Introduction
Drinking wine in moderation, in particular red wine,
may lower the incidence of cardiovascular diseases, presumably due
in part to the benefits of resveratrol in the wine (1). Polydatin is a glycoside derivative
of resveratrol, and widely present in various plant sources
including red wine, grapes skins and Japanese knotweed (2). Polydatin is an ingredient of many
herbal medications used for the treatment of cardiovascular
diseases in China. Similar to resveratrol, polydatin has
antioxidant (3),
anti-inflammatory (4) and
anti-ageing (5) effects.
It has been shown that oxidative stress promotes the
proliferation of vascular smooth muscle cells (VSMCs) and induces
blood vessel remodeling (6),
thereby contributing to the pathogenesis of atherosclerosis
(7,8). Consequently, inhibiting VSMC
proliferation may be used as a future therapeutic strategy for the
treatment of atherosclerosis. Previous studies have indicated that
polydatin may protect cardiac function from acute injury by
reducing oxidative stress and inhibiting sarcoplasmic reticulum
Ca2+ leakage (9),
inhibiting platelet aggregation and intercellular cell adhesion
molecule-1 (ICAM-1) expression, and weakening white blood
cell-endothelial cell adhesion (10). However, the effect of polydatin on
the oxidative stress-induced proliferation of VSMCs remains
unclear.
Silent information regulator 1 (SIRT1) is a key
factor that regulates cell responses to oxidative stress by
deacetylating non-histone targets (11). Inhibiting SIRT1 expression has
been demonstrated to accelerate cell senescence and promote cell
injury (12,13). Knockdown of SIRT1 by small
interfering RNA in HUVECs resulted in impaired antioxidant ability
(14). A previous study from this
laboratory revealed that the expression of SIRT1 was reduced in
ageing (15). The improvement of
endothelial function in the thoracic artery of ageing rats by
atorvastatin was associated with the increased expression of SIRT1
and endothelial nitric oxide synthase (eNOS) (15).
In the present study, we demonstrated that polydatin
inhibited the oxidative stress-induced proliferation of VSMCs. We
then examined the potential role of the eNOS/SIRT1 pathway with
EX527, a SIRT1 inhibitor, and L-NAME, an eNOS inhibitor.
Materials and methods
Cell culture
VSMCs were isolated from the thoracic aorta of
12-week-old male Sprague-Dawley rats (Experimental Animal Center,
Southern Medical University, Guangzhou, China) as previously
described (16). The present
study was performed in strict accordance with the Guide for the
Care and Use of Laboratory Animals (15). Experimental procedures were
approved by the Animal Research Committee of Nanfang Hospital. The
purity of the isolated VSMCs was verified using immunohistochemical
staining of smooth muscle α-actin. The cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY,
USA) containing 10% fetal bovine serum (FBS; PAA Laboratories GmbH,
Australia), in a humidified incubator containing 5% CO2
at 37°C. VSMCs at 4–6 passages, and at 40% confluence, were used
for the experiments.
Treatment
In the preliminary dose-finding experiments, VSMCs
were exposed to the vehicle (0.1% DMSO) and 100 µM
H2O2 (Sigma-Aldrich, St. Louis, MO, USA)
(14) in the absence or presence
of polydatin (purity ≥98%, HPLC) (10, 50 or 100 µM; Baoji
Herbest Bio-Tech Co., Ltd., Baoji, China) for 24 h prior to
performing a cell proliferation assay using a CCK-8 assay kit
(Beyotime Institute of Biotechnology, Nantong, China), as
previously described (17).
Absorbance was measured with an enzyme-linked microplate assay
reader (Thermo Fisher Scientific, Waltham, MA, USA) at 450 nm. In
the subsequent set of experiments, the cells were treated with 100
µM eNOS inhibitor L-NAME (18) or 10 µM SIRT1 inhibitor
EX527 (both from Sigma-Aldrich) (13) for 2 h prior to treatment with
H2O2 and polydatin (100 µM).
Measurement of reactive oxygen species
(ROS), superoxide dismutase (SOD) and nitric oxide (NO) levels
Re-suspended cells were incubated in pre-warmed DMEM
containing 2,7-dichlorofluorescein diacetate (H2DCFDA;
Gibco) fluorescent probe (5 µM) at 37°C for 30 min. The
cells were washed with PBS twice. Intracellular ROS was examined
using a spectrofluorometer (Agilent Technologies, Inc., Palo Alto,
CA, USA) at an excitation wavelength of 485 nm and an emission
wavelength of 530 nm, as previously described (19). A thiobarbituric acid method was
used to detect SOD with WST-1 assay kit (Jiancheng Bioengineering
Institute, Nanjing, China) (20).
NO in the supernatant was examined using a Total Nitric Oxide Assay
kit (Jiancheng Bioengineering Institute), as previously described
(21). Since NO is rapidly
converted to nitrite (NO2−) and further to
nitrate (NO3−), NO content was reflected by
nitrite plus nitrate, as measured with Griess reagent.
Western blot analysis
Total protein was measured using a bicinchoninic
acid (BCA) method. Samples containing 50 µg protein were
separated by 10% SDS-PAGE and transferred to PVDF membranes
(Millipore, Bedford, MA, USA). The membranes were blocked with 5%
non-fat milk in Tris-buffered saline containing 0.1% Tween-20 at
room temperature for 1.5 h, and then incubated overnight at 4°C
with anti-Kip1/p27 antibody (1:500), anti-cyclin B1 antibody
(1:500), anti-cyclin dependent kinase (Cdk)1 antibody (1:500),
anti-c-myc antibody (1:1,000), phosphorylated (p-) (Ser1177)-eNOS
antibody (1:250), anti-eNOS antibody (1:250) or anti-SIRT antibody
(1:500). Polyclonal primary antibodies against SIRT1,
p-(Ser1177)-eNOS and eNOS were purchased from Cell Signaling
Technology, Inc. (Boston, MA, USA) and Kip1/p27, cyclin B1, Cdk1
and c-myc were purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). Following incubation with a horseradish peroxidase
(HRP)-conjugated secondary antibody (Bio-Rad, Hercules, CA, USA)
for 1 h, the blots were visualized using an enhanced
chemiluminescence (ECL) method. Bands of interest were quantified
using Image J software (National Institutes of Health, Bethesda,
MD, USA). β-actin (1:1,000; ZSGB-BIO, Beijing, China) was used as
the loading control.
Cell cycle analysis
The cells were fixed with ice-cold 70% ethanol for 4
h, washed with PBS twice and stained with propidium iodide (PI). A
minimum of 1×103 cells/sample were counted. Cells in
distinct cell cycles were characterized by DNA amount (G0/G1 cells,
diploid; S cells, DNA synthesis between diploid and tetraploid; and
G2/M cells, tetraploid) using flow cytometry (FCM; Beckman Coulter,
Inc., Brea, CA, USA), as previously reported (22).
Reverse transcription
quantitative-polymerase chain reaction (RT-qPCR)
Total RNA was extracted with RNAiso Plus (Takara
Bio, Dalian, China) using a phenol-chloroform method. Reverse
transcription was carried out in 20 µl reaction mixture
containing 1 µg total RNA. The primers were designed using
GenBank sequences and Primer-BLAST (Table I). The resulting cDNA was
amplified using a SYBR-Green PCR method. Initial denaturation was
performed at 94°C for 5 min, followed by 40 cycles of denaturation
at 94°C for 30 sec, annealing at 60°C for 30 sec and extension at
72°C for 45 sec. SIRT1 and eNOS mRNA were calculated using a
comparative threshold cycle (Ct) method (ΔΔCt method) with β-actin
as a reference.
 | Table IPrimers used for RT-qPCR. |
Table I
Primers used for RT-qPCR.
| Target mRNA | Forward primer (5′
to 3′) | Reverse primer (5′
to 3′) |
|---|
| SIRT1 |
ACAACCTCCTGTTGGCTGATGAGA |
AGAATTGTTCGAGGATCGGTGCCA |
| eNOS |
GGATCCAGTGGGGGAAACTG |
TGGCTGAACGAAGATTGCCT |
| β-actin |
CCCATCTATGAGGGTTACGC |
TTTAATGTCACGCACGATTTC |
Statistical methods
Data were presented as the means ± standard
deviation (SD) for at least three sets of independent experiments
and analyzed using one-way analysis of variance (ANOVA), followed
by post-hoc analysis for pairwise comparison: LSD method upon
homogeneity of variance, Dunnett's t-test. P<0.05 (two-sided
test) was considered to indicate a statistically significant
difference. Statistical analyses were performed using SPSS 13.0
software (SPSS Inc., Chicago, IL, USA).
Results
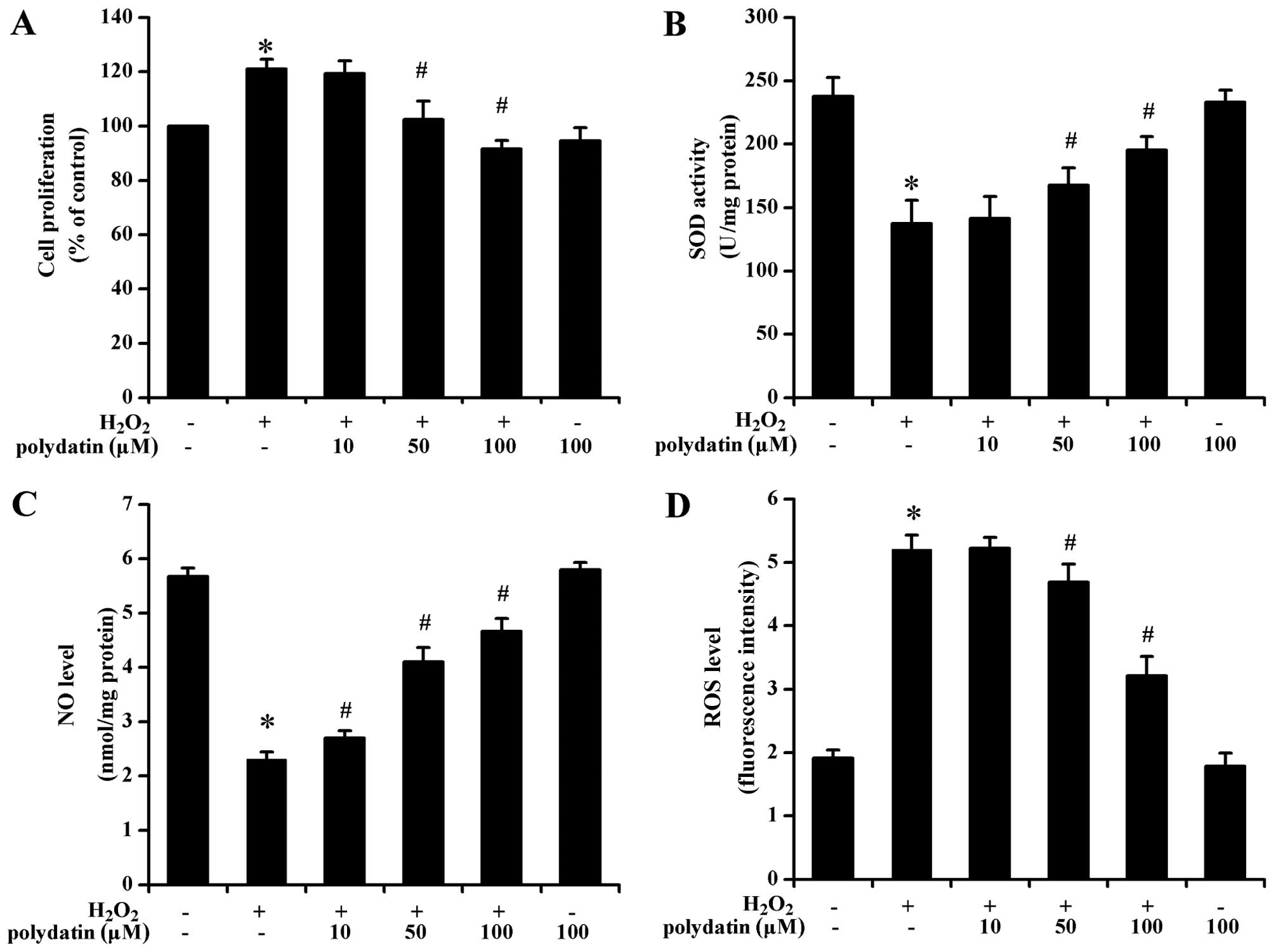
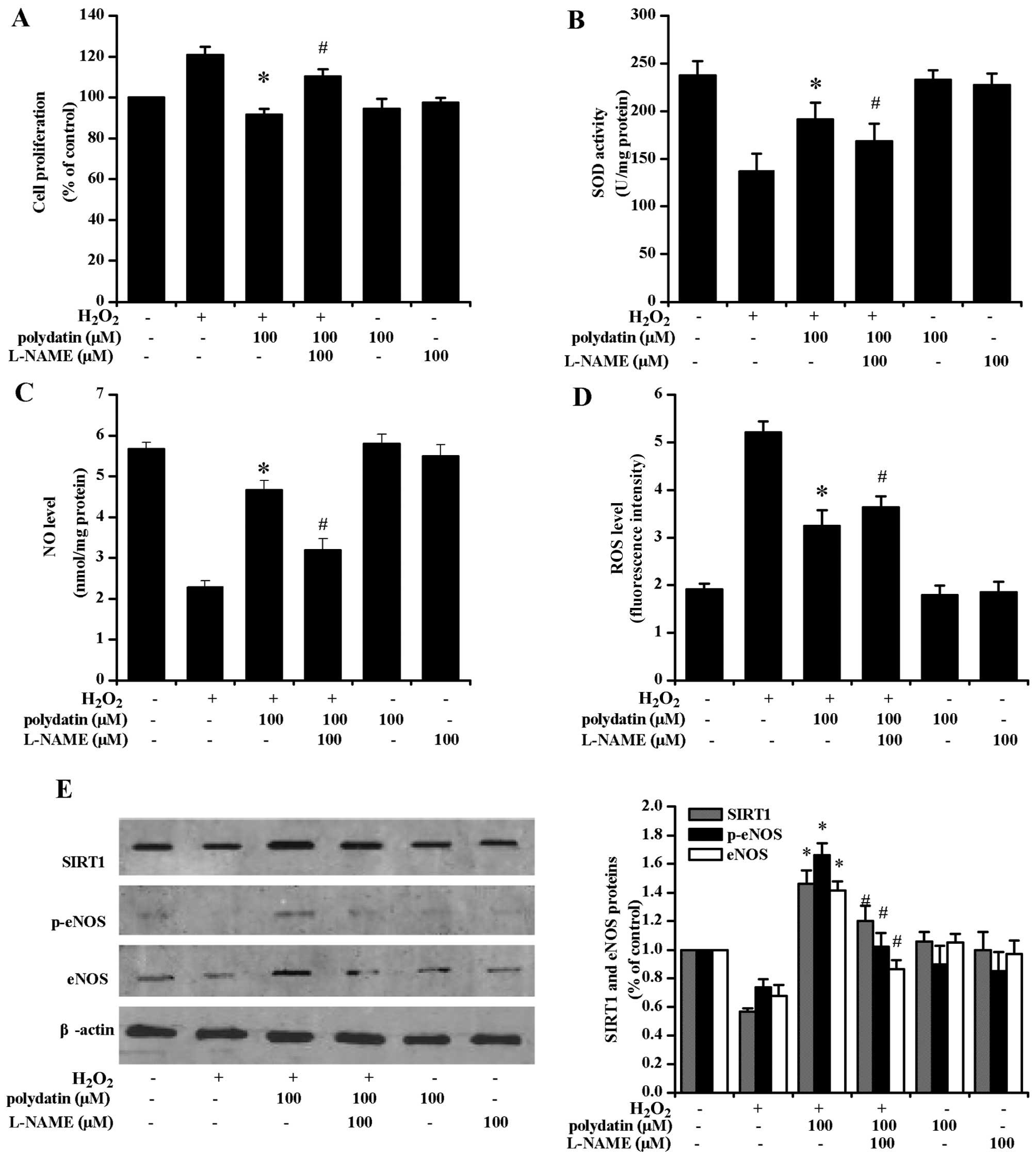
Effect of polydatin on proliferation
Polydatin attenuated
H2O2-induced VSMC proliferation in a
concentration-dependent manner (P<0.05 at 50 and 100 µM
vs. H2O2-treated group alone; Fig. 1A). Polydatin concentrations of 50
and 100 µM were selected for subsequent experiments.
Effects of polydatin on ROS, SOD, NO,
eNOS and SIRT1
At 50 and 100 µM, polydatin significantly
decreased the level of ROS, and increased the activity of SOD and
the level of NO (P<0.05 vs. H2O2-treated
group alone; Fig. 1B–D).
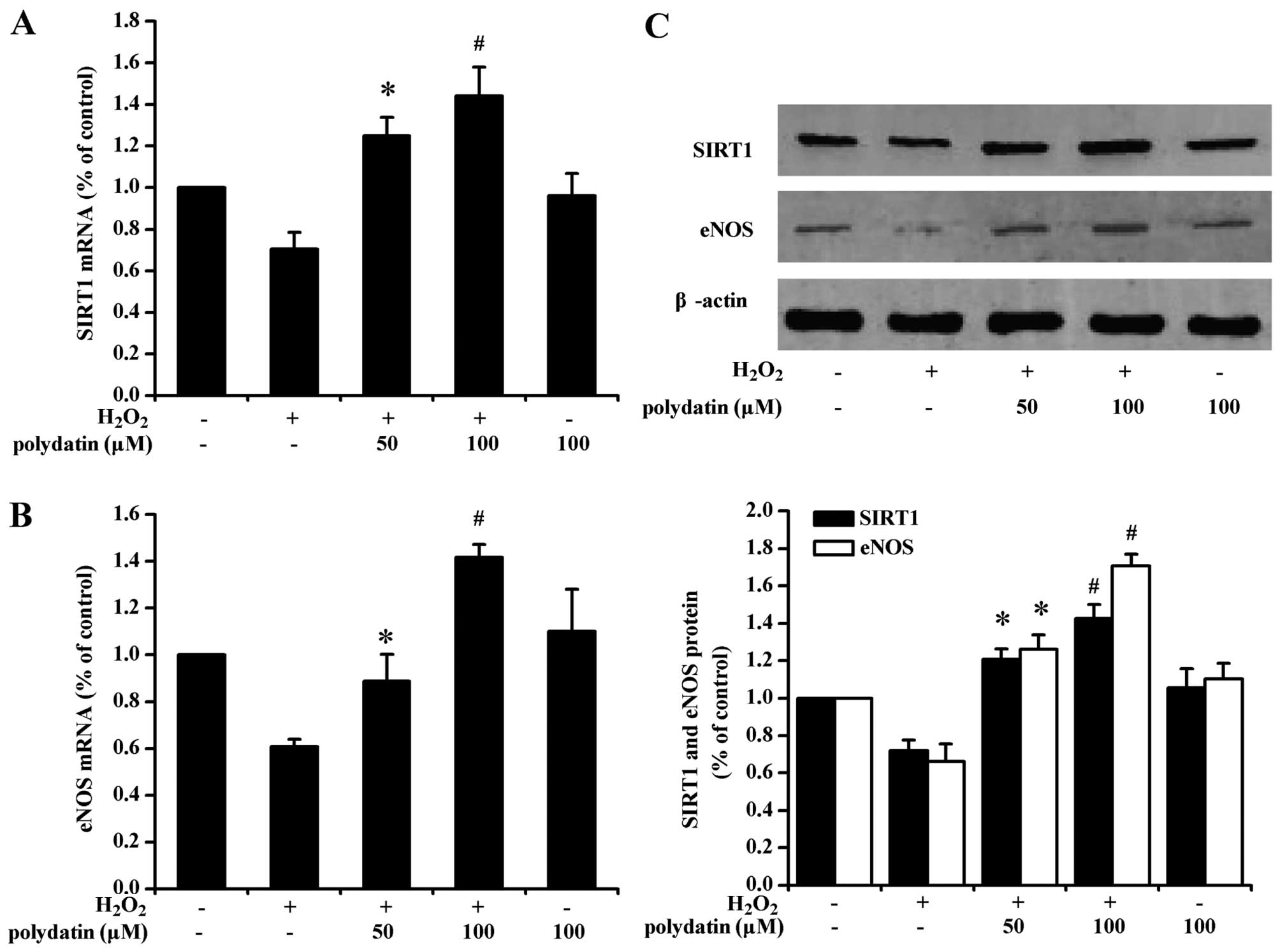
Polydatin (100 µM) increased the mRNA level of SIRT1 and
eNOS in cells exposed to H2O2 (P<0.05 vs.
50 µM polydatin-treated group; Fig. 2A and B). The results obtained with
western blot analysis were generally consistent with the mRNA
findings (Fig. 2C).
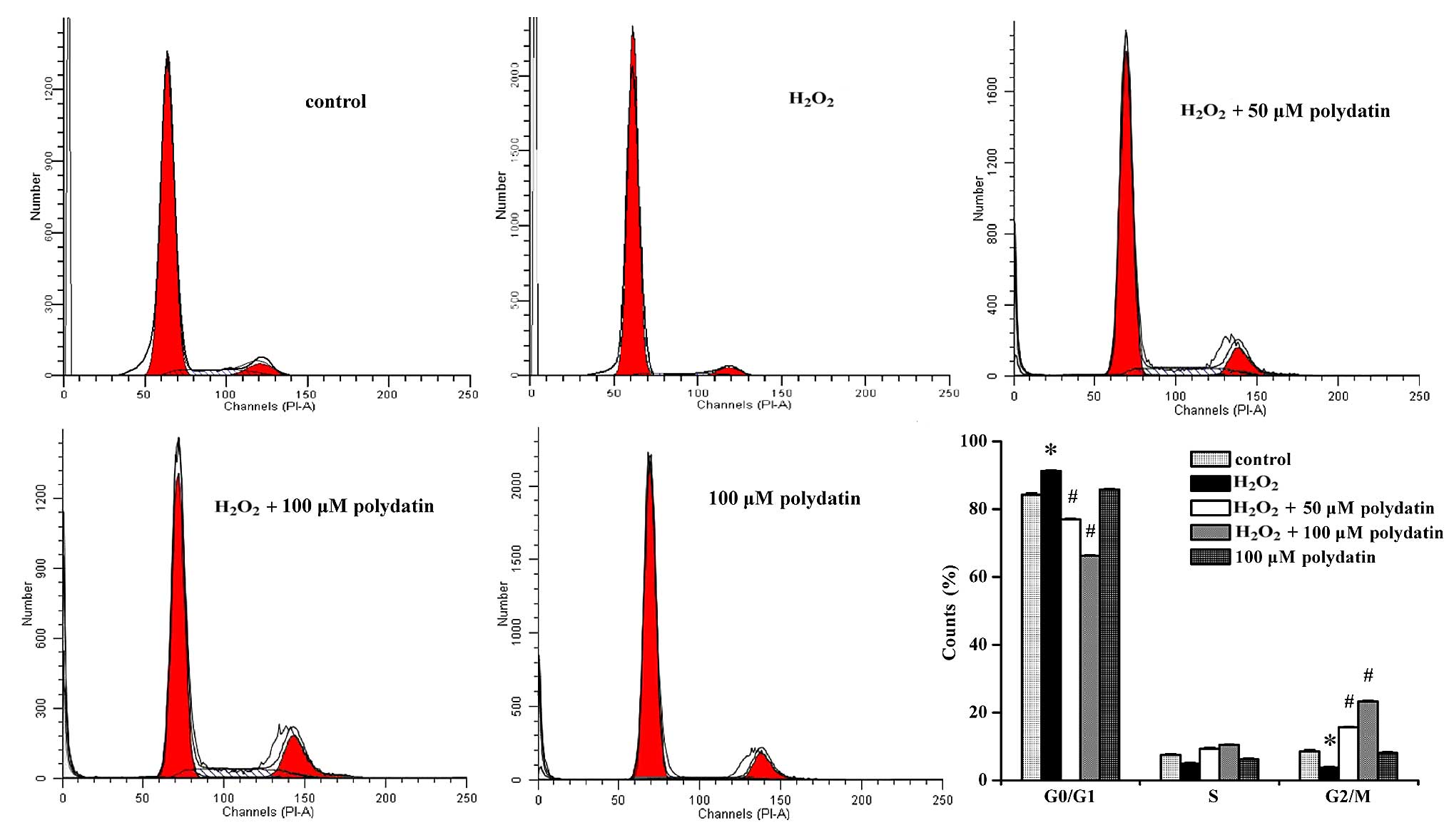
Effects of polydatin on the cell
cycle
Treatment with polydatin increased the number of
cells in the G2/M phase (from 3.78±0.26 to 15.62±0.22% and
23.31±0.34% at 50 and 100 µM, respectively) and decreased
the number of cells in the G0/G1 phase (from 92.15±0.19 to
77.02±0.31% and 66.30±0.18% at 50 and 100 µM, respectively)
(P<0.05 at 50 and 100 µM vs.
H2O2-treated group alone; Fig. 3).
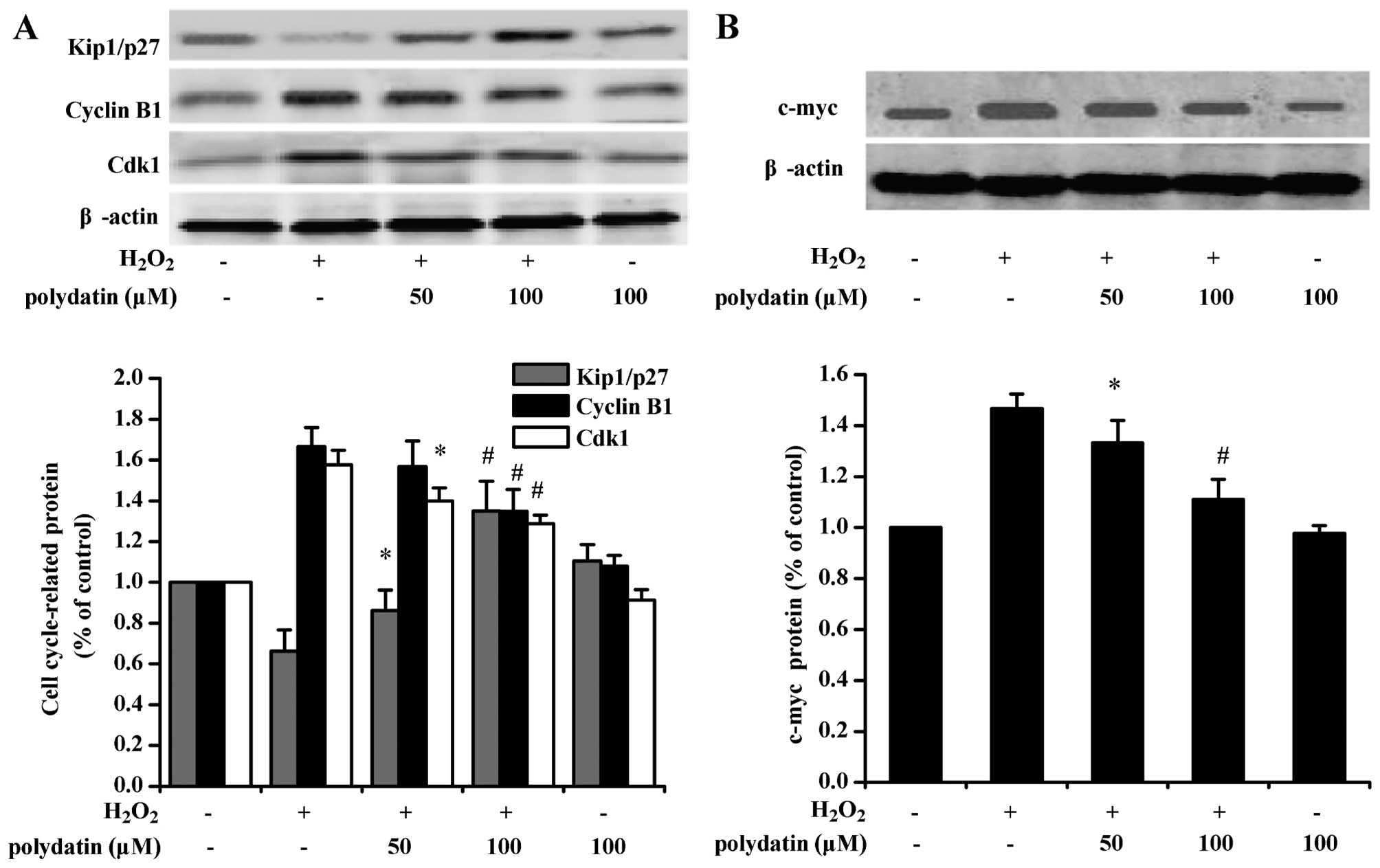
Effects of polydatin on the cell cycle
proteins
H2O2 treatment alone increased
cyclin B1 and Cdk1 and decreased Kip1/p27 (P<0.05 vs. the
control group; Fig. 4A).
Polydatin decreased cyclin B1 and Cdk1 expression and increased
Kip1/p27 expression in cells exposed to H2O2
(P<0.05 vs. the H2O2-treated group alone).
H2O2 increased the protein level of c-myc.
Polydatin inhibited such a response (P<0.05 vs. the
H2O2-treated group alone; Fig. 4B).
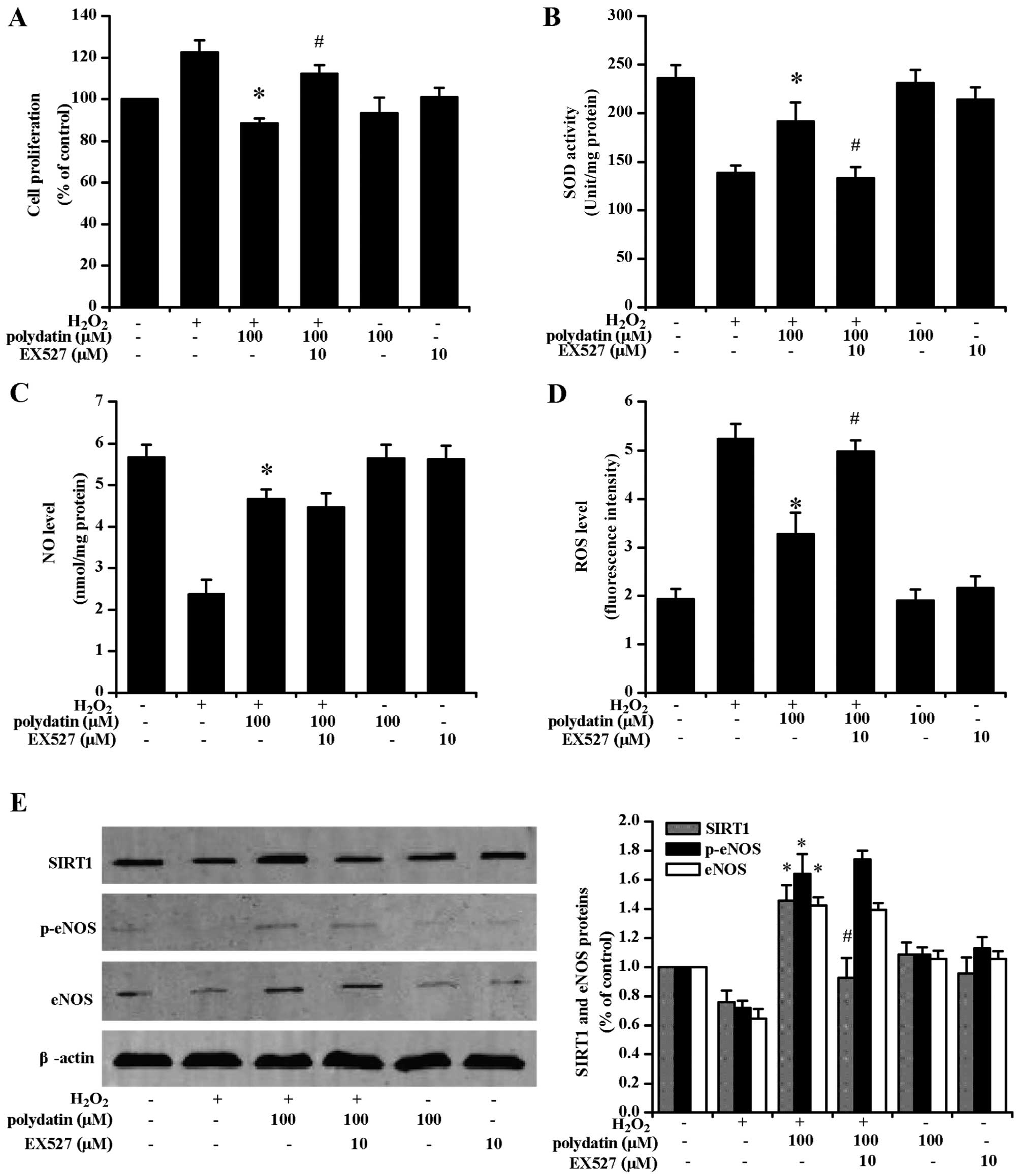
Effects of L-NAME and EX527
pre-treatment
The CCK-8 assay revealed that polydatin inhibited
VSMC proliferation (Fig. 5A).
L-NAME pre-treatment reversed the effects of polydatin on SOD
activity (Fig. 5B) and ROS levels
(Fig. 5D). Additionally, L-NAME
pre-treatment decreased NO levels (Fig. 5C). Polydatin (100 µM)
increased the phosphorylation of eNOS protein (p-eNOS) at Ser1177
of its catalytic subunit, and L-NAME treatment attenuated such a
response (P<0.05; Fig.
5E).
EX527 pre-treatment attenuated the
anti-proliferative effects of polydatin (Fig. 6A). EX527 pre-treatment also
decreased SOD activity (Fig. 6B)
and increased ROS levels (Fig.
6D). EX527 pre-treatment significantly decreased SIRT1
expression at the protein level (P<0.05). There was no decrease
in NO levels (Fig. 6C), and eNOS
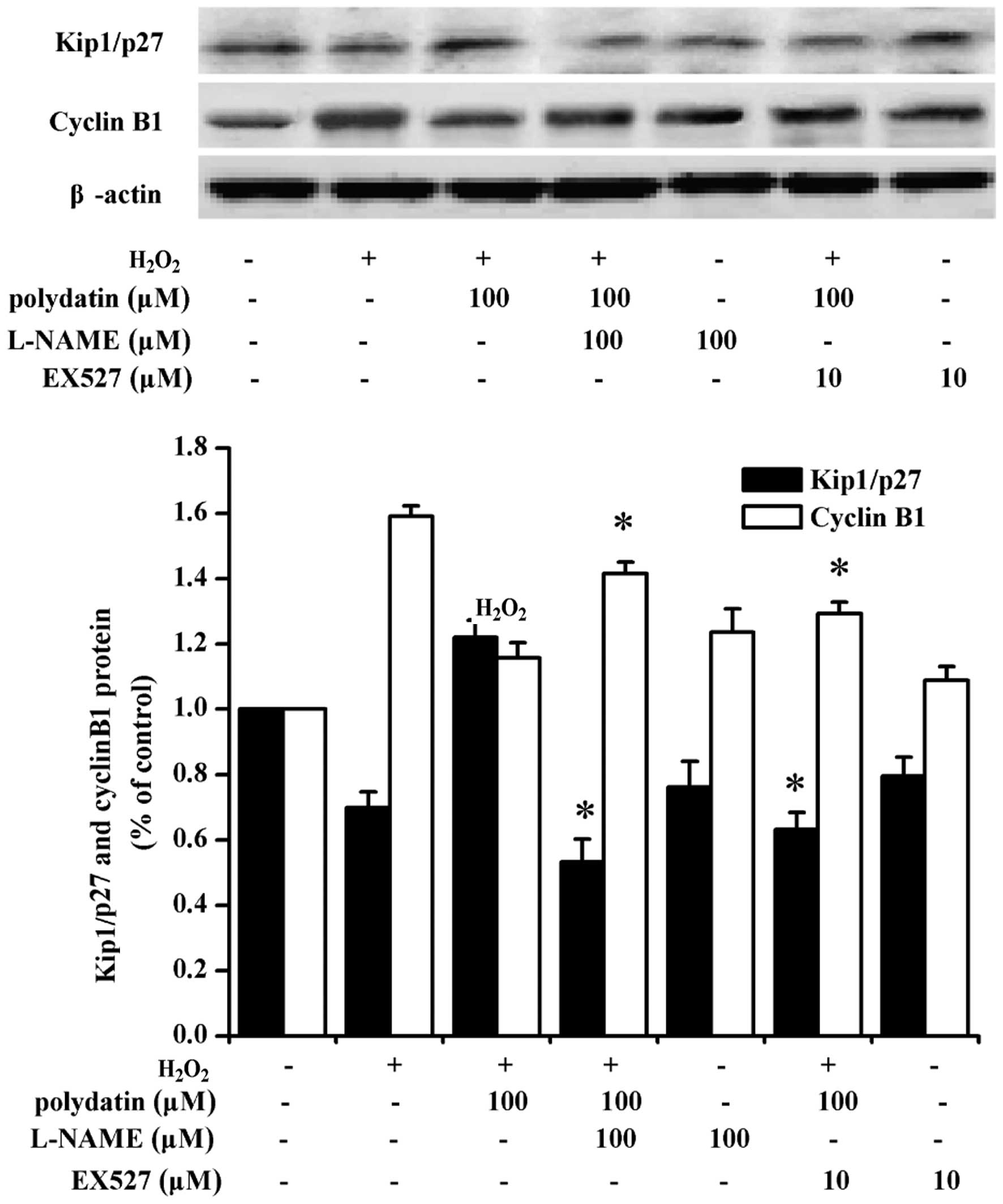
and p-eNOS expression at the protein level (Fig. 6E). L-NAME and EX527 pre-treatment
decreased the expression of Kip1/p27 at the protein level, and
increased cyclin B1 (Fig. 7).
Discussion
The results from the present study have shown that
polydatin, an analog of resveratrol, attenuated the proliferation
of VSMCs under oxidative stress. Such an effect may be attributed
to G2/M-phase arrest, an increased antioxidant capacity, and
activation of the eNOS-SIRT1 signaling pathway in the VSMCs.
Resveratrol has been previously reported to have
anti-proliferative (23),
antioxidant (24) and anti-aging
(25,26) properties in vitro and in
vivo. Polydatin, also known as piceid, is a derivative of
resveratrol. Polydatin and resveratrol have been demonstrated to
produce their effects via the stilbene synthesis pathway (27). Previous studies have indicated
that stilbene derivatives with more phenolic moieties and an
increased number of OH groups on the phenol ring have improved
biological effects compared with resveratrol (28), including lower toxicity, better
anticancer activities and sirtuin activation (29). Hydroxystilbene analogues with more
hydroxyl groups on the phenol rings of the stilbene structure tend
to have higher anti-radical activity than resveratrol (29). Polydatin has more potent
anti-proliferative effects on intestinal epithelial cells than
resveratrol (30), possibly due
to an increased number of hydroxyl groups on the
ring-structure.
Cell proliferation is accompanied by the activation
of cell cycle proteins (31). The
cell cycle is controlled by many factors, including cyclins/CDK
complexes and CDK inhibitors (CDKI) (32). High activity of the cyclin B1/CDK
complex allows the progression of cells through the G2/M phase, and
thus, is critical for mitosis (33). Previous findings have shown that
resveratrol inhibited VSMC proliferation by inducing cell cycle
arrest and increasing DNA synthesis (22). The results from the present study
indicated that the promotion of VSMC proliferation by oxidative
stress was accompanied by an increased expression of cyclin B1 and
Cdk1, and a decreased expression of Kip1/p27 (Fig. 4). A previous study indicated that
the downregulation of CDKI and Kip1/p27, interfered with G2/M
arrest in response to DNA injury stress, thereby increasing genetic
instability (34). Consistent
with those findings, our data indicated that polydatin inhibited
oxidative stress-induced VSMC proliferation, in part by arresting
the cell cycle at the G2/M phase (Fig. 3). This finding is associated with
the upregulation of CDKI and Kip1/p27, and the downregulation of
cyclin B1 and Cdk1. Pre-treatment with the eNOS inhibitor, L-NAME,
or the SIRT1 inhibitor, EX527, attenuated Kip1/p27 expression, and
increased cyclin B1 levels (Fig.
7). These results indicated that the effect of polydatin on
VSMC proliferation is mediated by eNOS and SIRT1.
The c-myc is an important oncogene that regulates
cell growth. Activation of c-myc promotes the proliferation of many
cells, including VSMCs (35). In
our study, c-myc expression was significantly increased by
H2O2 and this response was inhibited by
polydatin.
Resveratrol is a trihydroxy stilbene that is
effective against oxidative stress and in the treatment of
cardiovascular diseases (36).
Numerous stilbene analogues have been developed based on the
structure of resveratrol. A previous study indicated that polydatin
and resveratrol possess scavenging activity against hydroxyl
radicals in vitro (36).
To evaluate the effect of polydatin on
H2O2-treated VSMCs, we assessed NO, SOD, and
ROS levels. In our experiments, the levels of NO and SOD were
significantly increased following treatment with 50 and 100
µM polydatin, with a concomitant decrease in ROS levels. The
results suggested that polydatin plays an anti-oxidant role by
increasing the cellular oxidative tolerance to extracellular
oxidative environmental change, as well as by eliminating ROS
caused by oxidative stress in VSMCs.
eNOS regulates VSMC proliferation (37). Several protein kinases, including
Akt/PI3K and AMPK, activated eNOS by phosphorylating Ser1177 in
response to various stimuli (38,39). A previous study showed that
resveratrol increased eNOS mRNA and protein expression levels and
promoted NO production in endothelial cells (40). Resveratrol has been shown to
inhibit rat aortic VSMC proliferation through estrogen
receptor-dependent NO production (41). The present study demonstrated that
the inhibition of eNOS by H2O2 was
accompanied by a decreasing NO expression level. Although eNOS/NO
expression by polydatin was not equivalent to the restoration of
VSMC function in the normal control group in the present study.
Thus, polydatin may attenuate VSMC prolife ration. In our
experiments, L-NAME, a reversible eNOS inhibitor, downregulated the
expression of eNOS, SIRT1 and Kip1/p27, with the concomitant
upregulation of cyclin B1 protein expression. We hypothesized that
L-NAME reversed the inhibitory effects of polydatin on cell
proliferation by inhibiting eNOS expression.
SIRT1 regulates many pathways for nutrient
bioavailability, cellular energy status and various receptor
signaling pathways. SIRT1 has been proven to inhibit oxidative
stress (42), interact with eNOS
to improve vascular function and retard endothelial senescence
(43). Resveratrol induced VSMC
differentiation by stimulating SIRT1 and AMPK (44). SIRT1 was regulated by NO when
shuttled between the nucleus and cytosol, upregulated ROS
scavengers, Mn-SOD and catalase, and catabolized toxic
H2O2, which in turn was detoxified to water
(45). SIRT1 is a crucial
regulator of radical scavengers and transcriptional activation, and
inhibits inflammatory expression via induction of eNOS (46,47). Thus, the SIRT1-eNOS/NO signaling
pathway may be regarded as a potential target against
atherosclerosis (48). In the
present study, the expression of SIRT1 protein in VSMCs was
significantly induced by polydatin. The peak effect on SIRT1
expression at 100 µM polydatin positively correlated with
eNOS expression, suggesting that SIRT1 maybe involved in NO
synthesis.
EX527, a specific inhibitor of SIRT1, did not affect
the expression of eNOS in VSMCs. We hypothesize that SIRT1 may act
as antioxidant regulator by activating upstream eNOS/NO in VSMCs.
Taken together with the findings regarding cell cycle-related
proteins, results of the present study suggest that SIRT1 functions
as a vital regulator of the anti-proliferative effect of polydatin
in VSMCs.
There are some limitations to our study. Firstly, it
remains undetermined whether polydatin and resveratrol are capable
of enhancing the activation of the eNOS/SIRT1 pathway in VSMCs.
Secondly, the H2O2 model does not faithfully
represent the in vivo oxidative damage microenvironment.
Thirdly, the present study did not include the AMPK/eNOS pathway,
which is apparently important for cell proliferation.
In conclusion, our study has demonstrated that
polydatin improves oxidative stress-related changes by increasing
SOD levels, ameliorating the level of ROS, inhibiting cell
proliferation, upregulating eNOS/NO-SIRT1 expression during
H2O2-induced oxidative damage in VSMCs. The
present findings may contribute to the development of strategies
for the prevention and treatment of oxidative lesions in the
vasculature by targeting the eNOS/NO-SIRT1 pathway.
Acknowledgments
This study was supported by grants from the Science
and Technology Item of Guangdong Province (2010B031500013) and the
National Basic Research Program of China (2007CB507404).
References
|
1
|
Das S and Das DK: Resveratrol: a
therapeutic promise for cardiovascular diseases. Recent Patents
Cardiovasc Drug Discov. 2:133–138. 2007. View Article : Google Scholar
|
|
2
|
Chen H, Tuck T, Ji X, Zhou X, Kelly G,
Cuerrier A and Zhang J: Quality assessment of Japanese knotweed
(Fallopia japonica) grown on Prince Edward Island as a source of
resveratrol. J Agric Food Chem. 61:6383–6392. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Du QH, Peng C and Zhang H: Polydatin: a
review of pharmacology and pharmacokinetics. Pharm Biol.
51:1347–1354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen L, Lan Z, Lin Q, Mi X, He Y, Wei L,
Lin Y, Zhang Y and Deng X: Polydatin ameliorates renal injury by
attenuating oxidative stress-related inflammatory responses in
fructose-induced urate nephropathic mice. Food Chem Toxicol.
52:28–35. 2013. View Article : Google Scholar
|
|
5
|
Wen H, Gao X and Qin J: Probing the
anti-aging role of polydatin in Caenorhabditis elegans on a chip.
Integr Biol (Camb). 6:35–43. 2014. View Article : Google Scholar
|
|
6
|
Blanc A, Pandey NR and Srivastava AK:
Synchronous activation of ERK 1/2, p38mapk and PKB/Akt signaling by
H2O2 vascular smooth muscle cells: potential
involvement in vascular 2 2 in disease (Review). Int J Mol Med.
11:229–234. 2003.PubMed/NCBI
|
|
7
|
Jiang D, Li D and Wu W: Inhibitory effects
and mechanisms of luteolin on proliferation and migration of
vascular smooth muscle cells. Nutrients. 5:1648–1659. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lan TH, Huang XQ and Tan HM: Vascular
fibrosis in atherosclerosis. Cardiovasc Pathol. 22:401–407. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang X, Liu W, Deng J, Lan L, Xue X,
Zhang C, Cai G, Luo X and Liu J: Polydatin protects cardiac
function against burn injury by inhibiting sarcoplasmic reticulum
Ca2+ leak by reducing oxidative modification of
ryanodine receptors. Free Radic Biol Med. 60:292–299. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao KS, Jin C, Huang X, Liu J, Yan WS,
Huang Q and Kan W: The mechanism of polydatin in shock treatment.
Clin Hemorheol Microcirc. 29:211–217. 2003.
|
|
11
|
Salminen A, Kaarniranta K and Kauppinen A:
Crosstalk between oxidative stress and SIRT1: Impact on the aging
process. Int J Mol Sci. 14:3834–3859. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang H, Zhai Z, Wang Y, Zhang J, Wu H,
Wang Y, Li C, Li D, Lu L, Wang X, et al: Resveratrol ameliorates
ionizing irradiation-induced long-term hematopoietic stem cell
injury in mice. Free Radic Biol Med. 54:40–50. 2013. View Article : Google Scholar
|
|
13
|
Zarzuelo MJ, López-Sepúlveda R, Sánchez M,
Romero M, Gómez-Guzmán M, Ungvary Z, Pérez-Vizcaíno F, Jiménez R
and Duarte J: SIRT1 inhibits NADPH oxidase activation and protects
endothelial function in the rat aorta: implications for vascular
aging. Biochem Pharmacol. 85:1288–1296. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kao CL, Chen LK, Chang YL, Yung MC, Hsu
CC, Chen YC, Lo WL, Chen SJ, Ku HH and Hwang SJ: Resveratrol
protects human endothelium from H2O2-induced
oxidative stress and senescence via SirT1 activation. J Atheroscler
Thromb. 17:970–979. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gong X, Ma Y, Ruan Y, Fu G and Wu S:
Long-term atorvastatin improves age-related endothelial dysfunction
by ameliorating oxidative stress and normalizing eNOS/iNOS
imbalance in rat aorta. Exp Gerontol. 52:9–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Salabei JK, Cummins TD, Singh M, Jones SP,
Bhatnagar A and Hill BG: PDGF-mediated autophagy regulates vascular
smooth muscle cell phenotype and resistance to oxidative stress.
Biochem J. 451:375–388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ni L, Li T, Liu B, Song X, Yang G, Wang L,
Miao S and Liu C: The protective effect of Bcl-xl overexpression
against oxidative stress-induced vascular endothelial cell injury
and the role of the Akt/eNOS pathway. Int J Mol Sci.
14:22149–22162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
San Martin A, Foncea R, Laurindo FR,
Ebensperger R, Griendling KK and Leighton F: Nox1-based NADPH
oxidase- derived superoxide is required for VSMC activation by
advanced glycation end-products. Free Radic Biol Med. 42:1671–1679.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Botden IP, Oeseburg H, Durik M, Leijten
FP, Van Vark-Van Der, Zee LC, Musterd-Bhaggoe UM, Garrelds IM,
Seynhaeve AL, Langendonk JG, Sijbrands EJ, et al: Red wine extract
protects against oxidative-stress-induced endothelial senescence.
Clin Sci (Lond). 123:499–507. 2012. View Article : Google Scholar
|
|
20
|
Wang Q, Zhou H, Gao H, Chen SH, Chu CH,
Wilson B and Hong JS: Naloxone inhibits immune cell function by
suppressing superoxide production through a direct interaction with
gp91phox subunit of NADPH oxidase. J Neuroinflammation.
9:322012. View Article : Google Scholar
|
|
21
|
Miranda KM, Espey MG and Wink DA: A rapid,
simple spectrophotometric method for simultaneous detection of
nitrate and nitrite. Nitric Oxide. 5:62–71. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi Y, Hou X, Zhang X, Wang Y, Chen Y and
Zou J: Inhibition of oxidized-phospholipid-induced vascular smooth
muscle cell proliferation by resveratrol is associated with
reducing Cx43 phosphorylation. J Agric Food Chem. 61:10534–10541.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Prasad K: Resveratrol, wine, and
atherosclerosis. Int J Angiol. 21:7–18. 2012. View Article : Google Scholar :
|
|
24
|
Gutiérrez-Pérez A, Cortés-Rojo C,
Noriega-Cisneros R, Calderón-Cortés E, Manzo-Avalos S,
Clemente-Guerrero M, Godínez-Hernández D, Boldogh I and
Saavedra-Molina A: Protective effects of resveratrol on
calcium-induced oxidative stress in rat heart mitochondria. J
Bioenerg Biomembr. 43:101–107. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han X, Ling S, Gan W, Sun L, Duan J and Xu
JW: 2,3,5,4′-tetra-hydroxystilbene-2-O-β-d-glucoside ameliorates
vascular senescence and improves blood flow involving a mechanism
of p53 deacetylation. Atherosclerosis. 225:76–82. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park SJ, Ahmad F, Philp A, Baar K,
Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, et al:
Resveratrol ameliorates aging-related metabolic phenotypes by
inhibiting cAMP phosphodiesterases. Cell. 148:421–433. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Robb EL and Stuart JA: The stilbenes
resveratrol, pterostilbene and piceid affect growth and stress
resistance in mammalian cells via a mechanism requiring estrogen
receptor beta and the induction of Mn-superoxide dismutase.
Phytochemistry. 98:164–173. 2014. View Article : Google Scholar
|
|
28
|
Yang H, Baur JA, Chen A, Miller C, Adams
JK, Kisielewski A, Howitz KT, Zipkin RE and Sinclair DA: Design and
synthesis of compounds that extend yeast replicative lifespan.
Aging Cell. 6:35–43. 2007. View Article : Google Scholar
|
|
29
|
Szekeres T, Fritzer-Szekeres M, Saiko P
and Jäger W: Resveratrol and resveratrol analogues -
structure-activity relationship. Pharm Res. 27:1042–1048. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Storniolo CE, Quifer-Rada P,
Lamuela-Raventos RM and Moreno JJ: Piceid presents
antiproliferative effects in intestinal epithelial Caco-2 cells,
effects unrelated to resveratrol release. Food Funct. 5:2137–2144.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bicknell KA, Surry EL and Brooks G:
Targeting the cell cycle machinery for the treatment of
cardiovascular disease. J Pharm Pharmacol. 55:571–591. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bonelli P, Tuccillo FM, Borrelli A,
Schiattarella A and Buonaguro FM: CDK/CCN and CDKI alterations for
cancer pro gnosis and therapeutic predictivity. Biomed Res Int.
2014:3610202014. View Article : Google Scholar
|
|
33
|
Wang Z, Fan M, Candas D, Zhang TQ, Qin L,
Eldridge A, Wachsmann-Hogiu S, Ahmed KM, Chromy BA, Nantajit D, et
al: Cyclin B1/Cdk1 coordinates mitochondrial respiration for
cell-cycle G2/M progression. Dev Cell. 29:217–232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Payne SR, Zhang S, Tsuchiya K, Moser R,
Gurley KE, Longton G, deBoer J and Kemp CJ: p27kip1
deficiency impairs G2/M arrest in response to DNA damage, leading
to an increase in genetic instability. Mol Cell Biol. 28:258–268.
2008. View Article : Google Scholar :
|
|
35
|
Wang J, Liu K, Shen L, Wu H and Jing H:
Small interfering RNA to c-myc inhibits vein graft restenosis in a
rat vein graft model. J Surg Res. 169:e85–e91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Su D, Cheng Y, Liu M, Liu D, Cui H, Zhang
B, Zhou S, Yang T and Mei Q: Comparision of piceid and resveratrol
in anti-oxidation and antiproliferation activities in vitro. PLoS
One. 8:e545052013. View Article : Google Scholar
|
|
37
|
Huang J, Li LS, Yang DL, Gong QH, Deng J
and Huang XN: Inhibitory effect of ginsenoside Rg1 on vascular
smooth muscle cell proliferation induced by PDGF-BB is involved in
nitric oxide formation. Evid Based Complement Alternat Med.
2012:3143952012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gonon AT, Widegren U, Bulhak A, Salehzadeh
F, Persson J, Sjöquist PO and Pernow J: Adiponectin protects
against myocardial ischaemia-reperfusion injury via AMP-activated
protein kinase, Akt, and nitric oxide. Cardiovasc Res. 78:116–122.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Trott DW, Luttrell MJ, Seawright JW and
Woodman CR: Aging impairs PI3K/Akt signaling and NO-mediated
dilation in soleus muscle feed arteries. Eur J Appl Physiol.
113:2039–2046. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Takahashi S and Nakashima Y: Repeated and
long-term treatment with physiological concentrations of
resveratrol promotes NO production in vascular endothelial cells.
Br J Nutr. 107:774–780. 2012. View Article : Google Scholar
|
|
41
|
Ekshyyan VP, Hebert VY, Khandelwal A and
Dugas TR: Resveratrol inhibits rat aortic vascular smooth muscle
cell proliferation via estrogen receptor dependent nitric oxide
production. J Cardiovasc Pharmacol. 50:83–93. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hori YS, Kuno A, Hosoda R and Horio Y:
Regulation of FOXOs and p53 by SIRT1 modulators under oxidative
stress. PLoS One. 8:e738752013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Potente M and Dimmeler S: NO targets
SIRT1: A novel signaling network in endothelial senescence.
Arterioscler Thromb Vasc Biol. 28:1577–1579. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Thompson AM, Martin KA and Rzucidlo EM:
Resveratrol induces vascular smooth muscle cell differentiation
through stimulation of SirT1 and AMPK. PLoS One. 9:e854952014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tanno M, Kuno A, Horio Y and Miura T:
Emerging beneficial roles of sirtuins in heart failure. Basic Res
Cardiol. 107:2732012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Xia N, Strand S, Schlufter F, Siuda D,
Reifenberg G, Kleinert H, Förstermann U and Li H: Role of SIRT1 and
FOXO factors in eNOS transcriptional activation by resveratrol.
Nitric Oxide. 32:29–35. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Takizawa Y, Kosuge Y, Awaji H, Tamura E,
Takai A, Yanai T, Yamamoto R, Kokame K, Miyata T, Nakata R and
Inoue H: Up-regulation of endothelial nitric oxide synthase (eNOS),
silent mating type information regulation 2 homologue 1 (SIRT1) and
autophagy-related genes by repeated treatments with resveratrol in
human umbilical vein endothelial cells. Br J Nutr. 110:2150–2155.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ota H, Eto M, Ogawa S, Iijima K, Akishita
M and Ouchi Y: SIRT1/eNOS axis as a potential target against
vascular senescence, dysfunction and atherosclerosis. J Atheroscler
Thromb. 17:431–435. 2010. View Article : Google Scholar : PubMed/NCBI
|