Introduction
The 293 cells were thought to be derived from
primary human embryonic kidney cells by transformation with sheared
fragments of adenovirus 5 (Ad5) DNA and contain nucleotides 1-4344
of Ad5, comprising the early region 1 (E1) transforming sequences
integrated into chromosome 19 (1,2).
Since the isolation of these cells over 30 years ago, the 293 cell
line has been widely used for the production of E1-deleted Ad
vectors and in a number of transfection studies (3).
Although 293 cells express cytokeratins, which are
epithelial cell markers, a previous study used a combination of
immunostaining, immunoblot analysis and microarray analysis to
demonstrate that 293 cells express neurofilament (NF) subunits,
α-internexin, and several other proteins typically found in
neurons. These findings raised the possibility that the 293 cell
line was derived from human neuronal lineage cells transformed by
adenovirus (4). Although the
presence of keratin proteins is characteristic of the early stage
differentiation of epithelial cells and is unusual in neurons of
the human or the rodent central nervous systems, these proteins are
found in transformed lines of undoubted neuronal origin. For
example, the rat PC12 line, derived from the adrenal gland, and
NTera-2 cells derived from human embryonal carcinoma cells, express
keratins, all three NF triplet protein subunits and vimentin
(5–6). Human tumors, which contain NFs and
keratins, have also been described (8).
Despite extensive analysis of neural cell marker
expression in 293 cells, the expression of epithelial cell markers
other than cytokeratins has not yet been studied, to the best of
our knowledge. Herein, we examined the expression of E-cadherin,
zonula occludens (ZO)-1, occludin and desmoglein 2, which are
proteins involved in the formation and maintenance of cell-cell
junctions (9–20). These proteins are components of
the specialized junctional structure, consisting of the tight
junction (occludin and ZO-1), the adherens junction (E-cadherin),
and the desmosome (desmoglein 2). These structures are found at the
apical area of lateral cell-cell contacts (9). Tight junctions are located in the
most apical contact region, which constitutes the transepithelial
permeability barrier. This unique junction is formed by the
association of claudins and occludin (two transmembrane components
of tight junctions (10,11) with ZO-1 and other cytoskeletal
proteins (12,13). The adherens junction is located
proximal to the tight junction. The main adhesion receptors within
the adherens junctions are the classic cadherins (14), the cytoplasmic domain of which is
associated with β-catenin (15,16). β-catenin, in turn, associates with
α-catenin to generate a three protein complex (17,18). Desmosomes are multi-unit, protein
hetero-complexes that contain desmocollin and desmoglein, two
glycoproteins of the cadherin family (19,20). They are located basal to the
adherens junction and are associated with intermediate filaments.
In simple epithelia, these three junction structures are typically
aligned in the order described above, although desmosomes are also
independently distributed throughout other areas of the cell
membrane (19,20).
In the present study, we found that the 293 cells
expressed N-cadherin, a cell adhesion protein expressed in neural
cells. However, the 293 cells also expressed cytokeratins 5/8, as
well as desmoglein 2, which are epithelial cell markers. Low
expression levels of E-cadherin were detected in the 293 cells
using immunoblot analysis, but not by immunostaining. The cells
primarily cultured from the kidneys of Clawn miniature swine and
passaged 10–15 generations [termed porcine kidney epithelial (PKE)
cells], tested positive for the expression of cytokeratins and
N-cadherin. Thus, transformation by adenovirus was not necessary
for the cells to express N-cadherin. Occludin, an integral
component of tight junctions in epithelial and endothelial cells,
was detected in both the 293 and the PKE cells. Thus, the findings
of the present study demonstrate that 293 cells retain several
characteristics of epithelial cells.
Materials and methods
Cells and transfection
The 293 cells were provided by Dr Tatsuhiko Furukawa
(Department of Molecular Oncology, Kagoshima University, Kagoshima,
Japan). The cells were cultured in Dulbecco's modified Eagle medium
(DMEM) supplemented with 10% fetal calf serum. DLD1, a human
colorectal adenocarcinoma cell line, was provided by Dr Shintaro T.
Suzuki (Kwansei Gakuin University, Kobe, Japan). The PKE cells,
provided by Dr Takami Matsuyama (Department of Immunology,
Kagoshima University), were cells primarily cultured from the
kidneys of Clawn miniature swine [a swine strain established by
Japanese scientists (21)] and
passaged 10–15 generations. Madin-Darby canine kidney (MDCK) cells
were provided by Dr Yasushi Daikuhara (Kagoshima University Dental
School, Kagoshima, Japan). The expression vector encoding
hemagglutinin (HA)-tagged full-length E-cadherin was prepared as
previously described (22). The
vector contains neo gene, which confers G418-resistance. As a
control, an empty vector without E-cadherin gene was used yielding
nH-2 and nH-6 clones. Transfection of 293 cells with the HA-tagged
E-cadherin vector resulted in EH-5 and EH-13 clones. The cells
(5×105) were transfected with the expression vector (10
µg) using the calcium-phosphate transfection method as
previously described (15), and
stably transfected cells were selected in G418-containing medium.
Isolated G418-resistant clones were tested for the expression of
the transfected construct by immunofluorescence microscopy and
immunoblot analysis.
Antibodies and reagents
Mouse monoclonal antibodies (mAbs) against
E-cadherin (catalogue no. 610182), β-catenin (catalogue no.
610153), fibronectin (catalogue no. 610077) and plakoglobin
(γ-catenin; catalogue no. 610253) were obtained from BD
Transduction Laboratories (Lexington, KY, USA). Pan-cadherin mAb
(catalogue no. C1821-100UL) was obtained from Sigma-Aldrich (St.
Louis, MO, USA). Rabbit polyclonal anti-occludin (catalogue no.
71-1500), anti-ZO-1 (catalogue no. 61-7300) and mouse monoclonal
anti-vimentin (catalogue no. 18-0052) antibodies were purchased
from Zymed Laboratories (South San Francisco, CA, USA). A mouse mAb
specific for cytokeratins 5/8 (catalogue no. MAB3228) was acquired
from Merk Millipore Ltd. (Tokyo, Japan). A mouse mAb against
desmoglein 2 was obtained from Progen Biotechnik GmbH (Heidelberg,
Germany). A rat mAb (3F10; catalogue no. 11867423001) directed
against HA was purchased from Roche Applied Science (Mannheim,
Germany). All secondary antibodies (fluorescein-, rhodamine- and
peroxidase-conjugated) were obtained from Jackson ImmunoResearch
Laboratories, Inc. (West Grove, PA, USA).
Immunoblot analysis
Immunoblot analysis was performed essentially as
previously described (22).
Briefly, the cells were lysed by boiling in SDS sample buffer for 5
min. Proteins (30–50 µg) were separated by SDS-PAGE and then
transferred onto nitrocellulose membranes (Schleicher &
Schuell, Keene, NH, USA). The membranes were incubated with the
appropriate primary antibodies diluted at 1:1,000, followed by
incubation in horseradish peroxidase-conjugated secondary
anti-mouse (catalogue no. 315-036-045) or anti-rabbit (catalogue
no. 111-036-045) antibodies (Jackson ImmunoResearch Laboratories,
Inc.) diluted at 1:1,000. Proteins were visualized using the
enhanced chemiluminescence (ECL) system (Amersham Pharmacia
Biotech, Piscataway, NJ, USA).
Immunofluorescence staining
Immunofluorescence staining of the cells was
performed as previously described (23) with some modifications. The cells
were cultured on coverslips for 48 h prior to fixation. The cells
were then fixed with 3% paraformaldehyde in phosphate-buffered
saline (PBS) for 20 min at room temperature. Following 3 washes in
PBS containing 50 mM NH4Cl, the cells were permeabilized
using 0.1% Triton X-100 in PBS for 5 min. After washing in PBS, the
cells were soaked in blocking solution (PBS containing 5% fetal
calf serum) for 15 min, and then incubated for 30 min with primary
antibodies diluted in blocking solution. After washing 3 times in
PBS, the cells were incubated with rhodamine- or
fluorescein-conjugated secondary antibodies. The cells were
analyzed as previously described (24) using an Olympus microscope.
Results
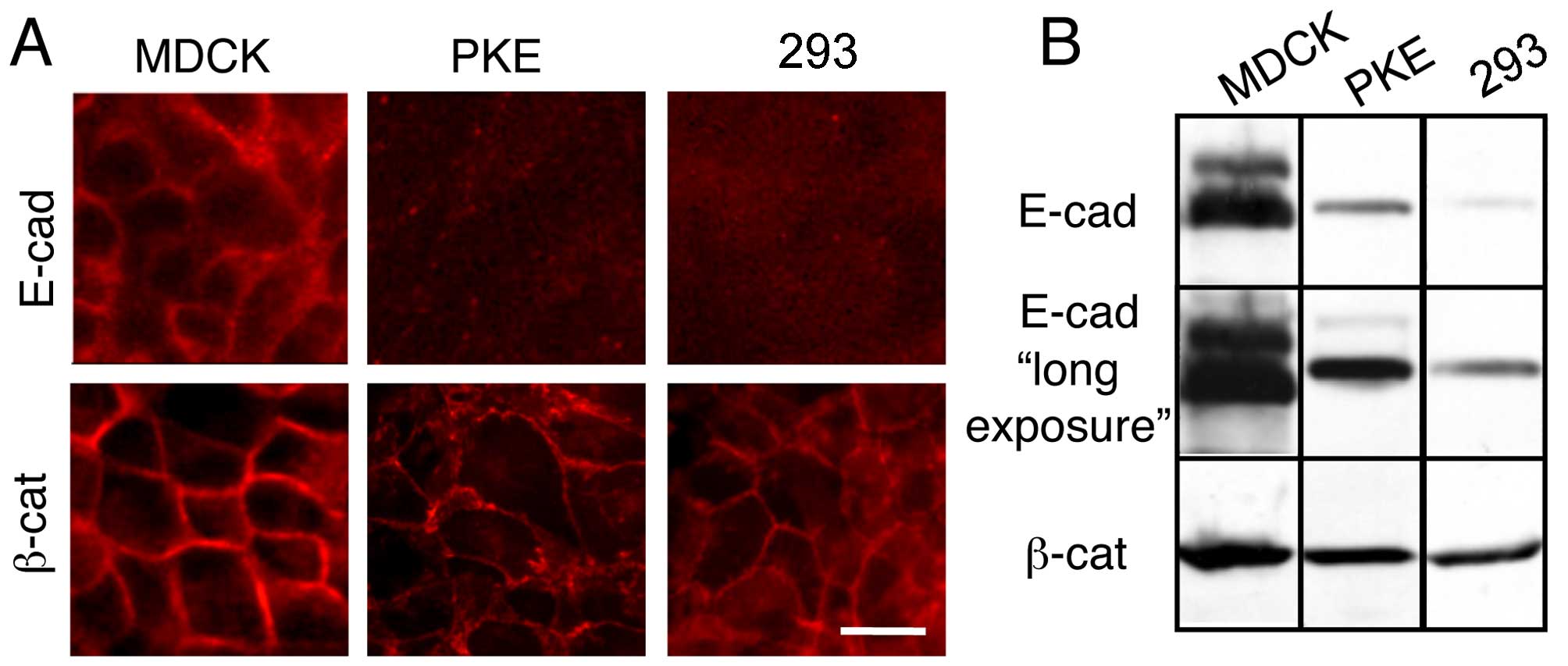
Compared with MDCK cells, PKE and 293
cells express reduced amounts of E-cadherin
E-cadherin is a major cell adhesion molecule in
epithelial cells and an epithelial cell marker (25). Although the immunofluorescence
staining of MDCK cells, a typical epithelial cell line, with
E-cadherin antibodies revealed strong membrane staining, the PKE
and the 293 cells exhibited no membrane staining (Fig. 1A, upper panels). In a control
experiment, we noted clear membrane staining for β-catenin, a
cytoplasmic subunit of the transmembrane cell adhesion cadherin
complex, in these 3 cell lines (Fig.
1A, lower panels).
The immunoblot detection of E-cadherin in these
cells revealed that E-cadherin expression in the PKE and the 293
cells was markedly decreased compared with its expression level in
the MDCK cells (Fig. 1B).
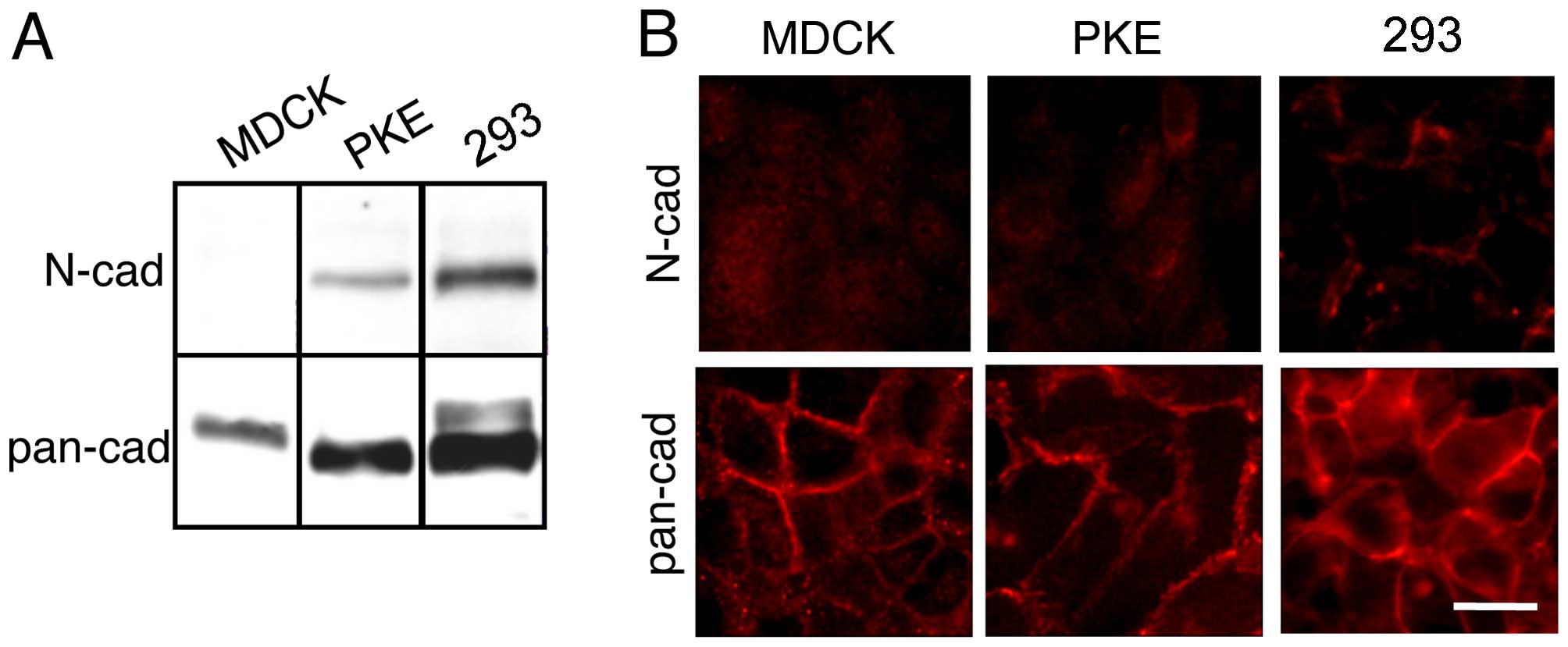
293 and PKE cells express N-cadherin
The immunoblot detection of N-cadherin in the PKE
and the 293 cells revealed high expression levels of N-cadherin
(Fig. 2A). The MDCK cells, on the
other hand, did not express N-cadherin (Fig. 2A), which was in agreement with
previous findings (26).
Immunoblot analysis using pan-cadherin antibodies raised against a
synthetic peptide corresponding to the C-terminal amino acids of
chicken N-cadherin, revealed that the PKE and the 293 cells
expressed a protein with the same electrophoretic mobility as
N-cadherin (Fig. 2A). Although
the MDCK cells do not express N-cadherin, they express a protein
that migrates slowly relative to N-cadherin. This protein has been
shown to be K-cadherin (23,27). The 293 cells also expressed a
protein with a slightly slower electrophoretic mobility (Fig. 2A). Although this protein has not
been identified definitively, we conjecture that this protein is
the precursor form of N-cadherin, containing the prosequence.
The immunofluorescence staining of the cells with
pan-cadherin antibodies revealed clear membrane staining (Fig. 2B). Thus, the proteins recognized
by the pan-cadherin antibodies were present on the membrane. No
heterogeneity of expression within the cell lines was noted. These
results are consistent with a previous observation that N-cadherin
is expressed endogenously at cell-cell contact sites in 293 cells
(28).
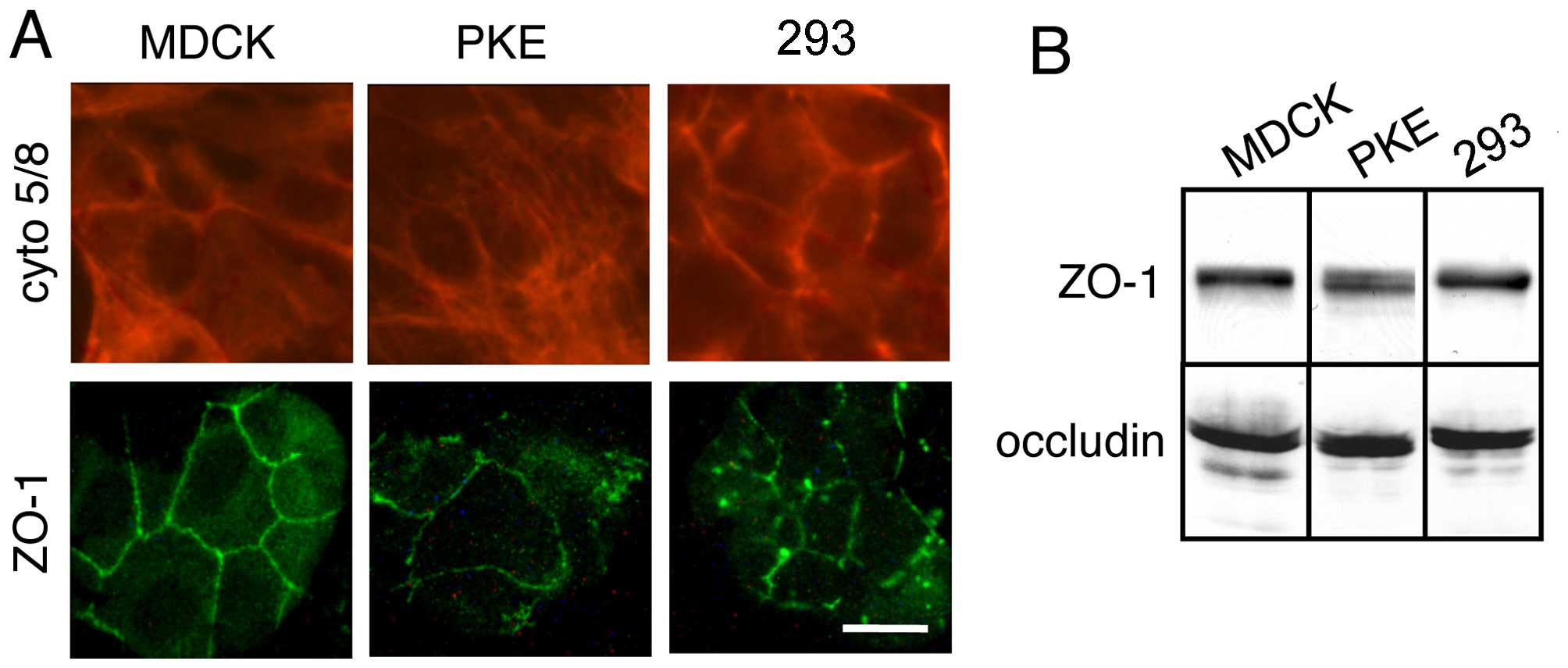
293, PKE and MDCK cells express
cytokeratins 5/8
The MDCK cells exhibited strong and marked
cytoplasmic and filamentous staining with a pan-keratin antibody
mix containing mAb specific for keratin 5 and mAb specific for
keratin 8 (Fig. 3A, upper
panels). This staining pattern was also observed in the PKE and the
293 cells (Fig. 3A, upper
panels). No heterogeneity of expression within the cell lines was
noted as expected for typical kidney-derived cell lines and the 293
cells.
293, PKE and MDCK cells express ZO-1 and
occluding
Although the decreased expression of E-cadherin and
the unexpected expression of N-cadherin argue against the
epithelial cell origin of 293 cells, the expression of keratin 5/8
supports the notion that they are epithelial cells. To examine the
epithelial nature of 293 cells, we determined the expression levels
of ZO-1 and occludin in these cells. These proteins are components
of tight junctions in epithelial and endothelial cells (11). Immunoblot analysis of the cells
revealed that the PKE and 293 cells, as well as the MDCK cells,
expressed ZO-1 and occludin (Fig.
3B).
Immunofluorescence staining of these cells with ZO-1
antibodies revealed strong membrane staining (Fig. 3A, lower panels). Thus, transport
of the tight junction component, ZO-1, from its site of
biosynthesis occurred. No heterogeneity of expression within the
cell lines was noted. Immunostaining for occludin revealed similar
membrane staining patterns (data not shown).
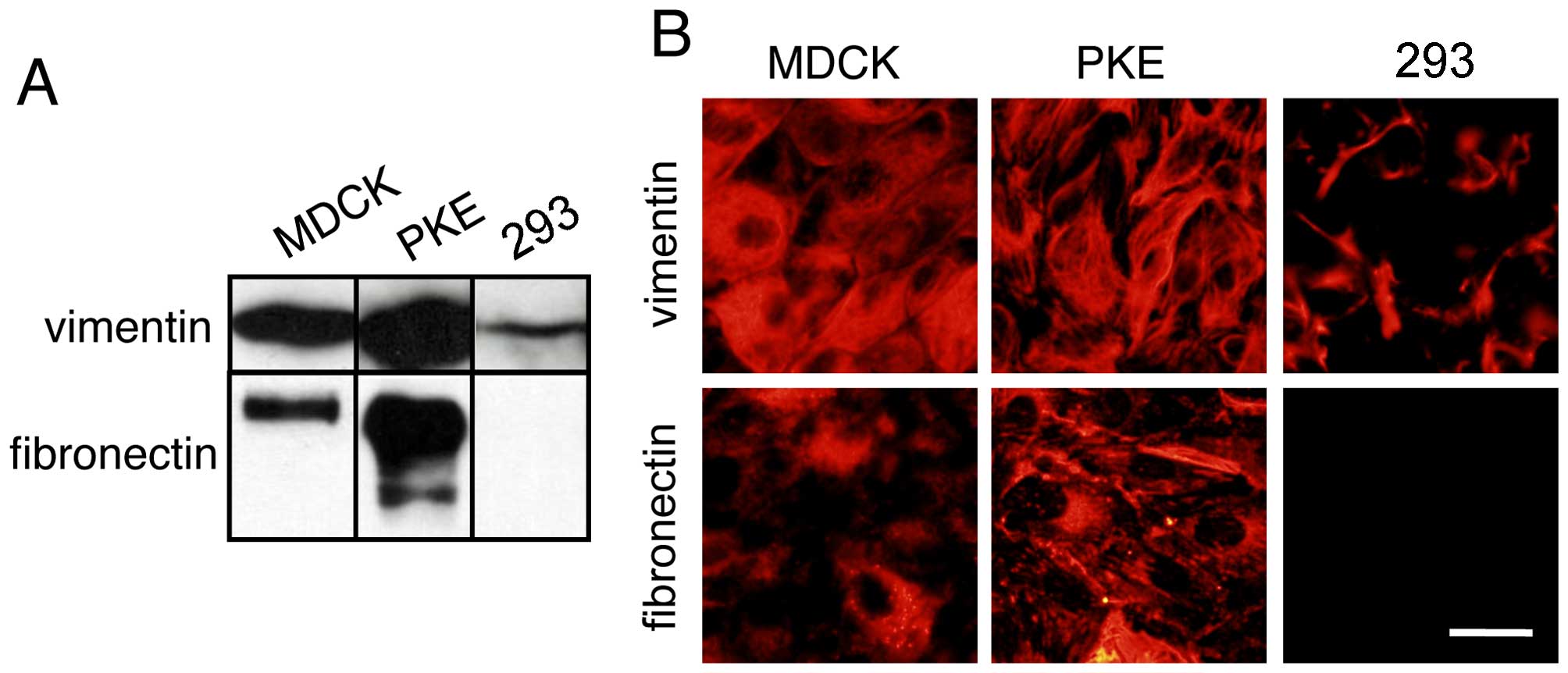
MDCK and PKE cells express the
mesenchymal markers, vimentin and fibronectin
The immunoblot detection of vimentin in the MDCK and
the PKE cells revealed high expression levels of vimentin (Fig. 4A). The 293 cells, on the other
hand, expressed vimentin at a reduced level (Fig. 4A). Immunoblot analysis of
fibronectin revealed that the PKE cells expressed a large amount of
fibronectin (Fig. 4A). Although
the MDCK cells expressed a small amount of fibronectin, the 293
cells did not express detectable amounts of fibronectin.
Consistent with the results of the immunoblot
analysis, immunofluorescence staining of the cells with vimentin
and fibronectin antibodies revealed that the PKE cells expressed
these proteins (Fig. 4B). The 293
cells expressed vimentin at a low level.
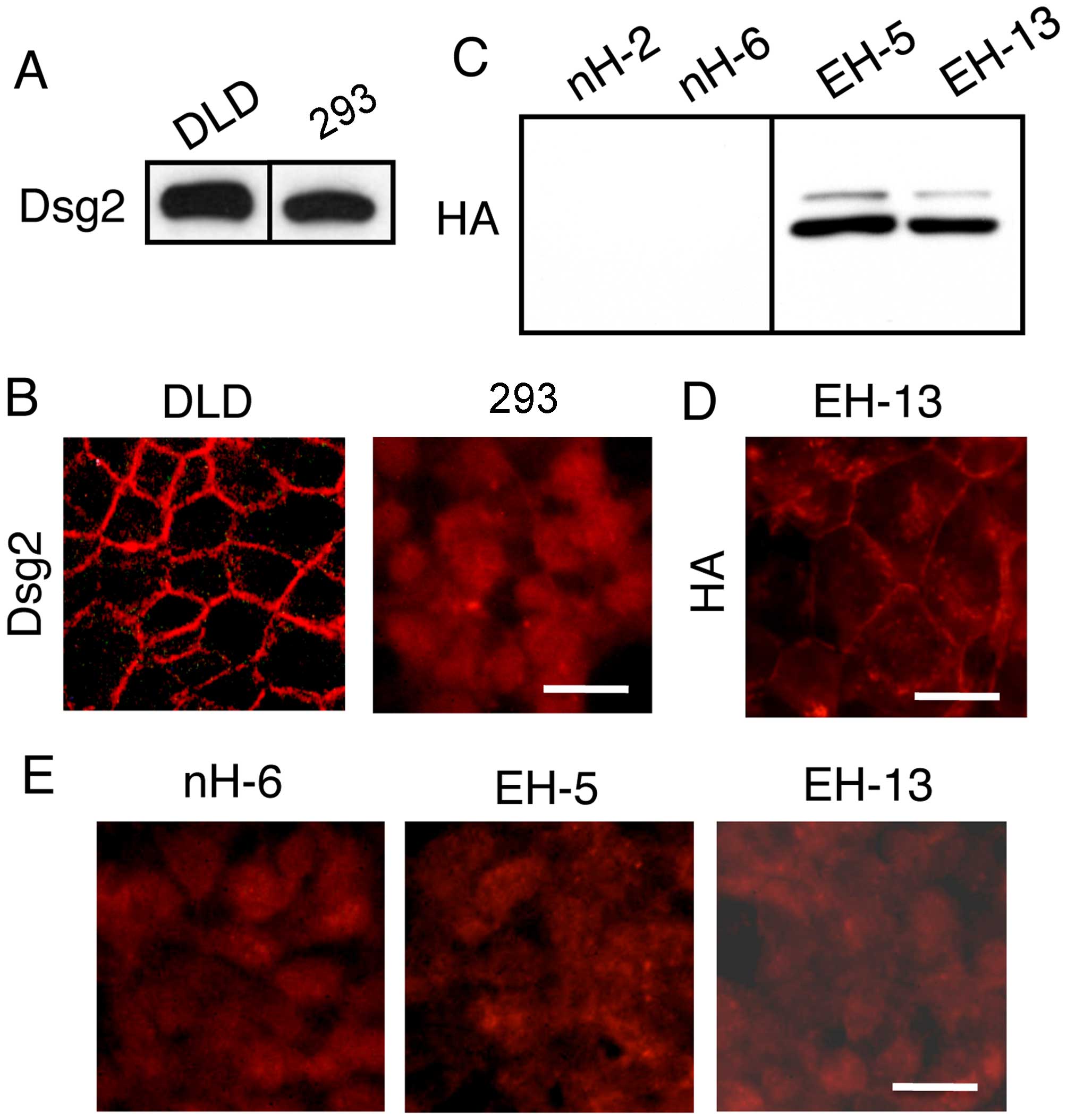
Although desmoglein 2 is expressed in the
293 cells, it remains localized in intracellular compartments
The immunoblot detection of desmoglein 2 in the 293
cells revealed high expression levels (Fig. 5A). Immunofluorescence staining of
the 293 cells, however, revealed no membrane staining, although the
staining of the DLD1 cells revealed clear membrane staining
(Fig. 5B). Thus, desmoglein 2
remained localized in intracellular compartments. No heterogeneity
of expression within the cell lines was noted.
These observations suggested that low E-cadherin
expression levels were responsible for the failure of desmosome
assembly. In an attempt to restore the localization of desmosomes
in the membrane through increased E-cadherin expression, the 293
cells were transfected with the expression vector for HA-tagged
E-cadherin and stable transfectants were isolated following
selection with G418 (Fig. 5C). In
these cells, E-cadherin was detected mainly on the surface membrane
as revealed by staining with anti-HA antibodies (Fig. 5D). Immunofluorescence staining of
these E-cadherin-expressing cells with desmoglein 2 antibodies
revealed no membrane staining (Fig.
5E). Thus, E-cadherin expression in 293 cells is insufficient
for the cell surface localization of desmoglein 2.
Discussion
A thorough analysis of the 293 cells, thought to
have been derived from human embryonic kidney cells that had been
transformed by adenovirus 5 (Ad5), notably revealed that these
cells express a variety of proteins (such as NF subunits) that are
typically found in neural cells (4). The transformation of cells with Ad5,
including the early region 1 (E1), has generated several human
embryonic retinal cell lines (29), suggesting that Ad5 E1 may
preferentially transform human neural lineages. Previous research
has demonstrated that the efficient transformation of primary human
amniocytes with the E1 gene of human Ad5 yielded stable cell lines
which exhibited the morphological features of epithelial cells
(30); a thorough
immunocytochemical analysis confirmed the expression of epithelial
cell markers and the analysis also revealed the expression of
neuronal and glial marker proteins, such as nestin, vimentin, A2B5
and glial fibrillary acidic protein (GFAP) (30). In agreement with previous studies
on 293 cells, these results suggest that epithelial and neuronal
marker proteins are co-expressed in E1-transformed human amniotic
fluid-derived cells. 293 cells exhibit chromosomal abnormalities,
containing less than three times the number of chromosomes of a
normal diploid human cell (31).
Structural genomic alterations produced during cultivation for
decades in different laboratories have been proposed to underlie
the sometimes different conclusions drawn from experimentation with
293 cell lines (31). Thus, these
differences may be the reason why we obtained the data
demonstrating that 293 cells have characteristics of epithelial
lineage cells.
In the present study, we examined the expression of
epithelial marker proteins in 293 cells. Moreover, epithelial
features were also investigated in non-transformed PKE cells, as
well as in MDCK cells as a control cell population. Our data
revealed that the 293 and PKE cells homogeneously expressed the
neural cell marker, N-cadherin. These cells were also found to
express various epithelial marker proteins, cytokeratins 5/8, ZO-1,
occludin and desmoglein 2. Strictly speaking, desmoglein 2 is not a
specific marker of epithelial cells, as it is expressed not only in
epithelial cells, but also in various non-epithelial cells, such as
myocardiac and Purkinje fiber cells of the heart (32). However, desmoglein 2 is found in
all cell types that possess desmosomes and is the only desmoglein
detected in diverse tissues, such as simple and transitional
epithelia (33). Non-epithelial
cell lines, including human fibroblast, rhabdomyosarcoma and glioma
cell lines, do not express desmoglein 2. To the best of our
knowledge, there is no evidence at present to suggest that neural
cells express desmoglein 2. As demonstrated by the present study
for the first time to the best of our knowledge, the epithelioid
characteristics of 293 cells were confirmed by the homogenous
expression of the epithelial markers, cytokeratins 5/8, as well as
by the expression of the epithelial-specific contact proteins, ZO-1
(33) and occludin (10). Although endothelial cells express
ZO-1 and occludin, they do not express E-cadherin, which is
detected in 293 cells.
In the assembly of epithelia, surface interactions
between adhesion molecules of the cadherin superfamily nucleate a
cascade of protein-protein interactions that leads to the formation
of additional junctions including desmosomes and tight junctions
(34). It is generally accepted
that before the extracellular domains of cadherins are capable of
mediating adhesion, the cytoplasmic domains must first bind to
catenins inside the cell (16,35). The formation of this molecular
complex confers adhesive strength by linking cadherins to the actin
cytoskeleton and by clustering cadherin molecules, thus increasing
the avidity of their interactions. We have previously demonstrated
that an E-cadherin-expressing human colon carcinoma cell line
lacking α-catenin expression failed to organize desmosomes, and
that the expression of α-catenin in these cells by transfection
resulted in the reorganization of desmosomes (36). Thus, the formation of desmosomes
is dependent on the integrity of E-cadherin-catenin complexes.
N-cadherin has been shown to rescue some, but not
all, functionalities of E-cadherin during selective embryogenic
events (37); gene replacement
experiments have revealed that the strength of cellular adhesion
provided by N-cadherin is sufficient to mediate morula compaction;
however, it is insufficient for the subsequent formation of a fully
polarized functional trophectoderm. A previous study also
demonstrated that the first desmosomes in mouse embryos are formed
between trophectoderm cells in early cavitating blastocysts
(38), and, therefore, we
conjectured that N-cadherin may not act as a substitute for
E-cadherin during desmosome formation. However, the attempted
rescue by the exogenous expression of E-cadherin in 293 cells,
failed to induce desmosome formation. Thus, it is clear that other
factors are required for induction. In conclusion, the findings of
the present study have established that 293 cells, by means of
their epithelioid properties described herein, are suitable for use
in studies on desmosome formation.
Acknowledgments
This study was supported by grants from the Ministry
of Education, Culture, Sports, Science and Technology of Japan and
a grant from Kodama Memorial Found for Medical Research. We wish to
thank the Joint Research Laboratory at Kagoshima University
Graduate School of Medical and Dental Sciences for the use of their
facilities.
Abbreviations:
|
Ad5
|
adenovirus 5
|
|
E1
|
early region 1
|
|
MDCK
|
Madin-Darby canine kidney
|
|
NF
|
neurofilament
|
|
PBS
|
phosphate-buffered saline
|
|
PKE
|
porcine kidney epithelial
|
References
|
1
|
Graham FL, Smiley J, Russell WC and Nairn
R: Characteristics of a human cell line transformed by DNA from
human adenovirus type 5. J Gen Virol. 36:59–74. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Louis N, Evelegh C and Graham FL: Cloning
and sequencing of the cellular-viral junctions from the human
adenovirus type 5 transformed 293 cell line. Virology. 233:423–429.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suzuki R, Sakamoto H, Yasukawa H, Masuhara
M, Wakioka T, Sasaki A, Yuge K, Komiya S, Inoue A and Yoshimura A:
CIS3 and JAB have different regulatory roles in interleukin-6
mediated differentiation and STAT3 activation in M1 leukemia cells.
Oncogene. 17:2271–2278. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shaw G, Morse S, Ararat M and Graham FL:
Preferential transformation of human neuronal cells by human
adenoviruses and the origin of HEK 293 cells. FASEB J. 16:869–871.
2002.PubMed/NCBI
|
|
5
|
Franke WW, Grund C and Achtstätter T:
Co-expression of cytokeratins and neurofilament proteins in a
permanent cell line: cultured rat PC12 cells combine neuronal and
epithelial features. J Cell Biol. 103:1933–1943. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Damjanov I, Clark RK and Andrews PW:
Cytoskeleton of human embryonal carcinoma cells. Cell Differ.
15:133–139. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee VM and Andrews PW: Differentiation of
NTERA-2 clonal human embryonal carcinoma cells into neurons
involves the induction of all three neurofilament proteins. J
Neurosci. 6:514–521. 1986.PubMed/NCBI
|
|
8
|
Schmidt U, Müller U, Metz KA and Leder LD:
Cytokeratin and neurofilament protein staining in Merkel cell
carcinoma of the small cell type and small cell carcinoma of the
lung. Am J Dermatopathol. 20:346–351. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Farquhar MG and Palade GE: Junctional
complexes in various epithelia. J Cell Biol. 17:375–412. 1963.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Furuse M, Hirase T, Itoh M, Nagafuchi A,
Yonemura S and Tsukita S and Tsukita S: Occludin: a novel integral
membrane protein localizing at tight junctions. J Cell Biol.
123:1777–1788. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Furuse M, Fujita K, Hiiragi T, Fujimoto K
and Tsukita S: Claudin-1 and -2: novel integral membrane proteins
localizing at tight junctions with no sequence similarity to
occludin. J Cell Biol. 141:1539–1550. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Furuse M, Itoh M, Hirase T, Nagafuchi A,
Yonemura S and Tsukita S and Tsukita S: Direct association of
occludin with ZO-1 and its possible involvement in the localization
of occludin at tight junctions. J Cell Biol. 127:1617–1626. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Itoh M, Furuse M, Morita K, Kubota K,
Saitou M and Tsukita S: Direct binding of three tight
junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH
termini of claudins. J Cell Biol. 147:1351–1363. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boller K, Vestweber D and Kemler R:
Cell-adhesion molecule uvomorulin is localized in the intermediate
junctions of adult intestinal epithelial cells. J Cell Biol.
100:327–332. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ozawa M, Baribault H and Kemler R: The
cytoplasmic domain of the cell adhesion molecule uvomorulin
associates with three independent proteins structurally related in
different species. EMBO J. 8:1711–1717. 1989.PubMed/NCBI
|
|
16
|
Ozawa M, Ringwald M and Kemler R:
Uvomorulin-catenin complex formation is regulated by a specific
domain in the cytoplasmic region of the cell adhesion molecule.
Proc Natl Acad Sci USA. 87:4246–4250. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aberle H, Butz S, Stappert J, Weissig H,
Kemler R and Hoschuetzky H: Assembly of the cadherin-catenin
complex in vitro with recombinant proteins. J Cell Sci.
107:3655–3663. 1994.PubMed/NCBI
|
|
18
|
Jou TS, Stewart DB, Stappert J, Nelson WJ
and Marrs JA: Genetic and biochemical dissection of protein
linkages in the cadherin-catenin complex. Proc Natl Acad Sci USA.
92:5067–5071. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Buxton RS and Magee AI: Structure and
interactions of desmosomal and other cadherins. Semin Cell Biol.
3:157–167. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garrod DR, Merritt AJ and Nie Z:
Desmosomal cadherins. Curr Opin Cell Biol. 14:537–545. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakanishi Y, Ogawa K, Yanagita K and
Yamauchi C: Body measurement and some characteristics of inbred
Clawn miniature pigs. Jpn J Swine Sci. 28:211–218. 1991. View Article : Google Scholar
|
|
22
|
Ozawa M: p120-independent modulation of
E-cadherin adhesion activity by the membrane-proximal region of the
cytoplasmic domain. J Biol Chem. 278:46014–46020. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miyashita Y and Ozawa M: A dileucine motif
in its cytoplasmic domain directs beta-catenin-uncoupled E-cadherin
to the lysosome. J Cell Sci. 120:4395–4406. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ozawa M and Kobayashi W: Cadherin
cytoplasmic domains inhibit the cell surface localization of
endogenous E-cadherin, blocking desmosome and tight junction
formation and inducing cell dissociation. PLoS One. 9:e1053132014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ohkubo T and Ozawa M: The transcription
factor Snail downregulates the tight junction components
independently of E-cadherin downregulation. J Cell Sci.
117:1675–1685. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stewart DB, Barth AI and Nelson WJ:
Differential regulation of endogenous cadherin expression in
Madin-Darby canine kidney cells by cell-cell adhesion and
activation of beta-catenin signaling. J Biol Chem. 275:20707–20716.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Frank M, Ebert M, Shan W, Phillips GR,
Arndt K, Colman DR and Kemler R: Differential expression of
individual gamma-protocadherins during mouse brain development. Mol
Cell Neurosci. 29:603–616. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fallaux FJ and Hoeben RC: Safety of
recombinant adenoviruses produced on adenovirus-transformed human
cells. Dev Biol (Basel). 106:489–497. 501–511. 2001.
|
|
30
|
Arnhold S, Post C, Glüer S, Hoopmann M,
Wenisch S, Volpers C and Addicks K: Neuronal characteristics of
amniotic fluid derived cells after adenoviral transformation. Cell
Biol Int. 32:1559–1566. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin YC, Boone M, Meuris L, Lemmens I, Van
Roy N, Soete A, Reumers J, Moisse M, Plaisance S, Drmanac R, et al:
Genome dynamics of the human embryonic kidney 293 lineage in
response to cell biology manipulations. Nat Commun. 5:47672014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schäfer S, Koch PJ and Franke WW:
Identification of the ubiquitous human desmoglein, Dsg2, and the
expression catalogue of the desmoglein subfamily of desmosomal
cadherins. Exp Cell Res. 211:391–399. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stevenson BR, Siliciano JD, Mooseker MS
and Goodenough DA: Identification of ZO-1: a high molecular weight
polypeptide associated with the tight junction (zonula occludens)
in a variety of epithelia. J Cell Biol. 103:755–766. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gumbiner B, Stevenson B and Grimaldi A:
The role of the cell adhesion molecule uvomorulin in the formation
and maintenance of the epithelial junctional complex. J Cell Biol.
107:1575–1587. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van Hengel J, Gohon L, Bruyneel E,
Vermeulen S, Cornelissen M, Mareel M and von Roy F: Protein kinase
C activation upregulates intercellular adhesion of
alpha-catenin-negative human colon cancer cell variants via
induction of desmosomes. J Cell Biol. 137:1103–1116. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Taniguchi T, Miyazaki M, Miyashita Y,
Arima T and Ozawa M: Identification of regions of alpha-catenin
required for desmosome organization in epithelial cells. Int J Mol
Med. 16:1003–1008. 2005.PubMed/NCBI
|
|
37
|
Kan NG, Stemmler MP, Junghans D, Kanzler
B, de Vries WN, Dominis M and Kemler R: Gene replacement reveals a
specific role for E-cadherin in the formation of a functional
trophectoderm. Development. 134:31–41. 2007. View Article : Google Scholar
|
|
38
|
Fleming TP, Garrod DR and Elsmore AJ:
Desmosome biogenesis in the mouse preimplantation embryo.
Development. 112:527–539. 1991.PubMed/NCBI
|