Introduction
Bone is a dynamic tissue that constantly undergoes
remodeling through a coupled process of bone formation by
osteoblasts and resorption by osteoclasts throughout life (1). Chronic osteomy-elitis, a persistent
infection of the bone, involves dysregulation of this process which
results in excessive bone resorption (2).
Interleukin (IL)-1 is a proinflammatory cytokine
that plays important roles in inflammation and host responses to
infection (3,4). Once released, IL-1 elicits a
multitude of effects on target cells that lead to and modulate the
inflammatory response as well as tissue damage (3). The two forms of IL-1, IL-1α and
IL-1β, have similar biological activities but different functional
roles in the body (3,5). Bone is highly sensitive to IL-1,
which is involved in the regulation of bone formation (6–8)
and bone resorption (9–12).
Mitogen-activated protein kinases (MAPKs, also known
as MAP kinases), including c-Jun N-terminal kinase (JNK) and p38,
are involved in the progression of inflammatory responses and in
the apoptosis of osteoblasts (13–15). Moreover, the mechanism responsible
for the effects of IL-1 on the differentiation and apoptosis of
osteoblasts, which may occur through the MAPK signaling pathways,
remains unclear. Thus, research into the effects of IL-1 on
osteoblast differentiation and function as well as the subsequent
development and evaluation of potential drugs suitable for
preventing bone destruction in infective bone diseases remains an
area of great interest.
The aim of the present study was to evaluate the
mechanism responsible for the effects of IL-1α on the
differentiation and apoptosis of osteoblasts. We also aimed to
elucidate the mechanism through which IL-1α induces MAPK
phosphorylation.
Materials and methods
Reagents
IL-1α was purchased from PeproTec (Rocky Hill, NJ,
USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT), 4-(2-hydroxyethyl)-1-piperazineethane-sulfonic acid (HEPES),
protease inhibitor cocktail, and the selective MAPK inhibitors
SB203580, PD98059 and SP600125 were all purchased from Calbiochem
(San Diego, CA, USA). An alkaline phosphatase (ALP) colorimetric
assay kit and a caspase-3 activity kit were purchased from Beyotime
Institute of Biotechnology (Shanghai, China). Dulbecco's modified
Eagle's medium (DMEM), fetal bovine serum (FBS) and
penicillin/streptomycin were purchased from Gibco (Rockville, MD,
USA). Antibodies to Bcl-2 (#2870), Bcl-xL (#2764), p38 MAPK
(#9212), phosphorylated (p-)p38 (#9211), extracellular
signal-regulated kinase (ERK)1/2 (#4695) and p-ERK1/2 (#4370) were
purchased from Cell Signaling Technology, Inc. (Beverly, MA, USA).
Antibodies to caspase-3 (sc-7148), Bax (sc-493), cytochrome
c (sc-13156), JNK1/2 (sc-571), p-JNK1/2 (sc-12882), osterix
(OSX; sc-393325), osteocalcin (OCN; sc-390877), ALP (sc-137213) and
β-actin (sc-47778) were purchased from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). Antibodies to runt-related
transcription factor 2 (Runx2; ab76956) were purchased from Abcam
(Cambridge, UK). The osteoblast-like cell line MC3T3-E1 was
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA). All other chemicals and reagents used in the present
study were of analytical grade.
Cell culture
The MC3T3-E1 cells were grown in DMEM supplemented
with 10% (v//v) FBS, 1% (v/v) penicillin-strep-tomycin
solution, 10 mM HEPES solution and incubated at 37°C in a 5%
CO2 humidified atmosphere in air. To evaluate the effect
of IL-1α on the differentiation and function of osteoblasts, the
MC3T3-E1 cells at a density of 5×104
cells/cm2 were cultured in osteogenic differentiation
medium (DMEM with 10% FBS, 10 mM HEPES, 50 µg/ml L-ascorbic
acid and 5 mM β-glycerophosphate (β-GP) for 2 days. On
differentiation day 3, the cells were treated with 0.5, 1, 2.5, 5
and 10 ng/ml IL-1α or without IL-1α for the indicated times. To
evaluate the effect of MAPK inhibitors on the phosphorylation of
MAPKs in osteoblasts induced by IL-1α, MAPK inhibitors were applied
for 2 h prior to IL-1α treatment.
Cell viability assay
Cell viability was measured using an MTT assay. The
MC3T3-E1 cells (5×103 cells/well) were plated in 96-well
culture plates in 0.2 ml culture medium and incubated overnight. To
evaluate the effect of IL-1α on cell viability, the cells were
treated with 0.5, 1, 2.5, 5 and 10 ng/ml IL-1α or without IL-1α for
1, 3 and 5 days. At the end of treatment, 20 µl MTT (5
mg/ml) was added to each well. The cells were cultured for an
additional 4 h, the supernatant was removed, and then 100 µl
dimethyl sulfoxide was added to each well. After shaking for 3 min,
absorbance was measured at 490 nm using a microplate reader (Thermo
MK3; Thermo Fisher Scientific Inc., Pittsburgh, PA, USA). The
experiment was performed in triplicate.
Determination of ALP activity
The MC3T3-E1 cells were cultured in 100-mm diameter
culture dishes in the presence or absence of IL-1α for 24 h. The
supernatant was discarded and then subjected to the intracellular
ALP enzyme assay as previously described (14). Briefly, the cells were detached
mechanically with a cell scraper in phosphate-buffered saline
(PBS), and then centrifuged at 15,000 × g for 8 min. Cell pellets
was placed in 300 µl of lysis buffer (Cytobuster protein
extraction reagent; Novagen, Darmstadt, Germany) with 1X protease
inhibitor cocktail. The ALP activity in the lysates was determined
by the measurement of p-nitrophenyl phosphate (PNPP) using a
commercial assay kit (Beyotime Institute of Biotechnology). The
absorbance of the reaction mixture was measured by
spectrophotometry (Eppendorf BioSpectrometer; Eppendorf AG,
Hamburg, Germany) at 405 nm. Three replicates from each group were
analyzed. Total protein concentrations were quantified by
spectrophotometry (Eppendorf BioSpectrometer). ALP activity was
normalized to the total protein in each sample.
Measurement of caspase-3 activity
The MC3T3-E1 cells were cultured in 100-mm-diameter
culture dishes in the presence or absence of IL-1α for 24 h. The
activity of caspase-3 was determined using the caspase-3 activity
kit (Beyotime Institute of Biotechnology) according to the
manufacturer's instructions, and then detected by spectrophotometry
(Eppendorf BioSpectrometer). Briefly, the cells were detached with
the aid of a cell scraper in PBS buffer, then centrifuged at 15,000
× g for 8 min and the cell pellets was placed in 300 µl of
lysis buffer (Cytobuster protein extraction reagent) with 1X
protease inhibitor cocktail. Total protein concentrations were
quantified by spectrophotometry (Eppendorf BioSpectrometer). The
samples were then incubated with a mixture provided in the kit,
containing Ac-DEVD-pNA, the substrate of caspase-3. The optical
density (coloration) resulting from the cleavage of the substrate
and the release of pNA, was detected and quantified by
spectrophotometry at 405 nm. A standard curve was also generated
using a series of diluted pNA of known concentrations. The
concentration was calculated by projecting the optical densities on
the standard curve. All the experiments were performed in
triplicate.
Analysis of the apoptosis of
osteoblasts
To measure apoptosis, we performed Hoechst 33258
staining (Beyotime Institute of Biotechnology) to visualize nuclear
morphology and nucleo-somal DNA fragmentation in osteoblasts. The
MC3T3-E1 cells (4×104 cells/cm2) were seeded
into 12-well plates and incubated overnight prior to the
experiment. The cells were treated with 0.5, 1, 2.5, 5 and 10 ng/ml
IL-1α or without IL-1α for 1 day. At the end of the treatment, the
cells were washed to remove non-adherent cells, and the adherent
cells were fixed in 4% paraformaldehyde solution for 10 min. After
washing with PBS, the cells were incubated with 0.2 mM Hoechst
33258 for 5 min in the dark. The cells with nuclei containing
clearly condensed chromatin or the cells with fragmented nuclei
were scored as apoptotic; the results were expressed as the number
of apoptotic cells. The images of apoptotic osteoblasts were
captured with a fluorescence microscope (Olympus IX71; Olympus
Optical, Tokyo, Japan).
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen, Grand Island, NY, USA) and quantified by
spectrophotometry. After isolation, 2 µg total RNA from each
sample was reverse transcribed utilizing the HiFi-MMLV cDNA kit
(Beijing CoWin Biotech Co., Ltd., Beijing, China) according to the
manufacturer's instructions. The primer sequences of the
osteoblast-associated genes, caspase-3, Bax and β-actin (Generay
Biotech Co., Ltd., Shanghai, China) and annealing temperatures used
in this study are listed in Table
I. qPCR was performed with SYBR® Premix Ex Taq™
(Takara, Dalian, China) according to the manufacturer's
instructions. All qPCR reactions were performed using the ABI 7300
sequence detection system (Applied Biosystems, Grand Island, NY,
USA). In each reaction, 1 µl cDNA, 10 µl
SYBR® Premix Ex Taq (Takara), and 0.4 µM forward
and reverse primer in a total volume of 20 µl were used. The
reaction conditions were as follows: 1 cycle of 95°C for 30 sec
followed by 40 cycles of 95°C for 5 sec and 60–66°C for 30 sec.
qPCR assays were run in triplicate for each sample. β-actin was
used as the internal control, and all results were analyzed using
the standard 2−ΔΔCT method as described previously
(16).
 | Table IList of primers used for RT-qPCR. |
Table I
List of primers used for RT-qPCR.
| Gene | Primer sequences
5′→3′ (forward/reverse) | Accession number | Annealing temperature
(°C) | Product size
(bp) |
|---|
| ALP |
GAGCGTCATCCCAGTGGAG
TAGCGGTTACTGTAGACACCC | NM_007433 | 62 | 158 |
| OCN |
CTGACCTCACAGATCCCAAGC
TGGTCTGATAGCTCGTCACAAG | NM_031368 | 62 | 187 |
| Runx2 |
TTCAACGATCTGAGATTTGTGGG
GGATGAGGAATGCGCCCTA | NM_001146038 | 62 | 221 |
| OSX |
ATGGCGTCCTCTCTGCTTG
TGAAAGGTCAGCGTATGGCTT | NM_130458 | 62 | 156 |
| Bax |
AGACAGGGGCCTTTTTGCTAC
AATTCGCCGGAGACACTCG | NM_007527 | 60 | 137 |
| Caspase-3 |
TGGTGATGAAGGGGTCATTTATG
TTCGGCTTTCCAGTCAGACTC | BC038825 | 60 | 105 |
| β-actin |
GGCTGTATTCCCCTCCATCG
CCAGTTGGTAACAATGCCATGT | NM_007393 | 60 | 154 |
Western blot analysis
At the end of treatment, the cell culture medium was
aspirated and the cells were detached in PBS by scraping. The
detached cells were centrifuged at 15,000 × g at 4°C for 8 min.
Cell pellets were lysed in 300 µl lysis buffer (Cytobuster
protein extraction reagent) with 25 mM NaF, 1 mM
Na3VO4, and 1X protease inhibitor cocktail.
Protein concentrations were quantified by spectrophotometry
(Eppendorf BioSpectrometer). For the western blot analysis, equal
amounts of protein from each sample were separated by SDS-PAGE and
electrotrans-ferred onto PVDF membranes (Millipore, Bedford, MA,
USA). The membranes were then blocked with 5% (w/v) bovine serum
albumin in TBST [10 mM Tris, 150 mM NaCl, and 0.1% (v/v) Tween-20,
pH 7.5] for 1 h at room temperature, and incubated with primary
antibodies: rabbit polyclonal anti-JNK, p-JNK, β-actin, capase-3,
Bax, mouse polyclonal anti-OSX, ALP, OCN, Runx2 and cytochrome
c at dilutions of 1:300 (all from Santa Cruz Biotechnology,
Inc.); rabbit polyclonal anti-ERK1/2, p-ERK1/2, p38, p-p38, Bcl-2
and Bcl-xL at dilutions of 1:800 (Cell Signaling Technology, Inc.).
The secondary antibodies used for detection were horseradish
peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin G (IgG)
and HRP-conjugated goat anti-mouse IgG (both from Santa Cruz
Biotechnology, Inc.). Enhanced chemiluminescence (ECL; Beijing
CoWin Biotech Co., Ltd.) was performed in order to detect
immunoreactive protein signals. Protein signals were visualized and
images were captured with a chemiluminescence detection system
(MiniChemi™ III; Beijing Sage Creation Science Co., Ltd., Beijing,
China) and quantified using ImageJ software (National Institutes of
Health, Bethesda, MD, USA). For re-probing, the PVDF membranes were
stripped with 0.2 M NaOH for 15 min prior to blocking with another
primary antibody. The expression of molecules of interest was
determined relative to β-actin.
Statistical analysis
The data are expressed as the means ± standard
deviation (SD) for three or more independent experiments.
Significant differences were determined using factorial analysis of
variance (ANOVA). Statistical analysis was performed using SPSS
13.0 software. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
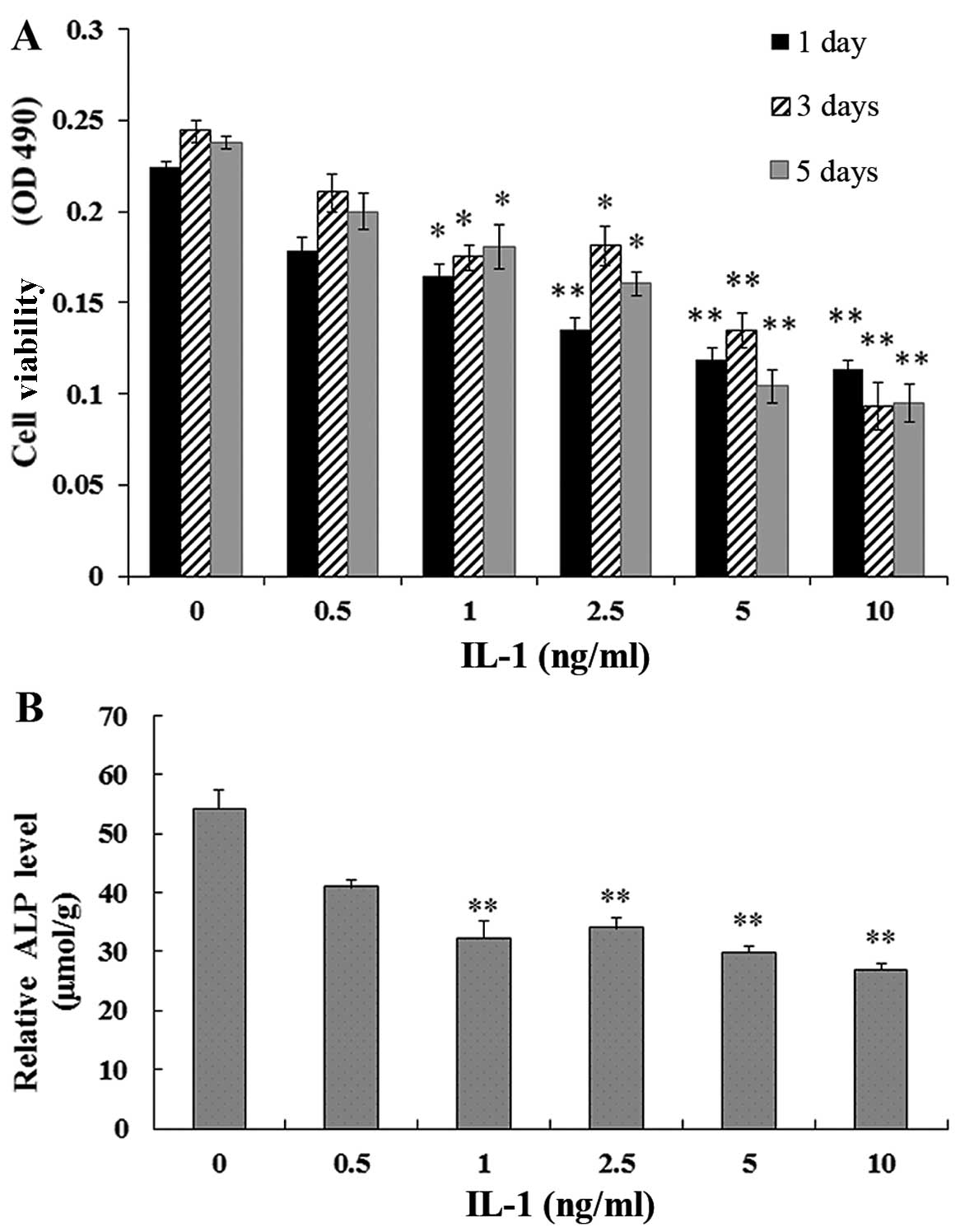
Effect of IL-1α on the viability of
MC3T3-E1 cells
The MC3T3-E1 cells were treated with IL-1α at
concentrations of 0.5, 1, 2.5, 5 and 10 ng/ml or without IL-1α for
1, 3 and 5 days. MTT assays showed that the viability of the
MC3T3-E1 cells exposed to IL-1α was significantly reduced compared
with that in the non-treated culture at 1, 3 and 5 days (P<0.01)
(Fig. 1A).
Effect of IL-1α on ALP activity in
MC3T3-E1 cells
The MC3T3-E1 cells were treated with IL-1α at
concentrations of 0.5, 1, 2.5, 5 and 10 ng/ml or without IL-1α for
24 h. The ALP activity in the MC3T3-E1 cells exposed to IL-1 was
signifi-cantly decreased in a dose-dependent manner compared with
that in the non-treated culture at 1 day (P<0.01) (Fig. 1B).
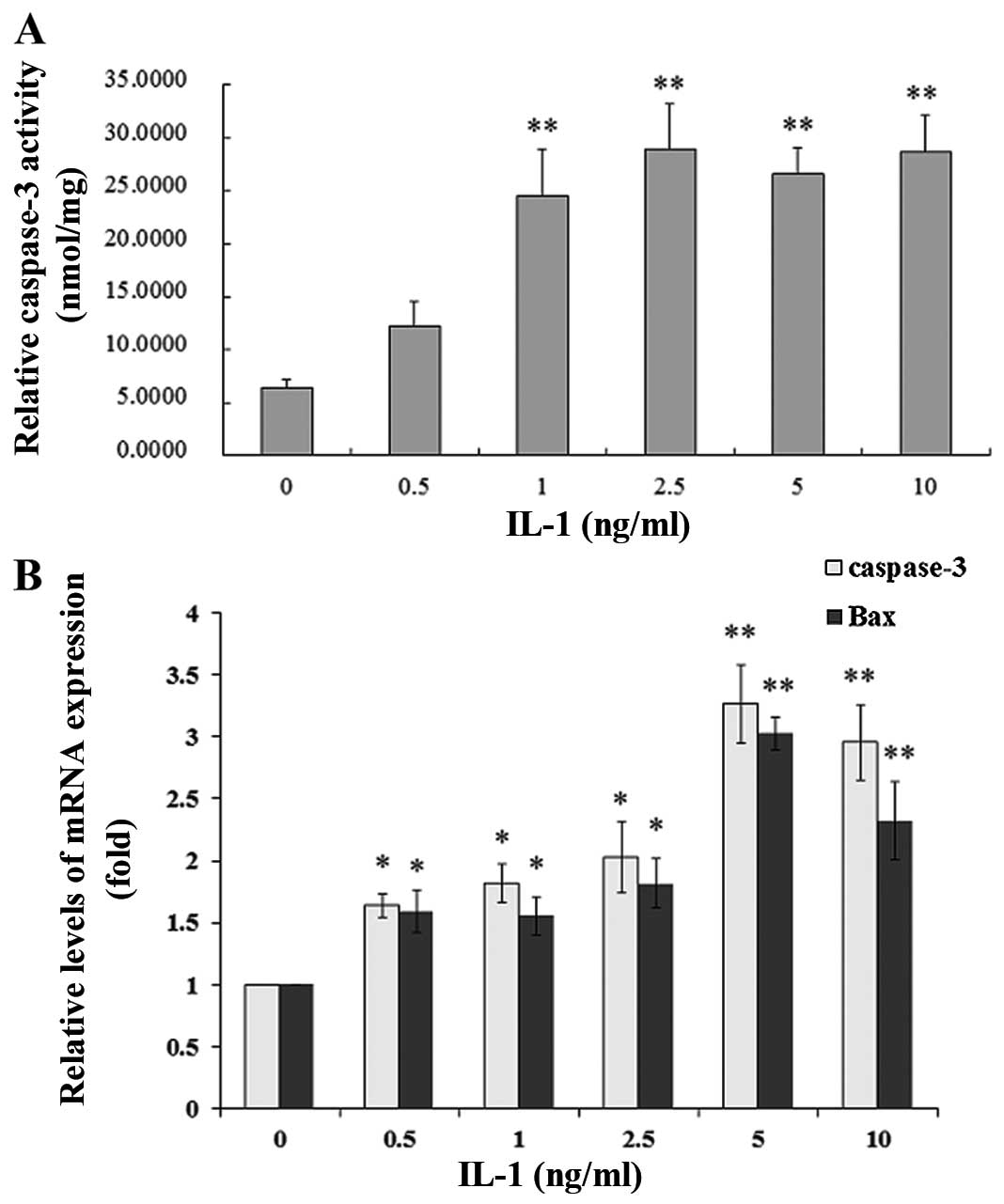
Effect of IL-1α on caspase-3 activity in
MC3T3-E1 cells
To evaluate IL-1α-induced caspase-3 activity, the
MC3T3-E1 cells were treated with IL-1α at concentrations of 0.5, 1,
2.5, 5 and 10 ng/ml or without IL-1α for 24 h. IL-1α significantly
increased caspase-3 activity in a dose-dependent manner compared
with that in the non-treated culture at 24 h (P<0.01) (Fig. 2A).
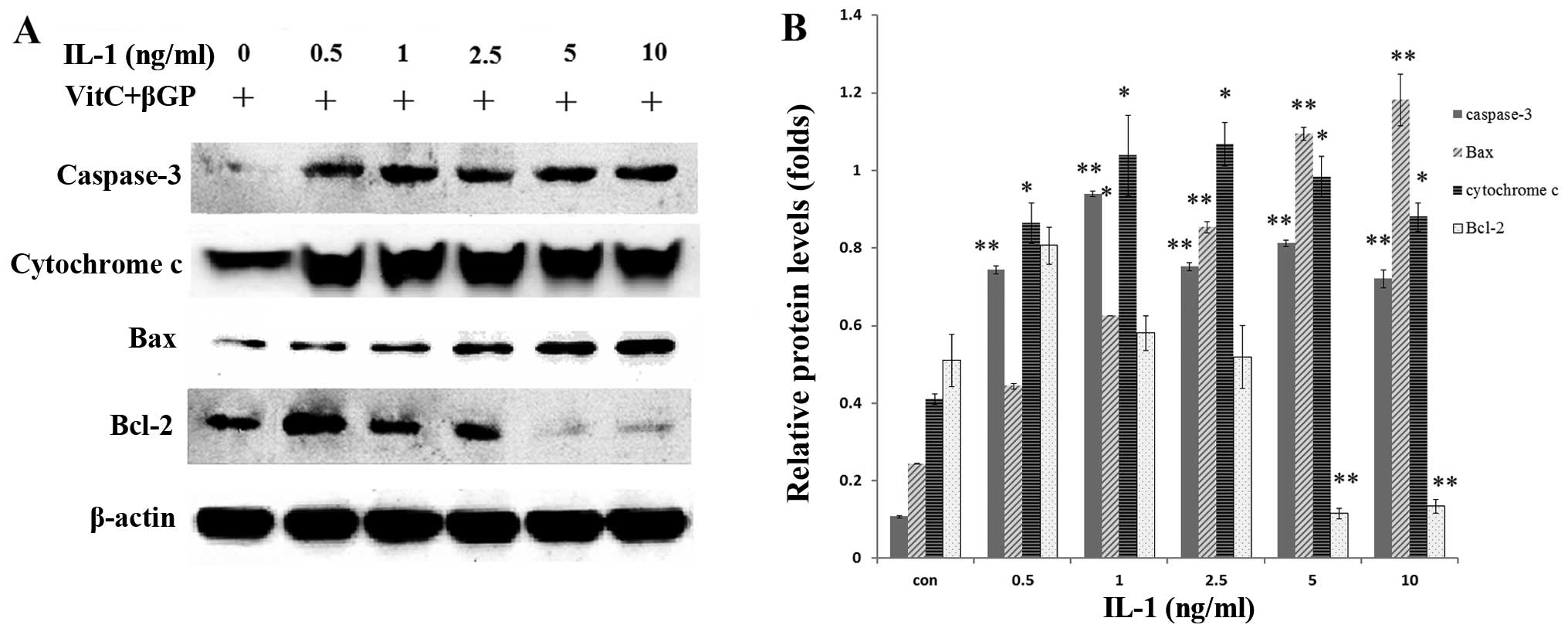
Effect of IL-1α on the mRNA and protein
expression of Bax, Bcl-2 and caspase-3 in MC3T3-E1 cells
The MC3T3-E1 cells were treated with IL-1α at
concentrations of 0.5, 1, 2.5, 5 and 10 ng/ml or without IL-1α for
24 h. IL-1 significantly increased the mRNA expression and the
protein levels of Bax and caspase-3 in a dose-dependent manner
compared with that in the non-treated culture at 24 h (P<0.01),
whereas Bcl-2 expression was decreased (P<0.01) in the MC3T3-E1
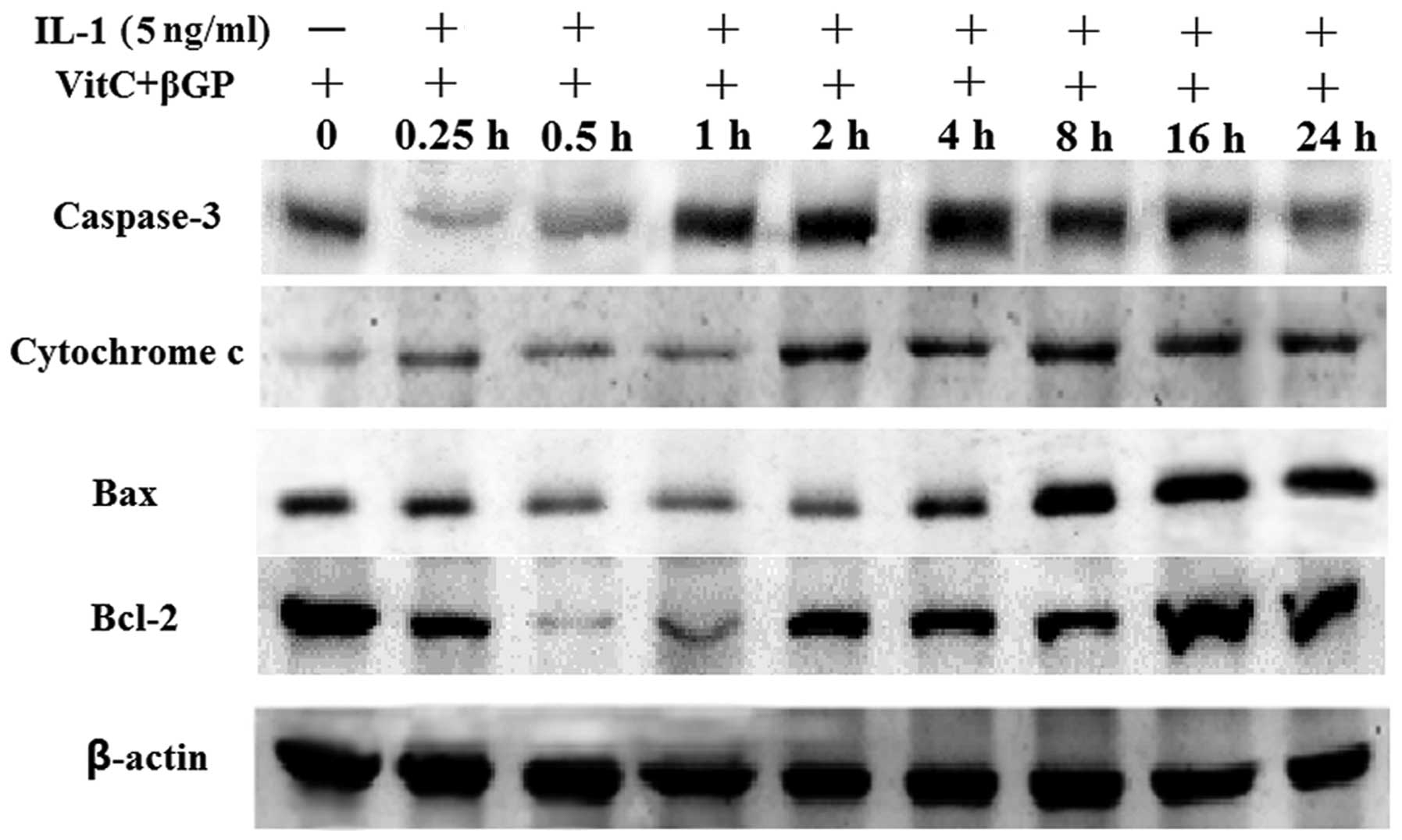
cells (Figs. 2B and 3). Following treatment with IL-1α at 5
ng/ml for 24 h, IL-1α significantly increased the protein
expression of Bax, cytochrome c and caspase-3 in a
time-dependent manner compared with that in the non-treated
culture, whereas Bcl-2 expression was unaffected (Fig. 4).
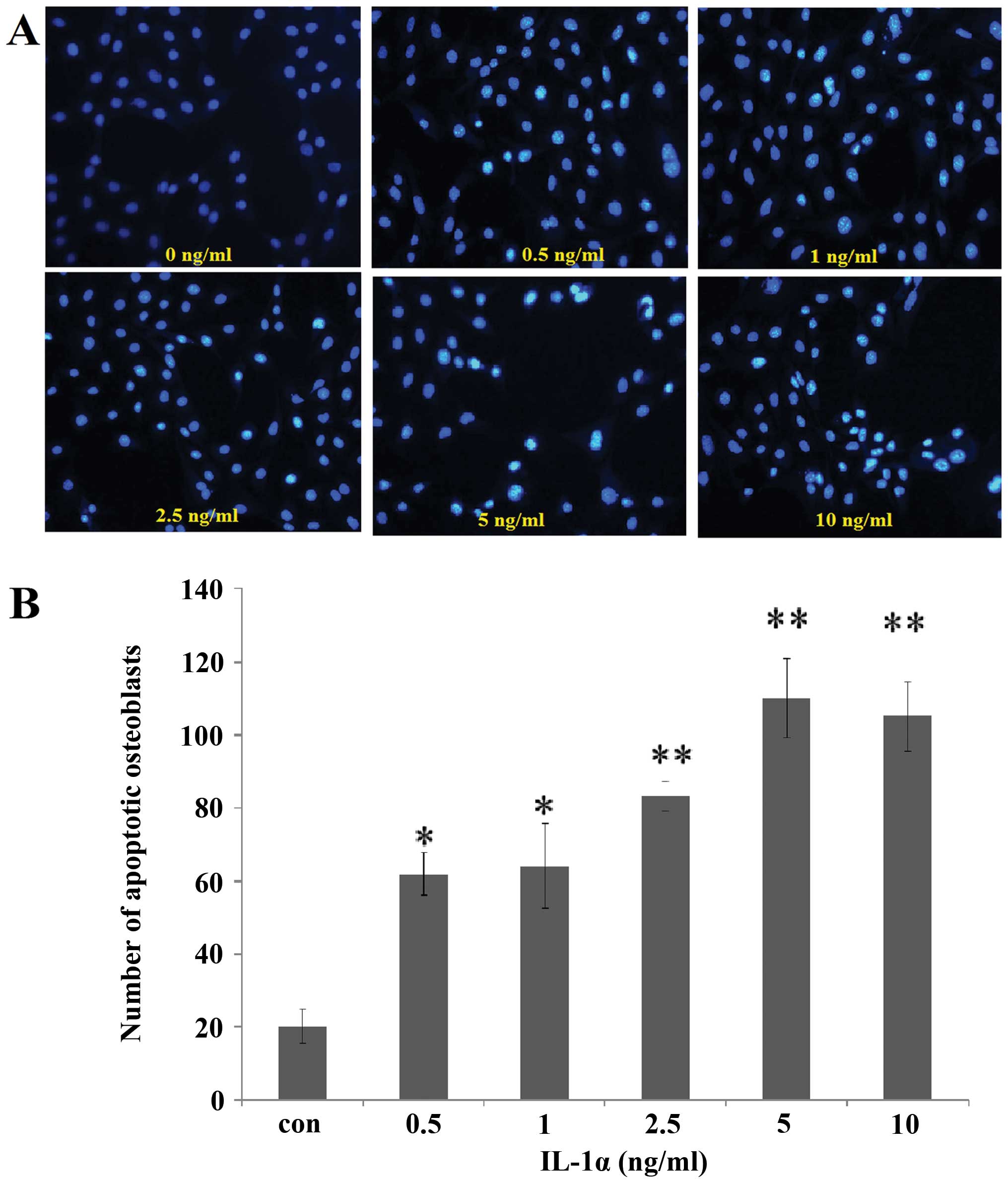
Effect of IL-1α on cell apoptosis
Following incubation with IL-1α at 0.5, 1, 2.5, 5
and 10 ng/ml or without IL-1α for 24 h, Hoechst 33258 staining of
the cells was analyzed under a fluorescence microscope. The cells
with nuclei containing condensed chromatin or the cells with
fragmented nuclei were defined as apoptotic cells. The number of
fluorescence-positive osteoblasts was increased by IL-1α treatment
in a dose-dependent manner compared with that in the
non-IL-1α-treated group at 24 h (Fig.
5).
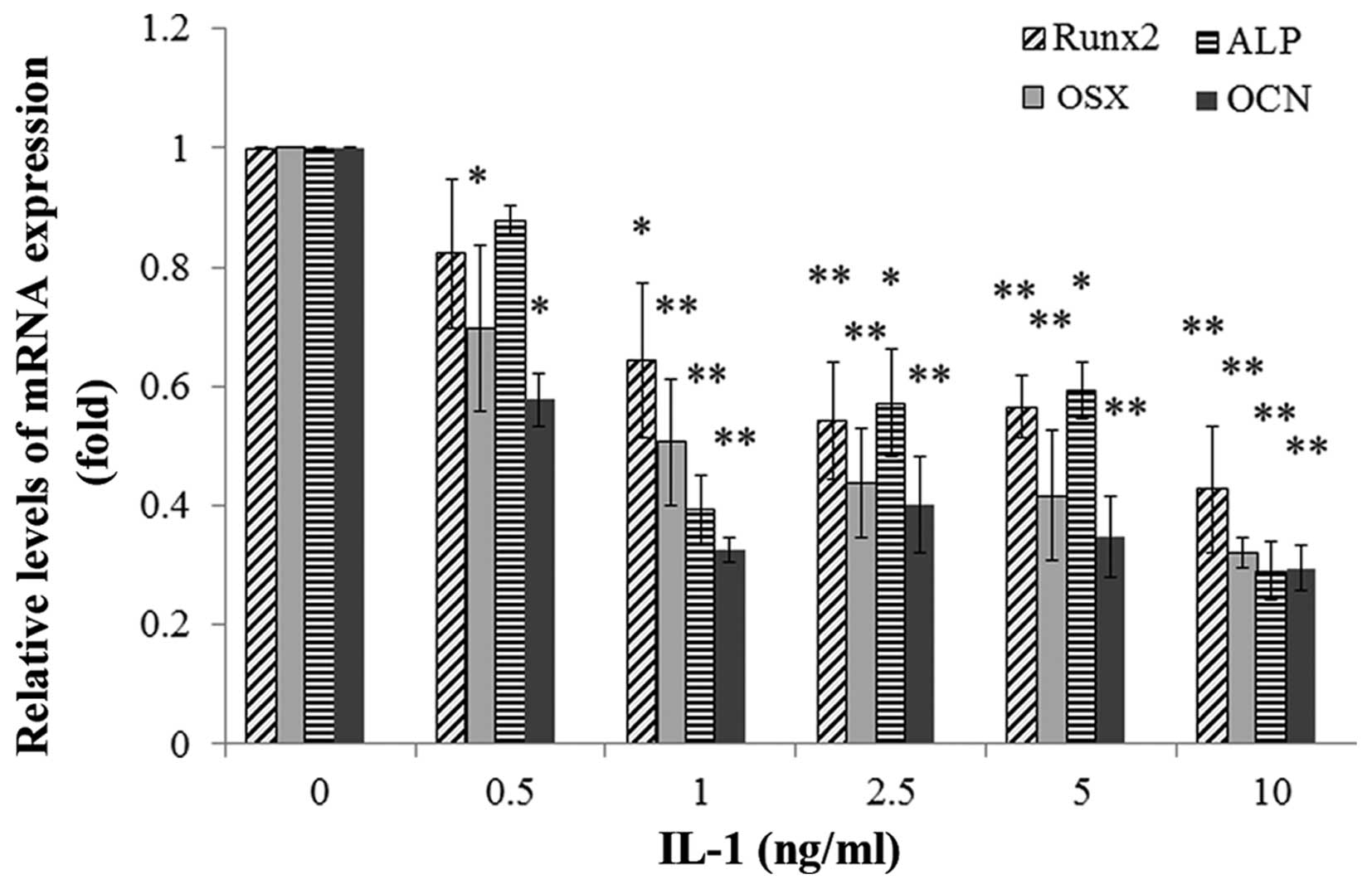
Effect of IL-1α on the mRNA and protein
expression of osteoblast-specific genes in MC3T3-E1 cells
The MC3T3-E1 cells were treated with IL-1α at
concentrations of 0.5, 1, 2.5, 5 and 10 ng/ml or without IL-1α for
24 or 48h. IL-1 significantly decreased the mRNA expression and the
protein levels of the osteoblast-specific genes Runx2, ALP, OSX and
OCN in the MC3T3-E1 cells in a dose-dependent manner compared with
those in the non-treated culture at 24 h (P<0.01) (Figs. 6 and 7).
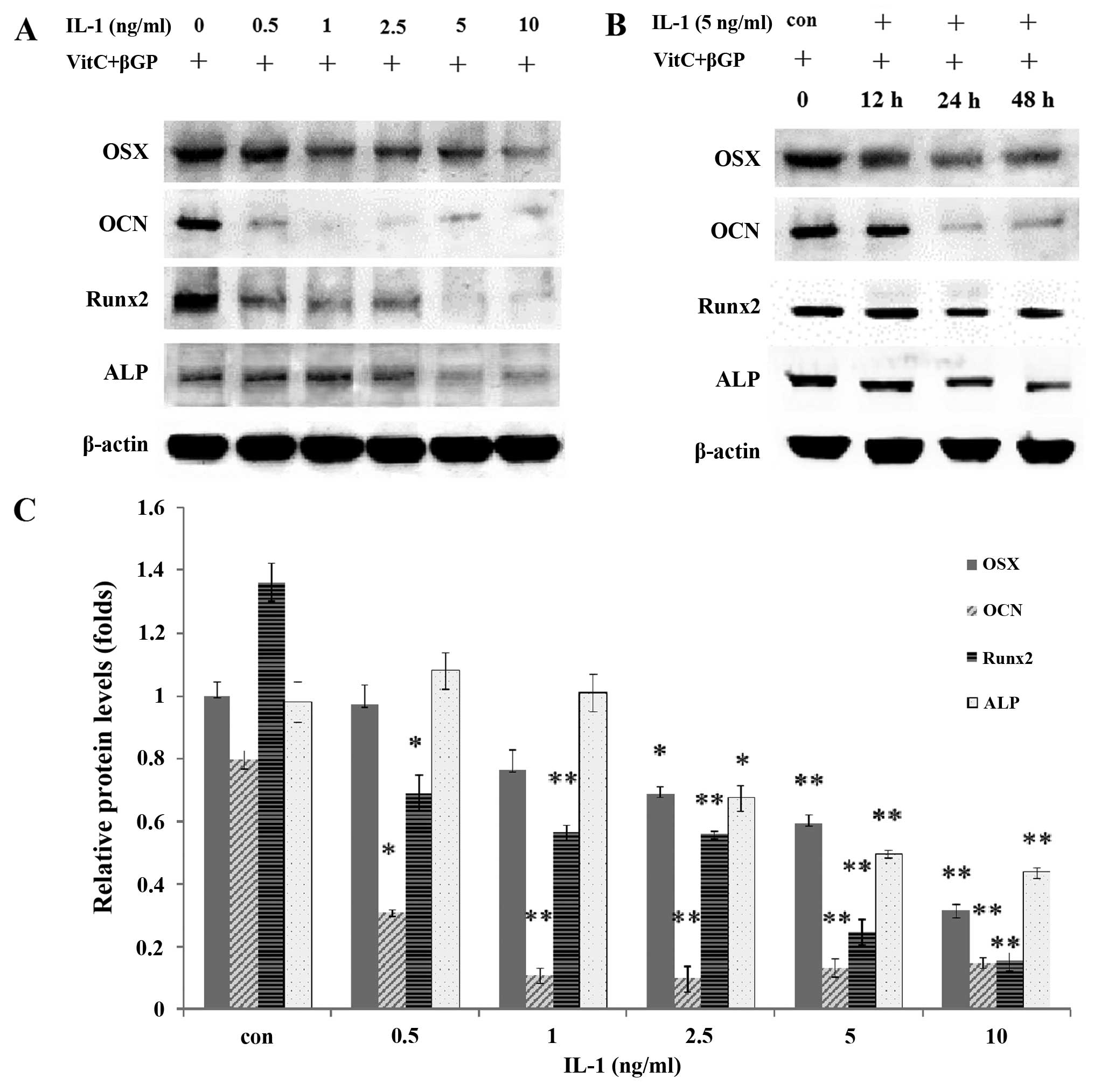 | Figure 7(A and C) Effect of interleukin
(IL)-1α on the protein expression of osterix (OSX), osteocalcin
(OCN), runt-related transcription factor 2 (Runx2) and alkaline
phosphatase (ALP) at 24 h after IL-1α treatment (0, 0.5, 1, 2.5, 5
or 10 ng/ml) in MC3T3-E1 cells. (B) Effect of IL-1α on the protein
expression of OSX, OCN, Runx2 and ALP at 0, 12, 24 and 48 h after
IL-1α treatment (5 ng/ml) in MC3T3-E1 cells. *P<0.05
and **P<0.01. |
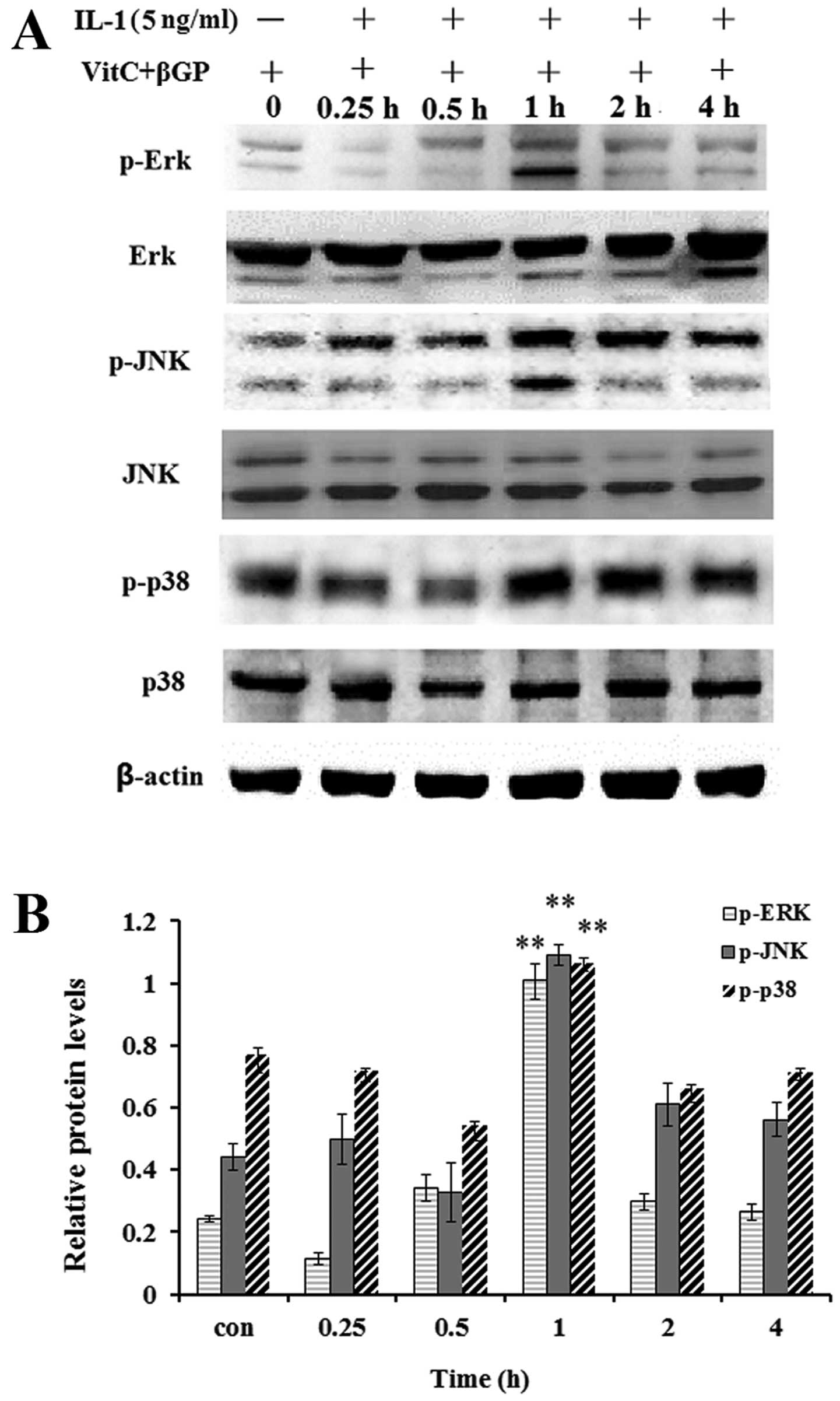
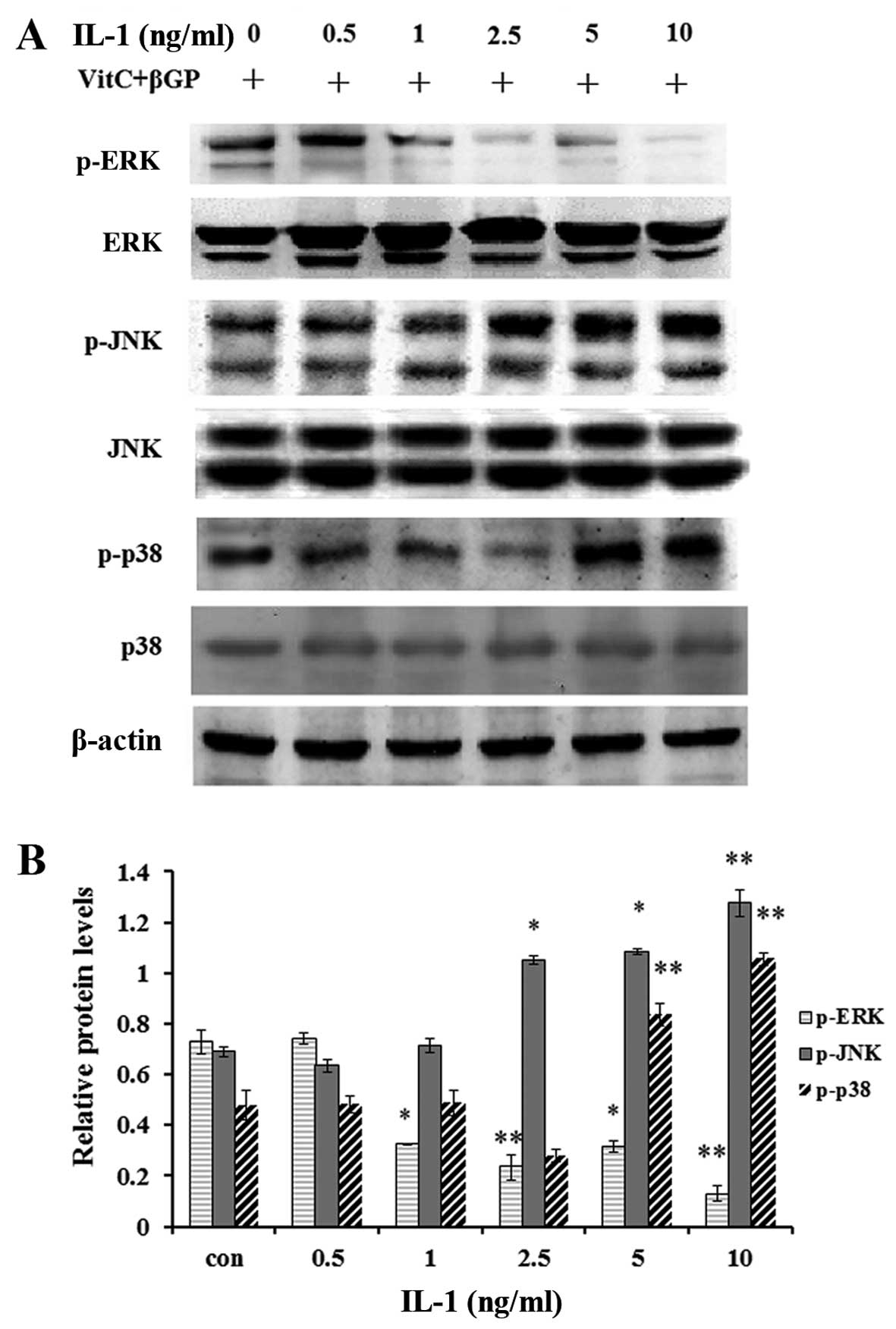
Effect of IL-1 on the activation of MAPK
in MC3T3-E1 cells
As MAP kinases are important regulators of
inflammatory mediators and osteoblast differentiation, we performed
western blot analysis to examine the effect of IL-1α on the
activation of MAPKs in the MC3T3-E1 cells. The results showed that
IL-1α at concentrations of 5 ng/ml significantly enhanced the
protein levels of phosphorylated p38 MAPK, JNK1/2 and ERK1/2 at the
1 h incubation time (Fig. 8);
moreover IL-1α at concentrations >5 ng/ml enhanced the protein
levels of p-p38 MAPK and p-JNK1/2, whereas it markedly inhibited
those of p-ERK1/2 (Fig. 9).
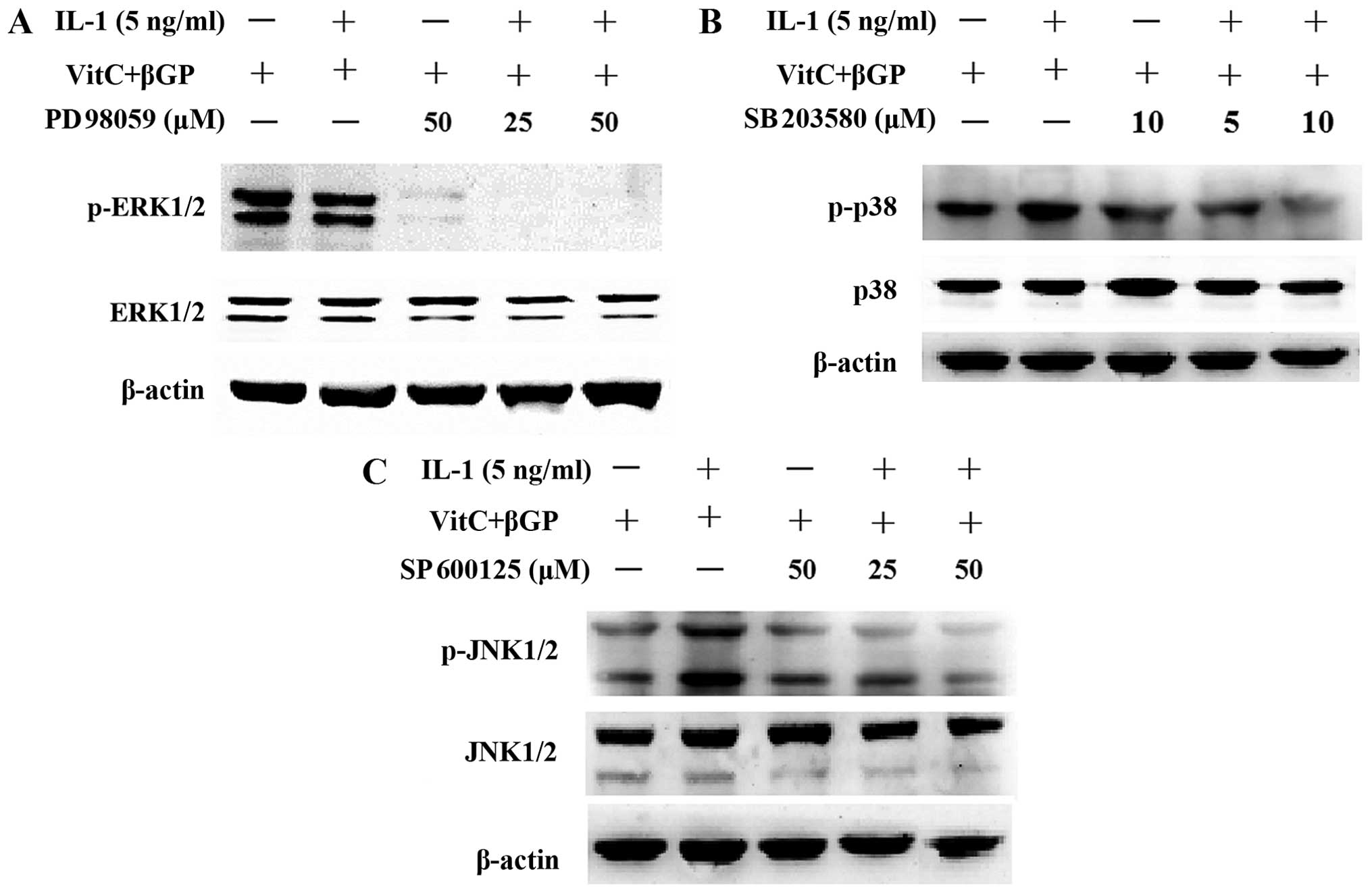
As controls, the MAPK inhibitors SP600125, PD98059
and SB203580 were applied for 2 h prior to IL-1α treatment and then
proteins were prepared at 1 or 24 h after IL-1α treatment in the
presence of MAPK inhibitors. The results showed that pre-treatment
with the MAPK inhibitors attenuated the phosphorylation of JNK and
p38 MAPK induced by IL-1α; however, pre-treatment with the PD98059
(ERK inhibitor) resulted in the further inhibition of the
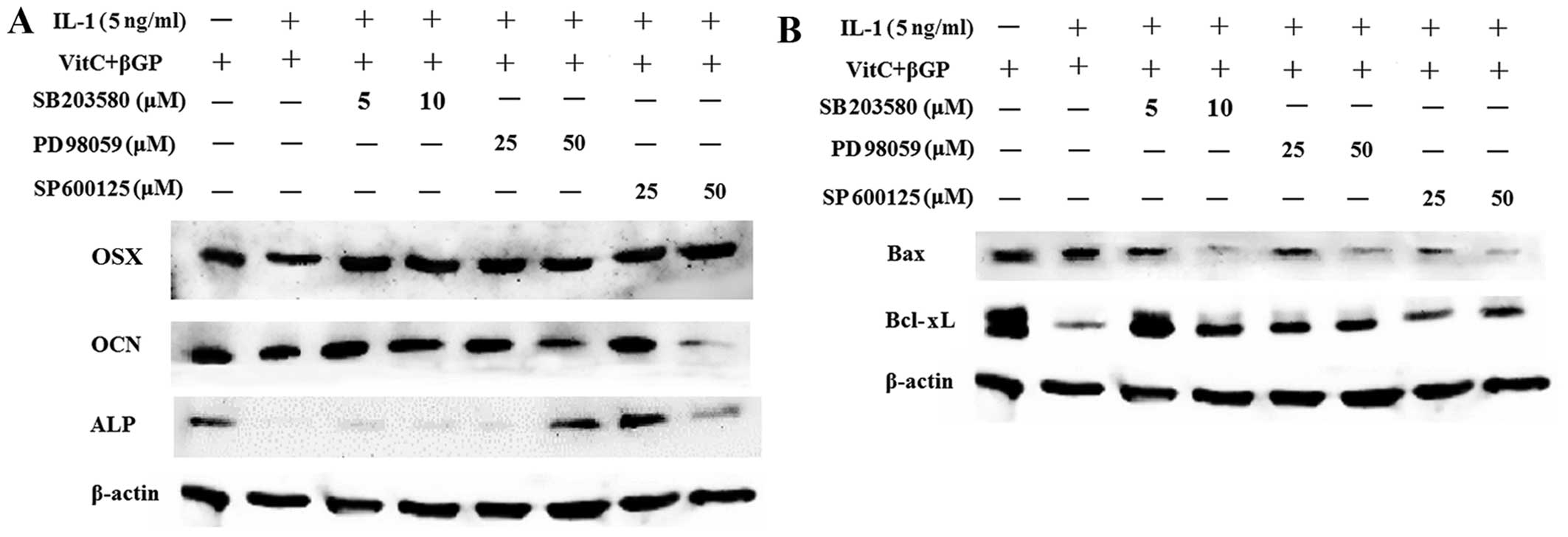
phosphorylation of ERK1/2 induced by IL-1α (Fig. 10). Moreover, pre-treatment with
the MAPK inhibitors decreased the protein expression of Bax induced
by IL-1α in the MC3T3-E1 cells. However, MAPK inhibitors markedly
increased the protein expression of osteoblast-related genes and
Bcl-xL in the MC3T3-E1 cells downregulated by IL-1α (Fig. 11).
Discussion
Excessive bone resorption in chronic inflammatory
diseases, such as septic arthritis, osteomyelitis and infected
orthopedic implant failure, is at least partially caused by the
activation of bacteria-induced inflammatory responses (2). IL-1, a proin-flammatory cytokine
that is important in inflammation and host responses to infection,
plays a well documented role in the modulation of osteoclastic bone
resorption (10–12) and bone formation (6–9).
However, the mechanism responsible for the effects of IL-1 on the
differentiation and function of osteoblasts remains unclear. In the
present study, IL-1α directly induced apoptosis and inhibited
osteoblast differentiation of MC3T3-E1 osteoblasts. Our results
confirmed that IL-1α inhibits osteoblast differentiation directly
(6–9).
The inhibitory effect of IL-1α on osteoblast
differentiation was confirmed by evaluating the expression of
osteoblast marker genes. Runx2 (Cbfa1) is required for mesenchymal
cell differentiation into preosteoblasts (17,18). OSX, downstream of Runx2, is an
osteoblast-specific transcription factor essential for osteoblast
differentiation and bone formation (19,20). ALP has been suggested to be
involved in the early-stage molecular events of osteoblast
differentiation, whereas OCN is involved in the late-stage
molecular events (13,21). In the present study, the mRNA
expression and protein levels of Runx2, ALP, OSX and OCN as well as
ALP activity in MC3T3-E1 cells were significantly downregulated by
IL-1α in a dose-dependent manner. Taken together, these findings
indicate that the inhibitory effect of IL-1α on osteoblast
differentiation may be due to the inhibition of osteoblast-related
genes.
The induction of osteoblast apoptosis results in
further bacteria-induced bone destruction (2). We evaluated the effect of IL-1α on
inducing the apoptosis of MC3T3-E1 cells. Our data show that IL-1α
significantly upregulated the expression of Bax, cytochrome
c and caspase-3 as well as increasing caspase-3 activity,
whereas Bcl-2 expression was decreased in the MC3T3-E1 cells. These
results indicated that IL-1 induced osteoblast apoptosis in a
dose-dependent manner compared with the non-treated cells.
Moreover, IL-1α enhanced the protein levels of p-JNK and p-p38 MAPK
in the MC3T3-E1 cells. Based on our results, we suggest that IL-1
induces the apoptosis of osteoblasts through a mitochondrial
pathway.
MAP kinases are activated by various stresses,
including proinflammatory cytokines, and affect apoptosis either
positively or negatively (22,23). In many cell types, JNK and p38
MAPK contribute to the induction of apoptosis, whereas ERK inhibits
apoptotic processes (23–26). In the present study, treatment
with IL-1α enhanced the protein levels of p-p38 and p-JNK whereas
it inhibited the phosphorylation of ERK. Pre-treatment with MAPK
inhibitors attenuated the phosphorylation of JNK and p38 enhanced
by IL-1α. Moreover, pre-treatment with MAPK inhibitors decreased
the protein expression of Bax induced by IL-1α in the MC3T3-E1
cells. However, MAPK inhibitors markedly increased the protein
expression of osteoblast-related genes and Bcl-xL in MC3T3-E1 cells
downregulated by IL-1α. This suggests that the JNK and the p38 MAPK
pathways play a vital role in regulating IL-1α-induced apoptosis
and osteoblast differentiation of MC3T3-E1 cells.
In conclusion, our data suggest that IL-1α induces
osteo-blast apoptosis and inhibits bone formation by activating the
JNK and the p38 MAPK pathways. These results indicate that agents
modulating the JNK and the p38 MAPK pathways may be of potential
therapeutic use for restoring osteoblast function in
bacteria-induced bone diseases.
Acknowledgments
The present study was supported by a research grant
from the National Natural Science Foundation of China (grant no.
81371981), the School Fund of Luohe Medical College (no.
2014-DF-002; 2013-S-LMC04) and the Fund of Henan Provincial Health
Bureau (no. 201203081).
References
|
1
|
Roodman GD: Advances in bone biology: the
osteoclast. Endocr Rev. 17:308–332. 1996.PubMed/NCBI
|
|
2
|
Nair SP, Meghji S, Wilson M, Reddi K,
White P and Henderson B: Bacterially induced bone destruction:
mechanisms and misconceptions. Infect Immun. 64:2371–2380.
1996.PubMed/NCBI
|
|
3
|
Dinarello CA: Biologic basis for
interleukin-1 in disease. Blood. 87:2095–2147. 1996.PubMed/NCBI
|
|
4
|
Nakae S, Asano M, Horai R and Iwakura Y:
Interleukin-1 beta, but not interleukin-1 alpha, is required for
T-cell-dependent antibody production. Immunology. 104:402–409.
2001. View Article : Google Scholar
|
|
5
|
Garlanda C, Dinarello CA and Mantovani A:
The interleukin-1 family: back to the future. Immunity.
39:1003–1018. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stashenko P, Dewhirst FE, Rooney ML,
Desjardins LA and Heeley JD: Interleukin-1 beta is a potent
inhibitor of bone formation in vitro. J Bone Miner Res. 2:559–565.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohmori Y, Hanazawa S, Amano S, Hirose K,
Kumegawa M and Kitano S: Effects of recombinant human interleukin I
alpha and interleukin 1 beta on cell growth and alkaline
phosphatase of the mouse osteoblastic cell line MC3T3-E1. Biochim
Biophys Acta. 970:22–30. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lacey DL, Grosso LE, Moser SA, Erdmann J,
Tan HL, Pacifici R and Villareal DT: IL-1-induced murine osteoblast
IL-6 production is mediated by the type 1 IL-1 receptor and is
increased by 1,25 dihydroxyvitamin D3. J Clin Invest. 91:1731–1742.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee YM, Fujikado N, Manaka H, Yasuda H and
Iwakura Y: IL-1 plays an important role in the bone metabolism
under physiological conditions. Int Immunol. 22:805–816. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gowen M, Wood DD, Ihrie EJ, McGuire MK and
Russell RG: An interleukin 1-like factor stimulates bone resorption
in vitro. Nature. 306:378–380. 1983. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lorenzo JA, Sousa SL, Alander C, Raisz LG
and Dinarello CA: Comparison of the bone-resorbing activity in the
supernatants from phytohemagglutinin-stimulated human peripheral
blood mononuclear cells with that of cytokines through the use of
an antiserum to interleukin 1. Endocrinology. 121:1164–1170. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakamura I and Jimi E: Regulation of
osteoclast differentiation and function by interleukin-1. Vitam
Horm. 74:357–370. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsuguchi T, Chiba N, Bandow K, Kakimoto
K, Masuda A and Ohnishi T: JNK activity is essential for Atf4
expression and late-stage osteoblast differentiation. J Bone Miner
Res. 24:398–410. 2009. View Article : Google Scholar
|
|
14
|
Guo C, Yuan L, Wang JG, Wang F, Yang XK,
Zhang FH, Song JL, Ma XY, Cheng Q and Song GH: Lipopolysaccharide
(LPS) induces the apoptosis and inhibits osteoblast differentiation
through JNK pathway in MC3T3-E1 cells. Inflammation. 37:621–631.
2014. View Article : Google Scholar
|
|
15
|
Guo C, Wang SL, Xu ST, Wang JG and Song
GH: SP600125 reduces lipopolysaccharide-induced apoptosis and
restores the early-stage differentiation of osteoblasts inhibited
by LPS through the MAPK pathway in MC3T3-E1 cells. Int J Mol Med.
35:1427–1434. 2015.PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
17
|
Komori T and Kishimoto T: Cbfa1 in bone
development. Curr Opin Genet Dev. 8:494–499. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Komori T, Yagi H, Nomura S, Yamaguchi A,
Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, et al:
Targeted disruption of Cbfa1 results in a complete lack of bone
formation owing to maturational arrest of osteoblasts. Cell.
89:755–764. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakashima K, Zhou X, Kunkel G, Zhang Z,
Deng JM, Behringer RR and de Crombrugghe B: The novel zinc
finger-containing transcription factor osterix is required for
osteoblast differentiation and bone formation. Cell. 108:17–29.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fu H, Doll B, McNelis T and Hollinger JO:
Osteoblast differentiation in vitro and in vivo promoted by
Osterix. J Biomed Mater Res A. 83:770–778. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim HK, Cho SG, Kim JH, Doan TK, Hu QS,
Ulhaq R, Song EK and Yoon TR: Mevinolin enhances osteogenic genes
(ALP, type I collagen and osteocalcin), CD44, CD47 and CD51
expression during osteogenic differentiation. Life Sci. 84:290–295.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pearson G, Robinson F, Beers Gibson T, Xu
BE, Karandikar M, Berman K and Cobb MH: Mitogen-activated protein
(MAP) kinase pathways: regulation and physiological functions.
Endocr Rev. 22:153–183. 2001.PubMed/NCBI
|
|
23
|
Kyriakis JM and Avruch J: Mammalian MAPK
signal transduction pathways activated by stress and inflammation:
a 10-year update. Physiol Rev. 92:689–737. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xia Z, Dickens M, Raingeaud J, Davis RJ
and Greenberg ME: Opposing effects of ERK and JNK-p38 MAP kinases
on apoptosis. Science. 270:1326–1331. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang C, Liang J, Qian J, Jin L, Du M, Li M
and Li D: Opposing role of JNK-p38 kinase and ERK1/2 in hydrogen
peroxide-induced oxidative damage of human trophoblast-like JEG-3
cells. Int J Clin Exp Pathol. 7:959–968. 2014.PubMed/NCBI
|
|
26
|
Fister S, Günthert AR, Aicher B, Paulini
KW, Emons G and Gründker C: GnRH-II antagonists induce apoptosis in
human endometrial, ovarian, and breast cancer cells via activation
of stress-induced MAPKs p38 and JNK and proapoptotic protein Bax.
Cancer Res. 69:6473–6481. 2009. View Article : Google Scholar : PubMed/NCBI
|