Introduction
The ATP-binding cassette (ABC) superfamily proteins
are important functional transporters in both prokaryotes and
eukaryotes, which are involved in the transport of compounds across
biological membranes. Almost all ABC transporters found in
eukaryotes are exporters (1). In
humans, the ABC family comprises 48 members (2). The abnormal function of ABC
transporters has been directly linked to the causes of several
human diseases, such as Tangier disease (ABCA1) (3,4),
Stargardt macular dystrophy (ABCA4) (5), pseudoxanthoma elasticum (ABCC6)
(6) and cystic fibrosis
(ABCC7/CFTR) (7). ABC
transporters have also frequently been associated with multidrug
resistance in cancer chemotherapy. For example, P-glycoprotein
(ABCB1) and MRP1 (ABCC1) actively efflux anticancer drugs out of
cells and thus decrease their cytotoxicity (8,9).
ABC transporters share a common structure of four
functional units or domains: two transmembrane domains (TMDs) and
two nucleotide binding domains (NBDs) that hydrolyze ATP to provide
energy for substrate transport (1). The TMDs typically contain 6
transmembrane α-helices. In eukaryotes, most ABC transporters are
constituted by a single polypeptide chain containing all four
functional units ('full transporters'). In the case of so-called
half transporters, the functional transporter is assembled from two
polypeptides, each containing one NBD and one TMD.
Contrary to ABC half-transporters, which
obligatorily form homo- or heterodimers in order to gain transport
activity, the functional unit of full ABC transporters is believed
to be the monomer (10). This
traditional view has changed over the last years. Accumulating
evidence points to the existence of dimers or even higher oligomers
in the case of several full transporters from different
subfamilies, among them ABCB1/P-glycoprotein, ABCC1/MRP1 and
ABCC7/CFTR (11–13). Oligomerization was related to the
transport activity of these transporters (10).
Members of the ABCA subfamily are mostly involved in
lipid transport (14). In the
case of the cholesterol transporter ABCA1, Denis et al
demonstrated that ABCA1 predominantly exists as a homotetramer
(15). ABCA3 is another member of
the ABC transporter subfamily A, transporting choline-phospholipids
and cholesterol into lamellar bodies in alveolar epithelial type II
cells (16,17). ABCA3 deficiency in humans leads to
acute respiratory distress syndrome in newborns and interstitial
lung disease in children and adults (18–20).
In the present study, we investigated a possible
oligomerization of ABCA3. We used a combination of classical in
vitro biochemical methods with molecular in vivo
techniques, i.e., bioluminescence resonance energy transfer (BRET),
to demonstrate that ABCA3 predominantly exists as a homooligomer.
Moreover, we showed that the arrest of ABCA3 in the endoplasmic
reticulum (ER), either through drug treatment or induced by
mutations in ABCA3, interferes with the ability of the
protein's to form dimers, pointing to a novel pathomechanism in
ABCA3-associated lung disease.
Materials and methods
Unless otherwise indicated, all chemicals were
purchased from Sigma-Aldrich (Taufkirchen, Germany).
Antibodies
The following antibodies were used: rat
anti-hemagglutinin (HA) (#11867423001; Roche, Grenzach-Wyhlen,
Germany), mouse anti-GFP (#632380; Clonetech, Mountain View, USA)
(for the detection of EYFP-tag, referenced as 'EYFP-antibody'
throughout the manuscript), mouse anti-LAMP3 (#561924; BD
Pharmingen, Heidelberg, Germany), Alexa Fluor 488 donkey anti-rat
IgG (#A-21208), Alexa Fluor 555 goat anti-mouse IgG (#A-21422)
(both from Thermo Fisher Scientific, Waltham, MA, USA), chicken
HRP-conjugated anti-β-actin (#sc-47778; Santa Cruz Biotechnology,
Inc., Heidelberg, Germany) and HRP-conjugated rabbit anti-rat IgG
(#P0450; Dako, Glostrup, Denmark). For the detection of mouse
primary antibodies, the Western Breeze Chemiluminescent
Immuno-detection system (Invitrogen, Karlsruhe, Germany) was
used.
Cell culture
293 cells (aka HEK293) were purchased from the
German Collection of Microorganisms and Cell Cultures (DSMZ,
Braunschweig, Germany). The cells were maintained in RPMI-1640
medium supplemented with 10% fetal bovine serum (FBS) at 37°C with
5% CO2. Plasmids encoding HA-and EYFP-tagged ABCA3 were
constructed as previously described (21,22). The 293 cells were transfected with
the pUB6-ABCA3-WT and pEYFP-ABCA3-WT vectors using ExGene 500
(Fermentas, St. Leon-Rot, Germany) according to the manufacturer's
instructions. Twenty-four hours post-transfection, the selection of
stable cells began by the addition of 6 µg/ml Blasticidin
(Invivogen, San Diego, CA, USA) or 500 µg/ml G-418 (Roth,
Karlsruhe, Germany). Single cell clones were obtained by
transferring single cells into the wells of a 96-well plate. For
transient transfected experiments, cells were seeded into 10 cm
dishes and grown for 48 h in RPMI-1640 supplemented with 10%
FBS.
Crude membrane preparation
Cells in 10 cm dishes were rinsed with
phosphate-buffered saline (PBS) once and covered with ice-cold
homogenization buffer [PBS supplemented with 1 mM EDTA and complete
protease inhibitor cocktail (Roche)] and scraped from the dish.
Subsequently, the cells were broken in a glass homogenizer with 30
strokes and sonicated on ice. The nuclei and cell debris were
removed by centrifugation (700 × g, 4°C, 10 min) and the
post-nuclear supernatant was centrifuged for 1 h at 100,000 × g and
4°C. The crude membrane fraction was resuspended in 30 µl
resuspension buffer (25 mM HEPES/NaOH pH 7.0, complete protease
inhibitor cocktail). The protein concentration of the post-nuclear
supernatant was determined with the Bio-Rad protein assay (Bradford
assay) using BSA as protein standard.
Blue native-polyacrylamide gel
electrophoresis (BN-PAGE) and western blot analysis
A total of 10 µg of crude membrane lysates
solubilized in 1% n-dodecyl-β-D-maltoside (DDM) or digitonin for 30
min at 37°C were loaded onto 3-12% NativePAGE Novex Bis-Tris
polyacrylamide gels (Invitrogen). Dithiothreitol (DTT) was added to
the samples before solubilization as indicated. For digestion of
contaminating DNA, MgCl2 was added to a final
concentration of 2 mM and lysates were treated with benzonase for
45 min at room temperature prior to solubilization. Following gel
electrophoresis, proteins were blotted to PVDF-membranes
(Millipore, Billerica, MA, USA). After transfer, the membranes were
blocked with 5% skim milk in TBS-T. The membranes were incubated
overnight with primary antibodies in blocking solution. After
washing the membranes three times with TBS-T, HRP-conjugated
secondary antibodies were applied for 1 h at room temperature.
Detection was performed using ECL reagent (GE Healthcare, Freiburg,
Germany).
Gel filtration
Gel filtration chromatography was performed using an
ÄKTA purifier system (Amersham Biosciences, Freiburg, Germany) with
a Superose 6 HR 10/30 column. A total of 200 µg of crude
membrane preparations were solubilized by 1% DDM in sample buffer
containing 50 mM Tris-HCl, pH 8.0 and 100 mM DTT for 30 min at room
temperature. Following centrifugation at 13,000 × g for 15 min, the
supernatants were injected onto the column, which was equilibrated
with 2.5 column volumes with running buffer (50 mM Tris-HCl, pH
7.4, 150 mM NaCl, 1 mM DTT and 0.1% DDM). The eluate was collected
in fractions of 1 ml each, concentrated using Amicon Ultra columns
50 K (Millipore) according to the manufacturers' instructions, and
subjected to sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) or BN-PAGE and western blot analysis as
described above. The column was standardized with the Gel
Filtration Calibration kit LMW (GE Healthcare) and the western blot
signal was quantified by measuring the intensity using GelAnalyzer
2010a software (Lazar Software).
Co-immunoprecipitation
Forty-eight hours after transfection with
pUB6-ABCA3-WT-HA, pEYFP-ABCA3-WT or both vectors, the 293 cells
were lysed in lysis buffer [50 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA,
1% (v/v) Triton X-100, pH 7.4] supplemented with complete protease
inhibitor. Clarified lysates were immunoprecipitated using anti-HA
antibody coupled with magnetic Dynabeads (Invitrogen) according to
the manufacturer's instructions. Precipitated proteins were eluted
with elution buffer (200 mM Glycin and 0.01% (v/v) Tween-20, pH
2.8). 4X LDS buffer (Invitrogen) and DTT (50 mM) were added and the
solution was incubated for 10 min at 70°C. Samples were then
separated by SDS-PAGE using NuPAGE Mini 3–8% Tris-Acetate gels
(Invitrogen). Following gel electrophoresis, proteins were blotted
to PVDF membranes (Millipore) and blocked with 5% skim milk in
TBS-T. Following overnight incubation with anti-HA or anti-EYFP
antibody, the membranes were washed and incubated with
HRP-conjugated secondary antibodies for 1 h or 30 min,
respectively. The detection was performed with ECL reagent (GE
Healthcare) or, in the case of the EYFP-antibody, with secondary
antibody solution (Invitrogen). Membranes were stripped with
Restore buffer (Pierce/Thermo Fisher Scientific) and re-stained
with the complementary antibody (anti-EYFP or anti-HA).
BRET
Wild-type and mutant hABCA3 (NM_001089.2), and
phenylalanine hydroxylase (PAH, U49897.1) were introduced into
pDONR™221 (Invitrogen) by recombinational cloning according to the
manufacturer's instructions. Expression clones coding for N- and
C-terminal fusion proteins with an improved version of YFP (EYFP)
or Renilla luciferase (Rluc) were generated by the
recombination of pDONRTM221 entry clones with the respective
destination vectors.
The interaction of proteins in living cells was
analyzed by BRET as previously described (23). BRET was performed in living 293
cells. Binary interactions were tested in all four possible
combinations of two proteins of interest either fused to Rluc
(energy donor) or EYFP (energy acceptor). Cells were co-transfected
with a total of 0.8 µg of DNA at an acceptor to donor ratio
of 3:1. BRET saturation experiments were performed to distinguish a
true positive interaction from bystander BRET resulting from random
collision. Cells were co-transfected at increasing acceptor to
donor ratios with a total of 2 µg DNA. All combinations were
tested in three independent experiments. The cells were incubated
at 30°C or 37°C, respectively. Following 48 h of incubation,
coelenterazine (15 µM, PJK) was added to the living cells
and light emission was collected in a 96-well microplate
luminometer (PHERAstar FS, BMG LABTECH) for 10 sec at 475 nm (Rluc
signal) and 535 nm (EYFP signal). The BRET ratio was calculated
based on R = IA/ID - cf,
where R is the BRET ratio, IA is the
intensity of light emission at 535 nm, ID is the
intensity of light emission at 475 nm, and cf is a
correction factor (BRETcontrol/Rluccontrol) with the control being
the co-transfection of donor fusion-proteins with EYFP in the
absence of a second protein of interest. In addition, the
background of a noDNA control was subtracted, general BRET
efficiency was tested by a EYFP-Rluc fusion protein, and assay
performance was evaluated using a standard protein interaction pair
(b-Jun and b-Fos). Relative affinities were calculated using the
non-linear regression model Y = BRETmax ×
(X/BRET50 + X), where
BRETmax is the maximal BRET ratio and
BRET50 is the acceptor to donor ratio required to
reach half-maximal BRET. For additional Brefeldin A (BFA)
treatment, the cells were treated after 24 h of incubation with BFA
(10 µg/ml in ethanol) for 24 h. Cells treated with ethanol
were used as the vehicle control, and untreated cells were used as
negative controls. BRET measurement was then performed as described
above.
Statistic analysis
Comparisons of multiple groups were carried out
using one-way repeated measure ANOVAs with Tukey's post hoc test.
The results are presented as mean ± SEM of a minimum of three
different experiments. P-values of <0.05 were considered to
indicate statistically significant differences. For saturation
experiments, nonlinear regression was applied. All tests were
performed using GraphPad Prism 5.0 (GraphPad Software, La Jolla,
CA, USA).
Results
Heterlogous expression of ABCA3
protein
Transfection of the 293 cells with the pUB6-ABCA3-WT
and pEYFP-ABCA3-WT vectors resulted in the robust expression of
ABCA3, as detected by immunofluorescence and western blot analysis.
Both HA- and EYFP-tagged ABCA3-WT were mainly localized in
LAMP3-positive intracellular vesicles in the 293 cells and yielded
two protein bands of approximately 220/190 and 180/150 kDa for
EYFP- and HA-tagged ABCA3, respectively (data not shown). These
features are in concordance with the findings of a previous study
(24).
Non-ionic detergent extraction and
BN-PAGE analysis of human ABCA3
Following solubilization, proteins were separated on
a 3–12% native PAGE Bis-Tris polyacrylamide gel in the presence of
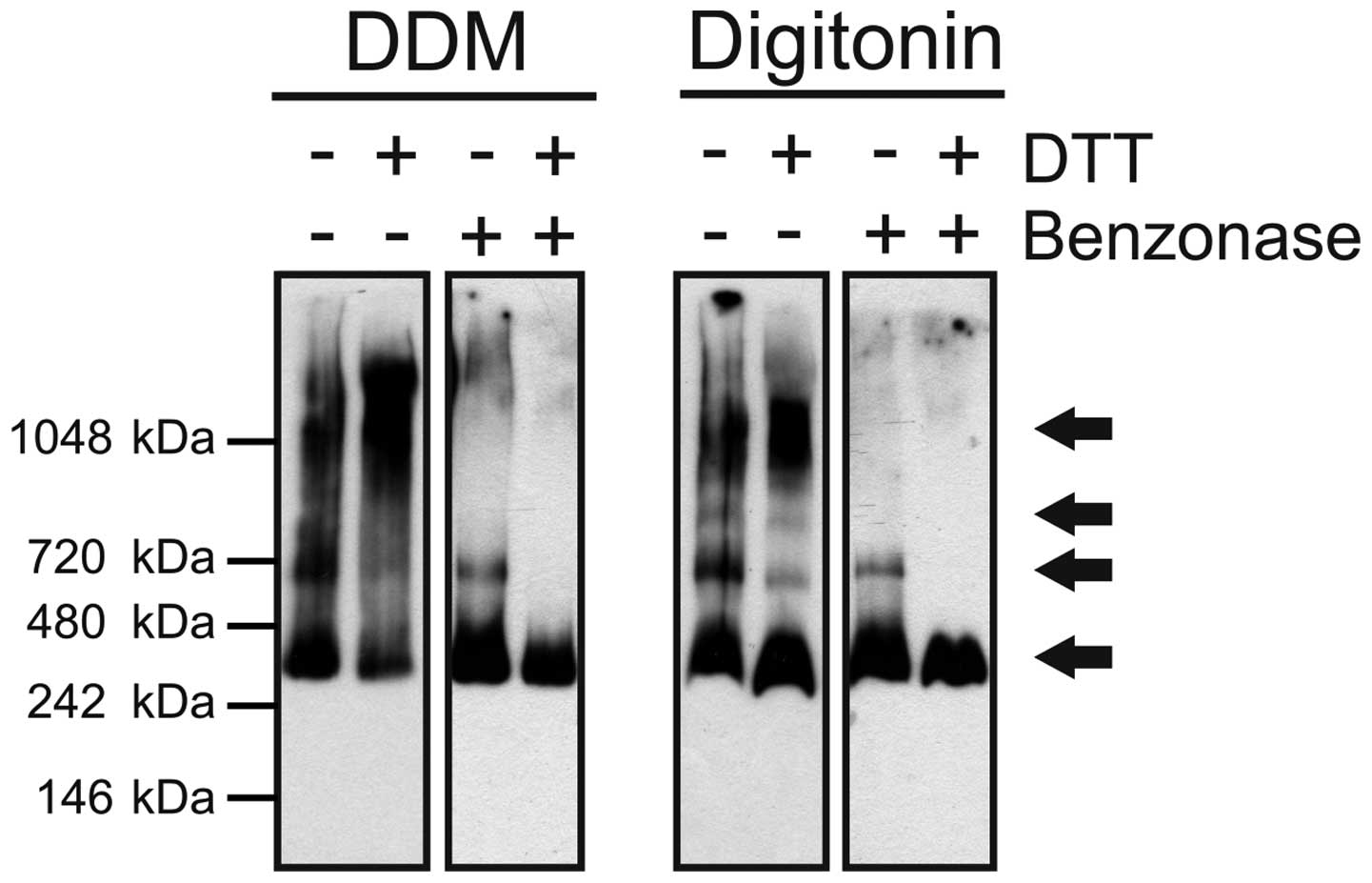
DDM or digitonin (Fig. 1).
BN-PAGE revealed roughly similar patterns of protein bands in both
of the solubilizates with the prevalence of one major band with the
size of the presumed monomer, particularly in the case of DDM
treatment. Additionally, next to the monomer, three higher
molecular mass forms were detectable, which likely represent
dimeric, trimeric and tetrameric ABCA3. The observed molecular
masses were 374.2±13.8 kDa for the monomer, 631.0±11.1 kDa for the
dimer, 864.0±34.4 kDa for the trimer/tetramer and 1213.8±89.5 kDa
for a higher oligomeric complex (Table I). The bands observed in the case
of the digitonin-treated samples (right panel) were similar in mass
(347.8±44.8 kDa monomer, 610.0±24.5 kDa dimer, 796.5±24.7 kDa
trimer/tetramer, 1079±26.9 kDa higher oligomer). The monomers (45%)
and tetramers (30%) were the most determined forms, followed by 20%
dimers and 5% trimers. Treatment with DTT, i.e., reduction of
disulphide bonds, led to the accumulation of high molecular weight
forms in the DDM- and digitonin-solubilized samples. In the case of
digitonin-treated samples, the band corresponding to the monomer
was more pronounced after DDT treatment. Since complete
dissociation to monomers was not achieved, benzonase endonuclease
digestion was performed to avoid protein streaking caused by a high
DNA content in samples. Benzonase treatment resulted in the almost
complete absence of high molecular weight forms, remaining only the
bands putatively corresponding to monomeric and dimeric ABCA3. In
the cells treated with DTT and benzonase, eventually all forms
other than the supposed monomer were absent.
 | Table IOverview of ABCA3 forms identified by
different methods. |
Table I
Overview of ABCA3 forms identified by
different methods.
| Method | Form | Estimated molecular
weight (kDa) | Estimated true
molecular weight (kDa)a | Percentage |
|---|
| SDS-PAGE in
literature (17,22) | Processed
monomer | 190 | | |
| Unprocessed
monomer | 150 | | |
| SDS-PAGE | Processed
monomer | 165.5±12.4 | | |
| Unprocessed
monomer | 192.5±10.5 | | |
| BN-PAGE | | | | |
| Detergent | | | | |
| DDM | Monomer | 374.2±13.8 | 207.9 | 45 |
| Dimer | 631.0±11.1 | 350.6 | 25 |
| Trimer | 894.0±34.4 | 496.7 | 2 |
| Tetramer | 1214±89.5 | 674.3 | 28 |
| Digitonin | Monomer | 347.8±44.8 | 193.2 | 44 |
| Dimer | 610.0±24.5 | 338.9 | 16 |
| Trimer | 796.5±24.7 | 442.5 | 7 |
| Tetramer | 1079±26.9 | 599.4 | 33 |
| Gel filtration | Comparison with standard
proteins | | | |
| Monomer | 247.3 | | |
| Dimer | 310.8 | | |
| Trimer | 456.0 | | |
| Tetramer | 774.5 | | |
| SDS-PAGE | | | |
| Processed
monomer | 150.2±14.4 | | |
| Unprocessed
monomer | 167.1±15.0 | | |
| BN-PAGE | | | |
| Monomer | 392.5±19.1 | 218.1 | |
| Dimer | 602.5±35.5 | 334.7 | |
| Trimer | 951.3±105 | 528.5 | |
| Tetramer | 1579±196 | 876.9 | |
Gel filtration chromatography analysis of
human ABCA3
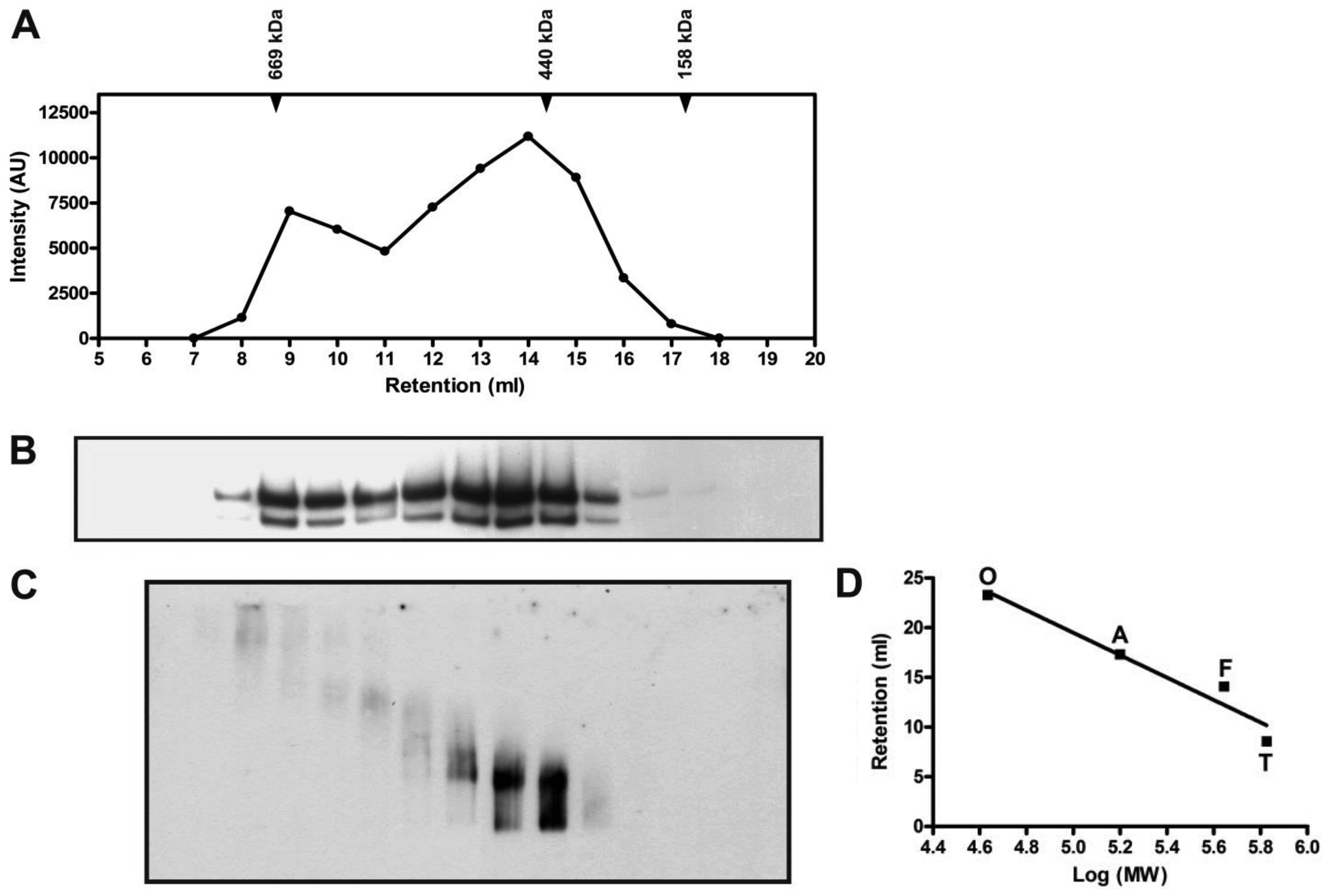
After gel filtration chromatography, ABCA3 was found
exclusively in fractions 8–17 in following SDS-PAGE (Fig. 2). When the western blot signal was
quantified by measuring the intensity plotted against the retention
volume (Fig. 2A), it was apparent
that two peaks were eluted, wherein the second peak was more
intense. The typical pattern with two ABCA3 bands remained in
SDS-PAGE (Fig. 2B).
When BN-PAGE subsequent to gel filtration was used
to analyze the oligomeric pattern, we found complex structures in
the early-eluted fractions 8 and 9 (Fig. 2C). Smaller oligomeric forms
supposed to represent ABCA3 tetramers, trimers or dimers were found
in fractions 10–15. Molecular weights estimated by comparison with
marker proteins are given in Table
I. The presumed monomeric form which has an estimated molecular
mass of 392.5±19.1 kDa was also present in fractions 14–16.
Co-immunoprecipitation of human
ABCA3
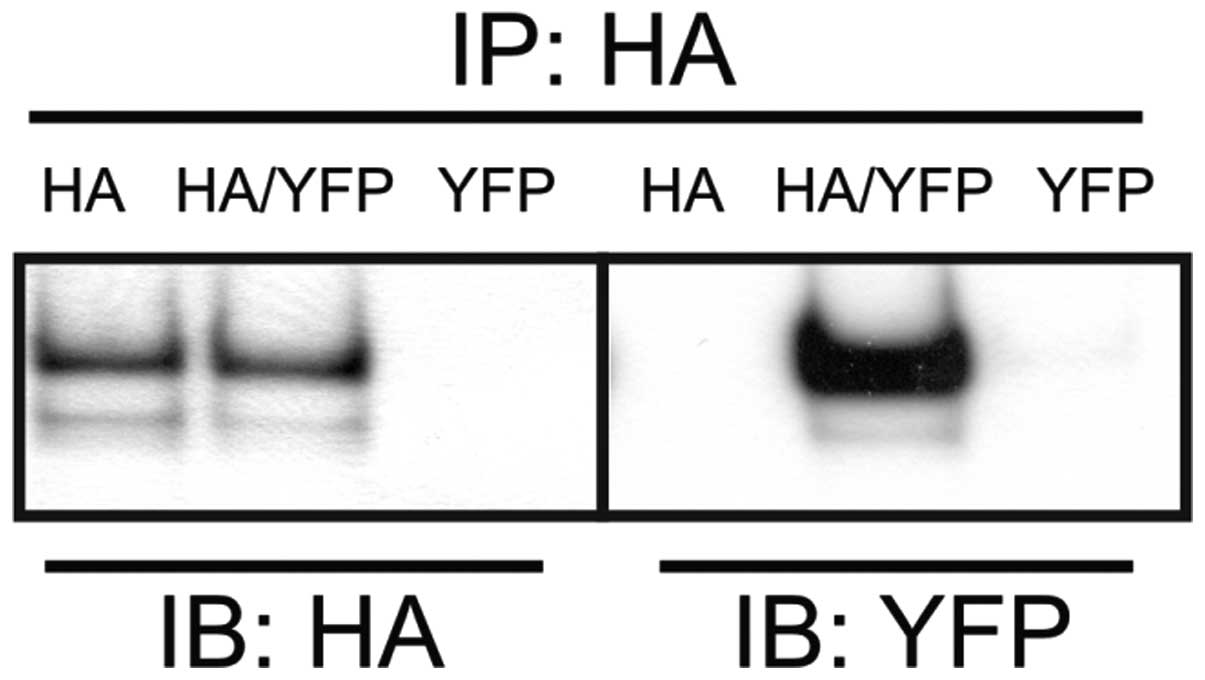
To further verify ABCA3 homooligomerization, we
performed co-immuno-precipitation with two differentially tagged
ABCA3 fusion proteins. For this purpose, EYFP-tagged ABCA3 was
transiently transfected into 293 cells that did or did not express
stable HA-epitope-tagged ABCA3. Following transfection, cell
lysates were prepared and co-immunoprecipitation was performed with
anti-HA antibody, followed by western blot analysis using both
anti-HA and anti-EYFP antibodies (Fig. 3). The EYFP-ABCA3 fusion protein
could be immunoprecipitated with the anti-HA antibody only in cells
that were co-transfected with both tagged proteins. Thus, the
oligomeric state of human ABCA3 is as least dimeric when expressed
in 293 cells.
BRET assay confirms the
homooligomerization of human ABCA3 in vivo
To confirm the homooligomerization of ABCA3 in
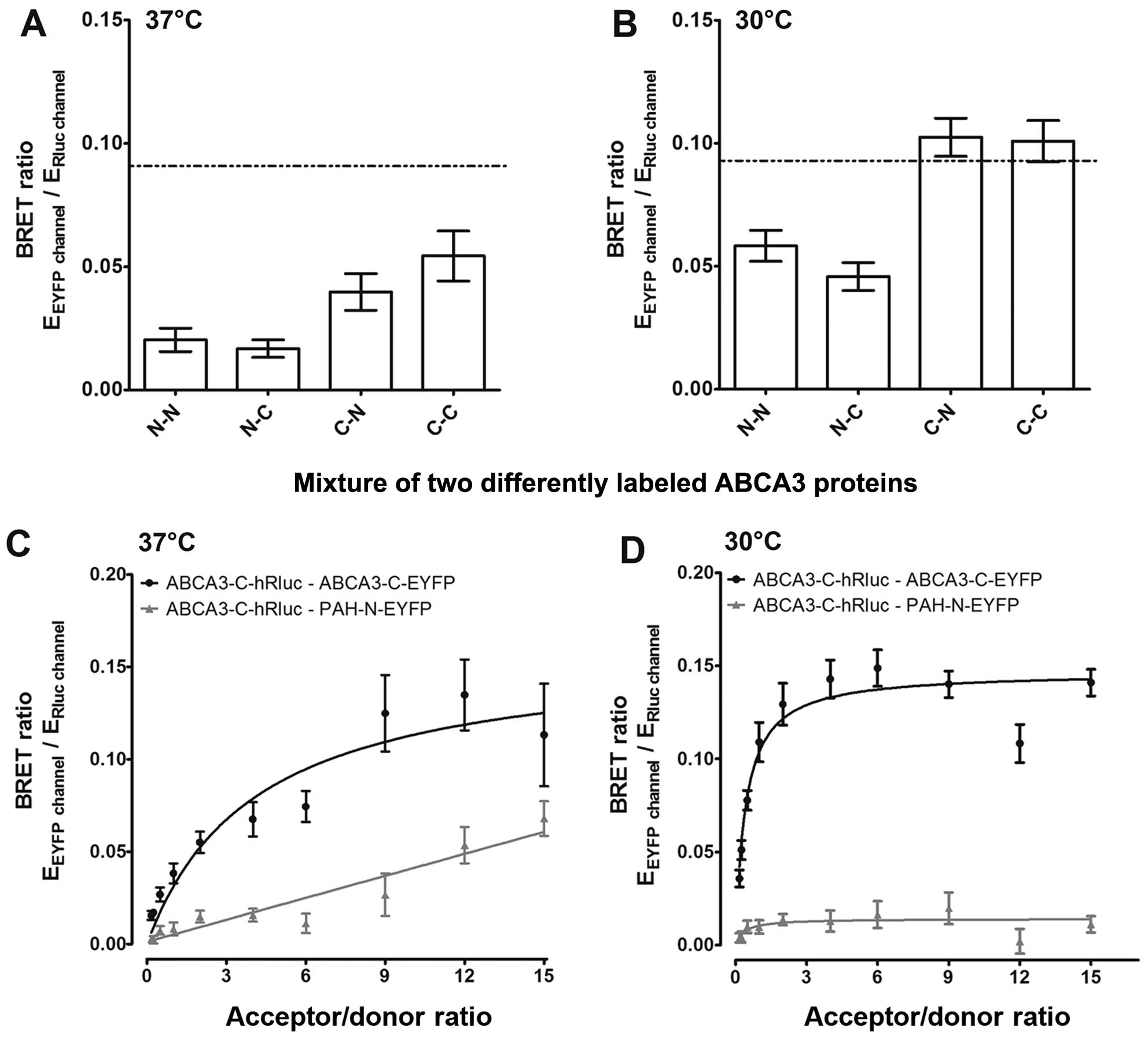
living cells, we performed BRET analyses. Cells were co-transfected
with ABCA3 carrying N- or C-terminal tags of Rluc or EYFP,
respectively, in four possible combinations (Fig. 4A and B). The correct cellular
localization of the N- and C-terminally tag fusion proteins was
verified by immunofluorescence microscopy (data not shown). Two out
of eight combinations resulted in BRET ratios above the background
level; however, BRET ratios did not exceed the method-specific
threshold for a positive protein-protein interaction of 0.094. Due
to better folding characteristics at lower temperatures (25), we performed BRET experiments at
30°C. Under these conditions, the respective combinations resulted
in BRET ratios above the method-specific threshold, indicating a
homooligomerization of ABCA3. To further substantiate homomeric
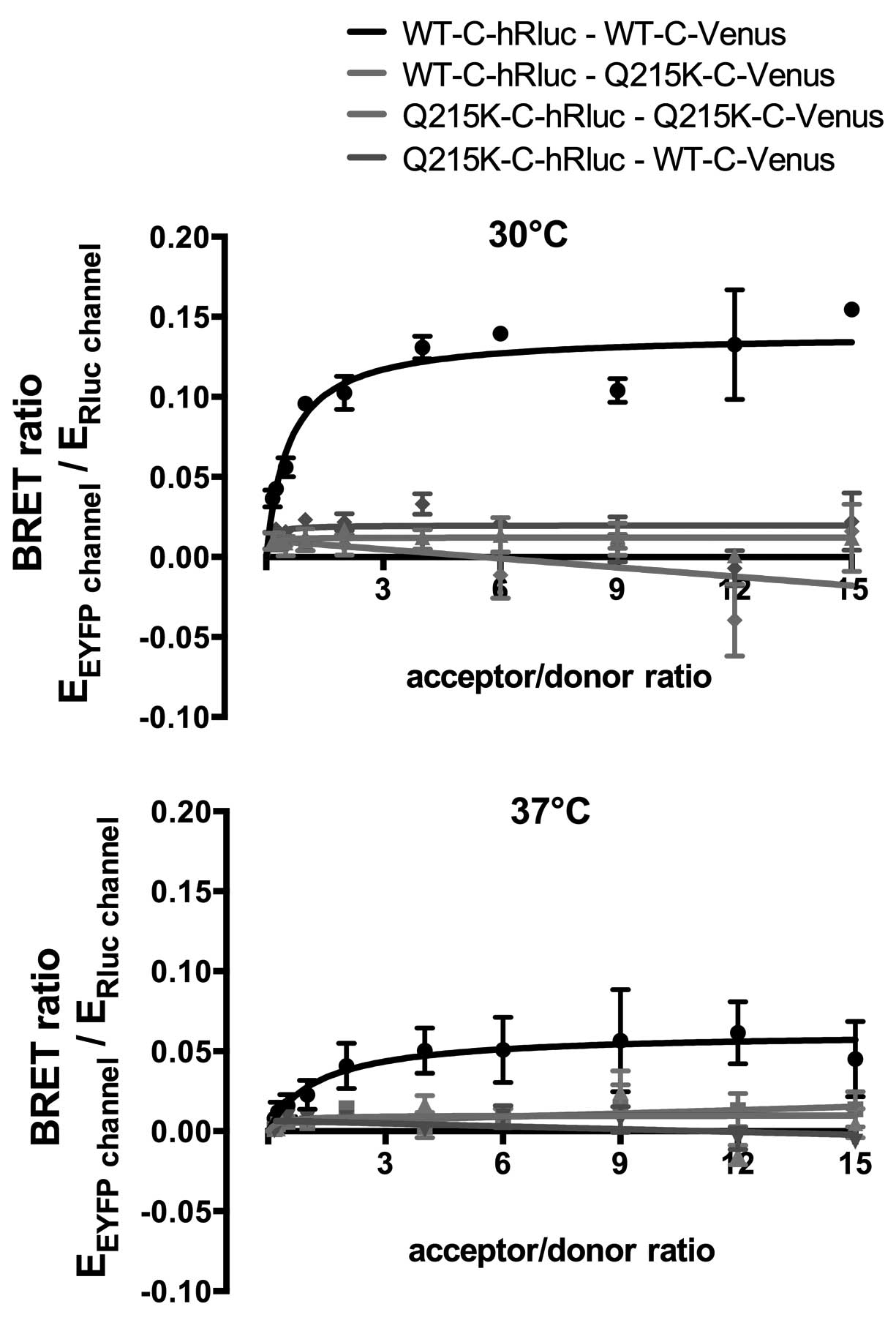
interaction of ABCA3 in living cells, we performed BRET saturation
experiments (Fig. 4C and D). For
specific protein-protein interactions, such as the formation of
homooligomeric states, a sequential increase in the ratio of
proteins carrying the EYFP tag over proteins carrying the Rluc tag
results in hyperbolic behavior of the BRET ratios (26). In addition, a relative binding
affinity index can be determined by use of the EYFP to Rluc ratio
(acceptor/donor ratio) at half-maximal BRET (BRET50).
Co-expression of homomeric protein pairs with C-terminal tags
resulted in hyperbolic BRET curves with a BRET50 value
of 4,091 at 37°C and 0,415 at 30°C. These data confirm the
homooligomerization of ABCA3 at 30°C and at physiological
conditions (37°C), with higher affinities of oligomer formation at
30°C. As a negative control, the co-transfection of ABCA3 with PAH
resulted in a curve best fitted by linear regression, which is
indicative for bystander BRET due to random collision.
Determination of the localization of the
ABCA3-ABCA3 interaction
To explore the intracellular localization of the
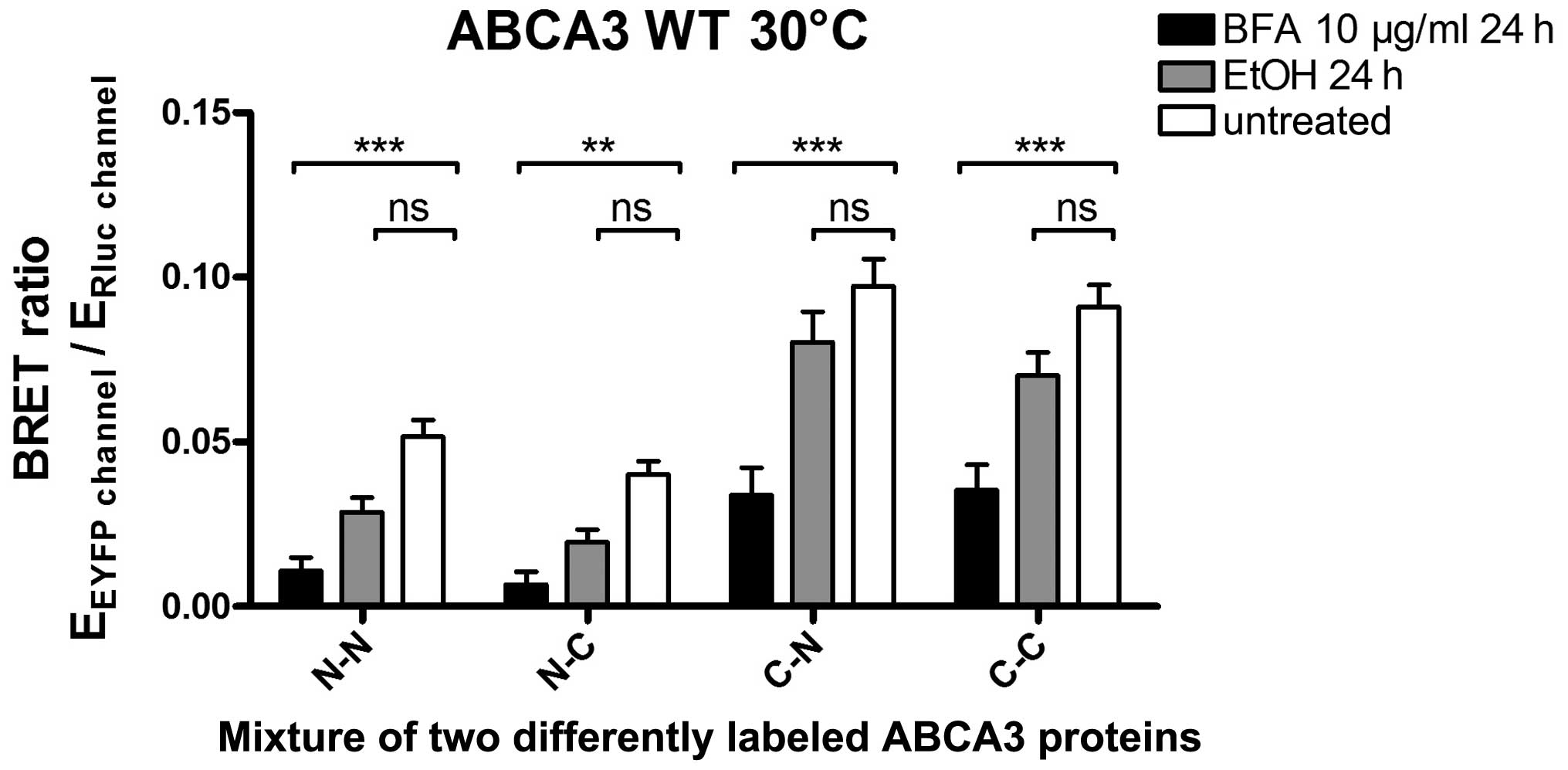
ABCA3 homooligomerization we used BFA treatment. BFA inhibits the
transportation of proteins from the ER to the Golgi apparatus,
resulting in protein accumulation in the ER (27). The cells were treated with BFA (10
µg/ml) or the vehicle (ethanol) for 24 h prior to BRET
measurement, which was done at 30°C (Fig. 5). Treatment with BFA resulted in a
significantly lower BRET ratio for the ABCA3-ABCA3 inter action
compared to the negative control, while no significant difference
was noted in the case of the vehicle control. This observation
suggested that the ABCA3-ABCA3 interaction (and thus the formation
of oligomers) takes place in a post-ER compartment.
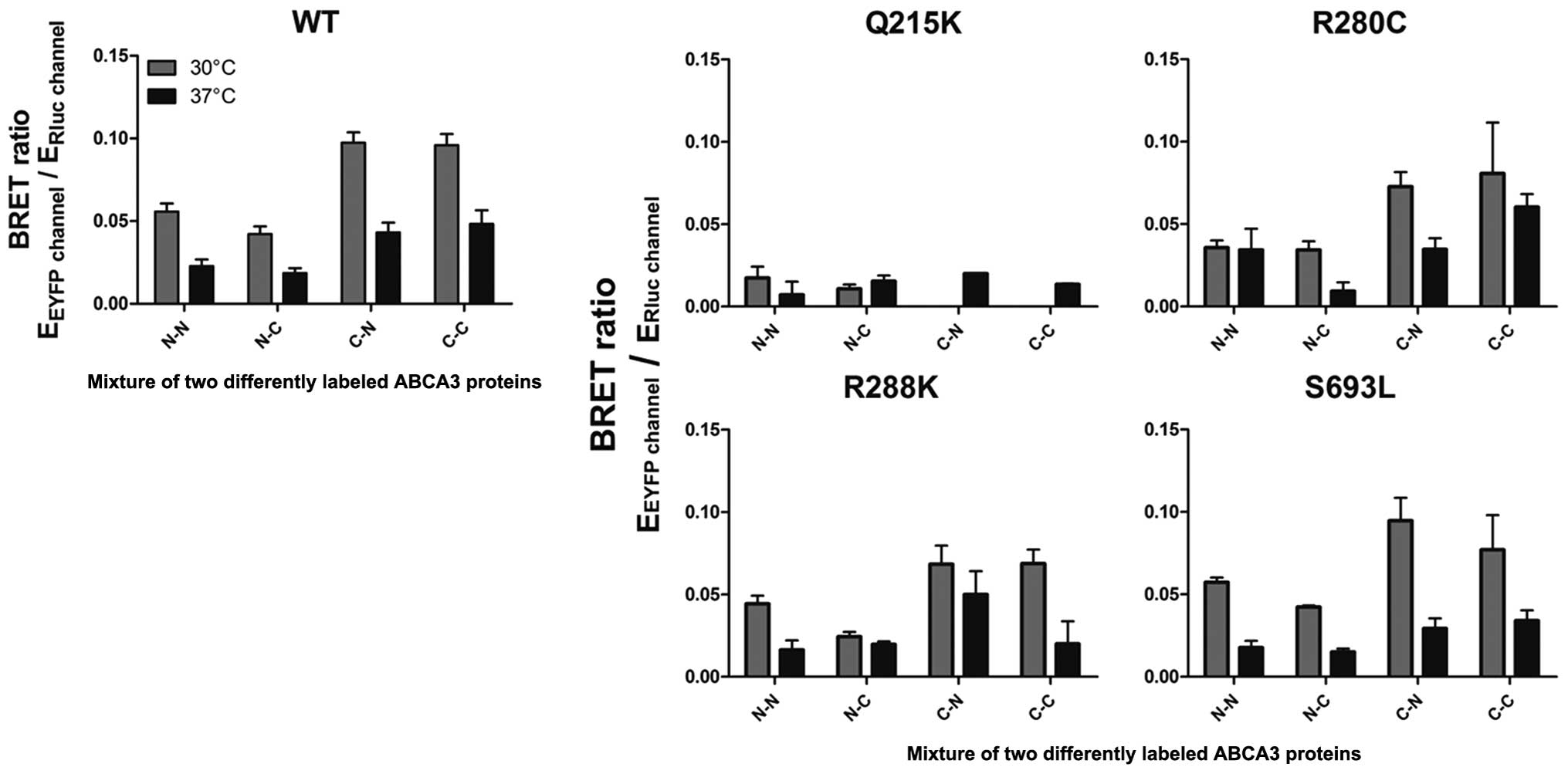
The mistrafficking mutation p.Q215K
interferes with the ABCA3-ABCA3 interaction
To determine whether ABCA3 mutations disrupt ABCA3
oligomer formation, BRET experiments were performed for the
wild-type protein compared to four different clinically relevant
mutations. All experiments were performed at 37°C and 30°C. While
in three of four tested mutations (R280C, R288K and S693L) BRET
ratios were nearly similar to wild-type ABCA3 for all
configurations tested, in the case of ABCA3-Q215K, BRET ratios were
hardly detectable (Fig. 6). To
confirm this, we again performed BRET saturation experiments
(Fig. 7). Low BRET ratios and a
lack of hyperbolic behavior of the BRET ratios confirmed that Q215K
leads to perturbation of the ABCA3 homooligomerization.
Since p.Q215K leads to the arrest of ABCA3 in the ER
(22), this result corroborates
the assumption that ABCA3-ABCA3 interaction takes place in a
post-ER compartment.
Discussion
In this study, using different complementary
approaches, we demonstrate that the lipid transporter ABCA3 forms
dimers and possibly also higher oligomers. Using BRET technology,
an independent method to assess protein-protein interactions in
living cells avoiding detergent solubilization, we demonstrate that
homooligomerization occurs in vivo. Moreover, we show that
mutations in ABCA3 can negatively affect oligomerization. Since
homooligomerization can be expected to be of functional
significance, these findings point to a new level of the regulation
of ABCA3 function. Our data may also introduce the perturbation of
oligomer formation as a new mechanism by which mutations interfere
with ABCA3 function.
To the best of our knowledge, the question of
whether ABCA3 forms oligomers has not been addressed by any study
to date. However, as stated above, oligomerization has been
demonstrated for the lipid transporter ABCA1 (15,28). Given the close association of
these two subclass A transporters, it does not come as a surprise
that ABCA3 behaves in a similar way and forms dimers and higher
oligomers in vitro and also in vivo. It therefore
adds to the growing list of ABC transporters for which
oligomerization has been demonstrated, suggesting a functional
relevance of oligomerization.
We aimed to reliably establish the
homooligomerization of ABCA3 by using various approaches
complementing each other. While co-immunoprecipation detects
ABCA3-ABCA3 interaction, BN-PAGE can be used to visualize the
oligomeric states of the protein. In addition, we used gel
filtration chromatography as an alternative approach for
quantifying the molecular masses of oligomeric forms. Since all
these biochemical methods may yield false-positive results due to
artifacts of the extraction process, the analysis of
protein-protein interaction by BRET circumvents this issue and
permits the measurement of interactions in vivo.
The accurate determination of the molecular mass and
thereby the oligomeric state of membrane proteins is a challenging
task. BN-PAGE is widely used for the analysis of the composition,
oligomeric state and molecular mass of non-dissociated membrane
protein complexes. However, when determining molecular masses of
proteins from BN-PAGE, one must take into account that membrane
proteins bind large amounts of Coomassie brilliant blue (CBB) dye.
Bound dye adds to the apparent masses of the proteins and results
in an overestimation. To address this issue, Heuberger et al
established that taking into account an average ratio of
CBB-protein-complex to pure protein of approximately 1.8, molecular
masses deduced from BN-PAGE via a soluble marker calibration curve
can be converted to provide a good estimate of the true molecular
mass (29). Using this
correction, we identified oligomeric ABCA3 forms including dimers,
trimers and tetramers. The molecular masses deduced from BN-PAGE
are in good agreement with those estimated from gel filtration
chromatography with subsequent BN-PAGE (Table I). Size estimation in gel
filtration with only standard proteins (thyroglobulin, ferritin and
aldolase) shows the same deviations as ready to use markers in
BN-PAGE owing to the fact that these proteins are cytosolic and
have a different running behavior in the column than membrane
proteins.
Using BRET, we demonstrated ABCA3-ABCA3 interaction
in vivo. In all experiments, interaction affinity was higher
at lower temperatures. This may be explained by more efficient
folding of the large ABCA3 protein at lower temperatures, when
overexpressed in cells (25,30). Since the C-C combination showed
the highest BRET ratio in our measurements (Fig. 4), we conclude that the C-termini
of ABCA3 are in close proximity. The C-terminal interaction of
ABCA3 is likely to be of functional relevance, since similar
C-terminal interaction sites were identified for ABCA1 and
ABCC7/CFTR (31,32).
How the oligomerization of ABC transporters is
related to their transport activities is still a matter of debate
and it is possible that both, monomeric and oligimeric, forms
co-exist and there might be a dynamic process of formation and
dissociation that can influence the substrate binding affinity and
may function as another level of transporter regulation (10). To this end, Trompier et al
showed that ABCA1 tetramers are formed upon binding of ATP, while
the transporter is present predominantly as a homodimer (28). This scenario, which may also apply
to ABCA3, implies that the transition of dimers into higher order
structures is an integral part of the protein's catalytic cycle and
thus crucially required for its activity. The assumption that
oligomerization is a prerequisite for transport activity also
implies possible detrimental effects of mutations affecting
oligomerization on ABCA3 function. It is thus important to
determine whether ABCA3 mutations that are found in patients
suffering from ABCA3-assosciated lung disease have any impact on
its oligomerization. Among the mutations we tested, three mutations
(p.R280C, p.R288K and p.S693L) showed BRET ratios comparable to the
wild-type protein, while in the case of p.Q215K the BRET signal was
almost absent.
We showed that the ABCA3 mutation p.Q215K, which was
associated with the arrest of the protein in the ER, practically
abolished oligomerization. While this is consistent with the view
that oligomerization is a prerequisite for export from the ER, one
could also argue that ER arrest induced by the p.Q215K mutation
blocks oligomerization, which is in line with diminished oligomer
formation caused by BFA treatment. However, it is also conceivable
that BFA treatment directly affects the oligomerization of ABCA3 by
blocking the ER to Golgi transport. Consecutive protein
accumulation may hamper the ABCA3-ABCA3 interaction required for
oligomerization. In addition, ABCA1 dimerization was shown to take
place in the ER (28). Thus, our
data are consistent with the notion that ABCA3 oligomerization
takes place in the ER and that the misfolding of ABCA3-p.Q215K
abrogates protein-protein interaction needed for formation of
oligomers and subsequent ER export.
Taken together, the findings of the present study
demonstrate that ABCA3 monomers form dimers and to a lesser extent,
also higher oligomers. We further demonstrate that the
oligomerization and trafficking of ABCA3 are mutually dependent,
and that mutations can interfere with this process. We have thus
unraveled a potential novel molecular mechanism for ABCA3-related
interstitial lung disease.
Acknowledgments
We would like to thank Claudia Bräu-Heberger,
Kathrin Schiffl and Andrea Schams for their excellent technical
assistance. This study was supported by a grant from the Deutsche
Forschungsgemeinschaft to M.G. (GR 970/8-1).
References
|
1
|
Wilkens S: Structure and mechanism of ABC
transporters. F1000Prime Rep. 7:142015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dean M, Hamon Y and Chimini G: The human
ATP-binding cassette (ABC) transporter superfamily. J Lipid Res.
42:1007–1017. 2001.PubMed/NCBI
|
|
3
|
Bodzioch M, Orsó E, Klucken J, Langmann T,
Böttcher A, Diederich W, Drobnik W, Barlage S, Büchler C,
Porsch-Ozcürümez M, et al: The gene encoding ATP-binding cassette
transporter 1 is mutated in Tangier disease. Nat Genet. 22:347–351.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brooks-Wilson A, Marcil M, Clee SM, Zhang
LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO,
et al: Mutations in ABC1 in Tangier disease and familial
high-density lipoprotein deficiency. Nat Genet. 22:336–345. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Allikmets R, Singh N, Sun H, Shroyer NF,
Hutchinson A, Chidambaram, Gerrard B, Baird L, Stauffer D, Peiffer
A, et al: A photoreceptor cell-specific ATP-binding transporter
gene (ABCR) is mutated in recessive Stargardt macular dystrophy.
Nat Genet. 15:236–246. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bergen AA, Plomp AS, Schuurman EJ, Terry
S, Breuning M, Dauwerse H, Swart J, Kool M, van Soest S, Baas F, et
al: Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat Genet.
25:228–231. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Riordan JR, Rommens JM, Kerem B, Alon N,
Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et
al: Identification of the cystic fibrosis gene: Cloning and
characterization of complementary DNA. Science. 245:1066–1073.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kartner N, Riordan JR and Ling V: Cell
surface P-glycoprotein associated with multidrug resistance in
mammalian cell lines. Science. 221:1285–1288. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cole SP: Multidrug resistance protein 1
(MRP1, ABCC1), a 'multitasking' ATP-binding cassette (ABC)
transporter. J Biol Chem. 289:30880–30888. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mo W and Zhang JT: Oligomerization of
human ATP-binding cassette transporters and its potential
significance in human disease. Expert Opin Drug Metab Toxicol.
5:1049–1063. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jetté L, Potier M and Béliveau R:
P-glycoprotein is a dimer in the kidney and brain capillary
membranes: Effect of cyclosporin A and SDZ-PSC 833. Biochemistry.
36:13929–13937. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Soszyński M, Kałuzna A, Rychlik B, Sokal A
and Bartosz G: Radiation inactivation suggests that human multidrug
resistance-associated protein 1 occurs as a dimer in the human
erythrocyte membrane. Arch Biochem Biophys. 354:311–316. 1998.
View Article : Google Scholar
|
|
13
|
Ramjeesingh M, Kidd JF, Huan LJ, Wang Y
and Bear CE: Dimeric cystic fibrosis transmembrane conductance
regulator exists in the plasma membrane. Biochem J. 374:793–797.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Albrecht C and Viturro E: The ABCA
subfamily - gene and protein structures, functions and associated
hereditary diseases. Pflugers Arch. 453:581–589. 2007. View Article : Google Scholar
|
|
15
|
Denis M, Haidar B, Marcil M, Bouvier M,
Krimbou L and Genest J: Characterization of oligomeric human ATP
binding cassette transporter A1. Potential implications for
determining the structure of nascent high density lipoprotein
particles. J Biol Chem. 279:41529–41536. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ban N, Matsumura Y, Sakai H, Takanezawa Y,
Sasaki M, Arai H and Inagaki N: ABCA3 as a lipid transporter in
pulmonary surfactant biogenesis. J Biol Chem. 282:9628–9634. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Matsumura Y, Sakai H, Sasaki M, Ban N and
Inagaki N: ABCA3-mediated choline-phospholipids uptake into
intracellular vesicles in A549 cells. FEBS Lett. 581:3139–3144.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shulenin S, Nogee LM, Annilo T, Wert SE,
Whitsett JA and Dean M: ABCA3 gene mutations in newborns with fatal
surfactant deficiency. N Engl J Med. 350:1296–1303. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bullard JE, Wert SE, Whitsett JA, Dean M
and Nogee LM: ABCA3 mutations associated with pediatric
interstitial lung disease. Am J Respir Crit Care Med.
172:1026–1031. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Campo I, Zorzetto M, Mariani F, Kadija Z,
Morbini P, Dore R, Kaltenborn E, Frixel S, Zarbock R, Liebisch G,
et al: A large kindred of pulmonary fibrosis associated with a
novel ABCA3 gene variant. Respir Res. 15:432014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weichert N, Kaltenborn E, Hector A,
Woischnik M, Schams A, Holzinger A, Kern S and Griese M: Some ABCA3
mutations elevate ER stress and initiate apoptosis of lung
epithelial cells. Respir Res. 12:42011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Engelbrecht S, Kaltenborn E, Griese M and
Kern S: The surfactant lipid transporter ABCA3 is N-terminally
cleaved inside LAMP3-positive vesicles. FEBS Lett. 584:4306–4312.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gersting SW, Lotz-Havla AS and Muntau AC:
Bioluminescence resonance energy transfer: An emerging tool for the
detection of protein-protein interaction in living cells. Methods
Mol Biol. 815:253–263. 2012. View Article : Google Scholar
|
|
24
|
Matsumura Y, Ban N, Ueda K and Inagaki N:
Characterization and classification of ATP-binding cassette
transporter ABCA3 mutants in fatal surfactant deficiency. J Biol
Chem. 281:34503–34514. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bischof JC and He X: Thermal stability of
proteins. Ann NY Acad Sci. 1066:12–33. 2005. View Article : Google Scholar
|
|
26
|
Hamdan FF, Percherancier Y, Breton B and
Bouvier M: Monitoring protein-protein interactions in living cells
by biolu-minescence resonance energy transfer (BRET). Curr Protoc
Neurosci. Chapter 5, Unit 5.23. 2006. View Article : Google Scholar
|
|
27
|
Nylander S and Kalies I: Brefeldin A, but
not monensin, completely blocks CD69 expression on mouse
lymphocytes: Efficacy of inhibitors of protein secretion in
protocols for intracellular cytokine staining by flow cytometry. J
Immunol Methods. 224:69–76. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Trompier D, Alibert M, Davanture S, Hamon
Y, Pierres M and Chimini G: Transition from dimers to higher
oligomeric forms occurs during the ATPase cycle of the ABCA1
transporter. J Biol Chem. 281:20283–20290. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heuberger EH, Veenhoff LM, Duurkens RH,
Friesen RH and Poolman B: Oligomeric state of membrane transport
proteins analyzed with blue native electrophoresis and analytical
ultra-centrifugation. J Mol Biol. 317:591–600. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matsumoto N, Tamura S, Furuki S, Miyata N,
Moser A, Shimozawa N, Moser HW, Suzuki Y, Kondo N and Fujiki Y:
Mutations in novel peroxin gene PEX26 that cause
peroxisome-biogenesis disorders of complementation group 8 provide
a genotype-phenotype correlation. Am J Hum Genet. 73:233–246. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fitzgerald ML, Okuhira K, Short GF III,
Manning JJ, Bell SA and Freeman MW: ATP-binding cassette
transporter A1 contains a novel C-terminal VFVNFA motif that is
required for its cholesterol efflux and ApoA-I binding activities.
J Biol Chem. 279:48477–48485. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang S, Yue H, Derin RB, Guggino WB and Li
M: Accessory protein facilitated CFTR-CFTR interaction, a molecular
mechanism to potentiate the chloride channel activity. Cell.
103:169–179. 2000. View Article : Google Scholar : PubMed/NCBI
|