Introduction
Sepsis is a systemic response to invasive microbial
infection or severe tissue damage, resulting in tissue necrosis,
multi-organ failure and death (1). After being triggered by overwhelming
initial stimuli, neutrophils and macrophages can produce excessive
pro-inflammatory mediators, leading to a dysregulated immune
response that characterizes sepsis (2). The dominant causes of acute kidney
injury (AKI) in hospitalized patients are sepsis and septic shock
(3). Lipopolysaccharide (LPS),
known as the major component of the outer membrane of Gram-negative
bacteria, is a potent activator for acute inflammatory response in
sepsis (4), and LPS signaling is
initiated by activation of Toll-like receptor 4 (TLR4) (5). Therefore, the blockade of TLR4
signaling has been considered as a promising therapeutic approach
for sepsis (6,7) and draws intensive attention from
scientists and clinicians worldwide. However, a recent randomized
double-blind controlled trial revealed that the application of a
specific TLR4 antagonist failed to reduce sepsis-induced mortality
(8). Thus, the further
identification of critical mediators and molecular targets in
sepsis is required. The developmetn of effective therapeutic
approaches is urgenly required.
Reportedly, following bacterial infection, the
massive recruitment and activation of neutrophils are presented
with the extracellular release of neutrophil elastase (NE). NE is a
serine protease that propagates persistent neutrophilic
inflammation by accelerating pro-inflammatory cytokine production
(9,10). By using mouse models of ischemic
AKI, Zager et al observed a marked increase in renal
cortical/isolated proximal tubule NE mRNA levels and a decrease in
NE protein levels (11). Their
study suggested that the downregulated protein expression of renal
NE correlated with the upregulation of endogenous α-1-antitrypsin,
which has protease inhibitor activity (11). From this previous study, it can be
postulated that the blockade of NE toxicity may exert
renoprotective effects. Sivelestat, a specific NE inhibitor, has
been demonstrated to mitigate lung injury, such as pulmonary
fibrosis (12) and acute lung
injury (13). Of note, Suda et
al found that sivelestat improved the survival of animals with
sepsis (14). However, they only
focused on the beneficial effects of sivelestat in attenuating lung
damage. Apart from lung damage, sepsis often leads to impaired
function in other vital organs, including the kidneys (15), liver (16) and heart (17). In order to fully evaluate the
potential therapeutic effects of a drug or an agent in sepsis,
assessing its effects on multiple organs is mandatory.
In the present study, we examined the effects of
sivelestat on sepsis-associated AKI in a rat model of sepsis
induced by cecal ligation and puncture (CLP) and also explored the
underlying mechanisms.
Materials and methods
Animals and ethics
Male Sprague-Dawley rats (weighing 200–250 g) were
obtained from the Changsheng Biotech Co., Ltd. (Beijing, China) and
housed under specific pathogen-free conditions at a constant
temperature of 20–22°C and humidity of 50–60% with a 12-h
light/dark cycle, and were allowed free access to food and water.
All animal experiments were performed in accordance with the
Guidelines for the Care and Use of Laboratory Animals and were
approved by the Institutional Animal Care and Use Committee of
China Medical University, Shenyang, China.
CLP procedure and animal grouping
CLP procedure was performed to initiate sepsis in
rats according to previously published protocols (18). In short, the rats were first
anaesthetized by the intraperitoneal (i.p.) administration of 10%
chloral hydrate (350 mg/kg; Sinopharm, Beijing, China), and a
ventral midline incision (1.5 cm in length) was made on the rats.
The caecum was exposed, ligated, punctured with a gauge needle 3
times, and then placed back into the abdomen. Rats that underwent
sham operation (the caecum was exposed by a ventral midline
incision, but was not ligated or punctured) were used as the
controls. Thereafter, the abdominal incision was closed in layers
with 3-0 surgical sutures, and the rats were allowed to recover
from the anaesthesia. These rats were randomly divided into 6
groups (n=8/group) as follows: i) group 1: the sham-operated group
(Sham); ii) group 2: the sham-operated group administered the low
dose of sivelestat (Sham + L-sivelestat); iii) group 3: the
sham-operated group administered the high dose of sivelestat (Sham
+ H-sivelestat); iv) group 4: the rats with sepsis who were not
treated (Sepsis); v) group 5: the rats with sepsis who were
administered the low dose of sivelestat (Sepsis + L-sivelestat);
and vi) group 6: the rats with sepsis who were administered the
high dose of sivelestat (Sepsis + H-sivelestat). The rats in groups
2 and 3, and 5 and 6, rats were administered an i.p. injection of
50 or 100 mg/kg body weight sivelestat (Ono Pharmaceutical Co.,
Osaka, Japan) immediately after the sham-operation or the
initiation of sepsis. The rats in groups 1 and 4 received an
infusion of normal saline (vehicle) into the intraperitoneal
cavity. The doses of sivelestat used in our study were selected
based on our preliminary experiments (data not shown) and a
previous study (12). Blood
samples from each rat were obtained at 6 and 24 h post-surgery, and
the rat kidneys were rapidly removed at 24 h post-surgery after
sacrifice (by an overdose of anesthetics) and immediately frozen in
liquid nitrogen or fixed in 10% formaldehyde (Sinopharm) for use in
subsequent experiments.
In order to determine whether sivelestat affects
animal survival, the mortalities were measured in another set of
rats treated with or without sivelestat up to 108 h after the
procedure (n=10/group). In addition, the mean arterial pressure
(MAP) in these rats was assessed using a non-invasive blood
pressure monitoring system at 24 h according to the manufacturer's
instructions (ALC-NIBP, Alcott Biotech Co., Ltd., Shanghai,
China).
Measurement of serum biochemical
parameters
Serum samples were centrifuged at 1,000 rpm for 20
min, and then subjected to the detection of blood urea nitrogen
(BUN), neutrophil gelatinase-associated lipocalin (NGAL) levels,
tumor necrosis factor α (TNF-α) and interleukin-1β (IL-1β) levels
and NE levels in the serum and renal tissues using commercially
available kits (Boster, Uscn Life Science, Inc., Wuhan, China)
according to the manufacturer's instructions.
Glomerular filtration rate (GFR) and
fractional excretion of sodium (FENa) assessments
The rats that underwent CLP or sham surgery were
anaesthetized at 24 h and subjected to the following assessments.
GFR was determined by measuring the inulin clearance. In brief,
inulin (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in normal
saline to a concentration of 5 mg/ml, and then administered through
the femoral vein at a dose of 1 ml/h/100 g body weight. Following
equilibration, the urinary bladder was cannulated to collect a
30-min urine sample. In addition, a blood sample (1 ml) was
collected at the midpoint of the urine collection period. Ten
minutes later, urine and blood samples were collected again. Inulin
concentrations in the urine and plasma were determined using the
anthrone method, while sodium and creatinine concentrations were
determined using commercial assay kits (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China), respectively. The values
of GFR and FENa were calculated through standard
formulas, as previously described (19–21): GFR = [(urine inulin) x (volume of
urine collected)]/[(plasma inulin) x (time of collection)], and
expressed as ml/min/100 g body weight; FENa = 100 x
[(urine sodium x plasma creatinine)/(plasma sodium x urine
creatinine)].
Hematoxylin and eosin (H&E) staining
and immunohisto-chemistry
The formaldehyde-fixed kidney tissues were embedded
in paraffin, cut into 5-µm-thick sections, and then
deparaffinized in xylene and hydrated in graded ethanol. For the
morphological examination, these slices were stained with
hematoxylin (Solarbio, Beijing, China) and eosin (Sinopharm). For
immunohistochemistry, these sections were heated in citrate buffer
at 100°C for 10 min to retrieve the antigen and then treated with
3% hydrogen peroxide (both from Sinopharm) at room temperature for
15 min to block the endogenous peroxidase activity. After being
blocked with normal goat serum at room temperature for 15 min,
these sections were incubated with rabbit polyclonal antibodies
against high-mobility group box 1 (HMGB1; 1:100 dilution,
bs-0664R), inducible nitric oxide (NO) synthase (iNOS; 1:100
dilution, bs-0162R) (both from Bioss, Beijing, China) and a mouse
monoclonal antibody against ED-1 (1:50 dilution, sc-59103; Santa
Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight. These
sections were then incubated with biotin-labeled goat anti-rabbit
IgG (1:200 dilution, A0277) or anti-mouse IgG (1:200 dilution,
A0286) at 37°C for 30 min, and treated with horseradish peroxidase
(HRP)-conjugated streptavidin (all from Beyotime, Shanghai, China)
for an additional 30 min. Finally, the tissue sections visualized
with 3,3′-diaminobenzidine (DAB) and counterstained with
hematoxylin (both from Solarbio), and evaluated under a light
microscope (Olympus, Tokyo, Japan).
Western blot analysis
Total protein samples were extracted from the kidney
tissues using RIPA buffer (Beyotime) and the protein concentrations
were evaluated by bicinchoninic acid assay. An equivalent amount of
each protein sample (40 µg) was loaded and separated through
sodium dodecyl sulphate (SDS)-polyacrylamide gel electrophoresis
(PAGE) and transferred onto polyvinylidene difluoride membranes
(PVDF; Millipore, Bedford, MA, USA). The membranes were then
blocked with 5% non-fat milk, and incubated overnight at 4°C with
rabbit polyclonal antibodies against NE (1:500 dilution, bs-6982R),
HMGB1 (1:500 dilution, bs-0664R), iNOS (1:500 dilution, bs-0162R),
serine/threonine kinase (Akt; 1:500 dilution, bs-6951R) and
phosphorylated Akt (p-Akt; 1:500 dilution, bs-0876R). All primary
antibodies were purchased from Bioss. Thereafter, the membranes
were incubated with HRP-conjugated secondary antibodies (1:5000
dilution, A0208; Beyotime) at 37°C for 45 min and visualized using
an enhanced chemiluminescence (ECL) system (Seven Sea Pharmtech
Co., Ltd., Shanghai, China). The protein expression levels were
expressed as a ratio to the endogenous control, β-actin.
Statistical analysis
All data are presented as the means ± standard
deviation (SD), and analyzed using GraphPad Prism version 6.0 or
SPSS version 20.0. One-way analysis of variance (ANOVA) was
performed on data obtained from the same time point, whilst two-way
ANOVA was performed on data from different time points. The
Bonferroni test was utilized for post hoc comparisons between
different groups. A P-value <0.05 was considered to indicate a
statistically significant difference. Survival data were analyzed
by the Kaplan-Meier curve and log-rank (Mantel-Cox) test.
Results
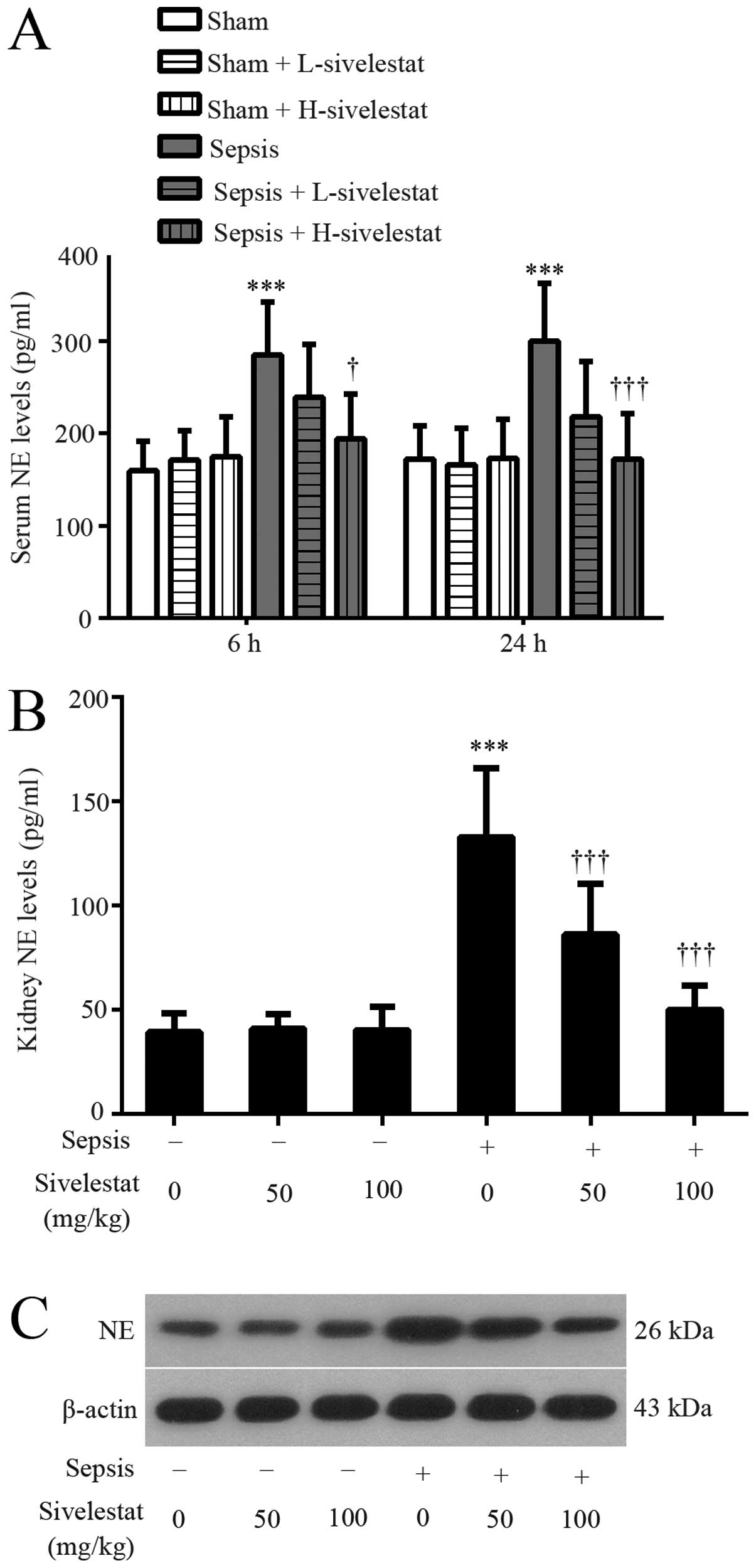
Sivelestat decreases NE expression in
rats with sepsis
We first evaluated whether the NE levels were
affected by the CLP procedure using an ELISA kit and western blot
analysis. Our results revealed that the administration of
sivelestat alone generated no changes in comparison with the
sham-operated rats (Fig. 1). The
serum NE level was significantly increased at both 6 and 24 h
post-CLP surgery, whereas decreased after sivelestat treatment
(Fig. 1A). In addition, renal NE
expression was significantly enhanced at 24 h post-CLP surgery,
whereas it was suppressed by treatment with sivelestat (Fig. 1B and C). The higher dose of
sivelestat was more effective than the lower dose.
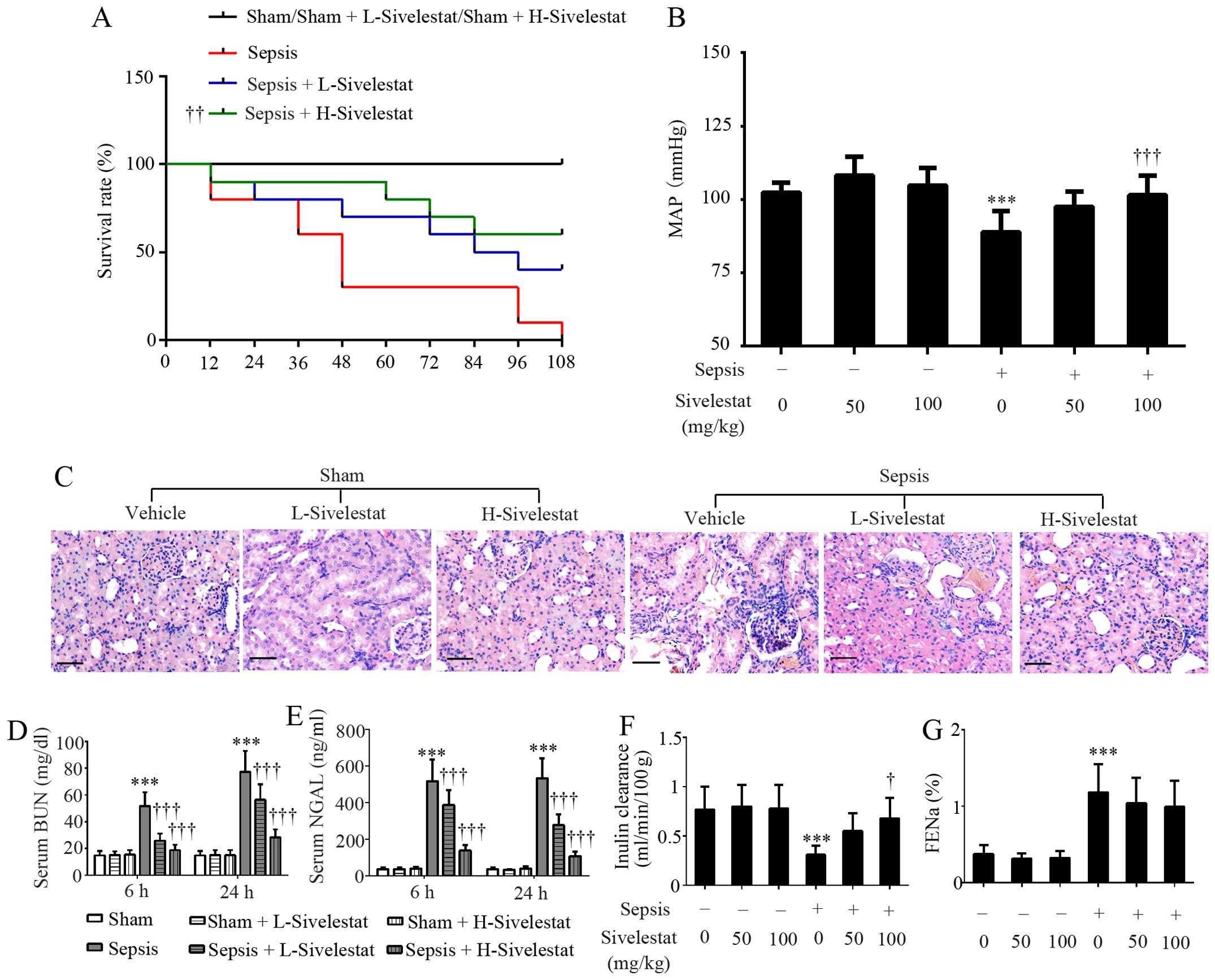
Sivelestat promotes the survival and
preserves the kidney function of rats with sepsis
Another set of rats (10 per group) was subjected to
survival analysis for 108 h. As indicated in Fig. 2A, no sham-operated rats treated
with 50 or 100 mg/kg sivelestat or the vehicle died during the
whole experimental period. All rats in the sepsis group were dead
at 108 h post-CLP surgery, while 4 and 6 septic rats survived for
108 h when treated with 50 (P=0.062 vs. sepsis group) and 100 mg/kg
sivelestat (P<0.01 vs. sepsis group), respectively (Fig. 2A). As compared with the
sham-operated group (104±5.8 mmHg), CLP induced a reduction in MAP
(89±7.2 mmHg) (P<0.001), which was restored by sivelestat
(Fig. 2B). Morphological changes
in the rat kidneys were determined by H&E staining and the
parameters related to renal function were detected using commercial
kits. Pathological alterations in the rat kidneys were
characterized by the loss of the brush border, tubular cell
flattening and tubular lumen dilation, while the administration of
sivelestat partly reversed these changes (Fig. 2C). In addition, sivelestat did not
affect the serum BUN levels, NGAL levels, inulin clearance and
FENa in the rats that underwent sham surgery (P>0.05;
Fig. 2D–G). However, CLP-induced
a significant upregulation in serum BUN and NGAL levels in the rats
and this effect was suppressed by sivelestat, with the higher dose
being more effective (Fig. 2D and
E). Additionally, the decreased inulin clearance (indicating
reduced GFR) in the rats with sepsis was partly reversed by
sivelestat (Fig. 2F). The
CLP-induced increase in FENa (indicating tubular
dysfunction) was slightly reduced by sivelestat, but failed to
reach statistical significance (Fig.
2G). Collectively, the administration of sivelestat protected
the rats against sepsis induced by multiple bacterial infection.
Since the lower dose of sivelestat did not induce mortality, kidney
distortion or renal dysfunction in the sham-operated rats, this
group was excluded from the following mechanism experiments.
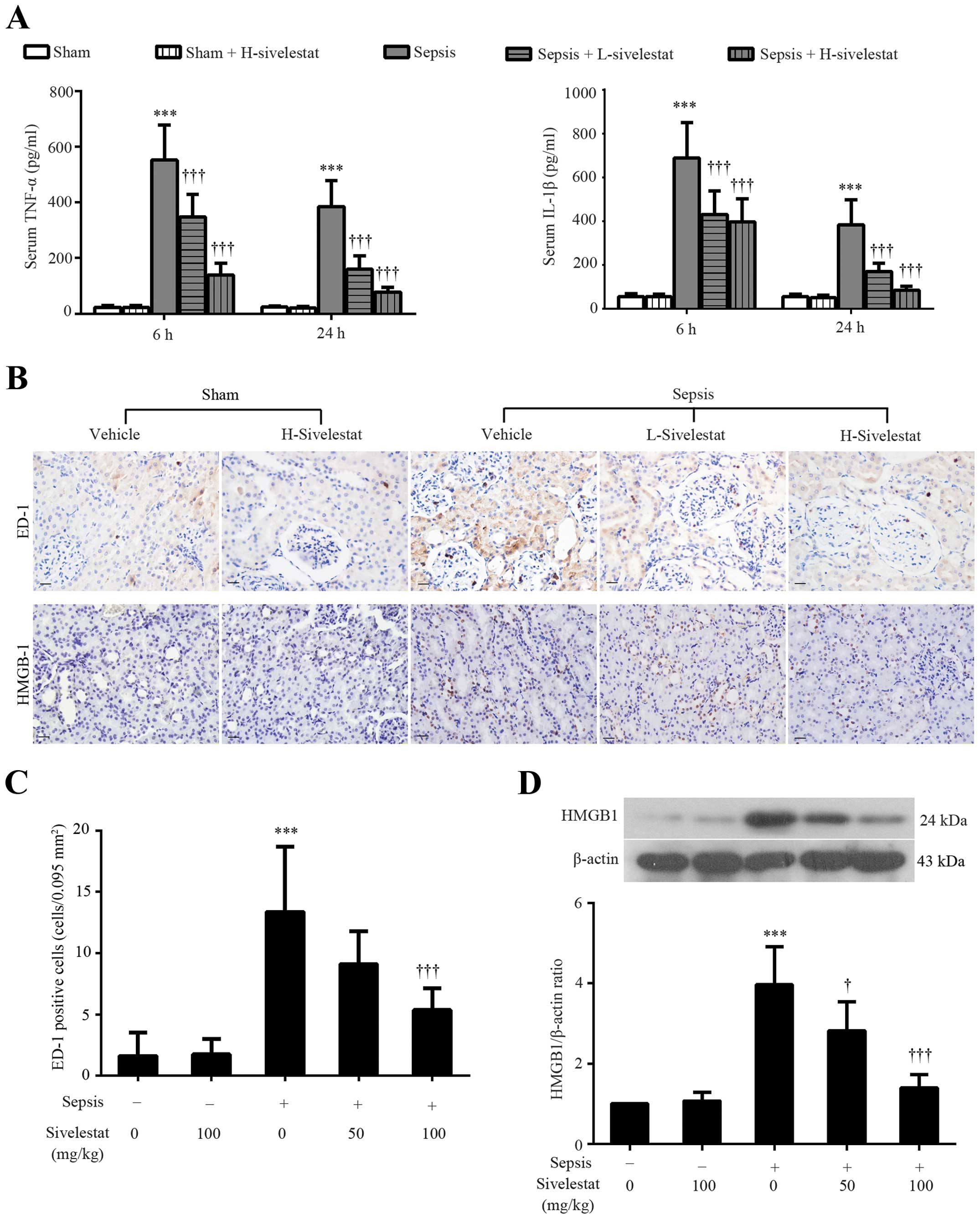
Sivelestat suppresses the release of
pro-inflammatory mediators and macrophage infiltration induced by
CLP in rats
The overproduction of pro-inflammatory mediators in
response to bacterial infection is one of the major characteristics
of sepsis (22). Therefore, the
serum levels of TNF-α and IL-1β were assessed in this study. We
found that the levels of these two pro-inflammatory cytokines were
significantly increased at 6 and 24 h post-CLP procedure (Fig. 3A). Although sivelestat alone
generated no changes in the serum levels of TNF-α and IL-1β as
compared to the sham-operated group (P>0.05), a marked
inhibitory effect of sivelestat on these two pro-inflammatory
cytokines in the rats with sepsis rats was observed (Fig. 3A). Immunohistochemistry was also
performed in the renal tissues to detect the ED-1-positive
macrophages (a marker of macrophage infiltration) at 24 h after the
surgical procedures (Fig. 3B). As
indicated in Fig. 3C, the number
of renal ED-1-positive cells was increased from 1.63±1.92 to
13.38±5.29 per 0.095 mm2 after CLP surgery, but was
decreased by the administration of sivelestat (9.13±2.64 by
L-sivelestat; 5.38±1.77 by H-sivelestat). Unlike TNF-α and IL-1β
that are released immediately in response to bacterial infection,
HMGB1 is a late pro-inflammatory mediator (23). We therefore wished to determine
whether sivelestat can reduce HMGB1 expression by detecting its
renal expression at 24 h after the sham or CLP procedure using
immunohistochemical staining and western blot analysis. A weak
nuclear expression of HMGB1 was observed in a few renal parenchymal
cells in the control kidney tissues (Fig. 3B). However, the number of
HMGB1-positive cells was significantly increased after the CLP
procedure, whereas it was decreased by treatment with sivelestat at
different doses (Fig. 3B). The
results from western blot analysis confirmed the
immunohistochemical results (Fig.
3D). The above-mentioned results indicate an anti-inflammatory
effect of sivelestat in a model of sepsis-related AKI.
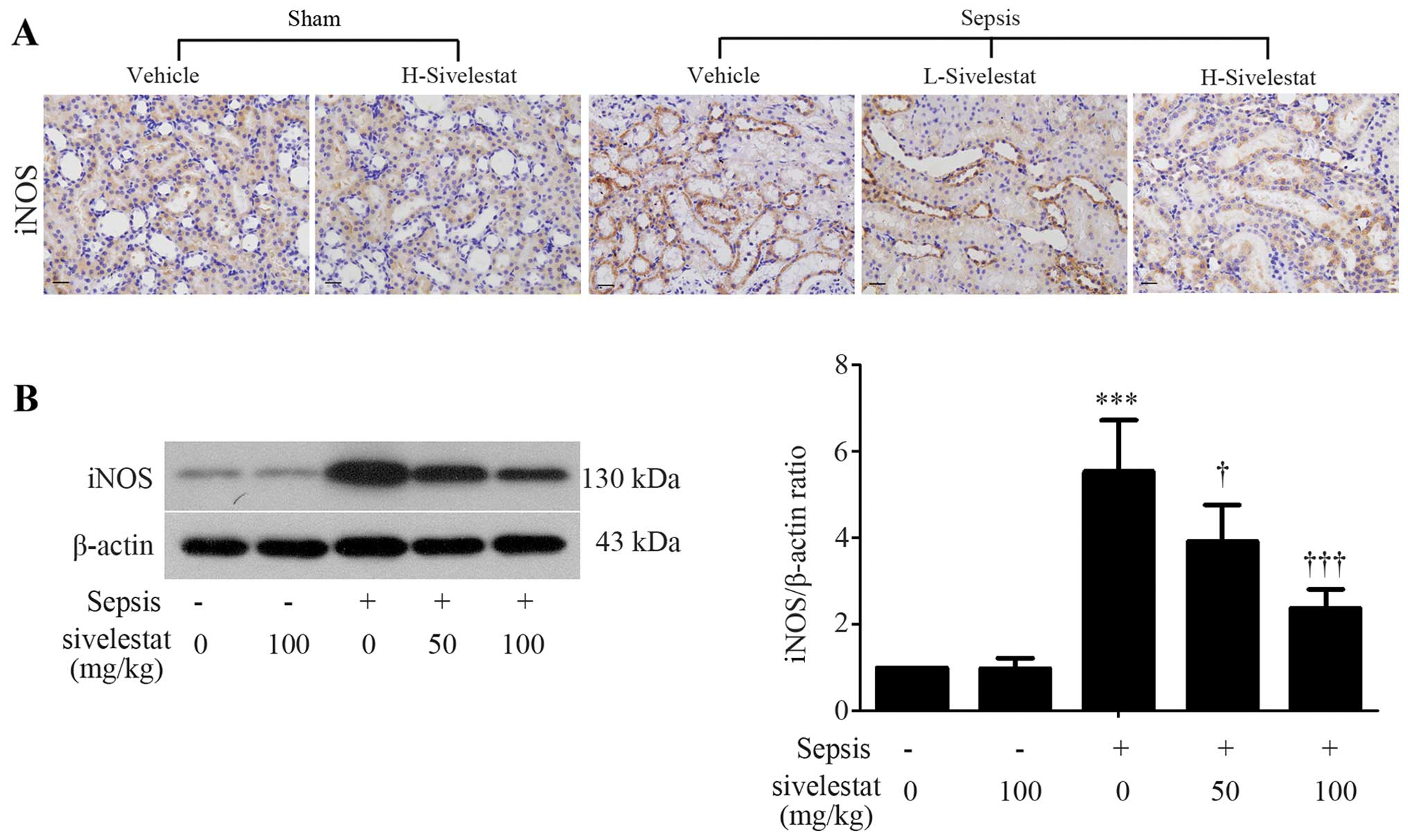
Sivelestat inhibits renal iNOS expression
in septic rats
The polymicrobial infection induced-overproduction
of pro-inflammatory mediators can accelerate the release of
powerful secondary mediators, such as NO (24). Given the fact that the production
of NO is predominantly mediated by iNOS (25), we evaluated renal iNOS protein
expression by using immunohistochemical staining and western blot
analysis. We found that iNOS was expressed in the cytoplasm of
renal tubule epithelial cells (Fig.
4A). As compared with the normal kidney tissues, the more
intense expression of iNOS was observed in the septic kidney
tissues (Fig. 4A). However, the
upregulation of iNOS expression was significantly inhibited by
treatment with sivelestat (Fig.
4A). These results were confirmed by western blot analysis
(Fig. 4B).
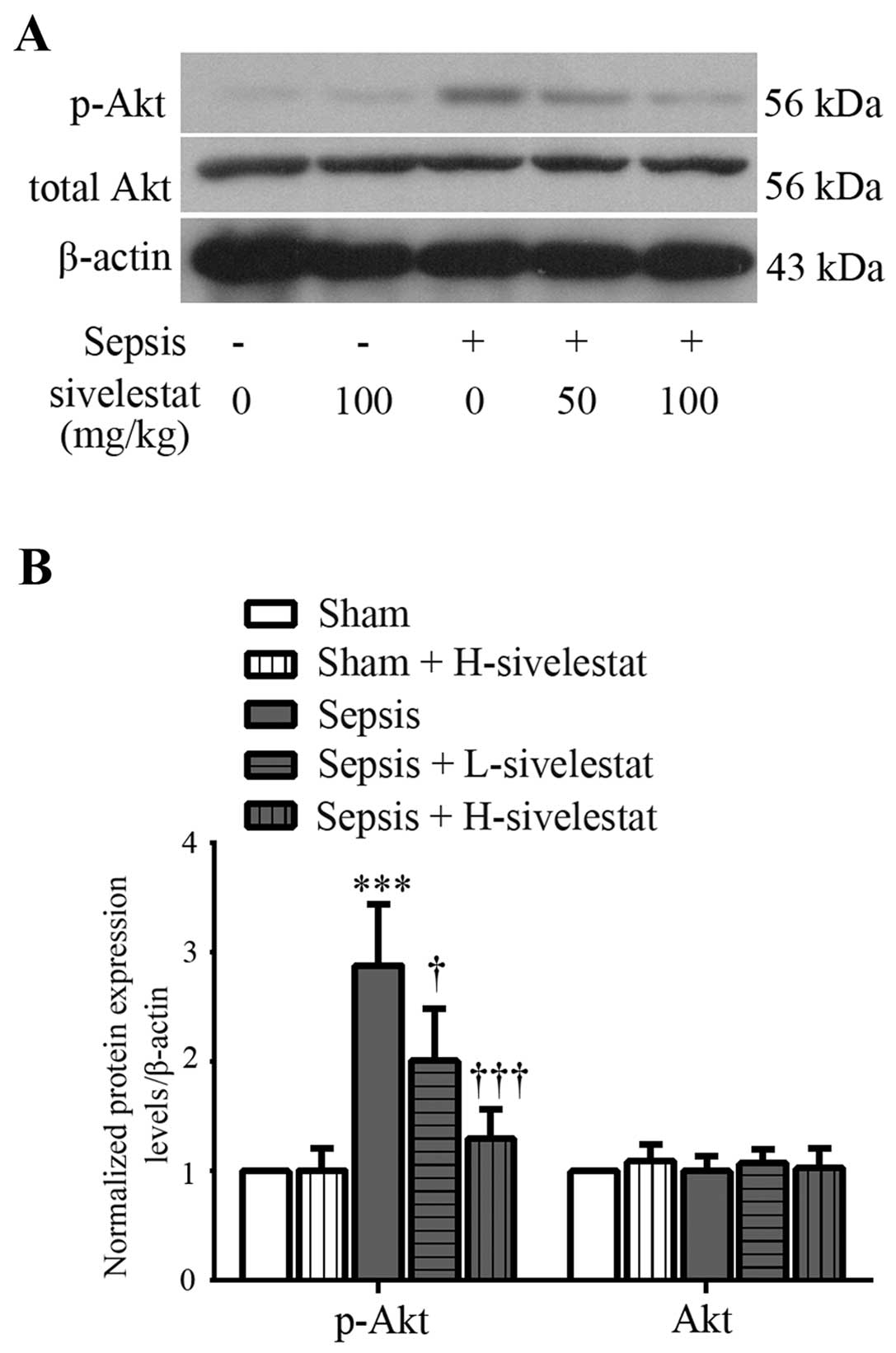
Sivelestat inhibits the activation of the
Akt signaling pathway in the kidney tissues of rats with
sepsis
The activation of the Akt pathway was assessed by
western blot analysis of the Akt phosphorylation product, p-Akt in
the rat kidney tissues at 24 h post-surgery. We noted that total
Akt protein expression in the kidney tissues remained unaltered in
the different experimental groups (Fig. 5). Treatment with sivelestat alone
had no significant effect on renal p-Akt expression in the rats
subjected to sham operation (Fig.
5). However, a marked increase in the phosphorylation levels of
Akt was observed in the septic renal tissues. The high
phosphorylation levels of Akt induced by sepsis were suppressed
after low-dose sivelestat treatment and the levels almost returned
to normal after high-dose sivelestat treatment (Fig. 5). Our data thus suggest that the
administration of sivelestat suppresses the CLP-induced activation
of Akt in rat kidneys.
Discussion
Despite improvements in the supportive care of
patients with sepsis, current therapeutic approaches are relatively
ineffective due to the protean nature of septic AKI (3,26).
A variety of animal models have been established to investigate the
specific molecular events occurring in sepsis (27). The most frequently used model is
the rodent model of CLP, in which sepsis originates from a
polymicrobial infection (18,28). While Hirche et al found
that the absence of NE increased the mortality of mice to
Pseudomonas aeruginosa infection (29), Suda et al found that the
inhibition of NE improved the survival rate of rats that uderwent
CLP (14). These earlier findings
suggest that NE mediates innate host protection against bacterial
infection; however, its on-going and excessive activation may have
adverse effects. Our present study showed that sivelestat improved
the survival of rats with sepsis and preserved their kidney
functions, revealing the therapeutic role of sivelestat in
sepsis-associated renal injury.
Sivelestat is a specific NE inhibitor first
synthesized by Kawabata et al in 1991 (30), and has been reported to attenuate
pulmonary inflammation and fibrosis in animal models (12,31). Its effects on lung and
cardiovascular diseases have been examined in several clinical
trials (32–34). Of note, apart from the therapeutic
effects of sivelestat in the lungs, it has also been demonstrated
to have potential to reduce inflammation-related lesions in the
liver (35) and pancreas
(36) in preclinical disease
models. Our study demonstrated that treatment with sivelestat
alleviated the dysregulation in BUN and NGAL levels in the rats
with sepsis. The CLP surgery-induced decrease in GFR and tubular
function were partly restored by sivelestat, indicating a
protective role of this NE inhibitor in sepsis-related kidney
injury.
We then focused on the anti-inflammatory effects of
sivelestat in septic AKI. Ischemia/reperfusion injury, bacterial
infection or the nephrotoxic agent-induced aberrant infiltration of
immune cells, such as neutrophils, macrophages and lymphocytes are
considered to contribute to the pathogenesis of AKI (37). Pro-inflammatory mediators, such as
TNF-α and IL-1β are released early in response to bacterial
infection and can be acutely toxic (38). Therapeutic agents being able to
reduce the release of TNF-α and/or IL-1β in murine septic models
are suggested to ameliorate sepsis (39–41). The study by Suda et al
revealed that sivelestat suppressed the aberrant release of TNF-α
(not statistically significant) and IL-1β (statistically
significant) in septic lungs (14). In this study, we found that
abnormal macrophage infiltration and TNF-α/IL-1β release were
reduced by sivelestat. In contrast to other sepsis-associated
cytokines, HMGB1 is a late pro-inflammatory cytokine released from
monocytes and/or apoptotic cells after the onset of sepsis that
further amplifies the inflammatory process (42). The neutralization of HMGB1 can
therapeutically reverse lethality in experimental sepsis (38,43). In this study, we demonstrated that
the administration of sivelestat markedly reduced the CLP-induced
upregulation of renal HMGB1. Such results were supported by an
earlier study showing that sivelestat treatment reduced LPS-induced
pulmonary HMGB1 upregulation in rats (44). Of note, most clinical trials
targeting TNF (45) or IL-1
(46) in sepsis have failed.
Therefore, further clinical studies examining the anti-inflammatory
effects of sivelestat in sepsis are urgently required.
The polymicrobial infection-induced overproduction
of pro-inflammatory mediators can accelerate the release of
powerful secondary mediators, such as reactive nitrogen/oxygen
species (47). The activation of
iNOS during sepsis results in increased NO levels that causes
tubular injury through the local generation of reactive nitrogen
species, and the selective inhibition of iNOS has been suggested as
a potential novel treatment for sepsis-induced AKI (24). In this study, we found that
sivelestat was able to reduce CLP-induced renal iNOS overexpression
in rats. Although previous studies have indicated an inhibitory
effect of sivelestat on iNOS (48,49), as far as we know, our study was
the first to show such an effect in septic kidneys.
Previous studies searching for novel
anti-inflammatory agents have suggested a critical role of
phosphatidylinositol 3-kinase (PI3K)/Akt signaling in sepsis. It is
worth noting that the phosphorylation of Akt referring to PI3K/Akt
pathway activation is enhanced in the lungs (50) and liver (51), but is weakened in the heart
(52) after the CLP procedure or
LPS challenge. Consequently, both the blockade and activation of
PI3K/Akt signaling transduction have been shown to improve outcome
in septic shock. Of note, a previous study by Sadhu et al
showed that the inactivation of the PI3K/Akt pathway with a
pharmaceutical inhibitor blocked TNF1α-stimulated NE exocytosis
(53). Since sivelestat can
suppress IL-1β-stimulated Akt phosphorylation in hepatocytes
(54), we explored whether
sivelestat also has an inhibitory effect on PI3K/Akt signaling
transduction in septic kidneys in this study. We found that
CLP-enhanced renal Akt phosphorylation was decreased by sivelestat.
Moreover, the LPS induction of the phosphorylation of Akt is
responsible for nuclear factor-κB (NF-κB) activation in human renal
mesangial cells (55), and the
inhibitory effects of sivelestat on NF-κB signals have been
previously reported (36). It is
likely that Akt signals are involved in the regulatory effects of
sivelestat on the NF-κB pathway. Further in vitro
experiments are thus being carried out by our group to study the
underlying mechanisms.
In conclusion, in this study, we demonstrate that
the administration of the NE inhibitor, sivelestat, mitigates
CLP-induced kidney injury, reduces inflammation, and suppresses the
activation of the Akt signaling pathway. Our study suggests that
sivelestat has potential to attenuate sepsis-induced kidney
injury.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (no. 81471847), the Natural
Science Foundation of Liaoning Province (no. 2014021003), and the
Science and Technology Project of Shenyang City (no.
F14-158-9-40).
References
|
1
|
Fry DE: Sepsis, systemic inflammatory
response, and multiple organ dysfunction: the mystery continues. Am
Surg. 78:1–8. 2012.PubMed/NCBI
|
|
2
|
Rittirsch D, Flierl MA and Ward PA:
Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 8:776–787.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morrell ED, Kellum JA, Pastor-Soler NM and
Hallows KR: Septic acute kidney injury: molecular mechanisms and
the importance of stratification and targeting therapy. Crit Care.
18:5012014. View Article : Google Scholar
|
|
4
|
Chen L, Yang S, Zumbrun EE, Guan H,
Nagarkatti PS and Nagarkatti M: Resveratrol attenuates
lipopolysaccharide-induced acute kidney injury by suppressing
inflammation driven by macrophages. Mol Nutr Food Res. 59:853–864.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shimazu R, Akashi S, Ogata H, Nagai Y,
Fukudome K, Miyake K and Kimoto M: MD-2, a molecule that confers
lipopolysaccharide responsiveness on toll-like receptor 4. J Exp
Med. 189:1777–1782. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Villar J, Cabrera N, Casula M, Flores C,
Valladares F, Muros M, Blanch L, Slutsky AS and Kacmarek RM:
Mechanical ventilation modulates toll-like receptor signaling
pathway in a sepsis-induced lung injury model. Intensive Care Med.
36:1049–1057. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bang BR, Kim SJ, Yagita H, Croft M and
Kang YJ: Inhibition of 4-1BBL-regulated TLR response in macrophages
ameliorates endotoxin-induced sepsis in mice. Eur J Immunol.
45:886–892. 2015. View Article : Google Scholar :
|
|
8
|
Opal SM, Laterre PF, Francois B, LaRosa
SP, Angus DC, Mira JP, Wittebole X, Dugernier T, Perrotin D,
Tidswell M, et al: ACCESS Study Group: Effect of eritoran, an
antagonist of MD2-TLR4, on mortality in patients with severe
sepsis: the ACCESS randomized trial. JAMA. 309:1154–1162. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Griffin KL, Fischer BM, Kummarapurugu AB,
Zheng S, Kennedy TP, Rao NV, Foster WM and Voynow JA: 2-O,
3-O-desulfated heparin inhibits neutrophil elastase-induced HMGB-1
secretion and airway inflammation. Am J Respir Cell Mol Biol.
50:684–689. 2014. View Article : Google Scholar :
|
|
10
|
Korkmaz B, Horwitz MS, Jenne DE and
Gauthier F: Neutrophil elastase, proteinase 3, and cathepsin G as
therapeutic targets in human diseases. Pharmacol Rev. 62:726–759.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zager RA, Johnson AC and Frostad KB: Rapid
renal alpha-1 antitrypsin gene induction in experimental and
clinical acute kidney injury. PLoS One. 9:e983802014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takemasa A, Ishii Y and Fukuda T: A
neutrophil elastase inhibitor prevents bleomycin-induced pulmonary
fibrosis in mice. Eur Respir J. 40:1475–1482. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ishii T, Doi K, Okamoto K, Imamura M, Dohi
M, Yamamoto K, Fujita T and Noiri E: Neutrophil elastase
contributes to acute lung injury induced by bilateral nephrectomy.
Am J Pathol. 177:1665–1673. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suda K, Takeuchi H, Hagiwara T, Miyasho T,
Okamoto M, Kawasako K, Yamada S, Suganuma K, Wada N, Saikawa Y, et
al: Neutrophil elastase inhibitor improves survival of rats with
clinically relevant sepsis. Shock. 33:526–531. 2010.
|
|
15
|
Schortgen F and Asfar P: Update in sepsis
and acute kidney injury 2014. Am J Respir Crit Care Med.
191:1226–1231. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan J and Li S and Li S: The role of the
liver in sepsis. Int Rev Immunol. 33:498–510. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Drosatos K, Lymperopoulos A, Kennel PJ,
Pollak N, Schulze PC and Goldberg IJ: Pathophysiology of
sepsis-related cardiac dysfunction: driven by inflammation, energy
mismanagement, or both? Curr Heart Fail Rep. 12:130–140. 2015.
View Article : Google Scholar :
|
|
18
|
Rittirsch D, Huber-Lang MS, Flierl MA and
Ward PA: Immunodesign of experimental sepsis by cecal ligation and
puncture. Nat Protoc. 4:31–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schick MA, Baar W, Flemming S, Schlegel N,
Wollborn J, Held C, Schneider R, Brock RW, Roewer N and Wunder C:
Sepsis-induced acute kidney injury by standardized colon ascendens
stent peritonitis in rats - a simple, reproducible animal model.
Intensive Care Med Exp. 2:342014. View Article : Google Scholar
|
|
20
|
Souza AC, Volpini RA, Shimizu MH, Sanches
TR, Camara NO, Semedo P, Rodrigues CE, Seguro AC and Andrade L:
Erythropoietin prevents sepsis-related acute kidney injury in rats
by inhibiting NF-κB and upregulating endothelial nitric oxide
synthase. Am J Physiol Renal Physiol. 302:F1045–F1054. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kadova Z, Dolezelova E, Cermanova J, Hroch
M, Laho T, Muchova L, Staud F, Vitek L, Mokry J, Chladek J, et al:
IL-1 receptor blockade alleviates endotoxin-mediated impairment of
renal drug excretory functions in rats. Am J Physiol Renal Physiol.
308:F388–F399. 2015. View Article : Google Scholar
|
|
22
|
King EG, Bauzá GJ, Mella JR and Remick DG:
Pathophysiologic mechanisms in septic shock. Lab Invest. 94:4–12.
2014. View Article : Google Scholar
|
|
23
|
Diener KR, Al-Dasooqi N, Lousberg EL and
Hayball JD: The multifunctional alarmin HMGB1 with roles in the
pathophysiology of sepsis and cancer. Immunol Cell Biol.
91:443–450. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Heemskerk S, Masereeuw R, Russel FG and
Pickkers P: Selective iNOS inhibition for the treatment of
sepsis-induced acute kidney injury. Nat Rev Nephrol. 5:629–640.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lowenstein CJ and Padalko E: iNOS (NOS2)
at a glance. J Cell Sci. 117:2865–2867. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fink MP and Warren HS: Strategies to
improve drug development for sepsis. Nat Rev Drug Discov.
13:741–758. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Buras JA, Holzmann B and Sitkovsky M:
Animal models of sepsis: setting the stage. Nat Rev Drug Discov.
4:854–865. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brooks HF, Osabutey CK, Moss RF, Andrews
PL and Davies DC: Caecal ligation and puncture in the rat mimics
the pathophysiological changes in human sepsis and causes
multi-organ dysfunction. Metab Brain Dis. 22:353–373. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hirche TO, Benabid R, Deslee G, Gangloff
S, Achilefu S, Guenounou M, Lebargy F, Hancock RE and Belaaouaj A:
Neutrophil elastase mediates innate host protection against
Pseudomonas aeruginosa. J Immunol. 181:4945–4954. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kawabata K, Suzuki M, Sugitani M, Imaki K,
Toda M and Miyamoto T: ONO-5046, a novel inhibitor of human
neutrophil elastase. Biochem Biophys Res Commun. 177:814–820. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoshikawa N, Inomata T, Okada Y, Shimbo T,
Takahashi M, Akita K, Uesugi Y and Narumi Y: Sivelestat sodium
hydrate reduces radiation-induced lung injury in mice by inhibiting
neutrophil elastase. Mol Med Rep. 7:1091–1095. 2013.PubMed/NCBI
|
|
32
|
Kohira S, Oka N, Inoue N, Itatani K,
Kitamura T, Horai T, Oshima H, Tojo K, Yoshitake S and Miyaji K:
Effect of additional preoperative administration of the neutrophil
elastase inhibitor sivelestat on perioperative inflammatory
response after pediatric heart surgery with cardiopulmonary bypass.
Artif Organs. 38:1018–1023. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nomura N, Asano M, Saito T, Nakayama T and
Mishima A: Sivelestat attenuates lung injury in surgery for
congenital heart disease with pulmonary hypertension. Ann Thorac
Surg. 96:2184–2191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tagami T, Tosa R, Omura M, Fukushima H,
Kaneko T, Endo T, Rinka H, Murai A, Yamaguchi J, Yoshikawa K, et
al: Effect of a selective neutrophil elastase inhibitor on
mortality and ventilator-free days in patients with increased
extravascular lung water: a post hoc analysis of the PiCCO
Pulmonary Edema Study. J Intensive Care. 2:672014. View Article : Google Scholar
|
|
35
|
Sakai S, Tajima H, Miyashita T, Nakanuma
S, Makino I, Hayashi H, Nakagawara H, Kitagawa H, Fushida S,
Fujimura T, et al: Sivelestat sodium hydrate inhibits neutrophil
migration to the vessel wall and suppresses hepatic
ischemia-reperfusion injury. Dig Dis Sci. 59:787–794. 2014.
View Article : Google Scholar
|
|
36
|
Cao J and Liu Q: Protective effects of
sivelestat in a caerulein-induced rat acute pancreatitis model.
Inflammation. 36:1348–1356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jang HR and Rabb H: Immune cells in
experimental acute kidney injury. Nat Rev Nephrol. 11:88–101. 2015.
View Article : Google Scholar
|
|
38
|
Yang H, Ochani M, Li J, Qiang X, Tanovic
M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, et al:
Reversing established sepsis with antagonists of endogenous
high-mobility group box 1. Proc Natl Acad Sci USA. 101:296–301.
2004. View Article : Google Scholar :
|
|
39
|
Lingaraju MC, Pathak NN, Begum J,
Balaganur V, Ramachandra HD, Bhat RA, Ram M, Singh V, Kandasamy K,
Kumar D, et al: Betulinic acid attenuates renal oxidative stress
and inflammation in experimental model of murine polymicrobial
sepsis. Eur J Pharm Sci. 70:12–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Carrino DA, Lidor C, Edelstein S and
Caplan AI: Proteoglycan synthesis in vitamin D-deficient cartilage:
recovery from vitamin D deficiency. Connect Tissue Res. 19:135–147.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang H, Wang W, Fang H, Yang Y, Li X, He
J, Jiang X, Wang W, Liu S, Hu J, et al: GSK-3β inhibition
attenuates CLP-induced liver injury by reducing inflammation and
hepatic cell apoptosis. Mediators Inflamm. 2014:6295072014.
View Article : Google Scholar
|
|
42
|
Scaffidi P, Misteli T and Bianchi ME:
Release of chromatin protein HMGB1 by necrotic cells triggers
inflammation. Nature. 418:191–195. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang H, Liao H, Ochani M, Justiniani M,
Lin X, Yang L, Al-Abed Y, Wang H, Metz C, Miller EJ, et al:
Cholinergic agonists inhibit HMGB1 release and improve survival in
experimental sepsis. Nat Med. 10:1216–1221. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hagiwara S, Iwasaka H, Togo K and Noguchi
T: A neutrophil elastase inhibitor, sivelestat, reduces lung injury
following endotoxin-induced shock in rats by inhibiting HMGB1.
Inflammation. 31:227–234. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Abraham E, Laterre PF, Garbino J,
Pingleton S, Butler T, Dugernier T, Margolis B, Kudsk K, Zimmerli
W, Anderson P, et al: Lenercept (p55 tumor necrosis factor receptor
fusion protein) in severe sepsis and early septic shock: a
randomized, double-blind, placebo-controlled, multicenter phase III
trial with 1,342 patients. Crit Care Med. 29:503–510. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fisher CJ Jr, Dhainaut JF, Opal SM,
Pribble JP, Balk RA, Slotman GJ, Iberti TJ, Rackow EC, Shapiro MJ,
Greenman RL, et al: Recombinant human interleukin 1 receptor
antagonist in the treatment of patients with sepsis syndrome.
Results from a randomized, double-blind, placebo-controlled trial.
Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA.
271:1836–1843. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cauwels A: Nitric oxide in shock. Kidney
Int. 72:557–565. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hagiwara S, Iwasaka H, Hidaka S, Hasegawa
A and Noguchi T: Neutrophil elastase inhibitor (sivelestat) reduces
the levels of inflammatory mediators by inhibiting NF-kB. Inflamm
Res. 58:198–203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Toda Y, Takahashi T, Maeshima K, Shimizu
H, Inoue K, Morimatsu H, Omori E, Takeuchi M, Akagi R and Morita K:
A neutrophil elastase inhibitor, sivelestat, ameliorates lung
injury after hemorrhagic shock in rats. Int J Mol Med. 19:237–243.
2007.PubMed/NCBI
|
|
50
|
He Z, Zhu Y and Jiang H: Inhibiting
toll-like receptor 4 signaling ameliorates pulmonary fibrosis
during acute lung injury induced by lipopolysaccharide: an
experimental study. Respir Res. 10:1262009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kim TH, Kim SJ and Lee SM: Stimulation of
the α7 nicotinic acetylcholine receptor protects against sepsis by
inhibiting toll-like receptor via phosphoinositide 3-kinase
activation. J Infect Dis. 209:1668–1677. 2014. View Article : Google Scholar
|
|
52
|
Li C, Hua F, Ha T, Singh K, Lu C,
Kalbfleisch J, Breuel KF, Ford T, Kao RL, Gao M, et al: Activation
of myocardial phos-phoinositide-3-kinase p110α ameliorates cardiac
dysfunction and improves survival in polymicrobial sepsis. PLoS
One. 7:e447122012. View Article : Google Scholar
|
|
53
|
Sadhu C, Dick K, Tino WT and Staunton DE:
Selective role of PI3K delta in neutrophil inflammatory responses.
Biochem Biophys Res Commun. 308:764–769. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Araki Y, Matsumiya M, Matsuura T, Kaibori
M, Okumura T, Nishizawa M and Kwon AH: Sivelestat suppresses iNOS
gene expression in proinflammatory cytokine-stimulated hepatocytes.
Dig Dis Sci. 56:1672–1681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wu F, Zhang W, Li L, Zheng F, Shao X, Zhou
J and Li H: Inhibitory effects of honokiol on
lipopolysaccharide-induced cellular responses and signaling events
in human renal mesangial cells. Eur J Pharmacol. 654:117–121. 2011.
View Article : Google Scholar
|