Introduction
Endothelial cells (ECs) play an essential role in
maintaining endothelial homeostasis, which is involved in many
vascular processes, including angiogenesis, vasoconstriction and
vasodilation (1,2). It has previously been reported that
endothelial dysfunction underlies the pathogenesis of various
cardiovascular diseases (3). EC
injury, accompanied by an increase in the rate of apoptosis, has
been noted as a common feature of endothelial dysfunction and in
the pathogenesis of cardiovascular diseases such as hypertension
(4). Angiotensin II (AngII), the
main effector peptide of the renin-angiotensin system (RAS), plays
a pivotal role in regulating cardiovascular function via its
specific receptors (5). Marked
endothelial dysfunction induced by AngII has been observed in
various cardiovascular diseases, including hypertension,
atherosclerosis and myocardial infarction (6). AngII triggers excessive expression
of reactive oxygen species (ROS), DNA damage, mitochondrial
dysfunction and inflammation, as well as eventually inducing
endothelial apoptosis (7–10). Notably, a previous study has
demonstrated that blockade of AngII signaling by specific
antagonists of the AngII type 1 receptor protects against
hypertensive vascular injury, mainly through the inhibition of EC
apoptosis (11). Although AngII
has long been recognized as a mediator of endothelial apoptosis,
the underlying mechanisms remain poorly understood.
p120 catenin (p120ctn) is a member of a subfamily of
armadillo repeat proteins, and was originally identified as a
substrate for Src kinase in 1989 (12). It has been proposed that it is an
important component of adherens junctions (13). Previous studies have demonstrated
that a number of proteins, including epithelial (E)-cadherin
(14,15), receptor tyrosine kinases (Src,
Yes, Fer and Fyn) (16),
transcription regulator Kaiso (17,18), Rho GTPases (19), and Wnt signaling proteins such as
glycogen synthase kinase-3β (GSK-3β) (20), are physically or functionally
regulated by p120ctn, and this is indicative of the critical role
played by p120ctn in the regulation of cell adhesion,
proliferation, migration and cancer progression (21,22). Interestingly, p120ctn deficiency
in ECs exacerbates inflammatory responses in endotoxin-induced lung
injury through activation of Toll-like receptor (TLR)4 signaling
(23). Moreover, knockdown of
p120ctn in ECs has been shown to activate the transcription of
pro-inflammatory adhesion molecules and to trigger an inflammatory
response (24), which is of
significance during the process of EC apoptosis. However, the
functional role of p120ctn in endothelial apoptosis had not been
elucidated prior to this study. Thus, in the present study, we
hypothesized that p120ctn plays an important role in the regulation
of EC apoptosis. Our results demonstrated that p120ctn inhibits the
apoptosis of human umbilical vein endothelial cells (HUVECs), which
was induced by AngII through modulation of the
mitochondria-dependent pathway. This suggests that p120ctn
expression is a novel strategy which could be used to prevent EC
apoptosis-related diseases, including hypertension.
Materials and methods
Cell culture
HUVECs were isolated upon collagenase treatment of
human umbilical cord veins as previously described (5). After obtaining approval from the
Medical Ethics Committee of Beijing Hospital and in accordance with
the Declaration of Helsinki, human umbilical cords were collected
following delivery (full-term pregnancies) and after obtaining
written informed consent from the mother. The cells were grown in
M199 medium supplemented with 20% fetal calf serum, 50 µg/ml
recombinant human endothelial cell growth factor, 25 U/ml heparin,
100 U/ml penicillin and 0.1 U/ml streptomycin (all from Gibco,
Carlsbad, CA, USA) at 37°C, in an atmosphere with 5%
CO2. HUVECs at passages 3–6 were used for all
experiments.
Adenoviral infection
Human p120ctn adenovirus (Ad-p120ctn) was purchased
from Cyagen Biosciences, Inc. (Santa Clara, CA, USA). The
adenovirus expressing a small hairpin RNA (shRNA) sequence (GenBank
accession no. NM_001331; 5′-GGACCUUACUGAAGUUAUUUU-3′) targeting
human p120ctn (Ad-shp1120ctn) was designed by and purchased from
Sunbio Medical Biotechnology Co., Ltd., (Shanghai, China). A
negative control (Lacz) was obtained from Clontech Laboratories
(Mountain View, CA, USA) and untreated cells were used as controls.
On the day prior to adenoviral infection, HUVECs were seeded in
6-well plates and reached 80% confluence. Ad-p120ctn or
Ad-shp120ctn, at a different multiplicity of infection (MOI), was
diluted in 2 ml M199 medium (Gibco) without serum and then added to
the cells. After 3 h, the cells were transferred into fresh culture
medium and incubated for 48 h before analysis.
Cell viability assay
Cell viability was measured using a Cell Counting
kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan) according to
the manufacturer's instructions. Briefly, HUVECs were seeded in
96-well plates at a density of 2×103 cells/well.
Following treatment with different concentrations of AngII (Sigma
Chemical Co., St. Louis, MO, USA) for 24 h, 10 µl CCK-8 was
added to each well, followed by incubation at 37°C for an
additional 3 h. A colorimetric assay was performed using a Spectra
Max 190 microplate reader (Molecular Devices LLC, Sunnyvale, CA,
USA) and absorbance was measured at a wavelength of 450 nm.
Cell apoptosis assay
Apoptosis of HUVECs was determined using an Accuri
C6 flow cytometer and the FITC-Annexin V and propidium iodide (PI)
double staining assay (both from BD Biosciences, San Jose, CA, USA)
according to the manufacturer's instructions. Briefly, the cells
were digested with trypsin at a density of 1×106/ml,
washed with phosphate-buffered saline (PBS) three times, and then
incubated with Annexin V and PI in the dark for 20 min at room
temperature. The cells were counted with the flow cytometer, and
the data were analyzed using CFlow Plus software (BD
Biosciences).
Western blot analysis
Western blot analysis was performed as previously
described (25). Briefly, HUVECs
were lysed in RIPA lysis buffer (Beyotime Biotech, Jiangsu, China)
supplemented with protease and phosphatase inhibitor cocktail
(Sigma Chemical Co.). The cell lysates were separated by 8–12%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE), and then transferred to polyvinylidene fluoride
membranes (Millipore Corp., Billerica, MA, USA). The membranes were
blocked in 5% non-fat milk for 1 h at room temperature, and then
incubated with the following primary antibodies: p120ctn (1:500;
612536; BD Biosciences); Bcl-2 (#4223), Bax (#5023), cytochrome
c (#11940), caspase-3 (#9669), and caspase-9 (1:2,000;
#9509; all from Cell Signaling Technology, Danvers, MA, USA);
Cox-IV (SC-69360), GAPDH (SC-365062), and β-actin (1:1,000;
SC-8430; all from Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA). After washing, secondary antibodies, namely HRP-conjugated
anti-rabbit (#7074) or anti-mouse (1:1,000; #7076; both from Cell
Signaling Technology), were incubated for 1 h at room temperature,
and the membranes were then visualized using a chemiluminescence
kit (Thermo Fisher Scientific, Inc., Rockford, IL, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis of p120ctn expression
Total RNA from HUVECs was isolated using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA) following the
manufacturer's instructions. The purity and concentration of RNA
were determined by nucleic acid quantitative instrument (Qubit 2.0;
Invitrogen). One micro-gram of total RNA was used to performed the
RT-PCR with a One Step RT-PCR kit (Qiagen, Inc., Valencia, CA,
USA). The reaction conditions were as follows: 95°C for 30 sec,
60°C for 45 sec, 72°C for 60 sec (32 cycles). The specific primers
for p120ctn and GAPDH were synthesized by Invitrogen as follows:
p120ctn, 5′-TACGCTCTCTCCTTCCTGCT-3′ and 5′-AGACATGGCTCCCTCAGGAT-3′;
and GAPDH, 5′-GGG CACGAAGGCTCATCATT-3′ and 5′-AGAAGGCTGGGG
CTCATTTG-3′. The amplified products were electrophoresed on 1%
agarose gels and the bands were analyzed using ImageJ software
(NIH, Bethesda, MD, USA).
Preparation of mitochondrial
fractions
Following treatment, HUVECs were harvested and
suspended in 4 ml mitochondrial isolation buffer (200 mM mannitol,
75 mM sucrose, 1 mM EDTA, 1% cocktail protease inhibitor, 5 mM
Tris/HCl pH 7.4). The homogenates were centrifuged at 2,500 rpm
twice for 5 min at 4°C, and the supernatant contained the cytosolic
proteins. The pellet was resuspended in mitochondrial isolation
buffer and placed on top of sucrose density gradient buffer (1.0,
1.2 and 1.5 M sucrose). The samples were centrifuged at 25,000 rpm
for 30 min at 4°C on a Sorvall TST60.4 rotor (Beckman Coulter,
Krefeld, Germany). Mitochondrial-enriched fractions were collected
at 1.2/1.5 M sucrose interphases and analyzed by western blot
analysis.
Examination of mitochondrial membrane
potential (MMP)
In the present study,
5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethyl-benza-midazolocarbocyanin
iodide (JC-1 dye; Molecular Probes™, Invitrogen, Carlsbad, CA, USA)
was used to measure MMP. JC-1 is a cationic dye that exhibits
potential-dependent accumulation in mitochondria, which is
indicated by red fluorescence (excitation, 550 nm; emission, 600
nm) in viable cells. During apoptosis, JC-1 exists in the cytoplasm
as a monomer, indicated by green fluorescence (excitation, 485 nm;
emission, 535 nm). Consequently, the red/green fluorescence
intensity ratio indicates changes to MMP.
Statistical analysis
In the present study, all data are expressed as the
means ± SEM, and n indicates the number of independent experiments.
A one-way analysis of variance (ANOVA) followed by Tukey's multiple
comparison post-hoc test was used in order to analyze the
differences of multiple groups. A P-value <0.05 was considered
to indicate a statistically significant difference in all
statistical tests. All statistical analyses were performed using
SPSS version 15 statistical software (SPSS Inc., Chicago, IL,
USA).
Results
Effects of AngII and p120ctn on HUVEC
viability
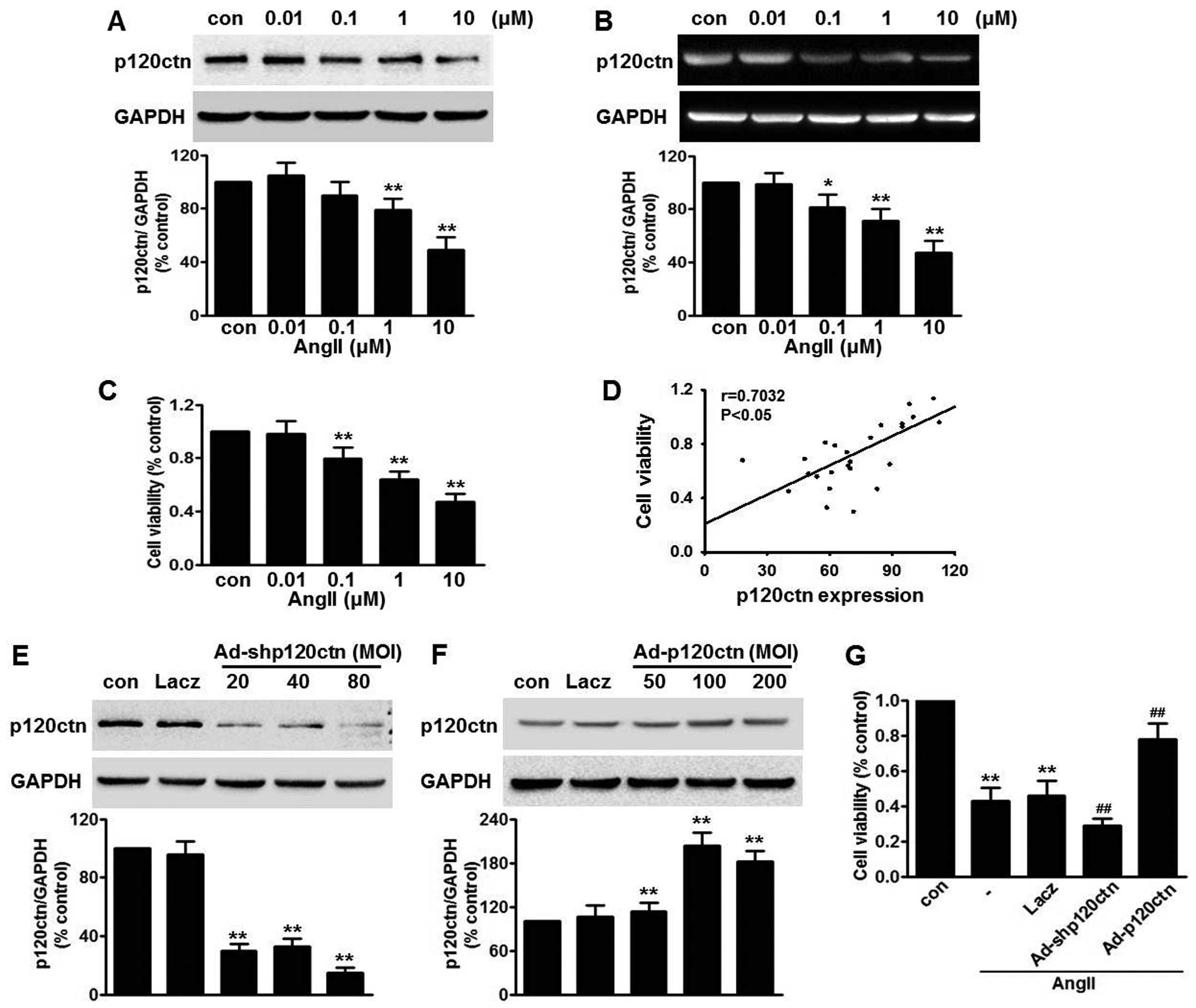
We first investigated the effect of AngII on p120ctn
expression in HUVECs. Western blot analysis revealed that p120ctn
protein expression decreased in a concentration-dependent manner
following treatment with AngII for 24 h. AngII at a concentration
of 10 µM significantly decreased p120ctn protein expression
to 54.7±6.9% compared with the control group (Fig. 1A). RT-PCR revealed a similar trend
in p120ctn mRNA expression after challenge with AngII (Fig. 1B), indicating that AngII decreased
p120ctn expression, at least in part, through a
post-transcriptional mechanism. The effect of AngII on cell
viability was measured by CCK-8 assay. AngII decreased cell
viability in a concentration-dependent manner (Fig. 1C). Compared with the control, at
concentrations of 0.1, 1 and 10 µM, cell viability was
reduced to 78.2±9.3, 63.5±7.8 and 45.2±3.3%, respectively. We found
p120ctn protein expression to be positively correlated with cell
viability after AngII treatment (Fig.
1D), suggesting that p120ctn expression is involved in
AngII-induced injury to HUVECs. In order to verify this
observation, we used adeno-viruses bearing shRNA or p120ctn cDNA to
knock down or overexpress p120ctn. Efficiency of infection was
detected by western blot analysis. Ad-shp120ctn at an MOI of 80
decreased endogenous p120ctn expression >80% compared with the
Lacz group (Fig. 1E). Ad-p120ctn
significantly increased p120ctn expression, and the peak increase
was observed at an MOI of 100, while Lacz produced no marked effect
(Fig. 1F). Thus, we selected
Ad-shp120ctn at an MOI of 80 and Ad-p120ctn at an MOI of 100 for
use in the following experiments. The CCK-8 assay revealed that
neither p120ctn knockdown nor overexpression exerted a significant
effect on cell viability (data not shown). However, Ad-shp120ctn
further augmented AngII-induced injury to HUVECs, reducing the cell
viability to 36.3±2.7% of the control group. As expected,
overexpression of p120ctn markedly inhibited the decrease in cell
viability caused by AngII (Fig.
1G).
p120ctn attenuates AngII-induced
apoptosis of HUVECs
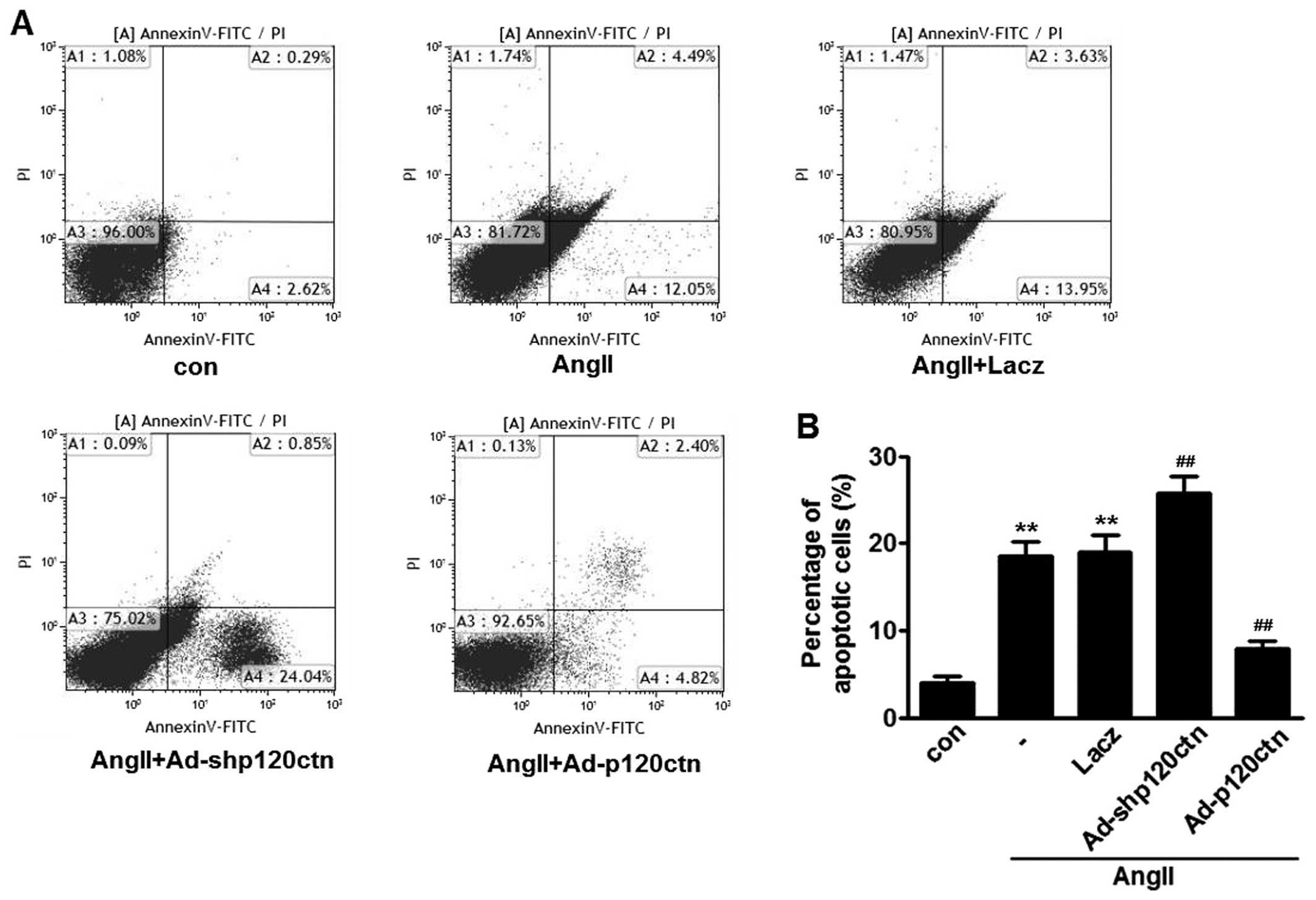
To determine whether decreased p120ctn is involved
in AngII-induced apoptosis of HUVECs, quantitative analysis of
apoptosis by flow cytometric analysis was performed. The Annexin
V-FITC/PI data revealed that incubation with AngII for 24 h
increased the apoptotic rate to 17.2±1.9% in HUVECs.
p120ctn-specific shRNA further increased the apoptotic cell
population to 26.1±2.7%, whereas p120ctn overexpression markedly
abrogated AngII-induced apoptosis of HUVECs. Lacz infection did not
alter the increase in apoptotic cells caused by transfection with
AngII (Fig. 2). These results
suggest that p120ctn exerts no effect on apoptosis of HUVECs at
basal levels; however, p120ctn attenuates AngII-induced
apoptosis.
Effect of p120ctn on AngII-induced
expression of the apoptosis-associated proteins, Bcl-2 and Bax
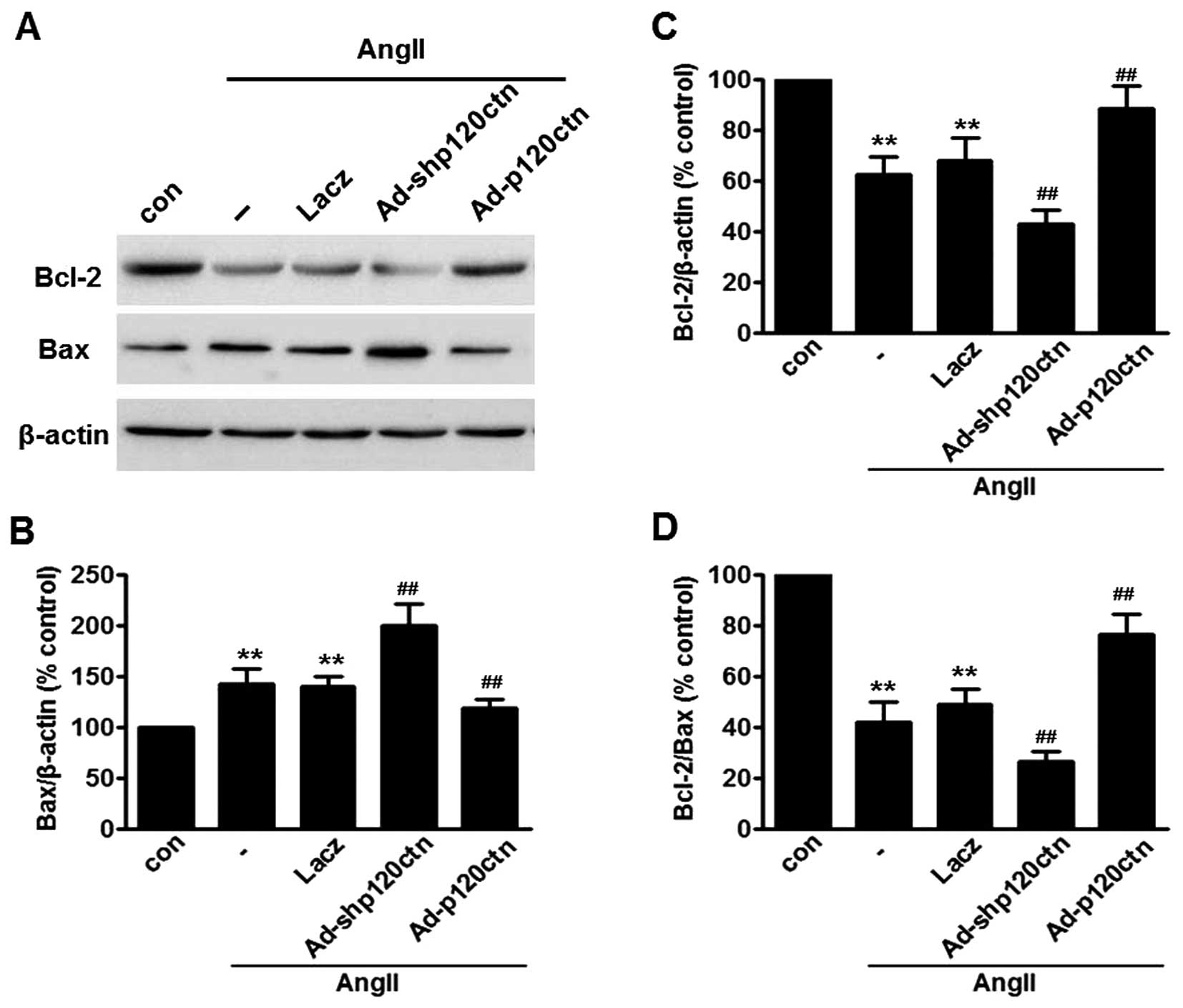
In order to investigate the mechanism by which
p120ctn regulates AngII-induced apoptosis of HUVECs, western blot
analysis was performed to examine the expression of Bcl-2 and Bax,
two Bcl-2 family members which are known to be important in the
regulation of apoptosis (27,32) (Fig.
3A). Low levels of Bcl-2 and high levels of Bax expression were
detected following AngII treat ment for 24 h. Furthermore, the
ratio of Bcl-2 to Bax, which appears to determine cell fate
(survival or death), was markedly reduced by AngII treatment. As
expected, knockdown of p120ctn further augmented the effect of
AngII on Bcl-2 and Bax expression, and the Bcl-2/Bax ratio.
Moreover, upregulation of p120ctn reversed the AngII-decreased
Bcl-2/Bax ratio, due to an increase in Bcl-2 expression and a
decrease in Bax expression (Fig.
3B–D).
Effect of p120ctn on AngII-induced MMP
depolarization and cytochrome c release
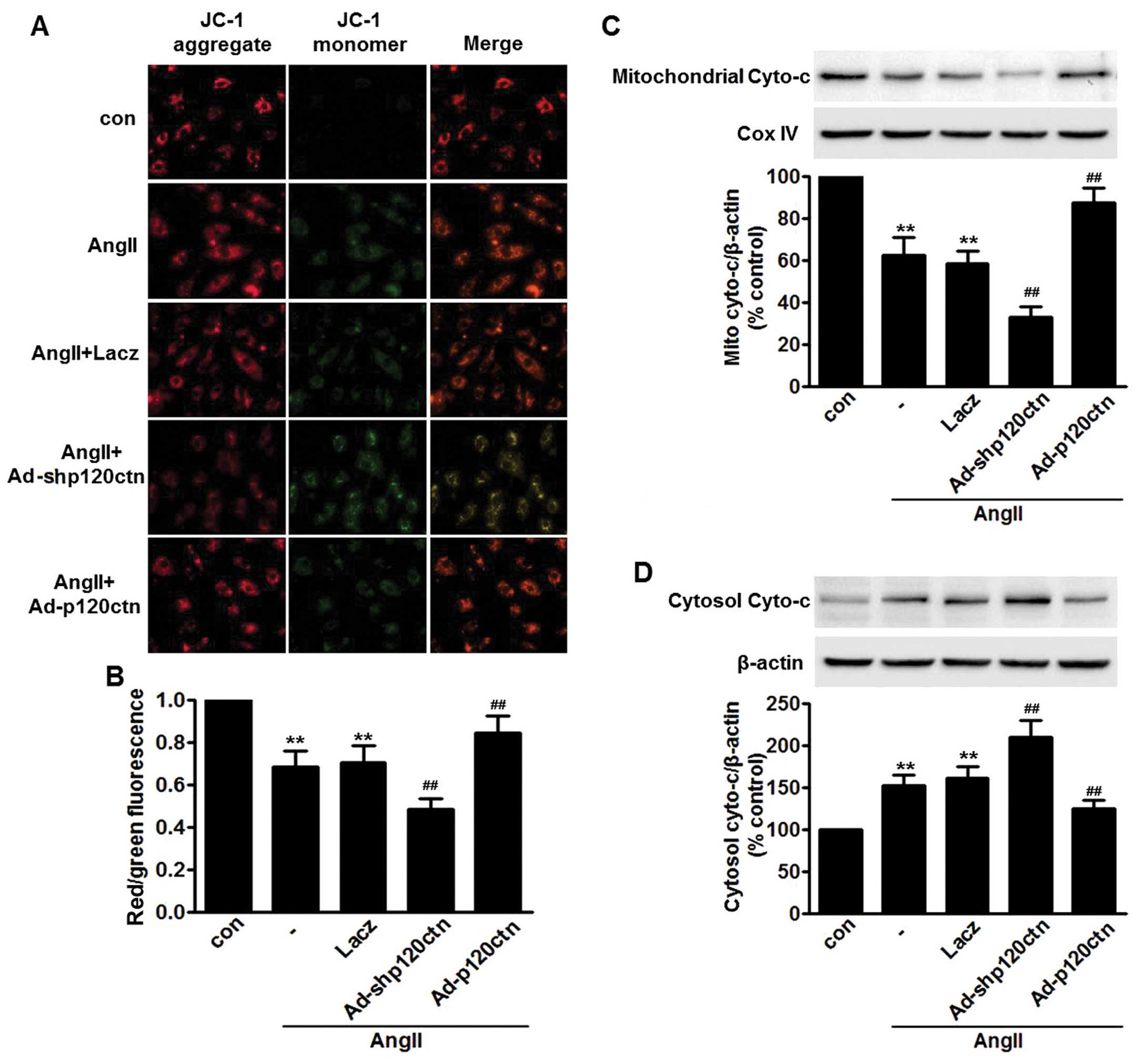
A previous study has shown that MMP plays an
essential role in the AngII-induced apoptosis of HUVECs (26). Consistent with this study, we
found that AngII induced loss of MMP in HUVECs. Confocal microscopy
revealed that AngII decreased red JC-1 fluorescence and increased
green JC-1 fluorescence, respectively, resulting in a decline in
the red/green fluorescence ratio (Fig. 4A and B). We further explored
whether p120ctn attenuated AngII-induced apoptosis of HUVECs by
regulating MMP. We noted that p120ctn knockdown markedly enhanced
the AngII-induced decrease in MMP, and in the Ad-p120ctn-treated
cells this decrease was reduced (Fig.
4A and B).
Impairment of the mitochondrial pathway has
previously been suggested to induce the release of cytochrome
c from mitochondrial into the cytoplasm (27). Following incubation with AngII for
24 h, the translocation of cytochrome c from mitochondria
into the cytoplasm was significantly increased, suggesting that the
increase of cytochrome c in cytoplasm may be involved in
apoptosis induced by AngII. The increased translocation of
cytochrome c into the cytoplasm was further enhanced in
Ad-shp120ctn-infected cells. However, we found that overexpression
of p120ctn markedly impaired AngII-induced translocation of
cytochrome c (Fig. 4C and
D).
Collectively, these data indicate that the
stabilization of MMP and the impairment of cytochrome c
translocation underlie, at least partially, the protective effect
of p120ctn on the AngII-induced apoptosis of HUVECs.
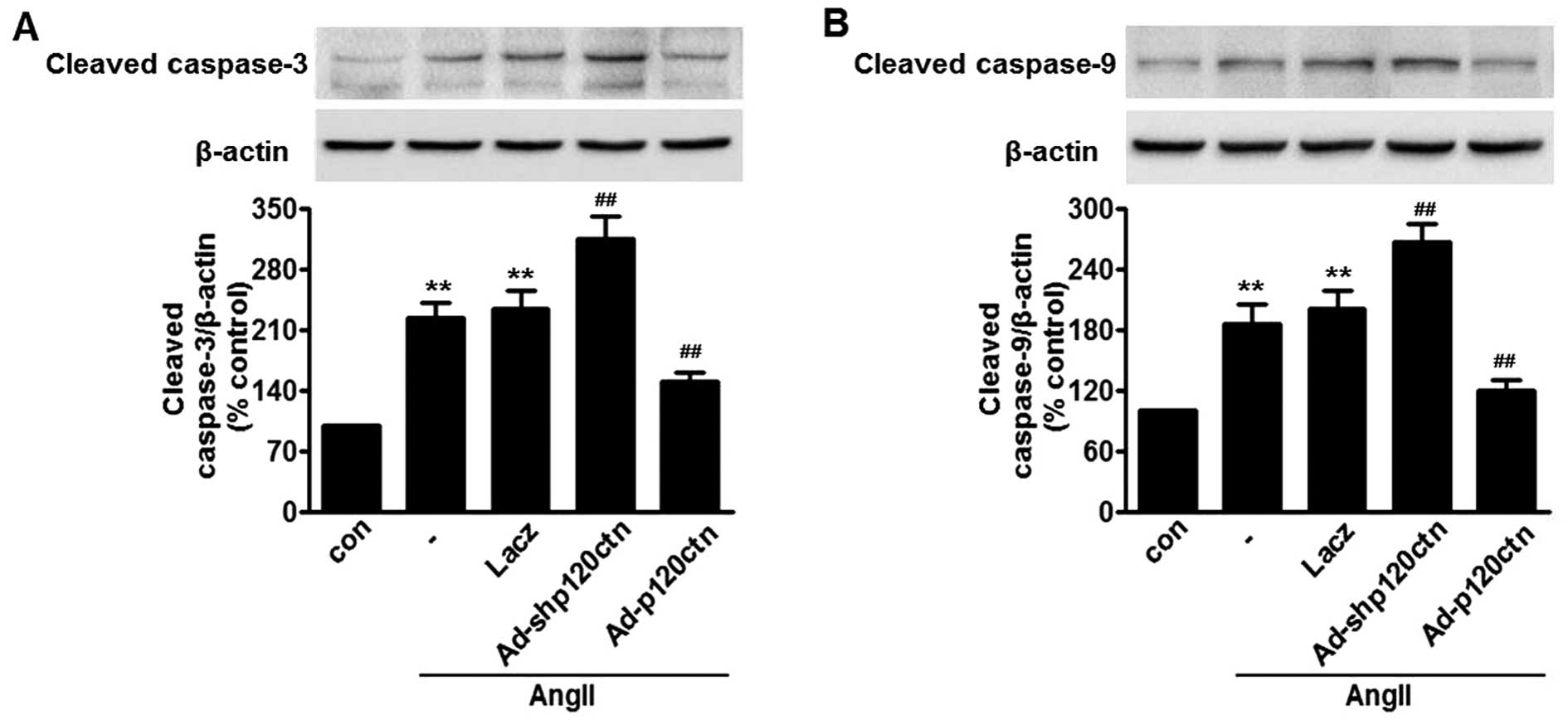
p120ctn abolishes AngII-induced
activation of caspase-3 and -9
Cytochrome c released from mitochondria
cleave and activate downstream apoptosis-associated proteins, such
as caspase-3 and caspase-9, which in turn initiate
mitochondria-dependent apoptosis (28). Our results showed that AngII
increased cleaved caspase-3 and caspase-9. The activation of
caspase-3 and caspase-9 was further enhanced in HUVECs transfected
with Ad-shp120ctn, whereas overexpression of p120ctn almost
abolished the caspase activation induced by AngII. The adenovirus
bearing Lacz did not markedly alter this activation (Fig. 5).
Discussion
p120ctn is best known for its role in the
inflammatory responses; previous studies of conditional targeting
of p120ctn in knockout mice have noted increased inflammatory cell
infiltration and pro-inflammatory cytokines, resulting from
activation of the nuclear factor-κB (NF-κB) pathway (25,29). Deficiency of p120ctn markedly
potentiates lipopolysac-charide (LPS)-induced inflammation in human
brain microvascular endothelial cells and human bronchial
epithelial cells (30,31). Notably, p120ctn also functions as
an endogenous anti-inflammatory molecule in ECs. While the role of
p120ctn with respect to endothelial inflammation has been
previously described, its role in inflammation-associated apoptosis
had not been explored in detail. In the present study, we
investigated the association between p120ctn and AngII-induced
apoptosis of HUVECs. We have reported, for the first time to the
best of our knowledge, that endogenous p120ctn expression was
decreased following AngII treatment. This reduction was accompanied
by a decrease in cell viability, in the same
concentration-dependent manner. Moreover, knockdown of p120ctn
augmented AngII-induced apoptosis of HUVECs and mitochondrial
dysfunction. By contrast, overexpression of p120ctn produced the
opposite effects. Thus, the data from the present study indicate
that p120ctn plays a protective role in EC apoptosis.
Apoptosis of vascular ECs has been shown to play a
critical role in endothelial dysfunction; importantly, EC apoptosis
is an initiating, early mechanism for hypertension, leading
directly to microvascular obliteration (33). It has been proved that the main
effector of the renin-angiotensin-aldosterone system, AngII, is
involved in the pathogenesis of hypertension and results in the
apoptosis of ECs (34,35). We suggest that inhibition of
AngII-induced apoptosis of ECs is an important strategy to prevent
cardiovascular diseases, including hypertension. In the present
study, we found that AngII treatment significantly decreased cell
viability and induced cell apoptosis, supporting the theory that
AngII contributes to endothelial dysfunction, as noted in previous
research (26). Of note, our
study has revealed, for the first time to the best of our
knowledge, that p120ctn expression was decreased after challenge
with AngII. AngII-induced apoptosis of HUVECs was found to be
accompanied by a decrease in p120ctn expression, indicating that
p120ctn may be a critical regulator of EC apoptosis in
AngII-related hypertension. AngII-induced apoptosis of HUVECs was
almost abolished after infection with p120ctn adenovirus, whereas
apoptosis was markedly enhanced in HUVECs transfected with p120ctn
shRNA. These data further confirm the essential role which p120ctn
plays in EC apoptosis.
We next explored the mechanism linking p120ctn and
AngII-induced apoptosis of ECs. It is known that the mitochondrial
pathway is a major signaling pathway involved in the apoptotic
process; changes in MMP usually correlate with alterations in Bcl-2
and Bax expression (36). These
two proteins are crucial to the apoptotic machinery and a wide
array of diverse upstream survival signals, thus determining the
fate of the cells (32,37). Upon apoptotic stimulation, the
balance of Bcl-2 and Bax is broken, the increased level of Bax in
mitochondria induces MMP depolarization, which is followed by
cytochrome c release from mitochondria into the cytosol,
which combines with caspase-9 to form a complex, and activates
caspase-3, triggering apoptosis (27,28). Consistent with a previous study
(26), AngII treatment of HUVECs
markedly decreased Bcl-2 and increased Bax expression,
concomitantly with the loss of MMP and the increase of caspase-3
and -9, suggesting that the mitochondrial pathway is involved in
AngII-induced apoptosis. The results of the present study have
demonstrated that p120ctn shRNA enhanced the decrease in the
Bcl-2/Bax ratio, the depolarization of MMP, the increase in
cytochrome c release into the cytoplasm and the activation
of capase-3 and -9 induced by AngII. However, overexpression of
p120ctn produced the opposite effects. These results indicate that
the effect of p120ctn on apoptosis in HUVECs is
mitochondria-dependent.
In conclusion, the present study provides evidence
that p120ctn attenuates AngII-induced apoptosis of ECs. The
anti-apoptotic effects of p120ctn are mainly due to inhibition of
the mitochondria-dependent pathway. These findings assist us in
gaining a better understanding of the role of p120ctn in apoptosis
and also provide a novel strategy for the prevention of endothelial
dysfunction in cardiovascular diseases, such as hypertension.
Acknowledgments
This study was supported by the Natural Science
Foundation (no. 51272181).
References
|
1
|
Lee R, Channon KM and Antoniades C:
Therapeutic strategies targeting endothelial function in humans:
clinical implications. Curr Vasc Pharmacol. 10:77–93. 2012.
View Article : Google Scholar
|
|
2
|
Shi H, Sheng B, Zhang F, Wu C, Zhang R,
Zhu J, Xu K, Kuang Y, Jameson SC, Lin Z, et al: Kruppel-like factor
2 protects against ischemic stroke by regulating endothelial blood
brain barrier function. Am J Physiol Heart Circ Physiol.
304:H796–H805. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wada K, Gerbaudo VH and Spector M: Effects
of PDGF-BB and OP-1 on mesenchymal stem cells in a porous mineral
block. Int J Periodontics Restorative Dent. 33:e72–e78. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xie F, Cai W, Liu Y, Li Y, Du B, Feng L
and Qiu L: Vaccarin attenuates the human EA.hy926 endothelial cell
oxidative stress injury through inhibition of Notch signaling. Int
J Mol Med. 35:135–142. 2015.
|
|
5
|
Wang C, He Y, Yang M, Sun H, Zhang S and
Wang C: Safflor yellow B suppresses angiotensin II-mediated human
umbilical vein cell injury via regulation of Bcl-2/p22(phox)
expression. Toxicol Appl Pharmacol. 273:59–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dimmeler S, Rippmann V, Weiland U,
Haendeler J and Zeiher AM: Angiotensin II induces apoptosis of
human endothelial cells. Protective effect of nitric oxide. Circ
Res. 81:970–976. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mendell JT and Olson EN: MicroRNAs in
stress signaling and human disease. Cell. 148:1172–1187. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Caporali A and Emanueli C: MicroRNA
regulation in angiogenesis. Vascul Pharmacol. 55:79–86. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang J, Sun P, Bao Y, Dou B, Song D and Li
Y: Vitamin E renders protection to PC12 cells against oxidative
damage and apoptosis induced by single-walled carbon nanotubes.
Toxicol In Vitro. 26:32–41. 2012. View Article : Google Scholar
|
|
10
|
Paravicini TM and Touyz RM: Redox
signaling in hypertension. Cardiovasc Res. 71:247–258. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sata M and Fukuda D: Crucial role of
renin-angiotensin system in the pathogenesis of atherosclerosis. J
Med Invest. 57:12–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reynolds AB, Roesel DJ, Kanner SB and
Parsons JT: Transformation-specific tyrosine phosphorylation of a
novel cellular protein in chicken cells expressing oncogenic
variants of the avian cellular src gene. Mol Cell Biol. 9:629–638.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang M, Li N, Li J, Ma Y, Li D, Qin L,
Wang X and Wu R: Involvement of p120 in LPS-induced NF-kappaB
activation and IL-8 production in human bronchial epithelial cells.
Toxicol Lett. 195:75–81. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reynolds AB, Daniel J, McCrea PD, Wheelock
MJ, Wu J and Zhang Z: Identification of a new catenin: the tyrosine
kinase substrate p120cas associates with E-cadherin complexes. Mol
Cell Biol. 14:8333–8342. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shibamoto S, Hayakawa M, Takeuchi K, Hori
T, Miyazawa K, Kitamura N, Johnson KR, Wheelock MJ, Matsuyoshi N,
Takeichi M and Fumiaki Ito: Association of p120, a tyrosine kinase
substrate, with E-cadherin/catenin complexes. J Cell Biol.
128:949–957. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Piedra J, Miravet S, Castaño J, Pálmer HG,
Heisterkamp N, García de Herreros A and Duñach M: p120
Catenin-associated Fer and Fyn tyrosine kinases regulate
beta-catenin Tyr-142 phosphorylation and beta-catenin-alpha-catenin
Interaction. Mol Cell Biol. 23:2287–2297. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim SW, Park JI, Spring CM, Sater AK, Ji
H, Otchere AA, Daniel JM and McCrea PD: Non-canonical Wnt signals
are modulated by the Kaiso transcriptional repressor and
p120-catenin. Nat Cell Biol. 6:1212–1220. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Prokhortchouk A, Hendrich B, Jørgensen H,
Ruzov A, Wilm M, Georgiev G, Bird A and Prokhortchouk E: The p120
catenin partner Kaiso is a DNA methylation-dependent
transcriptional repressor. Genes Dev. 15:1613–1618. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anastasiadis PZ, Moon SY, Thoreson MA,
Mariner DJ, Crawford HC, Zheng Y and Reynolds AB: Inhibition of
RhoA by p120 catenin. Nat Cell Biol. 2:637–644. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hong JY, Park JI, Cho K, Gu D, Ji H,
Artandi SE and McCrea PD: Shared molecular mechanisms regulate
multiple catenin proteins: canonical Wnt signals and components
modulate p120-catenin isoform-1 and additional p120 subfamily
members. J Cell Sci. 123:4351–4365. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reynolds AB: p120-catenin: past and
present. Biochim Biophys Acta. 1773:2–7. 2007. View Article : Google Scholar
|
|
22
|
Reynolds AB and Roczniak-Ferguson A:
Emerging roles for p120-catenin in cell adhesion and cancer.
Oncogene. 23:7947–7956. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang YL, Malik AB, Sun Y, Hu S, Reynolds
AB, Minshall RD and Hu G: Innate immune function of the adherens
junction protein p120-catenin in endothelial response to endotoxin.
J Immunol. 186:3180–3187. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
O'Donnell JJ III, Zhuge Y, Holian O, Cheng
F, Thomas LL, Forsyth CB and Lum H: Loss of p120 catenin
upregulates transcription of pro-inflammatory adhesion molecules in
human endothelial cells. Microvasc Res. 82:105–112. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Perez-Moreno M, Davis MA, Wong E, Pasolli
HA, Reynolds AB and Fuchs E: p120-catenin mediates inflammatory
responses in the skin. Cell. 124:631–644. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu J, Zhang FF, Li L, Yang J, Liu J, Guan
YY and Du YH: ClC-3 deficiency prevents apoptosis induced by
angiotensin II in endothelial progenitor cells via inhibition of
NADPH oxidase. Apoptosis. 18:1262–1273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ott M, Gogvadze V, Orrenius S and
Zhivotovsky B: Mitochondria, oxidative stress and cell death.
Apoptosis. 12:913–922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mignotte B and Vayssiere JL: Mitochondria
and apoptosis. Eur J Biochem. 252:1–15. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Perez-Moreno M, Song W, Pasolli HA,
Williams SE and Fuchs E: Loss of p120 catenin and links to mitotic
alterations, inflammation, and skin cancer. Proc Natl Acad Sci USA.
105:15399–15404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu N, Li AL, Zhou XP, Chen Q and Cao W:
P120 catenin attenuates lipopolysaccharide-induced blood-brain
barrier dysfunction and inflammatory responses in human brain
micro-vascular endothelial cells. Int J Clin Exp Pathol.
8:4204–4212. 2015.
|
|
31
|
Qin L, Qin S, Zhang Y, Zhang C, Ma H, Li
N, Liu L, Wang X and Wu R: p120 modulates LPS-induced NF-κB
activation partially through RhoA in bronchial epithelial cells.
Biomed Res Int. 2014:9323402014. View Article : Google Scholar
|
|
32
|
Ye QF, Zhang YC, Peng XQ, Long Z, Ming YZ
and He LY: Silencing Notch-1 induces apoptosis and increases the
chemo-sensitivity of prostate cancer cells to docetaxel through
Bcl-2 and Bax. Oncol Lett. 3:879–884. 2012.PubMed/NCBI
|
|
33
|
Teichert-Kuliszewska K, Tsoporis JN,
Desjardins JF, Yin J, Wang L, Kuebler WM and Parker TG: Absence of
the calcium-binding protein, S100A1, confers pulmonary hypertension
in mice associated with endothelial dysfunction and apoptosis.
Cardiovasc Res. 105:8–19. 2015. View Article : Google Scholar
|
|
34
|
Efrati S, Berman S, Goldfinger N, Erez N,
Averbukh Z, Golik A, Rotter V and Weissgarten J: Enhanced
angiotensin II production by renal mesangium is responsible for
apoptosis/proliferation of endothelial and epithelial cells in a
model of malignant hypertension. J Hypertens. 25:1041–1052. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rojas E, Rodríguez-Molina D, Bolli P,
Israili ZH, Faría J, Fidilio E, Bermúdez V and Velasco M: The role
of adiponectin in endothelial dysfunction and hypertension. Curr
Hypertens Rep. 16:4632014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Misiti F, Orsini F, Clementi ME, Lattanzi
W, Giardina B and Michetti F: Mitochondrial oxygen consumption
inhibition importance for TMT-dependent cell death in
undifferentiated PC12 cells. Neurochem Int. 52:1092–1099. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Reagan-Shaw S, Nihal M, Ahsan H, Mukhtar H
and Ahmad N: Combination of vitamin E and selenium causes an
induction of apoptosis of human prostate cancer cells by enhancing
Bax/Bcl-2 ratio. Prostate. 68:1624–1634. 2008. View Article : Google Scholar : PubMed/NCBI
|