Introduction
Insufficient blood supply to the myocardium may
result in myocardial ischemia, injury or infarction, or all three.
Myocardial injury (MI) leads to hypoxia and ultimately to
myocardial necrosis (1). Although
thrombolysis is one of the effective treatments currently
available, only limited numbers of patients are eligible for
treatment. It is therefore necessary to develop alternative
effective treatments. The increased production of reactive oxygen
species (ROS) is a major contributor to the initiation and
development of ischemic MI (2).
Excessive ROS production causes a number of adverse effects,
including DNA mutagenesis, cellular dysfunction and lipid
peroxidation, in the myocardium following ischemic injury (3). Thus, antioxidants are considered to
exert protective effects against MI. The peroxisome
proliferator-activated receptor gamma coactivator-1α (PGC-1α) acts
as a powerful suppressor of ROS production, and improves cardiac
energy metabolism and delays the progression of heart failure
(4–6). PGC-1α plays a critical role in
mitochondrial biogenesis, and is a major regulator of the nuclear
respiratory factors (7). Nuclear
factor erythroid 2-related factor 2 (Nrf2) upregulates the
transcription of cytoprotective genes, that reduce damage from
oxidative stress (8). The
PGC-1α/Nrf2 pathway is a promising therapeutic target for heart
disease (9,10).
According to the Compendium of Surger y (Wai Ke Da
Cheng), the combination of Carthamus tinctorius L. and
Boswellia serrata Roxb. ex Colebr. is widely used to protect
against ischemic diseases. The principal active constituent of
C. tinctorius is safflower yellow (SY) (11). Hydroxysafflor yellow A (HSYA), a
water-soluble monomer of SY, is responsible for the main beneficial
effects of SY (12). C.
tinctorius extract is reported to improve cardiac function
following myocardial ischemic injury by exerting antioxidant
effects (13,14). Acetyl-11-keto-β-boswellic acid
(AKBA) is the major organic acid component extracted from B.
serrata (15,16). It is a pentacyclic triterpene
which possesses antioxidant properties (17). In our previous study, we have
demonstrated that AKBA protects against cerebral
ischemia/reperfusion (I/R) injury in rats by activating the Nrf2
pathway in order to enhance the antioxidant capacity of brain
tissue (18). However, the
additional biochemical mechanisms responsible for the beneficial
effects of HSYA and AKBA in the treatment of MI remain unclear. In
addition, to the best of our knowledge, the synergistic
cardioprotective effects of HSYA and AKBA in combination have not
been investigated to date.
In the present study, we applied in vivo and
in vitro ischemic paradigms to analyze the protective
effects of HSYA and AKBA, alone and in combination. We aimed to
provide evidence to elucidate the mechanisms through which HSYA and
AKBA protect against MI.
Materials and methods
Materials
Isoproterenol hydrochloride (ISO) was purchased from
Sigma-Aldrich. (St. Louis, MO, USA). HSYA and AKBA were purchased
from the National Institute for the Control of Pharmaceutical and
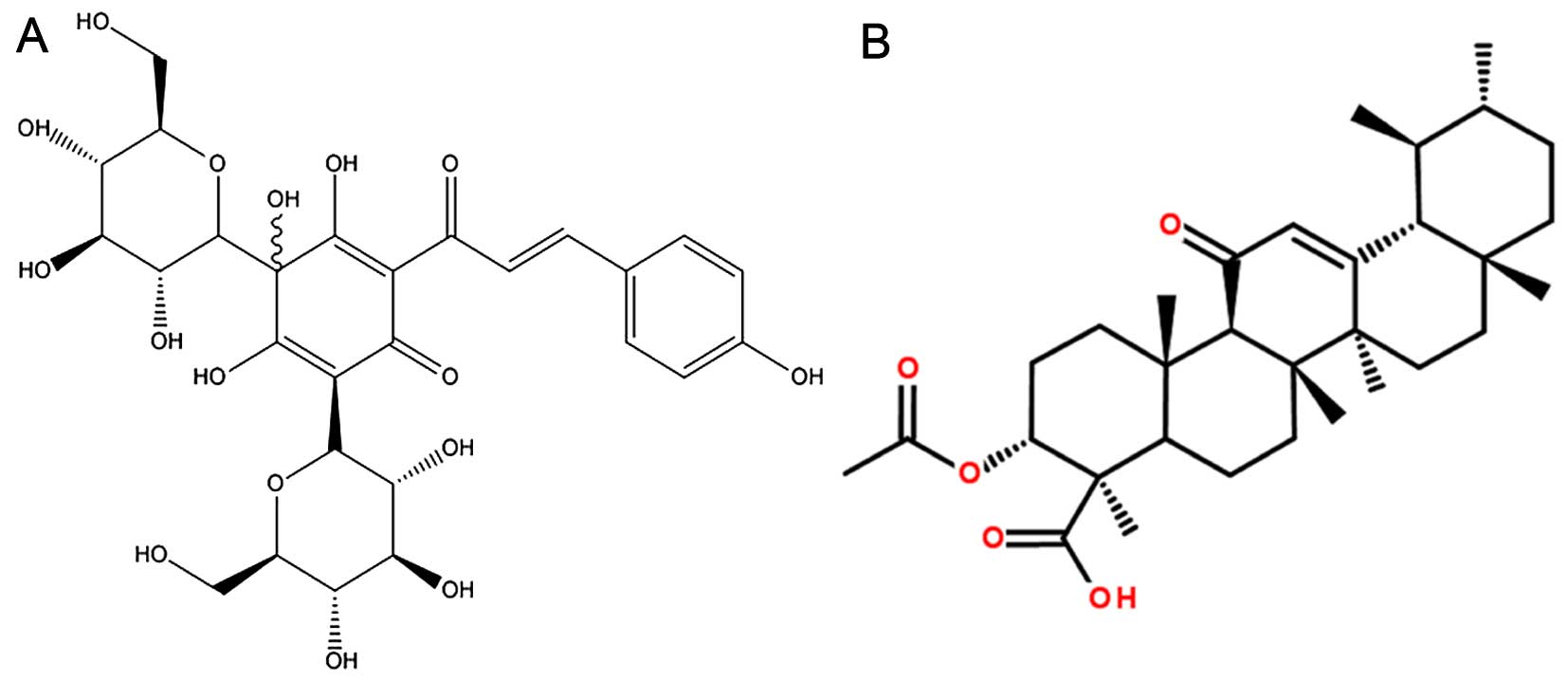
Biological Products (Beijing, China). The chemical structures of
HYSA and AKBA are shown in Fig.
1.
Animals
Six-week old male Sprague-Dawley rats (250±20 g)
were purchased from the Animal Research Center of the Fourth
Military Medical University (Xi'an, China). The animals were
maintained in air-conditioned animal quarters at a temperature of
22±2°C under a 12 h light/12 h dark cycle with unlimited access to
food and water. All procedures were approved by the Ethics
Committee for Animal Experimentation of the Fourth Military Medical
University. The rats were randomized into five groups each
consisting of six rats: i) sham group; ii) ISO + vehicle group;
iii) ISO + HSYA group; iv) ISO + AKBA group; v) ISO + HSYA + AKBA
group. A rat model of focal MI was established using the method of
ISO-induced myocardial necrosis. Briefly, ISO (100 mg/kg) was
dissolved in saline and injected subcutaneously into the rats at 24
h intervals for 2 days (19).
ISO-induced MI was confirmed by the measurement of elevated
activity levels of cardiac markers, compared with those in the
normal rats. AKBA and HSYA were first dissolved in 2 ml of 0.5%
dimethyl sulfoxide (DMSO) solvent, and then diluted with
physiological saline. The rats in the ISO + HSYA group were
administered HSYA (100 mg/kg) through intragastric tubes. The rats
in the ISO + AKBA group were administered AKBA (100 mg/kg) through
intragastric tubes. The rats in the ISO + HSYA + AKBA group were
administered HSYA (50 mg/kg) and AKBA (50 mg/kg) through
intragastric tubes. The doses of HSYA and AKBA was selected based
upon previous studies (20,21). The rats in the sham and ISO +
vehicle groups were administered orally 2 ml of 0.5% DMSO through
intragastric tubes. The rats were gavaged for 14 days, and then
subcutaneously injected with ISO at 24 h intervals for 2
consecutive days on the 15th and 16th day.
Cell culture
The rat H9C2 cardiomyocyte cell line was obtained
from the American Type Culture Collection (ATCC, Manassas, VA, USA)
and cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% (v/v) fetal bovine serum at 37°C in a
CO2 incubator. The medium was replaced every 2 days, and
the cells were subjected to experimental procedures at 80–90%
confluence.
Oxygen-glucose deprivation (OGD) and
reoxygenation in H9C2 cells
The H9C2 cells were randomly divided into five
groups: i) sham group without any treatment; ii) OGD + vehicle
group; iii) OGD + HSYA group (10µM); iv) OGD + AKBA group
(10 µM); v) OGD + HSYA + AKBA group (HSYA, 5 µM and
AKBA, 5 µM). The H9C2 cells were pre-treated with the
above-mentioned drugs for 24 h. To simulate ischemic-like
conditions in vitro, the cells were subjected to OGD for 4
h. The OGD procedures were based on a previously described method
(22). Briefly, the H9C2 cells
were washed with a solution (5 mM HEPES, 137 mM NaCl, 4 mM KCl, 1
mM MgCl2, 1.5 mM CaCl2, pH 7.0). The cells
were incubated in glucose-free DMEM and then placed in a hypoxic
incubator (37°C, 95% N2, 5% CO2)
(Billups-Rothenberg, Del Mar, CA, USA). After 4 h of anoxia, the
cells were subjected to reoxygenation and cultured under normal
conditions in a CO2 incubator for 20 h.
Histopathology
At the end of the experimental period, the rats were
anesthetized with hydral (400 mg/kg, i.p.). The hearts were rapidly
excised. Following removal, the cardiac apex was immediately fixed
in 4% paraformaldehyde, routinely processed and embedded in
paraffin wax. The cardiac apex was stained with hematoxylin and
eosin (H&E) and examined under a light microscope (Olympus
IX71; Olympus, Tokyo, Japan).
Measurement of MI markers in the
serum
Following experimental treatment, artexrial blood
was collected and centrifuged at 16,000 rpm for 10 min. The serum
was used to assay creatine kinase-MB (CK-MB) and lactate
dehydrogenase (LDH) activities. The levels of CK-MB and LDH were
assayed using commercial kits purchased from Jiancheng
Bioengineering Institute (Nanjing, China) according to the
manufacturer's instructions. An enzyme-linked immunosorbent assay
was used to detect CK-MB and LDH levels with a microplate reader at
450 and 490 nm (Thermo Fisher Scientific, Waltham, MA, USA). CK-MB
and LDH levels were also measured in the H9C2 cells. We determined
the levels of CK-MB and LDH in the culture medium.
TUNEL assay and Hoechst 33258 staining
for detection of cell apoptosis
Cell apoptosis was analyzed by performing the TUNEL
assay in vivo and Hoechst 33258 staining in vitro.
The TUNEL assay was performed using an In Situ Cell Death
Detection kit (KeyGen Biotech, Nanjing, China). The
paraffin-embedded tissue was washed twice with xylene for 10 min,
and then with distilled water and graded concentrations of ethanol
(absolute, 95, 90, 80, 70 and 50%). After washing with
phosphate-buffered saline (PBS), the slides were immersed in a
solution with 20 µg/ml proteinase K for 10 min at 37°C, and
then permeabilized in a solution with 0.1% Triton X-100 and 0.1%
sodium citrate for 5 min. The cells were washed once with
DAPI-methanol (1 µg/ml) and were then covered with
DAPI-methanol and incubated for 15 min at 37°C. After fluorescein
staining with terminal deoxynucleotidyl transferase (TdT) and
avidin-FITC, the individual nuclei were examined under a
fluorescence microscope. The number of apoptotic nuclei was
determined as a percentage of the total number of cells. An average
of 300-400 nuclei were analyzed from each slide.
The H9C2 cells were stained with Hoechst 33258 and
the nuclei were imaged. The AKBA/HSYA-treated H9C2 cells were
washed twice with PBS, and incubated with 10 µM Hoechst
33258 at room temperature for 30 min. After three washes, the cells
were grown on glass coverslips. The slides were examined for any
nuclear morphological alterations and apoptotic bodies under an
inverted fluorescence microscope (Olympus IX71; Olympus).
Detection of ROS
Mitochondrial ROS production in the H9C2 cells was
assessed using MitoSOX Red mitochondrial superoxide indicator
(Invitrogen, Carlsbad, CA, USA). The H9C2 cells were seeded in a
6-well plate and grown to a density of 1×106 cell/ml.
The cells were loaded with 5 µM MitoSOX Red for 15 min at
37°C in a CO2 incubator after 24 h of incubation with
HSYA and/or AKBA. The cells were then carefully washed twice with
PBS. Fluorescence was read at 510 nm (excitation) and 580 nm
(emission). The fluorescence intensity was quantified using ImageJ
software (National Institutes of Health, Bethesda, MD, USA).
Assessment of mitochondrial membrane
potential (ΔΨm or MMP)
ΔΨm was assessed using a flow cytometer (FACScan;
Becton-Dickinson, Frankin Lakes, NJ, USA) using
5,5′,6,6′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) dye
(KeyGen Biotech) according to the manufacturer's instructions. The
H9C2 cells were stained with JC-1 for 15 min at 37°C in a
CO2 incubator following 24 h of incubation with HSYA
and/or AKBA. Green fluorescence was analyzed in the FL-1 (FITC)
channel and red fluorescence was analyzed in the FL-2 (PE-A)
channel. The fluorescence intensity was acquired for 10,000 events.
The ratio of aggregated JC-1 and monomeric JC-1 represented the ΔΨm
of the H9C2 cells.
Evaluation of lipid peroxidation and
antioxidant enzyme levels
After experimental treatment, the homogenates were
centrifuged at 16,000 rpm for 10 minutes. The resulting supernatant
and the culture medium of the H9C2 cells was used to assay
malondialdehyde (MDA) levels and superoxide dismutase (SOD)
activity, according to the manufacturer's instructions, on a
microplate reader at 560 and 532 nm. The commercially available
assay kits were purchased from Jiancheng Bioengineering
Institute.
Immunohistochemical analysis
The paraffin sections (5 mm thickness) were
deparaffinaged in xylene, and then rehydrated with various grades
of ethanol (100, 95, 90, 80 and 70%). The sections were exposed to
3% H2O2 for 10 min at 37°C, and treated with
citrate-buffered saline for 15 min at 95°C. By incubating the
sections in 10% bovine serum albumin, non-specific binding of
immunoglobulins was blocked for 10 min. Subsequently, the sections
were incubated overnight at 4°C with primary antibodies, Nrf2
(ab31163, 1:1,000 dilution) or PGC-1α (ab54481, 1:1,000 dilution)
(both from Abcam, Cambridge, MA, USA) and then rinsed with PBS and
incubated for 1 h with peroxidase-conjugated secondary antibody.
The sections were visualized with 4′,6-diamidino-2-phenylindole
(DAPI) for nuclear counterstaining. Finally, the stained sections
were examined under a fluorescence microscope (Olympus).
Protein extraction and western blot
analysis
The cultured H9C2 cells were washed three times with
ice-cold PBS and then harvested by scraping and centrifugation
(16,000 × g for 15 min at 4°C). The sediment was lysed in Animal
Cell Lysis solution containing 1 mM protease inhibitor cocktail and
phosphatase inhibitor mix (Tiandz, Inc., Beijing, China) on ice for
30 min. The lysates were collected by centrifugation at 4°C for 15
min at 12,000 rpm. After quantifying the protein concentration
using a Coomassie (Bradford) Protein Assay kit (Tiangen Biotech,
Beijing, China) with a microplate reader at 595 nm (Thermo Fisher
Scientific), the lysates were boiled for 5 min at 100°C in loading
buffer. Total protein (20 µg) was subjected to 8% SDS-PAGE
and transferred onto nitrocellulose membranes (Millipore Corp.,
Billerica, MA, USA), and blocked for 30 min at 37°C with 5% skim
milk. The membranes were incubated with the respective primary
antibodies, namely Nrf2 (1:1,000 dilution) and PGC-1α (1:1,000
dilution) (both from Abcam) and β-actin (1:5,000 dilution;
Sigma-Aldrich) overnight at 4°C. After extensive washing, the
membranes were incubated with horseradish peroxidase-conjugated
secondary antibodies in TBST solution for 30 min at 37°C, and then
washed as described above. The bands were visualized with an
ECL-Plus chemiluminescence kit (Millipore Corp.) and the signals
were scanned and quantified by densitometric analysis (Bio-Rad,
Richmond, CA, USA). β-actin was used as a total protein loading
control.
Statistical analysis
Statistical analysis was performed by one-way
analysis of variance (ANOVA) followed by a least significant
difference (LSD) test for multiple comparisons, using SPSS version
13.0 statistical software. P<0.05 was considered to indicate a
statistically significant difference. All the results in this study
are presented as the means ± SD.
Results
HSYA and AKBA exert protective effects
against MI
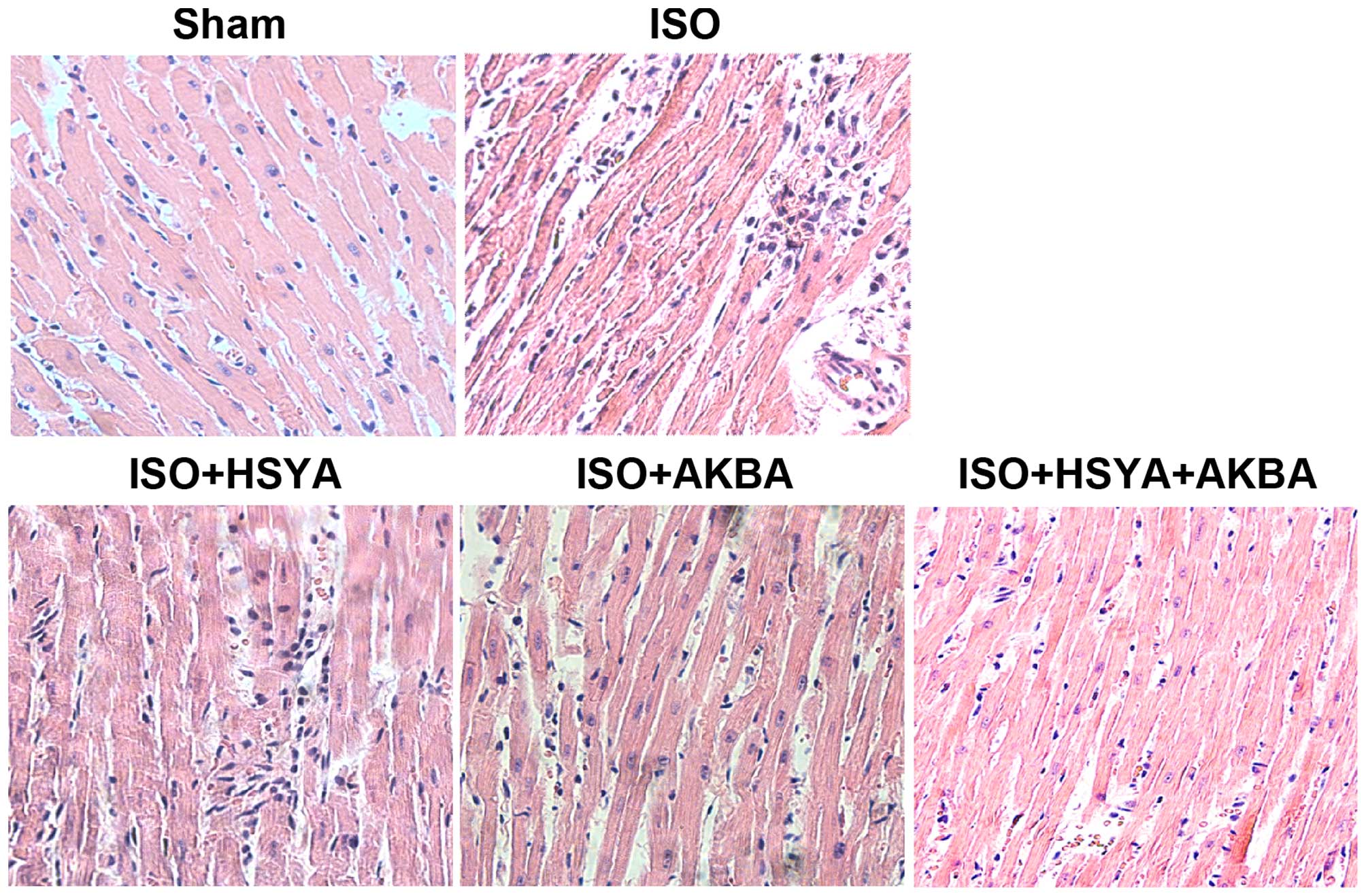
Histopathological analysis of the rat cardiac tissue
was performed under a light microscope. Normal cardiac muscle
fibers without necrosis, a branched appearance and continuity with
adjacent myofibrils were observed in the sham group. Marked
myofibrillar degeneration, necrosis, edema and infiltration with
neutrophil granulocytes were found in the ISO-exposed group.
However, HSYA and AKBA exerted protective effects against MI, and
the combination of HSYA and AKBA appeared to be more effective
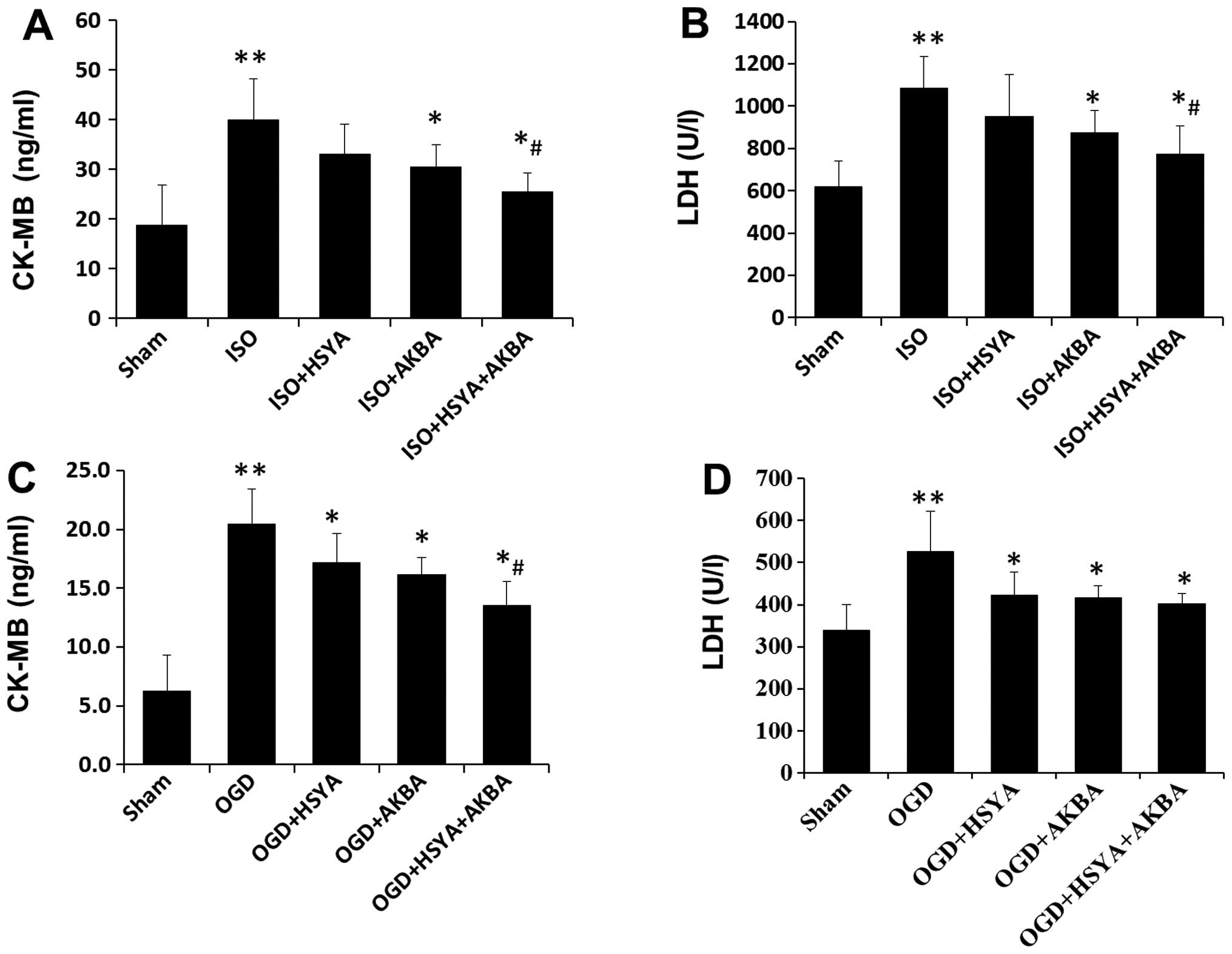
(Fig. 2). ISO-induced MI caused
the release of CK-MB and LDH into the bloodstream. Significant
increases in the levels of CK-MB and LDH were detected in the
ISO-exposed group compared with those in the sham group. Treatment
with HSYA or AKBA reduced the ISO-mediated increase in the levels
of CK-MB and LDH (Fig. 3A and B).
To model ischemic-like conditions in vitro, H9C2 cells were
subjected to transient OGD. HSYA and AKBA also markedly decreased
CK-MB and LDH levels in the H9C2 cells following OGD (Fig. 3C and D). A combination of HSYA and
AKBA exerted synergistic effects in vivo and in vitro
(Fig. 3).
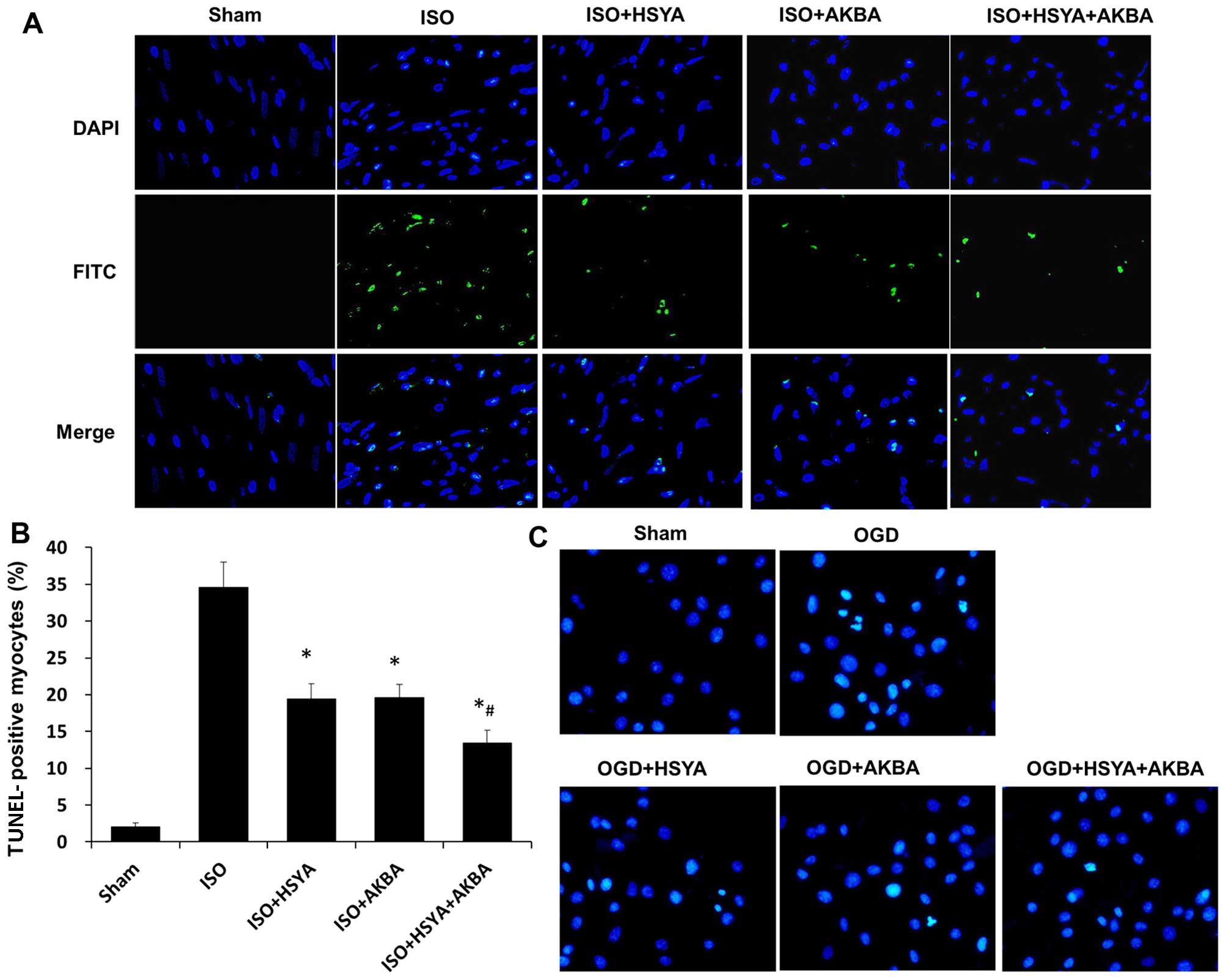
Furthermore, the protective effects of HSYA and AKBA
against myocardial damage were confirmed by TUNEL staining on
sections of the rat myocardium isolated 48 h after the induction of
ischemia by ISO (Fig. 4A). In the
ISO-exposed group, TUNEL-positive cells were densely distributed in
the myocardium. The HSYA- and AKBA-treated groups exhibited fewer
numbers of TUNEL-positive cells. The percentage of TUNEL-positive
cells in the ischemic myocardium decreased from 34.57 to 19.47% or
19.61% following treatment with HSYA or AKBA, respectively (n=6
rats/group; P<0.05) (Fig. 4B).
HSYA and AKBA in combination further decreased the percentage of
TUNEL-positive cells compared with that in the HSYA or AKBA groups
(P<0.05) (Fig. 4B).
Representative photomicrographs of Hoechst 33258 staining are shown
in Fig. 4C. The majority of the
H9C2 cells in the group subjected to OGD appeared shrunken with
triangulated, pycnotic nuclei. By contrast, the extent of cell
damage was substantially reduced in the HSYA- and AKBA-treated
groups (Fig. 4C).
HSYA and AKBA attenuate oxidative
stress
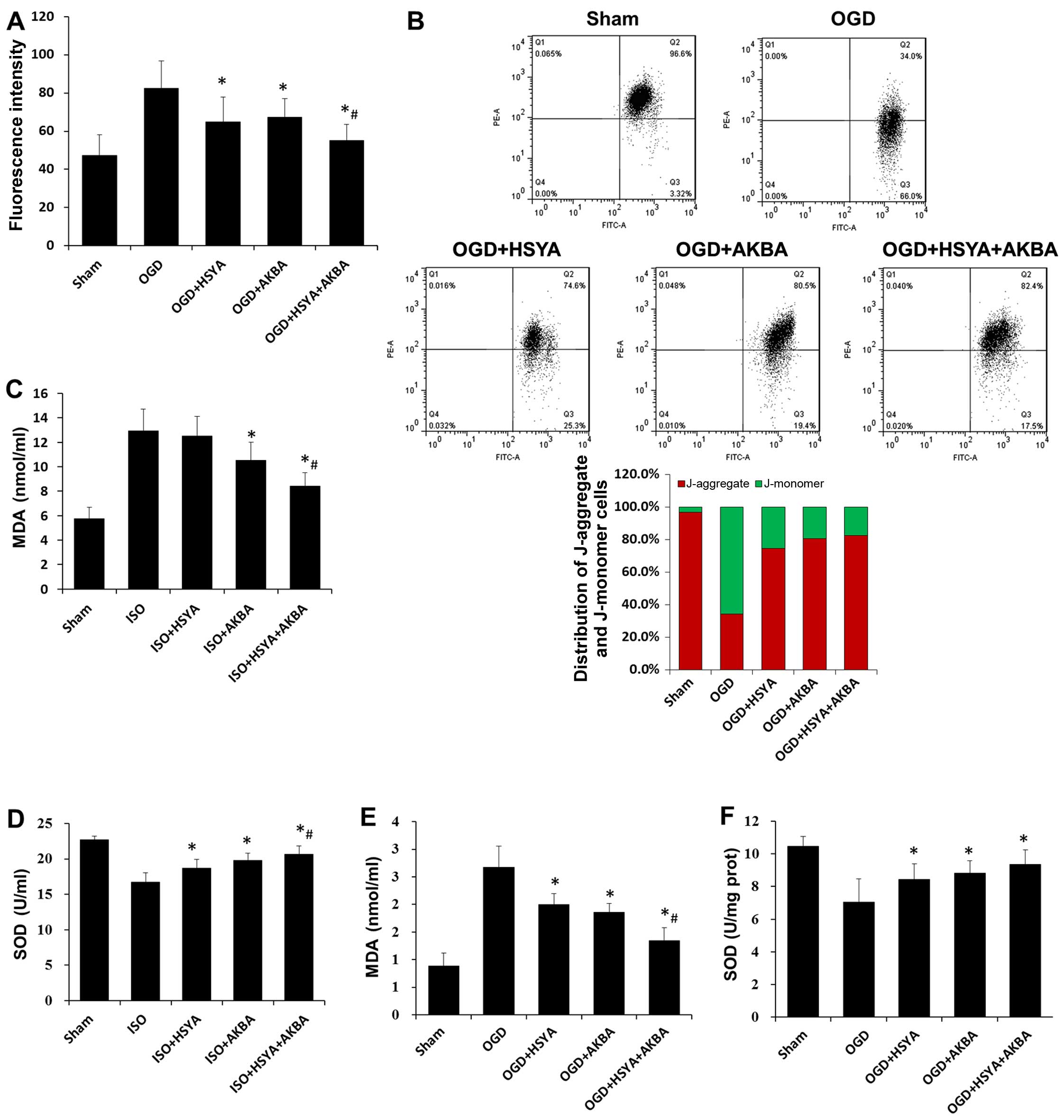
Assessment of the mitochondrial ROS levels
demonstrated that HSYA and AKBA effectively reduced the OGD-induced
increase in mitochondrial ROS levels (Fig. 5A). The OGD-induced decrease in ΔΨm
was partly prevented by HSYA or AKBA (Fig. 5B). A combination of HSYA and AKBA
induced a further decrease in ROS levels and an increase in ΔΨm
compared with the HSYA- or AKBA-treated H9C2 cells subjected to OGD
(Fig. 5A and B). The MDA level,
which is an index of lipid peroxidation, was increased in the
groups exposed to ISO or OGD conditions compared with that in the
sham groups. A reduction in the MDA level was observed in the HSYA-
or AKBA-treated groups (n=6 rats/group; P<0.05) (Fig. 5C and E). SOD activity was
decreased in the groups exposed to ISO or OGD conditions compared
with that in the sham groups, and was restored by HSYA or AKBA (n=6
rats/group; P<0.05) (Fig. 5D and
F). A combination of HSYA and AKBA induced a further decrease
in the MDA level and a further increase in SOD activity in the
groups which were exposed to ISO or OGD conditions (Fig. 5C–F).
HSYA and AKBA increase the expression of
PGC-1α and Nrf2
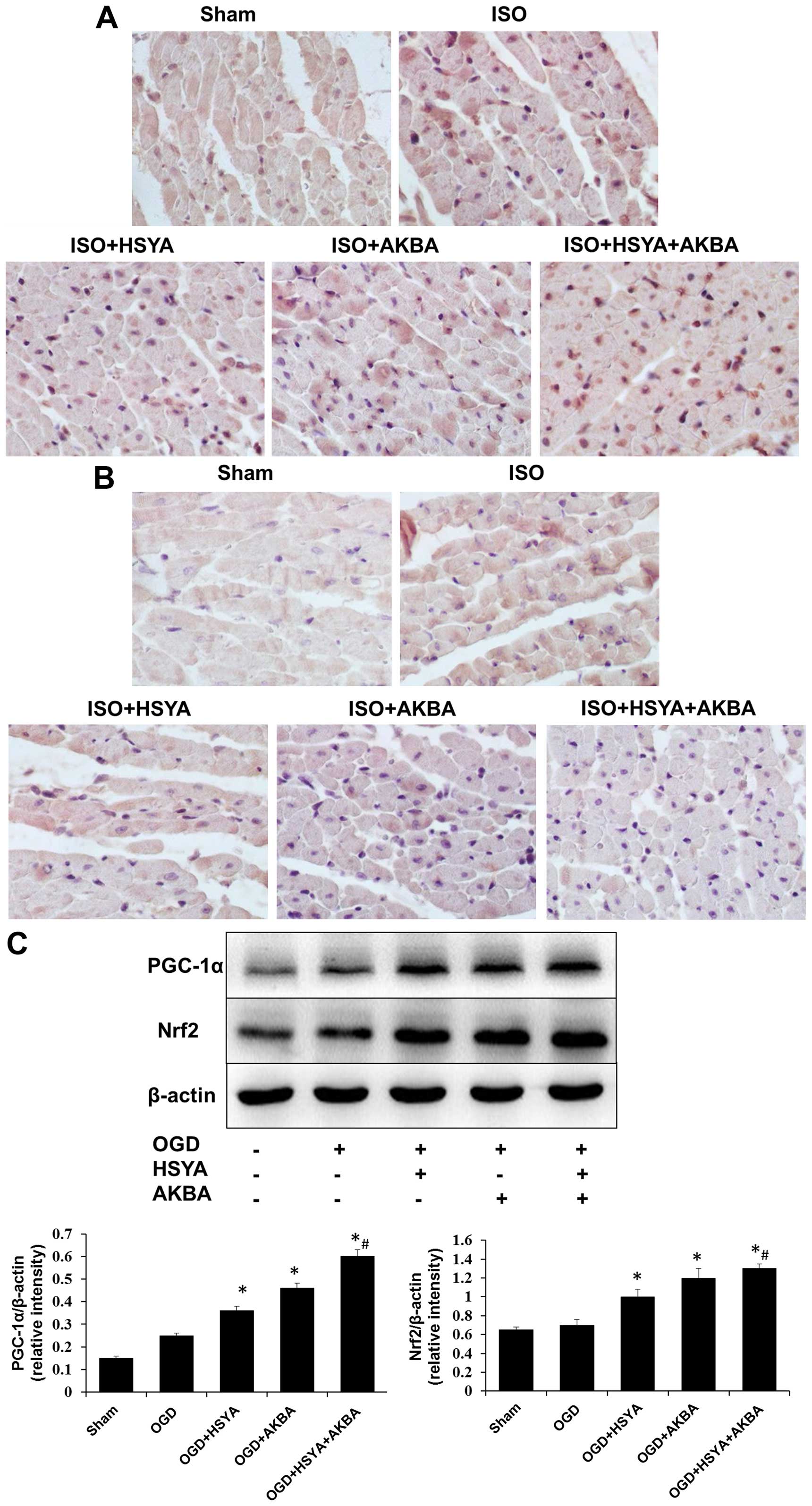
To determine whether PGC-1α/Nrf2 signaling is
involved in the cardioprotective effects exerted by HSYA or AKBA,
the expression of PGC-1α and Nrf2 in the rat myocardium and the
H9C2 cells was evaluated by immunohistochemistry and western blot
analysis, respectively. Immuhistochemical analysis of the
myocardium revealed that HSYA or AKBA increased the expression of
PGC-1α and Nrf2 following ISO exposure (Fig. 6A and B). This was consistent with
the results of western blot analysis which revealed that the
expression of PGC-1α and Nrf2 in the H9C2 cells was upregulated by
HSYA or AKBA following exposure to OGD conditions (Fig. 6C). HSYA and AKBA in combination
further enhanced the expression of PGC-1α and Nrf2 in the H9C2
cells compared with either HSYA or AKBA alone (n=6 rats/group;
P<0.05) (Fig. 6C).
Discussion
In the present study, we confirmed the protective
effects of HSYA and AKBA against MI. Pre-treatment with HSYA and
AKBA significantly inhibited tissue damage and cell death by
decreasing mitochondrial ROS levels due to the increased expression
of PGC-1α and Nrf2. In addition, HSYA and AKBA in combination
appeared to exert a synergistic cardioprotective effect.
Oxidative stress plays a critical role in the
pathophysiology of myocardial ischemic injury (23). HSYA and AKBA are capable of
neutralizing the increased mitochondrial ROS production (Fig. 5A). The first oxygen reduction
product generated in mitochondria is superoxide
(O2−), which may be converted to
H2O2 (24).
To determine whether intracellular O2− levels
were decreased by HSYA and AKBA following MI, we stained the cells
with MitoSOX Red, a superoxide-sensitive mitochondria-targeted
hydroethidine analog. Taken together, these findings demonstrated
that HSYA and AKBA enhanced the ability of cardiac mitochondria to
decompose H2O2. Mitochondria are the
principal source of endogenous ROS in the majority of mammalian
cell types. Mitochondrial alterations are closely associated with
ROS generation (25). During
ischemia, mitochondria generate large quantities of ROS. However,
excessive ROS production also damages mitochondria (26,27). The destruction of mitochondrial
integrity is associated with disruption of the ΔΨm. It has also
been demonstrated that the increased permeabilization of the
mitochondrial membrane may lead to increased intracellular ROS
generation (28). Mitochondria
are a key therapeutic target in MI. The results demonstrated that
HSYA and AKBA largely maintained the ΔΨm following OGD (Fig. 5B).
The antioxidant system is a compensatory mechanism
for hyperoxidation which protects against oxidative injury. The
effective ROS scavenger, SOD, is therefore essential for protective
tissue functions. SOD is one of the major
H2O2-inducible antioxidant enzymatic
defenses, which plays an important role in neutralizing oxygen free
radicals (29). MDA is not only
generated by oxidative stress-induced peroxidation but also by the
interaction between free radical or lipid peroxyl radicals
(LOO•) with lipid molecules (30). In the present study, the reduced
activity of SOD and the increased levels of MDA were due to
increased oxidative stress following ISO-induced MI. Reduced SOD
activity and increased MDA levels may be attributable to the
accumulation of free radicals in the heart and irreversible
depletion of the endogenous antioxidant system. Consistent with
previous findings (31), a
significant increase in SOD activity and a decrease in MDA levels
were observed in the HSYA or AKBA groups, in the present study
(Fig. 5). HYSA and AKBA reduced
ROS levels, enhanced SOD activity and inhibited lipid peroxidation,
therefore alleviating MI.
Mitochondria are the principal sites of ROS
production (32). PGC-1α and Nrf2
are the major regulators of mitochondrial biogenesis and activity
(6,7). PGC-1α and Nrf2 regulate the
expression of a set of antioxidant-related genes which remove ROS
through sequential enzymatic reactions (9,33).
Thus, they are the key components involved in the maintenance of
cellular redox homeostasis following oxidative stress-induced MI
(10). Consequently, compounds
that interfere with the PGC-1α/Nrf2 pathway may have the potential
to be used as cardioprotective agents. It has been previously
demonstrated that HSYA exerts neuroprotective effects against
cerebral I/R injury through its antioxidant action (34). HSYA has been reported to protect
H9C2 cardiomyocytes against apoptosis through the PI3K/Akt/Nrf2
pathway (14). Additionally, AKBA
was demonstrated to exert antioxidant effects thereby protecting
against cerebral ishemic injury in the rat brain through the
Nrf2/heme oxygenase-1 (HO-1) pathway (18). Consequently, it is important to
examine the antioxidant activity of HSYA and AKBA and the role of
these compounds in modulating the PGC-1α/Nrf2 pathway. In the
present study, HSYA and AKBA markedly increased the expression of
PGC-1α and nuclear Nrf2 in rats with ISO-induced MI as well as in
the H9C2 cells subjected to OGD. The activation of PGC-1α and Nrf2
is known to stimulate mitochondrial biogenesis and to activate
enzymes. Following nuclear translocation, PGC-1α promotes the
expression of its target genes and thus, maintains the basal and
inducible expression of a number of antioxidant genes. It has been
found that PGC-1α relies on docking to particular transcription
factors in order to perform different biological programs (35). The transcriptional activation of
mitochondrial biogenesis is dependent on docking of Nrf2. Increased
Nrf2 activity is associated with an enhanced antioxidant defenses
and tolerance to stress-induced tissue pathologies (36).
In clinical practice, the concept of multidrug
interventions for MI has received much attention (37–39). Drugs act synergistically when
lower concentrations of both drugs produce better efficacy than
monotherapy with either agent. The synergistic mode of action of
drugs should be given priority when multi-drug therapy is
indicated. In comparison with monotherapy, the potential advantages
of the combination drug therapy are: i) lower doses of drugs
improve treatment efficacy; ii) reduced adverse effects and drug
toxicities. The present study demonstrated the synergistic actions
of HSYA and AKBA to produce cardioprotective effects (Figs. 2Figure 3–4). In support of this notion, the
administration of HSYA and AKBA in combination exerted synergistic
antioxidant effects (Fig. 5).
Western blot analysis revealed the synergistic effects of AKBA and
HSYA on PGC-1α expression in vitro (Fig. 6C). HSYA and AKBA in combination
decreased mitochondrial superoxide production and largely
maintained ΔΨm, thus, leading to further protection against MI
compared with HSYA or AKBA alone.
Overall, the results of the present study have
demonstrated that HSYA and AKBA are effective in protecting against
MI by alleviating mitochondria-dependent oxidative stress, through
the enhanced expression of PGC-1α and Nrf2. In addition, HSYA and
AKBA appear to exert synergistic cardioprotective effects.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81373947 and
81201985) and the Key Technologies for New Drug Innovation and
Development of China (nos. 2011ZXJ09202-13 and 2012BAK25B00).
Abbreviations:
|
AKBA
|
acetyl-11-keto-β-boswellic acid
|
|
HSYA
|
hydroxysafflor yellow A
|
|
MI
|
myocardial injury
|
|
ROS
|
reactive oxygen species
|
|
SY
|
safflower yellow
|
|
ISO
|
isoproterenol
|
|
OGD
|
oxygen-glucose deprivation
|
|
ΔΨm or MMP
|
mitochondrial membrane potential
|
|
JC-1
|
5,5′,6,6′-tetraethylbenzimidazolylcarbocyanine iodide
|
|
PGC-1α
|
peroxisome proliferator-activated
receptor gamma coactivator-1α
|
|
Nrf2
|
nuclear factor erythroid 2-related
factor 2
|
|
CK-MB
|
creatine kinase-MB
|
|
LDH
|
lactate dehydrogenase
|
|
SOD
|
superoxide dismutase
|
|
MDA
|
malondialdehyde
|
References
|
1
|
Anversa P and Sonnenblick EH: Ischemic
cardiomyopathy: pathophysiologic mechanisms. Prog Cardiovasc Dis.
33:49–70. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion - from mechanism to translation. Nat Med. 17:1391–1401.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takimoto E and Kass DA: Role of oxidative
stress in cardiac hypertrophy and remodeling. Hypertension.
49:241–248. 2007. View Article : Google Scholar
|
|
4
|
Zhang J, Wei C, Wang H, Tang S, Jia Z,
Wang L, Xu D and Wu Y: Protective effect of qiliqiangxin capsule on
energy metabolism and myocardial mitochondria in pressure overload
heart failure rats. Evid Based Complement Alternat Med.
2013:3782982013.PubMed/NCBI
|
|
5
|
Wan Z, Root-McCaig J, Castellani L, Kemp
BE, Steinberg GR and Wright DC: Evidence for the role of AMPK in
regulating PGC-1alpha expression and mitochondrial proteins in
mouse epididymal adipose tissue. Obesity (Silver Spring).
22:730–738. 2014. View Article : Google Scholar
|
|
6
|
Lai L, Wang M, Martin OJ, Leone TC, Vega
RB, Han X and Kelly DP: A role for peroxisome
proliferator-activated receptor γ coactivator 1 (PGC-1) in the
regulation of cardiac mitochondrial phospholipid biosynthesis. J
Biol Chem. 289:2250–2259. 2014. View Article : Google Scholar :
|
|
7
|
Wu Z, Puigserver P, Andersson U, Zhang C,
Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC and
Spiegelman BM: Mechanisms controlling mitochondrial biogenesis and
respiration through the thermogenic coactivator PGC-1. Cell.
98:115–124. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cimino F, Speciale A, Anwar S, Canali R,
Ricciardi E, Virgili F, Trombetta D and Saija A: Anthocyanins
protect human endothelial cells from mild hyperoxia damage through
modulation of Nrf2 pathway. Genes Nutr. 8:391–399. 2013. View Article : Google Scholar :
|
|
9
|
Zhou S, Sun W, Zhang Z and Zheng Y: The
role of Nrf2-mediated pathway in cardiac remodeling and heart
failure. Oxid Med Cell Longev. 2014:2604292014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hybertson BM, Gao B, Bose SK and McCord
JM: Oxidative stress in health and disease: the therapeutic
potential of Nrf2 activation. Mol Aspects Med. 32:234–246. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nie PH, Zhang L, Zhang WH, Rong WF and Zhi
JM: The effects of hydroxysafflor yellow A on blood pressure and
cardiac function. J Ethnopharmacol. 139:746–750. 2012. View Article : Google Scholar
|
|
12
|
Feng ZM, He J, Jiang JS, Chen Z, Yang YN
and Zhang PC: NMR solution structure study of the representative
component hydroxysafflor yellow A and other quinochalcone
C-glycosides from Carthamus tinctorius. J Nat Prod. 76:270–274.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han SY, Li HX, Ma X, Zhang K, Ma ZZ and Tu
PF: Protective effects of purified safflower extract on myocardial
ischemia in vivo and in vitro. Phytomedicine. 16:694–702. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu SX, Zhang Y, Wang YF, Li XC, Xiang MX,
Bian C and Chen P: Upregulation of heme oxygenase-1 expression by
hydroxysafflor yellow A conferring protection from
anoxia/reoxygenation-induced apoptosis in H9c2 cardiomyocytes. Int
J Cardiol. 160:95–101. 2012. View Article : Google Scholar
|
|
15
|
Park B, Sung B, Yadav VR, Cho SG, Liu M
and Aggarwal BB: Acetyl-11-keto-β-boswellic acid suppresses
invasion of pancreatic cancer cells through the downregulation of
CXCR4 chemokine receptor expression. Int J Cancer. 129:23–33. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Sun Y, Wang C, Huo X, Liu P, Wang
C, Zhang B, Zhan L, Zhang H, Deng S, et al: Biotransformation of
11-keto-β-boswellic acid by Cunninghamella blakesleana.
Phytochemistry. 96:330–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Henkel A, Kather N, Mönch B, Northoff H,
Jauch J and Werz O: Boswellic acids from frankincense inhibit
lipopolysaccharide functionality through direct molecular
interference. Biochem Pharmacol. 83:115–121. 2012. View Article : Google Scholar
|
|
18
|
Ding Y, Chen M, Wang M, Wang M, Zhang T,
Park J, Zhu Y, Guo C, Jia Y, Li Y and Wen A: Neuroprotection by
acetyl-11-keto-β-Boswellic acid, in ischemic brain injury involves
the Nrf2/HO-1 defense pathway. Sci Rep. 4:70022014. View Article : Google Scholar
|
|
19
|
Stanely Mainzen Prince P and Roy AJ:
p-Coumaric acid attenuates apoptosis in isoproterenol-induced
myocardial infarcted rats by inhibiting oxidative stress. Int J
Cardiol. 168:3259–3266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tian Y, Yang ZF, Li Y, Qiao Y, Yang J, Jia
YY and Wen AD: Pharmacokinetic comparisons of hydroxysafflower
yellow A in normal and blood stasis syndrome rats. J
Ethnopharmacol. 129:1–4. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yadav VR, Prasad S, Sung B, Gelovani JG,
Guha S, Krishnan S and Aggarwal BB: Boswellic acid inhibits growth
and metastasis of human colorectal cancer in orthotopic mouse model
by down-regulating inflammatory, proliferative, invasive and
angiogenic biomarkers. Int J Cancer. 130:2176–2184. 2012.
View Article : Google Scholar
|
|
22
|
Woo AY, Cheng CH and Waye MM: Baicalein
protects rat cardiomyocytes from hypoxia/reoxygenation damage via a
prooxidant mechanism. Cardiovasc Res. 65:244–253. 2005. View Article : Google Scholar
|
|
23
|
Tomaselli GF and Barth AS: Sudden cardio
arrest: oxidative stress irritates the heart. Nat Med. 16:648–649.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cadenas E and Davies KJ: Mitochondrial
free radical generation, oxidative stress, and aging. Free Radic
Biol Med. 29:222–230. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Green DR and Kroemer G: The
pathophysiology of mitochondrial cell death. Science. 305:626–629.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Smith RA, Hartley RC, Cochemé HM and
Murphy MP: Mitochondrial pharmacology. Trends Pharmacol Sci.
33:341–352. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Q and Lesnefsky EJ: Depletion of
cardiolipin and cytochrome c during ischemia increases hydrogen
peroxide production from the electron transport chain. Free Radic
Biol Med. 40:976–982. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen YR and Zweier JL: Cardiac
mitochondria and reactive oxygen species generation. Circ Res.
114:524–537. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Warner DS, Sheng H and Batinić-Haberle I:
Oxidants, antioxidants and the ischemic brain. J Exp Biol.
207:3221–3231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Halliwell B and Gutteridge J: Free
Radicals in Biology and Medicine. 1st edition. Oxford University
Press; New York, NY: 2007
|
|
31
|
Hartmann RM, Morgan Martins MI, Tieppo J,
Fillmann HS and Marroni NP: Effect of Boswellia serrata on
antioxidant status in an experimental model of colitis rats induced
by acetic acid. Dig Dis Sci. 57:2038–2044. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li J, Ma X, Yu W, Lou Z, Mu D, Wang Y,
Shen B and Qi S: Reperfusion promotes mitochondrial dysfunction
following focal cerebral ischemia in rats. PLoS One. 7:e464982012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
St-Pierre J, Drori S, Uldry M, Silvaggi
JM, Rhee J, Jäger S, Handschin C, Zheng K, Lin J, Yang W, et al:
Suppression of reactive oxygen species and neurodegeneration by the
PGC-1 transcriptional coactivators. Cell. 127:397–408. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wei X, Liu H, Sun X, Fu F, Zhang X, Wang
J, An J and Ding H: Hydroxysafflor yellow A protects rat brains
against ischemia-reperfusion injury by antioxidant action. Neurosci
Lett. 386:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kressler D, Schreiber SN, Knutti D and
Kralli A: The PGC-1-related protein PERC is a selective coactivator
of estrogen receptor alpha. J Biol Chem. 277:13918–13925. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Joshi G and Johnson JA: The Nrf2-ARE
pathway: A valuable therapeutic target for the treatment of
neurodegenerative diseases. Recent Patents CNS Drug Discov.
7:218–229. 2012. View Article : Google Scholar
|
|
37
|
Ma X, Oyamada S, Gao F, Wu T, Robich MP,
Wu H, Wang X, Buchholz B, McCarthy S, Gu Z, et al:
Paclitaxel/sirolimus combination coated drug-eluting stent: In
vitro and in vivo drug release studies. J Pharm Biomed Anal.
54:807–811. 2011. View Article : Google Scholar
|
|
38
|
Gaziano TA, Galea G and Reddy KS: Scaling
up interventions for chronic disease prevention: the evidence.
Lancet. 370:1939–1946. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Robich MP, Chu LM, Oyamada S, Sodha NR and
Sellke FW: Myocardial therapeutic angiogenesis: a review of the
state of development and future obstacles. Expert Rev Cardiovasc
Ther. 9:1469–1479. 2011. View Article : Google Scholar : PubMed/NCBI
|