Introduction
Lung cancer is the leading cause of cancer-related
deaths worldwide, and studies on lung cancer have increased
significantly in recent years. Among all types of lung cancer,
non-small cell lung carcinoma (NSCLC) represents the highest number
of cases of diagnosed lung cancer (1). Surgical resection, chemotherapy and
radiotherapy are currently used for the treatment of NSCLC
(2). However, the long-term
survival for NSCLC patients remains poor. A lack of information
regarding the effects of genetic, epigenetic and environmental
factors in NSCLC has led to difficulties in elucidating the
developmental mechanisms of cancer cells, such as roles in
viability, aggressiveness and progression. To date, improvements in
the understanding of the genetic processes involved in NSCLC has
assisted in the diagnosis and the establishment of treatment
options. Unveiling the pathogenesis of lung cancer epigenetically
would also aid in identifying new diagnostic biomarkers as well as
in establishing new therapeutic strategies.
In cancer, the cellular genomes are unstable and
typically exhibit alterations including amplification and/or
deletion of cancer genes and epigenetic silencing (3). Epigenetic alterations including
microRNA (miRNA or miR) silencing, DNA methylation and histone
modification have been demonstrated to participate in cancer
progression (4). DNA
hypomethylation largely affects intergenic and intronic regions of
the genome as well as repeat sequences and transposable elements
(TEs) (5). In addition, the
methylation levels of oncogenes and tumor suppressor genes in
cancer cells are commonly aberrant (6). TEs as mobile factors in the genome
are widely distributed among all regions, causing deleterious
mutations, gene disruptions and chromosomal rearrangements
(7), and potentially lead to the
initiation and progression of cancer (8). To date, our understanding of the
association between TEs and human cancer is limited. In addition,
the correlation between the mobility of TEs and the cancer cell
environment remains unknown.
Recently, the piwi-interacting RNA (piRNA or piR)
pathway was identified in germline cells in the testis and
confirmed as an highly conserved, evolutionarily pathway in animals
(9). piRNAs are 26–32 nucleotides
in length and bind PIWI proteins and mediate the post-translational
activities of TEs as well as gene expression (10–12). Thus, the piRNA pathway plays a
crucial role in epigenetic change by regulating genome stability.
Several PIWI-like genes have been identified as mammalian paralogs,
including four paralogs in humans and three paralogs in the mouse
(13). The initial studies of
PIWI and piRNAs were focused on germ cell development, particularly
in the testis. A recent study by Yan et al using
high-throughput small RNA sequencing, showed widespread expression
of piRNAs in somatic tissues, which suggested new functions of the
piRNAs in addition to their roles in the development of gonads
(14). These results suggest that
the piRNA pathway has comprehensive functions during development,
both in germ cells and somatic cells, by regulating TEs and gene
expression.
Several studies have indicated that the piRNA
pathway is increased in different types of cancer, including lung
cancer, pancreatic cancer and ovarian cancer (15). The abundant expression of PIWI
proteins has been found in the breast, gastrointestinal tract,
stomach, and endometrium with cancer whereas no expression has been
found in normal tissues (16,17). Moreover, increasing Piwil1
enhanced tumor growth and mortality (18). Piwil2 is also widely expressed in
pre-cancerous stem cells, breast cancer cells and cervical cancer
cells, suggesting that Piwil2 is involved in tumorigenesis
(19). Piwil3 and Piwil4
expression has only been identified in colon cancer, to the best of
our knowledge (16). In addition
to PIWI proteins, piRNAs have also been found to be abnormally
expressed in cancer cells. Cheng et al showed that piR-651
was upregulated in gastric cancer tissues and was associated with
the tumor-node-metastasis (TNM) stage. The authors concluded that
piR-651 was involved in the development of gastric cancer and may
be a potential marker for diagnosis (20). In another study, Cheng et
al reported that piR-823 reduced tumor activity in gastric
cancer cells. These findings suggested that piRNAs mediate cancer
progression (21). However, the
precise roles and mechanisms of these piRNAs in different types of
cancer and processes require further study.
In the present study, we aimed to elucidate the role
of piR-651 in NSCLC by examining the expression and potential
function of piR-651 in NSCLC.
Materials and methods
Clinical lung cancer samples
Clinical lung cancer samples and adjacent normal
tissues from 78 patients with NSCLC were provided by the Xiangya
School of Medicine, Central South University (Changsha, China).
Informed consent was obtained for all samples, and the study was
approved by the institutional review board (Xiangya School of
Medicine Research Ethics Committee). All patients included in this
study had not received preoperative chemotherapy, radiotherapy or
any other therapy. Basic information was collected, including age,
gender, clinical manifestation and histological differentiation.
The follow-up was from December 1st 2012 and lasted for 24 months.
Tissue histology classification was determined according to
criteria from the World Health Organization classification of lung
tumors (22). The tumor stages
were confirmed following the pathological TNM (pTNM) classification
(7th edition) of the International Union against Cancer.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA, USA). RNA (1 µg) was used to
synthesize first-strand cDNA. To perform RT-qPCR of piRNA, the
miScript SYBR-Green PCR kit provided by Qiagen (Hilden, Germany)
was used for reverse transcription, and the PrimeScript™ II 1st
Strand cDNA Synthesis kit from Takara (Dalian, China) was used for
gene expression detection. The primers used for detecting piRNAs
and genes are listed in Table I.
The cDNAs were then analyzed using the Applied Biosystems 7500 qPCR
System (Life Technologies, Carlsbad, CA, USA). The PCR conditions
were as follows: 1 cycle of 94°C for 2 min; and 40 cycles of 94°C
for 15 sec and 60°C for 45 sec. The dissociation curves were
subsequently performed to verify whether the product was specific.
The quantity of the piRNA and gene expression were calculated using
the 2−ΔΔCt method.
 | Table IPrimer sequences used for
RT-qPCR. |
Table I
Primer sequences used for
RT-qPCR.
| Gene | Sense (5′→3′) | Antisense
(5′→3′) |
|---|
| piR-651 |
AGAGAGGGGCCCGTGCCTTG | Provided by
miScript SYBR-Green PCR kit |
| β-catenin |
ATGGAACCAGAGAAAAGC |
AAGGACTGAGAAAATCCCTG |
| cyclin D1 |
CGAGGAGCTGCTGCAAATGG |
GGTATCAAAATGCTCCGGAGAGG |
| cyclin D2 |
AGCAGCGGGAGAAGCTGTCTCTG |
ATGGACGCGTCTCTCTCTTTCGGCC |
| cyclin D3 |
TGGATGCTGGAGGTATGTG |
CGTGGTCGGTGTAGATGC |
| CDK2 |
ATCCGCCTGGACACTGAGACT |
TGGAGGACCCGATGAGAATG |
| CDK4 |
CACAGTTCGTGAGGTGGCTTTA |
TGTCCTTAGGTCCTGGTCTACATG |
| CDK8 |
CAAATCCCTTACCCAAAACGAG |
TCTGCGGCTGTGATGTGCT |
| NF-κB |
TAAGCAGAAGCATTAACTTCTCT |
CCTGCTTCTGTCTCTAGGAGAGTA |
| Caspase-3 |
TCTGACTGGAAAGCCGAAACTC |
TCCCACTGTCTGTCTCAATGCCAC |
| ICAM-1 |
GCAGACAGTGACCATCTACAGCTT |
CTTCTGAGACCTCTGGCTTCGT |
| BCL-2 |
TGGGATGCCTTTGTGGAACTAT |
AGAGACAGCCAGGAGAAATCAAAC |
| MIF |
ACCAGCTCATGGCCTTCG |
CTTGCTGTAGGAGCGGTT |
| MMP-9 |
ACGCAGACATCGTCATCCAGT |
GGACCACAACTCGTCATCGTC |
| KISS-1 |
GAGAACTCTTGAGACCGGGAGC |
TGGGCTCCCGGTCTCAAGAGTTCTC |
| RECK |
CACAGACCACATGGAGCACAAC |
GCTGCCAAGAGCGAAGGACA |
| EGFR |
ACTGCCAGAAACTGACCAAAATC |
GCCCTCGGGGTTCACATC |
| Raf kinase |
CTTCTTTGACTATGCGTCGTATGC |
GGTGAGGCTGATTCGCTGTG |
| myc |
ACATCATCATCCAGGACTGTATGTG |
GGCTGCCGCTGTCTTTGC |
| HER2 |
CCCCAAAGCCAACAAAGAAA |
GTGTACGAGCCGCACATCCT |
| p53 |
ATGGAGGAGCCGCAGTCAGATCCTA |
TAGGATCTGACTGCGGCTCCTCCAT |
| PTEN |
TGGAAAGGGACGAACTGGTG |
CATAGCGCCTCTGACTGGGA |
| APC |
ATGTACGGGCTTACTAATGACCACT |
CACTTCCAACTTCTCGCAACG |
| CD95 |
ATGCTGGGCATCTGGACCCT |
CAACATCAGATAAATTTATTGCCAC |
| ST5 |
GAGTGAGCCCAGCGCCTTCCTCAGG |
GAGTGAGCCCAGCGCCTTCCTCAGG |
| YPEL3 |
ATGTGTGTGGCCCAGGTCCTGACAG |
CTGTCAGGACCTGGGCCACACACAT |
| ST7 |
ATTCAATCCTCATGTGCCAAAAT |
GATTCAAAGCCCCTTCCACTC |
| ST14 |
ACCCTGAGCCCCATGGAGCCCCACG |
CGTGGGGCTCCATGGGGCTCAGGGT |
| GAPDH |
TTAGCACCCCTGGCCAAG |
GCCATCCACAGTCTTCTGGG |
piRNA northern blot analysis
Total RNA (30 µg) was separated in a 15%
denaturing polyacrylamide gel and then transferred onto a
GeneScreen Plus Hybridization Transfer membrane (NEN Life Science
Products, Inc., Boston, MA, USA) and blotted using a
32P-labeled oligonucleotide. The membrane was then
stripped in 0.1X SSPE and hybridized with the probe (piR-651,
5′-GAC GCU UUC CAA GGC ACG GGC CCC UCU CU-3′) overnight at 40°C.
The antisense oligonucleotide (5′-AAA ATA TGG AAC GCT TCA CGA-3′)
of U6 snRNA was used as a loading control. After washing with 2X
SSPE containing 1% sodium dodecyl sulfate (SDS), the membrane was
exposed to a PhosphorImager screen (Molecular Dynamics, Inc.,
Sunnyvale, CA, USA) for 20 min.
Fluorescent in situ hybridization
The oligonucleotide probe of piR-651 was synthesized
by Sangon Biotech, Co., Ltd. (Shanghai, China) according to the
following sequence: piR-651, 5′-GAC GCU UUC CAA GGC ACG-3′. Both
the 5′ and 3′ ends were modified using digoxigenin (DIG) (Life
Technologies). A control probe, 5′-AGC GUA UGG AAU UCA GAU CUC A-3′
was used as previously described (20).
In situ hybridization for piR-651 was
performed on fixed paraffin-embedded sections. Briefly, the samples
were fixed in 4% paraformaldehyde at 4°C overnight following
paraffin wax embedding and cut into 8-µm sections. After
xylene dewaxing, the samples were digested with 2 ng/ml proteinase
K in phosphate-buffered saline (PBS) buffer at 37°C for 10 min. The
samples were then hybridized with 0.5 µM piR-651 probes at
37°C for 24 h, and then washed with 4X saline sodium citrate (SSC)
containing 0.5% Tween-20 for 2 min at room temperature. Finally, an
ELF detection kit (Invitrogen) was used to detect green fluorescent
signals according to the manufacturer's instructions (Invitrogen).
4′,6-diamidino-2-phenylindole (DAPI; 1 µg/ml) (Invitrogen)
was used to stain the nuclei. The probe was visualized with DIG
combined with anti-DIG antibody conjugated to alkaline phosphatase
and the 5-bromo-4-chloro-3-indolyl-phosphate (BCIP)/nitro-blue
tetrazolium (NBT) kit (Invitrogen).
The tissue histology was shown by hematoxylin and
eosin (H&E) staining. The paraffin-embedded tissue samples were
prepared into 3-5-µm thick slices and were stained with
H&E (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China).
Cell culture and transfection
The A549 cell line was obtained from the American
Type Culture Collection (ATCC; Manassas, VA, USA). The experimental
and control vectors were constructed from the pcDNA3.1(t) plasmid
(pc3.1) (Invitrogen) and piRNAs. Briefly, the piR-651 sequence
5′-AGA GAG GGG CCC GUG CCU UGG AAA GCG UC-3′ was cloned into the
pc3.1 plasmid between the BamHI and EcoRI sites to
generate pc3.1-piR-651. The sequence 5′-CAG TAT TTT GTG TAG TAC
AA-3′ was used for the negative control to generate the
pc3.1-piR-control. Following vector construction, transfection was
performed using Lipofectamine™ 2000 (Invitrogen). The cells were
cultured in Dulbecco's modified Eagle's medium with 10% fetal
bovine serum (both from Gibco, Gaithersburg, MD, USA) at 37°C in an
incubator with 5% CO2. Following 24 h of transfection,
the cells were subjected to experimental analysis.
Colony formation assay
Colony formation was detected following
pc3.1-piR-651 transfection. For each group, the cells were plated
into 6-well plates at a density of 1×103 cells/well and
incubated for 2 weeks. After washing with PBS three times, the
cells were stained using 0.2% crystal violet (GE Healthcare Life
Sciences, Little Chalfont, UK) for 20 min at room temperature and
observed under an inverted microscope (Nikon Eclipse TS-100F;
Nikon, Tokyo, Japan).
Cell viability assay
Cell proliferation was assayed by
3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT)
kits (Invitrogen) according to the manufacturer's instructions.
Briefly, 2×103 cells/well were plated in 96-well plates.
After 24, 48, 72 or 96 h, MTT was added into the wells and plates
were incubated at 37°C for 2 h. Using a VMAX (Molecular Devices,
Sunnyvale, CA, USA), the absorbance at 570 and 690 nm (as
reference) was detected.
Flow cytometric analysis
To determine the effects of piR-651 on the cell
cycle, we performed flow cytometric analysis. Firstly, the cells
were fixed with 70% ethanol overnight. After resuspending the cells
with 50 µg/ml propidium iodide, the cells were treated with
0.5 µg/ml Annexin V-PE and 0.5 µg/ml
7-aminoactinomycin D (7-AAD) (BD Biosciences, Franklin Lakes, NJ,
USA) at room temperature for 15 min and assayed using a FACSCanto
flow cytometer (BD Biosciences). The signals of Annexin V, 7-AAD,
or both were detected and cell death was analyzed using
CellQuestPro software (BD Biosciences).
Western blot analysis
Tissues were homogenized using RIPA lysis buffer
(Beyotime, Wuhan, China). The total protein quantity of each sample
was analyzed using the bicinchoninic acid (BCA) method (Beyotime).
Protein samples (20 µg) were electrophoresed on a 12% SDS
polyacrylamide gel and the separated proteins were transferred onto
polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA).
The membranes were blocked using 4% skim milk for 1 h at room
temperature. Primary monoclonal antibodies (antibodies and
dilutions are listed in Table
II) were purchased from Santa Cruz Biotechnology, Inc., (Santa
Cruz, CA, USA) and were incubated with the membranes at 4°C
overnight. GAPDH was used as a loading control. After three washes
with TBST buffer (20 mM Tris-HCl, pH 7.6/137 mM NaCl/0.5%
Tween-20), the specific protein was detected by a secondary
horseradish peroxidase-conjugated goat anti-rabbit antibody
(1:2,000; Santa-Cruz Biotechnology, Inc., Heidelberg, Germany).
After three washes with TBST buffer, the signals were visualized
using an enhanced chemiluminescence detection system
(Millipore).
 | Table IIAntibodies and dilutions used in the
present study. |
Table II
Antibodies and dilutions used in the
present study.
| Protein name | Antibody product
code | Dilution
|
|---|
| Western blot |
Immunohistochemistry |
|---|
| Cyclin D1 | SAB4502603 | 1:500 | 1:100 |
| CDK4 | SAB4300695 | 1:500 | 1:100 |
| GAPDH | SAB2100894 | 1:1,000 | – |
Immunohistochemical analysis
Firstly, 8-µm-thick tissue sections were
prepared. The sections were dewaxed and blocked with 4% skim milk.
Following incubation with a monoclonal antibody (antibodies and
dilutions are listed in Table
II) at 4°C overnight and washing with TBST buffer, the sections
were processed with a secondary horseradish peroxidase
(HRP)-conjugated goat anti-rabbit antibody (1:200). The specific
protein was stained using HRP-conjugated streptavidin (Beyotime)
and exposed on photosensitive film (Kodak, Rochester, NY, USA) or
with the VersaDoc 3000 imaging system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Tumor formation in a nude mouse
model
Five-week-old female nude mice were used for the
tumor formation experiment. The mice were housed under specific
pathogen-free conditions. All animal protocols were approved and
monitored by the Ethics Committee of Xiangya School of Medicine,
Central South University (Changsha, China). The A549 cells
transfected with pc3.1-piR-651 and pc3.1-piR-control were
separately inoculated (2×106 cells) into the dorsal side
of the mouse. The tumor volumes were measured and calculated using
the equation V=0.5× (width × width × length). The mice were
sacrificed 35 days after cell inoculation by dislocation of the
cervical vertebrae and the tumor tissues were collected
subsequently. The tissues were assayed using RT-qPCR, western blot
analysis and immunohistochemistry.
Statistical analysis
All statistical analysis was performed using SPSS
v17.0 software. The significant difference among the groups was
confirmed by one-way analysis of variance. The correlation of the
associations between the expression of piRNAs and various
clinicopathologic parameters was calculated according to Spearman.
A P-value <0.05 was considered to indicate a statistically
significant difference. The Kaplan-Meier survival curves were
generated and analyzed with log rank tests with 95% confidence
intervals. For the Kaplan-Meier survival analysis over 24 months,
50 patients with relative expression between 1 and 3, as the low
expression group, and 50 patients with relative expression between
5 and 6, as the high expression group, were included.
Results
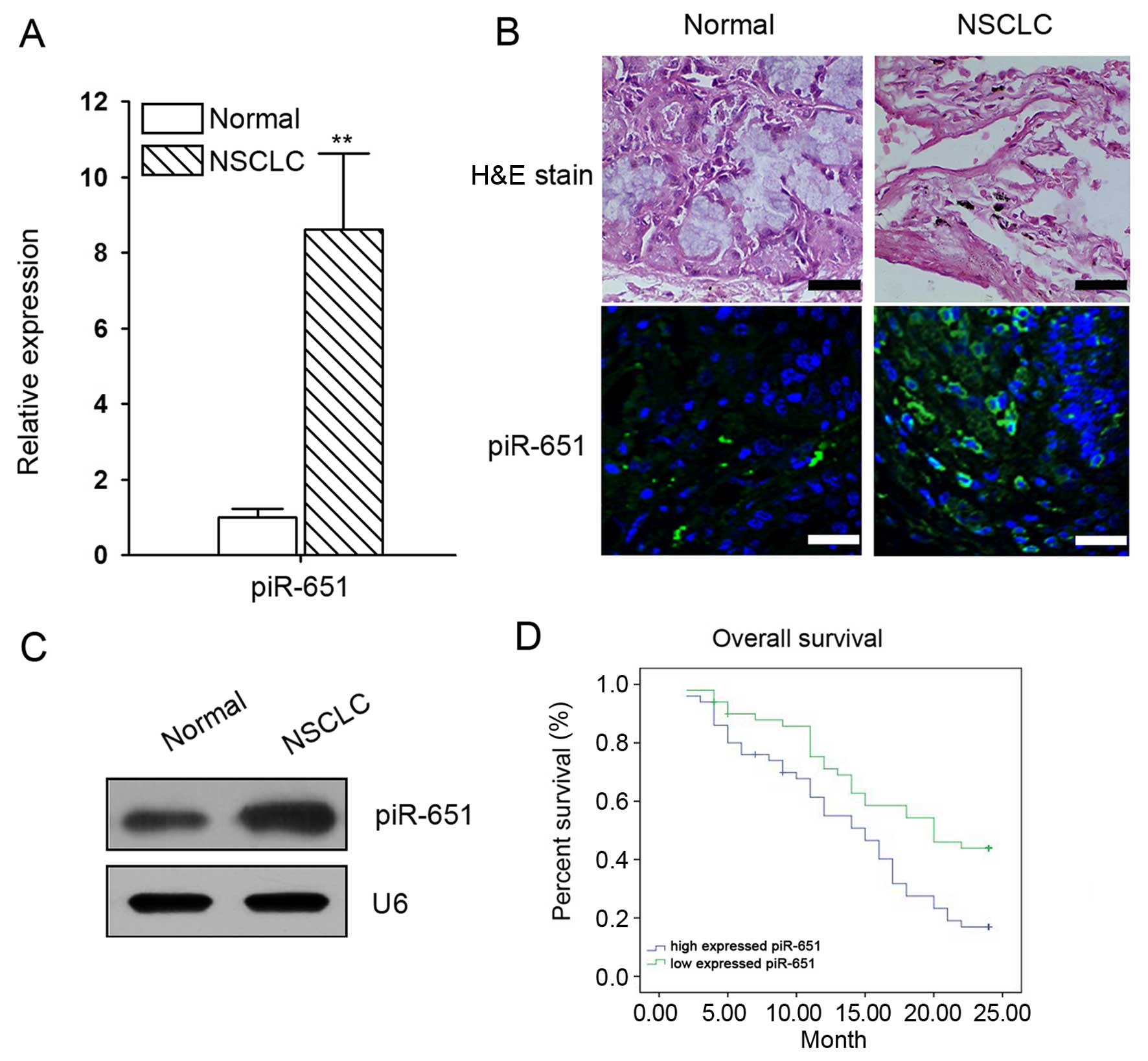
Aberrant expression of piR-651 in
NSCLC
The mRNA expression of piR-651 in normal and NSCLC
tissues was determined by RT-qPCR, northern blot analysis and in
situ hybridization. The results showed that piR-651 expression
in the cancer lesions of NSCLC patients was significantly higher
than that in the adjacent normal tissues (Fig. 1A–C). Furthermore, the upregulation
of piR-651 was associated with distal metastasis and disease
recurrence (Table III). The
Kaplan-Meier survival curves showed that increased piR-651
expression inversely correlated with overall survival (Fig. 1D).
 | Table IIIAnalysis of the correlation between
piR-651 expression in NSCLC tissues and the patient
clinicopathological characteristics. |
Table III
Analysis of the correlation between
piR-651 expression in NSCLC tissues and the patient
clinicopathological characteristics.
| Variable | N | Median piR-651
expression (range) | P-value
(piR-651) |
|---|
| Age (years) |
| ≤50 | 36 | 5.60
(0.89–12.42) | 0.89 |
| >50 | 42 | 4.41
(0.74–10.78) | |
| Gender |
| Male | 56 | 6.02
(0.89–10.78) | 0.45 |
| Female | 22 | 4.26
(0.74–12.42) | |
| T stage |
| T1/T2 | 52 | 3.18
(0.74–7.65) | 0.03 |
| T3/4 | 26 | 7.62
(3.62–12.42) | |
| Histologic
grade |
| I–II | 55 | 2.23
(0.74–4.69) | 0.02 |
| III–IV | 23 | 8.07
(4.54–12.42) | |
| Metastasis |
| No (M0) | 50 | 2.53
(0.74–4.49) | 0.01 |
| Yes (M1) | 28 | 9.62
(4.48–12.42) | |
| Tumor size |
| ≤5 cm | 63 | 3.88
(0.74–5.47) | 0.01 |
| >5 cm | 15 | 8.69
(4.49–12.42) | |
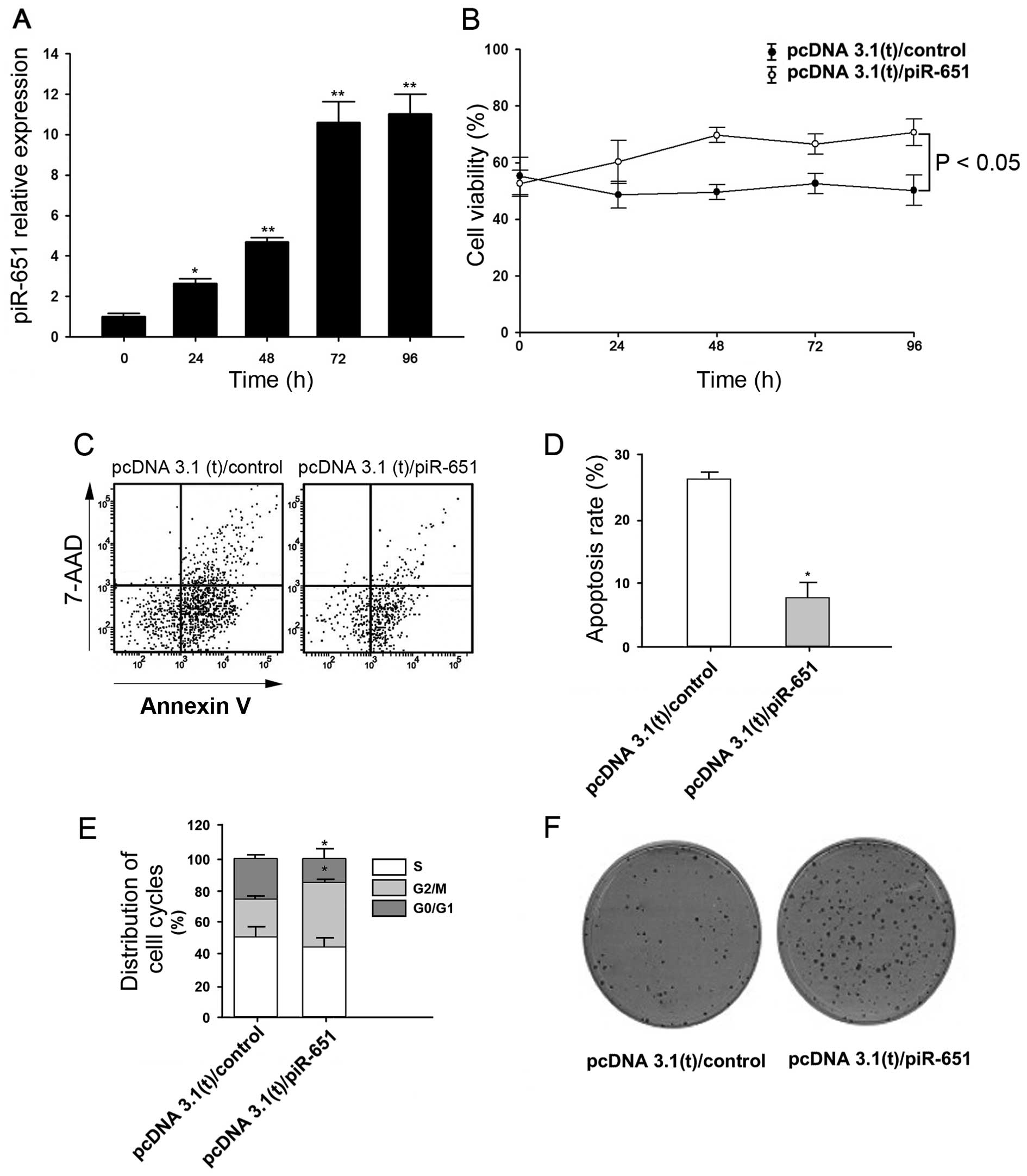
Effects of piR-651 on NSCLC cells
We then analyzed the effects of piR-651 upregulation
in NSCLC cells. Firstly, we confirmed that piR-651 expression was
increased in the cells transfected with a plasmid expressing
piR-651 (Fig. 2A). We then
studied cell viability using MTT assays. Cell viability increased
significantly following piR-651 overexpression compared with the
controls (P<0.05 after 96 h) (Fig.
2B). Additionally, flow cytometry showed that the cells
overexpressing piR-651 exhibited significantly reduced levels of
apoptosis compared with the controls (Fig. 2C and D). piR-651-overexpressing
cells displayed a significantly lower percentage of cells in the
G0/G1 phase and significantly higher percentage of cells in the
G2/M phase (Fig. 2E), indicating
that piR-651 overexpression promotes cell cycle progression. We
also found that piR-651-overexpressing cells had a stronger ability
to form colonies (Fig. 2F).
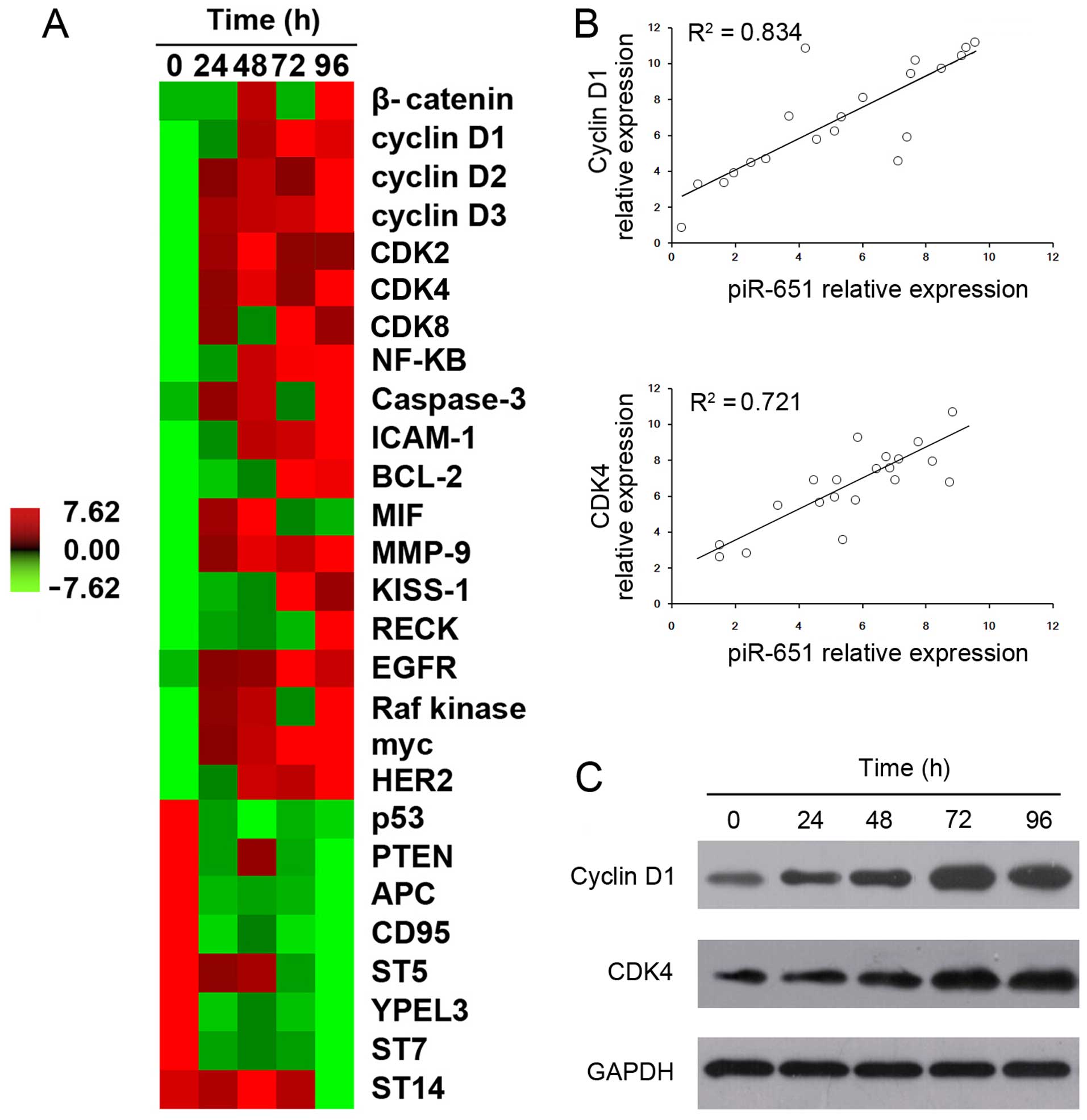
Correlation between piR-651 expression
and cyclin D1 and CDK4 expression in NSCLC cells
To elucidate the mechanism underlying the effects of
piR-651 in NSCLC cells, the expression patterns of lung
cancer-related genes were examined after overexpression of piR-651.
The cancer-promoting genes induced by piR-651 overexpression
included β-catenin, cyclin D, CDK, NF-κB, ICAM and MIF. Similarly,
several oncogenes, including cyclin D, CDK, MMP-9, KISS-1, RECK,
EGFR, Raf kinase, myc and HER2, showed significantly higher
expression after piR-651 overexpression. By contrast, the
expression levels of cancer suppressor genes, such as p53, PTEN,
APC, CD95, ST5, YPEL3, ST7 and ST14, were decreased with higher
expression of piR-651 (Fig.
3A).
Of all the examined genes, only cyclin D1 and CDK4
showed a high correlation with piR-651 (R2>0.5 and
P<0.05). Cyclin D1 expression positively correlated with piR-651
expression (R2=0.834 and P<0.05) (Fig. 3B). Similarly, CDK4 expression
positively correlated with piR-651 expression (R2=0.721
and P<0.05) (Fig. 3B). Western
blot analysis also revealed that the protein expression of cyclin
D1 and CDK4 increased following the upregulation of piR-651
(Fig. 3C).
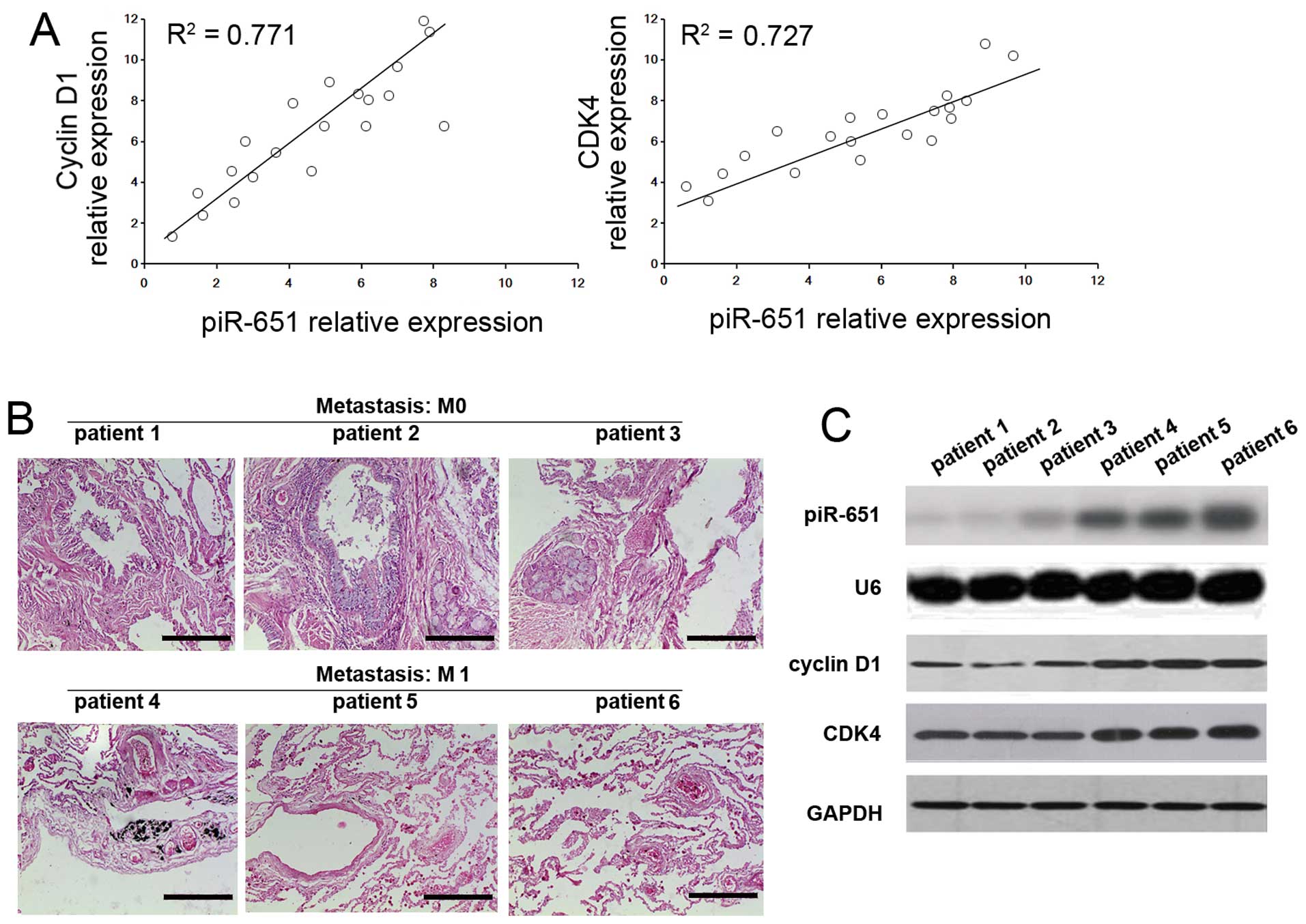
Correlation between piR-651 expression
and cyclin D1 and CDK4 expression in NSCLC tissues
A positive correlation (R2=0.771 and
P<0.05) between piR-651 expression and cyclin D1 was observed in
the NSCLC tissues from patients. Moreover a similar positive
correlation between piR-651 expression and CDK4
(R2=0.727 and P<0.05) was also shown in these tissues
(Fig. 4A). To determine whether
piR-651, cyclin D1 and CDK4 are involved in metastasis, the
expression levels from patients at the M0 and M1 stage (n=3) were
assayed (Fig. 4B). The expression
levels of piR-651, cyclin D1 and CDK4 were higher in the M1 stage
patients compared with the M0 stage patients (Fig. 4C), indicating that increased
levels of piR-651 induced cyclin D1 and CDK4 and thus, promoted
metastasis.
piR-651 promotes tumor growth in
vivo
In order to further examine the relevance of our
in vitro findings with regard to NSCLC tumorigenesis in
vivo, we injected A549 cells transfected with control sequences
or piR-651 plasmids into nude mice to generate xenograft models and
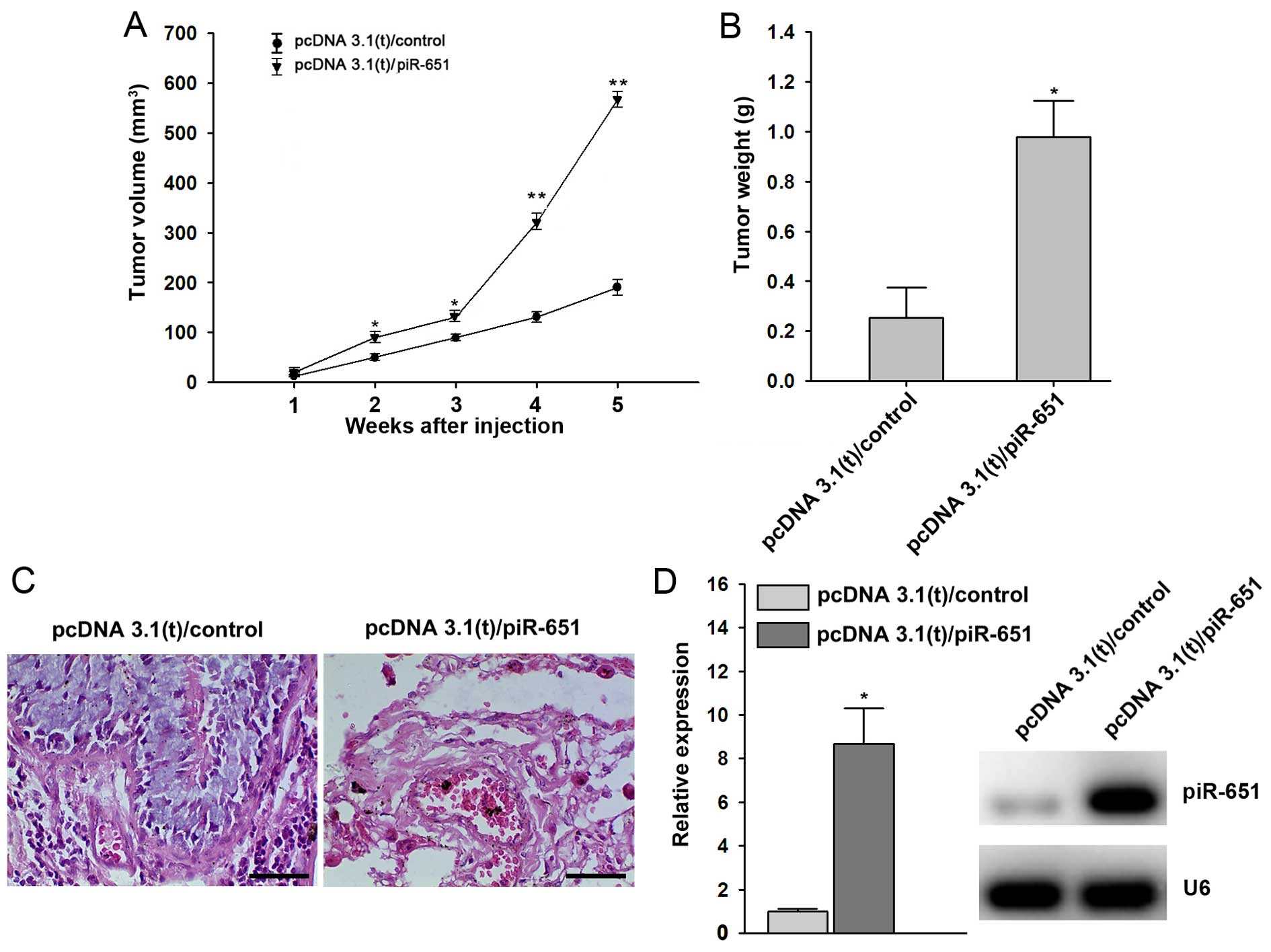
monitored tumor growth. In tumors injected with
piR-651-overexpressing cells, the tumor volume and tumor weight
were higher compared with the controls (P<0.05 after 5 weeks)
(Fig. 5A and B), which was
consistent with our in vitro findings. The tissue sections
also showed that piR-651 triggered NSCLC tumorigenesis in
vivo (Fig. 5C). At 5 weeks
after cell implantation, higher expression of piR-651 was observed
in tumor tissues evaluated by RT-qPCR and northern blot analysis
(Fig. 5D).
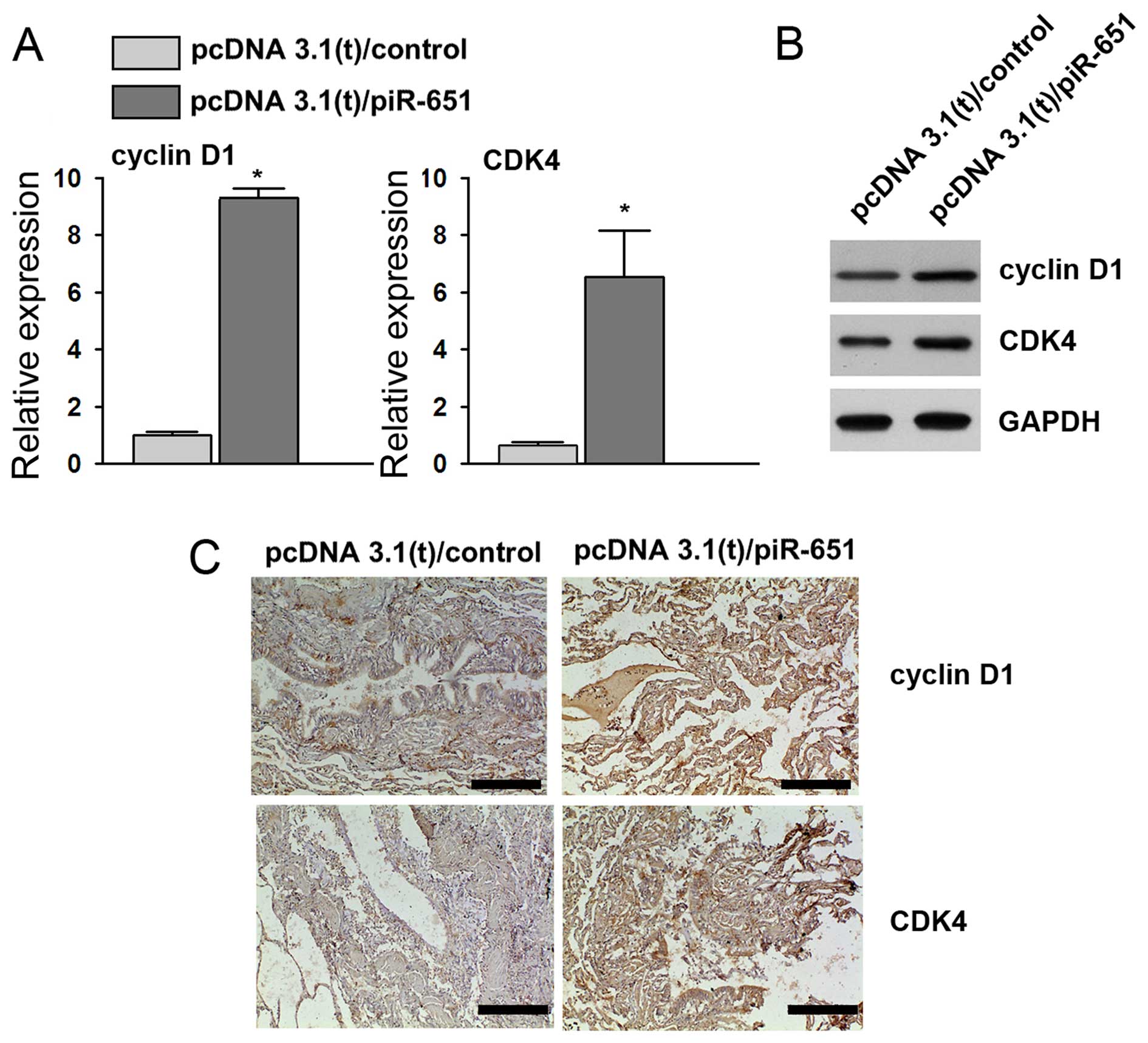
Consistent with our results described above, the
overexpression of piR-651 led to an increased level of cyclin D1
and CDK4 in the xenograft tumor tissues (Fig. 6). Thus, these results from the
nude mouse xenograft model demonstrate that piR-651 promotes NSCLC
tumorigenesis in vivo through the cyclin D1 and CDK4
pathway.
Discussion
A novel class of small RNAs, referred to as piRNAs,
have been identified in mouse germline cells (23,24). Notably, it has been demonstrated
that the overexpression of PIWI and piRNAs results in the abnormal
development of gonads in vertebrates (25,26). In humans, the increased expression
of Hiwi, a homolog of Piwil1, was found to correlate with the risk
of cancer-related death in the pancreas of male individuals
(27). A previous study has
suggested that piRNAs were highly expressed in gastric cancer
tissues (17). These findings
have demonstrated that piRNAs are potential diagnostic indicators
of gastric cancer. In the present study, our findings confirmed
that piR-651 participates in the development of NSCLC.
The present study demonstrated high piR-651
expression in patients with NSCLC. The overall survival curves
suggested that the high expression of piR-651 was associated with a
higher risk of death. These results are similar to the findings
from a study of gastric cancer showing that piR-651 was upregulated
in gastric cancer. In addition, by inhibiting piR-651, gastric
cancer cells were suppressed, which to the best of our knowledge,
was the first demonstration of a novel class of small RNAs capable
of regulating the progression of cancer (20). Based on the observations of
similar expression levels of piR-651 in patients with gastric
cancer and NSCLC, the regulatory mechanisms controlled by these
piRNAs need to be elucidated.
Our results showed that following the overexpression
of piR-651 in NSCLC cells, cell viability was significantly
increased. A previous study has demonstrated that upregulating
piR-651 may promote cancer metastasis in gastric cell lines and
downregulating piR-651 arrested the cells in the G2/M phase
(20). However, current
information regarding the effects of piR-651 on the activities of
cancer cells is limited. Whether the effects of piR-651 on cancer
cells are specific to different types of cancer remains unknown.
Our findings suggest that piR-651 exhibits similar effects in NSCLC
as in gastric cancer cells in terms of promoting cell viability. In
addition, upregulating piR-651 in NSCLC cells significantly reduced
the number of cells in the G0/G1 phase and increased the number of
cells in the G2/M phase, indicating a regulatory function for
piR-651 in cell proliferation.
Our results showed that the expression of oncogenes
was induced and that of the cancer suppressor genes was reduced by
piR-651. To the best of our knowledge, this is the first
demonstration that a single piRNA is capable of regulating the
expression of a wide panel of cancer-related genes. Furthermore,
the correlation between piR-651 expression and the genes was
analyzed. Notably, in vivo and in vitro studies
showed that only cyclin D1 and CDK4 positively correlated with
piR-651 expression. Cyclin D1 and CDK4 drive cell cycle progression
through the G1 checkpoint. The upregulation of cyclin D1 and CDK4
promoted cell cycle progression, which led to cell proliferation.
Thus, our findings revealed that piR-651 promotes the cell cycle by
regulating cyclin D1 and CDK4.
The mechanism of gene regulation by miRNAs has been
intensively investigated. However, as a novel class of non-coding,
small RNAs, the regulation of genes by piRNAs remains unclear.
Robine et al have reported the existence of a 3′
untranslated region (3′UTR) conserved pathway of piRNAs, suggesting
a similar transcriptional regulating function for piRNAs as for
miRNAs by acting on the 3′UTR (28). Nevertheless, there is no
evidence for any direct binding between piRNAs and transcripts. The
molecular mechanisms underlying the regulatory effects of piR-651
on cyclin D1 and CDK4 remain unknown and warrant further
investigation.
Finally, our results demonstrated that the injection
of piR-651-transfected cells significantly promoted increases in
tumor volume and tumor weight. The overexpression of piR-651 lasted
for 5 weeks. Thus, we propose that piR-651 may function as an
oncogene in NSCLC. Previous studies have demonstrated a role for
piR-651 in promoting tumorigenesis. However, whether single piRNAs
play a direct role in different types of cancer is currently
unknown. Notably, upregulation of cyclin D1 and CKD4 was found in
the group injected with piR-651-transfected cells, which confirmed
the function of piR-651 as a modulator of the cell cycle. Taken
together, this evidence indicates that piRNAs may play a crucial
role in human disease.
In conclusion, the present study has demonstrated
that piR-651 is associated with the progression of NSCLC. The high
expression of piR-651 was associated with a higher risk of death in
patients with NSCLC. The viability and cell cycle progression of
NSCLC cells were also regulated by piR-651. The expression levels
of cyclin D1 correlated with piR-651 expression. In addition, tumor
formation was promoted by injecting nude mice with
piR-651-overexpressing A549 cells. Our findings suggest that
piR-651 may have applications as a potential diagnostic indicator
and therapeutic target in the management of NSCLC.
Acknowledgments
This study was supported by the Hunan Provincial
Natural Science Fund of China (grant no. 13JJ6092).
References
|
1
|
Goldstraw P, Ball D, Jett JR, Le Chevalier
T, Lim E, Nicholson AG and Shepherd FA: Non-small-cell lung cancer.
Lancet. 378:1727–1740. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Suntharalingam M, Paulus R, Edelman MJ,
Krasna M, Burrows W, Gore E, Wilson LD and Choy H: Radiation
therapy oncology group protocol 02-29: a phase II trial of
neoadjuvant therapy with concurrent chemotherapy and full-dose
radiation therapy followed by surgical resection and consolidative
therapy for locally advanced non-small cell carcinoma of the lung.
Int J Radiat Oncol Biol Phys. 84:456–463. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharma S, Kelly TK and Jones PA:
Epigenetics in cancer. Carcinogenesis. 31:27–36. 2010. View Article : Google Scholar :
|
|
4
|
Sandoval J and Esteller M: Cancer
epigenomics: beyond genomics. Curr Opin Genet Dev. 22:50–55. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schlesinger F, Smith AD, Gingeras TR,
Hannon GJ and Hodges E: De novo DNA demethylation and noncoding
transcription define active intergenic regulatory elements. Genome
Res. 23:1601–1614. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lopez-Serra P and Esteller M: DNA
methylation-associated silencing of tumor-suppressor microRNAs in
cancer. Oncogene. 31:1609–1622. 2012. View Article : Google Scholar :
|
|
7
|
Levin HL and Moran JV: Dynamic
interactions between transposable elements and their hosts. Nat Rev
Genet. 12:615–627. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chénais B: Transposable elements and human
cancer: a causal relationship? Biochim Biophys Acta. 1835:28–35.
2013.
|
|
9
|
Senti KA and Brennecke J: The piRNA
pathway: a fly's perspective on the guardian of the genome. Trends
Genet. 26:499–509. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ambros V and Chen X: The regulation of
genes and genomes by small RNAs. Development. 134:1635–1641. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Houwing S, Kamminga LM, Berezikov E,
Cronembold D, Girard A, van den Elst H, Filippov DV, Blaser H, Raz
E, Moens CB, et al: A role for Piwi and piRNAs in germ cell
maintenance and transposon silencing in Zebrafish. Cell. 129:69–82.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aravin AA, Sachidanandam R, Girard A,
Fejes-Toth K and Hannon GJ: Developmentally regulated piRNA
clusters implicate MILI in transposon control. Science.
316:744–747. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seto AG, Kingston RE and Lau NC: The
coming of age for Piwi proteins. Mol Cell. 26:603–609. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan Z, Hu HY, Jiang X, Maierhofer V, Neb
E, He L, Hu Y, Hu H, Li N, Chen W and Khaitovich P: Widespread
expression of piRNA-like molecules in somatic tissues. Nucleic
Acids Res. 39:6596–6607. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Siddiqi S and Matushansky I: Piwis and
piwi-interacting RNAs in the epigenetics of cancer. J Cell Biochem.
113:373–380. 2012. View Article : Google Scholar
|
|
16
|
Li L, Yu C, Gao H and Li Y: Argonaute
proteins: potential biomarkers for human colon cancer. BMC Cancer.
10:382010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cui L, Lou Y, Zhang X, Zhou H, Deng H,
Song H, Yu X, Xiao B, Wang W and Guo J: Detection of circulating
tumor cells in peripheral blood from patients with gastric cancer
using piRNAs as markers. Clin Biochem. 44:1050–1057. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Al-Janabi O, Wach S, Nolte E, Weigelt K,
Rau TT, Stöhr C, Legal W, Schick S, Greither T, Hartmann A, et al:
Piwi-like 1 and 4 gene transcript levels are associated with
clinicopathological parameters in renal cell carcinomas. Biochim
Biophys Acta. 1842:686–690. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ye Y, Yin DT, Chen L, Zhou Q, Shen R, He
G, Yan Q, Tong Z, Issekutz AC, Shapiro CL, et al: Identification of
Piwil2-like (PL2L) proteins that promote tumorigenesis. PLoS One5.
e134062010. View Article : Google Scholar
|
|
20
|
Cheng J, Guo JM, Xiao B-X, Miao Y, Jiang
Z, Zhou H and Li QN: piRNA, the new non-coding RNA, is aberrantly
expressed in human cancer cells. Clin Chim Acta. 412:1621–1625.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng J, Deng H, Xiao B, Zhou H, Zhou F,
Shen Z and Guo J: piR-823, a novel non-coding small RNA,
demonstrates in vitro and in vivo tumor suppressive activity in
human gastric cancer cells. Cancer Lett. 315:12–17. 2012.
View Article : Google Scholar
|
|
22
|
Brambilla E, Travis WD, Colby TV, Corrinz
B and Shimosato Y: The new World Health Organization classification
of lung tumours. Eur Respir J. 18:1059–1068. 2001. View Article : Google Scholar
|
|
23
|
Girard A, Sachidanandam R, Hannon GJ and
Carmell MA: A germline-specific class of small RNAs binds mammalian
Piwi proteins. Nature. 442:199–202. 2006.PubMed/NCBI
|
|
24
|
Aravin A, Gaidatzis D, Pfeffer S,
Lagos-Quintana M, Landgraf P, Iovino N, Morris P, Brownstein MJ,
Kuramochi-Miyagawa S, Nakano T, et al: A novel class of small RNAs
bind to MILI protein in mouse testes. Nature. 442:203–207.
2006.PubMed/NCBI
|
|
25
|
Houwing S, Berezikov E and Ketting RF:
Zili is required for germ cell differentiation and meiosis in
zebrafish. EMBO J. 27:2702–2711. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pan Y, Hu M, Liang H, Wang JJ and Tang LJ:
The expression of the PIWI family members miwi and mili in mice
testis is negatively affected by estrogen. Cell Tissue Res.
350:177–181. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grochola LF, Greither T, Taubert H, Möller
P, Knippschild U, Udelnow A, Henne-Bruns D and Würl P: The stem
cell-associated Hiwi gene in human adenocarcinoma of the pancreas:
expression and risk of tumour-related death. Br J Cancer.
99:1083–1088. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Robine N, Lau NC, Balla S, Jin Z, Okamura
K, Kuramochi Miyagawa S, Blower MD and Lai EC: A broadly conserved
pathway generates 3′UTR-directed primary piRNAs. Curr Biol.
19:2066–2076. 2009. View Article : Google Scholar : PubMed/NCBI
|