Introduction
Nuclear receptor subfamily 0 group B member 1
(Nr0b1) is an orphan nuclear receptor expressed in the
ventromedial hypothalamus, pituitary gonadotropes, the adrenal
cortex, the testis and the ovary (1,2).
The duplication of a region of the X-chromosome spanning
NR0B1 results in dosage sensitive, male-to-female sex
reversal (3). Mutations of
NR0B1 cause an X-linked form of adrenal hypoplasia congenita
in males, who usually present with an adrenal crisis during the
first year of life (4). The
disorder is limited to males and is characterized by neonatal
adrenal insufficiency and failure to undergo puberty as a result of
hypogonadotropic hypogonadism. It has been demonstrated that the
targeted disruption of Nr0b1 in male mice results in
infertility, decreased testicular size, and degeneration of
germinal epithelium (5). A
thorough histological examination of the male reproductive tract
from Nr0b1 knockout (Nr0b1-KO) mice revealed that the
rete testis, the passageway for sperm to leave the testis, is
obstructed by ectopic clusters of Sertoli cells (SCs) (6). Despite these findings, the function
of Nr0b1 in the male reproductive system remains
unclear.
Androgen and its receptor, androgen receptor (AR),
play important roles in spermatogenesis and male fertility
(7–9). The AR belongs to a family of nuclear
transcription factors that mediate the action of androgens. It
contains an N-terminal transactivation domain, a central
DNA-binding domain (DBD), and a C-terminal ligand-binding domain
(LBD). Cytoplasmic AR, when bound by androgens, translocates to the
nucleus and binds to the androgen response elements (AREs) on
target genes. Testicular AR has been detected in Leydig cells,
peritubular cells and SCs (10).
Androgens affect spermatogenesis indirectly through AR-expressing
somatic cells, such as SCs or peritubular myoid cells (10). AR mutations cause a spectrum of
hereditary disorders, including androgen insensitivity syndrome and
male infertility (11).
Furthermore, male total AR-KO mice exhibited a typical female
external appearance, which was similar to a human
androgen-insensitivity syndrome or testicular feminization mutation
in mice (12).
In light of accumulating data regarding the function
and expression of NR0B1, we became interested in studying the
possible interactions between NR0B1 and AR. The AR is a member of
the steroid hormone receptor branch of the nuclear receptor
superfamily and, as a mediator of androgen signaling, it plays
important roles in coordinating gene expression in male
reproductive tissues (13–16).
A number of distinct co-regulatory factors are involved in the
regulation of AR signaling (17).
Although many of these factors function as bona fide co-activators
or co-repressors by directly communicating with chromatin and the
transcription machinery, additional co-regulators may exist that
function in an antagonistic manner by preventing, disrupting or
redirecting interactions with bona fide co-activators and
co-repressors.
In the present study, we have identified NR0B1 as an
inhibitory co-regulator of AR. To the best of our knowledge, we
have provided evidence of previously uncovered aspects of the
mechanisms of action of NR0B1 in mouse SCs. The data strongly
suggest that NR0B1 antagonism plays a physiological role in
modulating AR-dependent gene regulation in male reproductive
tissues.
Materials and methods
Reagents and medium
Rabbit polyclonal anti-NR0B1 antibody (ab60144),
anti-AR antibody (ab74272) and mouse monoclonal anti-GAPDH antibody
(ab8245) were purchased from Abcam (Cambridge, UK). Anti-HA
antibody (H3663) was purchased from Sigma-Aldrich (St. Louis, MO,
USA). Anti-SOX9 antibody was purchased from Millipore Corp.
(Billerica, MA, USA). Anti-rabbit-Cy3, anti-rabbit-Alexa Fluor 488,
anti-mouse-Cy3 and anti-mouse-Alexa Fluor 488 were purchased from
Invitrogen (Carlsbad, CA, USA). All restriction endonucleases were
purchased from New England BioLabs, Inc. (Ipswich, MA, USA). The
Dual-Luciferase reporter assay system and the pGL4.15 plasmid,
which was used to construct two recombinant plasmids
[pUBE2B(−882/−343)-LUC and pHSF1(−625/−390)-LUC], were obtained
from Promega Corp. (Madison, WI, USA). Lipofectamine 2000 was
obtained from Invitrogen.
Animals and tissue collection
One hundred mice (C57BL/6) were purchased from the
Southern Medical University Animal Center (Guangzhou, China). All
animals were treated according to the National Research Council
Guide for the Care and Use of Laboratory Animals. The study was
approved by the Ethics Committee of Peking University Shenzhen
Hospital (Shenzhen, China).
The mouse testes were individually collected from
the mice at 1, 2, 3, 4, 6 and 8 weeks, and 6 months of age after
sacrifice by cervical dislocation. Five mice were sacrificed in
each group at each time-point. Other organs, namely the brain,
heart, lung, liver, kidney, spleen, epididymis and bladder, were
obtained from adult mice at 8 weeks of age.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from mouse tissues and TM4
cells using TRIzol (Invitrogen) according to the manufacturer's
instructions. First strand cDNA was synthesized using oligo(dT)
primers (K1622; Fermentas, Waltham, MA, USA). The primers specific
for mouse Nr0b1 were 5′-CACAGAGCAGCCACAGATG-3′ (forward),
and 5′-AATGTTCAGACTCCAGCACTTG-3′ (reverse). The primers for Ube2b
were 5′-GCAGCTGCGGAGCATGTCGA-3′ (forward), and
5′-CAGATGGGGCGCCACTGACC-3′ (reverse); The Hsf1 primers were
5′-CTGGTCCGTGTCAAGCAA-3′ (forward), and 5′-GGCTACGCTGAGGCACTT-3′
(reverse). Mouse Gapdh was used as an internal control and
the primers for Gapdh were 5′-AGTGGCAAAGTGGAGATT-3′
(forward), and 5′-GTGGAGTCATACTGGAACA-3′ (reverse). RT-qPCR was
performed using the SYBR® Premix EX Taq™ II PCR kit
(RR820A; Takara, Dalian, China) according to the manufacturer's
instructions on the Roche LightCycler 480 Real-Time PCR system
(Mannheim, Germany). The data were calculated according to the
Applied Biosystems comparative (Ct) method (18).
Western blot analysis
The proteins were extracted from mouse tissues and
TM4 cells and separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
electrotransferred ontopolyvinylidene fluoride (PVDF) membranes
(Millipore Corp., Bedford, MA, USA). The membranes were blocked
with 10% (w/v) low-fat milk in TBST (20 mM Tris-HCl pH 7.5, 150 mM
NaCl, 0.1% Tween-20). Following incubation with the anti-NR0B1
antibody (1:1,000; #ab60144; Abcam), anti-UBE2B antibody (1:2,000;
#10733-1-AP; Proteintech, Chicago, IL, USA) and anti-HSF1 antibody
(1:2,000; #ABE1044; Millipore Corp.) overnight at 4°C, the
membranes were treated with HRP-labeled secondary antibody (ab6721;
Abcam) for 1 h at room temperature. Positive bands were detected
using an ECL kit (Thermo Fisher Scientific, Waltham, MA, USA).
Immunofluorescence analysis
The mouse testes were fixed in Tissue-Tek (Sakura
Finetek USA, Inc., Torrance, CA, USA) and sectioned with an
ultra-thin semiautomatic microtome (CUT4062; Leica Microsystems,
Bensheim, Germany). After being blocked in 10% goat serum,
incubated with anti-NR0B1 polyclonal antibody (1:50), anti-AR
antibody (1:50) and anti-SOX9 antibody (1:100) overnight at 4 °C,
the appropriate FITC- or TRITC-conjugated secondary antibodies were
used, and the slides were counterstained with Hoechst 33258 and
mounted with ProLong Gold antifade reagent (both from Invitrogen).
Primary antibody pre-incubated with neutralizing peptide was used
as a negative control. The results were observed under a laser
scanning confocal microscope (Zeiss, Oberkochen, Germany) and
analyzed using Image-Pro Plus 5.1 software.
Plasmid construction and cell
culture
The full length of Nr0b1 cDNA was amplified
by PCR with the primers, 5′-CCGGAATTCATGGCGGGTGAGGACCACC-3′
(forward), and 5′-CCGCTCGAGTCACAGCTTTGCACAGAGCATCTCC-3′ (reverse),
and then inserted into pCDNA3.1/HA plasmids using EcoR1 and
Xhol. The PCR products were cloned and sequenced. Three cell
lines, TM4, 15P1 and 293T, were obtained from the American Type
Culture Collection (ATCC; Manassas, VA, USA). The cells were
maintained in Dulbecco's modified Eagle's medium (DMEM; Life
Technologies, Rockville, MD, USA) supplemented with 10% fetal
bovine serum at 37°C in a humidified atmosphere with 5%
CO2.
RNA Interference
The siRNAs targeting mouse Nr0b1 (siNr0b1,
5′-UAUCUGAAAGGGACCGUGCTCTT-3′) were obtained from GenePharma Co.,
Ltd. (Shanghai, China). The TM4 cells were seeded in a 6-well plate
and transfected with 200-pmol pools of siNr0b1 using Lipofectamine
2000 (Invitrogen) according to the manufacturer's instructions.
Co-immunoprecipitation (Co-IP)
The cell cultures were prepared as described above.
NR0B1-pCDNA3.1/HA was transfected into 293T cells together with
AR-pCDNA3.1+ or the vector control using Lipofectamine
2000 transfection reagent according to the manufacturer's
instructions. The cells were harvested 48 h after transfection,
washed with TBS (50 mM Tris-HCl (pH 7.4) and 150 mM NaCl), and then
the cells were lysed on ice for 30 min with lysis buffer [50 mM
Tris-HCl (pH 7.4), 2 mM EGTA or CaCl2, 150 mM NaCl, 1%
(v/v) NP-40, protease inhibitor cocktail (P8340; Sigma-Aldrich),
and 1 mM PMSF]. The lysates were centrifuged at 13,000 × g for 15
min at 4°C. The supernatants were incubated with nProtein A
Sepharose 4 Fast Flow (GE Healthcare, Little Chalfont, UK)
pre-bound with anti-HA or anti-AR antibody at 4°C overnight with
gentle rotation. The beads were washed four times with lysis buffer
and prepared with SDS-PAGE sample buffer for western blot
analysis.
Luciferase assay
Two mouse SC lines, TM4 (6×104
cells/well) and 15P1 (8×104 cells/well), were seeded
into a 24-well plate and transfected with 200 ng
AR-pCDNA3.1+, 200 ng pMMTV-LUC or pUBE2B(−882/−343)-LUC
or pHSF1(−625/−390)-LUC reporter, and increasing amounts (50, 100,
200 and 400 ng) of NR0B1-pCDNA3.1/HA. The cells were treated with
10 nM testosterone (T; Sigma-Aldrich) or ethanol vehicle for 24 h
prior to performing the luciferase assay. Renilla and
firefly luciferase activities were measured using the
Dual-Luciferase reporter assay system. Firefly luciferase data were
normalized to Renilla luciferase data. After normalization
for transfection efficiency, the induction factors were calculated
as ratios of the average value of the luciferase value of the
T-stimulated samples vs. ethanol vehicle-treated samples.
Statistical analysis
All experiments were repeated at least three times.
Statistical analysis was calculated using GraphPad Prism version
5.0 (GraphPad Software Inc., San Diego, CA, USA). The data values
are expressed as the means ± SD. The Student's t-test was used to
compare the difference between two groups. A P-value <0.05 was
considered to indicate a statistically significant difference.
Results
Expression of NR0B1 in different mouse
tissues
To assess the expression pattern of NR0B1 in adult
mouse tissues, NR0B1 expression was examined in different mouse
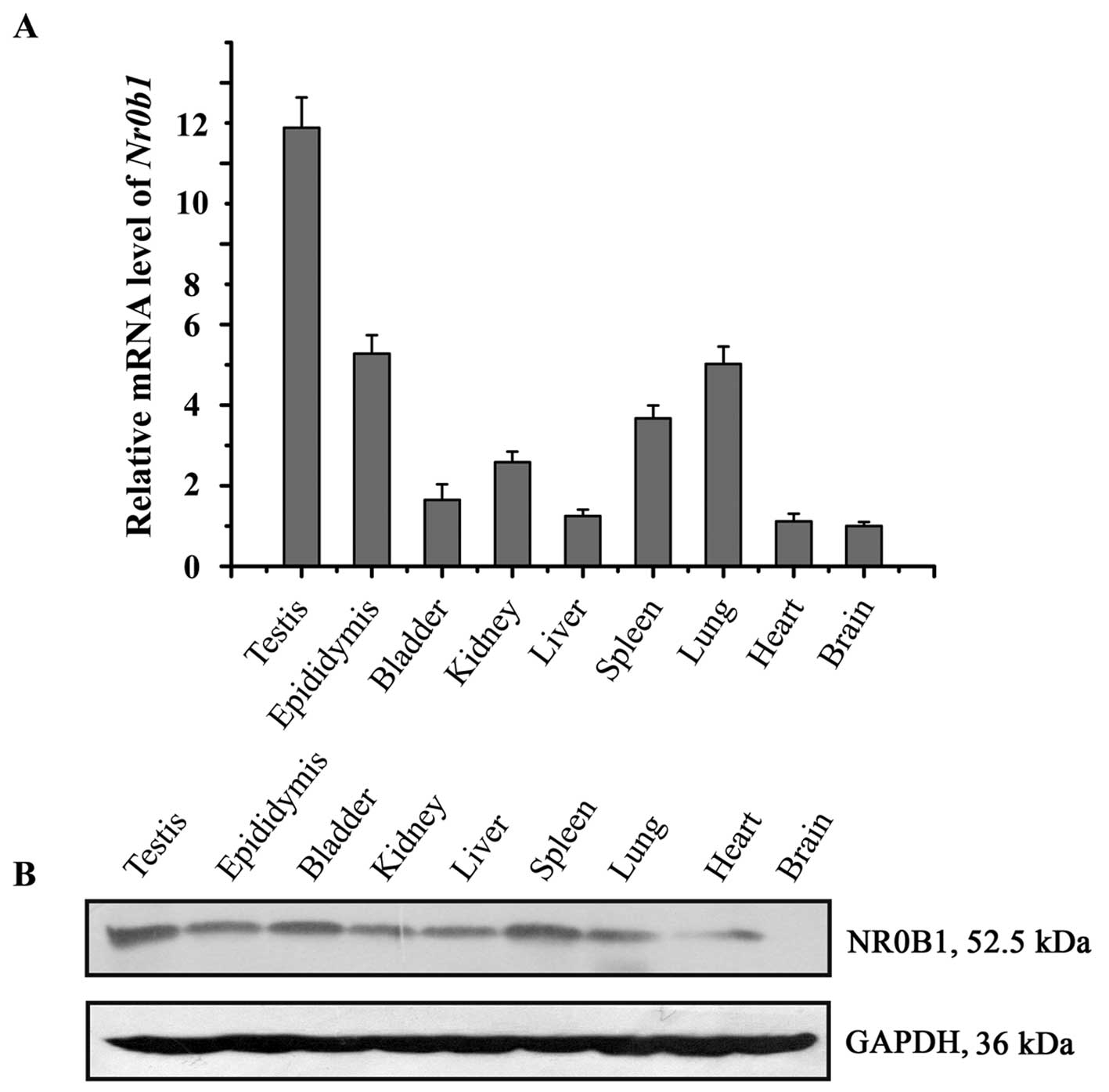
tissues using RT-qPCR and western blot analysis. The results showed
that Nr0b1 mRNA and its protein were highly expressed in the
testes compared with that in the other tissues (Fig. 1).
Temporal expression profile of NR0B1
during mouse testicular development
To detect the expression of NR0B1 during testicular
development, RT-qPCR and immunofluorescence analysis were
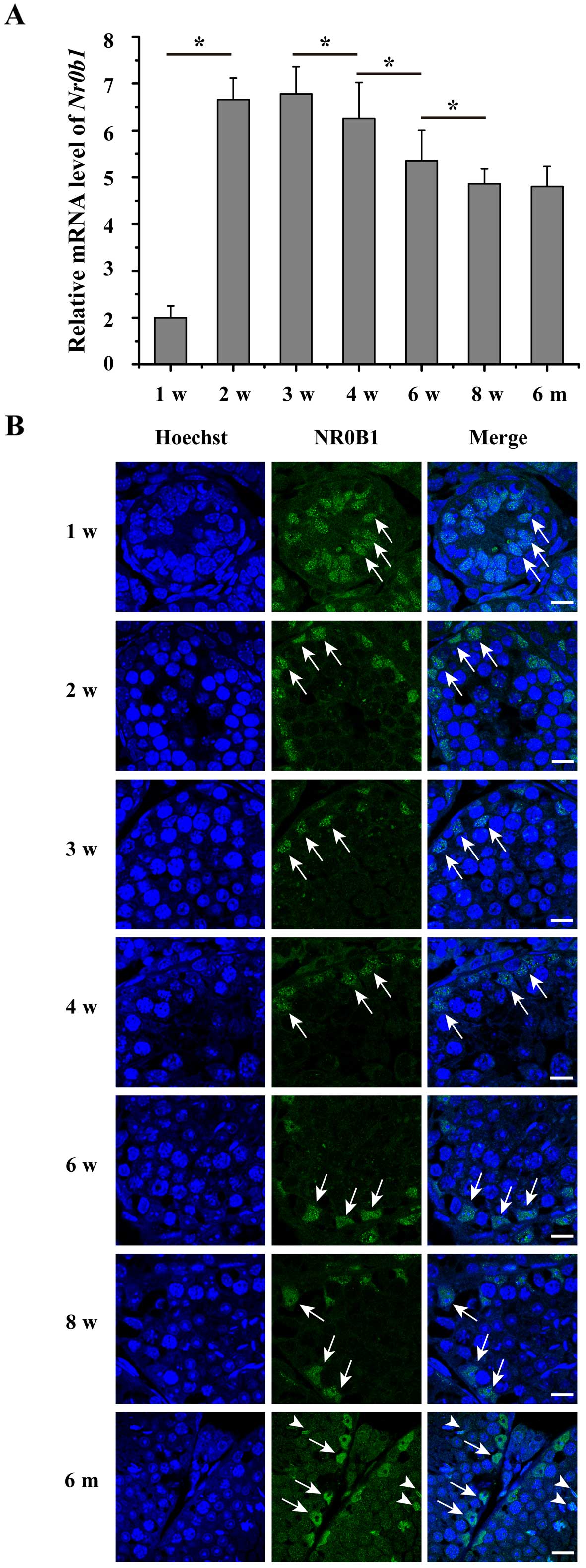
performed. The results showed that NR0B1 was expressed in the
testes during all the stages and decreased in a time-dependent
manner from 2–8 weeks postnatally (Fig. 2A). As shown in Fig. 2B, NR0B1 was mainly located in the
SCs at weeks 1–8, and was also expressed in the spermatids at 6
months.
Co-location of NR0B1 and AR in mouse
SCs
Previous studies have established that NR0B1
expression is associated with testis cord development in mice
(19,20). To further test the hypothesis that
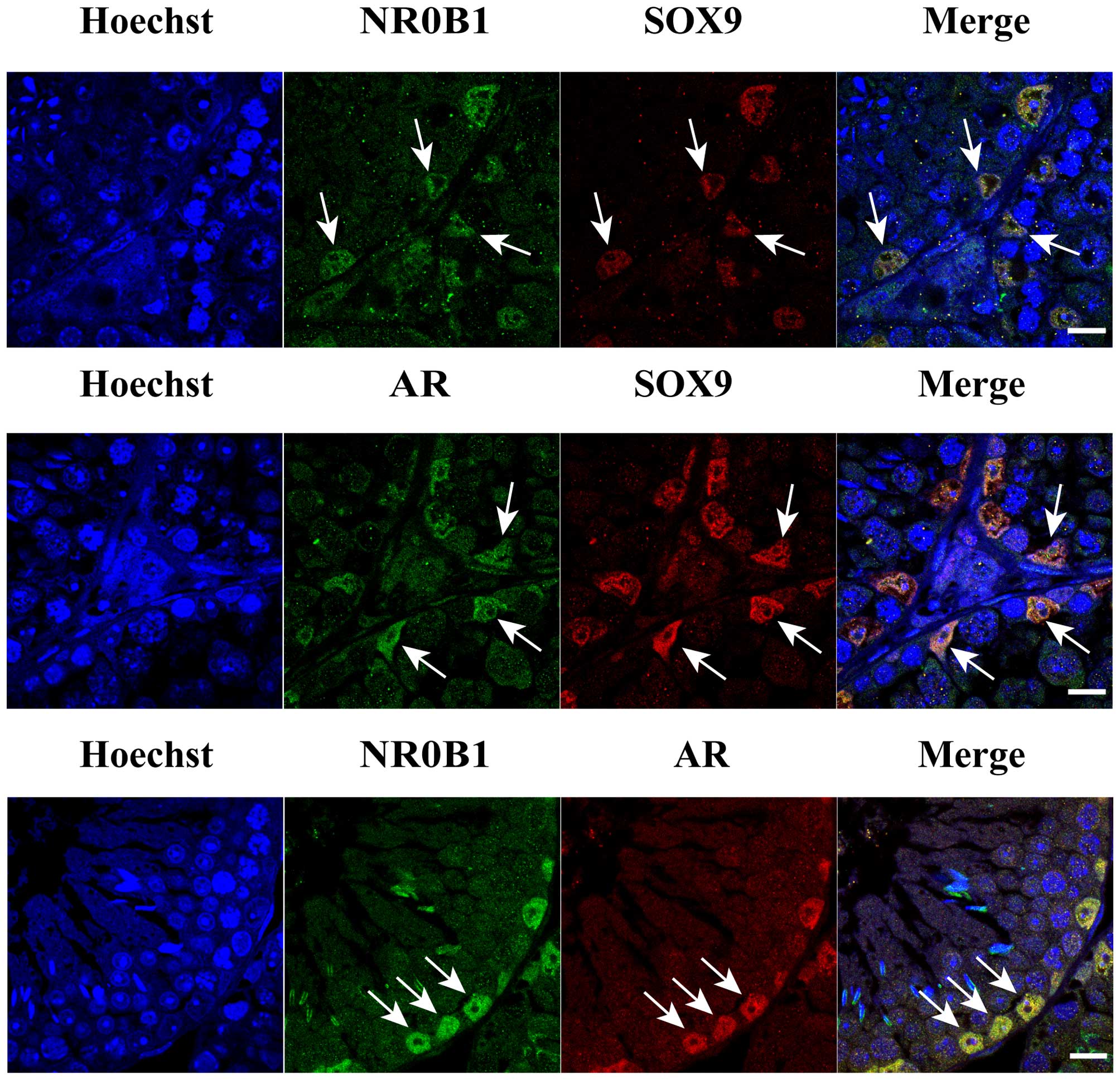
AR function may be inhibited by NR0B1 expression, it was necessary
to determine whether NR0B1 and AR were co-located in mouse SCs, as
the AR is known to be expressed in developing mouse SCs (21–23). We next performed immunostaining of
8-week-old adult mouse testes. We observed that NR0B1 and AR were
co-located in the mouse SCs (Fig.
3), as determined by co-immunostaining with a SC marker,
SOX9.
NR0B1 inhibits the effects of AR on the
expression of AR target genes in an androgen-dependent manner
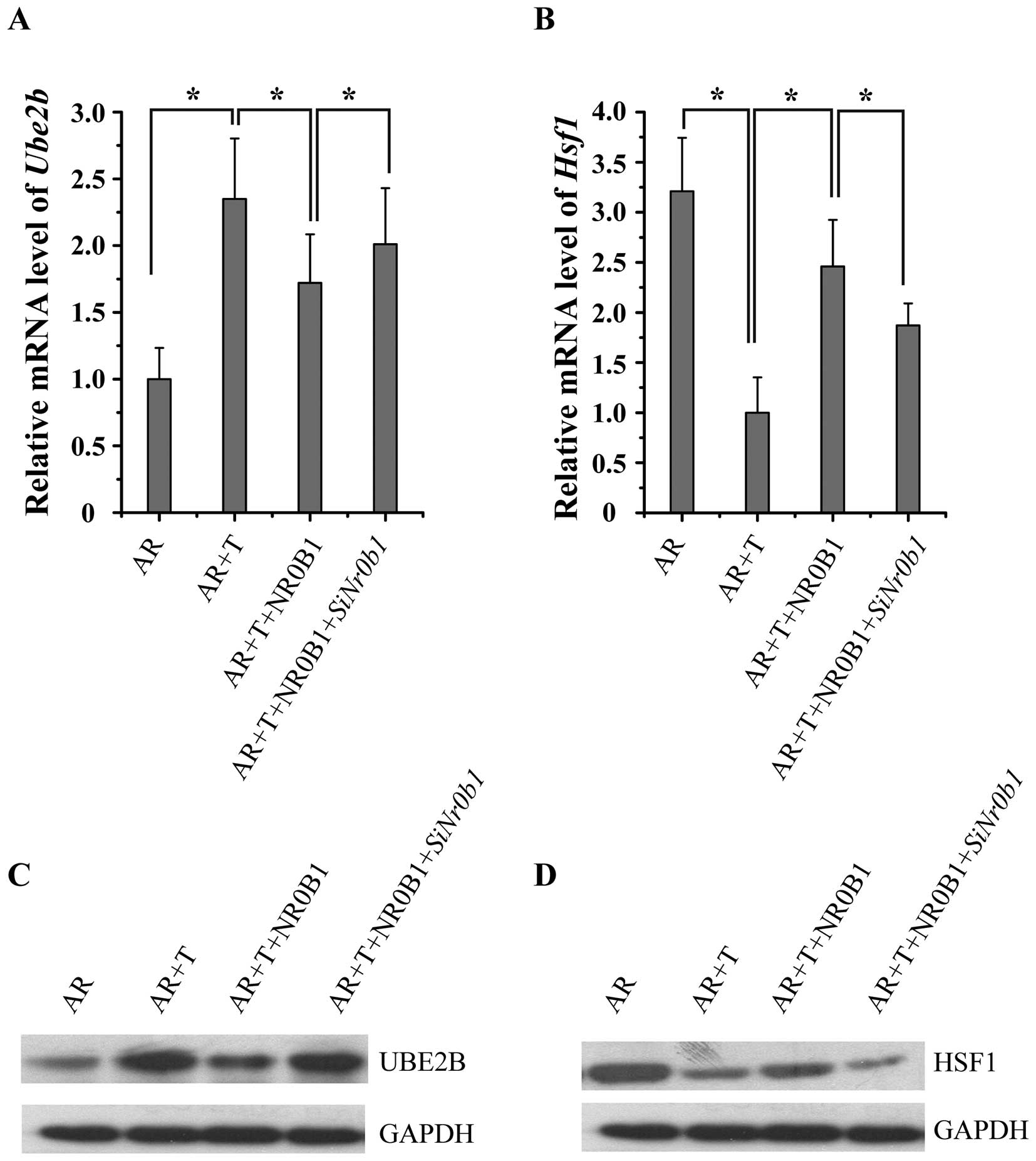
To determine whether NR0B1 is capable of inhibiting
AR target gene expression, NR0B1-pCDNA3.1/HA and
AR-pCDNA3.1+ vectors were transfected into TM4 cells,
following treatment with 10 nM T for 24 h. We determined the
expression of two AR target genes, ubiquitin-conjugating enzyme E2B
(UBE2B) and heat shock transcription factor 1 (HSF1) using RT-qPCR
and western blot analysis. The results showed that the
overexpression of NR0B1 inhibited UBE2B expression and promoted
HSF1 expression, while the knockdown of NR0B1 expression by siNr0b1
exerted opposite effects (Fig.
4), which indicated that NR0B1 reversed the effects of AR on
the expression of its target gene.
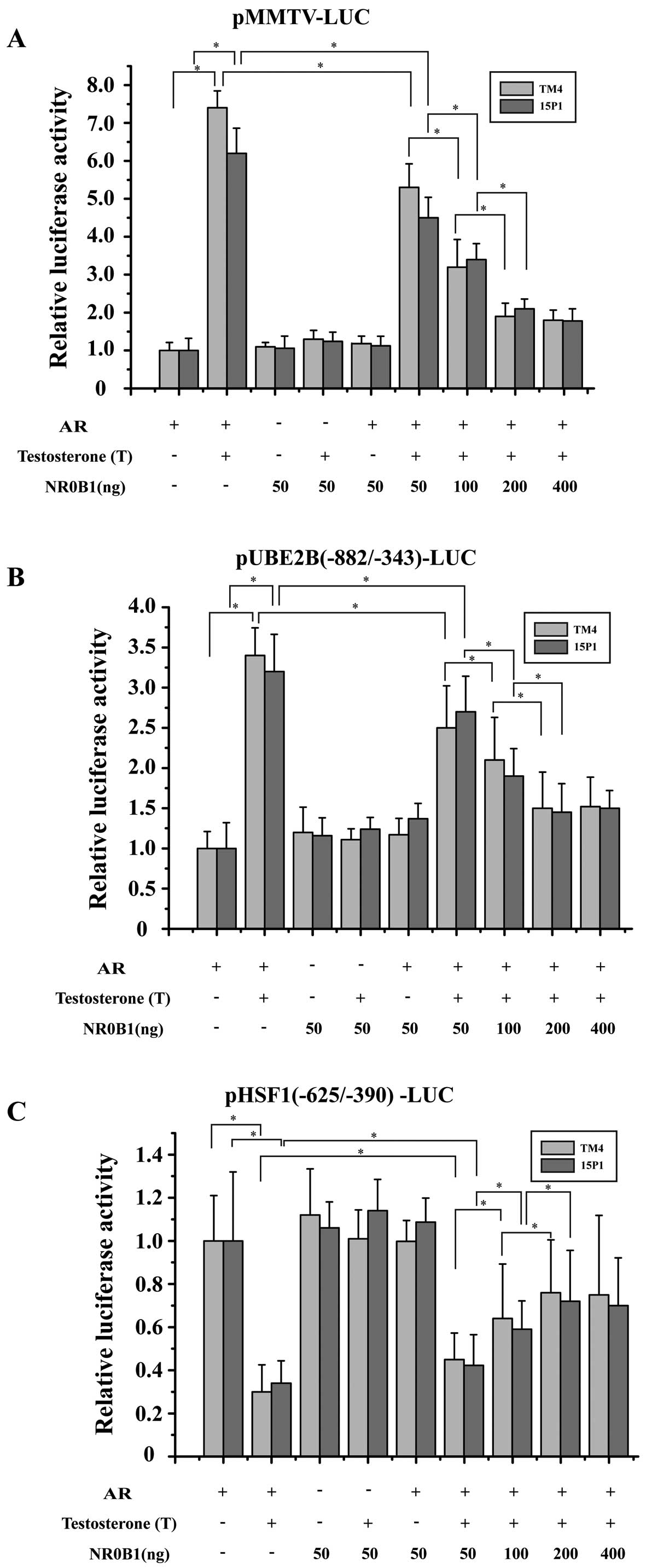
NR0B1 inhibition of transcriptional AR
activation
To determine whether NR0B1 inhibits the
transcriptional activity of AR, we performed transient transfection
studies using mouse SC lines (TM4 and 15P1). We used
androgen-responsive luciferase reporter constructs, namely
pMMTV-LUC containing mouse mammary tumor virus long terminal repeat
(LTR), in which there are AREs in front of a TATA box. In the
absence of NR0B1, AR activated pMMTV-LUC reporter in an
agonist-dependent fashion. The co-expression of increasing amounts
of NR0B1 decreased AR activity in a dose-dependent manner, and 200
ng NR0B1/well typically resulted in up to 70% inhibition of AR
activity (Fig. 5A). Furthermore,
NR0B1 did not directly inhibit the pMMTV-LUC reporter in the
absence of AR. These results indicate that NR0B1 potently inhibited
ligand-dependent transcriptional AR activation.
Transcriptional profiling studies with AR knockout
mouse models searching for androgen-regulated genes relevant to
spermatogenesis have identified many candidate target genes of ARs,
including Rhox5, Gpx5, Lcn5, Tubb3,
Crisp1 and Eppin (24–28). In our previous experiment, we have
identified Ube2b and Hsf1 as two critical target
genes of the AR in mouse SCs. To further demonstrate that NR0B1 may
inhibit the transcriptional activity of AR, we also performed
transient transfection studies using TM4 and 15P1 cell lines. We
used two other androgen-responsive luciferase reporter constructs,
namely pUBE2B(−882/−343)-LUC and pHSF1(−625/−390)-LUC, containing
an ARE in front of a TATA box (29,30). In the absence of NR0B1, AR
activated pUBE2B(−882/−343)-LUC reporter in an agonist-dependent
fashion (Fig. 5B) and activated
pHSF1(−625/−390)-LUC reporter in an antagonist-dependent fashion
(Fig. 5C). The co-expression of
increasing amounts of NR0B1 decreased AR activity in a
dose-dependent manner, and 200 ng of NR0B1/well typically resulted
in up to 55% inhibition of the AR activity (Fig. 5B). Furthermore, NR0B1 did not
directly inhibit pUBE2B(−882/−343)-LUC and pHSF1(−625/−390)-LUC
reporters in the absence of AR. All of these results strongly
suggest that NR0B1 antagonism plays a important role in modulating
AR-dependent gene regulation in mouse SCs.
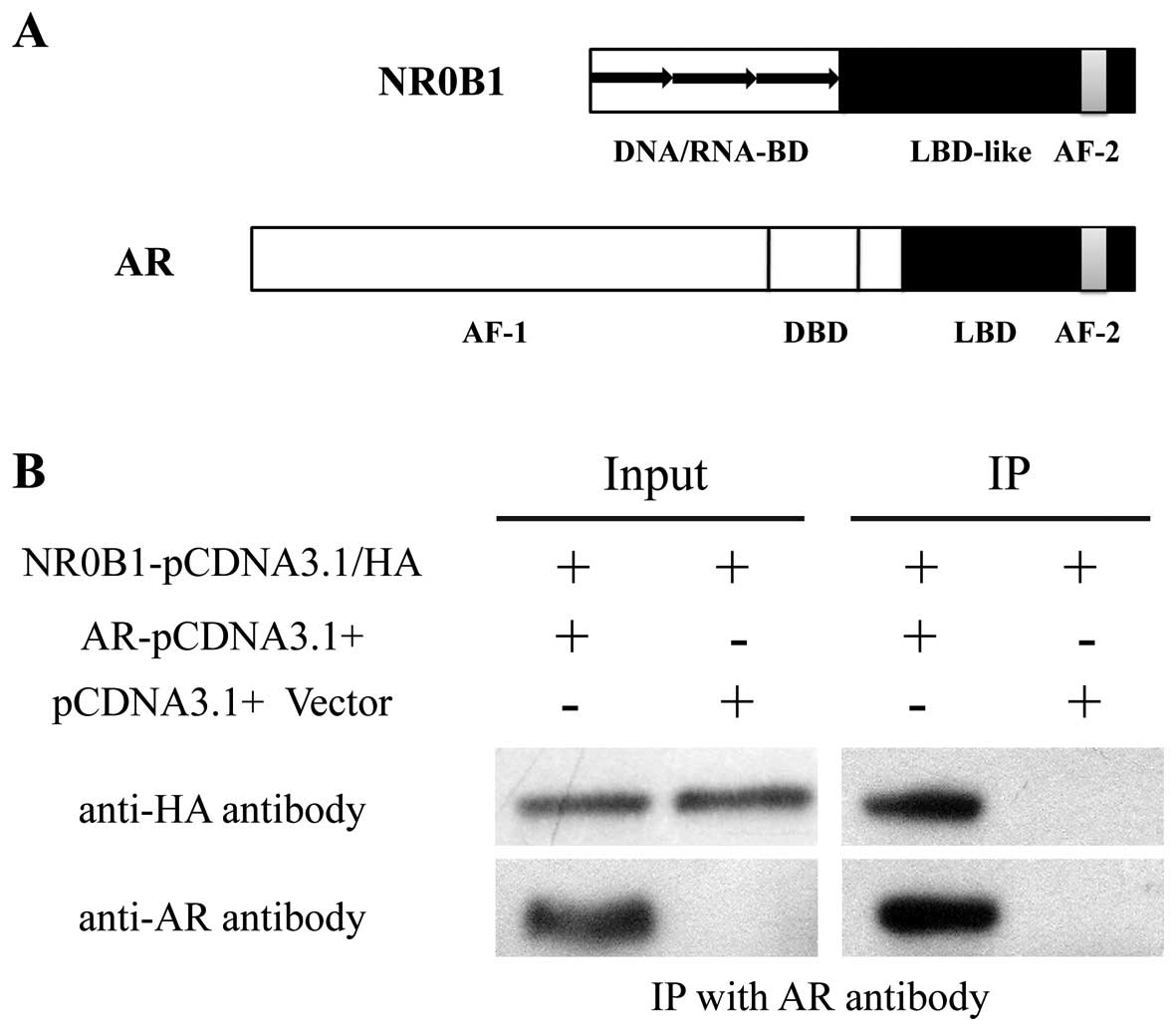
Interaction of NR0B1 and AR in vitro
Nr0b1 has been classified as an orphan member
of the nuclear receptor superfamily (31,32). The NR0B1 domain structure has an
amino-terminal domain (DNA/RNA-BD), a carboxy-terminal domain
(LBD-like) and an AF-2 transactivation domain (Fig. 6A). The amino-terminal domain has a
novel structure consisting of 3.5 alanine/glycine-rich repeats of a
65–70 amino acid motif that has no known homology to any other
proteins, with the exception of the related nuclear receptor
superfamily member, small heterodimer partner (SHP), encoded by
NR0B2 (33). ARs (also known as
dihydrotestosterone receptors) are nuclear hormone receptors of the
NR3C class. The AR domain structure has a AF-1 transactivation
domain, a DBD, a LBD and an AF-2 transactivation domain (Fig. 6A).
In our previous experiment, we found that NR0B1
inhibits the transcriptional activation of AR, and NR0B1 and AR
were co-located in mouse SCs. From these results, we speculated
that NR0B1 interacts with AR and then inhibits its transcriptional
activation. The interaction between NR0B1 and AR was determined
using an in vitro co-immunoprecipitation assay. The AR and
HA-fused NR0B1 vectors were transcribed as described above and
transfected into 293T cells. We found that HA-fused NR0B1 protein
was pulled down by anti-AR antibody, which demonstrated that NR0B1
interacted with AR in mouse SCs (Fig.
6B).
Discussion
Nr0b1 is an atypical member of the nuclear
receptor superfamily that is predominantly expressed in mouse
testes. In a previous study aiming to evaluate the role of
Nr0b1 in mice, Nr0b1 was expressed by the
Müllerian-inhibiting substance promoter (MIS-Nr0b1) in the
genetic background of the Nr0b1KO model (34). The expression of a Nr0b1
trans-gene was sufficient to partially rescue the primary
testicular defect of the male Nr0b1-deficient (KO) mouse.
Even so, the function of Nr0b1 in mouse SCs remains
unclear.
Androgen signaling via the AR may be represented as
a multistep cascade involving the dissociation of cytoplasmic
chaperone/heat shock protein complexes upon ligand-binding, nuclear
localization, DNA binding, and the association of the AR with
various bona fide co-activators, such as histone acetyltransferases
[p160s, cAMP response element binding protein (CREB)-binding
protein (CBP), p300, p300/CBP-associated factor (PCAF),
Tat-interacting protein 60 (TIP60)] and a number of unrelated
proteins, including protein inhibitor of activated STAT (PIAS)
proteins (35), AR-interacting
protein (ARIP)/small nuclear ring finger protein (SNURF)/RNF4s, and
AR-interacting nuclear protein kinase (ANPK) (17). Considerably less is known about
the mechanisms by which androgen-dependent transcription is
inhibited, and candidate co-repressors have only recently been
identified. They include the amino-terminal enhancer of split, a
member of the Groucho/transducin-like enhancer of split family of
co-repressors, that is not associated with histone deacetylases,
but instead functions through direct contact with the basal
transcription factor, TFIIE (36). Other repressors of androgen action
include cyclin D1, which directly antagonizes the
acetyltransferase, PCAF (37);
SMAD3, an intracellular mediator of the TGF-β pathway (38); and the protein kinases, PAK6 and
AKT, which presumably repress AR activity through direct
phosphorylation (39,40). Moreover, a novel covalent
modification of the AR by the attachment of small ubiquitin-related
modifier 1 in certain contexts inhibits the transcriptional
activity of the AR (41).
In this study, we have demonstrated that NR0B1
interacts with AR and inhibits the transcriptional activation of AR
in mouse SCs. The enforced expression of NR0B1 suppresses the
expression of target genes of AR, while the silencing of NR0B1
promotes the expression of these target genes. This is consistent
with our observation that a significant amount of NR0B1
co-localized with AR in the nuclei of mouse SCs. Although the
precise mechanisms responsible for these phenomena remain unclear,
NR0B1 most likely interferes with the events required for AR
activation in mouse SCs. This may include interference with the
association and dissociation of chaperones or interference with
nuclear import by masking the nuclear localization signals of AR.
In support of the general application of this mechanism, there is
preliminary evidence from several studies demonstrating that NR0B1
is also able to repress steroidogenic factor 1 (SF-1) and estrogen
receptor (ER)-mediated transactivation (42–45).
AR function is often altered in humans with
reproductive abnormalities, as well as in prostate cancer, due to
mutations within the LBD (15,46). While some of these mutations have
been demonstrated to affect the ligand-binding capacity and
specificity, others are proposed to affect inter-domain
communication or direct interactions with co-activators (47–49). Similarly, multiple NR0B1
mutations have been detected that primarily target the putative LBD
of NR0B1 (50–52). One of these mutations
(NR0B1 R267P) has been found to be less potent in ER
inhibition (42). However, as
this mutation presumably affects several features of the
NR0B1 LBD, further investigations are required in order to
determine whether mutated NR0B1 displays changes with
respect to intracellular tethering, co-activator competition or
co-repressor recruitment. AR and NR0B1 are the only two
reproductive nuclear receptors in which high numbers of natural
mutations have been detected in human males (53). Notably, the genes for both
AR and NR0B1 are located on the X-chromosome. Thus,
all mutations affecting the function yield a phenotype.
Interactions between NR0B1 and AR may be important for the proper
development of the male reproductive system.
In conclusion, we have identified NR0B1 as a new AR
co-repressor in mouse SCs. We have provided evidence, for the first
time to the best of our knowledge, for the novel roles of NR0B1 as
an inhibitory co-regulator of the AR in mouse SCs. These data
strongly suggest that NR0B1 antagonism plays a important role in
modulating AR-dependent gene regulation in the male reproductive
system.
Acknowledgments
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 31271244 and
31471344) and the Shenzhen Project of Science and Technology (nos.
XB201104220045A and JCYJ20140415162543017). We would also like to
thank Dr Qiaoxia Zhang for kindly supplying the AR overexpression
plasmid, and Dr Lisha Mou, Shenzhen Key Laboratory of Genitourinary
Tumor, Shenzhen Second People's Hospital, First Affiliated Hospital
of Shenzhen University for kindly supplying the reporter plasmids,
pMMTV-LUC, pUBE2B(−882/−343)-LUC and pHSF1(−625/−390)-LUC.
References
|
1
|
Goto M and Katsumata N: X-linked adrenal
hypoplasia congenita caused by a novel intronic mutation of the
DAX-1 gene. Horm Res. 71:120–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ikeda Y, Takeda Y, Shikayama T, Mukai T,
Hisano S and Morohashi KI: Comparative localization of Dax-1 and
Ad4BP/SF-1 during development of the hypothalamic-pituitary-gonadal
axis suggests their closely related and distinct functions. Dev
Dyn. 220:363–376. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bardoni B, Zanaria E, Guioli S, Floridia
G, Worley KC, Tonini G, Ferrante E, Chiumello G, McCabe ER,
Fraccaro M, et al: A dosage sensitive locus at chromosome Xp21 is
involved in male to female sex reversal. Nat Genet. 7:497–501.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Achermann JC, Meeks JJ and Jameson JL:
Phenotypic spectrum of mutations in DAX-1 and SF-1. Mol Cell
Endocrinol. 185:17–25. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu RN, Ito M, Saunders TL, Camper SA and
Jameson JL: Role of Ahch in gonadal development and gametogenesis.
Nat Genet. 20:353–357. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jeffs B, Meeks JJ, Ito M, Martinson FA,
Matzuk MM, Jameson JL and Russell LD: Blockage of the rete testis
and efferent ductules by ectopic Sertoli and Leydig cells causes
infertility in Dax1-deficient male mice. Endocrinology.
142:4486–4495. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heemers HV and Tindall DJ: Androgen
receptor (AR) coregulators: a diversity of functions converging on
and regulating the AR transcriptional complex. Endocr Rev.
28:778–808. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heinlein CA and Chang C: Androgen receptor
(AR) coregulators: an overview. Endocr Rev. 23:175–200. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patrão MT, Silva EJ and Avellar MC:
Androgens and the male reproductive tract: an overview of classical
roles and current perspectives. Arq Bras Endocrinol Metabol.
53:934–945. 2009. View Article : Google Scholar
|
|
10
|
De Gendt K, Swinnen JV, Saunders PT,
Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens
F, Lécureuil C, et al: A Sertoli cell-selective knockout of the
androgen receptor causes spermatogenic arrest in meiosis. Proc Natl
Acad Sci USA. 101:1327–1332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang RS, Yeh S, Tzeng CR and Chang C:
Androgen receptor roles in spermatogenesis and fertility: lessons
from testicular cell-specific androgen receptor knockout mice.
Endocr Rev. 30:119–132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H,
Huang KE, Lin H, Yeh SD, Altuwaijri S, Zhou X, et al: Generation
and characterization of androgen receptor knockout (ARKO) mice: an
in vivo model for the study of androgen functions in selective
tissues. Proc Natl Acad Sci USA. 99:13498–13503. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Quigley CA, De Bellis A, Marschke KB,
el-Awady MK, Wilson EM and French FS: Androgen receptor defects:
historical, clinical, and molecular perspectives. Endocr Rev.
16:271–321. 1995.PubMed/NCBI
|
|
14
|
Yong EL, Ghadessy F, Wang Q, Mifsud A and
Ng SC: Androgen receptor transactivation domain and control of
spermatogenesis. Rev Reprod. 3:141–144. 1998. View Article : Google Scholar
|
|
15
|
Culig Z, Hobisch A, Bartsch G and Klocker
H: Androgen receptor - an update of mechanisms of action in
prostate cancer. Urol Res. 28:211–219. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hughes IA: Minireview: sex
differentiation. Endocrinology. 142:3281–3287. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jänne OA, Moilanen AM, Poukka H, Rouleau
N, Karvonen U, Kotaja N, Häkli M and Palvimo JJ:
Androgen-receptor-interacting nuclear proteins. Biochem Soc Trans.
28:401–405. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meeks JJ, Crawford SE, Russell TA,
Morohashi K, Weiss J and Jameson JL: Dax1 regulates testis cord
organization during gonadal differentiation. Development.
130:1029–1036. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hanley NA, Hagan DM, Clement-Jones M, Ball
SG, Strachan T, Salas-Cortés L, McElreavey K, Lindsay S, Robson S,
Bullen P, et al: SRY, SOX9, and DAX1 expression patterns during
human sex determination and gonadal development. Mech Dev.
91:403–407. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Münsterberg A and Lovell-Badge R:
Expression of the mouse anti-müllerian hormone gene suggests a role
in both male and female sexual differentiation. Development.
113:613–624. 1991.
|
|
22
|
Kent J, Wheatley SC, Andrews JE, Sinclair
AH and Koopman P: A male-specific role for SOX9 in vertebrate sex
determination. Development. 122:2813–2822. 1996.PubMed/NCBI
|
|
23
|
Morais da Silva S, Hacker A, Harley V,
Goodfellow P, Swain A and Lovell-Badge R: Sox9 expression during
gonadal development implies a conserved role for the gene in testis
differentiation in mammals and birds. Nat Genet. 14:62–68. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Denolet E, De Gendt K, Allemeersch J,
Engelen K, Marchal K, Van Hummelen P, Tan KA, Sharpe RM, Saunders
PT, Swinnen JV and Verhoeven G: The effect of a Sertoli
cell-selective knockout of the androgen receptor on testicular gene
expression in prepubertal mice. Mol Endocrinol. 20:321–334. 2006.
View Article : Google Scholar
|
|
25
|
De Gendt K, Denolet E, Willems A, Daniels
VW, Clinckemalie L, Denayer S, Wilkinson MF, Claessens F, Swinnen
JV and Verhoeven G: Expression of Tubb3, a beta-tubulin isotype, is
regulated by androgens in mouse and rat Sertoli cells. Biol Reprod.
85:934–945. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou W, Wang G, Small CL, Liu Z, Weng CC,
Yang L, Griswold MD and Meistrich ML: Gene expression alterations
by conditional knockout of androgen receptor in adult Sertoli cells
of Utp14b jsd/jsd (jsd) mice. Biol Reprod. 84:400–408. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang QX, Zhang XY, Zhang ZM, Lu W, Liu L,
Li G, Cai ZM, Gui YT and Chang C: Identification of
testosterone-/androgen receptor-regulated genes in mouse Sertoli
cells. Asian J Androl. 14:294–300. 2012. View Article : Google Scholar
|
|
28
|
Eacker SM, Shima JE, Connolly CM, Sharma
M, Holdcraft RW, Griswold MD and Braun RE: Transcriptional
profiling of androgen receptor (AR) mutants suggests instructive
and permissive roles of AR signaling in germ cell development. Mol
Endocrinol. 21:895–907. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang L, Wang Y, Zhang Q, Lai Y, Li C,
Zhang Q, Huang W, Duan Y, Jiang Z, Li X, et al: Identification of
Hsf1 as a novel androgen receptor-regulated gene in mouse Sertoli
cells. Mol Reprod Dev. 81:514–523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mou L, Zhang Q, Wang Y, Zhang Q, Sun L, Li
C, Huang W, Yuan Y, Duan Y, Diao R, et al: Identification of Ube2b
as a novel target of androgen receptor in mouse sertoli cells. Biol
Reprod. 89:322013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Burris TP, Guo W and McCabe ER: The gene
responsible for adrenal hypoplasia congenita, DAX-1, encodes a
nuclear hormone receptor that defines a new class within the
superfamily. Recent Prog Horm Res. 51:241–260. 1996.PubMed/NCBI
|
|
32
|
Giguère V: Orphan nuclear receptors: from
gene to function. Endocr Rev. 20:689–725. 1999.PubMed/NCBI
|
|
33
|
Iyer AK and McCabe ER: Molecular
mechanisms of DAX1 action. Mol Genet Metab. 83:60–73. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jeffs B, Ito M, Yu RN, Martinson FA, Wang
ZJ, Doglio LT and Jameson JL: Sertoli cell-specific rescue of
fertility, but not testicular pathology, in Dax1 (Ahch)-deficient
male mice. Endocrinology. 142:2481–2488. 2001.PubMed/NCBI
|
|
35
|
Kotaja N, Vihinen M, Palvimo JJ and Jänne
OA: Androgen receptor-interacting protein 3 and other PIAS proteins
cooperate with glucocorticoid receptor-interacting protein 1 in
steroid receptor-dependent signaling. J Biol Chem. 277:17781–17788.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yu X, Li P, Roeder RG and Wang Z:
Inhibition of androgen receptor-mediated transcription by
amino-terminal enhancer of split. Mol Cell Biol. 21:4614–4625.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Reutens AT, Fu M, Wang C, Albanese C,
McPhaul MJ, Sun Z, Balk SP, Jänne OA, Palvimo JJ and Pestell RG:
Cyclin D1 binds the androgen receptor and regulates
hormone-dependent signaling in a p300/CBP-associated factor
(P/CAF)-dependent manner. Mol Endocrinol. 15:797–811. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hayes SA, Zarnegar M, Sharma M, Yang F,
Peehl DM, ten Dijke P and Sun Z: SMAD3 represses androgen
receptor-mediated transcription. Cancer Res. 61:2112–2118.
2001.PubMed/NCBI
|
|
39
|
Lin HK, Yeh S, Kang HY and Chang C: Akt
suppresses androgen-induced apoptosis by phosphorylating and
inhibiting androgen receptor. Proc Natl Acad Sci USA. 98:7200–7205.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang F, Li X, Sharma M, Zarnegar M, Lim B
and Sun Z: Androgen receptor specifically interacts with a novel
p21-activated kinase, PAK6. J Biol Chem. 276:15345–15353. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Poukka H, Karvonen U, Janne OA and Palvimo
JJ: Covalent modification of the androgen receptor by small
ubiquitin-like modifier 1 (SUMO-1). Proc Natl Acad Sci USA.
97:14145–14150. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang H, Thomsen JS, Johansson L,
Gustafsson JA and Treuter E: DAX-1 functions as an LXXLL-containing
corepressor for activated estrogen receptors. J Biol Chem.
275:39855–39859. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ito M, Yu R and Jameson JL: DAX-1 inhibits
SF-1-mediated transactivation via a carboxy-terminal domain that is
deleted in adrenal hypoplasia congenita. Mol Cell Biol.
17:1476–1483. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Altincicek B, Tenbaum SP, Dressel U,
Thormeyer D, Renkawitz R and Baniahmad A: Interaction of the
corepressor Alien with DAX-1 is abrogated by mutations of DAX-1
involved in adrenal hypoplasia congenita. J Biol Chem.
275:7662–7667. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Crawford PA, Dorn C, Sadovsky Y and
Milbrandt J: Nuclear receptor DAX-1 recruits nuclear receptor
corepressor N-CoR to steroidogenic factor 1. Mol Cell Biol.
18:2949–2956. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yong EL, Lim J, Qi W, Ong V and Mifsud A:
Molecular basis of androgen receptor diseases. Ann Med. 32:15–22.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Thompson J, Saatcioglu F, Jänne OA and
Palvimo JJ: Disrupted amino- and carboxyl-terminal interactions of
the androgen receptor are linked to androgen insensitivity. Mol
Endocrinol. 15:923–935. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Beilin J, Ball EM, Favaloro JM and Zajac
JD: Effect of the androgen receptor CAG repeat polymorphism on
transcriptional activity: Specificity in prostate and non-prostate
cell lines. J Mol Endocrinol. 25:85–96. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Slagsvold T, Kraus I, Bentzen T, Palvimo J
and Saatcioglu F: Mutational analysis of the androgen receptor AF-2
(activation function 2) core domain reveals functional and
mechanistic differences of conserved residues compared with other
nuclear receptors. Mol Endocrinol. 14:1603–1617. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Reutens AT, Achermann JC, Ito M, Ito M, Gu
WX, Habiby RL, Donohoue PA, Pang S, Hindmarsh PC and Jameson JL:
Clinical and functional effects of mutations in the DAX-1 gene in
patients with adrenal hypoplasia congenita. J Clin Endocrinol
Metab. 84:504–511. 1999.PubMed/NCBI
|
|
51
|
Muscatelli F, Strom TM, Walker AP, Zanaria
E, Récan D, Meindl A, Bardoni B, Guioli S, Zehetner G, Rabl W, et
al: Mutations in the DAX-1 gene give rise to both X-linked adrenal
hypoplasia congenita and hypogonadotropic hypogonadism. Nature.
372:672–676. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lalli E, Bardoni B, Zazopoulos E, Wurtz
JM, Strom TM, Moras D and Sassone-Corsi P: A transcriptional
silencing domain in DAX-1 whose mutation causes adrenal hypoplasia
congenita. Mol Endocrinol. 11:1950–1960. 1997. View Article : Google Scholar
|
|
53
|
Achermann JC and Jameson JL: Fertility and
infertility: genetic contributions from the
hypothalamic-pituitary-gonadal axis. Mol Endocrinol. 13:812–818.
1999. View Article : Google Scholar : PubMed/NCBI
|