Introduction
Hepatocellular carcinoma (HCC) is the most common
primary hepatic malignancy, which is associated with increasing
morbidity worldwide over the last decade (1). HCC is the third most common cause of
cancer death in the world, and it accounts for 90% of primary liver
cancers (2–5). Despite great efforts to develop
treatments for HCC, which have led to the implementation of several
relatively effective methods, the cure rate and survival of
patients with liver cancer are still not optimistic (6–9).
At present, liver resection and transplantation remain the
principal treatments for patients with early-stage HCC (10). However, tumor recurrence after
liver resection and a lack of appropriate donor organs are still
the major problems affecting patient survival (11–13). Thus, in-depth investigations of
the molecular mechanisms underlying the development of HCC are
urgently required, which may lead to novel therapies for HCC.
Astragalus polysaccharide (APS), the primary active
constituent extracted from a traditional Chinese medicinal herb
Astragalus membranaceus, has been shown to exhibit diverse
pharmacological and biological effects, including antioxidant,
immune-enhancing and antiviral effects as well as resistance to
immunosuppression (14–20). Several studies have demonstrated
the antiviral effects (specifically anti-duck hepatitis A virus
activity and anti-infectious bursal disease virus activity) of APS
and its sulfate (sAPS) in vitro (21,22). In the tumor microenvironment of
human HCC, it has been demonstrated that APS is capable of
restoring the cytokine balance and suppressing the expression of
FOXp3 mRNA, to inhibit the immunosuppressive effects of Treg cells
(23). Furthermore, APS exerts a
marked inhibitory effect on a number of types of solid tumors
(24–26). A previous study suggested that
Astragalus injection can suppress apoptosis of mesothelial cells,
which has the ability to prevent cancer invasion, and thus revealed
that Astragalus may be used in treatment of gastric cancer
(25). Astragalus saponin extract
induced growth inhibition and apoptosis in human colon cancer cells
and a tumor xenograft model in nude mice (27). APS exerted a synergistic antitumor
effect with adriamycin in H22 tumor-bearing mice by promoting the
expression of interleukin (IL)-1α, IL-2, IL-6 and tumor necrosis
factor (TNF)-α, and by suppressing the expression of IL-10,
MDR1 mRNA and P-glycoprotein (P-GP) (28). Taken together, these studies show
that despite evidence of APS exerting inhibitory effects on human
solid tumors, and the use of APS as an adjuvant treatment in
combination with other anticancer drugs in order to reduce
side-effects and increase sensitivity, its effect on the
progression of HCC as well as the underlying regulatory mechanism
remain unclear.
Recent findings have indicated that the ectopic
expression of Notch1 was associated with increased metastatic
capacity, improved survival times and vasculogenic mimicry in HCC
cell lines (29,30). Notch proteins (Notch 1–4) are
transmembrane receptors, which have an extracellular domain for
binding to specific ligands and an intracellular domain involved in
transcriptional regulation (31).
Previous research has demonstrated that the Notch signaling pathway
plays a number of important roles in cancer development (32–35). Notch1 signaling has been
demonstrated to regulate cell proliferation, apoptosis and
differentiation in lung carcinoma (34). The downregulation of Notch1
inhibited cell growth and induced apoptosis in A2780 ovarian cancer
cells (35). A chemically
sulfated polysaccharide derived from Grifola frondosa
induced HepG2 cell apoptosis through the Notch1/nuclear factor
(NF)-κB/p65-mediated caspase pathway (36). A recent study indicated that
Notch1 is a potential therapeutic target for APS-induced apoptosis
in non-small cell lung carcinoma cell lines (37). Based on these findings, we
hypothesized that APS may be involved in the regulation of tumor
growth and metastasis through Notch1 signaling.
In the present study, we aimed to examine the
effects of APS on the survival of an HCC cell line (H22) as well as
the underlying regulatory mechanism responsible for these effects.
Our results revealed that APS decreased cell viability and induced
cell apoptosis in HCC cells by decreasing the expression of Notch1.
Notch1 may be a potential therapeutic target for the treatment of
HCC.
Materials and methods
Samples and cell culture
Human HCC samples were collected from patients who
underwent surgery at Jinshan Hospital Affiliated to Fudan
University (Shanghai, China). All the patients provided written
informed consent. Dissected samples were frozen immediately after
surgery and stored at -80°C until use. All procedures involved
clinical specimens were approved by the Ethics Committee of Jinshan
Hospital Affiliated to Fudan University. The mouse HCC cell line
(H22) was obtained from the Shanghai Cell Bank (Chinese Academy of
Sciences) and cultured in RPMI-1640 medium (Invitrogen, Carlsbad,
CA, USA) supplemented with 10% fetal bovine serum (FBS;
Invitrogen), 100 µg/ml streptomycin and 100 U/ml penicillin
solutions (both from Beyotime Institute of Biotechnology, Haimen,
China). The cells were incubated at 37°C in humidified air with 5%
CO2. The medium buffer was replaced daily.
Reagents
APS was purchased from Hongsheng Biotech Co. (Xi'an,
China) and was diluted in H2O immediately prior to
administration.
Assays of cell viability
The cells were plated at a density of
1×104 cells per well in a 96-well plate. Following
incubation, the cells were treated with or without APS. Cell
viability was quantified using a cell counting kit-8 (CCK-8;
Dojindo Laboratories, Kumamoto, Japan) for 3 consecutive days after
infection. Each data point was obtained in triplicate.
Analysis of cell apoptosis
The rate of apoptosis was measured by Annexin-V FITC
and PI staining, followed by flow cytometry (flow cytometer from
Becton-Dickinson, Franklin Lakes, NJ, USA). Briefly, the cells were
trypsinized and suspended in 500 µl of binding buffer
containing 5 μl Annexin V FITC and 5µl PI
(Sigma-Aldrich Chemie Gmbh, Munich, Germany). Following incubation
in the dark for 1 h, the cells were subjected to flow cytometry and
the rate of cell apoptosis was determined.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells using TRIzol
reagent (Invitrogen) according to the manufacturer's instructions.
Reverse transcription was performed using a SuperScript III Reverse
Transcriptase kit (Invitrogen) in accordance with the
manufacturer's instructions. Quantitative (real-time) PCR (qPCR)
was performed using an Applied Biosystems 7300 Sequence Detection
system (Applied Biosystems, Foster City, CA, USA). The 20 µl
PCR reaction included 1 µl of cDNA, 10 µl of 2X
TaqMan gene expression master mix and 1 µl of TaqMan gene
expression assays reagent (Applied Biosystems). The reactions were
incubated in 96-well optical plates at 95°C for 10 min, followed by
40 cycles of 95°C for 15 sec and 60°C for 1 min. qPCR reactions
were performed in triplicate. The expression levels of the relative
genes were calculated using the 2−ΔΔCT method. GAPDH
served as an internal control. The primer sets used were as
follows: Notch1 forward, 5′-GATGACCTGGGCAAGTC-3′ and reverse,
5′-CCCTGTTGTTCTGCATATCT-3′; GAPDH forward,
5′-GCACCGTCAAGCTGAGAAC-3′ and reverse, 5′-GAGCAGCGTCTTCAGAGACAG-3′;
Bcl-2 forward, 5′-CTGAGTACCTGAACCGGCATC-3′ and reverse,
5′-TGGTGAAGACGCCAGTGGA-3′; BAX forward, 5′-GTTTCATCCAGGATCGAGCAG-3′
and reverse, 5′-AGCTGAGCGAGTGTCTCCGGCG-3′. The PCR products were
separated on a 1.2% agarose gel and identified after ethidium
bromide staining (both from Tiangen Biotech (Beijing) Co., Ltd,
Beijing, China).
Western blot analysis
Protein extraction and western blot analysis were
performed as previously described (29). β-actin was used as the loading
control protein. Primary antibodies against Notch1, Bcl-2, BAX,
caspase-3, caspase-8, E-cadherin, matrix metallopeptidase 9
(MMP-9), cyclooxygenase-2 (COX-2) and β-actin were all purchased
from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All secondary
antibodies were obtained from Univ-Bio Inc. (Shanghai, China).
Transfection of small interfering RNA
(siRNA)
Three different siRNAs targeting Notch1
(si-Notch1-1, si-Notch1-2 and si-Notch1-3) and a scramble siRNA
(siScramble) were synthesized by Santa Cruz Biotechnology and
transfected into the HCC cells using Lipofectamine 2000
(Invitrogen) as previously described by Zhou et al (29). The sequences of the siRNAs were as
follows: si-Notch1-1 sense, 5′-GCUCCCUCAACUUCAAUGAUU-3′ and
antisense, 3′-UUCGAGGGAGUUGAAGUUACU-5′; si-Notch1-2 sense,
5′-GCCUGGACAAGAUCAAUGAUU-3′ and antisense,
3′-UUCGGACCUGUUCUAGUUACU-5′; si-Notch1-3 sense,
5′-CAGGGAGCAUGUGUAACAUUU-3′ and antisense,
3′-UUGUCCCUCGUACACAUUGUA-5′.
Statistical analysis
Statistical analysis was conducted using SPSS
software version 16.0 (SPSS, Inc., Chicago, IL, USA). All
experiments were performed at least three times, and the data were
summarized and are presented as the means ± SD. The t-test was used
to compare two independent groups. Inter-group differences were
analyzed by one-way ANOVA followed by Tukey's multiple comparison
test as a post-test to compare the group means. P<0.05 was
considered to indicate a statistically significant difference.
Results
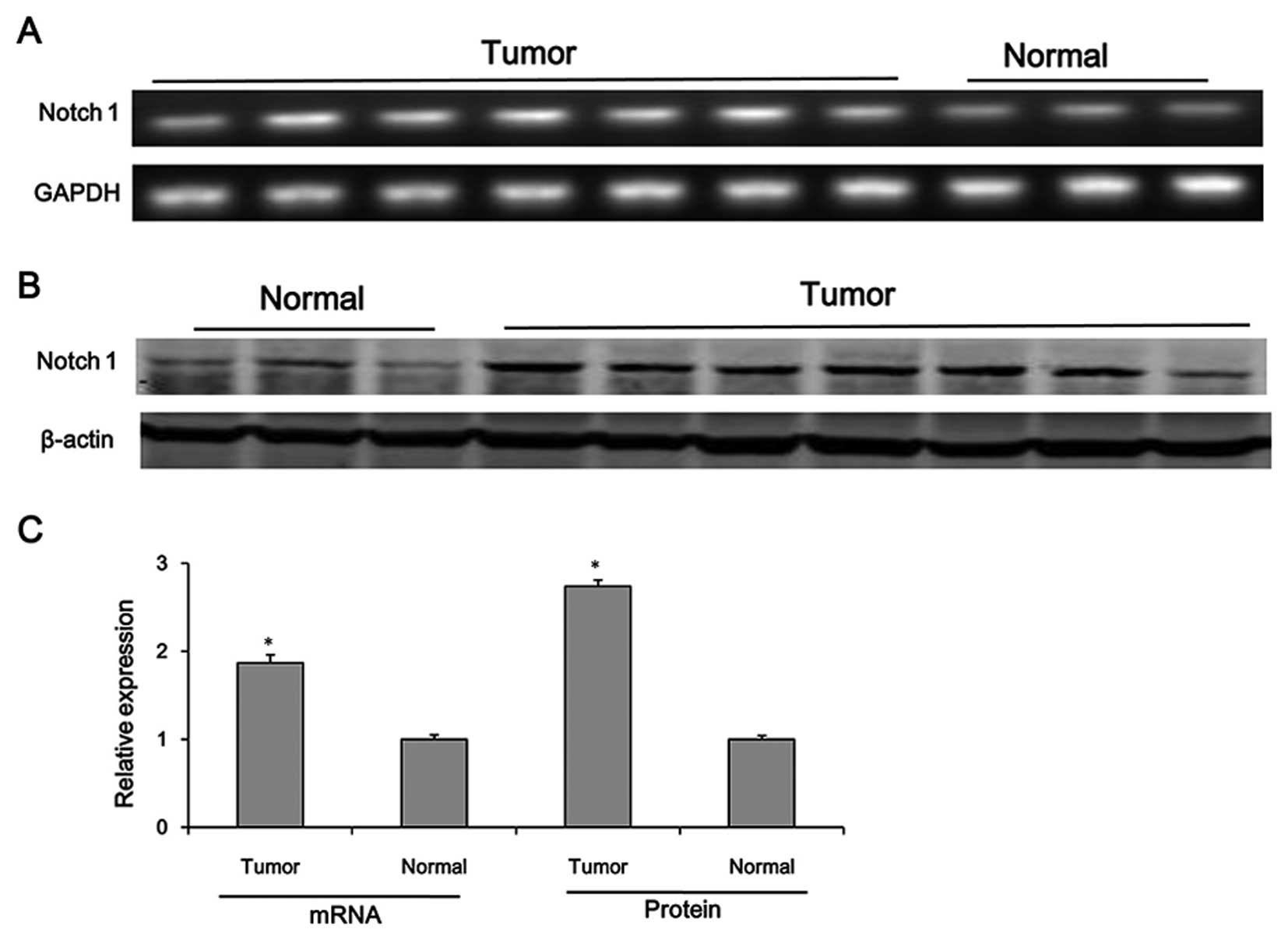
Notch1 is upregulated in HCC tissues
According to a study by Zhou et al (29), the results of immunohistochemical
analysis revealed that the high expression of Notch1 in HCC tissues
correlated with tumor size, tumor grade, metastasis and venous
invasion. In the present study, we determined the protein and mRNA
levels of Notch1 in seven HCC tissues and three normal liver
tissues. As shown in Fig. 1, the
protein and mRNA levels of Notch1 were significantly upregulated in
the HCC tissues compared with those in normal tissues
(P<0.05).
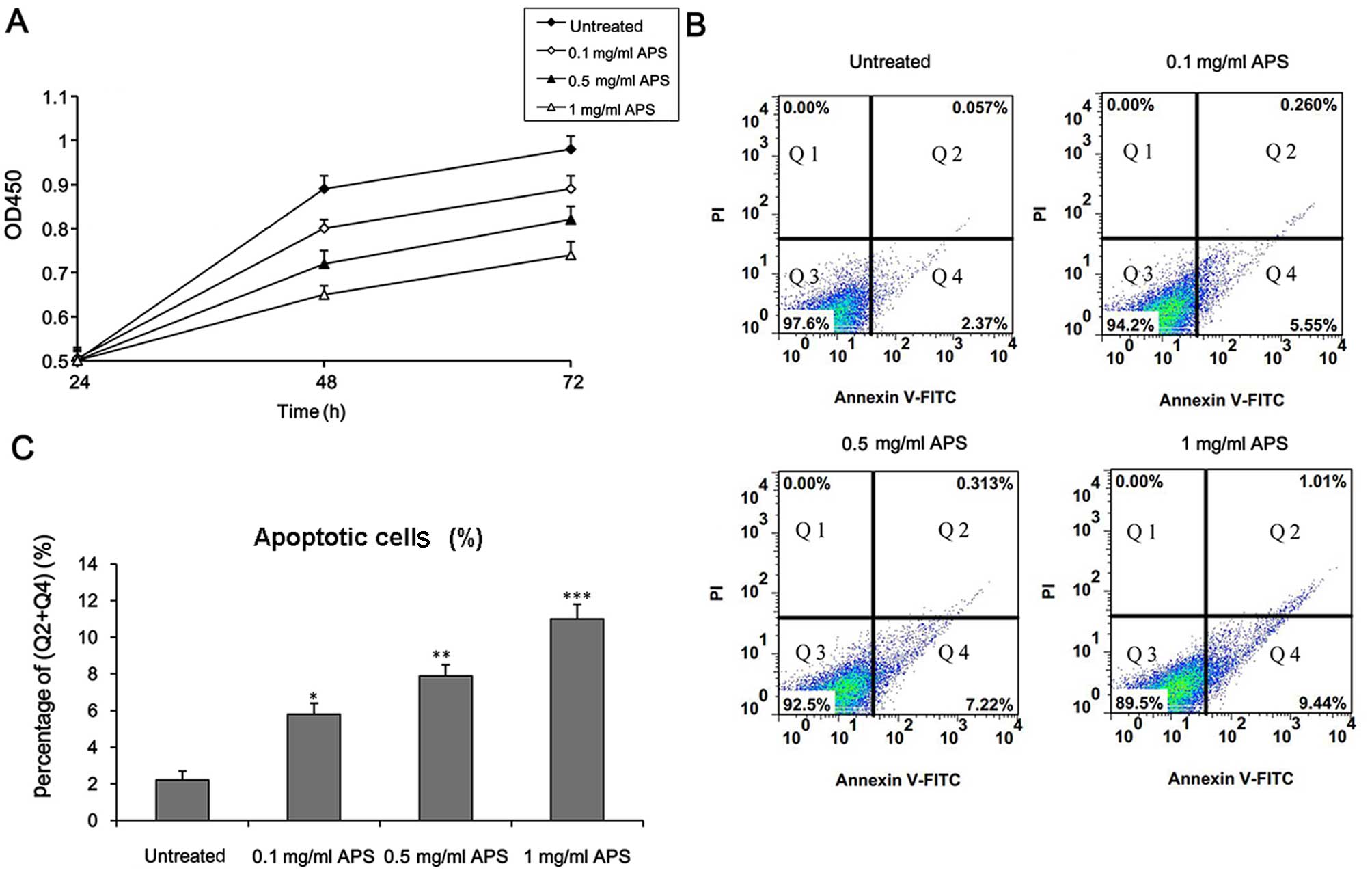
APS decreases cell viability and induces
cell apoptosis in HCC cells
It was previously demonstrated that APS inhibited
the growth and proliferation of Treg cells in the tumor
microenvironment of HCC and exerted an antitumor effect (23,28). To determine whether APS exerts an
effect on the regulation of HCC cell growth, H22 cells were
incubated with different concentrations of APS. Firstly, we
examined the viability of the H22 cells. The results showed that
APS treatment decreased cell survival in a concentration-dependent
manner (Fig. 2A).
To determine whether APS induced cell death through
an apoptotic mechanism, apoptosis was detected by Annexin V/PI
double staining. As shown in Fig. 2B
and C, the apoptotic rate was significantly increased in the
H22 cells following APS treatment in a concentration-dependent
manner. To further explore the effect of APS on apoptosis in the
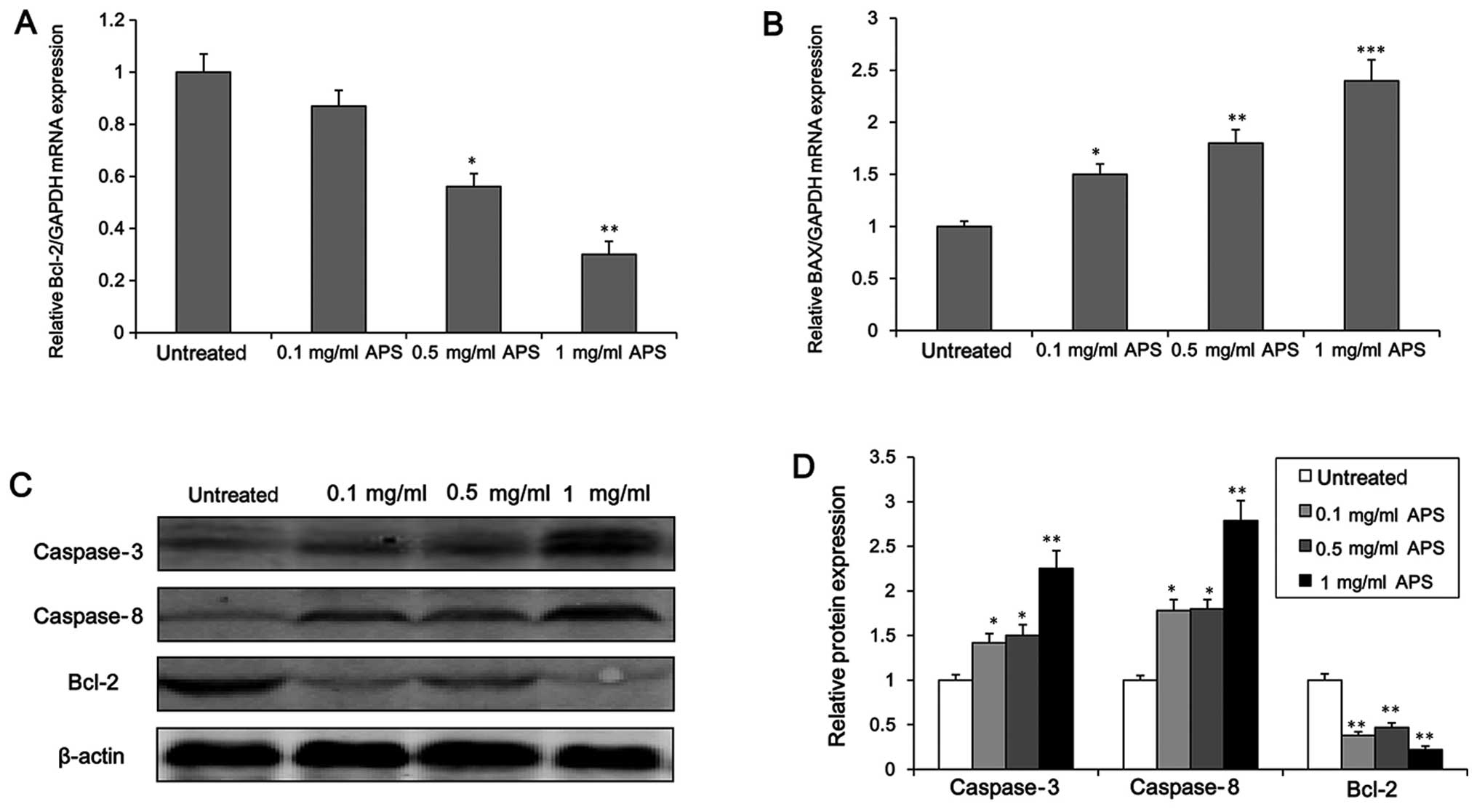
HCC cells, we measured the expression level of apoptosis-related
genes (Bcl-2 and BAX). It was found that at concentrations >0.1
mg/ml, APS significantly inhibited the mRNA level of the apoptosis
suppressor gene Bcl-2 (Fig. 3A).
Similarly, the protein level of Bcl-2 was down-regulated markedly
following the incubation of the H22 cells with APS (Fig. 3C and D, P<0.01). In addition,
the mRNA level of BAX, a pro-apoptotic gene, was markedly increased
by APS in a concentration-dependent manner (Fig. 3B). Furthermore, the expression of
the apoptosis-related proteases (caspase-3 and -8) was also
affected by APS. Following APS treatment, the protein levels of
caspase-3 and -8 were significantly enhanced in the H22 cells
(Fig. 3C and D). Taken together,
these findings suggest that APS decreases HCC cell survival through
an apoptotic mechanism.
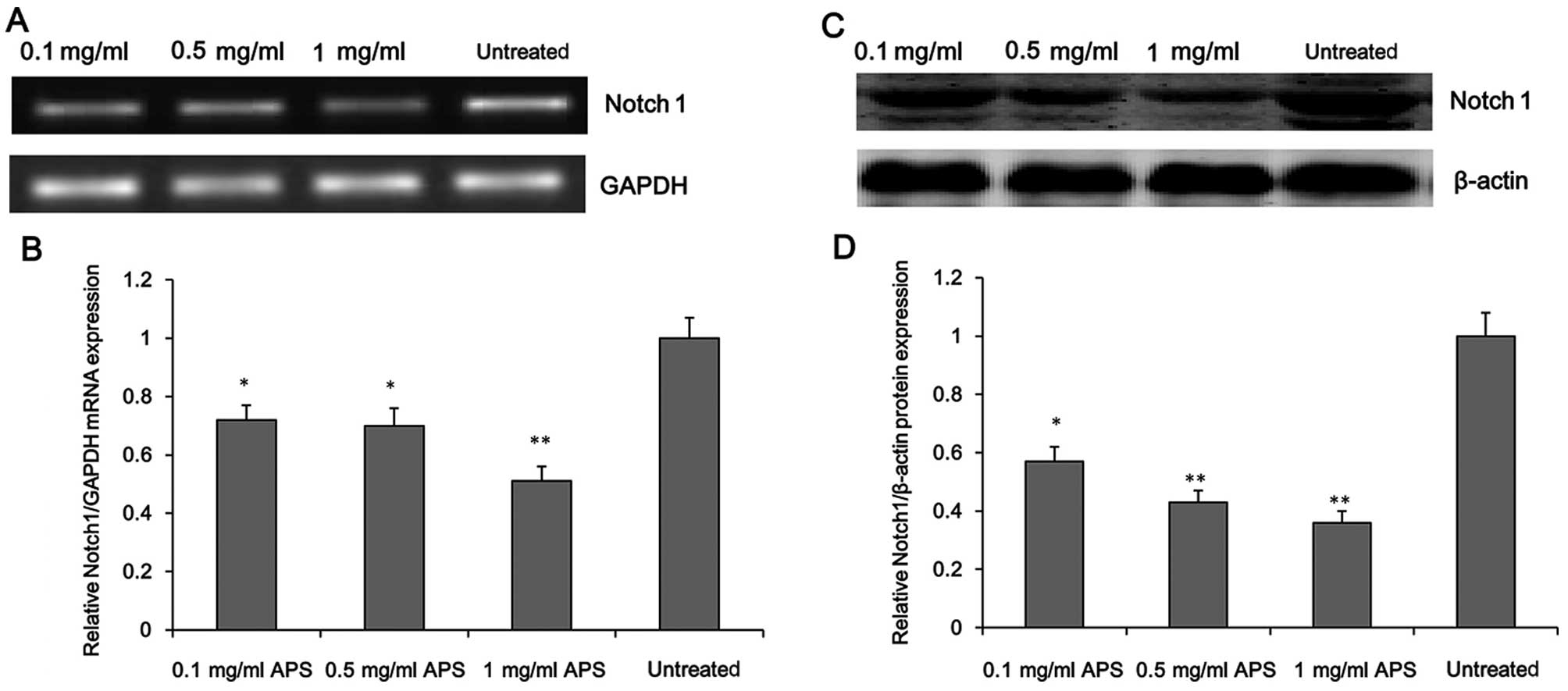
APS inhibits Notch1 expression in
HCC
To further investigate the molecular mechanism
underlying the inhibitory effect of APS on HCC cell survival, we
evaluated the expression level of Notch1 in the APS-treated H22
cells. As shown in Fig. 4, APS
treatment significantly decreased the mRNA and protein levels of
Notch1 in the H22 cells in a concentration-dependent manner, which
suggests that Notch1, is a potential target of APS.
Notch1 knockdown suppresses the survival
and metastasis of HCC cells
After clarifying the association between APS and
Notch1, we further intended to explore the effects of Notch1 on
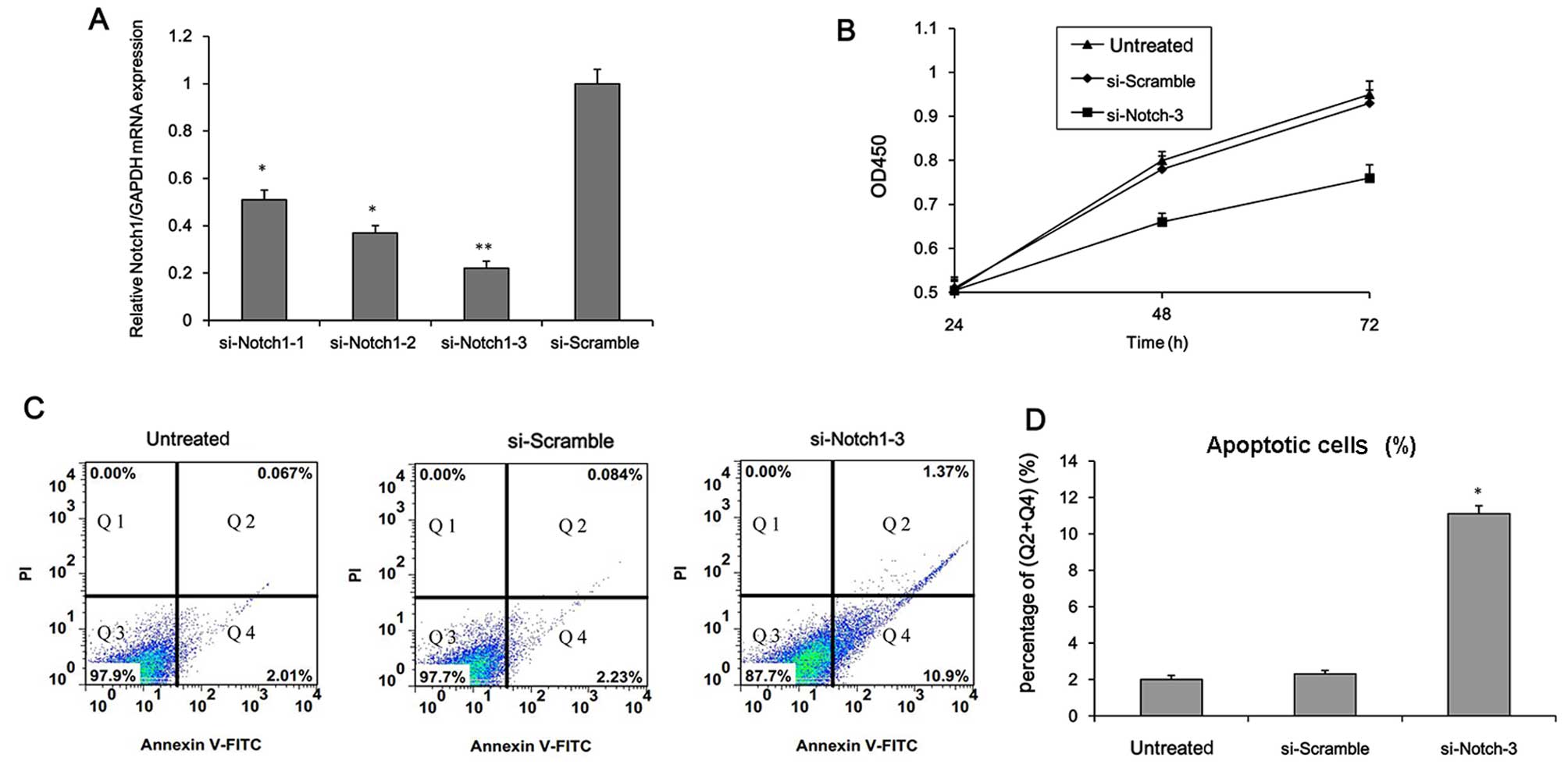
cell growth. Firstly, Notch1 was knocked down in the H22 cells.
Three different siRNAs targeting Notch1 were transfected into the
H22 cells. The Notch1 mRNA level was markedly down-regulated and
si-Notch1-3 exhibited the strongest inhibitory effect (Fig. 5A). Consequently, si-Notch1-3 was
selected for use in subsequent experiments. As shown in Fig. 5B–D, Notch1 knockdown reduced cell
viability and significantly enhanced apoptosis (P<0.05). The
next experiment examined changes in the expression of
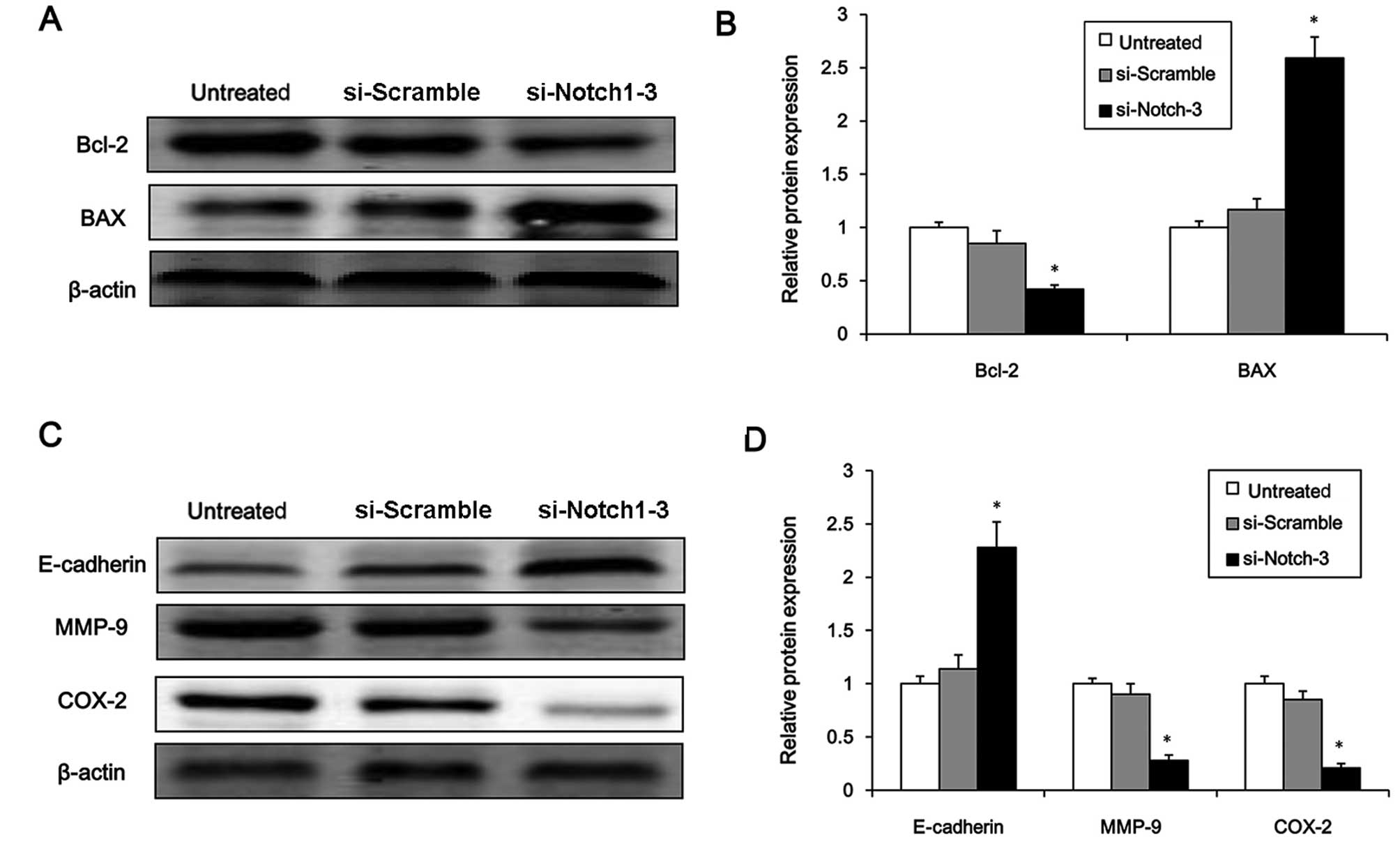
apoptosis-related genes. Notch1 knockdown significantly suppressed
the protein expression of the apoptosis suppressor gene, Bcl-2, and
enhanced the protein expression of the pro-apoptotic gene, BAX
(Fig. 6A and B, P<0.05).
Finally, the protein expression levels of metastasis-associated
molecules including E-cadherin, MMP-9 and COX-2 were examined to
determine whether Notch1 affects the metastasis of HCC cells. The
results showed that the protein expression of E-cadherin was
significantly increased by Notch1 knockdown, whereas the levels of
MMP-9 and COX-2 were markedly reduced (Fig. 6C and D, P<0.05). These results
suggest that Notch1 plays a role in the regulation of HCC cell
survival and metastasis.
Discussion
In the present study, we evaluated changes in the
expression of Notch1 in human HCC tissues compared with that in the
normal tissues, and found that Notch1 was overexpressed in the HCC
tissues. We further demonstrated that APS decreased the survival of
HCC cells through an apoptotic mechanism. Furthermore, we showed
that Notch1 knockdown decreased cell viability and enhanced the
apoptosis and metastatic capacity of HCC cells. We suggest that APS
induced HCC cell apoptosis by suppressing the expression of
Notch1.
The root of A. membranaceus, known as Huang
Qi in Mandarin, was widely used in traditional Chinese medicine.
APS, an extract of A. membranaceus, was extensively used in
the clinic due to a number of beneficial effects including
stimulation of the immune response, antiviral and antioxidant
effects, protection against organ damage and inhibition of cancer
cell proliferation (14–20,38). In the present study, we found that
APS decreased H22 cell survival and induced apoptosis in a
concentration-dependent manner (Fig.
2). Furthermore, the expression of apoptosis-related genes
(Bcl-2 and BAX) and proteases (caspase-3 and -8) was regulated by
APS treatment (Fig. 3). APS
decreased HCC cell survival through an apoptotic mechanism. A
previous study reported that Astragalus saponin extract exerted
anti-apoptotic effects on human peritoneal mesothelial cells during
peritoneal gastric cancer metastasis and thus revealed that
Astragalus may be used as an adjuvant chemotherapeutic agent in
gastric cancer therapy (25).
Astragalus saponin extract inhibited cell proliferation through
cell cycle arrest, and promoted apoptosis in human colon cancer
cells (HT-29) through caspase-3 activation and poly(ADP-ribose)
polymerase cleavage (27). APS
exerted a synergistic antitumor effect with adriamycin in H22
tumor-bearing mice through regulating cytokine and P-GP expression
(28). Compound Astragalus
and Salvia miltiorrhiza extract inhibited cell invasion by
modulating transforming growth factor (TGF)-β/Smad signaling in
HepG2 cells (24). These findings
suggest that APS has the potential to act as an effective
chemotherapeutic agent in HCC treatment, and also to be used as an
adjuvant in combination with other orthodox chemotherapeutic drugs
in order to reduce side-effects.
In recent years, there have been several studies
examining the biological activity of polysaccharides. However, to
the best of our knowledge there have been limited reports regarding
the regulatory mechanism underlying the antitumor effect of
polysaccharides. Wang et al (36) demonstrated that S-GFB, a
chemically sulfated polysaccharide obtained from Grifola
frondosa, induced HepG2 cell proliferation and apoptosis
through the Notch1/NF-κB/p65-mediated caspase pathway. Previous
research has indicated that Notch1 signaling modulated cell
proliferation, apoptosis and differentiation in lung carcinoma and
was associated with the progression of glioma (33,34). Notably, it was found that Notch1
expression was significantly upregulated in the HCC tissues
compared with that in the normal tissues (Fig. 1). Based on these findings, we
speculated that APS may regulate the progression of HCC through
Notch1 signaling. Our results confirmed this hypothesis. Firstly,
APS treatment significantly decreased the mRNA and protein levels
of Notch1 in the H22 cells in a concentration-dependent manner
(Fig. 4), suggesting that APS
induces apoptosis through decreasing Notch1 expression. In
addition, Notch1 knockdown decreased cell viability and
significantly increased the apoptosis of H22 cells by decreasing
the expression of the apoptosis suppressor gene Bcl-2 and
increasing the expression of the pro-apoptotic gene BAX (Figs. 5 and 6). Additonally, the protein level of
E-cadherin was significantly upregulated by Notch1 knockdown,
whereas the levels of MMP-9 and COX-2 were markedly reduced
(Fig. 6C and D). It has been
reported that in some types of cancer, COX-2 regulates the
expression of E-cadherin (29).
E-cadherin and MMP-9 are common metastasis-associated molecules.
Our results demonstrated that Notch1 regulated the survival and
metastatic capacity of HCC cells. APS induced the apoptosis of HCC
cells by suppressing the expression of Notch1. Notch1 may be a
potential therapeutic target for the treatment of HCC.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Serag HB and Mason AC: Rising incidence
of hepatocellular carcinoma in the United States. N Engl J Med.
340:745–750. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Farazi PA and DePinho RA: Hepatocellular
carcinoma pathogenesis: from genes to environment. Nat Rev Cancer.
6:674–687. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fares N and Peron JM: Epidemiology,
natural history, and risk factors of hepatocellular carcinoma. Rev
Prat. 63:216–217. 220–222. 2013.In French.
|
|
6
|
Cusnir M and Patt YZ: Novel systemic
therapy options for hepatocellular carcinoma. Cancer J. 10:97–103.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takayasu K, Arii S, Ikai I, Omata M, Okita
K, Ichida T, Matsuyama Y, Nakanuma Y, Kojiro M, Makuuchi M and
Yamaoka Y; Liver Cancer Study Group of Japan: Prospective cohort
study of transarterial chemoembolization for unresectable
hepatocellular carcinoma in 8510 patients. Gastroenterology.
131:461–469. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han SH, Reddy KR, Keeffe EB,
Soldevila-Pico C, Gish R, Chung RT, Degertekin B and Lok A; NIH HBV
OLT Study Group: Clinical outcomes of liver transplantation for
HBV-related hepatocellular carcinoma: data from the NIH HBV OLT
study. Clin Transplant. 25:E152–E162. 2011. View Article : Google Scholar
|
|
9
|
Rahbari NN, Mehrabi A, Mollberg NM, Müller
SA, Koch M, Büchler MW and Weitz J: Hepatocellular carcinoma:
current management and perspectives for the future. Ann Surg.
253:453–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hassan T, El-Shafie M, Helmy A, Ammar S
and Attyia M: Liver resection for early stage hepatocellular
carcinoma. SECI Oncology. 2015. View Article : Google Scholar
|
|
11
|
Forner A, Hessheimer AJ, Isabel Real M and
Bruix J: Treatment of hepatocellular carcinoma. Crit Rev Oncol
Hematol. 60:89–98. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Silva MF and Wigg AJ: Current
controversies surrounding liver transplantation for hepatocellular
carcinoma. J Gastroenterol Hepatol. 25:1217–1226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sapisochin G, Castells L, Dopazo C, Bilbao
I, Minguez B, Lázaro JL, Allende H, Balsells J, Caralt M and Charco
R: Single HCC in cirrhotic patients: liver resection or liver
transplantation? Long-term outcome according to an
intention-to-treat basis. Ann Surg Oncol. 20:1194–1202. 2013.
View Article : Google Scholar
|
|
14
|
Guo L, Liu J, Hu Y, Wang D, Li Z, Zhang J,
Qin T, Liu X, Liu C, Zhao X, et al: Astragalus polysaccharide and
sulfated epimedium polysaccharide synergistically resist the
immunosuppression. Carbohydr Polym. 90:1055–1060. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li HQ, Reeve-Johnson L and Wang JD: Effect
of Astragalus polysaccharides on erythrocyte immune adherence of
chickens inoculated with infectious bursal disease virus. Agric Sci
China. 6:1402–1408. 2007. View Article : Google Scholar
|
|
16
|
Li R, Chen WC, Wang WP, Tian WY and Zhang
XG: Antioxidant activity of Astragalus polysaccharides and
antitumour activity of the polysaccharides and siRNA. Carbohydr
Polym. 82:240–244. 2010. View Article : Google Scholar
|
|
17
|
Jin M, Zhao K, Huang Q and Shang P:
Structural features and biological activities of the
polysaccharides from Astragalus membranaceus. Int J Biol Macromol.
64:257–266. 2014. View Article : Google Scholar
|
|
18
|
Wang T, Sun Y, Jin L, Xu Y, Wang L, Ren T
and Wang K: Enhancement of non-specific immune response in sea
cucumber (Apostichopus japonicus) by Astragalus membranaceus and
its polysaccharides. Fish Shellfish Immunol. 27:757–762. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yin X, Chen L, Liu Y, Yang J, Ma C, Yao Z,
Yang L, Wei L and Li M: Enhancement of the innate immune response
of bladder epithelial cells by Astragalus polysaccharides through
upregulation of TLR4 expression. Biochem Biophys Res Commun.
397:232–238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhong R, Yu M, Liu H, Sun H, Cao Y and
Zhou D: Effects of dietary Astragalus polysaccharide and Astragalus
membra-naceus root supplementation on growth performance, rumen
fermentation, immune responses, and antioxidant status of lambs.
Anim Feed Sci Technol. 174:60–67. 2012. View Article : Google Scholar
|
|
21
|
Chen Y, Song M, Wang Y, Xiong W, Zeng L,
Zhang S, Xu M, Du H, Liu J, Wang D, et al: The anti-DHAV activities
of Astragalus polysaccharide and its sulfate compared with those of
BSRPS and its sulfate. Carbohydr Polym. 117:339–345. 2015.
View Article : Google Scholar
|
|
22
|
Huang X, Wang D, Hu Y, Lu Y, Guo Z, Kong X
and Sun J: Effect of sulfated astragalus polysaccharide on cellular
infectivity of infectious bursal disease virus. Int J Biol
Macromol. 42:166–171. 2008. View Article : Google Scholar
|
|
23
|
Li Q, Bao JM, Li XL, Zhang T and Shen XH:
Inhibiting effect of Astragalus polysaccharides on the functions of
CD4+CD25 highTreg cells in the tumor microenvironment of
human hepatocellular carcinoma. Chin Med J (Engl). 125:786–793.
2012.
|
|
24
|
Liu X, Yang Y, Zhang X, Xu S, He S, Huang
W and Roberts MS: Compound Astragalus and Salvia miltiorrhiza
extract inhibits cell invasion by modulating transforming growth
factor-β/Smad in HepG2 cell. J Gastroenterol Hepatol. 25:420–426.
2010. View Article : Google Scholar
|
|
25
|
Na D, Liu FN, Miao ZF, Du ZM and Xu HM:
Astragalus extract inhibits destruction of gastric cancer cells to
mesothelial cells by anti-apoptosis. World J Gastroenterol.
15:570–577. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu ZY, Liu RQ, Si CL, Zhou F, Wang YX,
Ding LN, Jing C, Liu AJ and Zhang YM: Structural analysis and
anti-tumor activity comparison of polysaccharides from Astragalus.
Carbohydr Polym. 85:895–902. 2011. View Article : Google Scholar
|
|
27
|
Tin MM, Cho CH, Chan K, James AE and Ko
JK: Astragalus saponins induce growth inhibition and apoptosis in
human colon cancer cells and tumor xenograft. Carcinogenesis.
28:1347–1355. 2007. View Article : Google Scholar
|
|
28
|
Tian QE, Li HD, Yan M, Cai HL, Tan QY and
Zhang WY: Astragalus polysaccharides can regulate cytokine and
P-glycoprotein expression in H22 tumor-bearing mice. World J
Gastroenterol. 18:7079–7086. 2012. View Article : Google Scholar
|
|
29
|
Zhou L, Zhang N, Song W, You N, Li Q, Sun
W, Zhang Y, Wang D and Dou K: The significance of Notch1 compared
with Notch3 in high metastasis and poor overall survival in
hepatocellular carcinoma. PLoS One. 8:e573822013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu MS, Xu LB, Zeng H, Shi XD, Wu WR and
Liu C: Association of Notch1 with vasculogenic mimicry in human
hepatocellular carcinoma cell lines. Int J Clin Exp Pathol.
7:5782–5791. 2014.PubMed/NCBI
|
|
31
|
Yoshida R, Nagata M, Nakayama H,
Niimori-Kita K, Hassan W, Tanaka T, Shinohara M and Ito T: The
pathological significance of Notch1 in oral squamous cell
carcinoma. Lab Invest. 93:1068–1081. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Roy M, Pear WS and Aster JC: The
multifaceted role of Notch in cancer. Curr Opin Genet Dev.
17:52–59. 2007. View Article : Google Scholar
|
|
33
|
Jiang L, Wu J, Chen Q, Hu X, Li W and Hu
G: Notch1 expression is upregulated in glioma and is associated
with tumor progression. J Clin Neurosci. 18:387–390. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wael H, Yoshida R, Kudoh S, Hasegawa K,
Niimori-Kita K and Ito T: Notch1 signaling controls cell
proliferation, apoptosis and differentiation in lung carcinoma.
Lung Cancer. 85:131–140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang M, Wu L, Wang L and Xin X:
Down-regulation of Notch1 by gamma-secretase inhibition contributes
to cell growth inhibition and apoptosis in ovarian cancer cells
A2780. Biochem Biophys Res Commun. 393:144–149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang CL, Meng M, Liu SB, Wang LR, Hou LH
and Cao XH: A chemically sulfated polysaccharide from Grifola
frondosa induces HepG2 cell apoptosis by notch1-NF-κB pathway.
Carbohydr Polym. 95:282–287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang JX, Han YP, Bai C and Li Q: Notch1/3
and p53/p21 are a potential therapeutic target for APS-induced
apoptosis in non-small cell lung carcinoma cell lines. Int J Clin
Exp Med. 8:12539–12547, eCollection. 2015.PubMed/NCBI
|
|
38
|
Yuan CH, Liu YF and Cheng Y: Experimental
study on effect of Astragalus extractum on canine isolated kidney
during hypothermia perfusion and preservation. Zhongguo Zhong Xi Yi
Jie He Za Zhi. 23:291–293. 2003.In Chinese. PubMed/NCBI
|