Introduction
Prostate cancer (PCa) is the second most frequently
diagnosed cancer in Europe (1)
and the second leading cause of cancer mortality in males in the
USA (2). Although a complete
understanding of the causes of PCa remains elusive, obesity,
advancing age and family history have been established as the
principal risk factors (3).
Genetic alterations may also increase the risk of developing PCa,
as suggested by associations with specific gene variants. Mutations
in BRCA1 and BRCA2 (4),
hereditary PCa gene 1, the androgen receptor and the vitamin D
receptor (5), as well as fusion
between TMPRSS2 and ETS family members, have also been implicated
in PCa (6). Despite recent
advances, the molecular mechanisms involved in the development and
progression of PCa remain unclear.
DEAD (Asp-Glu-Ala-Asp) box polypeptide 20 (DDX20), a
member of the DEAD box protein family, encodes an RNA helicase.
Recently, the roles of DDX20 have been reported in hepatocellular
carcinoma (HCC) (7) and breast
cancer (8). In HCC, a deficiency
of DDX20 was demonstrated to impair the functioning of miRNA-140
and lead to hepatocarcinogenesis. However, in breast cancer, DDX20
was identified as a biomarker and an oncogenic driver of
metastasis. These contradictory findings prompted us to explore the
role of DDX20 in PCa.
In the present study, we demonstrated that the
expression of DDX20 is frequently increased in PCa. As DDX20 is
reportedly associated with the metastasis of breast cancer, we
examined whether DDX20 affects the same biological activities in
PCa. The results of the present study indicate that DDX20 plays a
similar role in PCa.
Materials and methods
Patients and sample collection
Twenty-four patients with PCa, who underwent
surgical resection at Chengdu Military General Hospital (Chengdu,
China) between 2008 and 2014, were enrolled into the present study.
All samples were frozen in liquid nitrogen immediately following
surgical resection and stored at −80°C until RNA extraction.
Ethics statement
All fresh tumor tissues and matched adjacent tissues
were collected from patients with pathologically and clinically
confirmed PCa. All human tumor tissues were obtained with written
informed consent from patients. The Institutional Review Board of
Chengdu Military General Hospital approved the use of the tumor
sample in this study.
Immunohistochemistry (IHC)
A PCa tissue microarray (TMA; HPro-Ade180PG-01)
containing tissues from 99 cases (81 paired carcinoma and adjacent
tissues as well as 18 cancer specimens) was purchased from Outdo
Biotech (Shanghai, China). Staining of the TMA was performed
according to standard IHC protocols. Following deparaffinization
and dehydration of the TMA, endogenous peroxidase activity was
blocked with 0.3% hydrogen peroxide (Sangon, Shanghai, China) at
37°C for 30 min, and then antigen retrieval was performed by
boiling in 10 mM citrate buffer (pH 6.0) for 15 min. The sections
were blocked with 10% bovine serum albumin (BSA; Sangon) for 1 h
and incubated with DDX20 antibody (1:1,000; 11324-1-AP) and matrix
metallopeptidase 9 (MMP9) antibody (1:1,000; 10375-2-AP) (both from
ProteinTech, Chicago, IL, USA) overnight at 4°C. The next day, the
TMA was incubated with horseradish peroxidase (HRP)-labeled
anti-mouse secondary antibody (1:200; Dako, Carpinteria, CA, USA)
for 1 h at room temperature. Antibody binding was detected using
3,3′-diaminobenzidine (DAB) in substrate chromogen solution (Dako).
The TMA was counterstained with hematoxylin (Beyotime, Nantong,
China) prior to dehydration and mounting. The final expression
level of DDX20 was designated as a low and high expression group:
score 0–1, low expression; 2–3, high expression. DDX20 expression
was quantified by two independent pathologists. The slides were
visualized using a Primostar FL2 microscope (Carl Zeiss,
Oberkochen, Germany).
Cell culture
The PCa cell lines LNCaP, PC-3 and DU145 were
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA). All of the cell lines were maintained in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% fetal bovine
serum (FBS), 100 IU/ml penicillin and 100 µg/ml streptomycin
(all from Gibco, Carlsbad, CA, USA) at 37°C in a 5% humidified
CO2 atmosphere.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The surgical specimens were homogenized using a
Mixer Mill MM 300 homogenizer (Qiagen, Chatsworth, CA, USA). Total
RNA from these tissues and the PCa cell lines was then isolated
using TRIzol reagent (Takara Bio, Inc., Otsu, Japan) and reverse
transcribed using the PrimeScript RT-PCR kit (Takara Bio, Inc.)
according to the manufacturer's instructions. Target gene
expression was determined by performing qPCR with a SYBR Premix Ex
Taq kit (Takara Bio, Inc.) and an ABI 7500 real-time PCR system
(Applied Biosystems, Foster City, CA, USA). The primers for qPCR
were designed as follows: DDX20 forward, 5′-CCGGGGAGAGGAAGAAA
ATA-3′ and reverse, 5′-ACTTCCACATCCCAATCCAC-3′; MMP9 forward,
5′-ACGACGTCTTCCAGTACCGA-3′ and reverse, 5′-GCACTGCAGGATGTCATAGG-3′;
and GAPDH forward, 5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′. GAPDH was amplified as an internal
control. We performed the assays according to the following
program: 95°C for 30 sec, followed by 40 cycles at 95°C for 5 sec
and 60°C for 31 sec, and finally 95°C for 15 sec, 60°C for 1 min
and 95°C for 15 sec. The relative expression of DDX20 and MMP9 was
calculated using the 2−ΔΔCT method with 18S RNA as the
control.
DDX20 RNA interference (RNAi)
Specific small interfering RNAs (siRNAs; GenePharma,
Shanghai, China) were designed to silence DDX20 and for the purpose
of avoiding off-target effects, we used an siRNA pool which
contained three siRNAs. The DU145 and the PC-3 cells were seeded at
at a density of 30×104 cells in a final volume of 1.5 ml
in 6-well plates. Pooled siRNAs were transfected at a final
concentration of 10 nM with Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA). The sequences for the DDX20 siRNAs were as
follows: siNC, 5′-UUCUCCGAACGUGUCACGUTT-3′; si-1,
5′-TCTTTATTCTTGATGAA-3′; si-2, 5′-GTGGATGATCGTATTT-3′ and si-3,
5′-GTATTACAAAGTTGTCAA-3′.
Ectopic expression of DDX20
The expression plasmid containing the open reading
frame of DDX20 and empty vector was purchased from GeneCopoeia
(Guangzhou, China). The LNCaP cells (30×104 cells) were
seeded in a 6-well plate and transfected with 2 µg plasmid
using Lipofectamine 2000 (Invitrogen). After a 48 h incubation,
stably transfected cells were selected using 1 mg/ml G418 (Gibco)
in DMEM and grown for 2 weeks. The G418-resistant colonies were
isolated by a limited dilution approach. They were expanded and
then maintained in regular growth medium containing 1 mg/ml
G418.
Western blot analysis
The total protein was extracted using RIPA lysis
buffer (Beyotime, Haimen, China) according to the manufacturer's
instructions and 50 µg protein was separated by reducing
SDS-PAGE, and transferred onto a nitrocellulose membrane. The
membrane was then blocked in TBS buffer containing 5% BSA (Sangon)
for 1 h. The membrane was incubated with primary antibodies for
DDX20 (1:1,000; 11324-1-AP); MMP9 (1:1,000; 10375-2-AP), and GAPDH
(1:5,000; 10494-1-AP, all from ProteinTech) overnight, and then
followed by HRP-linked secondary antibody (#7074; Cell Signaling
Technology, Danvers, MA, USA). Immobilon™ Western Chemiluminescent
HRP Substrate kit (Millipore Corp, Darmstadt, Germany) was used for
detection.
Cell counting kit-8 (CCK-8) assay of cell
viability
To evaluate changes in cell viability, CCK-8 assays
were performed. The transfected cancer cells were seeded at a
density of 4×104 cells/well in 96-well plates at a final
volume of 100 µl medium/well. Cell viability was quantified
by adding 10 µl CCK-8 (Dojindo, Kumamoto, Japan). After a
1.5 h incubation, the plates were monitored at specific time points
using a PowerWave XS Microplate reader (BioTek, Winooski, VT, USA),
which measured absorbance at 450 nm.
Wound healing assay
The transfected cancer cells were seeded onto
12-well plates and cultured until confluent. Wounds were generated
using a sterile 200 µl pipette tip (Axygen, Union City, CA,
USA). The cells were then cultured for an additional 72 h. Wound
closure was assessed using an IX71 inverted microscope (Olympus
Corp., Tokyo, Japan). The cell migration distance was measured
using Adobe Illustrator CS5 software and compared with baseline
measurements. Each experiment was performed in triplicate.
Invasion assay
For the Transwell migration assay, the transfected
cells (4×104) were placed in the top chamber of each
insert chamber (Millipore Corp.) with the Matrigel-coated membrane.
The cells were trypsinized and resuspended in serum-free DMEM and
700 µl complete medium was injected into the lower chamber.
The plates were incubated for 48–72 h, and then the medium and the
cells remaining in the top chambers were removed. After fixation
and staining with 0.1% crystal violet (Beyotime, Nantong, China),
the cells that had migrated to the lower membrane of the inserts
were counted and images were captured under an IX71 inverted
microscope (Olympus Corp.).
Luciferase reporter assays
To perform luciferase reporter assays, the
transfected cells were seeded in 96-well plates and transfected
with a mixture of 100 ng NF-κB reporter plasmids and 10 ng
Renilla (Promega, Madison, WI, USA) according to the
manufacturer's instructions for the Lipofectamine 2000 transfection
system. Following 48 h of incubation, firefly and Renilla
luciferase activities were measured sequentially in the cell
lysates using the Dual-Luciferase Reporter Assay system
(Promega).
Statistical analysis
Statistical analysis was conducted using SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA). We performed Chi-square
tests using cross-tabulation analysis in order to assess the
relationships between the expression levels of DDX20 and
clinicopathological factors. Overall survival (OS) was calculated
using the Kaplan-Meier method. The survival distributions were
compared through the log-rank test. The Student's t-test was used
for comparisons between groups. A P-value <0.05 was considered
to indicate a statistically significant difference.
Results
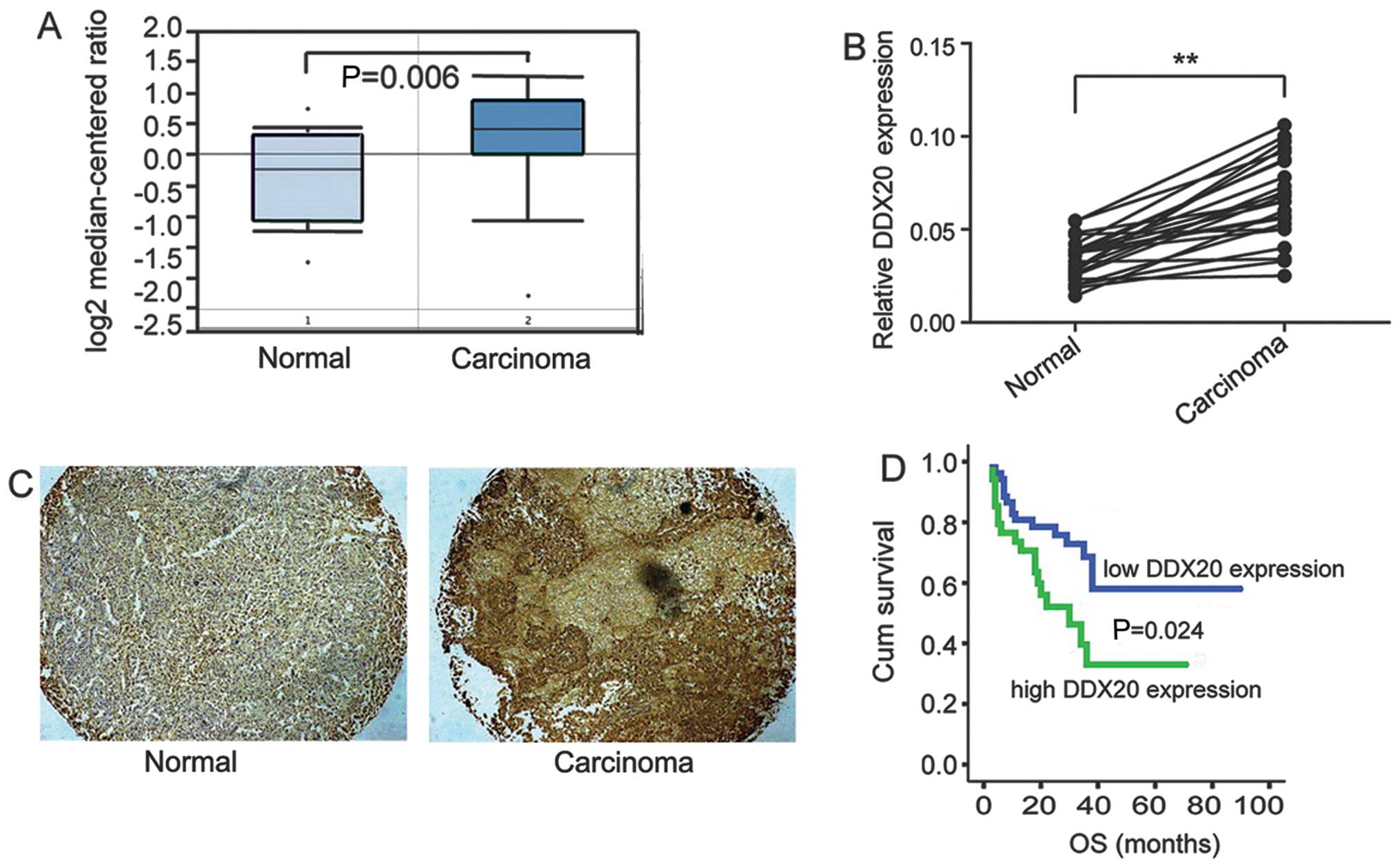
Expression of DDX20 is significantly
upregulated in PCa
Recently, a study showed that DDX20 was highly
expressed in human breast cancer and is associated with tumor
metastasis (8). This prompted us
to explore the functions of DDX20 in PCa. A query of the Oncomine
database revealed that notably DDX20 gene expression is
significantly upregulated in PCa tissue compared with that in
normal prostate gland tissue (Fig.
1A). To further delineate the expression profile of DDX20 in
fresh PCa and adjacent tissues, we examined the mRNA levels of
DDX20 in 24 paired adjacent and malignant tissues. As shown in
Fig. 1B, the mRNA expression of
DDX20 was significantly increased in the carcinomas compared with
that in the adjacent tissues. Furthermore, we detected DDX20 in a
TMA which contained 99 malignant tissues and 81 non-malignant
adjacent tissues. We found that DDX20 was more commonly detected in
the malignant tissues, as shown in Fig. 1C and this was indicated by
stronger DDX20 staining in the carcinoma tissues than in the
adjacent tissues. We then analyzed the correlation between DDX20
expression and patient prognosis and found that the patients with
high DDX20 expression had lower OS (P=0.024) (Fig. 1D). The analysis of the correlation
between DDX20 expression and key clinicopathological features is
presented in Table I and shows
that DDX20 expression is associated with tumor size, local
infiltration and Gleason grade.
 | Table ICorrelation between DDX20 expression
and key clinicopathological features. |
Table I
Correlation between DDX20 expression
and key clinicopathological features.
| Variable | DDX20 (n=99)
|
|---|
| Low | High | P-value |
|---|
| Age (years) | | | |
| ≤50 | 34 | 13 | 0.183 |
| >50 | 31 | 21 | |
| Tumor size (cm) | | | |
| ≤5 | 28 | 28 | 0.014a |
| >5 | 11 | 32 | |
| Local
infiltration | | | |
| Yes | 3 | 63 | 0.041a |
| No | 6 | 27 | |
| Gleason grade | | | |
| I | 2 | 1 | 0.018a |
| II–III | 10 | 40 | |
| IV–V | 4 | 42 | |
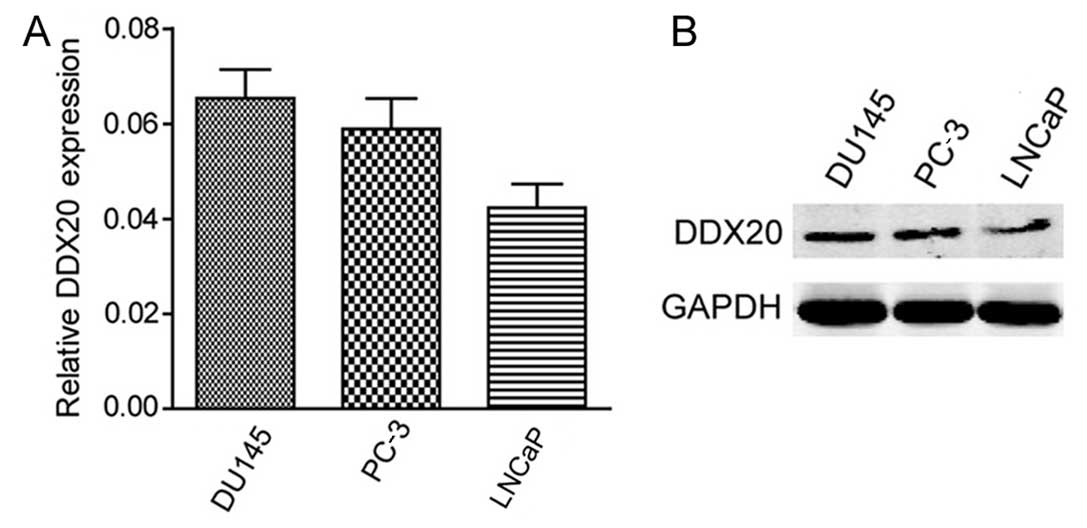
Human PCa cell lines express DDX20
The significant correlation between DDX20 expression
and clinicopathological features prompted us to investigate the
effect of DDX20 on various cellular functions during PCa
development. Firstly, we examined the expression of DDX20 using
RT-qPCR; the DU145 and PC-3 cells exhibited higher expression
levels of DDX20 compared with the LNCaP cells (Fig. 2A). To confirm the protein
expression of DDX20 in the three cell lines, we performed western
blot analysis and the results showed the same pattern as the
RT-qPCR results (Fig. 2B).
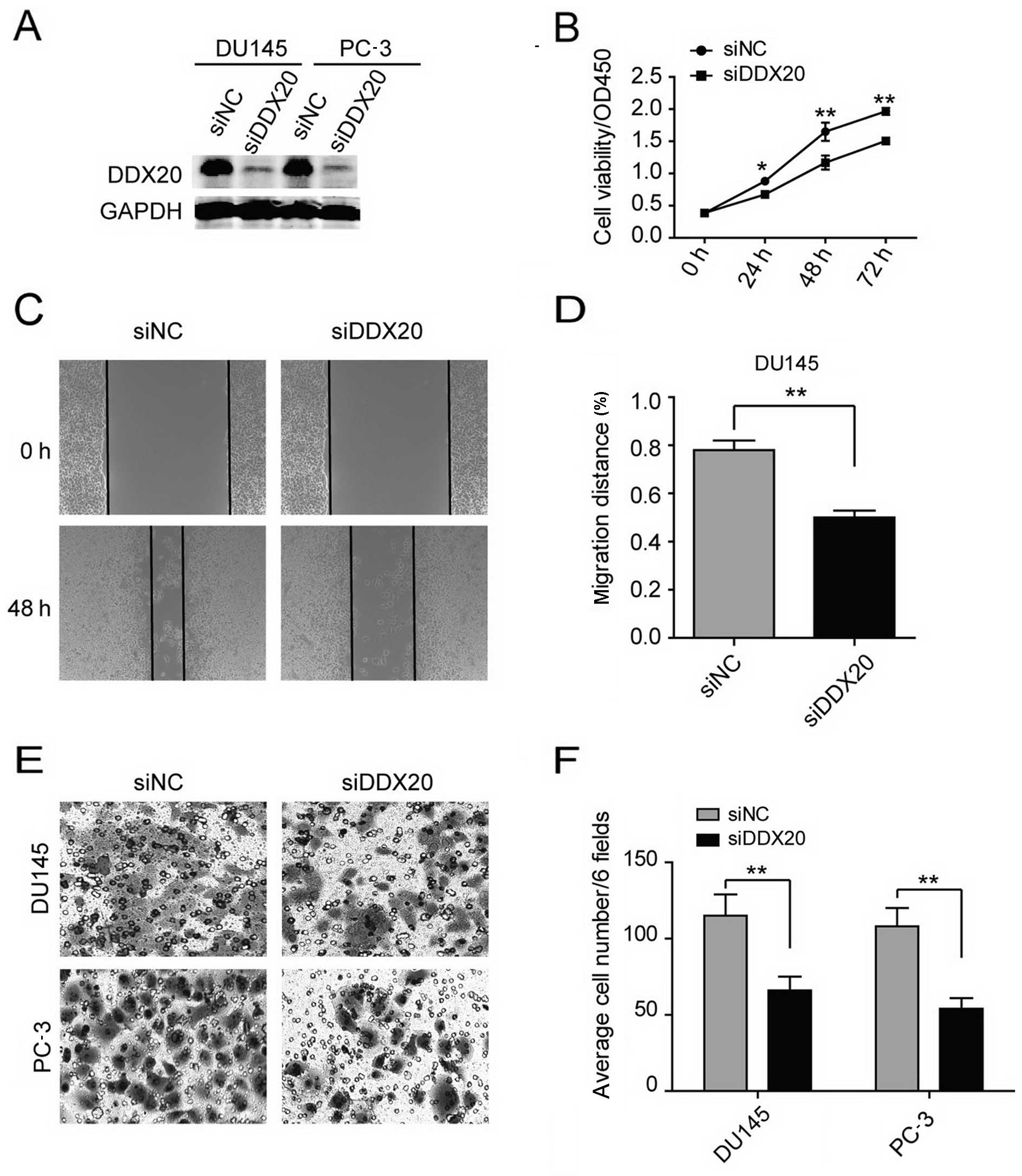
DDX20 contributes to the proliferation,
migration and invasiveness of human PCa cells
We selected the DU145 and PC-3 cell lines for
further RNAi analysis and the LNCaP cell line for use in
overexpression experiments in order to examine DDX20 expression.
The transfection of the DU145 and PC-3 cells with DDX20 siRNA
reduced DDX20 expression (Fig.
3A). We studied various cellular functions, namely
proliferation, migration and invasion, following siRNA treatment.
To examine cell proliferation, we performed CCK-8 cell viability
assays and found that cell viability decreased significantly in the
DDX20- silenced cells (Fig. 3B).
We then assessed cell migratory behavior. After silencing DDX20, we
performed wound-healing assays and found that DDX20 depletion led
to decreased migration (Fig. 3C and
D). Given that the suppression of DDX20 affects cell motility,
we next explored the effects of decreasing DDX20 expression on the
invasiveness of the malignant cells by performing the
Matrigel-coated Transwell invasion assay. In the DU145 and PC-3
cells, knockdown of DDX20 resulted in 40–50% fewer cells invading
through the Matrigel-coated inserts (Fig. 3E and F). Taken together, these
results demonstrate that DDX20 depletion led to the inhibition of
cell proliferation, migration and invasion.
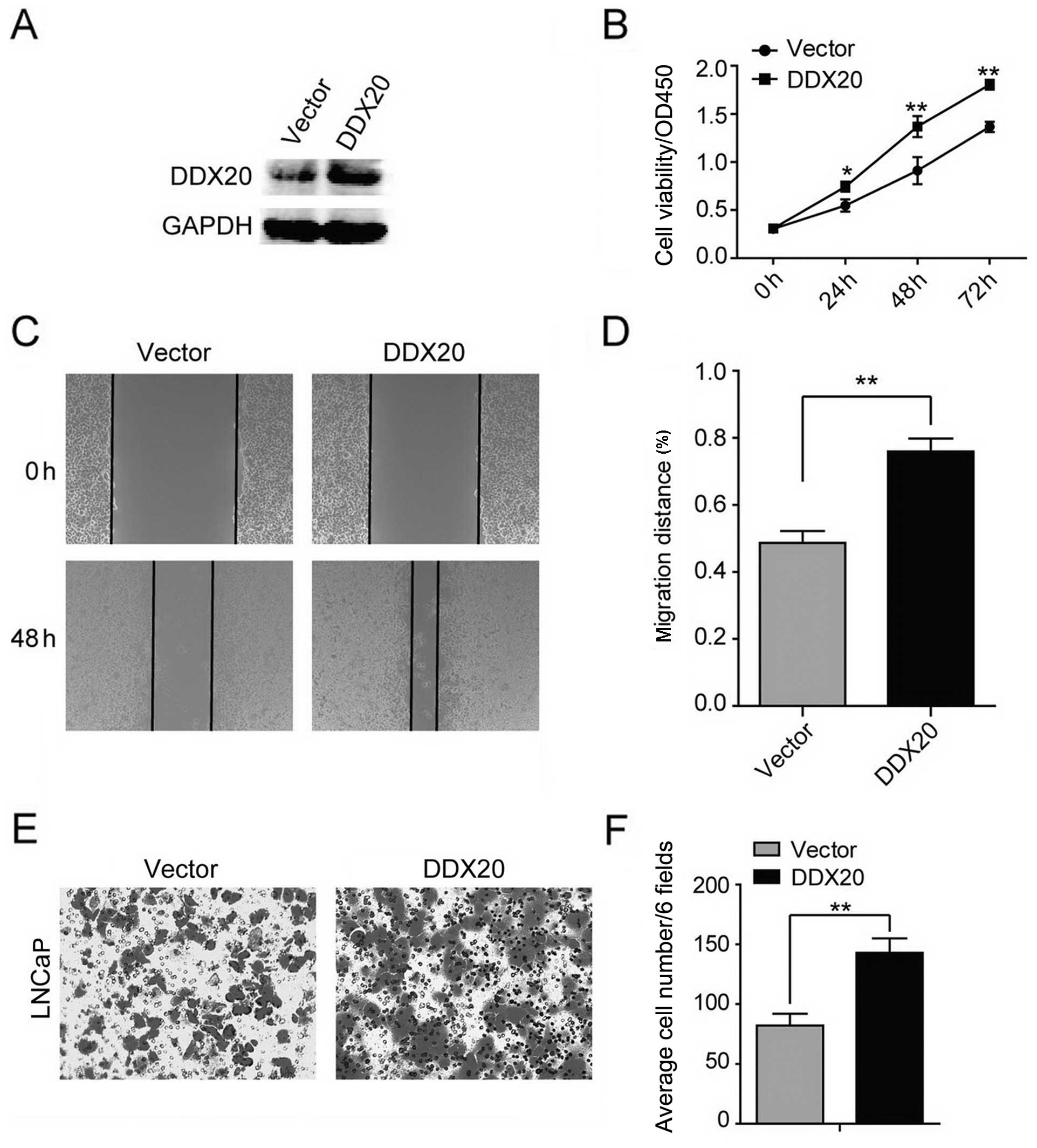
Ectopic expression of DDX20 contributes
to cell proliferation, migration and invasiveness
To further substantiate our findings, we then
evaluated whether the ectopic expression of DDX20 had the potential
to adversely affect LNCaP cells. We found that the ectopic
expression of DDX20 markedly elevated the expression of DDX20
(Fig. 4A). The ectopic expression
of DDX20 increased LNcaP cell viability (Fig. 4B). In addition, the ectopic
expression of DDX20 in the LNCaP cells promoted cell motility and
enhanced the invasive capacity of the LNCaP cells (Fig. 4C–F). Taken together, both loss-of-
and gain-of-function experiments demonstrate that DDX20 expression
concurrently affects the proliferation, migration and invasiveness
of PCa cells.
DDX20 expression correlates with MMP9
levels in PCa tissues
Taking into account the promigratory effect of DDX20
in PCa cell lines and the associated mechanisms reported in other
types of cancer, we decided to analyze the expression of MMP9,
which has been reported to play a pivotal role in the
DDX20-metastasis axis (8), in the
same cohorts used in Fig. 1B.
Similarly to DDX20, we observed higher mRNA expression levels of
MMP9 in the PCa tissues compared with those in the adjacent tissues
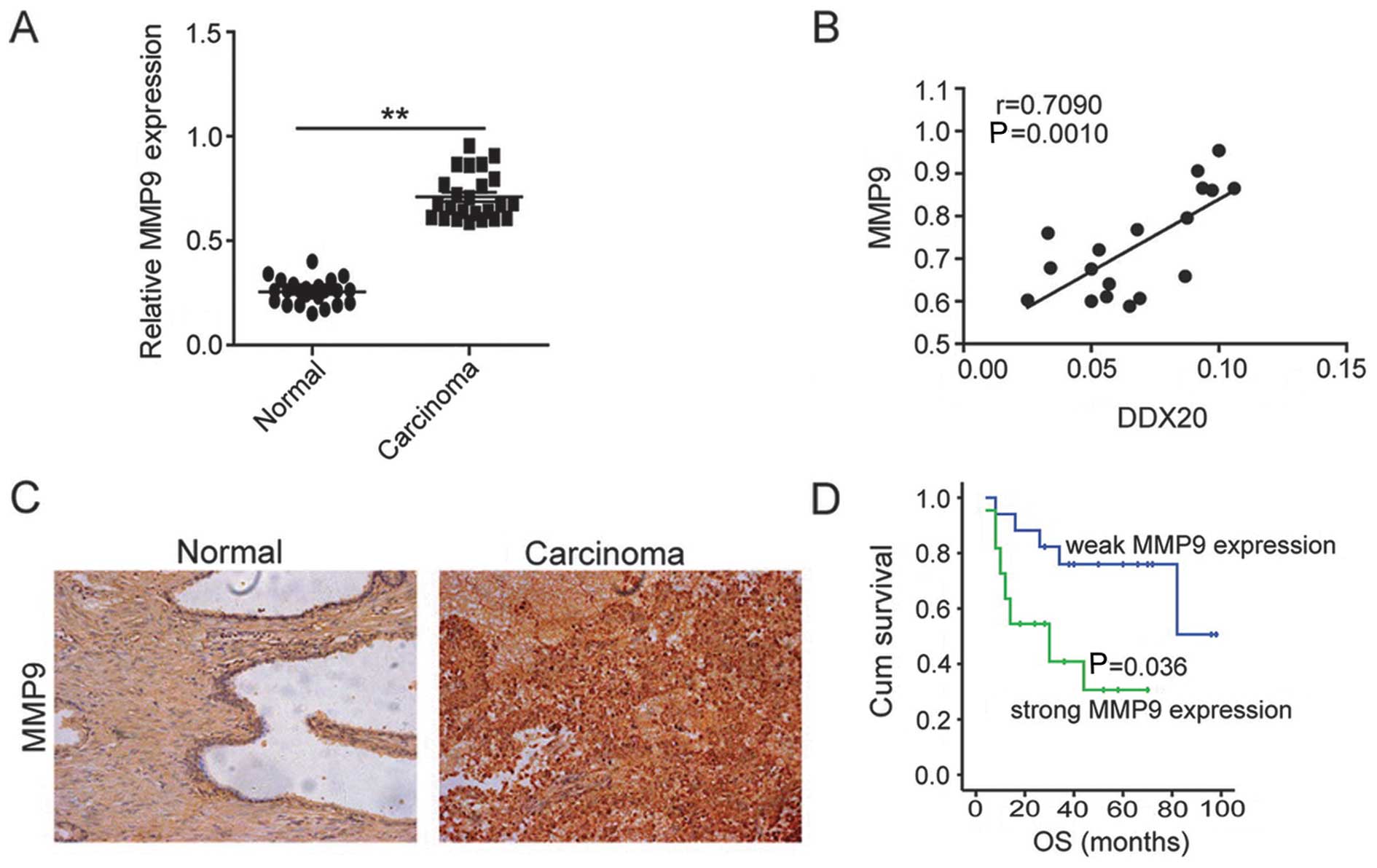
(Fig. 5A) and there was a
positive correlation between DDX20 and MMP9 mRNA levels (Fig. 5B). To further substantiate this
finding, we performed immunohistochemical analysis to examine the
expression of MMP9 in the same cohort used in Fig. 1C. Notably, similarly to DDX20, the
group of PCa patients with high expression of MMP9 were more likely
to have a poor prognosis (P=0.036) (Fig. 5D) and similar clinicopathological
features were also obtained from the same TMA (Table II) and the same
clinicopathological features significantly correlated with MMP9
expression. Taken together, the findings of the present study
indicate that DDX20 may also exert effects in PCa through MMP9.
 | Table IICorrelation between MMP9 expression
and key clinicopathological features. |
Table II
Correlation between MMP9 expression
and key clinicopathological features.
| Variable | MMP9 (n=99)
|
|---|
| Low | High | P-value |
|---|
| Age (years) | | | |
| ≤50 | 24 | 23 | 0.294 |
| >50 | 32 | 20 | |
| Tumor size (cm) | | | |
| ≤5 | 36 | 20 | 0.026a |
| >5 | 18 | 25 | |
| Local
infiltration | | | |
| Yes | 11 | 23 | 0.002a |
| No | 42 | 23 | |
| Gleason grade | | | |
| I | 2 | 1 | 0.040a |
| II–III | 16 | 34 | |
| IV–V | 26 | 20 | |
DDX20 regulates NF-κB signaling in PCa
cell lines
Given the strong correlation between DDX20 and MMP9
in PCa tissues, we then examined whether silencing or ectopically
expressing DDX20 would reduce or increase the levels of MMP9,
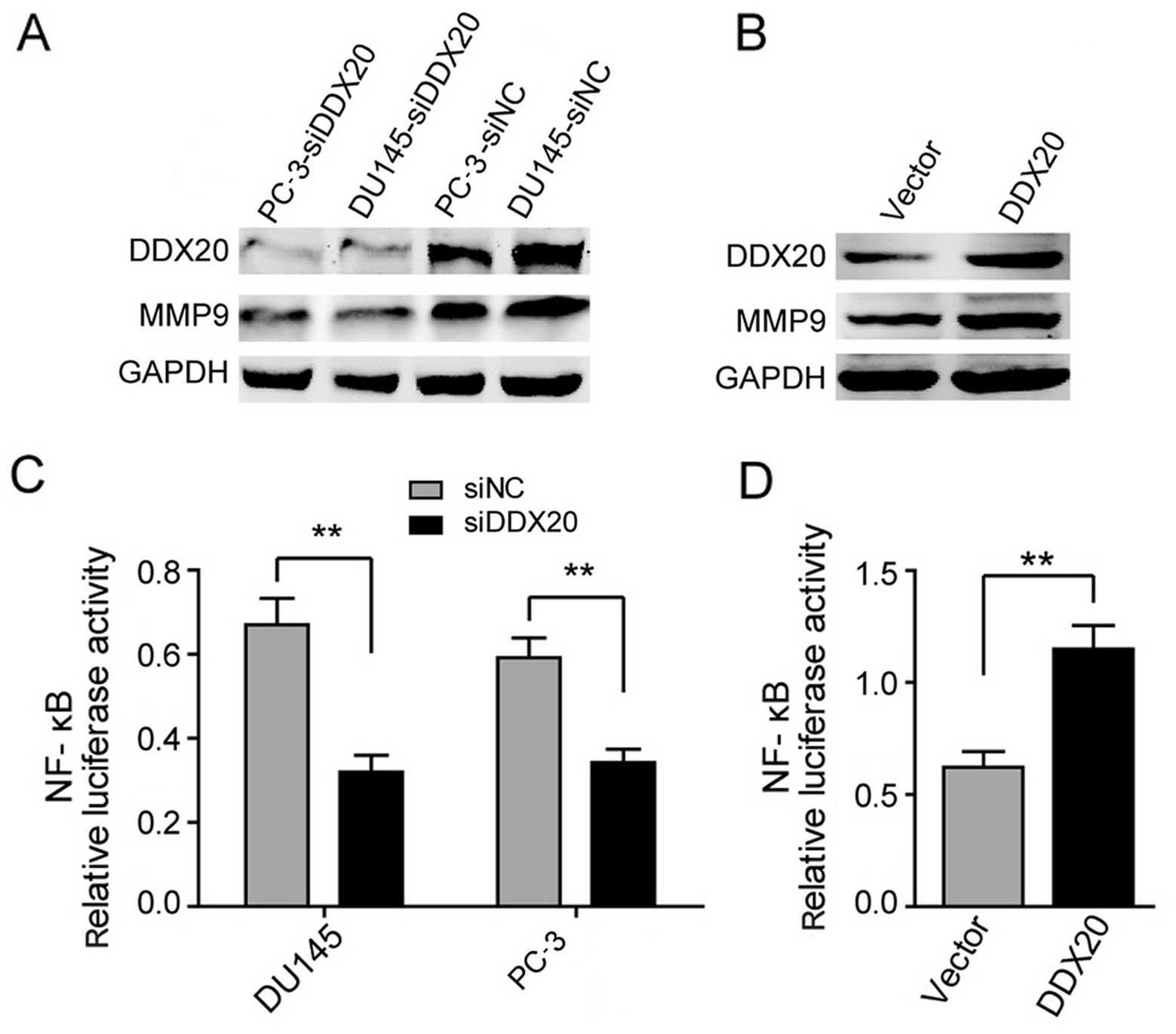
respectively. We found that silencing DDX20 resulted in the
inhibition of MMP9 protein levels in the DU145 and PC-3 cell lines
(Fig. 6A). Conversely, the
ectopic expression of DDX20 resulted in the increased expression of
MMP9 in the LNCaP cell line (Fig.
6B). These findings suggest that DDX20 may regulate MMP9
expression in order to enhance the migration and invasiveness of
PCa cells. As NF-κB is considered to be the major transcriptional
regulator of MMP9 (9,10), we then examined whether the
alterations in MMP9 expression following changes in DDX20 levels,
are due to NF-κB activity. We performed luciferase reporter assays
and the results are shown in Fig. 6C
and D; the suppression of DDX20 in the DU145 and PC-3 cells led
to the downregulation of NF-κB activity (Fig. 6C). By contrast, the overexpression
of DDX20 elevated NF-κB activity (Fig. 6D). Taken together, our findings
suggest that DDX20 promotes the migration and invasiveness of PCa
cells by regulating the NF-κB-MMP9 axis.
Discussion
In the present study, we report that DDX20 is
elevated in the majority of PCa tissue samples and the high
expression of DDX20 negatively correlates with patient prognosis.
In addition, we have demonstrated that PCa cells with high
expression of DDX20 are more prone to be proliferative and
invasive.
It has been previously reported that DDX20 increases
MMP9 levels which are associated with metastasis and invasion in
breast cancer through the activation of NF-κB (8). In the present study, a positive
correlation between DDX20 and MMP9 expression was observed in both
PCa tissues and cell lines. In addition, the findings of the
luciferase reporter assays suggested that DDX20 also exerted
promigratory effects through NF-κB signaling in PCa cells. Taken
together, these results provide further evidence that DDX20 acts as
an oncogenic protein in PCa as well as in breast cancer.
Notably, two other studies have reported that DDX20
acts as a tumor suppressor in HCC. Zender et al employed an
oncogenomics-based in vivo RNAi screen that identified DDX20
as a potential tumor suppressor in liver cancer (11). Takata et al revealed that
DDX20 suppresses NF-κB activity by regulating miRNA-140 function in
order to inhibit the progression of liver cancer (7,12).
We are unable to explain the contradictory results observed in
studies of DDX20 in different types of cancer. However, given that
it exerts disparate effects in various types of cancer through the
same pathway, namely the MMP9 and NF-κB signaling pathway, we
suggest that the ways in which DDX20 regulates the expression of
MMP9 and NF-κB are dependent on the type of cancer. As PCa and
breast cancer are gender-related cancers, we suggest that there may
be an association between the effects of DDX20 and sex hormones;
however, further studies are warranted.
In conclusion, we have demonstrated that there is
high expression of DDX20 in PCa tissues and it may be useful as a
potential prognostic marker in PCa. In addition, we have examined
the biological functions of DDX20 in PCa cell lines. Furthermore,
we have provided preliminary evidence for the molecular mechanisms
responsible for the effects of DDX20. The contradictory effects of
DDX20 in different types of cancer suggest that it may play a key
role in the development and progression of cancer and therefore be
a potential therapeutic target.
References
|
1
|
Ferlay J, Autier P, Boniol M, Heanue M,
Colombet M and Boyle P: Estimates of the cancer incidence and
mortality in Europe in 2006. Ann Oncol. 18:581–592. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hankey BF, Feuer EJ, Clegg LX, Hayes RB,
Legler JM, Prorok PC, Ries LA, Merrill RM and Kaplan RS: Cancer
surveillance series: interpreting trends in prostate cancer - part
I: Evidence of the effects of screening in recent prostate cancer
incidence, mortality, and survival rates. J Natl Cancer Inst.
91:1017–1024. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Struewing JP, Hartge P, Wacholder S, Baker
SM, Berlin M, McAdams M, Timmerman MM, Brody LC and Tucker MA: The
risk of cancer associated with specific mutations of BRCA1 and
BRCA2 among Ashkenazi Jews. N Engl J Med. 336:1401–1408. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gallagher RP and Fleshner N: Prostate
cancer: 3. Individual risk factors. CMAJ. 159:807–813.
1998.PubMed/NCBI
|
|
6
|
Beuzeboc P, Soulié M, Richaud P, Salomon
L, Staerman F, Peyromaure M, Mongiat-Artus P, Cornud F, Paparel P,
Davin JL and Molinié V: Fusion genes and prostate cancer. From
discovery to prognosis and therapeutic perspectives. Prog Urol.
19:819–824. 2009.In French. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takata A, Otsuka M, Yoshikawa T, Kishikawa
T, Hikiba Y, Obi S, Goto T, Kang YJ, Maeda S, Yoshida H, et al:
MicroRNA-140 acts as a liver tumor suppressor by controlling NF-κB
activity by directly targeting DNA methyltransferase 1 (Dnmt1)
expression. Hepatology. 57:162–170. 2013. View Article : Google Scholar
|
|
8
|
Shin EM, Hay HS, Lee MH, Goh JN, Tan TZ,
Sen YP, Lim SW, Yousef EM, Ong HT, Thike AA, et al: DEAD-box
helicase DP103 defines metastatic potential of human breast
cancers. J Clin Invest. 124:3807–3824. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chou YC, Sheu JR, Chung CL, Chen CY, Lin
FL, Hsu MJ, Kuo YH and Hsiao G: Nuclear-targeted inhibition of
NF-kappaB on MMP-9 production by N-2-(4-bromophenyl) ethyl
caffeamide in human monocytic cells. Chem Biol Interact.
184:403–412. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ricca A, Biroccio A, Del Bufalo D, Mackay
AR, Santoni A and Cippitelli M: bcl-2 over-expression enhances
NF-kappaB activity and induces mmp-9 transcription in human
MCF7(ADR) breast-cancer cells. Int J Cancer. 86:188–196. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zender L, Xue W, Zuber J, Semighini CP,
Krasnitz A, Ma B, Zender P, Kubicka S, Luk JM, Schirmacher P, et
al: An oncogenomics-based in vivo RNAi screen identifies tumor
suppressors in liver cancer. Cell. 135:852–864. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takata A, Otsuka M, Yoshikawa T, Kishikawa
T, Kudo Y, Goto T, Yoshida H and Koike K: A miRNA machinery
component DDX20 controls NF-κB via microRNA-140 function. Biochem
Biophys Res Commun. 420:564–569. 2012. View Article : Google Scholar : PubMed/NCBI
|