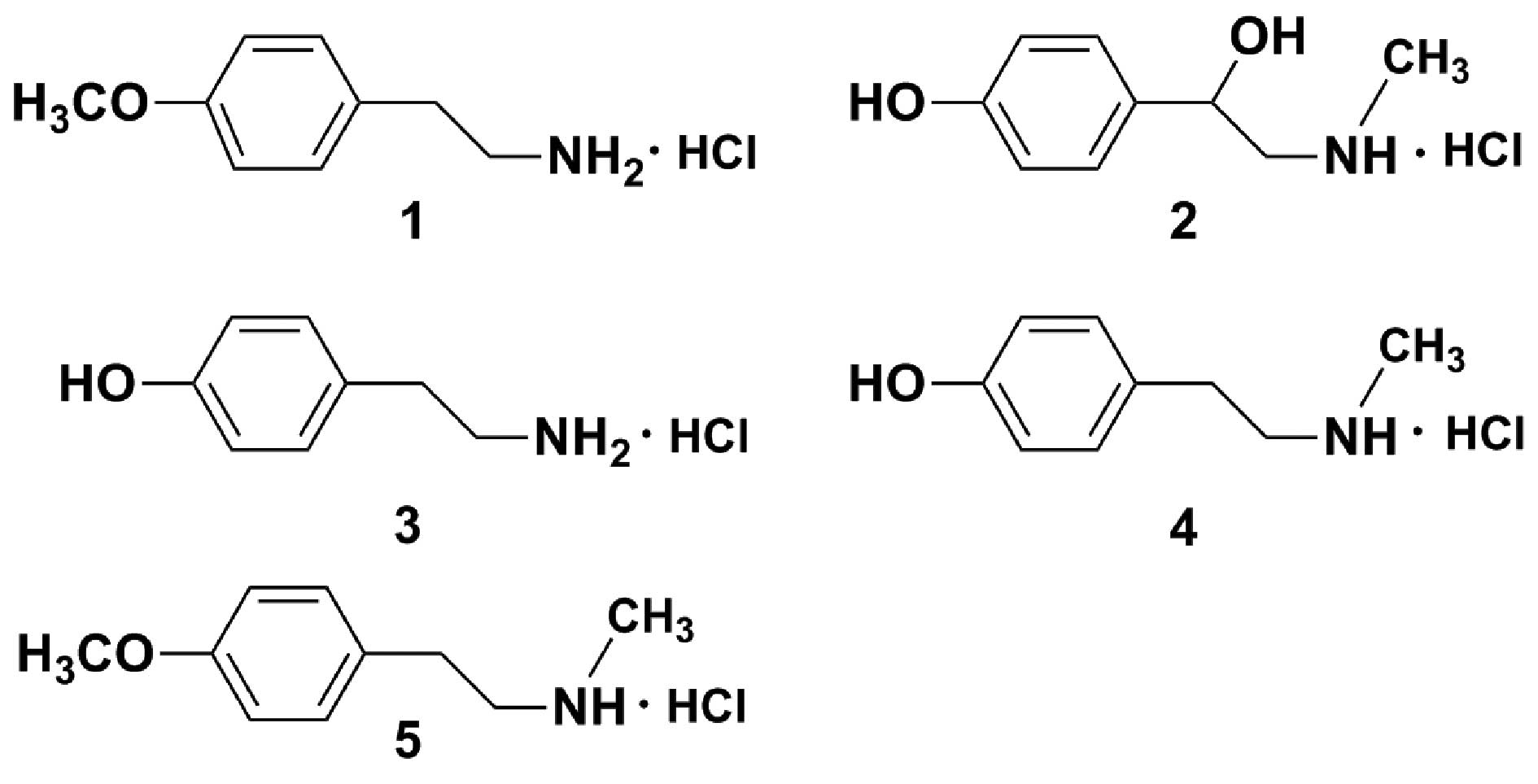
4 (18 mg) and 5 (20 mg). The structures of the isolated amines
(4-methoxy-phenethylamine, synephrine, para-tyramine,
N-methyltyramine and
N-methyl-4-methoxyphenethylamine) were determined based on
detailed comparisons of proton NMR (1H-NMR) spectra and
MS data with those of commercially available samples. For the
animal experiments, the commercially available amine (Tokyo
Chemical Industry, Tokyo, Japan) was used.
Preparation of amine hydrochlorides
To improve the solubility of the amines, each of the
above-mentioned amines (amines 1–5) was dissolved in methanol (5
mg/ml), and 10% HCl methanol solution was added (1:2, v/v).
Subsequently, the solution was completely evaporated under reduced
pressure to provide each amine hydrochloride for bioassays.
Cell culture and cell viability
assay
The hELFs used in the present study were obtained
from the Shanghai Institutes for Biological Sciences, Chinese
Academy of Sciences (Shanghai, China). The procedure for cell
culture followed our previously reported method (21). In brief, the hELFs were grown in
DMEM containing 10% FBS and 1% penicillin-streptomycin. The
logarithmically growing cells were detached with 0.25% trypsin PBS
and centrifuged (100 × g, 5 min, 4°C), and the cell concentration
was then adjusted to 5×104/ml using culture medium. Each
amine salt was dissolved in the medium and then diluted to the
desired concentrations. The amine and hELF cell solutions (each 100
μl) were added to 96-well culture plates and incubated for
48 h. Subsequently, an MTT assay was used to evaluate cell
viability.
Lactate dehydrogenase (LDH) release
assay
To evaluate the cytotoxicity of the amine
hydrochlorides, an LDH release assay was performed using an LDH
cytotoxicity detection kit from Nanjing Keygen Biotech Co., Ltd.
(Nanjing, China). hELF cell culture was conducted according to the
same protocol as described above, except that the culture period
was 24 h. Following culture, the LDH concentration was measured
following the manufacturer's instructions. The relative LDH release
was calculated as the ratio of the LDH release over the positive
control, and the positive control was treated with only 1% NP-40
and set as 100% LDH release, as previously reported (23). All cultures were kept in a
CO2 incubator under moist conditions with 5%
CO2, at 37°C.
Animals
The rats used in the present study were purchased
from the Laboratory Animal Center, Nanjing University of Chinese
Medicine (Nanjing, China). Pathogen-free mature male Sprague-Dawley
(SD) rats (weighing 180–200 g) were used in our experiments. The
rats were housed in a conventional animal facility with a 12-h
light/dark cycle, and had access to standard pelleted food and
water ad libitum. The rats were allowed to acclimatize for 7
days prior to their use in our experiments.
All of the procedures involving animals and their
care were approved by the Jiangsu Animal Care and Use Committee and
followed the national and institutional rules regarding animal
experiments.
Model of bleomycin-induced pulmonary
fibrosis
The SD rats were randomly divided into 6 groups (n=6
per group). The rat model of bleomycin-induced pulmonary fibrosis
was established following our previously reported method (23). Briefly, the rats were anesthetized
by an intraperitoneal injection of pentobarbital, followed by a
single intratracheal instillation of 5 mg/kg of bleomycin in 2.0
ml/kg PBS. The animals in the normal group received an
intratracheal injection of an equal volume of the PBS instead of
bleomycin. There were 4 groups for drug treatment: the rats were
orally administered 4-methoxyphenethylamine hydrochloride
(designated as amine hydrochloride 1) once daily at doses of 5
(treatment group 1; 1–5), 10 (treatment group 2; 1–10) and 20
(treatment group 3; 1–20) mg/kg (in distilled water) and prednisone
(treatment group 4) at a dose of 5 mg/kg once per day. The animals
in the normal (group 5) and control (group 6) groups received an
equal volume of distilled water only. The experimental period
lasted for 4 weeks. At the end of the experiment, the rats were
euthanized by pentobarbital administration, and the serum and lung
tissues were collected for bioassays. The blood was centrifuged at
2,500 rpm for 20 min, and then kept at −20°C until use. The lung
vasculature was perfused to free the blood. The right lung was
fixed in neutral buffered 10% formalin (pH 7.4) for
histopathological analysis and analysis of TGF-β1 expression. The
left lung was frozen in liquid nitrogen and stored at −80°C for
hydroxyproline level measurements.
Histopathological evaluation of lung
tissues
The right lung tissue was fixed by using 10%
neutralized buffered formalin on the trachea, and embedded in
paraffin. Serial sections (4-μm-thick) were acquired and
stained with hematoxylin and eosin (H&E) and Masson's trichrome
staining, following the manufacturer's instructions for light
microscopic evaluation. The histological grading of alveolitis and
fibrosis in the lung specimens was performed by experienced
pathologists in a blinded manner, and recorded in grades from − to
+++ and corresponding scores from 0 to 3, as previously described
(24).
Assay for hydroxyproline in serum and
lungs
The hydroxyproline content was determined as an
index of the collagen content in the serum and left lung tissues as
previously described (20).
Briefly, 0.5 ml of serum or 30–100 mg lung tissue samples were
respectively hydrolyzed in 1 ml lysis buffer solution (pH 7.4, 10
mM Tris-HCl, 0.1 mM EDTA-2Na, 10 mM saccharose, 0.8% sodium
chloride solution) at 100°C for 20 min. Hydroxyproline was then
measured using the test kit according to the manufacturer's
instructions. The absorbance of colored products was measured at
550 nm.
TGF-β1 protein expression
assay
The semi-quantification of the TGF-β1
protein levels in the right lungs of rats was assessed with an SABC
immunohistochemical test kit following the manufacturer's
instructions as previously described (20). Briefly, paraffin-embedded lung
sections were dewaxed with xylene and dehydrated with a series of
ethanol. Endogenous peroxidase was inactivated by treatment with 3%
hydrogen peroxide (H2O2) for 10 min at room
temperature. Non-specific binding was blocked with normal goat
serum for 20 min. The sections were then incubated with primary
antibody rabbit anti-TGF-β1 for 2 h at 37°C, and successively with
biotinylated goat anti-rabbit secondary antibody for 20 min at
25°C, followed by SABC for 20 min at 25°C. Immunoreactivity was
detected by the addition of diaminobenzidine (DAB). The sections
were counterstained with hematoxylin, dehydrated and mounted with
permont. As the negative controls, each primary antibody was
substituted with PBS. The protein expression of TGF-β1 was assessed
using a quantitative image analysis system.
Statistical analysis
Data are presented as the means ± standard deviation
(SD). Statistical analysis was undertaken by analysis of variance
(ANOVA) followed by appropriate post hoc tests, including multiple
comparison tests (LSD and t-test). The alveolitis and fibrosis
scores of lung tissues were evaluated using the Mann-Whitney test.
The SPSS 13.0 software package (SPSS, Inc., Chicago, IL, USA) was
used for the analysis. A p-value <0.05 was considered to
indicate a statistically significant difference.
Results
Amines and their hydrochloride
preparations
The alkaline extract of C. reticulata (10 g)
was first subjected to normal- and medium-pressure silica gel
column chromatographies repeatedly, and subsequently 5 amines,
4-methoxyphenethylamine, synephrine, para-tyramine,
N-methyltyramine and N-methyl-4-methoxyphenethylamine
were isolated. The structures of the isolated amines were then
determined, based on detailed comparisons of 1H-NMR
spectra and MS data with those of commercially available samples
(data not shown).
To improve the aqueous solubility of the isolated
amines, the amine hydrochlorides, 4-methoxyphenethylamine
hydrochloride (amine hydrochloride 1), synephrine hydrochloride
(amine hydrochloride 2), para-tyramine hydrochloride (amine
hydrochloride 3), N-methyltyramine hydrochloride (amine
hydrochloride 4) and N-methyl-4-methoxyphenethylamine
hydrochloride (amine hydrochloride 5) were prepared with 10% HCl
methanol solution (Fig. 1), and
used for all of the following bioassays.
Inhibitory effects and cytotoxicity to
hELFs
To screen the inhibitory activity of the amine
hydrochlorides, hELFs were used. All amine hydrochlorides were
dissolved in medium, and their inhibitory activity on the
proliferation of hELFs was firstly assayed at 10 μM. As
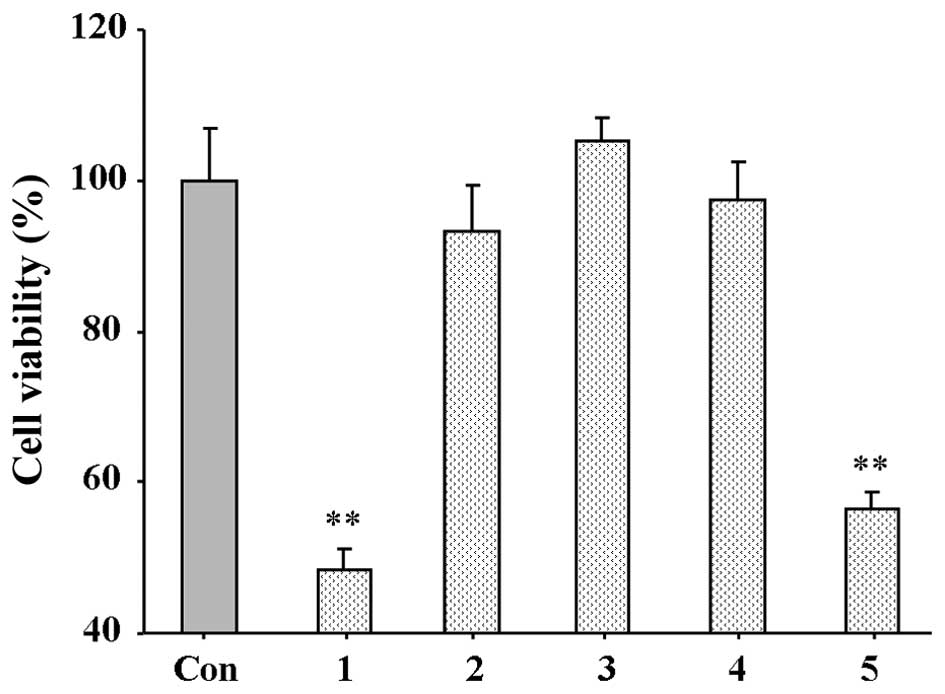
shown in Fig. 2, amine
hydrochlorides 1 and 5 significantly inhibited hELF proliferation
following 48 h of incubation, and it should be noted that amine
hydrochloride 1 caused a 51.63% inhibition of cell viability. Amine
hydrochloride 3 exerted no inhibitory effect, whereas amine
hydrochlorides 2 and 4 exerted a weak inhibitory effect. Due to the
fact that compound 1 displayed the most potent effect, further
detailed tests on its minimal half inhibitory concentration
(IC50) were performed, and the IC50 of
compound 1 was noted as 12.7 μM.
The cytotoxicity of amine hydrochlorides 1 and 5 on
hELF viability was evaluated by an LDH release assay. The results
of the assay revealed that at concentrations up to 20 μM,
amine hydrochlorides 1 and 5 did not cause cytotoxicity to the
cells (data not shown).
Effects of amine hydrochloride 1 on
bleomycin-induced weight loss in rats
As shown in Table
I, the body weight of the rats decreased significantly on the
7th day following the administration of bleomycin and decreased
gradually thereafter throughout the whole experiment period
compared to the normal rats (p<0.01). Even though the rats in
the control group gained weight slightly throughout the
experimental period, their weight was lower than the rats in the
normal group. Prednisone, a clinically available drug used to treat
IPF was used as a positive control, did not have much of a positive
impact on weight loss. However, body weight was significantly
increased following treatment with amine hydrochloride 1 at doses
of 10 and 20 mg/kg/day from the 14th to 28th day compared to the
bleomycin-treated group.
 | Table IEffects of amine hydrochloride 1 and
bleomycin on the body weight of rats. |
Table I
Effects of amine hydrochloride 1 and
bleomycin on the body weight of rats.
| Group | Day 0 | Day 7 | Day 14 | Day 21 | Day 28 |
|---|
| Normal | 202.1±14.5 | 236.2±5.1 | 246.7±20.8 | 290.2±30.9 | 306.5±40.1 |
| Control | 201.8±10.5 | 195.3±7.9a | 210.2±27.3a | 225.8±29.9a | 245.3±33.4a |
| Prednisone | 190.3±14.7 | 213.1±12.8b | 219.7±27.1 | 228.2±26.6 | 240.2±38.7 |
| 1–5 | 195.6±13.4 | 205.4±7.9 | 218.6±25.0 | 239.5±27.3 | 246.1±41.6 |
| 1–10 | 199.2±9.5 | 207.4±4.7b | 248.0±20.3c | 286.0±28.1c | 297.1±44.8c |
| 1–20 | 201.3±13.6 | 198.4±3.2 | 238.3±24.8b | 276.1±24.2c | 294.0±34.2c |
Effects of amine hydrochloride 1 on
bleomycin-induced collagen deposition and hydroxyproline expression
in rats
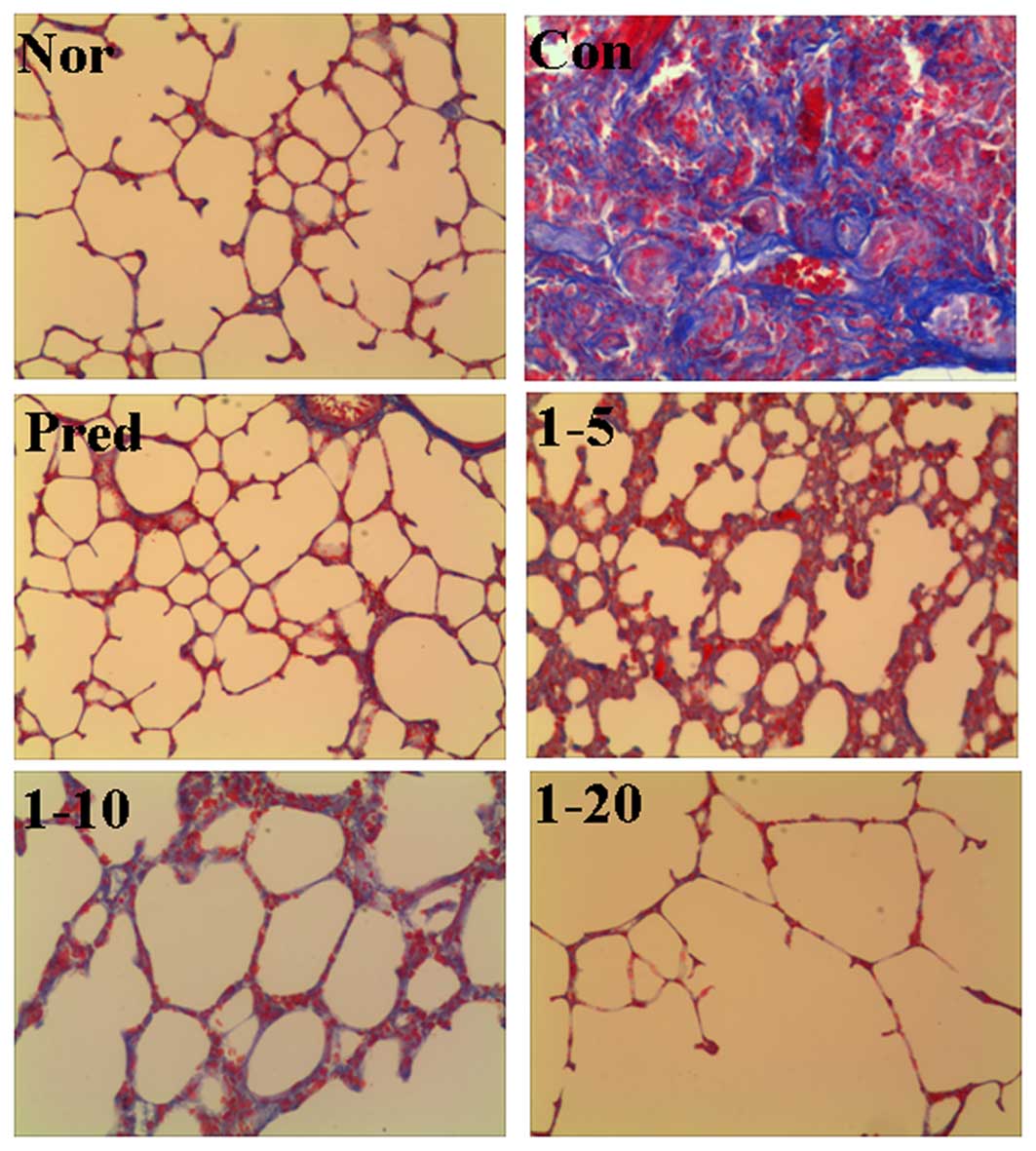
The effects of amine hydrochloride 1 on collagen
deposition in the rats with IPF were first evaluated by light
microscopy using lung tissue sections stained with Masson's
trichrome staining. As shown in Fig.
3, in the normal group, only a small amount of collagen fibers
in the alveolar septum were observed. In the lungs of the
bleomycin-treated rats, we noted an extended web of
collagen-positive stained areas in an irregular pattern (control
group). However, the lungs of the rats treated with amine
hydrochloride 1 displayed less collagen accumulation compared with
those of the rats in the control group, particularly in the rats
treated with amine hydrochloride 1 at 20 mg/kg/day, where only a
mild deposition of collagen fibers in the alveolar septum was
observed.
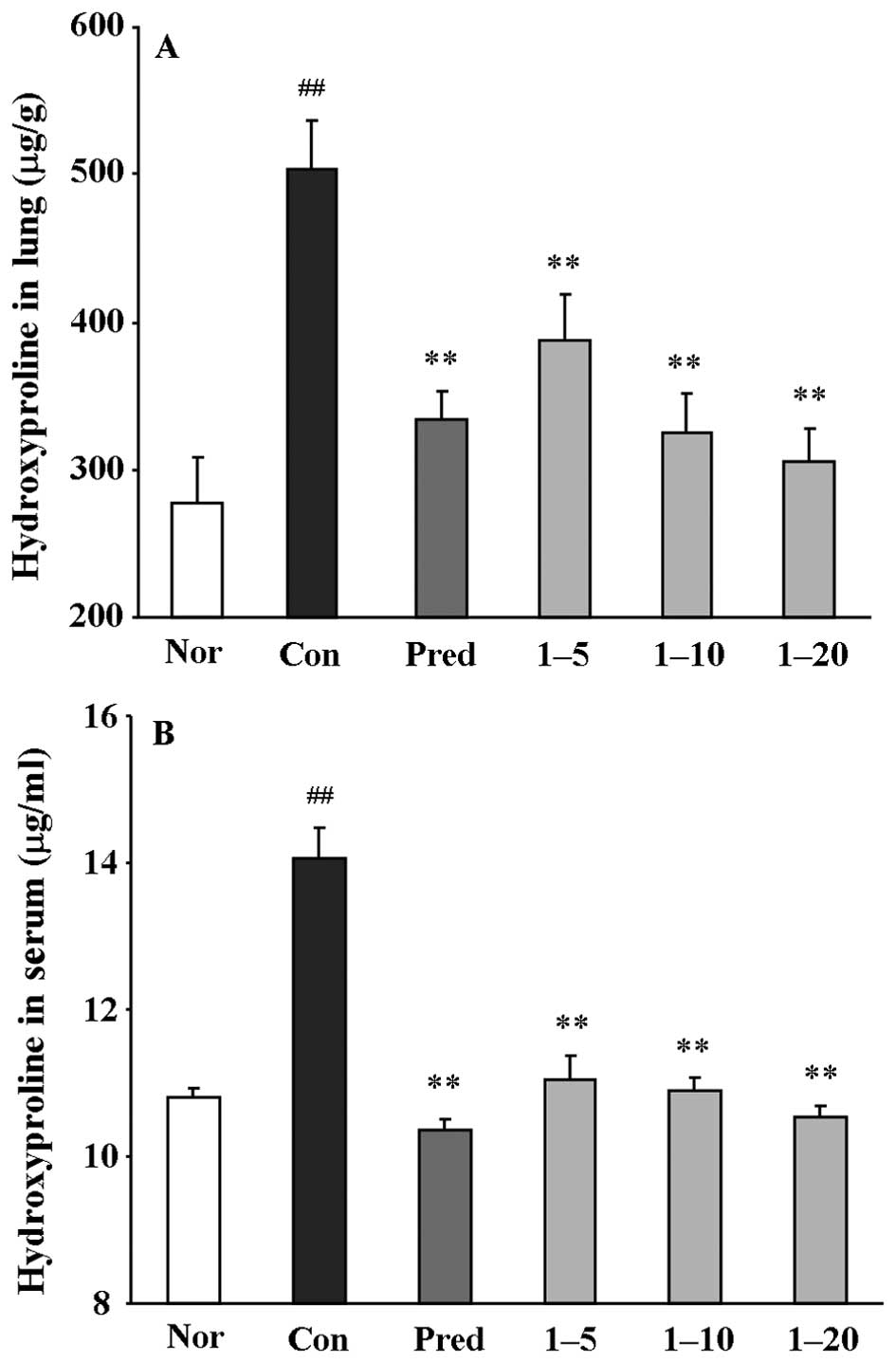
Subsequently, the hydroxyproline content in the
lungs and serum was determined. The rats treated with bleomycin
alone (the control group) showed a significant increase in
hydroxyproline content, by 1.8-fold in the lungs (μg/g,
p<0.01) and 1.36-fold in the serum (μg/ml, p<0.01),
compared with those of normal rats (Fig. 4). Treatment with amine
hydrochloride 1 at all doses significantly decreased the
hydroxyproline contents in both the lung and serum compared with
the control group. These results clearly indicated that amine
hydrochloride 1 inhibited collagen deposition.
Effect of amine hydrochloride 1 on
bleomycin-induced pathological changes
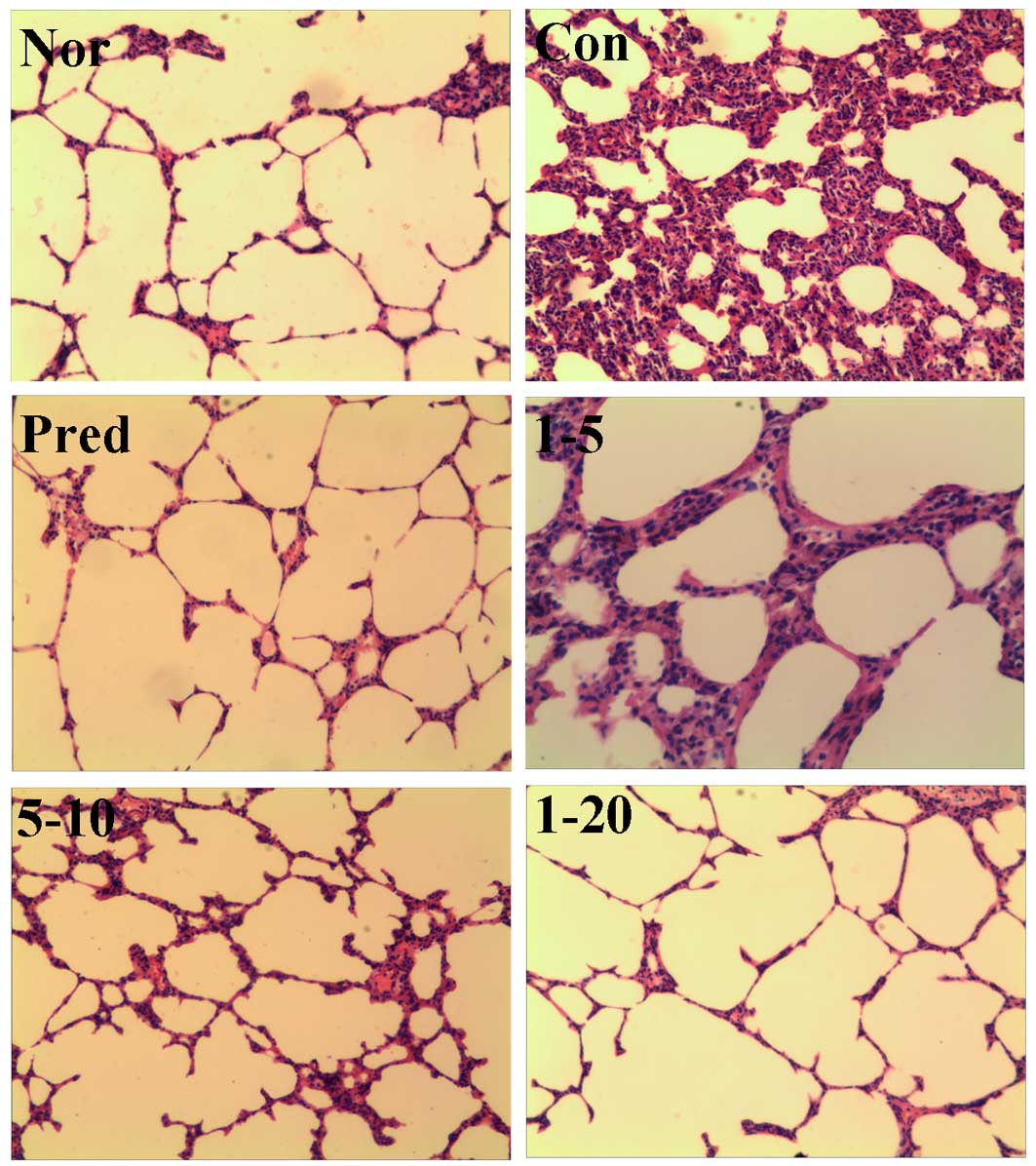
Lung fibrosis was further scored by
histopathological observations of the lung sections on the 28th
day. H&E staining of the lung tissues from the normal rats
revealed a normal histological appearance in terms of bronchi,
bronchioles and alveoli (Fig. 5,
Nor). The administration of bleomycin caused increased thickness of
the alveolar wall, alveolar and vascular congestion, and severe
infiltration of inflammatory cells in the alveolar septa and
interstitium in lung tissues were observed (Fig. 5, Con). The prednisone group
displayed only slightly thickened alveolar walls with some
inflammatory cells. Treatment with amine hydrochloride 1
significantly reduced bleomycin-induced inflammatory cell
infiltration, ameliorated the thickening of the interalveolar
septum with edema and restored the alveolar architecture (Fig. 5, 1Figure 2Figure 3Figure 4–5, 1–10
and 1–20).
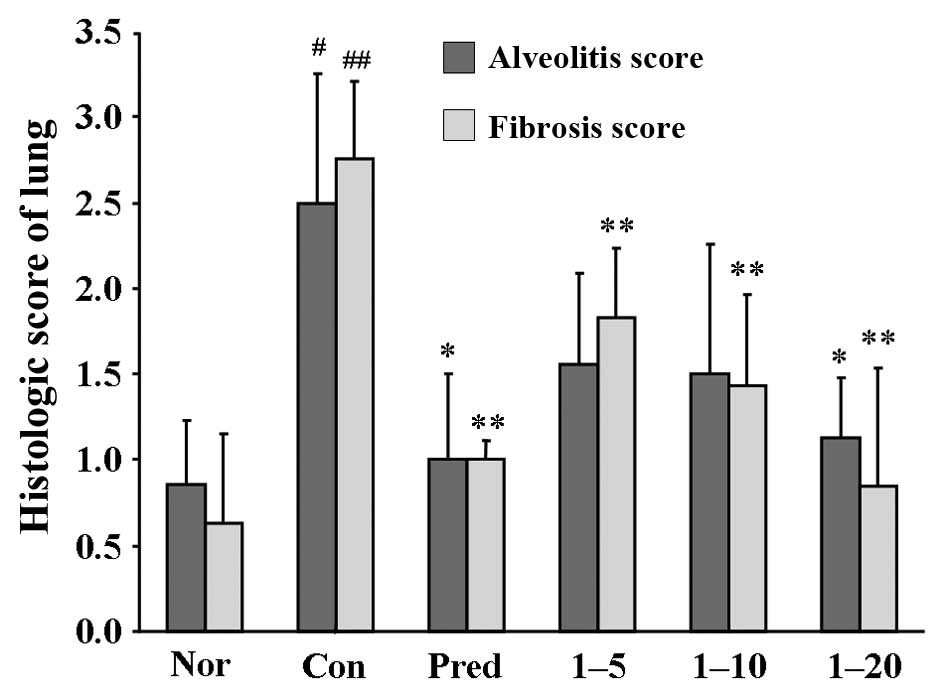
As shown in Fig.
6, the semi-quantitative assessment of the lung sections
revealed that an approximately 3-fold score increase in alveolitis,
and 4.4-fold in fibrosis, was observed in the control groups
compared to the normal group. Both the alveolitis and fibrosis
scores were significantly decreased in the prednisone-treated
groups. The lungs of the rats treated with amine hydrochloride 1
only at 20 mg/kg/day exhibited reduced pathological alveolitis
scores, and the fibrosis scores were markedly attenuated at all
doses.
Effect of amine hydrochloride 1 on
bleomycin-induced TGF-β1 protein expression
Since TGF-β1 appears to be closely
associated with fibroblast-to-myofibroblast activation and drives
pulmonary fibrosis in the current rat model (21), TGF-β1 protein
expression was assessed using immunohistochemical staining. The
results of the protein expression of TGF-β1 in the lungs
is shown in Table II. As
expected, the administration of bleomycin markedly induced
TGF-β1 protein expression, while the positive control,
prednisone, significantly suppressed its expression. Treatment of
the rats with amine hydrochloride 1 significantly repressed
TGF-β1 protein overexpression at all doses, particularly
the dose of 20 mg/kg/day, which decreased the expression to values
close to those of the normal group.
 | Table IIEffects of amine hydrochloride 1 and
bleomycin on TGF-β1 expression in lung tissues. |
Table II
Effects of amine hydrochloride 1 and
bleomycin on TGF-β1 expression in lung tissues.
| Group | Area
(μm2) | Integral
density |
|---|
| Normal | 249.09±44.92 | 19.20±10.23 |
| Control |
450.62±56.21a | 86.43±11.04a |
| Prednisone |
306.27±43.63b | 20.03±9.02b |
| 1–5 |
357.66±52.19b | 64.23±8.94b |
| 1–10 |
330.57±67.82b | 45.98±11.02b |
| 1–20 |
268.34±62.24b | 21.01±10.52b |
Discussion
As described in a previous study of ours, the
alkaline extract from the pericarp of C. reticulata exerted
inhibitory effects on pulmonary fibrosis in vitro and in
vivo (23). The amine
possesses alkalinity, and thus the chemical properties of the
alkaline extract, and elucidation of isolation and its structure,
focusing on amine components, were performed. As a result, 5 small
amines were obtained from the alkaline extract. As the amines
possessed poor solubility in aqueous solution, to improve their
solubility, their hydrochlorides were prepared.
Fibroblasts are mesenchymal cells which are derived
from embryonic mesodermal tissue, and their activation plays a
vital role in wound healing (25). One of the key characteristics of
IPF is a dysregulated wound-healing response that leads to the
fatal accumulation of fibroblasts in the lungs, and expansion of
the fibroblast population with the development of fibrotic lesions
known as fibroblast 'foci' in the lungs is believed to contribute
to the progression of fibrosis (26). The inhibition of lung fibroblast
differentiation thus has potential therapeutic benefits for IPF.
Therefore, in the present study, hELFs were used to screen the
inhibitory activity of the amine hydrochlorides. The results
indicated that amine hydrochlorides 1 and 5 exerted inhibitory
effects, whereas others exerted almost no effect. On the basis of
these data, we suggest that the methoxyl group, at the benzene ring
of the amines, is essential for this activity.
To verify that the inhibitory activity of amine
hydrochloride 1 and 5 on hELF was not due to its cytotoxicity, the
effects of the amines on cell viability were evaluated by an LDH
release assay. The data demonstrated that the inhibitory activity
was not due to their cytotoxicity.
To better understand the anti-fibrotic effects of
the tested compounds and the pathogenesis of lung fibrosis
disorders, multiple animal models have been developed, such as
silica-, radiation-induced fibrosis (27). Among currently applied models of
experimentally induced pulmonary fibrosis, the administration of
bleomycin is the most commonly used in studies on experimental lung
fibrosis (27). Furthermore, the
administration of bleomycin resulted in notable weight loss.
Fibroblast proliferation and extracellular matrix collagen
deposition constitute one of the key factors of IPF development,
and accumulation of the newly formed collagen in the lung
interstitium leads to the thickening of the alveolar septum and
lung dysfunction. Thus, the level of collagen is a hallmark of
bleomycin-induced IPF (28).
Since collagen contains a high ratio of hydroxyproline, the
estimation of the total hydroxyproline content is widely considered
a reliable index for collagen deposition (28). Therefore, both of them are
necessary to reflect collagen accumulation in IPF. The delivery of
bleomycin via the intratracheal route also results in a direct cell
injury via DNA damage (27).
Subsequently, histopathological abnormalities, such as a thickened
alveolar wall, collapsed alveolar spaces and focal honeycombing,
infiltration of inflammatory cells are observed (27,29). In the present study, amine
hydrochloride 1 significantly increased the rat body mass and
lowered the hydroxyproline content in both lung tissue and serum,
which revealed that amine hydrochloride 1 exerted an inhibitory
effect on collagen deposition. In terms of the pathological changes
to the lungs, amine hydrochloride 1 markedly improved the scores
for alveolitis and fibrosis, and inhibited lung inflammation. These
results clearly demonstrate that amine hydrochloride 1 suppresses
both the acute inflammatory response and fibrogenic changes that
are key factors of IPF.
Although multiple signaling pathways are currently
known to be involved in the development and progression of IPF, the
TGF-β family comprises multifunctional cytokines which regulate
cell growth, apoptosis, inflammation and extracellular matrix (ECM)
synthesis (30). The TGF-β
signaling pathway plays important roles in wound healing and organ
fibrosis, depending on the cellular context (30). Moreover, TGF-β1 appears
to be the most prevalent isoform, which is considered as a key
pro-fibrotic agent since it stimulates extracellular matrix
production, fibroblast-to-myofibroblast differentiation and
inhibition of autophagy in fibroblasts (31,32). In the present study, on the 28th
day following the administration of bleomycin, the protein level of
TGF-β1 was clearly increased, while treatment with amine
hydrochloride 1 inhibited the overexpression of TGF-β1
protein. These results suggest that the anti-pulmonary fibrotic
effect of amine hydrochloride 1 should be ascribed, at least
partially, to the downregulation of the protein expression of
TGF-β1.
In conclusion, the findings of the present study
demonstrate that amine hydrochloride 1 is a highly promising agent
which can be used for the prevention of lung inflammation and
fibrosis, and the anti-fibrotic effects seem to be mediated, at
least partially, through the inhibition of TGF-β1
protein expression. The present results also revealed that the
amines from the pericarp of Citrus fruits have therapeutic
potential for the prevention of IPF. Further studies are required
to clarify the detailed mechanisms of action of Citrus fruits, and
are currently being undertaken in our laboratory.
Acknowledgments
The present study was supported by the Key
Discipline of Jiangsu Province Administration of Traditional
Chinese Medicine (JS1302).
Abbreviations:
|
IPF
|
idiopathic pulmonary fibrosis
|
|
hELFs
|
human embryonic lung fibroblasts
|
|
TGF-β1
|
transforming growth factor-β1
|
|
1H-NMR
|
proton nuclear magnetic resonance
|
|
MS
|
mass spectrum
|
|
LDH
|
lactate dehydrogenase
|
|
H&E
|
hematoxylin and eosin
|
|
ECM
|
extracellular matrix
|
References
|
1
|
Raghu G, Collard HR, Egan JJ, Martinez FJ,
Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et
al ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis: An
official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis:
evidence-based guidelines for diagnosis and management. Am J Respir
Crit Care Med. 183:788–824. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nalysnyk L, Cid-Ruzafa J, Rotella P and
Esser D: Incidence and prevalence of idiopathic pulmonary fibrosis:
review of the literature. Eur Respir Rev. 21:355–361. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hilberg O, Simonsen U, du Bois R and
Bendstrup E: Pirfenidone: significant treatment effects in
idiopathic pulmonary fibrosis. Clin Respir J. 6:131–143. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
O'Connell OJ, Kennedy MP and Henry MT:
Idiopathic pulmonary fibrosis: treatment update. Adv Ther.
28:986–999. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Armanios M: Telomerase and idiopathic
pulmonary fibrosis. Mutat Res. 730:52–58. 2012. View Article : Google Scholar :
|
|
6
|
Rafii R, Juarez MM, Albertson TE and Chan
AL: A review of current and novel therapies for idiopathic
pulmonary fibrosis. J Thorac Dis. 5:48–73. 2013.PubMed/NCBI
|
|
7
|
Spagnolo P, Maher TM and Richeldi L:
Idiopathic pulmonary fibrosis: recent advances on pharmacological
therapy. Pharmacol Ther. 152:18–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Richeldi L, du Bois RM, Raghu G, Azuma A,
Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y,
et al INPULSIS Trial Investigators: efficacy and safety of
nintedanib in idiopathic pulmonary fibrosis. N Engl J Med.
370:2071–2082. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
King TE Jr, Bradford WZ, Castro-Bernardini
S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM,
Kardatzke D, Lancaster L, et al ASCEND Study Group: A phase 3 trial
of pirfenidone in patients with idiopathic pulmonary fibrosis. N
Engl J Med. 370:2083–2092. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cottin V: Current approaches to the
diagnosis and treatment of idiopathic pulmonary fibrosis in Europe:
the AIR survey. Eur Respir Rev. 23:225–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Velasco R and Licciardello C: A genealogy
of the citrus family. Nat Biotechnol. 32:640–642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Medina S, Ferreres F, García-Viguera C,
Horcajada MN, Orduna J, Savirón M, Zurek G, Martínez-Sanz JM, Gil
JI and Gil-Izquierdo A: Non-targeted metabolomic approach reveals
urinary metabolites linked to steroid biosynthesis pathway after
ingestion of citrus juice. Food Chem. 136:938–946. 2013. View Article : Google Scholar
|
|
13
|
Nile SH and Park SW: Bioactive components
and health-promoting properties of Yuzu (Citrus ichangensis x C.
reticulate). Food Rev Int. 30:155–167. 2014. View Article : Google Scholar
|
|
14
|
Yadav L and Kalidhar SB: Chemical
components of Mandarin species: a review. J Indian Chem Soc.
88:911–926. 2011.
|
|
15
|
Khan MK, Huma ZE and Dangles O: A
comprehensive review on flavanones, the major citrus polyphenols. J
Food Compos Anal. 33:85–104. 2014. View Article : Google Scholar
|
|
16
|
Vieira SM, Theodoro KH and Glória MBA:
Profile and levels of bioactive amines in orange juice and orange
soft drink. Food Chem. 100:895–903. 2007. View Article : Google Scholar
|
|
17
|
Stohs SJ, Preuss HG and Shara M: The
safety of Citrus aurantium (bitter orange) and its primary
protoalkaloid p-synephrine. Phytother Res. 25:1421–1428. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaats GR, Miller H, Preuss HG and Stohs
SJ: A 60day double-blind, placebo-controlled safety study involving
Citrus aurantium (bitter orange) extract. Food Chem Toxicol.
55:358–362. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stohs SJ and Preuss HG: Stereochemical and
pharmacological differences between naturally occurring
p-synephrine and synthetic p-synephrine. J Funct Foods. 4:2–5.
2012. View Article : Google Scholar
|
|
20
|
Zhou XM, Zhang GC, Li JX and Hou J:
Inhibitory effects of Hu-qi-yin on the bleomycin-induced pulmonary
fibrosis in rats. J Ethnopharmacol. 111:255–264. 2007. View Article : Google Scholar
|
|
21
|
Zhou XM, Huang MM, He CC and Li JX:
Inhibitory effects of citrus extracts on the experimental pulmonary
fibrosis. J Ethnopharmacol. 126:143–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
China Pharmacopoeia Committee: Citri
Reticulatae Pericarpium. Chinese Pharmacopeia 2010. Chemical
Industry; Press, Beijing: pp. 176–177. 2010
|
|
23
|
Zhou XM, Wen GY, Zhao Y, Liu YM and Li JX:
Inhibitory effects of alkaline extract of Citrus reticulata on
pulmonary fibrosis. J Ethnopharmacol. 146:372–378. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Szapiel SV, Elson NA, Fulmer JD,
Hunninghake GW and Crystal RG: Bleomycin-induced interstitial
pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis.
120:893–899. 1979.PubMed/NCBI
|
|
25
|
Camelo A, Dunmore R, Sleeman MA and Clarke
DL: The epithelium in idiopathic pulmonary fibrosis: breaking the
barrier. Front Pharmacol. 4:1732014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kendall RT and Feghali-Bostwick CA:
Fibroblasts in fibrosis: novel roles and mediators. Front
Pharmacol. 5:1232014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
B Moore B, Lawson WE, Oury TD, Sisson TH,
Raghavendran K and Hogaboam CM: Animal models of fibrotic lung
disease. Am J Respir Cell Mol Biol. 49:167–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kliment CR, Englert JM, Crum LP and Oury
TD: A novel method for accurate collagen and biochemical assessment
of pulmonary tissue utilizing one animal. Int J Clin Exp Pathol.
4:349–355. 2011.PubMed/NCBI
|
|
29
|
Huaux F, Noel S, Dhooghe B, Panin N, Lo Re
S, Lison D, Wallemacq P, Marbaix E, Scholte BJ, Lebecque P and Leal
T: Dysregulated proinflammatory and fibrogenic phenotype of
fibroblasts in cystic fibrosis. PLoS One. 8:e643412013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yan Z, Kui Z and Ping Z: Reviews and
prospectives of signaling pathway analysis in idiopathic pulmonary
fibrosis. Autoimmun Rev. 13:1020–1025. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Klingberg F, Hinz B and White ES: The
myofibroblast matrix: implications for tissue repair and fibrosis.
J Pathol. 229:298–309. 2013. View Article : Google Scholar
|
|
32
|
Della Latta V, Cecchettini A, Del Ry S and
Morales MA: Bleomycin in the setting of lung fibrosis induction:
from biological mechanisms to counteractions. Pharmacol Res.
97:122–130. 2015. View Article : Google Scholar : PubMed/NCBI
|