Introduction
Endometrial cancer (EC) is the most frequently
diagnosed malignancy of the female reproductive tract and the
fourth most common malignancy in women in Poland, after breast and
lung cancer. In 2012 the incidence of endometrial cancer in Polish
women was estimated at 5,426 of new cases and 1,162 of deaths
(1).
In 1983, Bokhman et al (2) proposed a dualistic model of
endometrial carcinogenesis based on clinical and prognostic
factors. Type I, estrogen-dependent endometrioid carcinomas (EEC)
comprises the sporadic cancers with a low grade and favorable
prognosis (3,4). The precursor of this group of
malignancies is atypical hyperplasia of the endometrium and
progressive mutation/methylation in mismatch repair genes [MutL
homolog 1 (MLH1), MutS homolog 2 (MSH2) and MutS
homolog 6 (MSH6)], and the oncogenes Kirsten rat sarcoma
viral oncogene homolog (KRAS) and catenin beta-1
(CTNNB1). Inactivation of phosphatase and tensin homolog
(PTEN) and loss of expression of the cadherin 1
(CDH1) gene are also typical for this type of neoplastic
lesion (5,6).
Type II, non-endometrioid endometrial carcinomas
(NEECs), are on the contrary not associated with oestrogen
stimulation. These high-grade tumors progress from the atrophic
endometrium and are typified by serous papillary or clear-cell
morphology, an aggressive clinical course and poor prognosis,
resulting from their high potential for deep myometrial invasion
and lymphatic spread (3,7). NEECs are characterized by mutations
of the tumor protein P53 (TP53) tumor suppressor gene,
leading to accumulation of non-functional p53 protein and thus
deregulation of cell cycle control (4,7).
As for oncogenic alterations, intensification of oncogenic signals
and subsequent excessive cell growth and differentiation due to
overexpression of human epidermal growth factor receptor 2
(HER2)/neu has been reported in type II EC (4). Moreover, NEECs have been found to
present significant genomic instability at a chromosomal level,
which is caused by telomere shortening and results in a high level
of aneuploidy, regardless of the active response of the MMR system
(6,7).
The WWOX tumor suppressor gene, encoding the
WW domain containing oxidoreductase, is also known as FOR, fragile
site FRA16D oxidoreductase. The WWOX gene is localized in
region 16q23.3-24.1, also referred to as common fragile site
FRA16D. The WWOX protein contains two N-terminal WW-domains, and
one central SDR domain. The first WW domain (WW1) of the WWOX
protein possesses the ability to associate with proteins containing
a specific proline-rich motif, PPxY. Since WWOX has been found to
generate such interactions with molecules involved in
transcriptional regulation or signal transduction e.g. via SMAD3
(8), RUNX2 (9), c-Jun (10), p73 (11), AP-2 α/γ (12), ERBB4 (13,14), and HIF1α (15), it is thus thought to participate
in the process of carcinogenesis (16). Alterations in the WWOX
tumor suppressor gene have been observed, in cases of cancer, of
many hormone-regulated tissues, including those of the breast,
ovary, prostate and testis (17–20). It has been shown that loss of
WWOX expression is correlated with unfavorable factors, such
as grade, stage, lymph node metastasis (6,19,21) and a lower degree of cancer cell
differentiation (20).
Additionally, our previous analysis conducted on normal and EC
samples revealed a decrease in WWOX protein level in tissues with
acquired cancer phenotype. We have also observed a tendency of
WWOX gene mRNA to decrease between grade 1 and 2, FIGO stage
1 and 2, and thus it is correlated with deeper myometrial invasion
(22). Much data from previous
studies demonstrate WWOX protein participation and regulation of
various processes involved in tumor development and progression
(23–27). One of these processes is
epithelial-mesenchymal transition (EMT), Yan and Sun indicated that
the WWOX gene may reverse the EMT in ovarian cancer stem
cells by regulating the expression of two EMT factors, Elf5 and
Snail (28). In our previous
study we also observed the influence which WWOX exerted on
the EMT process via modulation of cell motility and suppression of
the main mezenchymal marker, i.e., vimentin (VIM) (29).
The aim of the present study was to analyze the
impact of differential WWOX expression on biological
cancer-related processes in endometrial cell lines, both
non-cancerous and cancerous, which varied also in cellular
differentiation status. Accordingly, we silenced WWOX
expression in the human normal endometrial stromal cell line THESC
and two EC cell lines with different statuses of differentiation:
Ishikawa (grade 1; well-differentiated, expressing both estrogen
and progesterone receptors), and MFE296 (grade 2; moderately
differentiated, lacking the expression of estrogen receptors but
susceptible to androgen-induced inhibition of proliferation).
Materials and methods
Cell culture
MFE296, THESC and Ishikawa cell lines were obtained
from the Leibniz Institut DSMZ, German Collection of Microorganisms
and Cell Cultures GmbH (Braunschweig, Germany), American Type
Culture Collection (ATCC, Manassas, VA, USA) and Sigma-Aldrich
(Poznań, Poland), respectively. The MFE296 cells were cultured in
minimum essential medium supplemented with 10% fetal bovine serum
(FBS), 2 mM L-glutamine, 2 mM sodium pyruvate and 1% PSN antibiotic
mixture (penicillin 50 µg/ml, streptomycin 50 µg/ml,
neomycin 100 µg/ml; Life Technologies, Carlsbad, CA, USA).
The Ishikawa cells were cultured in minimum essential medium
containing 1% non-essential amino acids and supplemented with 5%
FBS, 2 mM L-glutamine, 2 mM sodium pyruvate and 1% PSN antibiotic
mixture. The THESC cell line was cultured in DMEMF-12 medium
without phenol red with 3.1 g/l glucose and 1 mM sodium pyruvate
supplemented with 1.5 g/l sodium bicarbonate, 1% ITS + Premix (cat.
no. 354352; BD Biosciences, Franklin Lakes, NJ, USA), 500 ng/ml
puromycin, 10% and charcoal/dextran treated FBS (cat. no.
SH30068.03; HyClone, Logan, UT, USA).
shRNA-mediated silencing of the WWOX
gene
GIPZ Lentiviral™ shRNA technology (Thermo Fisher
Scientific, Waltham, MA, USA) was used to modulate WWOX
expression in all cell lines. A mix of commercially available
lentiviral particles (V2LHS_115633, V2LHS_255229 and V2LHS_411864;
Thermo Fisher Scientific), comprising shRNA complementary to the
sequence of WWOX mRNA, was introduced into serum-starved cells
according to the manufacturers' instructions (MOI of 5). Polybrene
(5 µg/ml; Sigma-Aldrich) was added to the transduction
medium to increase efficiency of the process. Cells transduced with
non-silencing lentiviral shRNA vector (Thermo Fisher Scientific),
containing an shRNA sequence with no homology to known mammalian
genes, served as a negative control. Selection of the transduced
cells was based on resistance to puromycin (1 µg/ml; Sigma
Aldrich). Additionally, the lentiviral vector which was used
contained GFP protein, and observation, using a fluorescence
microscope (Life Technologies), of TurboGFP expression was used as
a control method for transduction.
Protein extraction and western blot
analysis
Protein samples were obtained using RIPA protein
extraction buffer supplemented with protease, phosphatase inhibitor
coctail and PMSF (Sigma-Aldrich). Protein concentration was
measured using the Bradford method (Bio-Rad Laboratories, Warsaw,
Poland), and samples were subsequently separated in 10% SDS-PAGE
gel electrophoresis and transferred to PVDF membranes
(Sigma-Aldrich). The membranes were blocked in 5% non-fat milk in
TBST for 1 h at room temperature and then incubated with a primary
antibody to goat anti-human WWOX, 1:100 (sc:20529; Santa Cruz
Biotechnology Inc., Santa Cruz, CA, USA) for 19 h at 4°C.
Subsequently, the membranes were washed three times with TBST
buffer and incubated with secondary antibodies conjugated with
alkaline phosphatase (Sigma-Aldrich) for 1 h. Membranes washed with
TBST were then developed using Novex® AP Chromogenic
Substrate (Invitrogen, Carlsbad, CA, USA).
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) at a dilution of
1:1,000 (sc-59540; Santa Cruz Biotechnology, Inc.) served as the
internal control.
Cell-culture assays
Due to the limited number of divisions, the normal
endometrial cell line THESC underwent only an adhesion assay after
silencing. All biological tests were performed in a minimum of
three replicates.
Adhesion assay
Adhesiveness of both variants (with and without
WWOX silencing) of MFE-296, Ishikawa and THESC cells was
evaluated by means of a plate coated with fibronectin, collagen
type I and IV, laminin type I and fibrinogen (CytoSelect™ 48-well
cell adhesion assay kit; Cell Biolabs, Inc., San Diego, CA, USA)
according to the manufacturer's instructions. BSA-coated wells
served as the negative controls. Cells were seeded on the plate
(1.5×105/well) and incubated for 90 min at 37°C in 5%
CO2 to enable interaction with the extracellular matrix
components. Cellular adhesion was quantified spectrophotometrically
at 560 nm (EL808; BioTek, Bedfordshire, UK).
Integrin expression test
The α/β integrin-mediated cell adhesion array combo
kit (Chemicon, Temecula, CA, USA) was used to analyze the adhesion
of MFE-296 and Ishikawa cells to anti-integrin antibodies,
according to the manufacturer's instructions. Both variants of cell
lines were seeded on a plate (1.5×105 cells/well) and
incubated for 2 h at 37°C in an atmosphere with 5% CO2.
Absorbance was measured at 560 nm with a BioTek plate reader.
Invasion assay
The CytoSelect™ Cell Invasion Assay kit (Cell
Biolabs, San Diego, CA, USA) was used to assess the invasiveness of
MFE-296 and Ishikawa cell lines according to the manufacturer's
instructions. Complete culture medium was added to wells, and the
cells suspended in starving medium were seeded
(3×105/well) onto the inner compartment of each insert.
The plate was incubated for 48 h at 37°C in 5% CO2. The
cells attached to the bottom of the membrane were stained with
0.005% crystal violet, and the number of cells was assessed
spectrophotometrically at 560 nm (EL808; BioTek). The test was
performed in triplicate for each cell variant.
Soft agar assay
Anchorage-independent proliferation potential of
both variants of MFE-296/Ishikawa cells was estimated using a soft
agar colony formation assay. A 2-ml layer of 0.9% agar in complete
culture medium was poured into a 6-well plate. Subsequently, the
cells of each variant were suspended in 0.3% agar medium and
inoculated (1×104/well) on the top of the base layer.
The plate was then incubated (for 14 days, at 37°C, in an
atmosphere with 5% CO2), allowing for the observation of
cell growth and colony formation in the semisolid medium. After
incubation, the colonies were stained with 0.005% crystal violet
(15 min, RT) and counted using ImageJ software (National Institutes
of Health, Bethesda, MD, USA; http://rsb.info.nih.gov/ij/). The test was performed
in triplicate for each cell variant.
Clonogenic assay
The MFE296 and Ishikawa cells of each variant were
seeded (5×102/well) onto a 6-well plate in complete
culture medium. After incubation (for 10 days, at 37°C, in an
atmosphere with 5% CO2), the cells were fixed with 4%
paraformaldehyde solution in phosphate-buffered saline (PBS).
Colonies were stained with 0.005% crystal violet (15 min, at room
temperature) and counted using ImageJ software. The test was
performed in triplicate for each cell variant.
Zymography
Cells were seeded on 6-well plates
(1.5×106/well), cultured to 80% confluence and
serum-starved 24 h prior to medium collection. Protein
concentration in the obtained cell culture supernatants was
measured using a Qubit protein assay and Qubit 2.0 fluorometer
(both from Invitrogen Life Technologies). Protein extracts (2
µg) were separated in 10% SDS-PAGE gel, supplemented with
gelatin (2 mg/ml). After electrophoresis, the gel was washed with
Triton X-100 (2×30 min) and incubated in a developing buffer (0.5 M
Tris-HCl, 2 M NaCl, 50 mM CaCl2, pH 7.5) overnight at
37°C. Subsequently, the gel was incubated alternately with
Coomassie Brilliant Blue R-250 staining and destaining solution
(methanol:acetic acid:water, 3:1:6) until clear bands were observed
over the dark background. Gelatinolytic activity of enzymes in
samples appeared in the form of clear bands and was further
evaluated by means of ImageJ software based on the band area.
RT-qPCR
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA). cDNA synthesis was
performed as previously described (22). Gene expression was analyzed using
LightCycler 480 (Roche Diagnostics, Warsaw, Poland), with
SYBR®-Green I and a qPCR Core kit for
SYBR®-Green I (Eurogentec, Southampton, UK). Expression
levels of studied genes were normalized to the mean expression of
reference genes ribosomal protein S17 (RPS17), ribosomal
protein, large, P0 (RPLP0) and H3 histone, family 3A (H3F3A)].
Primer sequences, PCR reaction conditions and length of the
obtained products are listed in Table
I. Each reaction was performed in duplicate and Universal Human
Reference RNA (Stratagene, Perlan Technology, Warszaw, Poland) was
used as the calibrator. Gene expression levels were calculated
according to Roche algorithm (30).
 | Table IRT-qPCR primer sequences. |
Table I
RT-qPCR primer sequences.
| Gene | Primer
sequences | Product (bp) | Annealing (°C) | Reading (°C) |
|---|
| Reference
genes |
| RPS17 |
5′-AAGCGCGTGTGCGAGGAGATCG-3′ | | | |
|
5′-TCGCTTCATCAGATGCGTGACATAACCTG-3′ | 87 | 64 | 72 |
| H3F3A |
5′-AGGACTTTAAAAGATCTGCGCTTCCAGAG-3′ | | | |
|
5′-ACCAGATAGGCCTCACTTGCCTCCTGC-3′ | 76 | 65 | 72 |
| RPLP0 |
5′-ACGGATTACACCTTCCCACTTGCTAAAAGGTC-3′ | | | |
|
5′-AGCCACAAAGGCAGATGGATCAGCCAAG-3′ | 69 | 65 | 72 |
| Analyzed genes |
| CDH1 |
5′-TCCCCCGGTATCTTCCCCGCCCTG-3′ | | | |
|
5′-AGTTCAGGGAGCTCAGACTAGCAGCTTCGG-3′ | 168 | 63 | 82 |
| CTNNB1 |
5′-AAAATGGCAGTGCGTTTAG-3′ | | | |
|
5′-TTTGAAGGCAGTCTGTCGTA-3′ | 100 | 58 | 72 |
| ZEB1 |
5′-GGAAATCAGGATGAAAGACA-3′ | | | |
|
5′-CACACAAATCACAAGCATAC-3′ | 136 | 63 | 72 |
| VIM |
5′-AGCCGAAAACACCCTGCAAT-3′ | | | |
|
5′-CGTTCAAGGTCAAGACGTC-3′ | 72 | 58 | 72 |
| EZR |
5′-CTCACCGTATGGCTGCACTG-3′ | | | |
|
5′-CTTCATCCTCCTTGCGCCTC-3′ | 153 | 55 | 72 |
| PTEN |
5′-CGAACTGGTGTAATGATATGT-3′ | | | |
|
5′-CATGAACTTGTCTTCCCGT-3′ | 330 | 55 | 72 |
| SPARC |
5′-TGGACTACATCGGGCCTTGCAAATACATC-3′ | | | |
|
5′-TTCTTGAGCCAGTCCCGCATGCAG-3′ | 91 | 65 | 72 |
Statistical analysis
Statistical analysis of the data was performed using
Statistica 8.0 (StatSoft). The Aspin-Welsch test was applied to
determine the differences between data obtained for both cell
variants in cell-culture assays. A p-value <0.05 was considered
to indicate a statistically significant difference.
Results
Successful transduction was determined by positive
rates of TurboGFP fluorescence observed in both variants (those
which received WWOX silencing and those which did not) of
the cell lines.
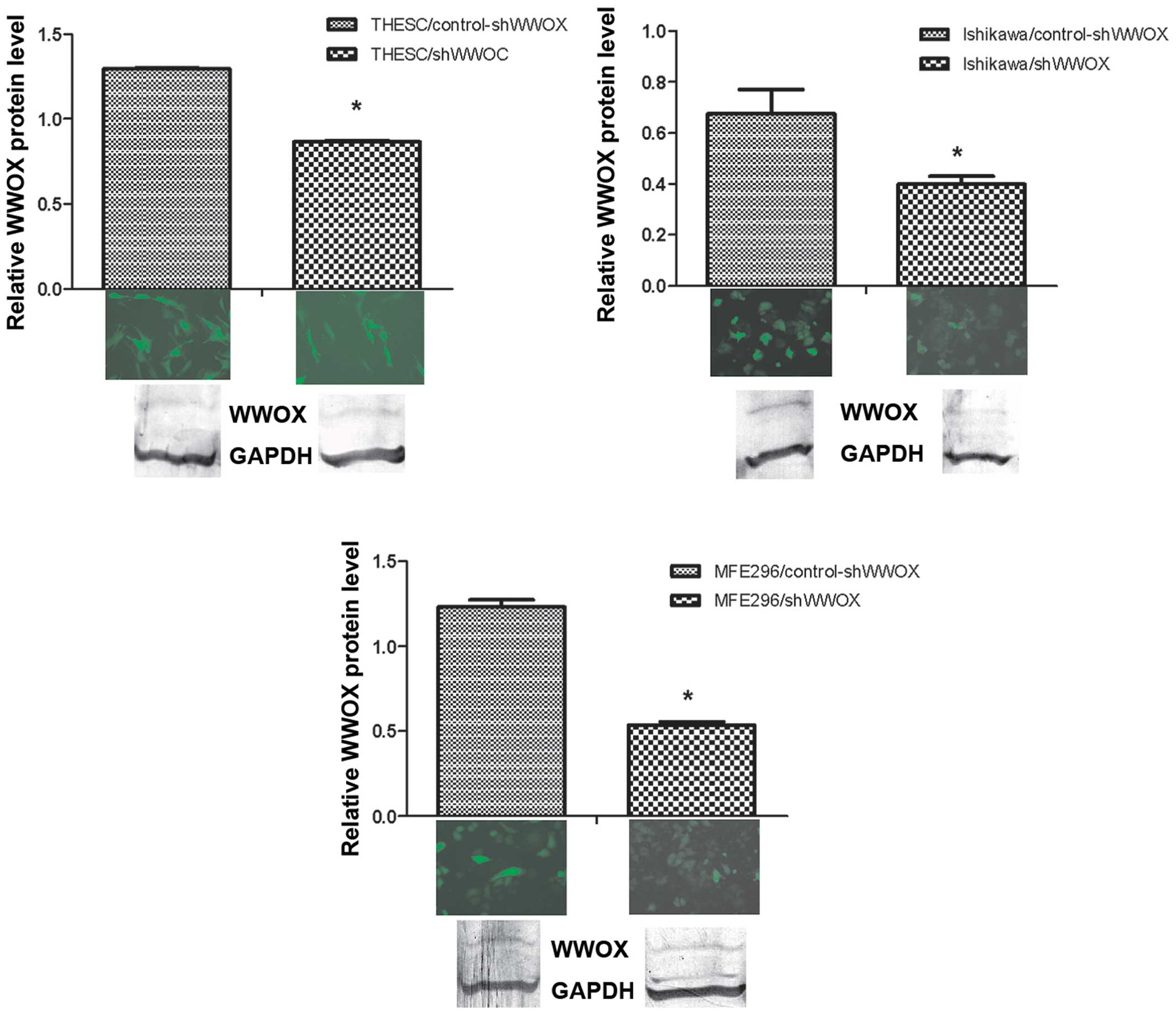
Silencing of the WWOX gene was confirmed also
at protein level, by western blot analysis. The WWOX protein was
suppressed by 66.9% in THESC (p<0.001), 59.2% in Ishikawa
(p=0.029) and 56% in MFE296 (p=0.014) cell lines (Fig. 1).
As a result of WWOX gene silencing, we
observed changes in the behaviour of the cell lines, but the
observed changes did not always follow the same direction.
The invasion assay, which was performed with basal
membrane-coated inserts, demonstrated that the silencing of
WWOX gene expression in MFE296 cells caused a moderate (by
27.6%) decrease of invasive ability (p=0.036) in comparison to the
control cells. By contrast, in the Ishikawa (ISH) cell line, this
resulted in insignificant modulation of their invasive potential
(Fig. 2).
Moreover, the amount of active matrix
metallopro-teinase-2 (MMP-2) in MFE296/shWWOX was dem onstrated to
be significantly reduced (arbitrary units of enzyme: 15185±154.7
vs. 9790±420.2; downregulation approximately 36%, p=0.002) when
compared to control cells.
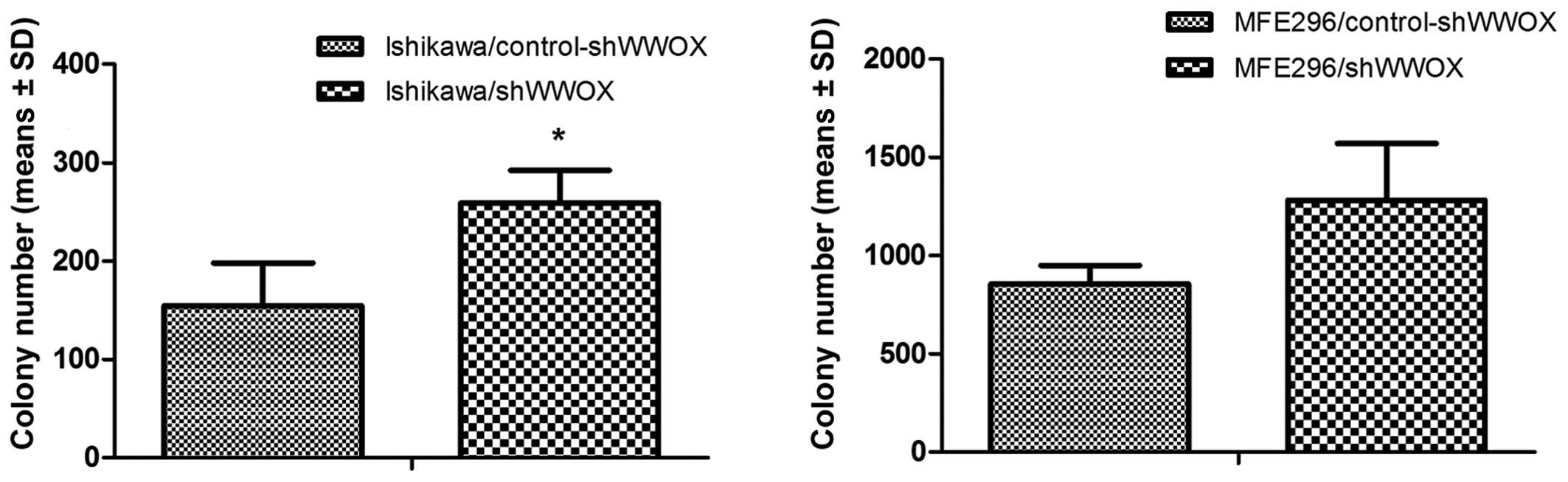
We also tested anchorage-independent growth.
Therefore, we used soft agar and colony formation assays, which
were conducted in order to assess whether WWOX silencing
affected the clonogenicity of EC cells as well as their ability to
grow in suspension, which is the characteristic feature of
malignant cancer cells. We noted that ectopic WWOX silencing
resulted in an increased ability to form colonies for the variants
with silenced WWOX expression in both cancerous cell lines
examined. It is worth noting that MFE296 cells presented higher
clonogenicity than the well-differentiated ISH cell line
(MFE296/control-shRNA variant formed 5.5-times more colonies than
the Ishikawa/control-shRNA variant). However, the increased ability
to form colonies in suspension was more evident in Ishikawa cells
than in MFE296 cells (by 68%, p=0.03 and by 50.5%, p=0.11,
respectively) (Fig. 3). On the
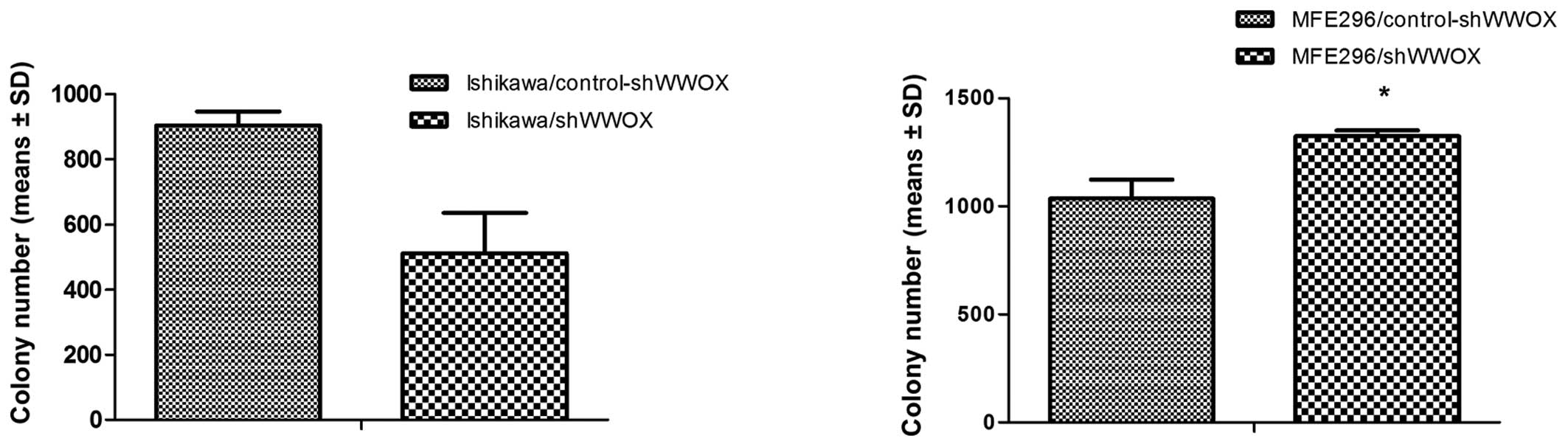
other hand, the soft agar assay demonstrated different effects on
anchorage-independent proliferation after WWOX silencing in
the two EC cell lines. A substantial, yet not statistically
significant, decrease of growth in semisolid medium was observed in
Ishikawa cells (43.4%, p=0.09), while a significant 27.7% increase
was observed in the MFE296/shWWOX variant (p=0.019) (Fig. 4).
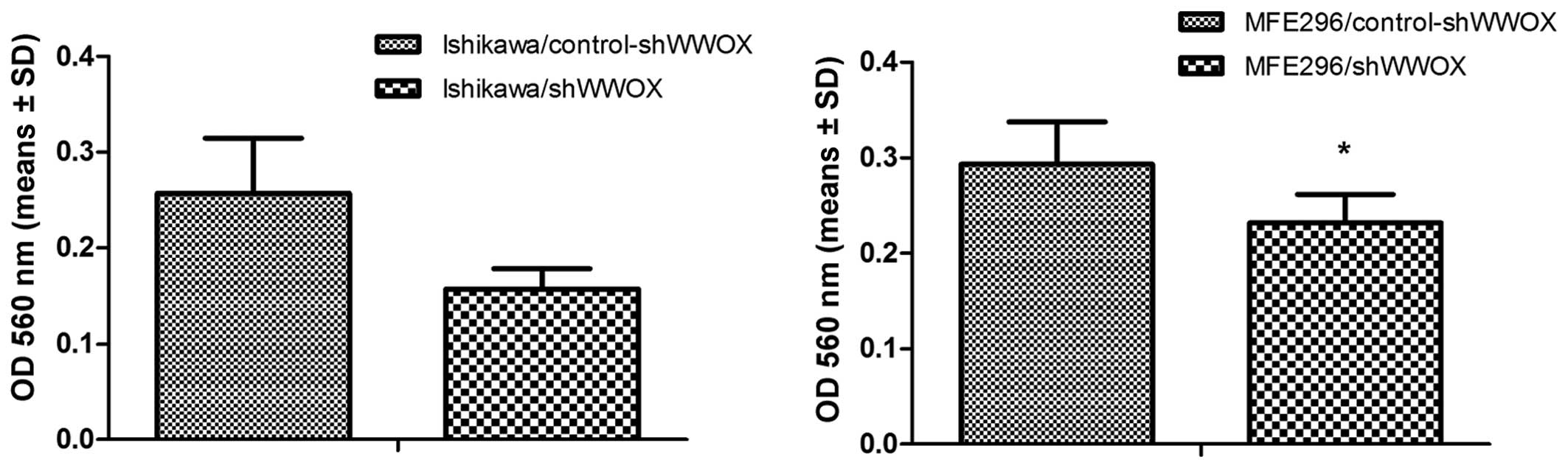
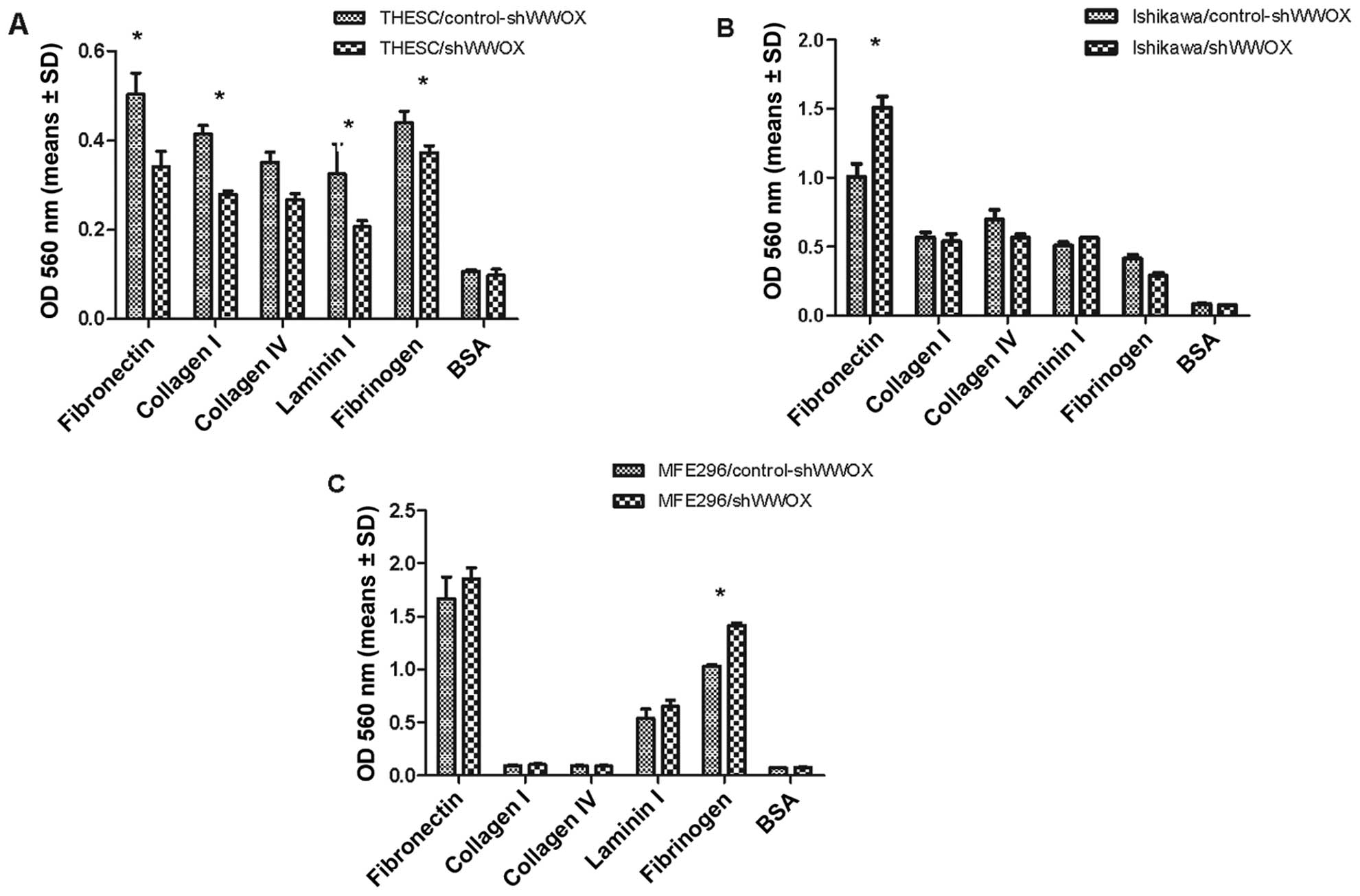
Subsequently, the adhesion to the extracellular
matrix (ECM) and its response to WWOX gene silencing was
evaluated in the normal endometrial cell line and both cancer cell
lines (Fig. 5).
The silencing of the WWOX gene in the THESC
cell line caused a significant decrease in adhesion to four ECM
proteins: fibronectin, collagen 1, laminin 1 and fibrinogen
(p<0.005).
The subsequent analysis of the adhesion assay
revealed significant differences between the two EC cell lines. In
Ishikawa cells, silencing caused a 49% increase (p=0.05) in
fibronectin adhesion, and in MFE296 cells it resulted in a 37%
increase in adhesiveness to fibrinogen (p=0.006).
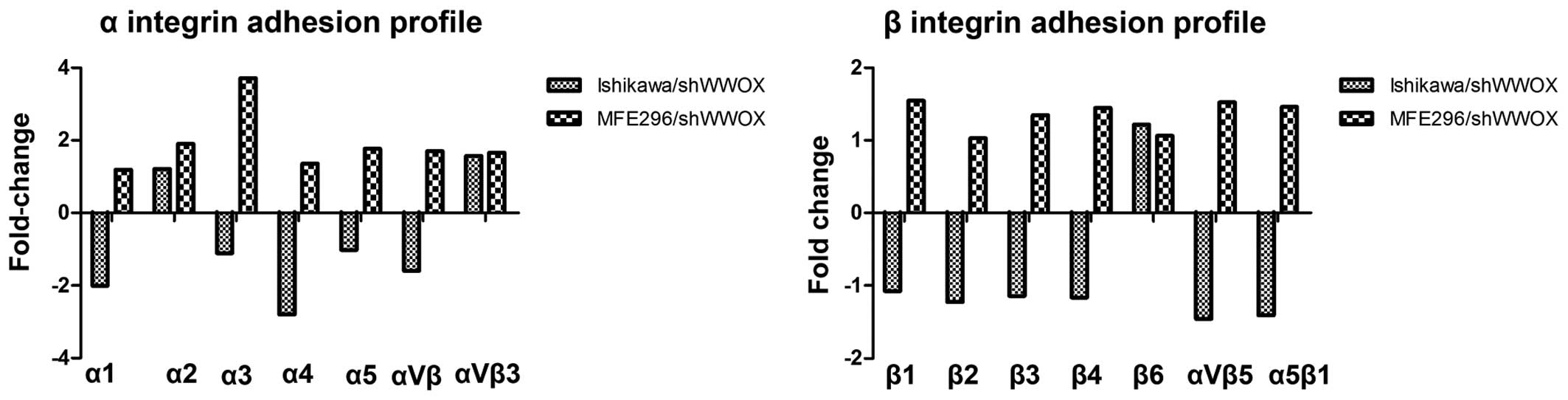
To emphasize the effect that decreased WWOX
expression had on the regulation of adhesion properties, its
influence on cell surface integrin expression and integrin-mediated
cell adhesion was also assessed. Discordant results were observed
for both cancer cell lines. In MFE296 cells, it resulted in
>2-fold increase in the adhesion mediated by subunit α3 integrin
(3.7-fold). On the other hand, in Ishikawa cells it resulted in
decreased adhesion mediated by two α subunits of integrins (between
2.0- and 2.8-fold for integrin α1 and α4, respectively) (Fig. 6).
RT-qPCR was performed in order to evaluate the
expression of genes involved in carcinogenesis-regulating
processes: CTNNB1 and zinc finger E-box binding homeobox 1
(ZEB1) (gene transcription), CDH1 and ezrin
(EZR) (cell adhesion), VIM (structural proteins) as
well as PTEN (tumor suppression) and secreted protein,
acidic, cysteine-rich (osteonectin) (SPARC) (cell growth
regulation). We noted that in both cell lines the silencing of the
WWOX tumor suppressor gene resulted in an increase (>2
fold-change) in the expression of EZR. However, only in the
MFE296 cell line was this association statistically significant
(p=0.04). Only in MFE296 cells did it cause >2-fold increase in
the expression of CDH1, EZR and SPARC
(p<0.05) (complete data shown in Table I).
Discussion
WWOX is a tumor suppressor gene; its
differential expression, protein presence, and elevation have been
proven in previous research to be connected with better prognosis
and survival in various types of cancer, such as gastric (31), ovarian (32), cervical (33) and also breast cancer (21,34–36). Nonetheless, its contribution to
carcinogenesis of the endometrium has not been well defined.
We evaluated the results of WWOX silencing in
a normal endometrial cell line (THESC) and two EC cell lines
(Ishikawa and MFE296) in relation to representative cancer-related
features, such as adhesion potential, growth in soft agar and
invasiveness. The endometrial phenomenon of cell-motility changes
is fundamental for many physiological processes, including
implantation, embryogenesis, immune responses, wound healing, as
well as the pathology of endometriosis, tumor invasion and
metastasis (37). Thus, the
examination of this process, concentrating on WWOX, which is the
global gene expression modulator, is rational. The motility
mechanism depends on the interactions of integrins with ECM
proteins and the differential expression of extracellular proteins
(38,39). We observed as a result of
WWOX silencing significant changes in adhesion potential in
all examined cell lines. In the noncancerous endometrial THESC
cells, a reduction of adhesion to fibronectin, collagen 1, laminin
1 and fibrinogen was noted; different effects were noted in the EC
cell lines, and increased adhesion to fibronectin and fibrinogen
was noted in the MFE29 cells, and increased adhesion to fibronectin
in the Ishikawa cells. Our observations, showing that WWOX
modulates adhesion to fibronectin are consistent with previous
findings from research using ovarian and breast cancer cell lines
(40–42); in these studies, WWOX restoration
resulted in decreased attachment to this ECM component, which has
been linked to peritoneal metastasis. The association between WWOX
signaling and fibrinogen in cell-ECM interaction therefore seems to
be an important feature in promoting cancer cell survival (43).
Integrins, the molecules which are essential for
processes of cellular adhesion, differentiation and motility,
interact from the cell side with ECM proteins. In the endometrium,
the expression of α1, α4, αv and β3 integrin molecules are observed
periodically during the menstrual cycle (44).
The data of the present study showed that in the
moderately differentiated MFE296 cell line, ectopically silenced
WWOX meant higher expression of the α3 subunit of integrin,
while in the well-differentiated Ishikawa cell line lower levels of
α1 and α4 expression were observed after WWOX knockdown.
The involvement of the α4 integrin in tumor
progression and metastasis has been previously demonstrated in
different tumor types. Several authors have demonstrated that
integrin α4 is also essential for angiogenesis (45,46). As reported by Jin et al,
integrin α4 is required for recruitment of circulating progenitor
cells and circulating mononuclear cells to the site of
neovas-cularization. Moreover, this integrin participates in
interaction between the progenitor cell and the tumor endothelium
(47). Also, Garmy-Susini et
al stated that the α4β1 integrin complex facilitates the
intercellular adhesion and survival of endothe-lial cells and
pericytes during blood vessel formation (48). However, as the Ishikawa cell line
is a well-differentiated one, WWOX silencing and the
resulting downregulation of the α4 integrin subunit, which were
noted in the present study, may be part of a loss of differentiated
features, leading to further phenotype changes. This hypothesis is
partially confirmed by studies on EC biology, which have
demonstrated the role which integrin differentiation plays in
relation to tumor invasiveness. As demonstrated by Prifti et
al, integrin dimers α4β1, α5β1 and α6β1 are formed in a number
of EC cell lines, including the Ishikawa cell line, and they are
involved in processes associated with cellular adhesion (49). Moreover, a study by Lessey et
al revealed an inverse correlation between the types and
differential dimerization of integrins and the histological grade
of the tumor, suggesting that EC cells lose certain specific
integrins while gaining a more undifferentiated morphology, and
consequently a metastatic phenotype. Namely, they noted that the
expression of α6 was decreased in the lowest number of tumors of
the endometrium, while α3 was found to be the subunit with the most
frequently declining expression level in EC (50). On the other hand, Gourley et
al demonstrated that WWOX modulates cell-ECM interaction
by lowering the expression of integrin α3 and fibronectin (41). We noted such effects in MFE296
cells, where downregulation of WWOX resulted in the
increased expression of the integrin α3 subunit and a strong
tendency to increased adhesion of fibronectin.
Another aspect of cancer cells modulated by
WWOX and examined in the present study was invasiveness. We
studied the invasion through a basement membrane assay and assessed
MMP-2 expression.
We observed in the MFE296 cell line (with silenced
WWOX) a reduction in MMP-2 activity and invasion through the
basement membrane. Furthermore, in these cells we also noted
increased growth ability in suspension. Our results are consistent
with the assumption that WWOX expression raises motility
potential, with a concurrent reduction in the malignant features of
cancer cells. In colon, breast and EC cell lines the overexpression
of WWOX resulted in an increased invasiveness but reduced
ability to grow in soft agar (26,29,51).
On the other hand, we observed in the present study
that in the well-differentiated Ishikawa cell line, silencing of
the WWOX gene resulted in an increased ratio of
proliferation without mobility and anchorage-independent growth
changes. Similar results have also been observed in the
non-cancerous/normal breast cell line MCF10 (8).
The ability of cells to invade and metastasize is
the result of changes in the expression of genes modulating
cell-cell, cell-matrix interactions and signal transduction
(38,52). In the present study, we revealed
that WWOX differentially modulates the expression of genes
involved in cancer progression. We observed that WWOX
silencing resulted in increased expression of EZR,
CDH1 and SPARC in the undifferentiated MFE296 cell
line.
The SPARC gene can act both as an oncogene
and a tumor suppressor gene depending on the cancer type (53). Yusuf et al reported that
SPARC gene expression was present in poorly differentiated
endometrioid adenocarcinoma tissues but not in normal endometrial
tissues; furthermore, it was also noted that overexpression of the
SPARC gene reinforces the expression of the EMT protein
fibronectin and increased migration activity (54).
Thus, increased expression of SPARC as a
probable result of downregulation of WWOX expression
reinforces the hypothesis that WWOX silencing during cancer
progression is involved in the regulation of EMT.
Moreover, in MFE296/shWWOX cells we also noticed
increased expression of the CDH1 gene, which is involved in
the cell-cell junction and is an important epithelial marker which
is suppressed during EMT.
This adverse correlation between WWOX and
CDH1 was observed in our previous epidemiological EC study,
but the correlation was noted in relation to the other
well-differentiated EC cell line ECC1 (22,29).
In the present study, both in the well- and
moderately differentiated cell lines, negative correlation was
noted between the WWOX and EZR gene associated with
anchoring actin to the cell membrane. Increased expression of
EZR is connected with reduced overall survival in FIGO stage
I (55) and is an essential
driver of metastatic potential in EC (56).
In conclusion, the results of our present study
strongly suggest that the WWOX gene is involved in the
process of endometrial carcinogenesis. Our observations demonstrate
that the WWOX gene modulates the adhesion process in healthy
endometrial cells as well as in tumor cells of varying
differentiation. Moreover, we suggest that in the moderately
differentiated EC cell line, MFE296, the WWOX gene, despite
increasing the motility of cells, exerts a tumor-suppressing
function by inhibiting growth. In addition, in this type of cell,
we suggest that WWOX is involved in regulation of
CDH1, EZR and SPARC pathways.
However, its influence appears to depend on the
stage of carcinogenesis, but further studies should be conducted in
order to better understand the complexity of the functioning of the
WWOX gene.
Acknowledgments
This study was funded by the National Center of
Sciences N N407 168940.
References
|
1
|
Ministry of Health: National Cancer
Registry of Poland: Reports based on data of National Cancer
Registry of Poland. (updated 19-11-2010). http://epid.coi.waw.pl/krn/english/index.asp.
Accessed June 30, 2012.
|
|
2
|
Bokhman JV: Two pathogenetic types of
endometrial carcinoma. Gynecol Oncol. 15:10–17. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Doll A, Abal M, Rigau M, Monge M, Gonzalez
M, Demajo S, Colás E, Llauradó M, Alazzouzi H, Planagumá J, et al:
Novel molecular profiles of endometrial cancer-new light through
old windows. J Steroid Biochem Mol Biol. 108:221–229. 2008.
View Article : Google Scholar
|
|
4
|
Ryan AJ, Susil B, Jobling TW and Oehler
MK: Endometrial cancer. Cell Tissue Res. 322:53–61. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O'Hara AJ and Bell DW: The genomics and
genetics of endometrial cancer. Adv Genomics Genet. 2012:33–47.
2012.PubMed/NCBI
|
|
6
|
Okuda T, Sekizawa A, Purwosunu Y,
Nagatsuka M, Morioka M, Hayashi M and Okai T: Genetics of
endometrial cancers. Obstet Gynecol Int. 2010:9840132010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Merritt MA and Cramer DW: Molecular
pathogenesis of endometrial and ovarian cancer. Cancer Biomark.
9:287–305. 2010.
|
|
8
|
Ferguson BW, Gao X, Zelazowski MJ, Lee J,
Jeter CR, Abba MC and Aldaz CM: The cancer gene WWOX behaves as an
inhibitor of SMAD3 transcriptional activity via direct binding. BMC
Cancer. 13:5932013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kurek KC, Del Mare S, Salah Z, Abdeen S,
Sadiq H, Lee SH, Gaudio E, Zanesi N, Jones KB, DeYoung B, et al:
Frequent attenuation of the WWOX tumor suppressor in osteosarcoma
is associated with increased tumorigenicity and aberrant RUNX2
expression. Cancer Res. 70:5577–5586. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gaudio E, Palamarchuk A, Palumbo T,
Trapasso F, Pekarsky Y, Croce CM and Aqeilan RI: Physical
association with WWOX suppresses c-Jun transcriptional activity.
Cancer Res. 66:11585–11589. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aqeilan RI, Pekarsky Y, Herrero JJ,
Palamarchuk A, Letofsky J, Druck T, Trapasso F, Han SY, Melino G,
Huebner K, et al: Functional association between Wwox tumor
suppressor protein and p73, a p53 homolog. Proc Natl Acad Sci USA.
101:4401–4406. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aqeilan RI, Palamarchuk A, Weigel RJ,
Herrero JJ, Pekarsky Y and Croce CM: Physical and functional
interactions between the Wwox tumor suppressor protein and the
AP-2gamma transcription factor. Cancer Res. 64:8256–8261. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aqeilan RI, Donati V, Palamarchuk A,
Trapasso F, Kaou M, Pekarsky Y, Sudol M and Croce CM: WW
domain-containing proteins, WWOX and YAP, compete for interaction
with ErbB-4 and modulate its transcriptional function. Cancer Res.
65:6764–6772. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aqeilan RI, Donati V, Gaudio E, Nicoloso
MS, Sundvall M, Korhonen A, Lundin J, Isola J, Sudol M, Joensuu H,
et al: Association of Wwox with ErbB4 in breast cancer. Cancer Res.
67:9330–9336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abu-Remaileh M and Aqeilan RI: Tumor
suppressor WWOX regulates glucose metabolism via HIF1α modulation.
Cell Death Differ. 21:1805–1814. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aqeilan RI and Croce CM: WWOX in
biological control and tumorigenesis. J Cell Physiol. 212:307–310.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aqeilan RI, Hagan JP, de Bruin A, Rawahneh
M, Salah Z, Gaudio E, Siddiqui H, Volinia S, Alder H, Lian JB, et
al: Targeted ablation of the WW domain-containing oxidoreductase
tumor suppressor leads to impaired steroidogenesis. Endocrinology.
150:1530–1535. 2009. View Article : Google Scholar :
|
|
18
|
Bednarek AK, Laflin KJ, Daniel RL, Liao Q,
Hawkins KA and Aldaz CM: WWOX, a novel WW domain-containing protein
mapping to human chromosome 16q23.3-24.1, a region frequently
affected in breast cancer. Cancer Res. 60:2140–2145.
2000.PubMed/NCBI
|
|
19
|
Lan C, Chenggang W, Yulan B, Xiaohui D,
Junhui Z and Xiao W: Aberrant expression of WWOX protein in
epithelial ovarian cancer: A clinicopathologic and
immunohistochemical study. Int J Gynecol Pathol. 31:125–132. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qin HR, Iliopoulos D, Nakamura T,
Costinean S, Volinia S, Druck T, Sun J, Okumura H and Huebner K:
Wwox suppresses prostate cancer cell growth through modulation of
ErbB2-mediated androgen receptor signaling. Mol Cancer Res.
5:957–965. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Płuciennik E, Kusińska R, Potemski P,
Kubiak R, Kordek R and Bednarek AK: WWOX--the FRA16D cancer gene:
Expression correlation with breast cancer progression and
prognosis. Eur J Surg Oncol. 32:153–157. 2006. View Article : Google Scholar
|
|
22
|
Płuciennik E, Kośla K, Wójcik-Krowiranda
K, Bieńkiewicz A and Bednarek AK: The WWOX tumor suppressor gene in
endometrial adenocarcinoma. Int J Mol Med. 32:1458–1464. 2013.
|
|
23
|
Cui Z, Lin D, Cheng F, Luo L, Kong L, Xu
J, Hu J and Lan F: The role of the WWOX gene in leukemia and its
mechanisms of action. Oncol Rep. 29:2154–2162. 2013.PubMed/NCBI
|
|
24
|
Ishii H, Vecchione A, Furukawa Y,
Sutheesophon K, Han SY, Druck T, Kuroki T, Trapasso F, Nishimura M,
Saito Y, et al: Expression of FRA16D/WWOX and FRA3B/FHIT genes in
hematopoietic malignancies. Mol Cancer Res. 1:940–947.
2003.PubMed/NCBI
|
|
25
|
Liu SY, Chiang MF and Chen YJ: Role of WW
domain proteins WWOX in development, prognosis, and treatment
response of glioma. Exp Biol Med (Maywood). 240:315–323. 2015.
View Article : Google Scholar
|
|
26
|
Nowakowska M, Pospiech K, Lewandowska U,
Piastowska-Ciesielska AW and Bednarek AK: Diverse effect of WWOX
overexpression in HT29 and SW480 colon cancer cell lines. Tumour
Biol. 35:9291–9301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie B, Zen Q, Wang X, He X, Xie Y, Zhang Z
and Li H: ACK1 promotes hepatocellular carcinoma progression via
downregulating WWOX and activating AKT signaling. Int J Oncol.
46:2057–2066. 2015.PubMed/NCBI
|
|
28
|
Yan H and Sun Y: Evaluation of the
mechanism of epithelial-mesenchymal transition in human ovarian
cancer stem cells transfected with a WW domain-containing
oxidoreductase gene. Oncol Lett. 8:426–430. 2014.PubMed/NCBI
|
|
29
|
Płuciennik E, Nowakowska M, Pospiech K,
Stępień A, Wołkowicz M, Gałdyszyńska M, Popęda M, Wójcik-Krowiranda
K, Bieńkiewicz A and Bednarek AK: The role of WWOX tumor suppressor
gene in the regulation of EMT process via regulation of
CDH1-ZEB1-VIM expression in endometrial cancer. Int J Oncol.
46:2639–2648. 2015.
|
|
30
|
Pfaffl MW, Horgan GW and Dempfle L:
Relative expression software tool (REST) for group-wise comparison
and statistical analysis of relative expression results in
real-time PCR. Nucleic Acids Res. 30:e362002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Aqeilan RI, Kuroki T, Pekarsky Y, Albagha
O, Trapasso F, Baffa R, Huebner K, Edmonds P and Croce CM: Loss of
WWOX expression in gastric carcinoma. Clin Cancer Res.
10:3053–3058. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nunez MI, Rosen DG, Ludes-Meyers JH, Abba
MC, Kil H, Page R, Klein-Szanto AJ, Godwin AK, Liu J, Mills GB, et
al: WWOX protein expression varies among ovarian carcinoma
histotypes and correlates with less favorable outcome. BMC Cancer.
5:642005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qu J, Lu W, Li B, Lu C and Wan X: WWOX
induces apoptosis and inhibits proliferation in cervical cancer and
cell lines. Int J Mol Med. 31:1139–1147. 2013.PubMed/NCBI
|
|
34
|
Bednarek AK, Keck-Waggoner CL, Daniel RL,
Laflin KJ, Bergsagel PL, Kiguchi K, Brenner AJ and Aldaz CM: WWOX,
the FRA16D gene, behaves as a suppressor of tumor growth. Cancer
Res. 61:8068–8073. 2001.PubMed/NCBI
|
|
35
|
Nunez MI, Ludes-Meyers J, Abba MC, Kil H,
Abbey NW, Page RE, Sahin A, Klein-Szanto AJ and Aldaz CM: Frequent
loss of WWOX expression in breast cancer: Correlation with estrogen
receptor status. Breast Cancer Res Treat. 89:99–105. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Göthlin Eremo A, Wegman P, Stål O,
Nordenskjöld B, Fornander T and Wingren S: Wwox expression may
predict benefit from adjuvant tamoxifen in randomized breast cancer
patients. Oncol Rep. 29:1467–1474. 2013.PubMed/NCBI
|
|
37
|
Schwenke M, Knöfler M, Velicky P, Weimar
CH, Kruse M, Samalecos A, Wolf A, Macklon NS, Bamberger AM and
Gellersen B: Control of human endometrial stromal cell motility by
PDGF-BB, HB-EGF and trophoblast-secreted factors. PLoS One.
8:e543362013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Desgrosellier JS and Cheresh DA: Integrins
in cancer: biological implications and therapeutic opportunities.
Nat Rev Cancer. 10:9–22. 2010. View Article : Google Scholar
|
|
39
|
Li X, Regezi J, Ross FP, Blystone S, Ilić
D, Leong SP and Ramos DM: Integrin alphavbeta3 mediates K1735
murine melanoma cell motility in vivo and in vitro. J Cell Sci.
114:2665–2672. 2001.PubMed/NCBI
|
|
40
|
Abdeen SK, Salah Z, Khawaled S and Aqeilan
RI: Characterization of WWOX inactivation in murine mammary gland
development. J Cell Physiol. 228:1391–1396. 2013. View Article : Google Scholar
|
|
41
|
Gourley C, Paige AJ, Taylor KJ, Ward C,
Kuske B, Zhang J, Sun M, Janczar S, Harrison DJ, Muir M, et al:
WWOX gene expression abolishes ovarian cancer tumorigenicity in
vivo and decreases attachment to fibronectin via integrin α3.
Cancer Res. 69:4835–4842. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang JQ, Li L, Song HL, Paige A and Gabra
H: Effect of WWOX gene on the attachment and adhesion of ovarian
cancer cells. Zhonghua Fu Chan Ke Za Zhi. 44:529–532. 2009.In
Chinese. PubMed/NCBI
|
|
43
|
Palumbo JS and Degen JL: Fibrinogen and
tumor cell metastasis. Haemostasis. 31(Suppl 1): S11–S15. 2001.
|
|
44
|
Lessey BA, Castelbaum AJ, Buck CA, Lei Y,
Yowell CW and Sun J: Further characterization of endometrial
integrins during the menstrual cycle and in pregnancy. Fertil
Steril. 62:497–506. 1994.PubMed/NCBI
|
|
45
|
Rebhun RB, Cheng H, Gershenwald JE, Fan D,
Fidler IJ and Langley RR: Constitutive expression of the alpha4
integrin correlates with tumorigenicity and lymph node metastasis
of the B16 murine melanoma. Neoplasia. 12:173–182. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Holzmann B1, Gosslar U and Bittner M:
Alpha 4 integrins and tumor metastasis. Curr Top Microbiol Immunol.
231:125–141. 1998.PubMed/NCBI
|
|
47
|
Jin H, Aiyer A, Su J, Borgstrom P, Stupack
D, Friedlander M and Varner J: A homing mechanism for bone
marrow-derived progenitor cell recruitment to the neovasculature. J
Clin Invest. 116:652–662. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Garmy-Susini B, Jin H, Zhu Y, Sung RJ,
Hwang R and Varner J: Integrin α4β1-VCAM-1-mediated adhesion
between endothelial and mural cells is required for blood vessel
maturation. J Clin Invest. 115:1542–1551. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Prifti S, Zourab Y, Koumouridis A,
Bohlmann M, Strowitzki T and Rabe T: Role of integrins in invasion
of endometrial cancer cell lines. Gynecol Oncol. 84:12–20. 2002.
View Article : Google Scholar
|
|
50
|
Lessey BA, Albelda S, Buck CA, Castelbaum
AJ, Yeh I, Kohler M and Berchuck A: Distribution of integrin cell
adhesion molecules in endometrial cancer. Am J Pathol. 146:717–726.
1995.PubMed/NCBI
|
|
51
|
Lewandowska U, Żelazowski M, Seta K,
Byczewska M, Płuciennik E and Bednarek AK: WWOX, the tumour
suppressor gene affected in multiple cancer. J Physiol Pharmacol.
60(Suppl 1): 47–56. 2009.
|
|
52
|
Martin TA and Jiang WG: Loss of tight
junction barrier function and its role in cancer metastasis.
Biochim Biophys Acta. 1788:872–891. 2009. View Article : Google Scholar
|
|
53
|
Neuzillet C, Tijeras-Raballand A, Cros J,
Faivre S, Hammel P and Raymond E: Stromal expression of SPARC in
pancreatic adenocarcinoma. Cancer Metastasis Rev. 32:585–602. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yusuf N, Inagaki T, Kusunoki S, Okabe H,
Yamada I, Matsumoto A, Terao Y, Takeda S and Kato K: SPARC was
overexpressed in human endometrial cancer stem-like cells and
promoted migration activity. Gynecol Oncol. 134:356–363. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Köbel M, Langhammer T, Hüttelmaier S,
Schmitt WD, Kriese K, Dittmer J, Strauss HG, Thomssen C and
Hauptmann S: Ezrin expression is related to poor prognosis in FIGO
stage I endometrioid carcinomas. Mod Pathol. 19:581–587. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ohtani K, Sakamoto H, Rutherford T, Chen
Z, Kikuchi A, Yamamoto T, Satoh K and Naftolin F: Ezrin, a
membrane-cytoskeletal linking protein, is highly expressed in
atypical endometrial hyperplasia and uterine endometrioid
adenocarcinoma. Cancer Lett. 179:79–86. 2002. View Article : Google Scholar : PubMed/NCBI
|